Abstract
The objective of this study was to investigate the effect of large granulated lactose carrier particle systems on aerosol performance of dry powder inhaler formulations. Granulated lactose carriers with average sizes ranging from 200 to 1,000 μm were prepared and subsequently fractionated into separate narrow size powders. The fractionated granulated lactose (GL) samples were characterized in terms of size, specific surface area, surface roughness, morphology, density, flowability, and solid-state. The in vitro aerosolization performance was performed on the different size fractions of GL samples from a commercial inhaler device (Aerolizer®) with a model formulation (2% w/w salbutamol sulfate). The cascade impaction parameters employed were 60 or 90 L/min with standard (aperture size, 0.6 mm) or modified piercing holes (aperture size, 1.2 mm) of the inhaler loaded capsules. It was shown that the largest size fraction formulation (850–1000 μm) had a slight improvement in the fine particle fraction (FPF) compared to immediately preceding size fractions, explained by a smaller adhesive force between drug and carrier. Compared to commercial piercing holes, enlarged piercing holes generated a slight decreasing trend of FPF as the lactose powder sizes increased from 200–250 μm to 600–850 μm, perhaps due to the reduced detachment force by flow forces. The size, surface roughness, density, and flowability of lactose carrier as well as device design all contributed to the aerosol dispersion performance of granulated lactose-based adhesive mixtures. It was concluded that poorer or enhanced redispersion performance is not an inherent property to the significantly large size of granulated lactose carriers as previously contended.
KEY WORDS: adhesive force, carrier roughness, carrier size, DPI formulations, granulated lactose
INTRODUCTION
Development of dry powder inhaler (DPI) formulations is generally challenging due to the need for a good product performance and the necessity of uniformity and quality. The active ingredients incorporated in DPI formulations are in micronized form with an aerodynamic diameter less than 5 μm which enables adequate deposition in the lung. It is well known that the high surface area/volume ratio of the micronized drug particles results in strong interactive forces (1). As a result, micronized drugs are generally highly cohesive and exhibit poor flow, which makes downstream processing, accurate dose metering, and handling of the drugs problematic. Therefore, in order to facilitate adequate powder performance, DPI formulations are frequently formulated with larger coarse “carriers” to form homogenous binary or tertiary mixtures (2). These blends are then required to be redispersed into primary particles upon inhalation by patients via the inhaler device.
α-lactose monohydrate is the most commonly used coarse carrier for DPI formulations due to its well-established safety profile, stable physicochemical properties, and compatibility with most available low molecular weight APIs (3). The particle size, size distribution (4), morphology, surface roughness (5), surface area, flowability (6), and surface energy (7) of lactose carriers all have been shown to have an influence on the DPI formulation performance. Amongst, the size and roughness of lactose carriers have been extensively investigated (2,4,8–10).
Several mechanisms are postulated to explain the effect of carrier size on the dispersion performance of adhesive mixtures for dry powder inhalation. Generally, to achieve a good aerosolization performance, the detachment forces generated from the inspiratory flow and the interactions of the flow and formulation with the inhaler device should be large enough to overcome the adhesive force between API particle and the coarse carrier particle to efficiently redisperse the primary drug particles (11,12). However, it has been determined previously that, in general, there are more surface asperities on the larger lactose carrier particles compared to smaller size fractions (13). These surface discontinuities, clefts, and depressions, where drugs are not fully exposed to the flow stream, are proposed to prevent detachment by fluid flow mechanisms. Additionally, larger lactose particles may exhibit higher press-on forces (defined as the adhesive forces between drug and carrier particles) (14) during mixing with the micronized API due to larger mass and inertia force, resulting in a stronger adhesive force between drug and lactose carrier (15). Also, as the size of lactose carrier is increased, the amount of fine lactose (<32 μm) present in the powder tends to decrease (16). Fine lactose components have been shown to increase the redispersion of APIs, explained mainly by “active site theory,” and “agglomeration theory.” In the “active site theory,” fine lactose particles occupy the high energy surface areas of lactose carrier particle, thus leaving the surface with lower energy binding sites for the API to occupy. It is also proposed that fine lactose facilitates the formation of agglomerate mixtures, which are more susceptible to detachment force.
As a result of these research findings, it was widely believed that carrier particles with smaller diameters were preferable to maximize aerosolization efficiency, with the consensus that increasing diameters and surface roughness hinder efficient drug dispersion performance (4,14,16–20). However, it was found recently that lactose carriers with large size fraction can also improve aerosol performance, especially when combined with significantly rough surface (13). This improvement is explained by the switch of predominant detachment mechanism from turbulence flow to impaction force. Impaction force (mechanical force) arises from the abrupt momentum transfer generated from the collisions between coarse carriers and the inhaler device during inhalation. The momentum relies on particle mass, thus detachment by impaction force is proportional to the cube of the carrier particle size such that large particles will have strong detachment force (13). It was found that Aerolizer® used in previous study actively promotes particle collisions with the inhaler wall, especially, with significantly large particle diameter (21). Additionally, different from carriers with flat surface, larger carriers with significantly rough surface would shelter drugs within asperities, and drug detachment depends more on impaction force (13). Therefore, the hypothesis in this research is that larger lactose carriers can improve DPI aerosol performance as the result of the major detachment mechanism switch from turbulence flow to impaction force.
Previously, the carrier particles studied have had a particle size less than 300 μm as a result of the sizes limitations of commercially available lactose (22). In this study, the aim was to evaluate the powder and aerosol performance of lactose carrier with significantly large size ranging from 200 to 1,000 μm. We manufactured granulated lactose and performed the physicochemical (e.g., solid-state form, density, specific surface area, and flowability) and impaction studies. To the author’s knowledge, for the first time we studied the significantly large granulated lactose carriers across a wide range of narrow sieve fractions in the size range of 200–1,000 μm and correlated with the physicochemical properties of these large granulated lactose carriers with the in vitro aerosol dispersion from an Aerolizer® DPI.
METHODS
Materials
α-Lactose monohydrate, Pharmatose 100 M, was supplied from DFE Pharma (Princeton, NJ, USA). Micronized salbutamol sulfate (Fig. 1) was purchased from LETCO MEDICAL. Deionized water was provided by MilliQ (Millipore).
Fig. 1.
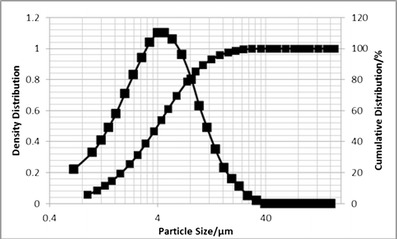
Particle size distribution (PSD) of salbutamol sulfate
Manufacture of Lactose Granules
Wet granulation was used to manufacture lactose granules with large diameter from Pharmatose 100 M (d10; 63 μm, d50; 150 μm, and d90; 250 μm). Granulation is a process to generate large aggregates from small primary powders to improve the flowability of the powders (23). Wet granulation of lactose usually employs polymeric binders that are not approved for inhalation (24–27). To solve this problem, the granulation process in this research involved merely water as the binding solvent. Briefly, a batch size of 50 g starting lactose was introduced into the granulator (Robot Coupe USA, Inc.) followed by addition of 50 mL water merely as the granulating solvent. Subsequently, the granulated lactose carriers were pan dried in the oven overnight at 80°F.
Fractionation of Granulated Lactose Carrier Particles
Different size fractions of lactose granules were obtained by separation of the bulk granulated material using a sieve tower with cut off sizes as follows: 1,000, 850, 600, 425, 300, 250, and 212 μm and a metal collection pan. A vibrating auto sieve shaker (Gilson Company Inc., OH, USA) was employed. The granulated lactose was poured on the top of the vibrating sieve shaker and sieved through the sieves for 30 min. All analysis described were performed on the sieved samples.
Particle Size Measurement
Particle size analysis of fractionized granulated lactose was evaluated by the Sympatec laser diffraction (Sympatec GmbH). The theoretical-specific surface area (based on volume, assuming an ideal spherical smooth surface of the particles) was calculated by the software installed in the Sympatec. The span of the samples, which is the width of the distribution based on the 10, 50, and 90% quintile, was calculated according to Eq. 1,
| 1 |
Scanning Electron Microscopy
The scanning electron microscopy (SEM; Supra 40VP, Zeiss, Germany) was used to visually assess the particle size and morphology of the granulated lactose. The coating conditions prior to SEM for all the granulated lactose were 20 nm of Pd/Pt via sputter coating.
Differential Scanning Calorimetry
Differential scanning calorimetry (DSC) of the lactose granules with different size fraction was conducted with modulated temperature DSC (MTDSC), Model 2920 (TA Instruments, New Castle, DE), which was equipped with a refrigerated cooling system. The flow rate of purge gas through the DSC cell was 40 mL/min. Lactose granules of 5–10 mg were weighted in aluminum crimped pans (PerkinElmer Instruments, Norwalk, CT). An empty sample pan was used as the reference. Samples were heated at a ramp rate of 10°C/min from 25 to 250°C with modulation temperature amplitude of 1°C per 60 s for all granule size fractions.
BET Analysis
The specific surface area of a powder could be determined by the amount of the monomolecular layer of adsorbate gas on the surface of the solid, calculated according to Brunauer, Emmett and Teller (BET) theory. The specific surface area of the lactose populations was determined via nitrogen adsorption with a single-point BET method using a Monosorb® surface area analyzer (Quantachrome, FL, USA). All BET analysis was performed in triplicate. Surface roughness was calculated by Eq. 2.
| 2 |
True, Bulk, and Tapped Density
True density of the granulated lactose and primary lactose was determined by helium pycnometry (Quantachrome, FL, USA).
The lactose samples were filled into a 10 mL gradual cylinder, and the volume was recorded as the bulk volume. Then the cylinder was tapped 750 times and the new volume was recorded (tapped volume). The bulk density, tapped density, and Carr’s index (CI) (Eq. 3) were calculated. To measure the angle of repose, certain amount of lactose samples was passed through the tunnel with a fixed height relative to the base. The angle of repose was calculated according to Eq. 4. Carr’s index and angle of repose were both used as the indicators of powder flowability. The Carr index, also called Carr’s index or Carr’s Compressibility Index, is an indication of the compressibility and flowability of a powder (28).
| 3 |
| 4 |
Preparation of Salbutamol Sulfate/Granulated Lactose Binary Blends
Salbutamol sulfate (SS) and fractionated granulated lactose were mixed in a ratio of 1:50 (w/w) to obtain a 500 mg 2% binary mixture. All formulations were blended at a constant speed of 46 RPM for 40 min with a Turbula® orbital mixer (Glen Mills, NJ, USA). The granules still maintained the initial shape after completion of blending. Prior to any further analysis, the blended formulations were stored in the dessicator for 5 days.
Drug Uniformity Test
Five of randomly selected samples (20 ± 1 mg) were taken for measurement of salbutamol sulfate content uniformity. The coefficient of variation (CV%) was used to determine the blending uniformity. The test was performed three times. The potency of formulations was calculated by the APIs percent amount to the nominal dose.
In Vitro Aerosolisation Study
About 20 (±1) mg mixture powders were filled into size 3 Vcaps HPMC capsules. The in vitro drug deposition of all formulations was assessed using Aerolizer® inhaler device (Novartis, Switzerland) of which the mixtures were loaded in the capsules pierced with four standard holes (0.6 mm) (29) at each end of the capsule, referred as Capsule40.6 in the results and discussion part. The impaction study was performed through a Next Generation Impactor (NGI; MSP Corporation, Shoreview, MN) at a volumetric flow rate of 60 L and 90 L/min, corresponding to 4 kPa pressure drop and 4 L air volume across the device.
Another 20 (±1) mg mixture powders of all formulations were filled into the same size 3 Vcaps HPMC capsules. After loading the mixtures in the capsules, one 1.2 mm hole instead was punctured at each end of the capsule, followed by in vitro impaction study with Aerolizer® inhaler device (Novartis, Switzerland) through the same next generation cascade at a volumetric flow rate of 90 L/min only. The dispersion method is referred as Capsule1.2 in the results and discussion part.
A 1% (w/v) solution of silicon oil in hexane was applied to precoat the NGI stages for particle re-entrainment prevention. Amounts of salbutamol sulfate deposited on the capsule, inhaler, mouthpiece adaptor, induction port, pre-separator, and NGI stages were measured and quantified. The drug content was measured by the ultraviolet visible absorption spectroscopy (Infinite M200, TECAN) at 230 nm. The parameters used to evaluate salbutamol sulfate deposition performance were emitted fraction (EF) (Eq. 5), fine particle fraction (FPF) (Eq. 6), respirable fraction (Fig. 2) mass median aerodynamic diameter (MMAD), and geometric standard deviation (GSD).
| 5 |
| 6 |
| 7 |
Fig. 2.
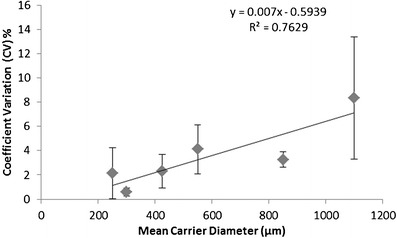
Blending uniformity of lactose granules-based DPI formulations as function of the mean carrier diameter. (n = 5 × 3)
Statistics Analysis
Statistical significance between aerosol performance values was determined with one-way TTESTs between groups (* indicates P < 0.05).
RESULTS
Particle Size Distribution of Lactose Granules with Different Size Fractions
The particle size of GL fractions fells into the nominal sieve size range, as confirmed by the SEM pictures (Fig. 3) and laser diffraction (Fig. 4a). As expected, the size of GL particles increased progressively with increased aperture size of sieves. The volume distribution of the particles at 10% (d10%), 50% (d50%), and 90% (d90%) (Fig. 4a), as well as the equivalent diameter from image analysis (Fig. 4b) generally agreed with each other. Slight differences obtained using the different methods of analysis are due to the different mechanisms of size determination, which is explained in the discussion section below. The equivalent diameter of GL 650–850 μm and GL 850–1000 μm from Image Analysis (Fig. 4a) was more accurate than the laser diffraction results for these powders and was therefore used in interpretation of other results.
Fig. 3.
SEM micrographs of a Pharmatose 100 M, b GL 212–250 μm granulated lactose, c GL 425–600 μm granulated lactose, d GL 850–1,000 μm granulated lactose sieve fractions. Scale bars denote 200 μm
Fig. 4.
Particle size obtained by a laser diffraction (d10, d50, and d90%) and b by image analysis SEM pictures
Particle size distribution width (as measured by span) and the fine lactose fractions (fraction size less than 5 μm) could also be determined from laser diffraction results (Fig. 4a). The span value of all GLs was less than one, indicating relatively narrow size distributions for all samples. The fine lactose fraction was less than 1% for all sieved GL samples, indicating that sieving efficiently removed fine lactose from the granulated powders. This is important, as fine lactose has been shown to significantly improve DPI performance and is generally present in different amounts for different size lactose fractions and could confound results if not sufficiently removed from the granulated lactose (8).
Morphology of Lactose Carrier Particles
The particle morphology and surface roughness of pre-granulated lactose particles (commercial Pharmatose 100 M) and lactose granules were visualized by SEM. As shown in Fig. 3a, commercial Pharmatose 100 M had a tomahawk shape, the typical morphology of alpha monohydrate lactose crystals (30). Figure 3b–d shows the micrographs of lactose granules with different size fractions: b: GL 212–250 μm, c: 425–600 μm, and d: 850–1,000 μm. Unlike the commercial Pharmatose 100 M, the shape of these lactose granules was elongated aggregates comprised of the primary lactose particles. Obviously, the number of primary lactose particles comprising each granule was different for the six size fractions. For example, less than ten primary lactose particles were observed to be contained in each GL 212–250 μm granule compared to hundreds observed for GL 850–1,000 μm.
Thermal Analysis
Figure 5 is the DSC thermographs obtained for the lactose particles. There were two endothermic peaks (220 and 150°C) observed for all granulated lactose, consistent with reports of commercial alpha monohydrate lactose . The peak at 220°C is a melt endotherm, while the peak at 150°C corresponded to the distinctive dehydration of crystalline hydrate water (bound water) as previously reported. The vaporization temperature of bound water (150°C) is significantly greater than 100°C, explained by thermodynamically favorable H-bonding of the water molecules in the lactose crystal lattice (31). There was no dehydration process from unbound water, indicated by no endothermic peak at ~100°C. This demonstrated that there was no excessive unbound water in the lactose granules as prepared. The distinct thermographs showed that all granulated lactose fractions had similar solid-state and was composed with alpha monohydrate lactose. This is reasonable because no special solvent other than water was used and there was no heating process above 93.5°C involved in the granulation process (32).
Fig. 5.
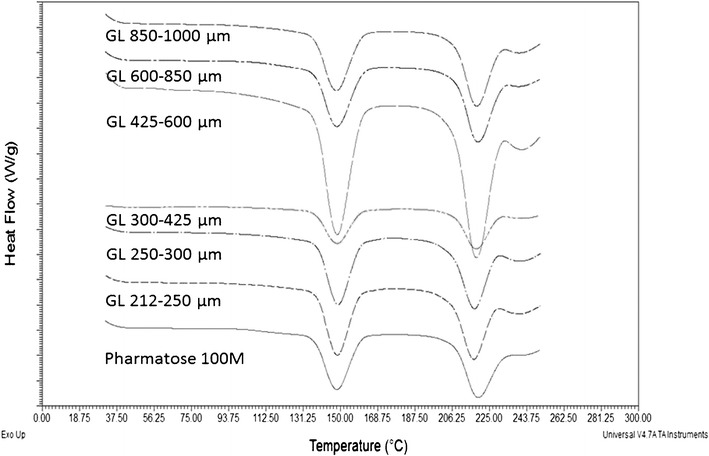
The DSC thermographs of all granulated lactose
Specific Surface Area
Despite different size fractions for the granulated lactose samples, all samples had similar specific surface area (~0.29 m2/g) (mean), which was similar to the specific surface area of the primary lactose carriers (0.30 ± 0.06 m2/g) (Fig. 6a). These findings are explained and discussed below.
Fig. 6.
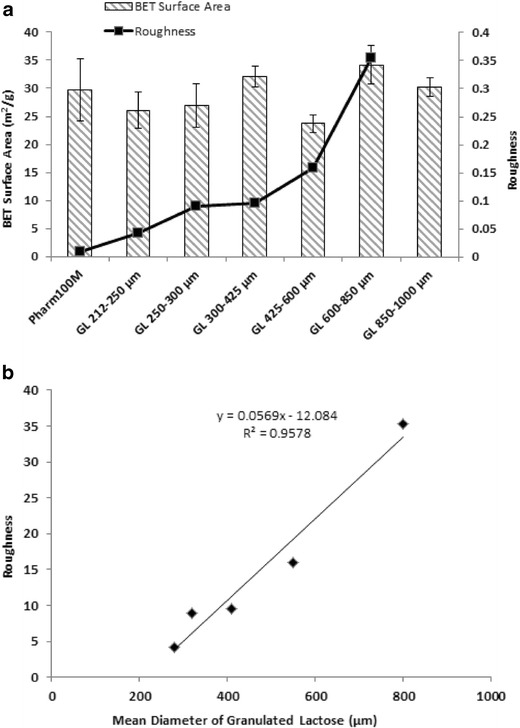
a BET surface area and roughness for granulated lactose carriers with different size fractions: GL 212–250 μm, GL 250–300 μm, GL 300–425 μm and GL 425–600 μm, GL 600–850 μm and GL 850–1000 μm; b and roughness of GL particles. All GL particles have similar specific surface area and larger GL particles have rougher surfaces
Density and Flowability
All granulated lactose demonstrated similar true density with commercial pharmatose 100 M (1.545 g/cm3, P > 0.05). Thus, the size of lactose granules had negligible effect on true density, which is expected and related to their similar solid-state form (Fig. 5).
The bulk and tapped densities of the lactose powders are shown in Table I. According to the results, an inverse relationship was observed between bulk/tapped density and lactose size. The bulk density decreased from 0.68 to 0.44 g/ml with increasing granule size. The tapped density decreased from 0.82 to 0.48 g/ml with increasing granule size.
Table I.
True Density, Bulk Density and Tapped Density (Mean ± SD, n = 3) of Granulated Lactose Carriers GL 212–250 μm, GL 250–300 μm, GL 300–425 μm and GL 425–600 μm, GL 600–850 μm and GL 850–1,000 μm. Bulk Density and Tapped Density Increased with Decreasing Particle Size of GLs
| Pharmatose 100 M | GL 212–250 μm | GL 250–300 μm | GL 300–425 μm | GL 425–600 μm | GL 600–850 μm | GL 850–1,000 μm | |
|---|---|---|---|---|---|---|---|
| True density (g/ml) | 1.546 | 1.544 | 1.544 | 1.542 | 1.546 | 1.547 | 1.544 |
| Bulk density (g/ml) | 0.71 (0.02) | 0.68 (0.01) | 0.61 (0.01) | 0.56 (0.00) | 0.48 ± 0.00 | 0.45 ± 0.00 | 0.44 ± 0.01 |
| Tapped density (g/ml) | 0.94 (0.99) | 0.82 (0.01) | 0.71 (0.01) | 0.65 (0.00) | 0.58 ± 0.00 | 0.53 ± 0.03 | 0.48 ± 0.01 |
| Carr’s Index (%) | 23.82 (1.81) | 16.66 (1.78) | 14.12 (1.76) | 14.23 (1.02) | 15.97 ± 0.11 | 14.95 ± 3.57 | 9.50 ± 3.03 |
| Angle of Repose (°) | 37.2 (1.5) | 31.1 (3.0) | 30.2 (1.5) | 32.0 (1.5) | 30.0 ± 0.7 | 29.4 ± 0.0 | 27.3 ± 1.8 |
Carr’s Index may be used as an indicator of powder flow. According to USP <1174> Powder Flow, powder with Carr’s Index smaller than 16–20% is considered to have good flow. Carr’s Index results for the powders in this study are listed in Table I and show that flowability of the lactose granules (Carr’s Index ≤ 16%) was good while that of the primary pharmatose 100 M powder is only above 20%. The lactose powder and granule flow shown by the angle of repose were consistent with the data obtained from Carr’s Index.
Aerosol Performance
In Vitro Aerosolization Performance with Capsules40.6 (0.6 mm, 4 holes) at 60 L/min
Firstly, the aerosol performance of the six DPI formulations with different granular lactose size fractions was evaluated by in vitro impaction study at 60 L/min using capsules with holes created by the standard pins used in the Aerolizer®, that is, 0.6 mm hole diameter, four holes at each end of the capsule (abbreviated now as Capsules40.6). Experimental parameters for the cascade impaction studies are tabulated in Table II.
Table II.
In Vitro Aerosolization Performance with Capsules40.6 in Aerolizer at 60 L/min. Emitted Fraction (EF), Fine Particle Fraction (FPF), Respiratory Fraction (RF), Mass Median Aerodynamic Diameter (MMAD), and Geometric Standard Deviation (GSD) Obtained from Formulations Containing SBS Blended with Granulated Lactose Carriers GL 212–250 μm, GL 250–300 μm, GL 300–425 μm, GL 425–600 μm, GL 600–850 μm, and GL 850–1,000 μm (mean ± SD, n = 3)
| GL 212–250 μm | GL 250–300 μm | GL 300–425 μm | GL 425–600 μm | GL 600–850 μm | GL 850–1000 μm | |
|---|---|---|---|---|---|---|
| EF (%) | 87.5 (2.8) | 82.3 (7.8) | 71.9 (18.1) | 63.3 (18.1) | 62.5 (10.4) | 67.0* (11.9) |
| FPF (%) | 21.0 (2.9) | 19.2 (0.4) | 20.5 (5.2) | 19.9 (1.8) | 20.4 (4.6) | 26.7* 5.7) |
| RF (%) | 19.2 (2.8) | 15.8 (1.5) | 15.3 (6.8) | 12.6 (2.4) | 13.0 (4.6) | 17.5 (2.6) |
| MMAD (μm) | 2.04 (0.13) | 1.90 (0.04) | 2.00 (0.14) | 1.81 (0.08) | 1.93 (0.13) | 1.93 (0.10) |
| GSD | 1.8 (0.2) | 1.7 (0.1) | 1.7 (0.2) | 1.7 (0.1) | 1.8 (0.3) | 1.7 (0.2) |
*P < 0.05
According to the results, all formulations yielded a similar aerodynamic particle size for the deposited SS in terms of the mass median aerodynamic diameter (MMAD) (1.9 ± 0.1 μm). The geometric standard deviations (GSD) (1.7 ± 0.1) for the SS aerosols were also similar between formulations. There were no significant differences for the fine particle fractions (FPF) detected between the different formulations, except for GL 850–1000 μm, which demonstrated higher dispersion efficiency (Table II). An increasing trend for FPF was also noted as the size of the carrier particles increased from 425–600 μm to 850–1000 μm. However, as the 4-pin piercing mechanism employed in the commercial Aerolizer® produced piercing holes with 0.6 mm diameter, the emitted fraction (EF) of lactose carriers with larger sizes (i.e., GL 425–600 μm, GL 600–850 μm, and GL 850–1,000 μm) was significantly reduced (Table II), ranging only between 62.5 and 67.0%. As a result, the respirable fraction for these larger size fractions GL 425–600 μm, GL 600–850 μm, and GL 850–1,000 μm was reduced, although not significantly different from smaller counterparts.
In Vitro Aerosolization Performance with Capsules40.6 (0.6 mm, four holes) at 90 L/min
A greater flow rate of 90 L/min was utilized under the same device and capsule design (Capsules40.6) for the impaction study to further investigate the detachment mechanisms in the granulated carriers. Results are shown in Table III. Similar trends were observed in this study as observed at the lower flow rates (Table II), although different magnitudes for each parameter were obtained. At 90 L/min compared to 60 L/min, decreased MMAD value (1.7 μm) and a broader GSD (1.9 ± 0.1) (Table III) were observed. Additionally, compared to flow rate at 60 L/min (FPF 19.3–26.7%, RF 12.6–19.3%), the higher flow rate (90 L/min) increased FPF (32.8–41.6%) and RF (25.1–30.4%).
Table III.
In Vitro Aerosolization Performance with Capsules40.6 in Aerolizer at 90 L/min. Emitted Fraction (EF), Fine Particle Fraction (FPF), Respiratory Fraction (RF), Mass Median Aerodynamic Diameter (MMAD), and Geometric Standard Deviation (GSD) Obtained from Formulations Containing SBS Blended with Granulated Lactose Carriers GL 212–250 μm, GL 250–300 μm, GL 300–425 μm, GL 425–600 μm, GL 600–850 μm, and GL 850–1,000 μm (mean ± SD, n = 3)
| GL 212–250 μm | GL 250–300 μm | GL 300–425 μm | GL 425–600 μm | GL 600–850 μm | GL 850–1,000 μm | |
|---|---|---|---|---|---|---|
| EF (%) | 83.2 (3.5) | 83.6 (5.5) | 81.1 (8.0) | 76.7 (2.8) | 73.2 (7.3) | 69.6*(11.0) |
| FPF (%) | 36.5 (3.1) | 33.8 (5.2) | 35.3 (7.0) | 32.8 (3.7) | 36.8 (6.0) | 41.6* (5.6) |
| RF (%) | 30.4 (3.7) | 28.5 (6.6) | 29.0 (8.3) | 25.1 (2.7) | 27.1 (6.5) | 29.3 (8.4) |
| MMAD (μm) | 1.69 (0.03) | 1.68 (0.11) | 1.65 (0.04) | 1.67 (0.03) | 1.58 (0.03) | 1.66 (0.01) |
| GSD | 1.8 (0.1) | 1.8 (0.2) | 1.8 (0.2) | 1.8 (0.1) | 1.9 (0.0) | 2.0 (0.2) |
*P < 0.05
In Vitro Aerosolization Performance with Capsules1.2 (1.2 mm, one hole)
In order to potentially increase EF of larger size formulations, another cascade impaction study at 90 L/min with larger pierced holes (1.2 mm, one hole) was performed. Small holes in the capsule may result in particle deaggregation by several mechanisms such as shear force break up and also higher collisions of the particles with the capsule. For example, a previous study demonstrated more efficient powder dispersion resulting from forcing powder agglomerates through the capsule holes that, in turn, induced improved powder break up (29). As our hypothesis for these studies centers around the particle collisions with the device as a deaggregation mechanism, modified piercing holes (1.2 mm) were used in the present study. These holes were significantly greater than the size of the larger carrier formulations to eliminate confounding deaggregation mechanisms that may have contributed to the aerosol dispersion studies presented above.
According to Table IV, using capsules with 1.2 mm pierced holes, all formulations had EFs around or higher than 85%. The larger piercing aperture size also reduced the variability of all measured parameters of the dispersion studies, especially in the powders with larger carrier particles (Table IV). As expected, the larger piercing holes employed resulted in a slight decrease in FPF of GL 425–600 μm, GL 600–850 μm, and GL 850–1,000 μm formulations (Tables III and IV), though it was not statistically significant.
Table IV.
In Vitro Aerosolization Performance with Capsules1.2 in Aerolizer at 90 L/min. Emitted Fraction (EF), Fine Particle Fraction (FPF), Respiratory Fraction (RF), Mass Median Aerodynamic Diameter (MMAD), and Geometric Standard Deviation (GSD) Obtained from Formulations Containing SBS Blended with Granulated Lactose Carriers GL 212–250 μm, GL 250–300 μm, GL 300–425 μm, GL 425–600 μm, GL 600–850 μm, and GL 850–1,000 μm (Mean ± SD, n = 3)
| GL 212–250 μm | GL 250–300 μm | GL 300–425 μm | GL 425–600 μm | GL 600–850 μm | GL 850–1,000 μm | |
|---|---|---|---|---|---|---|
| EF (%) | 89.0 (1.7) | 89.2 (0.8) | 89.1 (1.4) | 90.0 (1.2) | 88.9 (2.1) | 84.6 (3.8) |
| FPF (%) | 37.1 (4.5) | 38.2 (6.1) | 34.9 (3.3) | 30.7 (2.6) | 31.2 (2.9) | 37.8*(4.2) |
| RF (%) | 33.0 (4.3) | 34.1 (5.2) | 31.1 (2.8) | 27.6 (2.6) | 27.7 (2.6) | 32.0 (4.5) |
| MMAD (μm) | 1.73 (0.02) | 1.68 (0.04) | 1.70 (0.06) | 1.60 (0.04) | 1.62 (0.07) | 1.64 (0.06) |
| GSD | 1.7 (0.0) | 1.7 (0.0) | 1.7 (0.1) | 1.7 (0.0) | 1.8 (0.0) | 1.8 (0.1) |
*P < 0.05
Although aerosol performance of the largest lactose formulation (i.e., GL 850–1,000 μm) decreased when larger piercing holes were used, the FPF was not significantly different from the smallest lactose formulations (Capsule1.2, FPF90L/min: 37.8 vs. 37.1% for the largest and smallest lactose formulations, respectively). Meanwhile, although not statically significantly different, a slight decreasing trend in FPF was noticed as the granular lactose size was increased from 212–250 μm to 600–850 μm.
DISCUSSION
It is well documented that the particle size, size distribution (4), morphology (33,34), surface roughness (23,35), surface area, flowability (6), density, and surface energy (7) of lactose carriers all have influence on the DPI formulations. The granulated lactose carrier particles produced in this study had similar solid-state, flowability, surface area, and true density, but different size, morphology, roughness, and bulk/tap density.
Particle Size Distribution
Generally, the size distributions of the lactose granules from laser diffraction (Fig. 4a) corresponded to the data obtained from image analysis (Fig. 4b), with the exception of GL 600–850 μm and GL 850–1,000 μm due to measurement limit of laser diffraction instrument (Fig. 4a). It is interesting to note that the particle size distribution of the sieved granulated lactose samples using laser diffraction and image analysis of SEM micrographs did not exactly match the sieve fraction value. This difference in the size of the sieved granules may be ascribed to their non-spherical elongated shape (36). The smallest cross-sectional dimensions of the particles determine their passage through the sieve mesh (37), whereas diameters obtained from laser diffraction and image analysis are different (38).
Specific Surface Area
Of all the granulated lactose samples with different size fractions, the specific surface area remained a constant value similar to the primary lactose carriers, contradictory to theoretical surface area of smooth spherical particles in which surface area is calculated to decrease progressively with increased particle size (13). The constant surface area observed in spite of different granule sizes is related to particle roughness (Fig. 6a) and confirmed by a linear relationship (r2 = 0.9787) between mean diameter of lactose granules and roughness value (Fig. 6b). Moreover, as a result of the constant surface area across the different carrier particle systems, the average drug load per unit calculated surface area (carrier surface payload) is therefore expected to be the same or most possibly slightly decrease with increasing carrier diameter (11). The slight decrease is explained by the distribution of the micronized drugs mainly on the surface of the lactose granules, instead of the both exterior and interior portions, which however also accounts for the calculation of the specific surface area of the lactose granules. In the previous studies, when carriers of different sizes were compared, the findings are often confounded with surface area coverage of the carrier particle by the drug and therefore according to the “active site theory” differences in drug adhesion may happen. In the present studies, therefore, we postulate that the difference in potential active sites could be very small between the different carrier size fractions (39).
Density and Flowability
The granulated lactose density measurements had a decreasing trend with size, which may be explained by the increased interparticle space as granule size is increased. As a result, compared to smaller lactose, granulated lactose powders with large sizes may have a fewer number of particle–particle contact points with neighboring particles.
Theoretically, powder flow and mean particle size are positively correlated (40). Interestingly, when the size of the lactose granules was increased from 200 to 850 μm, the flowability of the lactose granules did not improve significantly demonstrated by both Carr’s Index and Angle of Repose (Table I). Additionally, in previous study, the Carr’s Index of spray dried mannitol (90–125 μm) was around 13% (41), much lower than that of lactose granules (Carr’s Index, 17%) even with a larger size fraction (200–250 μm). The reason of the not significantly improved flow may be the mechanical interlocking among the rough lactose particles, which prevents powder motion (42).
Aerosol Performance
Adhesion Forces and Bulk Powder Properties
The bulk powder properties characterized above can influence the balance between adhesion forces and separation forces for lactose granules-based adhesive mixtures. It has been well studied that the size of the interactive forces between carrier particle and drug particle significantly influence the redispersion efficiency of micronized drugs during inhalation while fixed device is used.
During powder mixing, frictional and inertial press on are related to the adhesion of drug particles on the carrier surface (14). The differences of these forces in the different lactose size fractions may explain the improvement of aerosol redispersion observed with the coarsest fraction of granulated lactose. In our study, larger size lactose carrier which had lower bulk density (Table I) may have a fewer number of contacts with neighboring particles, resulting in smaller effective surface area involved in particle–particle collisions and thus less magnitude of triboelectrification during mixing. Furthermore, the flow properties as the function of lactose granule size didn’t improve significantly (Table I). As a result, it is speculated that the frequency of impacted friction and inertial press on force of granular lactose during mixing would not increase significantly with increasing size fractions. Additionally, although at the beginning of mixing process, drugs are randomly distributed over the carrier surface, they tend to accumulate in carrier surface irregularities (steep slopes and clefts) (18,43). Such surface cavities are not necessarily “active sites” with high adhesive forces (39). The drug particles hidden in carrier surface discontinuities are subjected to lower or fewer press on forces than drug particles that are attached to the flat exposed carrier particle surfaces (44). As observed in Fig. 7, most drug particles were accumulated in the surface discontinuities, likely forming weak agglomerates between the primary particles. According to a previous study (13), intensive mixing of the drug particles with relatively large lactose granules would break up hard natural drug agglomerates and render holes for weak drug agglomerates. The weak agglomerates also were demonstrated by the relatively small aerodynamic size of the dispersed drugs (Tables II, III, and IV), especially compared to the d50% of the micronized salbutamol sulfate particle size distribution (Fig. 1). These weak drug agglomerates have higher inertial force in collision, so could be redispersed easily into primary drug particles during inhalation. Therefore, although the size of press on forces generally will increase with increasing carrier diameter due to inertia mass, the effective surface area available for press on effect decreases and consequently the effectiveness of the press on force and the adhesive force decreases. Strong enough adhesive force is required such that drug preferentially adheres to the carrier during mixing, so as to achieve adequate blend homogeneity (45). So, the relatively poor blending uniformity observed with GL 850–1,000 μm (Fig. 2) partially supports the lower adhesive force in the larger lactose size fraction mixtures. Taking into account that blending uniformity is not the most efficient method, adhesive forces determined by more direct methods such as centrifugation and sieving would be investigated in the future study. The reduced adhesive forces between the carrier and drug may also be supported by the improved aerosol dispersion observed (Tables II, III, and IV) for GL 850–1,000 μm.
Fig. 7.
SEM pictures of granulated lactose GL 212–250 μm blended with 2% SBS. Scale bar denotes 20 μm
Since the granulated lactose had relatively rough surface, it is likely that press on forces would be reduced as these surface irregularities will shelter drug particles during contact between the lactose carriers. A greater quantity of drug powder would be required to fill the cavities on the surface of the granulated lactose before press on forces would become important and limit aerosol performance. It is postulated, therefore, granulated lactose may be useful in making high drug loaded DPI formulations with acceptable aerosol performance. In the present study, 2% drug was incorporated in the blends, in which it was observed that the lactose carrier surface discontinuities were not fully occupied or saturated (Fig. 7). Since the surface roughness of granulated lactose increased with increasing size fractions (Fig. 6b), the larger lactose could be expected to provide a larger volume of surface pores to shelter a significantly higher amount of drugs from press on forces during mixing (14). As a result, larger granulated lactose particles could be potential carriers for high drug loading dry powder inhalation formulations.
Separation Forces and Bulk Powder Properties
The in vitro cascade impaction aerosol dispersion studies of adhesive mixtures for inhalation do not solely reflect the effectiveness of the adhesion forces, but also incorportate the separation forces generated in the inhaler. Detachment by fluid flow forces and detachment by impaction/collision forces are the two major mechanisms involved in drug dispersion from large carrier particles (11,12). Detachment by flow is preferred for carriers with relatively flat surface, such that drug particles could be exposed to the flow stream without obstructed path. Detachment by flow also facilitates the dispersion of larger drug particles, either primary drug particles or drug agglomerates, due to the increased drug surface area interacting with flow stream. Since the surface roughness of the granulated lactose increased significantly with size (Fig. 6), according to the theory, the detachment force from flow stream would decrease with the increased granulated lactose size fractions.
Different from detachment by flow, detachment by impaction arises from the abrupt collisions between carrier particles and device walls and/or grids. Previous studies have hypothesized that there would be a transition from flow detachment to impaction detachment with larger (and significantly rough) lactose carriers. It was also shown that larger lactose particles with significantly rough surface exhibited greater aerosol performance than smaller particles (13). However, the carrier particle diameter used in the previous studies was around or less than 300 μm, so it may be improper to extrapolate the improved mechanical impaction detachment force and aerosol performance for carrier particles larger than 300 μm as used in our study. In addition, as shown in the previous study, even though the mechanical impaction force was still the predominant detachment force, the aerosol performance of the 250–300 μm granular lactose formulations was not significantly greater than the size fraction immediately preceding it (212–250 μm) due to relatively heavy mass of large carrier particles. It was speculated that mechanical impaction force of the granular lactose would not continuously increase with increasing carrier diameter/mass (13). Our observations in these studies of similar mass median aerodynamic diameter (MMAD) of the deposited drug (Tables II, III and IV) agreed with this assumption. In another study investigating the relationship between mechanical impaction force and particle detachment it was shown that a greater impaction force was needed for drug particles with decreasing diameter (46). It follows that the magnitude of impaction force could be similar, if similar aerodynamic diameters of deposited drugs were generated during inhalation of different formulations. As shown in Tables II, III, and IV, MMAD values of the deposited drugs didn’t significantly decrease with increasing granulated lactose size fractions under the same impaction parameters. Thus, the mechanical impaction force may not significantly increase when lactose size increased from 212–250 μm to 850–1,000 μm. However, previous study showed that the impaction force increases with increasing carrier size (13). Also, similar significantly low MMAD values were achieved under different carrier diameters compared to the value of d50% of micronized salbutamol sulfate (Fig. 1), which indicates that lower impaction force was already enough to disperse down to primary size of the micronized drug particles. Thus, it is quite possible that impaction force did vary with increasing carrier diameter, but detachment due to mechanical impaction force didn’t increase significantly.
Accordingly, for the separation force of the different lactose size fractions in this study, detachment by flow decreases significantly and mechanical impaction force increases with increasing carrier diameter and surface roughness, but doesn’t contribute to the increasing detachment. As a result, the detachment forces are not as effective for larger and coarser granular lactose as for smaller ones, confirmed by a decrease trend of fine particle fraction from GL 212–250 μm to GL 600–850 μm (Table IV). The improved aerosol performance of GL 600–850 μm is explained, on the other hand, by the reduced adhesive force as discussed in the “adhesion forces and bulk powder properties” section.
CONCLUSION
To our knowledge, this study for the first time systemically studied the physicochemical properties and aerosol performance of significantly large lactose carriers (>200 μm) across a wide range of narrow sieve fractions, In our study, the particle size of the lactose granules had no significant effect on solid-state, specific surface area, true density, and flowability of the granulated lactose carriers. However, larger lactose granules had rougher particle surfaces and smaller bulk and tapped densities. This suggested that roughness, flowability, bulk, and tapped density as well as the size of lactose could play a role together on the adhesive and removal force between drug particle and lactose particle.
In summary, the coarser size fractions of lactose (850–1,000 μm) had a slight improvement of in vitro deposition under both 60 and 90 L/min with and without enlarged piercing holes (Tables II, III, and IV) compared to the immediately preceding size fractions (600–850 μm). A slight decreasing trend of FPF for the lactose carriers ranging from 212–250 μm to 600–850 μm was observed at 90 L/min with larger piercing holes (1.2 mm) (Table IV). The surface roughness, size, bulk/tapped density as well as unusual powder flow are speculated to result in poor adhesion force of the largest and coarsest lactose granules, which could cause a slight increase of FPF. Meanwhile, surface roughness limits the strength of detachment by flow, which could explain a slight decreasing trend of FPF with size increasing from 212–250 μm to 600–850 μm with enlarged piercing holes, although not significantly different. Past studies, which prefer smaller carrier diameters, contribute the increased aerosol performance to increased specific surface area of smaller carriers and increased fluid stream across drug particles on flat carrier surface. However, the larger granulated carrier particles in this study possess different physical properties, with similar specific surface area over increasing size fractions and different predominate detachment mechanism, which is detachment by impaction force instead of fluid flow. Taken account of the different properties of the granulated lactose, poorer or enhanced aerosolization performance is not an inherent property to large size of granulated lactose carriers. The significantly large granulated lactose as the DPI carriers leads to a new way to investigate and optimize DPI formulations.
Acknowledgments
Conflict of Interest
The authors report no conflicts of interest.
References
- 1.Hickey AJ. Pharmaceutical inhalation aerosol powder dispersion—an unbalancing act. Am Pharm Rev. 2003;6:106–10. [Google Scholar]
- 2.Smyth HD, Hickey AJ. Carriers in drug powder delivery. Am J Drug Deliv. 2005;3:117–32. doi: 10.2165/00137696-200503020-00004. [DOI] [Google Scholar]
- 3.Adi H, Larson I, Stewart PJ. Adhesion and redistribution of salmeterol xinafoate particles in sugar-based mixtures for inhalation. Int J Pharm. 2007;337:229–38. doi: 10.1016/j.ijpharm.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 4.Steckel H, Muller BW. In vitro evaluation of dry powder inhalers. 2. Influence of carrier particle size and concentration on in vitro deposition. Int J Pharm. 1997;154:31–7. doi: 10.1016/S0378-5173(97)00115-4. [DOI] [Google Scholar]
- 5.Flament MP, Leterme P, Gayot A. The influence of carrier roughness on adhesion, content uniformity and the in vitro deposition of terbutaline sulphate from dry powder inhalers. Int J Pharm. 2004;275:201–9. doi: 10.1016/j.ijpharm.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 6.Pitchayajittipong C, Price R, Shur J, Kaerger JS, Edge S. Characterisation and functionality of inhalation anhydrous lactose. Int J Pharm. 2010;390:134–41. doi: 10.1016/j.ijpharm.2010.01.028. [DOI] [PubMed] [Google Scholar]
- 7.Jiang RG, Zhang PW, Wang LQ, Liu H, Pan WS, Wang CL. Effect of surface modification on surface energy of lactose and performance of dry powder inhalations. Yao Xue Xue Bao. 2005;40:373–6. [PubMed] [Google Scholar]
- 8.Guenette E, Barrett A, Kraus D, Brody R, Harding L, Magee G. Understanding the effect of lactose particle size on the properties of DPI formulations using experimental design. Int J Pharm. 2009;380:80–8. doi: 10.1016/j.ijpharm.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 9.Kougoulos E, Marziano I, Miller PR. Lactose particle engineering: Influence of ultrasound and anti-solvent on crystal habit and particle size. J Cryst Growth 312:3509–20.
- 10.Ferrari F, Cocconi D, Bettini R, Giordano F, Santi P, Tobyn M, et al. The surface roughness of lactose particles can be modulated by wet-smoothing using a high-shear mixer. AAPS PharmSciTech. 2004;5:e60. doi: 10.1208/pt050460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Boer AH, Hagedoorn P, Gjaltema D, Goede J, Frijlink HW. Air classifier technology (ACT) in dry powder inhalation. Part 1. Introduction of a novel force distribution concept (FDC) explaining the performance of a basic air classifier on adhesive mixtures. Int J Pharm. 2003;260:187–200. doi: 10.1016/S0378-5173(03)00250-3. [DOI] [PubMed] [Google Scholar]
- 12.Voss A, Finlay WH. Deagglomeration of dry powder pharmaceutical aerosols. Int J Pharm. 2002;248:39–50. doi: 10.1016/S0378-5173(02)00319-8. [DOI] [PubMed] [Google Scholar]
- 13.Donovan MJ, Smyth HD. Influence of size and surface roughness of large lactose carrier particles in dry powder inhaler formulations. Int J Pharm. 2010;402:1–9. doi: 10.1016/j.ijpharm.2010.08.045. [DOI] [PubMed] [Google Scholar]
- 14.Dickhoff BH, de Boer AH, Lambregts D, Frijlink HW. The interaction between carrier rugosity and carrier payload, and its effect on drug particle redispersion from adhesive mixtures during inhalation. Eur J Pharm Biopharm. 2005;59:197–205. doi: 10.1016/j.ejpb.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 15.Grasmeijer F, Hagedoorn P, Frijlink HW, de Boer AH. Drug content effects on the dispersion performance of adhesive mixtures for inhalation. PLoS One. 2013;8:e71339. doi: 10.1371/journal.pone.0071339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Islam N, Stewart P, Larson I, Hartley P. Lactose surface modification by decantation: are drug-fine lactose ratios the key to better dispersion of salmeterol xinafoate from lactose-interactive mixtures? Pharm Res. 2004;21:492–9. doi: 10.1023/B:PHAM.0000019304.91412.18. [DOI] [PubMed] [Google Scholar]
- 17.Hickey AJ, Mansour HM, Telko MJ, Xu Z, Smyth HD, Mulder T, et al. Physical characterization of component particles included in dry powder inhalers. I. Strategy review and static characteristics. J Pharm Sci. 2007;96:1282–301. doi: 10.1002/jps.20916. [DOI] [PubMed] [Google Scholar]
- 18.de Boer AH, Hagedoorn P, Gjaltema D, Goede J, Kussendrager KD, Frijlink HW. Air classifier technology (ACT) in dry powder inhalation. Part 2. The effect of lactose carrier surface properties on the drug-to-carrier interaction in adhesive mixtures for inhalation. Int J Pharm. 2003;260:201–16. doi: 10.1016/S0378-5173(03)00264-3. [DOI] [PubMed] [Google Scholar]
- 19.Louey MD, Razia S, Stewart PJ. Influence of physico-chemical carrier properties on the in vitro aerosol deposition from interactive mixtures. Int J Pharm. 2003;252:87–98. doi: 10.1016/S0378-5173(02)00621-X. [DOI] [PubMed] [Google Scholar]
- 20.Cline D, Dalby R. Predicting the quality of powders for inhalation from surface energy and area. Pharm Res. 2002;19:1274–7. doi: 10.1023/A:1020338405947. [DOI] [PubMed] [Google Scholar]
- 21.Donovan MJ, Kim SH, Raman V, Smyth HD. Dry powder inhaler device influence on carrier particle performance. J Pharm Sci. 2012;101:1097–107. doi: 10.1002/jps.22824. [DOI] [PubMed] [Google Scholar]
- 22.Littringer EM, Mescher A, Schroettner H, Achelis L, Walzel P, Urbanetz NA. Spray dried mannitol carrier particles with tailored surface properties—the influence of carrier surface roughness and shape. Eur J Pharm Biopharm. 2012;82:194–204. doi: 10.1016/j.ejpb.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 23.Kawashima Y, Serigano T, Hino T, Yamamoto H, Takeuchi H. Effect of surface morphology of carrier lactose on dry powder inhalation property of pranlukast hydrate. 1998;172:188.
- 24.Li J, Tao L, Buckley D, Tao J, Gao J, Hubert M. The effect of the physical state of binders on high-shear wet granulation and granule properties: a mechanistic approach to understand the high-shear wet granulation process. Part IV. The impact of rheological state and tip-speeds. J Pharm Sci. 2013;102:4384–94. doi: 10.1002/jps.23750. [DOI] [PubMed] [Google Scholar]
- 25.Fujiwara M, Dohi M, Otsuka T, Yamashita K, Sako K. Influence of binder droplet dimension on granulation rate during fluidized bed granulation. Chem Pharm Bull (Tokyo) 2013;61:320–5. doi: 10.1248/cpb.c12-00969. [DOI] [PubMed] [Google Scholar]
- 26.Ogawa T, Uchino T, Takahashi D, Izumi T, Otsuka M. Pharmaceutical production of tableting granules in an ultra-small-scale high-shear granulator as a pre-formulation study. Drug Dev Ind Pharm. 2012;38:1390–3. doi: 10.3109/03639045.2011.652637. [DOI] [PubMed] [Google Scholar]
- 27.Mangwandi C, Adams MJ, Hounslow MJ, Salman AD. An investigation of the influence of process and formulation variables on mechanical properties of high shear granules using design of experiment. Int J Pharm. 2012;427:328–36. doi: 10.1016/j.ijpharm.2012.02.029. [DOI] [PubMed] [Google Scholar]
- 28.Singh I, Kumar P. Preformulation studies for direct compression suitability of cefuroxime axetil and paracetamol: a graphical representation using SeDeM diagram. Acta Pol Pharm. 2012;69:87–93. [PubMed] [Google Scholar]
- 29.Coates MS, Fletcher DF, Chan HK, Raper JA. The role of capsule on the performance of a dry powder inhaler using computational and experimental analyses. Pharm Res. 2005;22:923–32. doi: 10.1007/s11095-005-4587-y. [DOI] [PubMed] [Google Scholar]
- 30.Zeng XM, Martin GP, Marriott C, Pritchard J. The influence of crystallization conditions on the morphology of lactose intended for use as a carrier for dry powder aerosols. J Pharm Pharmacol. 2000;52:633–43. doi: 10.1211/0022357001774462. [DOI] [PubMed] [Google Scholar]
- 31.Li X, Mansour HM. Physicochemical characterization and water vapor sorption of organic solution advanced spray-dried inhalable trehalose microparticles and nanoparticles for targeted dry powder pulmonary inhalation delivery. AAPS PharmSciTech. 2011;12:1420–30. doi: 10.1208/s12249-011-9704-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaialy W, Ticehurst MD, Murphy J, Nokhodchi A. Improved aerosolization performance of salbutamol sulfate formulated with lactose crystallized from binary mixtures of ethanol-acetone. J Pharm Sci. 2011;100:2665–84. doi: 10.1002/jps.22483. [DOI] [PubMed] [Google Scholar]
- 33.Adi S, Adi H, Tang P, Traini D, Chan HK, Young PM. Micro-particle corrugation, adhesion and inhalation aerosol efficiency. Eur J Pharm Sci. 2008;35:12–8. doi: 10.1016/j.ejps.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 34.Adi H, Traini D, Chan HK, Young PM. The influence of drug morphology on aerosolisation efficiency of dry powder inhaler formulations. J Pharm Sci. 2008;97:2780–8. doi: 10.1002/jps.21195. [DOI] [PubMed] [Google Scholar]
- 35.Islam N, Stewart P, Larson I, Hartley P. Surface roughness contribution to the adhesion force distribution of salmeterol xinafoate on lactose carriers by atomic force microscopy. J Pharm Sci. 2005;94:1500–11. doi: 10.1002/jps.20381. [DOI] [PubMed] [Google Scholar]
- 36.Young PM, Edge S, Traini D, Jones MD, Price R, El-Sabawi D, et al. The influence of dose on the performance of dry powder inhalation systems. Int J Pharm. 2005;296:26–33. doi: 10.1016/j.ijpharm.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 37.Kaialy W, Martin GP, Larhrib H, Ticehurst MD, Kolosionek E, Nokhodchi A. The influence of physical properties and morphology of crystallised lactose on delivery of salbutamol sulphate from dry powder inhalers. Colloids Surf B: Biointerfaces. 2012;89:29–39. doi: 10.1016/j.colsurfb.2011.08.019. [DOI] [PubMed] [Google Scholar]
- 38.Sinko PJ. Martin’s physical pharmacy and pharmaceutical sciences. Philadelphia, PA, USA: Lippincott Williams & Wilkins; 2005. [Google Scholar]
- 39.Dickhoff BH, de Boer AH, Lambregts D, Frijlink HW. The effect of carrier surface and bulk properties on drug particle detachment from crystalline lactose carrier particles during inhalation, as function of carrier payload and mixing time. Eur J Pharm Biopharm. 2003;56:291–302. doi: 10.1016/S0939-6411(03)00109-7. [DOI] [PubMed] [Google Scholar]
- 40.Liu LX, Marziano I, Bentham AC, Litster JD, White ET, Howes T. Effect of particle properties on the flowability of ibuprofen powders. Int J Pharm. 2008;362:109–17. doi: 10.1016/j.ijpharm.2008.06.023. [DOI] [PubMed] [Google Scholar]
- 41.Kaialy W, Hussain T, Alhalaweh A, Nokhodchi A. Towards a more desirable dry powder inhaler formulation: large spray-dried mannitol microspheres outperform small microspheres. Pharm Res. 2013. [DOI] [PubMed]
- 42.Chew NYK, Bagster DF, Chan HK. Effect of particle size, air flow and inhaler device on the aerosolisation of disodium cromoglycate powders. Int J Pharm. 2000;206:75–83. doi: 10.1016/S0378-5173(00)00516-0. [DOI] [PubMed] [Google Scholar]
- 43.de Boer AH, Chan HK, Price R. A critical view on lactose-based drug formulation and device studies for dry powder inhalation: Which are relevant and what interactions to expect? Adv Drug Deliv Rev. 2012;64:257–74. doi: 10.1016/j.addr.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 44.Podczeck F. Assessment of the mode of adherence and the deformation characteristics of micronized particles adhering to various surfaces. Int J Pharm. 1996;145:65–76. doi: 10.1016/S0378-5173(96)04718-7. [DOI] [Google Scholar]
- 45.Le VN, Hoang Thi TH, Robins E, Flament MP. Dry powder inhalers: Study of the parameters influencing adhesion and dispersion of fluticasone propionate. AAPS PharmSciTech. 2012;13:477–84. doi: 10.1208/s12249-012-9765-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Concessio NM, VanOort MM, Knowles MR, Hickey AJ. Pharmaceutical dry powder aerosols: Correlation of powder properties with dose delivery and implications for pharmacodynamic effect. Pharm Res. 1999;16:828–34. doi: 10.1023/A:1018865717374. [DOI] [PubMed] [Google Scholar]


