Abstract
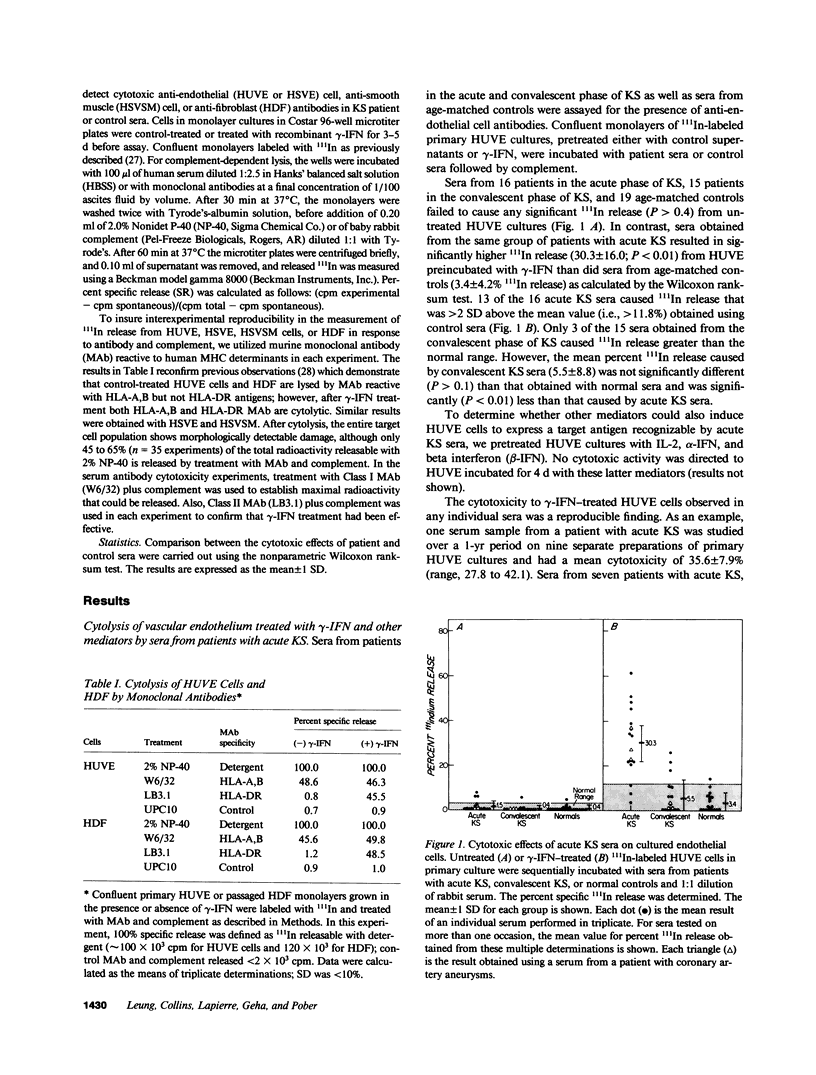
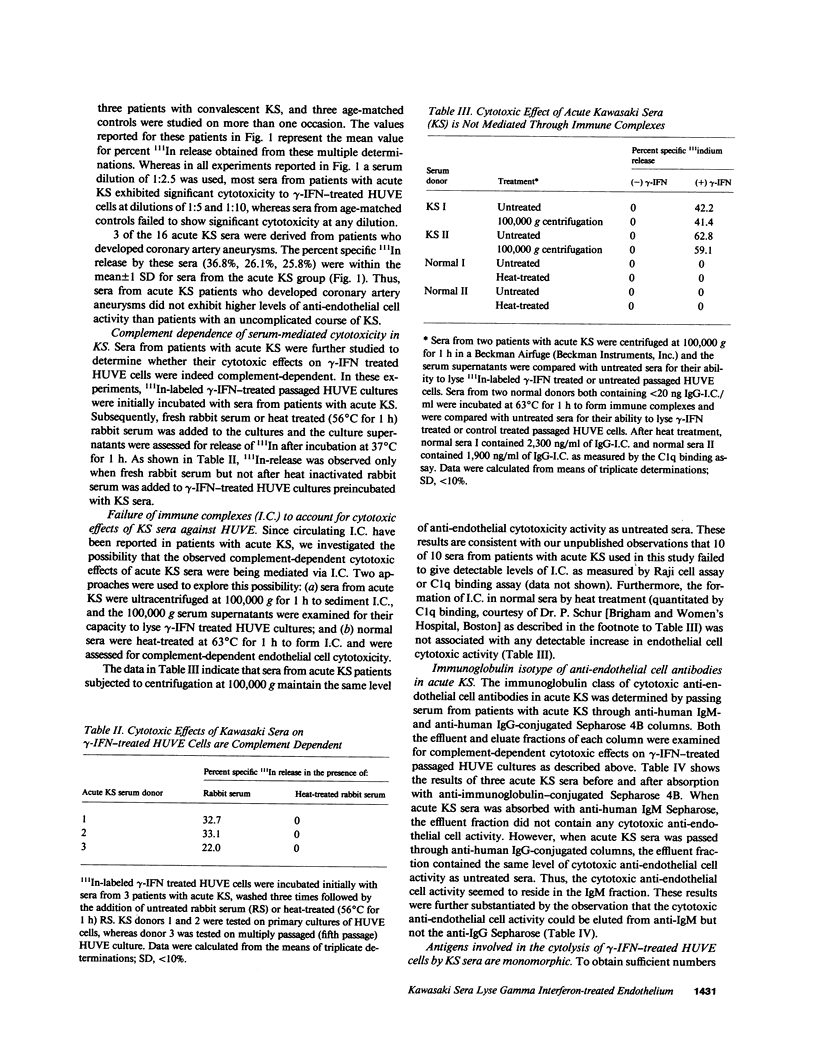
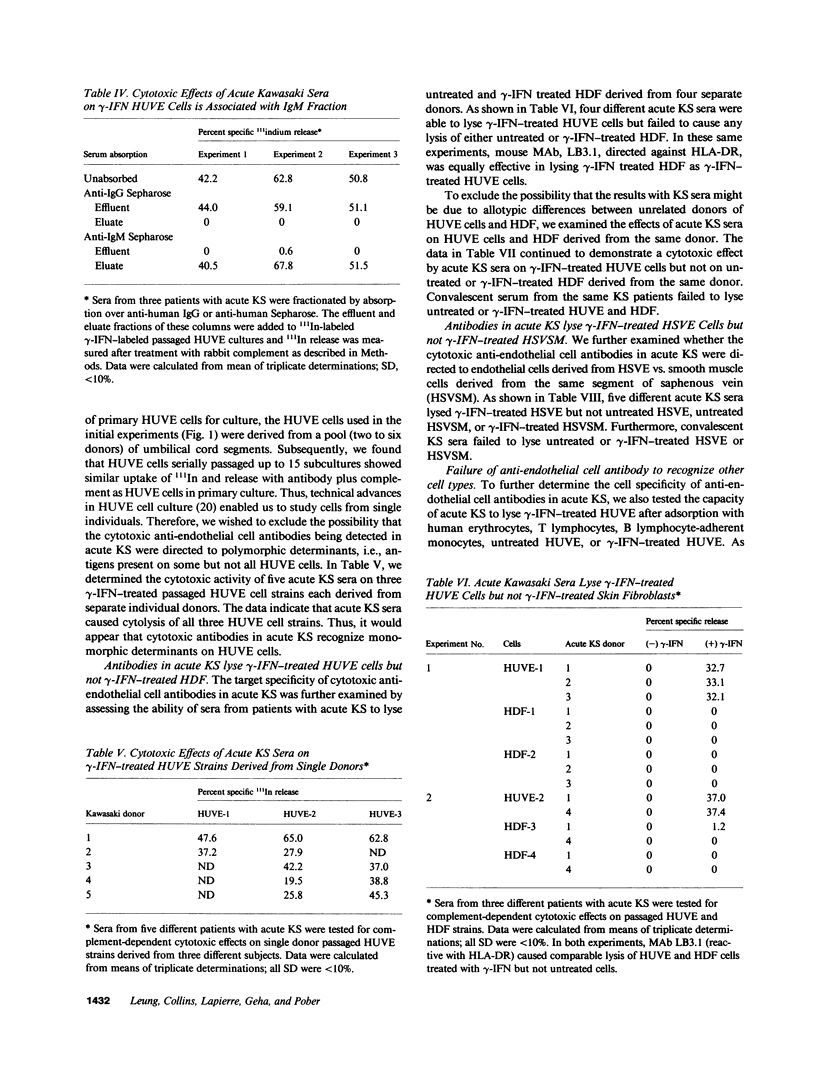
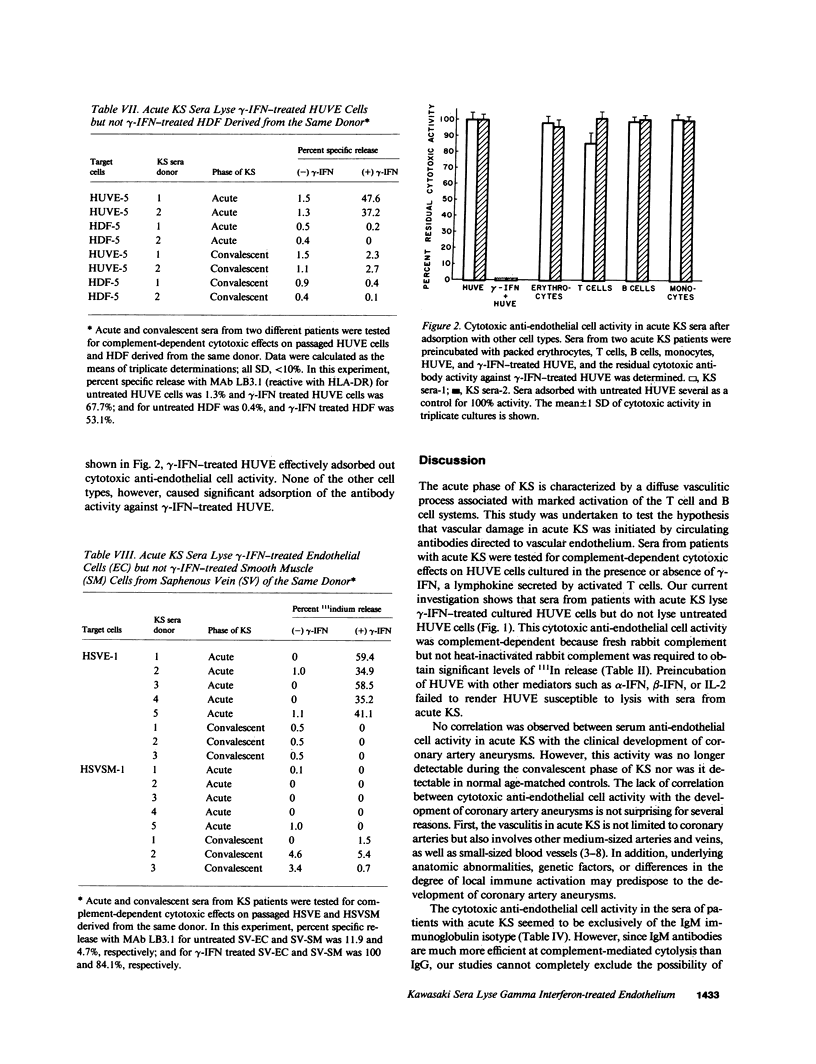
Kawasaki syndrome (KS) is characterized by diffuse vasculitis and marked T cell and B cell activation. In this study, sera from 16 patients with acute KS, 15 patients in the convalescent phase of KS, and 19 age-matched controls were assessed for complement dependent cytotoxic activity against 111In-labeled human umbilical vein endothelial (HUVE) cells, Neither sera from patients with KS nor sera from controls had cytotoxic effects on HUVE cells cultivated under standard conditions. Since activated T cells such as those present in acute KS secrete gamma interferon (gamma-IFN), we also examined the effects of sera from acute KS on HUVE cells preincubated with gamma-IFN. We report here that immunoglobulin M (IgM) antibodies in sera from patients with acute KS cause significant (P less than 0.01) killing of gamma-IFN-treated HUVE cells. Pretreatment with interleukin 2, gamma-IFN, or beta-IFN failed to render HUVE susceptible to lysis with acute KS sera. The observed effects were not mediated via immune complexes. The cytotoxic antibodies in acute KS seem to be directed against inducible monomorphic antigenic determinants present on gamma-IFN-treated HUVE cells but not on control or gamma-IFN treated autologous human dermal fibroblasts (HDF). Similarly, acute KS sera also induced lysis of gamma-IFN-treated human saphenous vein endothelial (HSVE) cells but not gamma-IFN treated human saphenous vein smooth muscle (HSVSM) cells. Since gamma-IFN induces the same level of class I and class II major histocompatibility complex (MHC) antigen expression on HDF, HUVE, HSVE, and HSVSM cells, our results suggest that the anti-endothelial cell antibodies in acute KS are directed to gamma-IFN-inducible molecules other than MHC determinants. These observations are further substantiated by the failure of human B cells or monocytes to absorb the anti-endothelial cell activity. Since most vasculitides, including acute KS, are characterized both by marked immune activation and the secretion of lymphokines, antibodies directed to gamma-IFN-inducible endothelial cell antigens may represent a general mechanism for vascular injury.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnstable C. J., Bodmer W. F., Brown G., Galfre G., Milstein C., Williams A. F., Ziegler A. Production of monoclonal antibodies to group A erythrocytes, HLA and other human cell surface antigens-new tools for genetic analysis. Cell. 1978 May;14(1):9–20. doi: 10.1016/0092-8674(78)90296-9. [DOI] [PubMed] [Google Scholar]
- Cerilli J., Brasile L. Endothelial cell alloantigens. Transplant Proc. 1980 Sep;12(3 Suppl 1):37–42. [PubMed] [Google Scholar]
- Cines D. B., Lyss A. P., Reeber M., Bina M., DeHoratius R. J. Presence of complement-fixing anti-endothelial cell antibodies in systemic lupus erythematosus. J Clin Invest. 1984 Mar;73(3):611–625. doi: 10.1172/JCI111251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins T., Korman A. J., Wake C. T., Boss J. M., Kappes D. J., Fiers W., Ault K. A., Gimbrone M. A., Jr, Strominger J. L., Pober J. S. Immune interferon activates multiple class II major histocompatibility complex genes and the associated invariant chain gene in human endothelial cells and dermal fibroblasts. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4917–4921. doi: 10.1073/pnas.81.15.4917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fossard C., Thompson R. A. Mucocutaneous lymph-node syndrome (Kawasaki disease): probable soluble-complex disorder. Br Med J. 1977 Apr 2;1(6065):883–883. doi: 10.1136/bmj.1.6065.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara H., Hamashima Y. Pathology of the heart in Kawasaki disease. Pediatrics. 1978 Jan;61(1):100–107. [PubMed] [Google Scholar]
- Gillis S., Ferm M. M., Ou W., Smith K. A. T cell growth factor: parameters of production and a quantitative microassay for activity. J Immunol. 1978 Jun;120(6):2027–2032. [PubMed] [Google Scholar]
- Gimbrone M. A., Jr Culture of vascular endothelium. Prog Hemost Thromb. 1976;3:1–28. [PubMed] [Google Scholar]
- Hirose S., Hamashima Y. Morphological observations on the vasculitis in the mucocutaneous lymph node syndrome. A skin biopsy study of 27 patients. Eur J Pediatr. 1978 Aug 17;129(1):17–27. doi: 10.1007/BF00441370. [DOI] [PubMed] [Google Scholar]
- Jaffe E. A., Hoyer L. W., Nachman R. L. Synthesis of antihemophilic factor antigen by cultured human endothelial cells. J Clin Invest. 1973 Nov;52(11):2757–2764. doi: 10.1172/JCI107471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato H., Koike S., Yamamoto M., Ito Y., Yano E. Coronary aneurysms in infants and young children with acute febrile mucocutaneous lymph node syndrome. J Pediatr. 1975 Jun;86(6):892–898. doi: 10.1016/s0022-3476(75)80220-4. [DOI] [PubMed] [Google Scholar]
- Kato S., Kimura M., Tsuji K., Kusakawa S., Asai T., Juji T., Kawasaki T. HLA antigens in Kawasaki disease. Pediatrics. 1978 Feb;61(2):252–255. [PubMed] [Google Scholar]
- Kawasaki T. [Acute febrile mucocutaneous lymphonodus syndrome and sudden death]. Nihon Shonika Gakkai Zasshi. 1971 Jun;75(6):433–434. [PubMed] [Google Scholar]
- Kawasaki T. [Acute febrile mucocutaneous syndrome with lymphoid involvement with specific desquamation of the fingers and toes in children]. Arerugi. 1967 Mar;16(3):178–222. [PubMed] [Google Scholar]
- Krensky A. M., Berenberg W., Shanley K., Yunis E. J. HLA antigens in mucocutaneous lymph node syndrome in New England. Pediatrics. 1981 May;67(5):741–743. [PubMed] [Google Scholar]
- Landing B. H., Larson E. J. Are infantile periarteritis nodosa with coronary artery involvement and fatal mucocutaneous lymph node syndrome the same? Comparison of 20 patients from North America with patients from Hawaii and Japan. Pediatrics. 1977 May;59(5):651–662. [PubMed] [Google Scholar]
- Leung D. Y., Chu E. T., Wood N., Grady S., Meade R., Geha R. S. Immunoregulatory T cell abnormalities in mucocutaneous lymph node syndrome. J Immunol. 1983 May;130(5):2002–2004. [PubMed] [Google Scholar]
- Leung D. Y., Parkman R., Feller J., Wood N., Geha R. S. Cell-mediated cytotoxicity against skin fibroblasts in atopic dermatitis. J Immunol. 1982 Apr;128(4):1736–1741. [PubMed] [Google Scholar]
- Leung D. Y., Siegel R. L., Grady S., Krensky A., Meade R., Reinherz E. L., Geha R. S. Immunoregulatory abnormalities in mucocutaneous lymph node syndrome. Clin Immunol Immunopathol. 1982 Apr;23(1):100–112. doi: 10.1016/0090-1229(82)90075-7. [DOI] [PubMed] [Google Scholar]
- Luster A. D., Unkeless J. C., Ravetch J. V. Gamma-interferon transcriptionally regulates an early-response gene containing homology to platelet proteins. Nature. 1985 Jun 20;315(6021):672–676. doi: 10.1038/315672a0. [DOI] [PubMed] [Google Scholar]
- Mason W. H., Jordan S. C., Sakai R., Takahashi M., Bernstein B. Circulating immune complexes in Kawasaki syndrome. Pediatr Infect Dis. 1985 Jan-Feb;4(1):48–51. doi: 10.1097/00006454-198501000-00012. [DOI] [PubMed] [Google Scholar]
- Moraes J. R., Stastny P. A new antigen system expressed in human endothelial cells. J Clin Invest. 1977 Aug;60(2):449–454. doi: 10.1172/JCI108795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morens D. M., Anderson L. J., Hurwitz E. S. National surveillance of Kawasaki disease. Pediatrics. 1980 Jan;65(1):21–25. [PubMed] [Google Scholar]
- Muchmore A. V., Megson M., Decker J. M., Knudsen P., Mann D. L., Broder S. Inhibitory activity of antibodies to human Ia-like determinants: comparison of intact and pepsin-digested antibodies. J Immunol. 1983 Aug;131(2):725–730. [PubMed] [Google Scholar]
- Okudaira K., Goodwin J. S., Williams R. C., Jr Anti-Ia antibody in the sera of normal subjects after in vivo antigenic stimulation. J Exp Med. 1982 Jul 1;156(1):255–267. doi: 10.1084/jem.156.1.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okudaira K., Searles R. P., Ceuppens J. L., Goodwin J. S., Williams R. C., Jr Anti-Ia reactivity in sera from patients with systemic lupus erythematosus. J Clin Invest. 1982 Jan;69(1):17–24. doi: 10.1172/JCI110428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul L. C., Carpenter C. B. Antibodies against renal endothelial alloantigens. Transplant Proc. 1980 Sep;12(3 Suppl 1):43–48. [PubMed] [Google Scholar]
- Paul L. C., van Es L. A., Baldwin W. M., 3rd Antigens in human renal allografts. Clin Immunol Immunopathol. 1981 May;19(2):206–223. doi: 10.1016/0090-1229(81)90064-7. [DOI] [PubMed] [Google Scholar]
- Pober J. S., Collins T., Gimbrone M. A., Jr, Cotran R. S., Gitlin J. D., Fiers W., Clayberger C., Krensky A. M., Burakoff S. J., Reiss C. S. Lymphocytes recognize human vascular endothelial and dermal fibroblast Ia antigens induced by recombinant immune interferon. Nature. 1983 Oct 20;305(5936):726–729. doi: 10.1038/305726a0. [DOI] [PubMed] [Google Scholar]
- Scahill S. J., Devos R., Van der Heyden J., Fiers W. Expression and characterization of the product of a human immune interferon cDNA gene in Chinese hamster ovary cells. Proc Natl Acad Sci U S A. 1983 Aug;80(15):4654–4658. doi: 10.1073/pnas.80.15.4654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott H., Brandtzaeg P., Hirschberg H., Solheim B. G., Thorsby E. Vascular and renal distribution of HLA--DR-like antigens. Tissue Antigens. 1981 Sep;18(3):195–202. doi: 10.1111/j.1399-0039.1981.tb01382.x. [DOI] [PubMed] [Google Scholar]
- Tanaka N., Sekimoto K., Naoe S. Kawasaki disease. Relationship with infantile periarteritis nodosa. Arch Pathol Lab Med. 1976 Feb;100(2):81–86. [PubMed] [Google Scholar]
- Thornton S. C., Mueller S. N., Levine E. M. Human endothelial cells: use of heparin in cloning and long-term serial cultivation. Science. 1983 Nov 11;222(4624):623–625. doi: 10.1126/science.6635659. [DOI] [PubMed] [Google Scholar]
- Weil J., Epstein C. J., Epstein L. B., Sedmak J. J., Sabran J. L., Grossberg S. E. A unique set of polypeptides is induced by gamma interferon in addition to those induced in common with alpha and beta interferons. Nature. 1983 Feb 3;301(5899):437–439. doi: 10.1038/301437a0. [DOI] [PubMed] [Google Scholar]
- Yanagihara R., Todd J. K. Acute febrile mucocutaneous lymph node syndrome. Am J Dis Child. 1980 Jun;134(6):603–614. doi: 10.1001/archpedi.1980.02130180059017. [DOI] [PubMed] [Google Scholar]



