Abstract
The aim of this study was to investigate olanzapine (OZ) systemic absolute bioavailability after intranasal (i.n.) administration in vivo to conscious rabbits. Furthermore, the study investigated the potential use of chitosan nanoparticles as a delivery system to enhance the systemic bioavailability of olanzapine following intranasal administration. Olanzapine-loaded chitosan nanoparticles were prepared through ionotropic gelation of chitosan with tripolyphosphate anions and studied in terms of their size, drug loading, and in vitro release. The OZ nanoparticles were administered i.n. to rabbits, and OZ plasma concentration at predetermined time points was compared to i.n. administration of OZ in solution. The concentrations of OZ in plasma were analyzed by ultra performance liquid chromatography mass spectroscopy (UPLC/MS). OZ-loaded chitosan nanoparticles significantly (p < 0.05) enhanced systemic absorption with 51 ± 11.2% absolute bioavailability as compared to 28 ± 6.7% after i.n. administration of OZ solution. The results of the present study suggest that intranasal administration of OZ-loaded chitosan nanoparticles formulation could be an attractive modality for delivery of OZ systemically.
KEY WORDS: bioavailability, intranasal, nanoparticles, olanzapine, pharmacokinetic
INTRODUCTION
Olanzapine (OZ) (2-methyl-4-(4-methylpiperazin-1-yl)-5H-thieno [3,2-c] [1,5] benzodiazepine) (Fig. 1) is an atypical antipsychotic drug used for treatment of schizophrenia and bipolar I disorder (1,2). Olanzapine is presently available in oral tablet form, which demonstrated extensive first-pass metabolism with around 40% of the drug metabolized before reaching systemic circulation. To overcome the bioavailability issues, orally disintegrating tablets and intramuscular injections, which have a more rapid rate of absorption, are also available (3). Orally disintegrating tablets are easier for patients to take and have more rapid absorption than traditional tablets; however, orally disintegrating tablets were shown to give similar bioavailability to standard tablets (4,5).
Fig. 1.
Chemical structure of olanzapine
Many schizophrenia patients struggle with medication compliance due to the nature of their symptoms (6). Some patients may be in denial of illness while others suffer delusions that lead to noncompliance (7,8). Highly agitated, noncompliant patients require dosage methods that are easily controlled by their physician and family (9). Furthermore, difficult patients may reject oral medications; alternatively, they could be dosed by intramuscular injections. However, the latter could require health-care personnel for administration, and depot injections often used with noncompliant patients cause pain that could lead to medication refusal (10). Thus, the intranasal dosage forms could be an attractive drug delivery modality and could meet that necessity for an easy to administer dosage form. Dose-related side effects can be minimized by utilizing an alternative dosing route with increased bioavailability. It was reported that some antipsychotics, including OZ, could increase weight gain with higher doses (11,12) that could further lead to noncompliance (13). In addition to weight gain, preliminary evidence indicates a dose-dependent relationship between OZ and metabolic issues such as diabetes (14–16).
With those considerations, it appears there is a need for an alternative route of administration for OZ to overcome the low bioavailability and the side effects. Intranasal drug delivery through the nasal mucosa is a useful method to avoid hepatic first-pass metabolism (17–19). Furthermore, intranasal administration offers a practical, noninvasive, alternative route of administration that could improve drug bioavailability resulting in a better safety profile by reducing dose-related side effects.
The aim of this study was to investigate the nasal drug delivery of OZ solution and the potential of chitosan nanoparticles as a delivery system to enhance the systemic absorption of OZ following intranasal administration in conscious rabbits. Chitosan is a mucoadhesive cationic polysaccharide that is produced from partially acetylated chitin (20). To maximize drug permeation through the nasal mucosa, it is critical for intranasal drug delivery systems to have enhanced nasal absorption (21). Chitosan has been shown to increase nasal absorption due to its properties of mucoadhesion and ability to increase membrane permeability (22–25). To our knowledge, this is one of the first reported studies to investigate the nasal absorption of a psychotic drug such as OZ using mucoadhesive polymer-based nanotechnology in a conscious, nonanesthetized rabbit animal model.
MATERIALS AND METHODS
Materials
Olanzapine, chitosan (low-molecular-weight 40,000 Da), and pentasodium tripolyphosphate (TPP) were purchased from Sigma-Aldrich (MO, USA). All other chemicals and HPLC grade solvents were obtained from Fisher Scientific (NJ, USA). Catheters were purchased from J&J Medical (NJ, USA); injection adaptors were purchased from Medex (OH, USA); plasma tubes with EDTA were purchased from Tyco Healthcare Group (MA, USA); saline was purchased from Baxter Healthcare Corporation (IL, USA); microcentrifuge tubes, vials, and tips were purchased from Fisher Scientific (NJ, USA); and AErrane™ Isoflurane was provided by VMC Anesthesia Ohmeda (WI, USA).
Preparation of Chitosan Nanoparticles
Chitosan nanoparticles (CS NP) were prepared based on the ionotropic gelation of chitosan with tripolyphosphate (TPP) anions. Chitosan (0.20% w/v) was dissolved in 1% (v/v) acetic acid, and then, the pH was raised to 4.7 with 10 N NaOH. Nanoparticles were obtained upon the addition of a TPP aqueous solution (0.5 mL, 0.2% w/v in 0.1 N NaOH) to chitosan solution (2 mL, 0.2% w/v) under magnetic stirring at room temperature. For the association of OZ to chitosan nanoparticles, OZ was first incubated for 20–40 s in the CS solution. The TPP solution was then added into the chitosan and OZ mixture. Nanoparticles loaded with OZ were obtained as described above. The concentration of OZ in the TPP solution was adjusted in order to obtain chitosan nanoparticles containing 20 and 60% (w/w) of OZ. Nanoparticles were concentrated by centrifugation at 8,000×g for 30 min in centrifuge tubes with a 10-μL glycerol bed in the bottom of the tube, which aids in the prevention of nanoparticles aggregation. Supernatant was discarded and the nanoparticles were re-suspended in phosphate buffer (pH 6.4) for further testing.
Ultra Performance Liquid Chromatography Mass Spectroscopy Assay
Chromatography was performed on a Waters Acquity UPLC® BEH Shield (2.1 mm × 100 mm, 1.7 μm) RP18 column with a mobile phase consisting of solvent A (acetonitrile) and solvent B (0.1% formic acid) with gradient elution program. The flow-rate was set at 0.1 mL/min. Selected ion monitoring (SIM) was performed in the positive mode for OZ and mirtazapine internal standard (IS) at M+ = 313 and 266 m/z, respectively. The capillary voltage and cone voltage were maintained at 0.3 kV and 34 V, respectively. Nitrogen was used as both the cone gas and the desolvation gas (500 L/h). Mass chromatograms and mass spectral data were acquired and processed by MassLynx software (Waters).
Characterizations of OZ-Loaded Chitosan Nanoparticles
The morphology and size distribution of the OZ-loaded chitosan nanoparticles were observed using Philips Tecnai 12 Biotwin microscope with an accelerating voltage of 100 kV at the University of Kentucky Medical Center Imaging Facility. A dried nanoparticle sample was dispersed directly into distilled water, and then, a copper grid coated with a carbon film was put into the previous suspension several times and left to incubate for 2.0 min at room temperature. After drying and removal of excess fluid, the samples were negatively stained with 2% uranyl acetate, and the grids were examined and recorded with the transmission electron microscope (TEM) images. The amount of entrapped OZ in nanoparticles was detected in triplicate by ultra performance liquid chromatography mass spectroscopy (UPLC/MS) analysis. The nanoparticles were separated from the aqueous medium by ultracentrifugation at 18,000×g for 30 min. The OZ concentration was analyzed by UPLC/MS as described above. The amount of OZ loaded into the nanoparticles was calculated as the difference between the total amount used to prepare the nanoparticles and the amount that was found in the supernatant. The encapsulation efficiency (EE) of OZ in nanoparticles was determined as the mass ratio of the entrapped OZ in nanoparticles to the theoretical amount of OZ used in the preparation.
In Vitro Release of OZ from OZ-CS Nanoparticles
Aliquots of the nanoparticle suspension containing 1 mg of OZ were diluted in 10 mL of purified water and incubated, under agitation, at 37°C, in order to assess sink conditions during the release studies. At different time intervals, samples were centrifuged (18,000×g for 30 min) and the released OZ was determined by UPLC/MS, as described above.
In Vivo Nasal Bioavailability Studies
Male New Zealand albino rabbits weighing between 4.0 and 4.5 kg (Myrtle’s Rabbitry Inc., Thompson Station, TN) were used. All research and testing activities related to this work were reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) prior to the initiation of this research and during its execution. A comparative determination of the blood levels of OZ delivered via both intravenous (i.v.) and intranasal (i.n.) administration was carried out in a random cross-over design. Two weeks were allowed between treatments. Prior to intranasal administration, each spray device was primed by activating the pump ten times. The nasal formulations were administered by pumping a single spray into each nostril while the rabbits head was held in an upright position, such that a total dose of 1 mg/kg was administered. In order to determine the absolute bioavailability of the nasal dose, rabbits received 0.5 mg/kg OZ intravenously. Animals were conscious throughout the duration of the experiment and were held in rabbit restrainers during blood sampling. Blood samples were collected at predetermined time points. Plasma (200 mL) was separated by centrifugation at 3,000 rpm for 10 min and was frozen at −20°C in polypropylene tubes until the time of analysis.
Preparation of Samples
Plasma samples (90 μL) were spiked with 10 μL mirtazapine IS and then vortexed for 2 min. The extraction was carried out using 200 μL of methylene chloride. The samples were vortexed for another 2 min, then centrifuged for 15 min at 10,000 rpm. The bottom organic layer was collected carefully and injected into LCMS.
Statistical Analysis
Pairs of groups were compared by Student’s t test. Differences between groups were considered significant at p < 0.05. Values for all measurements are expressed as means ± SD.
RESULTS
Nanoparticles were prepared based on ionotropic gelation process with 20 or 60% loading. Characterization for particle size, loading, and encapsulation efficiency is illustrated in Table I. Actual loading of OZ in the nanoparticles was similar to the prepared theoretical loading of 20 and 60% (17.2 and 52.3%, respectively) with an encapsulation efficiency of nearly 90%. Average particle size ranged from 179 to 237 nm (20% loading) and 304 to 340 nm (60% loading). The lower size nanoparticles with the 20% loading were further used for in vivo testing.
Table I.
Physicochemical Properties of Chitosan Nanoparticles Prepared with Different Amounts of OZ
| OZ theoretical loading in CS NPs | Particle size (nm) | Loading (%) | Encapsulation efficiency (%) |
|---|---|---|---|
| 20% | 208 ± 29 | 17.2 ± 1.4 | 86.7 ± 7.1 |
| 60% | 322 ± 18 | 52.3 ± 3.1 | 87.6 ± 5.2 |
OZ olanzapine, CS chitosan, NP nanoparticle
The in vitro release profile of OZ chitosan nanoparticles under sink conditions is shown in Fig. 2. Initial release of OZ from nanoparticles occurred rapidly, with more than 80% released during the first 30 min, followed by the remaining drug releasing slowly for the duration of the experiment.
Fig. 2.
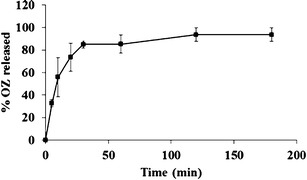
In vitro release profile of OZ from the OZ-loaded CS nanoparticles in dissolution medium
OZ was administered intravenously at 0.5 mg/kg while intranasal solution and chitosan nanoparticles were administered at 1.0 mg/kg. Blood plasma concentration of OZ versus time following i.v. and i.n. administration of solution and OZ-loaded nanoparticles is shown in Fig. 3. At 5 min following i.v. injection, OZ attained a high concentration of 364.22 ng/mL ± 163.19, declined quickly, and then decline slowed. Initial blood plasma concentrations of both i.n. formulations were significantly lower than those after i.v (p < 0.05). Using the AUC from blood plasma concentrations, absolute bioavailability was calculated. Absolute bioavailability of OZ i.n. solution when compared to i.v. administration was equal to 28 ± 6.7% (Table II), and the absolute bioavailability of i.n. OZ CP-NPs was 51 ± 11.2%.
Fig. 3.
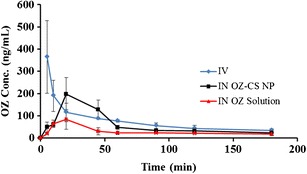
Plasma concentration versus time profile following 0.5 mg/kg intravenous and nasal administration of OZ solution and OZ loaded in chitosan nanoparticles at 1 mg/kg dose (n = 4)
Table II.
Dose-Normalized Area Under the Curve (AUC) and Absolute Bioavailability of OZ Formulations in Rabbit (n = 4)
| Route | Dose (mg/kg) | AUC0min-∞ (ng min kg/mL/mg) | Absolute bioavailability (%) |
|---|---|---|---|
| Mean ± SD | Mean ± SD | ||
| Intravenous solution | 0.5 | 38,862 | 100 |
| Intranasal solution | 1.0 | 10,636 | 28 ± 6.7 |
| Intranasal nanoparticles | 1.0 | 19,937 | 51 ± 11.2 |
AUC area under the curve, SD standard deviation
The pharmacokinetic parameters following i.v. and i.n. administration of OZ are shown in Table III. Both intranasal chitosan nanoparticles and intranasal solution achieved Tmax at 20 min with Cmax at 197.0 ± 75.19 and 84.04 ± 44.38 ng/mL, respectively. The i.v. solution had a Tmax of 5 min and Cmax of 364.22 ± 163.19 ng/mL. The half-life (T1/2) of OZ in i.v. solution, i.n. nanoparticles, and i.n. solution was 101.46 ± 33.07, 109.00 ± 20.20, and 160.53 ± 51.96 min, respectively. K elimination (Ke) was similar for the different preparations with i.n. solution (0.005) differing slightly from that of i.v. solution and i.n. nanoparticles (0.007 for both).
Table III.
Pharmacokinetic Parameters Following Intravenous and Intranasal Administration of OZ Formulations to Rabbits (n = 3)
| Parameter | i.v. solution | i.n. OZ-CS NP | i.n. OZ solution |
|---|---|---|---|
| K e(min−1) | 0.007 ± 0.002 | 0.007 ± 0.001 | 0.005 ± 0.002 |
| C max (ng/mL) | 364.22 ± 163.19 | 197.00 ± 75.19 | 84.04 ± 44.38 |
| T max (min) | 5 | 20 | 20 |
| T 1/2 (min) | 101.46 ± 33.07 | 109.0 ± 20.02 | 160.53.0 ± 51.96 |
K e K elimination, T 1/2 half-life, T max peak time, C max peak concentration, i.v. intravenous, i.n. intranasal, OZ-CS olanzapine-chitosan, NP nanoparticle
DISCUSSION
OZ was prepared as chitosan nanoparticles based on ionotropic gelation of chitosan with TPP anions. Chitosan has been shown as an effective delivery system (26,27), and therefore makes a promising carrier for OZ nanoparticles. To avoid irritation with the nasal formulations, solutions were kept within the pH range of nasal mucosa (pH 5.5–6.5) (28).
The characterization of the chitosan nanoparticles demonstrated actual loading of the nanoparticles to be comparable to the theoretical loading with encapsulation efficiency roughly around 85% at both loading values. Additionally, increased loading of OZ in nanoparticles produced a statistically significant (p < 0.05) increased particle size. An increased ratio between charges has been shown to increase nanoparticle size (29). Increased nanoparticle loading from 20 to 60% alters the charge ratio between the mixture of chitosan and OZ, which is considered a weak base (30), and TPP polyanion to favor the electrostatic interaction between the oppositely charged components.
The initial rapid in vitro dissolution of OZ chitosan nanoparticles under sink conditions indicates that, due to the hydrophilic nature of chitosan, the aqueous dissolution medium permeated the nanoparticles and dissolved the trapped OZ. Chitosan has been demonstrated to improve dissolution of poorly soluble drugs (31,32). Therefore, it could be proposed that the major factor governing OZ release is the chitosan polymer dissolution rate in the release medium.
Chitosan nanoparticles significantly enhanced in vivo OZ systemic absorption after i.n. administration (absolute bioavailability = 51 ± 11.2) when compared to i.v. dosing of OZ solution. Chitosan nanoparticles also increased bioavailability over intranasal OZ solution (p < 0.05). These results clearly evidence the ability of chitosan nanoparticles to enhance the nasal absorption of OZ. Kumar (33) found that OZ nanoparticles prepared with chitosan administered to rats produced a higher concentration in blood than other OZ i.n. preparations. Chitosan nanoparticles likely improve absorption through increased surface area, the demonstrated ability to adhere to mucosal tissues (34) and the capacity to transiently open the tight junctions (35).
To investigate the potential of using chitosan nanoparticles administered intranasally, the pharmacokinetics of OZ was compared after i.n. and i.v. administration to rabbits. Absorption of OZ prepared with chitosan nanoparticles was significantly higher than that of i.n. OZ in solution (p < 0.05), though both i.n. chitosan nanoparticles and i.n. solution were absorbed equally rapidly. Rapid absorption through the nasal mucosa would be beneficial when attempting to quickly dose an uncooperative patient while avoiding injections. Some clinical trials have indicated rapid absorption of OZ administered sublingually (36), suggesting OZ to be a good candidate for transmucosal drug delivery. Thus, correlating well with the rapid absorption through nasal mucosa demonstrated in the current study.
The use of anesthesia during intranasal administration can affect absorption (37). Some of the studies investigating intranasal administration of OZ used conscious rats (38,39), though one did not (33). Each of these studies used rats as a model, but rats are a difficult subject for conscious administration due to their small nasal capacity and body size. However, rabbits may be a better candidate for intranasal drug delivery studies. Rabbit nostrils can hold a larger i.n. dose, and tests with i.n. insulin showed rabbits to have similar blood level profiles to humans (40). Also, the nasal cavity of rabbits was reported to be similar to that of humans (41). Although animal experiments utilizing conscious animals would reflect more active mucociliary clearance conditions resulting in lower absolute bioavailability, this is more likely to reflect the real clinical conditions than data collected from animals under anesthesia.
Finally, due to the sensitivity of nasal mucosa, nasal dosage of OZ should not be considered for chronic dosing and might be considered after further clinical evaluation for adjunct therapy with other delivery systems when needed to support a missed dose or patient compliance cases.
CONCLUSION
The results of the present study indicate that OZ administered via intranasal chitosan nanoparticles has potential to improve the efficacy of systemic absorption. Chitosan nanoparticles enhanced absorption and could offer an efficient method of administration in noncompliant patients. Thus, the safety and clinical benefits of this method of administration should be investigated further.
REFERENCES
- 1.Tollefson GD, Taylor CC. Olanzapine: preclinical and clinical profiles of a novel antipsychotic agent. CNS Drug Rev. 2000;6:303–63. doi: 10.1111/j.1527-3458.2000.tb00155.x. [DOI] [Google Scholar]
- 2.Bhana N, Perry CM. Olanzapine—a review of its use in the treatment of bipolar I disorder. CNS Drugs. 2001;15:871–904. doi: 10.2165/00023210-200115110-00005. [DOI] [PubMed] [Google Scholar]
- 3.Keck PE, McElroy SL. Clinical pharmacodynamics and pharmacokinetics of antimanic and mood-stabilizing medications. J Clin Psychiatry. 2002;63:3–11. [PubMed] [Google Scholar]
- 4.Montgomery W, Treuer T, Karagianis J, Ascher-Svanum H, Harrison G. Orally disintegrating olanzapine review: effectiveness, patient preference, adherence, and other properties. Patient Prefer Adherence. 2012;6:109–25. doi: 10.2147/PPA.S27344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seager H. Drug-delivery products and the Zydis fast-dissolving dosage form. J Pharm Pharmacol. 1998;50:375–82. doi: 10.1111/j.2042-7158.1998.tb06876.x. [DOI] [PubMed] [Google Scholar]
- 6.Wilson JD, Enoch MD. Estimation of drug rejection by schizophrenic in-patients with analysis of clinical factors. Br J Psychiatry. 1967;113:209–11. doi: 10.1192/bjp.113.495.209. [DOI] [PubMed] [Google Scholar]
- 7.Owen RR, Fischer EP, Booth BM, Cuffel BJ. Medication noncompliance and substance abuse among patients with schizophrenia. Psychiatr Serv. 1996;47:853–8. doi: 10.1176/ps.47.8.853. [DOI] [PubMed] [Google Scholar]
- 8.Vanputten T. Drug refusal in schizophrenia—causes and prescribing hints. Hosp Community Psychiatry. 1978;29:110–2. doi: 10.1176/ps.29.2.110. [DOI] [PubMed] [Google Scholar]
- 9.Thomas P, Alptekin K, Gheorghe M, Mauri M, Olivares JM, Riedel M. Management of patients presenting with acute psychotic episodes of schizophrenia. CNS Drugs. 2009;23:193–212. doi: 10.2165/00023210-200923030-00002. [DOI] [PubMed] [Google Scholar]
- 10.Bloch Y, Mendlovic S, Strupinsky S, Altshuler A, Fennig S, Ratzoni G. Injections of depot antipsychotic medications in patients suffering from schizophrenia: do they hurt? J Clin Psychiatry. 2001;62:855–9. doi: 10.4088/JCP.v62n1104. [DOI] [PubMed] [Google Scholar]
- 11.de Leon J, Diaz FJ, Josiassen RC, Cooper TB, Simpson GM. Weight gain during a double-blind multiclosage clozapine study. J Clin Psychopharmacol. 2007;27:22–7. doi: 10.1097/JCP.0b013e31802e513a. [DOI] [PubMed] [Google Scholar]
- 12.Nemeroff CB. Dosing the antipsychotic medication olanzapine. J Clin Psychiatry. 1997;58:45–9. doi: 10.4088/JCP.v58n1008c. [DOI] [PubMed] [Google Scholar]
- 13.Kurzthaler I, Fleischhacker WW. The clinical implications of weight gain in schizophrenia. J Clin Psychiatry. 2001;62:32–7. [PubMed] [Google Scholar]
- 14.Fuller MA, Shermock KM, Secic M, Grogg AL. Comparative study of the development of diabetes mellitus in patients taking risperidone and olanzapine. Pharmacotherapy. 2003;23:1037–43. doi: 10.1592/phco.23.8.1037.32876. [DOI] [PubMed] [Google Scholar]
- 15.Yood MU, DeLorenze GN, Quesenberry CP, Jr., Oliveria SA, Tsai A-L, Kim E, et al. Association between second-generation antipsychotics and newly diagnosed treated diabetes mellitus: does the effect differ by dose? Bmc Psychiatry. 2011; 11: 197. [DOI] [PMC free article] [PubMed]
- 16.Simon V, van Winkel R, De Hert M. Are weight gain and metabolic side effects of atypical antipsychotics dose dependent? A literature review. J Clin Psychiatry. 2009;70:1041–50. doi: 10.4088/JCP.08r04392. [DOI] [PubMed] [Google Scholar]
- 17.Al-Ghananeem AM, Traboulsi AA, Dittert LW, Hussain AA. Targeted brain delivery of 17b-estradiol via nasally administered water soluble prodrugs. AAPS PharmSciTech. 2002;3:E5. doi: 10.1208/pt030105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hussain AA. Intranasal drug delivery. Adv Drug Deliv Rev. 1998;29:39–49. doi: 10.1016/S0169-409X(97)00060-4. [DOI] [PubMed] [Google Scholar]
- 19.Turker S, Onur E, Ozer Y. Nasal route and drug delivery systems. Pharm World Sci. 2004;26:137–42. doi: 10.1023/B:PHAR.0000026823.82950.ff. [DOI] [PubMed] [Google Scholar]
- 20.Sonia TA, Sharma CP. Chitosan and its derivatives for drug delivery perspective. In: Jayakumar R, Prabaharan M, Muzzarelli RAA, editors. Chitosan for biomaterials I. Advances in polymer science. 243. Berlin: Springer-Verlag Berlin; 2011. p. 23–53.
- 21.Sinswat P, Tengamnuay P. Enhancing effect of chitosan on nasal absorption of salmon calcitonin in rats: comparison with hydroxypropyl- and dimethyl-beta-cyclodextrins. Int J Pharm. 2003;257:15–22. doi: 10.1016/S0378-5173(03)00090-5. [DOI] [PubMed] [Google Scholar]
- 22.Dyer AM, Hinchcliffe M, Watts P, Castile J, Jabbal-Gill I, Nankervis R, et al. Nasal delivery of insulin using novel chitosan based formulations: a comparative study in two animal models between simple chitosan formulations and chitosan nanoparticles. Pharm Res. 2002;19:998–1008. doi: 10.1023/A:1016418523014. [DOI] [PubMed] [Google Scholar]
- 23.Yu SY, Zhao Y, Wu FL, Zhang X, Lu WL, Zhang H, et al. Nasal insulin delivery in the chitosan solution: in vitro and in vivo studies. Int J Pharm. 2004;281:11–23. doi: 10.1016/j.ijpharm.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 24.Al-Ghananeem AM, Saeed H, Florence R, Yokel RA, Malkawi AH. Intranasal drug delivery of didanosine-loaded chitosan nanoparticles for brain targeting; an attractive route against infections caused by aids viruses. J Drug Target. 2010;18:381–8. doi: 10.3109/10611860903483396. [DOI] [PubMed] [Google Scholar]
- 25.Al-Ghananeem AM, Malkawi AH, Crooks PA. Bioavailability of Delta(9)-tetrahydrocannabinol following intranasal administration of a mucoadhesive gel spray delivery system in conscious rabbits. Drug Dev Ind Pharm. 2011;37:329–34. doi: 10.3109/03639045.2010.513009. [DOI] [PubMed] [Google Scholar]
- 26.Hinchcliffe M, Jabbal-Gill I, Smith A. Effect of chitosan on the intranasal absorption of salmon calcitonin in sheep. J Pharm Pharmacol. 2005;57:681–7. doi: 10.1211/0022357056073. [DOI] [PubMed] [Google Scholar]
- 27.Patil S, Babbar A, Mathur R, Mishra A, Sawant K. Mucoadhesive chitosan microspheres of carvedilol for nasal administration. J Drug Target. 2010;18:321–31. doi: 10.3109/10611861003663523. [DOI] [PubMed] [Google Scholar]
- 28.England RJA, Homer JJ, Knight LC, Ell SR. Nasal pH measurement: a reliable and repeatable parameter. Clin Otolaryngol. 1999;24:67–8. doi: 10.1046/j.1365-2273.1999.00223.x. [DOI] [PubMed] [Google Scholar]
- 29.Dragan ES, Mihai M, Schwarz S. Complex nanoparticles based on chitosan and ionic/nonionic strong polyanions: formation, stability, and application. ACS Appl Mater Interfaces. 2009;1:1231–40. doi: 10.1021/am900109u. [DOI] [PubMed] [Google Scholar]
- 30.Nielsen MKK, Johansen SS. Determination of olanzapine in whole blood using simple protein precipitation and liquid chromatography-tandem mass spectrometry. J Anal Toxicol. 2009;33:212–7. doi: 10.1093/jat/33.4.212. [DOI] [PubMed] [Google Scholar]
- 31.Portero A, Remunan-Lopez C, Vila-Jato JL. Effect of chitosan and chitosan glutamate enhancing the dissolution properties of the poorly water soluble drug nifedipine. Int J Pharm. 1998;175:75–84. doi: 10.1016/S0378-5173(98)00245-2. [DOI] [Google Scholar]
- 32.Illum L. Chitosan and its use as a pharmaceutical excipient. Pharm Res. 1998;15:1326–31. doi: 10.1023/A:1011929016601. [DOI] [PubMed] [Google Scholar]
- 33.Kumar M, Misra A, Mishra AK, Mishra P, Pathak K. Mucoadhesive nanoemulsion-based intranasal drug delivery system of olanzapine for brain targeting. J Drug Target. 2008;16:806–14. doi: 10.1080/10611860802476504. [DOI] [PubMed] [Google Scholar]
- 34.He P, Davis SS, Illum L. In vitro evaluation of the mucoadhesive properties of chitosan microspheres. Int J Pharm. 1998;166:75–88. doi: 10.1016/S0378-5173(98)00027-1. [DOI] [Google Scholar]
- 35.Illum L, Jabbal-Gill I, Hinchcliffe M, Fisher AN, Davis SS. Chitosan as a novel nasal delivery system for vaccines. Adv Drug Deliv Rev. 2001;51:81–96. doi: 10.1016/S0169-409X(01)00171-5. [DOI] [PubMed] [Google Scholar]
- 36.Markowitz JS, DeVane CL, Malcolm RJ, Gefroh HA, Wang JS, Zhu HF, et al. Pharmacokinetics of olanzapine after single-dose oral administration of standard tablet versus normal and sublingual administration of an orally disintegrating tablet in normal volunteers. J Clin Pharmacol. 2006;46:164–71. doi: 10.1177/0091270005283839. [DOI] [PubMed] [Google Scholar]
- 37.Mayor SH, Illum L. Investigation of the effect of anaesthesia on nasal absorption of insulin in rats. Int J Pharm. 1997;149:123–9. doi: 10.1016/S0378-5173(96)04858-2. [DOI] [Google Scholar]
- 38.Salama HA, Mahmoud AA, Kamel AO, Hady MA, Awad GAS. Brain delivery of olanzapine by intranasal administration of transfersomal vesicles. J Liposome Res. 2012;22:336–45. doi: 10.3109/08982104.2012.700460. [DOI] [PubMed] [Google Scholar]
- 39.Abdelbary GA, Tadros MI. Brain targeting of olanzapine via intranasal delivery of core-shell difunctional block copolymer mixed nanomicellar carriers: in vitro characterization, ex vivo estimation of nasal toxicity and in vivo biodistribution studies. Int J Pharm. 2013;452:300–10. doi: 10.1016/j.ijpharm.2013.04.084. [DOI] [PubMed] [Google Scholar]
- 40.Gizurarson S. The relevance of nasal physiology to the design of drug absorption studies. Adv Drug Deliv Rev. 1993;11:329–47. doi: 10.1016/0169-409X(93)90015-V. [DOI] [Google Scholar]
- 41.Dondeti P, Zia HS, Needham TE. In-vivo evaluation of spray formulations of human insulin for nasal delivery. Int J Pharm. 1995;122:91–105. doi: 10.1016/0378-5173(95)00045-K. [DOI] [Google Scholar]


