Abstract
The main objective of the current work is to demonstrate the process of passive lateral diffusion in the human nail plate and its effect on the passive transungual permeation of antifungal drug ciclopirox olamine (CPO). A water soluble dye, methyl red sodium salt (MR) was used to visualize the process of lateral diffusion using a novel suspended nail experiment. The decline in concentration of CPO correlates with that of concentration of MR from the proximal to the distal end of the nail in suspended nail study. Three toenails each were trimmed to 5 mm × 5 mm (25 mm2), 7 mm × 7 mm (49 mm2), and 9 mm × 9 mm (81 mm2) to study the extent and effect of lateral diffusion of the CPO on its in vitro transungual permeation. The permeation flux of CPO decreased as the surface area of the toenail increased. There was a positive correlation between the concentrations of CPO and MR in the area of application and in the peripheral area of the toenails of the three surface areas, confirming the findings in the suspended nail experiment. Profound lateral diffusion of CPO was demonstrated and shown to reduce the in vitro passive transungual drug permeation and prolong the lag-time in human toenails. The study data implies that during passive in vitro transungual permeation experiments, the peripheral nail around the area of drug application has to be kept to a minimum, in order to get reliable data which mimics the in vivo situation.
KEY WORDS: ciclopirox olamine, lateral diffusion, passive, topical, transungual
INTRODUCTION
The barrier properties of the nail plate and its thickness pose the greatest challenge towards permeation of any topically applied antifungal drug (1–3). One of the approaches to improve the passive transungual drug delivery is the use of chemical penetration enhancers. Various methods have been investigated and reported to screen these penetration enhancers (4–19). Although these screening methods are high throughput and give reasonable estimate on the ability of the penetration enhancers to increase drug delivery into and across the human nail, the results have to be confirmed in a transungual permeation study. This is achieved in vitro, using the Franz diffusion cells with nail adapters. Human cadaver toenails in the in vitro transungual permeation studies serve as the best membrane to simulate the in vivo transungual permeation and determine the effect of the penetration enhancers on the nail barrier properties. It is expected that the data generated in this in vitro study will reflect the performance of the penetration enhancers in vivo. The lateral movement of topically applied drug molecules is reported as an observation in iontophoretic transungual permeation (20,21) and in passive skin permeation (22,23). However, the effects of lateral diffusion on passive transungual permeation of drugs have not been investigated in detail. In case of iontophoresis, the drug permeation is attained with the aid of electric current, whereas in passive drug delivery the concentration gradient across the thickness of the nail (from dorsal to ventral surface) acts as the only driving force for drug permeation. Therefore, the effect of lateral diffusion on the passive transungual permeation is profound.
It was hypothesized that the lateral movement of drug molecules around the site of application on the nail or lateral diffusion, during an in vitro experiment reduces the overall permeation of the drug molecule across the nail plate (Fig. 1). When there are simultaneous lateral and transungual permeation during the in vitro permeation experiment, the generated data underestimates the potential of the penetration enhancer or a prototype formulation. This data can therefore lead to the early elimination of a potential penetration enhancer or formulation from further investigation. Therefore, the main objective of the current work was to demonstrate the process of passive lateral diffusion in the human nail plate and determine its effect on the passive transungual permeation of antifungal drug ciclopirox olamine (CPO). The studies were performed in the presence of thiourea (TU), the nail penetration enhancer selected for CPO in our previous report (4).
Fig. 1.
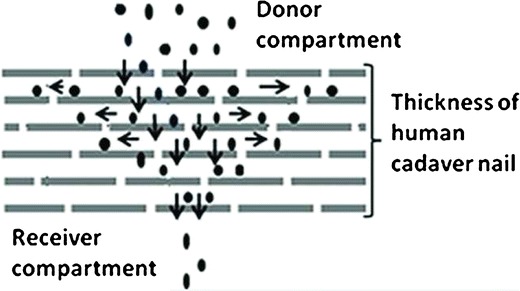
Theoretical representation of lateral diffusion of drug molecules in human nails. The horizontal and the vertical arrows denote the lateral movement of the molecules and the transungual diffusion, respectively
MATERIALS AND METHODS
CPO (99.0%) was purchased from Haorui Pharma-Chem Inc., Edison, NJ, USA. Potassium phosphate monobasic (99.99%), sodium hydroxide (ACS reagent, ≥97.0%), gentamicin sulfate (Potency ≥590 μg Gentamicin base per milligrams of salt), methyl red sodium salt (MR) (95% dye content), dimethyl sulfate (≥99.8%), and triethylamine (for HPLC, 99.0%) were bought from Sigma Aldrich. TU (ACS Grade, ≥99%) and sodium phosphate dibasic (Anhydrous, ACS reagent, ≥99%) were obtained from Acros Organics. Soft stainless steel wire with 0.02 gauge size was bought from Malin Co. (Brookpark, OH). Franz diffusion cells and neoprene nail adapters were purchased from PermeGear. The human nail clippings were donated by healthy volunteers (20–50 year) in the department of pharmaceutical sciences, Temple University. Human cadaver toenails were purchased from Anatomy Gifts Registry (Hanover, MD). Nanopure water was used for preparation of 10 mM pH 7.4 phosphate buffered saline. HPLC grade acetonitrile from Fischer scientific was used in preparation of mobile phase. All the chemicals were used as received without any additional purification.
Sample Preparation for HPLC and UV/Vis Analysis (4)
In order to quantify the amounts of CPO within the nail, a modification of the reported derivatization method (24–26) for CPO was used. The assay of CPO in the nail was performed by first dissolving the nails in 0.5 mL of 1 M sodium hydroxide (NaOH) solution. The nail solutions were filtered through Restek® 0.45 μ syringe filters (cellulose acetate membrane). These solutions were diluted sufficiently to reduce the strength of 1 M NaOH to 0.1 M before derivatization. For the derivatization of CPO, 25 μL of dimethyl sulfate was added to 0.2 mL of the dilute nail solution, and vortexed for 5 min. These solutions were maintained at 37 ± 1°C for 20 min. Twenty five microliters of triethylamine were added and vortexed for 1 min to terminate the reaction. The solutions were then filtered through Restek® 0.22 μ syringe filters (cellulose acetate membrane) and analyzed using the HPLC method. In order to quantify the CPO in the buffer, a similar pre-column derivatization process was used. To 0.2 mL of the samples, 50 μL of 0.1 M NaOH and 25 μL of dimethyl sulfate were added and vortexed for 5 min. The solutions were maintained at 37 ± 1°C for 20 min prior to the addition of 25 μL of triethylamine. The solutions were vortexed, filtered through Restek® 0.22 μ syringe filters (cellulose acetate membrane), and analyzed using HPLC.
High Pressure Liquid Chromatography
CPO in the derivatized samples was quantified using HPLC (24–26). An Agilent 1100 series HPLC with autosampler and dual wavelength detector was used. The mobile phase was acetonitrile to water (50:50 v/v) with a flow rate of 1 mL/min. The column was a 150 by 4.6 mm Eclipse Plus C18 column (Agilent). The sample injection volume was 50 μL. The wavelength of detection for derivatized CPO was 298 nm. The calibration curve was constructed by injecting ten concentrations (0.09, 0.18, 0.36, 0.73, 1.46, 2.92, 5.84, 11.68, 23.36, and 46.73 μg/mL) of derivatized CPO in triplicate. Six different sample solutions of the concentration, 2.92 μg/mL, were injected on the same day to determine the intra-day precision. The inter-day precision was estimated by injecting the same sample solution on three consecutive days. The intra-day and inter-day precision values are expressed as % CV.
UV/Vis Spectroscopy
MR level in the nail and the buffer was quantified using visible spectroscopy at 480 nm. The absorbance of solution of nails dissolved in 1 M NaOH, without MR was used to correct the absorbance of the samples. A standard curve for MR in the nail solution was obtained using a concentration range of 6.77 to 108.33 μg/mL.
Recovery of CPO from Human Nails (4)
Solutions of CPO in pH 7.4 phosphate buffered saline (PBS) with concentrations 0.0253, 0.0505, 0.202, 0.809, 3.24, and 6.47 mg/mL were prepared. Approximately 20 mg (19–23 mg) of human nail clippings were weighed for each sample. Each concentration was studied in triplicate. The nail clippings were treated with 200 μL of each of these solutions for 3 h at 32 ± 1°C. Two hundred microliters of 1 M NaOH were added to dissolve the nail clippings. The nail solutions were diluted to reduce the concentration of NaOH solution from 1 to 0.1 M and the sample preparation method was followed using the dilute nail solution to quantify the concentration of CPO. The standard solutions were derivatized as shown above and quantified using HPLC method described in the previous section. The percent recovery was calculated using the ratio of the recovered concentration of the nail sample to that of the corresponding standard solution. The use of human nail clippings in the study was approved by the Temple University Institutional Review Board (Protocol number 13671).
Lateral Diffusion of CPO in the Nail
MR was used to visualize the lateral diffusion in human cadaver toenails. A 5% w/w solution of TU was prepared using 10 mM phosphate buffered saline (pH 7.4), containing 0.01% w/v gentamicin sulfate as the antimicrobial agent (PBS-GS). This buffer was used to prepare a 3 mg/ml solution of MR. An excess amount of CPO was added to the above solution and equilibrated for 72 h to form a saturated solution of CPO in presence of MR and TU (C-MR-TU). Two cadaver toenails were hydrated at 100% RH for 24 h prior to the start of the experiment. Each of the toenails was cut longitudinally to produce two equal pieces. Each nail piece was suspended vertically with the proximal end suspended 2 mm into the C-MR-TU solution. Figure 2 shows the schematic of the experimental method. The study was performed in duplicate. The duration of the experiment was determined by the time required for MR to diffuse to the distal end of the nail, when observed visually. At the end of experiment, the nails were dismounted and cut into different sections (based on the color gradient of the dye). The weights, thicknesses, and the lengths (x1 to x6) of each section were measured. Each of the sections was dissolved in 0.2 mL of 1 M NaOH solution. The nail solution was derivatized and analyzed using HPLC. The absorbance of each of the undiluted nail solutions, prior to derivatization of CPO, was quantified using the process described in the previous sections.
Fig. 2.
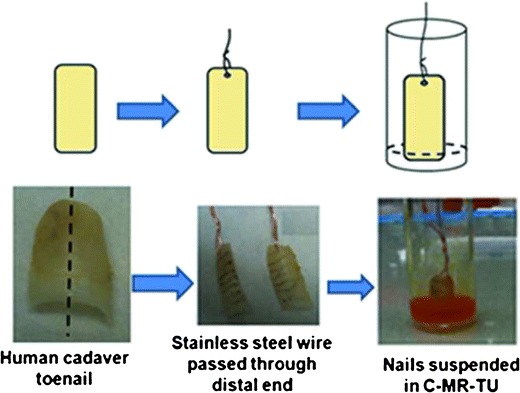
Schematic of the suspended nail experiment to show lateral movement of CPO in human nails
In Vitro Transungual Permeation of CPO
The C-MR-TU solution was prepared as described in the previous section. The cadaver toenails were pre-hydrated at 100% RH for 24 h. The thickness of the nails was measured using digital calipers (Marathon), prior to mounting on 3 mm diameter nail adapters (PermeGear) (Fig. 3a). Three toenails each were trimmed to the sizes 5 mm × 5 mm (25 mm2), 7 mm × 7 mm (49 mm2), and 9 mm × 9 mm (81 mm2) (Fig. 4). These different nail sizes were used to study the extent of lateral diffusion of CPO during the in vitro transungual permeation. The receiver compartment of the Franz cell (Fig. 3b) was filled with 3 mL of PBS-GS (pH 7.4), and the temperature was maintained at 32 ± 1°C. To mimic daily dosing, the nails were dosed with 21.4 μL of C-MR-TU and kept occluded for 40 days. The solution from the previous day was removed from the donor compartment, prior to application of subsequent doses of C-MR-TU. On days 5, 10, 15, 20, 25, 30, 35, and 40, a sample of 0.5 mL of receiver medium was removed and an equal volume of fresh pH 7.4 PBS-GS was added. Additionally on day 40, the nails were dismounted and the area of drug application and the surrounding sections of the nail periphery were cut (Fig. 4) for separate analysis. These sections were separately dissolved in 0.1 mL of 1 M NaOH. The levels of CPO and MR in the nail were quantified using HPLC (298 nm) and visible light spectroscopy (480 nm), respectively. Similarly, the samples withdrawn at the different time points were quantified for CPO. The experiment was performed in triplicate for each nail size.
Fig. 3.
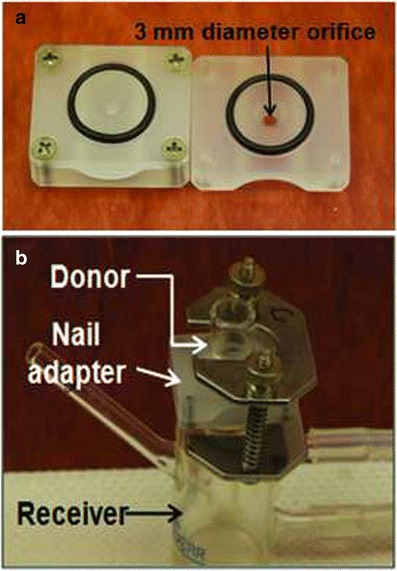
a Neoprene nail adapters (PermeGear) with an orifice diameter of 3 mm. b Franz diffusion cell with nail adapter
Fig. 4.
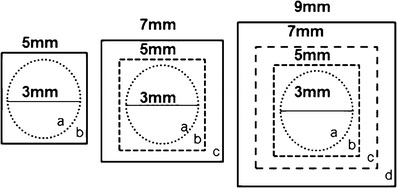
Sectioning the nails after the transungual permeation with section “a” under the orifice and peripheral sections “b”, “c” and “d” around the orifice
Statistical Analysis of Data
The transungual permeation studies were performed in triplicate and one way analysis of variance (ANOVA) was used to determine statistical difference (Minitab 16 software). The Tukey’s post hoc test was used to make one-way multiple comparisons and a p value < 0.05 was considered to be significantly different.
RESULTS
High Pressure Liquid Chromatography
A linear response was attained for CPO in the range of 0.09 to 46.73 μg/mL with a correlation coefficient of 0.9996. The retention time for the derivatised CPO was 4.6 min. The limit of detection (LOD) was 25 ng/mL and the limit of quantification (LOQ) was 90 ng/mL. The intra-day and inter-day precision was found to be 0.4 and 1.24%, respectively.
Visible Spectroscopy
A linear response with a correlation coefficient of 0.9981 was obtained for MR in the concentration range of 6.77 to 108.33 μg/mL.
Recovery of CPO from the Nail
A recovery of 86–97% was obtained for CPO in the concentration range of 0.25 μg/mg nail to 64.7 μg/mg nail after dissolving in 1 M NaOH and the derivatization process.
The Lateral Diffusion of Ciclopirox Olamine and Methyl Red in Nail
Under saturated condition in pH 7.4 PBS, concentration of CPO was 23.92 ± 0.71 mg/mL. Figure 5a shows the lateral diffusion of MR in the suspended toenail and the sectioning of the toenails for analysis. The levels of CPO from the proximal to the distal end revealed its migration along the lengths of the two toenails A and B (Fig. 5b). The lines did not show any standard deviation because the nails A and B had different lengths. The difference in lengths would result in a different concentration gradient for each nail. Therefore, the concentration at each distance would be different as expected. A positive correlation was observed between the concentrations of CPO and MR in each of the toenails (Fig. 5c). This correlation was not expected and it indicates that MR can be used as a surrogate marker to visualize the lateral diffusion of CPO in the human toenails. This was also validated in the in vitro transungual permeation of CPO and MR.
Fig. 5.
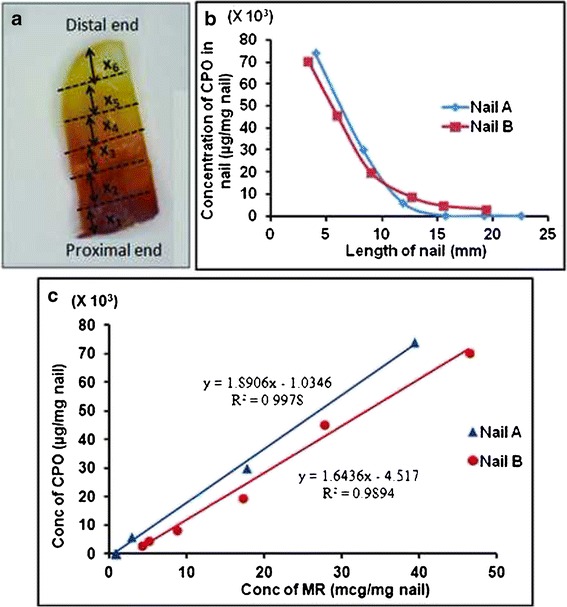
a Cadaver toenail showing the color gradient of MR from proximal to distal end. b Decline in concentration (μg/mg nail) of CPO from the proximal to the distal end c Correlation between the concentrations of CPO and MR salt in the suspended nail
In Vitro Transungual Permeation
Human cadaver toenails were trimmed to obtain three different dimensions (5 mm × 5 mm (25 mm2), 7 mm × 7 mm (49 mm2), and 9 mm × 9 mm (81 mm2)) in order to study the effect of the size of the peripheral areas (around a constant area of drug application) on lateral diffusion. Transungual permeation fluxes of 4.94 ± 2.61, 1.16 ± 0.57, and 0.81 ± 0.41 μg/cm2/day were attained for the toenails of the dimensions 5 mm × 5 mm (25 mm2), 7 mm × 7 mm (49 mm2), and 9 mm × 9 mm (81 mm2), respectively (Fig. 6a). The permeation fluxes attained with the 5 mm × 5 mm (25 mm2) and the 9 mm × 9 mm (81 mm2) surface areas are significantly different (p value < 0.05). The lag-times for the 5 mm × 5 mm (25 mm2) and the 7 mm × 7 mm (49 mm2) nail pieces were 6.4 ± 1.9 and 6.3 ± 0.2 days, respectively. These values are not significantly different (p value > 0.05). A longer lag-time of 8.8 ± 2.6 days was obtained for the nail surface area of 9 mm × 9 mm (81 mm2). The concentrations of CPO in the area of drug application and each of the subsequent sections of the periphery are shown in Fig. 6b. The drug concentrations within the area of drug application for each of the three dimensions of the toenails are not significantly different (p value > 0.05). The CPO concentration in the section b (shown in Fig. 4) for each of the three nail sizes was not significantly different (p value > 0.05). Similarly, the drug concentration in the section c (Fig. 4) of the nails with sizes 7 mm × 7 mm (49 mm2) and 9 mm × 9 mm (81 mm2) are not significantly different (p value > 0.05).
Fig. 6.
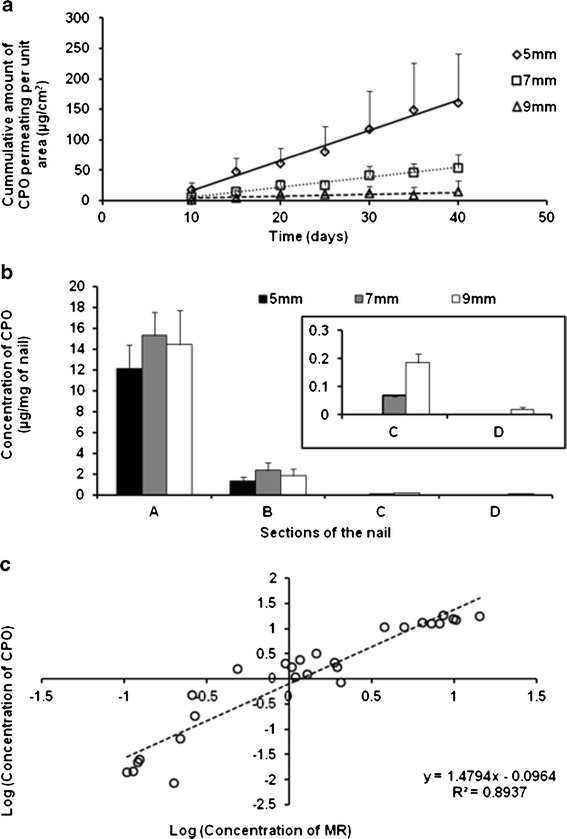
a Transungual permeation of CPO through the three sizes of human toenails. b Concentration of CPO in the area of application (a) and subsequent peripheral nail sections (b, c, d) in each of the three sizes of the nails after the transungual permeation study. c Correlation between the log concentrations of CPO and MR in the nail sections (a, b, c, d) after transungual permeation study
Figure 6c shows the correlation between the log concentrations (μg/mg of nail) of CPO and MR in the area of drug application and the sequential peripheral layers for each nail dimension studied. This confirms our initial observation that MR concentrations correlate with the CPO concentrations in each section of the nail and that it acts as a surrogate marker for CPO lateral diffusion.
DISCUSSION
The process of lateral diffusion was studied using MR as a visual aid in a suspended nail experiment. The original purpose for the use of the MR was to estimate the length of time needed for the experiment. MR was selected to visualize the process of lateral diffusion in the human toenail because it is water soluble, has a molecular weight (291.62 g/mol) similar to that of CPO (268.36 g/mol), and is readily taken up by the nail. It did not affect the stability of the drug, and it remained stable in presence of 5% w/w TU (unpublished data). Lateral diffusion was studied in presence of TU because in the preformulation screening studies 5% w/w TU had shown the greatest enhancement in penetration of CPO into the nail (4).
The lateral diffusion of the CPO around the area of application on the dorsal surface of the human nail was studied firstly using the suspended nail experiment. The utility of MR to visualize the diffusion process along the length of the nail was established in the suspended nail experiment. This lateral movement of MR was also evident when it was applied on the dorsal surface of the human nail during the transungual permeation. The movement of CPO molecules (in solution) along the length of the suspended nail is also speculated to be due to the orientation of the keratin fibers in a single plane and the hydrophilic nature of the nail. The intermediate layer is the thickest and softest layer of nail plate (27,28). In the intermediate layer, the keratin fibers are arranged in a single plane, parallel to the free end of the nail (29). The keratin fibers orient in both parallel and perpendicular direction in the dorsal and ventral layers of the nail (29).
The similarity in the trends of decline in concentrations of CPO and MR laterally in the toenails indicates similar lateral movement of CPO and MR. This was also evident from the strong correlation between the concentrations of CPO and MR in each of the two toenails suspended in the drug solution. One of the contributors to this similarity in lateral movement of MR and CPO is similarity in their respective binding abilities with keratin (unpublished data). MR can therefore be used to visualize the lateral diffusion of CPO. Previously, Lucifer yellow (fluorescent dye) was used to show the presence of a lateral diffusion pathway in the skin, which is suggested to have a physiological role in the transport of lymphokines and inflammatory mediators (22). The physiological role if any and effect lateral diffusion on antifungal therapy in human nail needs to be explored further. Similar study was performed using saturated solution of CPO in pH 7.4 PBS (in absence of 5% w/w TU) to evaluate the effect of the penetration enhancer on the lateral movement of CPO in the nail (unpublished data). There was no significant increase in the lateral movement of CPO and MR in presence of 5% TU in the suspended nail. This observation was different from the literature report that in the presence of 5% urea as the skin penetration enhancer for ibuprofen, there was increase in both penetration and lateral distribution of ibuprofen in the skin (23). This difference could be attributed to the difference of the physical and chemical composition of the skin and the nail.
Lateral diffusion of drugs in nails has been reported as an observation by researchers during iontophoretic transungual drug delivery of terbinafine hydrochloride (13,19–21). Because a higher drug flux is attained by iontophoresis compared to passive diffusion across the nail, the effects of passive lateral diffusion to reduce transungual drug permeation would be expected to be minimal. However, in passive topical delivery, this lateral diffusion can have a significant impact on the in vitro transungual permeation. It is therefore important to understand the effect of lateral diffusion during passive transungual permeation. To date, this is the only study on lateral diffusion which provides an insight on its effect on passive transungual permeation during in vitro studies. Longer lag-time and lower permeation flux values were observed in the transungual permeation of CPO through the nail dimension 9 mm × 9 mm (81 mm2). This indicates that a longer lag-time is obtained for a larger surface area of toenail using the same donor solution and same area of drug exposure. Therefore, the flux and the lag-times are a function of the extent of lateral diffusion which in turn, depends on the surface area of the toenail used. This indicates that lateral diffusion in the nail has to be minimized to obtain permeation flux and lag-time values from in vitro transungual permeation studies that are predictive of in vivo performance.
The orifice diameter of the nail adapters is only 3 mm and therefore the constant area of drug exposure is 7.07 mm2. The nail piece with a measurement of 5 mm × 5 mm (25 mm2) helps to attain the smallest peripheral area while maintaining a leak proof seal within the nail adapter. The other extreme, 9 mm × 9 mm (81 mm2) is the largest measurement of toenails which can be used to maintain the duration of the experiments within practical limits. A strong correlation exists between the permeation flux of CPO and ratio of drug exposure area to total nail area (Ao/An) (Fig. 7a). The 5 mm × 5 mm (25 mm2) nail has the highest Ao/An and also shows the greatest permeation flux for CPO. If a toenail with dimension of 11 mm × 11 mm (121 mm2) is used, this ratio falls to 0.0584. Based on the correlation in Fig. 7a, the increase in nail area will lead to a significant drop in permeation of CPO through the nail. In order to get a measurable quantity of the drug, the experiment may have to be continued for an extended duration of time. In a preliminary experiment (unpublished data), when untrimmed nails were used, no permeation was observed after 27 days (The duration of the experiment). Hence, the largest surface area of toenail in the study was limited to 9 mm × 9 mm (81 mm2).
Fig. 7.
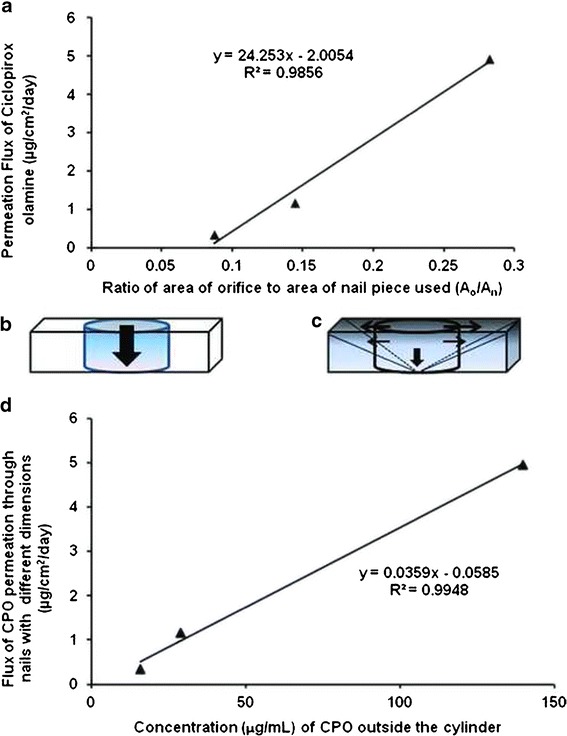
a Correlation between the permeation flux value and the ratio (A o /A n). b The ideal cylinder formed under the orifice during passive transungual permeation in absence of lateral diffusion. c The pyramid formed due to lateral diffusion of the drug molecules around the area of drug application. d Correlation between permeation flux and the concentration in the nail around the area of application
The CPO concentrations at the site of application on all the three nail surface areas were much greater than the reported minimum inhibitory concentration (MIC) of CPO against the dermatophytes (0.004 to 1.0 μg/mL) (30–34). The 3 mm orifice provides 7.065 mm2 of surface area for permeation of the drug and the dye. This area is 28.26, 14.42, and 8.72% of the total area of the nail piece with dimensions 5 mm × 5 mm (25 mm2), 7 mm × 7 mm (49 mm2), and 9 mm × 9 mm (81 mm2), respectively. When cadaver skin is placed between the donor and the receiver compartments of the Franz diffusion cell, there is a tight seal and the pressure exerted by the clamp on the skin greatly limits the lateral diffusion of the molecules beyond the area of drug application. However, the human nail plate is a hard, rigid structure which does not get compressed within the nail adapter. Hence, lateral diffusion cannot be prevented in the nail in vitro. CPO and MR showed lateral diffusion around the area of application in the in vitro transungual permeation studies. Previously, a photothermal imaging technique was reported to show the lateral distribution of the drug into the proximal end of the nail when the formulation was applied on the proximal nail fold (35).
When the nail under the orifice is treated with the drug, some drug molecules diffuse into the upper layers of the nail plate. Ideally, if there is no lateral diffusion, the drug molecules should diffuse through the entire thickness of the nail (h) under the orifice (Fig. 7b). As the permeation process continues, a concentration gradient of the drug is formed in this cylinder with the height of h and diameter of 3 mm and eventually CPO diffuses out from the ventral side of the nail plate. However, it is evident from Fig. 6b that, as the drug fills up the upper layers of the nail under the orifice, it also moves laterally from the cylinder to the periphery. The drug from the upper saturated layers of the nail then diffuses into the layers underneath. Simultaneously, the drug is also moving from the central part of cylinder towards the periphery. Thus, lateral diffusion, which itself is a passive diffusion process, is dependent on the concentration gradient formed between the cylinder and the area surrounding the cylinder. As this process continues, there is formation of a three dimensional figure which can be best approximated as a pyramid (Fig. 7c).
The volume of this pyramid (Vp) is given by:
| 1 |
where area of base = total area of nail and height = thickness of nail.
The volume of the ideal cylinder (Vc) is given by:
| 2 |
where radius of the nail adapter orifice, r = 1.5 mm and height = thickness of nail.
Volume of the nail outside the central cylinder, which contains the laterally diffused drug, is given by:
| 3 |
The area of drug application was found to contain the same amounts of drug in all the three nail sizes (Fig. 6b). The volume Vn was used to calculate the concentration of CPO in the nail around the central cylinder. The flux of transungual permeation of CPO correlates positively with the concentration of CPO in the nail surrounding the cylinder (Fig. 7d). This correlation shows that the highest flux is observed in the nail dimension, where the concentration developing in the peripheral layer immediately outside the central cylinder is highest. This concentration decreases drastically as the peripheral area and Vn increases. In the presence of lateral diffusion, the required driving force for transungual permeation is the high CPO concentration in the orifice and the periphery. As the size of the nail increases from 5 mm × 5 mm (25 mm2) to 7 mm × 7 mm (49 mm2) and finally to 9 mm × 9 mm (81 mm2), the concentration of CPO in the peripheral area of the nail drops significantly due to increased value of Vn, resulting in decline in transungual permeation. The concentration drop may be caused by the gradient that forms between the treated area and the peripheral area of the nail. A large peripheral area would lengthen the gradient and drop the peripheral concentration.
Lateral diffusion in human nail plate cannot be completely prevented, only its effect on in vitro transungual permeation of drugs can be controlled by minimizing the available peripheral surface area. The lag-times for 5 mm × 5 mm (25 mm2) and 7 mm × 7 mm (49 mm2) nail pieces were not significantly different. This indicates that the two dimensions did not affect the lag-time as much as it affected the permeation of CPO. However, when 9 mm × 9 mm (81 mm2) nail size was used, the lag-time increased by 2 days relative to the two smaller nail sizes. Additionally, untrimmed nails used in a similar experimental design showed the lag-times greater than the duration of the experiment (27 days). There was no measurable amount of drug permeating the nails during the 27 days. A similar increase in lag-time as a consequence of lateral diffusion was seen with ibuprofen in the skin (23). Longer lag-times mean longer time before detectable levels of CPO can reach the receiver compartment.
These results show that lateral diffusion occurs in the nail, and this can have a profound impact on the experimental data acquired from in vitro drug permeation studies. The decision of selecting a penetration enhancer or elimination of a potential formulation from further studies is dependent on this in vitro experimental data. If lateral diffusion overrides the permeation process, an enhancer or an optimum formulation may be falsely eliminated from the future experiments. Hence, it is important to select the appropriate size of nails, considering the area of the orifice on the nail adapters for the in vitro experiments. When the patient applies the topical transungual formulation, it covers the entire surface area of the nail and greatly reduces the area available for lateral diffusion. The lateral diffusion that would occur in the nail during therapy would actually be advantageous because this would increase the drug concentration in the unexposed nail underneath the nail folds. It is expected that the passive transungual permeation in vivo will be closer to the ideal cylinder in Fig. 7b. This in vivo scenario can be mimicked in the in vitro setting by using the appropriate size of the nail which is just enough to provide a tight, leak-proof seal around the orifice in the nail adapter. However, if there is no control on the nail sizes used in an experiment, the passive lateral diffusion competes with the passive transungual permeation, causing a shift from the cylinder to the pyramid. This leads to an overestimation of lag-time and underestimation of permeation flux. Thus, understanding the effect of lateral diffusion on passive transungual permeation of drugs will help to better relate to the permeation flux in vivo, aid in selection of penetration enhancers, and to optimize formulations.
CONCLUSION
It was shown that lateral diffusion occurs in the nail and can reduce the in vitro passive transungual drug permeation. MR is a useful surrogate marker for drugs such as CPO for visualizing their lateral diffusion in the human nail. The effect of lateral diffusion in in vitro transungual experiments can result in markedly lower drug permeation and underestimation of drug permeation in vivo. It is important to control the dimension of the toenails during in vitro passive drug delivery experiments to minimize variability in the permeation data and to obtain results which best relate to the in vivo permeation. It is therefore recommended that the cadaver toenails used for the permeation experiments be trimmed just enough to cover the orifice in the nail adapter and provide a leak-proof seal.
ACKNOWLEDGMENTS
The study was funded by cGMP services, Temple University.
REFERENCES
- 1.Hao J, Smith K, Li K. Iontophoretically enhanced ciclopirox delivery into and across human nail plate. J Pharm Sci. 2009;98(10):3608–3616. doi: 10.1002/jps.21664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hui X, Wester RC, Barbadillo S, Lee C, Patel B, Wortzmman M, et al. Ciclopirox delivery into the human nail plate. J Pharm Sci. 2004;93:2545–2548. doi: 10.1002/jps.20159. [DOI] [PubMed] [Google Scholar]
- 3.Monti D, Saccomani L, Chetoni P, Burgalassi S, Tampucci S, Mailland F. Validation of bovine hoof slices as a model for infected human toenails: in vitro ciclopirox transungual permeation. Br J Dermatol. 2011;165(1):99–105. doi: 10.1111/j.1365-2133.2011.10303.x. [DOI] [PubMed] [Google Scholar]
- 4.Palliyil B, Lebo DB, Patel PR. A preformulation strategy for the selection of penetration enhancers for a transungual formulation. AAPS PharmSciTech. 2013 doi: 10.1208/s12249-013-9954-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chouhan P, Saini TR. Hydration of nail plate: a novel screening model for transungual drug permeation enhancers. Int Pharm. 2012;436(1–2):179–182. doi: 10.1016/j.ijpharm.2012.06.020. [DOI] [PubMed] [Google Scholar]
- 6.Hao J, Smith KA, Li KS. Chemical method to enhance transungual transport and iontophoresis efficiency. Int J Pharm. 2008;357(1–2):61–69. doi: 10.1016/j.ijpharm.2008.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khengar R, Jones S, Turner R, Forbes B, Brown M. Nail swelling as a pre-formulation screen for the selection and optimisation of ungual penetration enhancers. Pharm Res. 2007;24(12):2207–2212. doi: 10.1007/s11095-007-9368-3. [DOI] [PubMed] [Google Scholar]
- 8.Kobayashi Y, Miyamoto M, Sugibayashi K, Morimoto Y. Enhancing effect of N-acetyl-L-cysteine or 2-Mercaptoethanol on the in vitro permeation of 5-fluorouracil or tolnaftate through the human nail plate. Chem Pharm Bull. 1998;46(11):1797–1802. doi: 10.1248/cpb.46.1797. [DOI] [PubMed] [Google Scholar]
- 9.Malhotra GG, Zatz JL. Investigation of nail permeation enhancement by chemical modification using water as a probe. J Pharm Sci. 2002;91(2):312–323. doi: 10.1002/jps.10058. [DOI] [PubMed] [Google Scholar]
- 10.Mohorcic M, Torkar A, Friedrich J, Kristl J, Murdan S. An investigation into keratinolytic enzymes to enhance ungual drug delivery. Int J Pharm. 2007;332(196–201):196. doi: 10.1016/j.ijpharm.2006.09.042. [DOI] [PubMed] [Google Scholar]
- 11.Murdan S. Enhancing the nail permeability of topically applied drugs. Expert Opin Drug Deliv. 2008;5(11):1267–1282. doi: 10.1517/17425240802497218. [DOI] [PubMed] [Google Scholar]
- 12.Murthy SN, Vaka SR, Sammeta SM, Nair AB. TranScreen-N: method for rapid screening of trans-ungual drug delivery enhancers. J Pharm Sci. 2009;98(11):4264–4271. doi: 10.1002/jps.21743. [DOI] [PubMed] [Google Scholar]
- 13.Nair AB, Sammeta SM, Vaka SR, Narasimha MS. A study on the effect of inorganic salts in transungual drug delivery of terbinafine. J Pharm Pharmacol. 2009;61(4):431–437. doi: 10.1211/jpp.61.04.0003. [DOI] [PubMed] [Google Scholar]
- 14.Nair AB, Sammeta SM, Kim HD, Chakraborty B, Friden PM, Murthy N. Alteration of the diffusional barrier property of the nail leads to greater terbinafine drug loading and permeation. Int J Pharm. 2009;375:22–27. doi: 10.1016/j.ijpharm.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 15.Nair AB, Chakraborty B, Murthy SN. Effect of polyethylene glycols on the trans-ungual delivery of terbinafine. Curr Drug Deliv. 2010;7(5):407–414. doi: 10.2174/156720110793566308. [DOI] [PubMed] [Google Scholar]
- 16.Quintanar-Guerrero D, Ganem-Quintanar A, Tapia-Olquin P, Kalia YN, Buri P. The effect of keratolytic agents on the permeability of three imidazole antimycotic drugs through the human nail. Drug Dev Ind Pharm. 1998;24(7):685–690. doi: 10.3109/03639049809082373. [DOI] [PubMed] [Google Scholar]
- 17.Shivakumar HN, Juluri A, Desai BG, Murthy N. Ungual and transungual drug delivery. Drug Dev Ind Pharm. 2012;38(8):901–911. doi: 10.3109/03639045.2011.637931. [DOI] [PubMed] [Google Scholar]
- 18.Traynor MJ, Turner RB, Evans CR, Khengar RH, Jones SA, Brown MB. Effect of a novel penetration enhancer on the ungual permeation of two antifungal agents. J Pharm Pharmacol. 2010;62(6):730–737. doi: 10.1211/jpp.62.06.0009. [DOI] [PubMed] [Google Scholar]
- 19.van Hoogdalem EJ, van den Hoven WE, Terpstra IJ, van Zijtveld J, Verschoor JSC, Visser JN. Nail penetration of the antifungal agent oxiconazole after repeated topical application in healthy volunteer, and the effect of acetylcysteine. Eur J Pharm Sci. 1997;5(3):119–127. doi: 10.1016/S0928-0987(97)00270-4. [DOI] [Google Scholar]
- 20.Nair AB, Kim HD, Chakraborty B, Singh J, Zaman M, Gupta A, et al. Ungual and trans-ungual iontophoretic delivery of terbinafine for the treatment of onychomycosis. J Pharm Sci. 2009;98(11):4130–4140. doi: 10.1002/jps.21711. [DOI] [PubMed] [Google Scholar]
- 21.Nair AB, Kim HD, Davis SP, Etheredge R, Barsness M, Friden PM, et al. An ex vivo toe model used to assess applicators for the iontophoretic ungual delivery of terbinafine. Pharm Res. 2009;26(9):2194–2201. doi: 10.1007/s11095-009-9934-y. [DOI] [PubMed] [Google Scholar]
- 22.Mansbridge J, Knapp M. Penetration of Lucifer Yellow into human skin: a lateral diffusion channel in the stratum corneum. J Histochem Cytochem. 1993;41(6):909–914. doi: 10.1177/41.6.8315281. [DOI] [PubMed] [Google Scholar]
- 23.Schicksnus G, Muller-Goymann CC. Lateral diffusion of Ibuprofen in human skin during permeation studies. Skin Pharmacol Physiol. 2004;17:84–90. doi: 10.1159/000076018. [DOI] [PubMed] [Google Scholar]
- 24.Escarrone AL, Bittencourt C, Laporta L, dos Santos M, Primel E, Caldas S. LC–UV method with pre-column derivatization for the determination of ciclopirox olamine in raw material and topical solution. Chromatographia. 2008;67:967–971. doi: 10.1365/s10337-008-0623-5. [DOI] [Google Scholar]
- 25.Lehr K-H, Damm P. Quantification of ciclopirox by high-performance liquid chromatography after pre-column derivatization. J Chromatogr. 1985;339:451–456. doi: 10.1016/S0378-4347(00)84680-0. [DOI] [PubMed] [Google Scholar]
- 26.Myoung Y, Choi H-K. Permeation of ciclopirox across porcine hoof membrane: effect of pressure sensitive adhesives and vehicles. Eur J Pharm Sci. 2003;20:319–325. doi: 10.1016/j.ejps.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 27.Kobayashi Y, Miyamoto M, Sugibayashi K, Morimote Y. Drug permeation through the three layers of the human nail plate. J Pharm Pharmacol. 1999;51:271–278. doi: 10.1211/0022357991772448. [DOI] [PubMed] [Google Scholar]
- 28.Murdan S. Drug delivery to the nail following topical application. Int J Pharm. 2002;236:1–26. doi: 10.1016/S0378-5173(01)00989-9. [DOI] [PubMed] [Google Scholar]
- 29.Garson J, Baltenneck F, Leroy F, Riekel C, Müller M. Histological structure of human nail as studied by synchrotron X-ray microdiffraction. Cell Mol Biol. 2000;46(6):1025–1034. [PubMed] [Google Scholar]
- 30.Gupta AK, Kohli Y. In vitro susceptibility testing of ciclopirox, terbinafine, ketoconazole and itraconazole against dermatophytes and nondermatophytes, and in vitro evaluation of combination antifungal activity. Br J Dermatol. 2003;149(2):296–305. doi: 10.1046/j.1365-2133.2003.05418.x. [DOI] [PubMed] [Google Scholar]
- 31.Kokjohn K, Bradley M, Griffiths B, Ghannoum M. Evaluation of in vitro activity of ciclopirox olamine, butenafine HCl and econazole nitrate against dermatophytes, yeasts and bacteria. Int J Dermatol. 2003;42(S1):11–17. doi: 10.1046/j.1365-4362.42.s1.4.x. [DOI] [PubMed] [Google Scholar]
- 32.Shehata AS, Mukherjee PK, Ghannoum MA. Comparison between the standardized clinical and laboratory standards institute M38-A2 method and a 2,3-Bis(2-Methoxy-4-Nitro-5-[(Sulphenylamino)Carbonyl]-2H-Tetrazolium hydroxide based method for testing antifungal susceptibility of dermatophytes. J Clin Microbiol. 2008;46(11):3668–3671. doi: 10.1128/JCM.01242-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Singh J, Zaman M, Gupta AK. Evaluation of microdilution and disk diffusion methods for antifungal susceptibility testing of dermatophytes. Med Mycol. 2007;45(7):595–602. doi: 10.1080/13693780701549364. [DOI] [PubMed] [Google Scholar]
- 34.Subissi A, Monti D, Togni G, Maillan F. Ciclopirox : recent nonclinical and clinical data relevant to its use as a topical antimycotic agent. Drugs. 2010;70(16):2133–2152. doi: 10.2165/11538110-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 35.Gotter B, Faubel W, Neubert RHH. Photothermal imaging in 3D surface analysis of membrane drug delivery. Eur J Pharm Biopharm. 2010;74:26–32. doi: 10.1016/j.ejpb.2009.05.014. [DOI] [PubMed] [Google Scholar]


