Abstract
Amorphous drugs are used to improve the solubility, dissolution, and bioavailability of drugs. However, these metastable forms of drugs can transform into more stable, less soluble, crystalline counterparts. This study reports a method for evaluating the effect of commonly used excipients on the surface crystallization of amorphous drugs and its application to two model amorphous compounds, nifedipine and indomethacin. In this method, amorphous samples of the drugs were covered by excipients and stored in controlled environments. An inverted light microscope was used to measure in real time the rates of surface crystal nucleation and growth. For nifedipine, vacuum-dried microcrystalline cellulose and lactose monohydrate increased the nucleation rate of the β polymorph from two to five times when samples were stored in a desiccator, while d-mannitol and magnesium stearate increased the nucleation rate 50 times. At 50% relative humidity, the nucleation rates were further increased, suggesting that moisture played an important role in the crystallization caused by the excipients. The effect of excipients on the crystal growth rate was not significant, suggesting that contact with excipients influences the physical stability of amorphous nifedipine mainly through the effect on crystal nucleation. This effect seems to be drug specific because for two polymorphs of indomethacin, no significant change in the nucleation rate was observed under the excipients.
KEY WORDS: amorphous, drugs, growth rate, nucleation rate, tablet excipients
INTRODUCTION
Amorphous formulations have become very popular as a method to overcome the poor aqueous solubility of potentially therapeutically important drugs. Examples of commercially available amorphous drugs include zafirlukast in Accolate®, cefuroxime axetil in Ceftin®, quinapril hydrochloride in Accupril®, nelfinavir mesylate in Viracept®, nabilone in Cesamet®, griseofulvin in Gris-PEG®, verapamil in Isoptin®, lopinavir/ritonavir in Kaletra®, itraconazole in Sporanox®, and troglitazone in Rezulin®. One concern of amorphous drug formulations is the stability of the amorphous drug against crystallization back to the poorly water-soluble crystalline form or forms (1).
Until recently, crystallization of amorphous solids has been treated mainly as a bulk crystallization process and correlated to their bulk properties and storage conditions (2–5). It is now recognized that crystallization mechanisms of amorphous solids involves surface crystallization (6,7). Surface crystal growth has been observed to be orders of magnitude faster than bulk crystal growth, a phenomenon attributed to the enhanced surface mobility of molecules (8–10). These findings demonstrate the importance of surface crystallization in the overall crystallization rate of amorphous materials (11).
During the formulation of solid dosage forms, excipients are added into tablets or capsules. These excipients can potentially change the stability of the amorphous drug. There have been studies relating the effect of polymer additives on the crystallization of amorphous drugs (12–14). A key finding from these studies is that polymer additives introduced into the bulk of an amorphous drug can strongly inhibit bulk crystal growth but have less effect on surface crystallization (15,16). Few studies have examined the effect of contact with excipients on the surface crystallization of amorphous drugs. In a previous study, we reported that polymer nanocoating on the surface of amorphous indomethacin inhibited the overall surface crystallization which has important implications for amorphous stability and preserving the advantages of the amorphous material such as higher solubility (11). Besides this report, little is known about the effect of surface contact with excipients on the crystallization of amorphous drugs.
If excipients can change the physical stability of amorphous drugs, it is important that formulation scientists find a way to determine their effect on the complicated crystallization of amorphous materials. During formulation studies, one of the first steps in the process is to determine drug-excipient compatibility. This usually involves using thermal analysis (DSC and isothermal microcalorimetry) and chromatographic techniques (UV, HPLC, and MS) to determine the effect of excipients on the chemical stability of the drug (17–19). However, to date, no simple screening method has been reported to ascertain the effect of excipients on the crystallization of amorphous materials.
In this study, we report a simple yet effective microscopic method for studying the effect of common tablet excipients on the surface crystallization of amorphous drugs. The method was used to study the model amorphous compounds nifedipine (NIF) and indomethacin (IMC) in contact with the excipients microcrystalline cellulose (MCC), lactose monohydrate (hereafter lactose), d-mannitol (hereafter mannitol), and magnesium stearate (MgSt). Surface crystal nucleation rate and growth rate were measured for samples stored in controlled environments. For NIF, the excipients increased the nucleation rate from 2 to 50 times in a desiccator with mannitol and magnesium stearate having the largest effect. At 50% relative humidity (RH), the nucleation rates were even further increased. In contrast, the growth rate of NIF crystals was only slightly changed under the excipients. For IMC, the method was able to measure the crystallization under excipients of two polymorphic forms. No significant change in the nucleation rate was observed for this drug.
MATERIALS AND METHODS
Materials
Nifedipine was purchased from Tokyo Kasei Kogyo Co. LTD. (Tokyo, Japan) and used as received. Indomethacin, lactose monohydrate, d-mannitol, and magnesium stearate were purchased from Sigma-Aldrich (St. Louis, MO, USA). Microcrystalline cellulose (Avicel® PH-101) was a kind gift from FMC BioPolymer (Philadelphia, PA, USA).
Instruments
Light microscopy was performed with a Nikon Diaphot inverted microscope equipped with bottom illumination (Melville, NY, USA), an Olympus BH2-UMA microscope and an Olympus BX53 microscope (Center Valley, PA, USA). Powder X-ray diffraction was performed with a Bruker D8 Advance diffractometer (Madison, WI, USA) using Cu Kα radiation, voltage of 40 kV, and current of 40 mA. Samples were put on a zero-background silicon (510) sample holder and scanned from 2 to 40° 2θ at a speed of 1.2°/min and a step size of 0.02°. Raman microscopy with a 10 mW 780 nm laser (Thermo Scientific DXR, Waltham, MA, USA) was used to distinguish crystal polymorphs. Experimental temperature and humidity was monitored in the real-time (Extech RHT10 Datalogger, Waltham, MA, USA).
Preparation of Amorphous Substrate and Contact with Excipients
Samples of amorphous drugs were prepared by melt quenching. In this method, 6–8 mg of drug powder on a clean 18-mm square cover slip was melted and kept at 183°C (NIF) or 175°C (IMC) for 2 min, covered with a 15-mm diameter round cover slip, and quenched to room temperature (22(1)°C) by contact with an aluminum block. The amorphous drug layer thus formed was about 30 μm thick and confirmed to be free of crystals by polarized light microscopy. The 18-mm square cover slip was detached from the amorphous drug layer at room temperature by gently bending its center toward the round cover slip. This exposed a free surface of the amorphous drug on the 15-mm round cover slip for studying surface crystallization (7,15,16). The resulting samples were placed in the 15-mm-diameter wells of a 24-well nontreated cell culture plate with the surface of the amorphous drug facing upward (Scheme 1).
Scheme 1.

Experimental setup
In order to control the moisture, excipients were vacuum dried for at least 12 h before use. Both the amorphous samples in the well-plate and the dried excipients were transferred into a N2-purged glove bag at room temperature. After 1 h of equilibration (<5% RH), 0.4 g of excipient was poured onto each amorphous sample inside its well (due to its low bulk density, 0.2 g of MgSt was used). A 200 g, steel cylinder press was used to gently press the top of the excipient to ensure a good contact with the amorphous drug surface (Scheme 1). For growth rate and NR measurements, the closed well-plate containing the excipient-covered amorphous samples was removed from the glove bag and quickly transferred into the designated test environment. For comparison, as-received excipients (without vacuum drying) were also tested using this procedure without N2 purging.
Measuring the Crystal Growth Rate and Nucleation Rate
Here, we take the model drug NIF to illustrate our experimental procedure. This procedure, with some adjustments as described in the “RESULTS AND DISCUSSION” section, was also used for evaluating IMC. For measuring nucleation rate, well-plates containing freshly made amorphous NIF samples, covered with excipients, were stored in a Drierite-charged desiccator (<5% RH) or a humidity chamber equilibrated with saturated magnesium nitrate solution (50(2)% RH) at 30(1)°C. The equilibration time after each opening was estimated to be 1 h for the desiccator and 3 h for the humidity chamber. After 12 h, the samples were taken out of the well-plate, and the excipients were removed by gently blowing air over the sample. The nucleation rate of NIF was determined by counting the number of crystals using polarized light microscopy. Based on the density of crystals, the counting was carried out on a smaller or larger area, from 10 to 40 mm2. Sample edges (2 mm from the perimeter) were excluded from counting, since nucleation may be induced by handling.
For measuring growth rate, amorphous NIF samples without excipients were allowed to crystallize partially in a desiccator (<5% RH) at 40(1)°C. Samples were kept at a higher temperature than the testing temperature (30°C) to induce crystallization, which provided distinguishable crystals for the growth rate measurements. Compared with spontaneously nucleated samples at 30°C which were used in the nucleation rate measurements, crystal induction at a higher temperature saved time, avoided glass aging, while having no significant effect on the growth rate at 30°C (15,16). After about 9 h of induction, the NIF samples were covered with excipients as described previously. The bottom-illuminated inverted microscope with N2 purge to control the moisture was used to identify several patches on the surface of each partially crystallized sample for further observation (Scheme 1). For growth rate measurement in dry condition, the well-plate was stored in the 30°C desiccator between observations. To determine the growth rate, photomicrographs of the chosen patches were taken every 3 h. For each measurement, samples were exposed for less than 10 min to room temperature with N2 purge. The growth rate of NIF was determined by tracking the radii of NIF crystal patches for up to 12 h. After the removal of excipients by gently blowing air over the sample, a clear view of the selected patches observed under the polarized light microscope was used to determine the crystalline boundary, and to validate the previously measured radius under the excipients.
For measuring growth rate in the presence of moisture, the storage time inside the 30°C humidity chamber was 6 or 12 h. After storage, selected patches were measured again under the microscope, and the growth rate was determined from the radii of patches before and after storage (2 points growth rate). A clear view of the selected patches after the removal of excipients was again used to help determine the crystalline boundary and to validate the previously measured radius.
RESULTS AND DISCUSSION
Effect of Contact with Dried Excipients on the Crystal Nucleation of Nifedipine Glasses
Figure 1 shows the effects of contact with different excipients on the crystallization of amorphous nifedipine. After 12 h of coverage by dried excipients inside a desiccator, control samples (no excipient coverage) and samples under MCC or lactose remained largely amorphous, while samples under mannitol were partially crystallized, and samples under MgSt were fully crystallized (Fig. 1, left column). Although most excipients could be removed by gently blowing air onto the sample, some particles, especially those of MgSt still adhered to the surface (20). Closer views of samples under a polarized light microscope (Fig. 1, right column) revealed different densities and sizes of crystals on the surface. Later, in Fig. 3, we show that these crystals grew over time, which further demonstrate the ability to observe NIF crystals under excipient particles. Since, at 30°C, the spontaneous nucleation of NIF crystals in the bulk was substantially longer, and the growth rate of bulk crystals is much slower (log u = −9.4 m/s) compared with surface crystals (as shown later in Table I) (15), we conclude that all the crystals observed in this study are surface crystals. Although NIF has three known polymorphs, previous studies showed that only the β polymorph was observed at the surface of amorphous samples below Tg (42°C) (7,15,21). Similarly, in this study, only the β polymorph was identified by Raman microscopy of the crystal patches and XRPD (13). Therefore, contact with excipients did not induce the growth of other NIF polymorphs.
Fig. 1.

Effect of contact with dried excipients on the surface crystallization of amorphous NIF at 30°C in a desiccator. Storage time is 12 h. Left The appearance of whole NIF samples after excipient coverage. The samples are 15 mm in diameter. Right Photomicrographs of crystal nuclei at the center of NIF samples. For control samples, the morphology of a typical crystal after 24 h of storage was shown
Fig. 3.
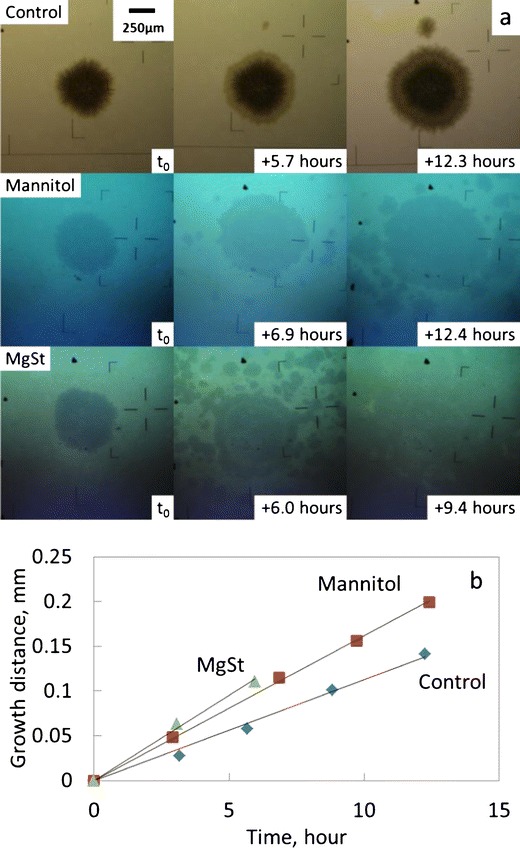
Crystal growth on NIF glasses at 30°C in a desiccator. a Photomicrographs of crystal growth. t 0 is the time to start tracking crystal growth. The Man and MgSt groups of photo were taken under excipients. b Typical data of growth distance vs. time. The data are shifted vertically to coincide at the origin for better comparison
Table I.
Nucleation Rate (n) and Growth Rate (u) of Amorphous NIF Under Different Excipients at 30°C for 12 or 6 h
| Excipient | Desiccator (<5% RH) | 50% RH | ||
|---|---|---|---|---|
| n, #/cm2 | log u, m/s | n, #/cm2 | log u, m/s | |
| Control | 19 (10) | −8.56 (0.07) | 130 (60) | −8.55 (0.07) |
| MCC | 39 (11) | −8.48 (0.07) | 380 (100) | −8.37 (0.07) |
| Lactose | 68 (27) | −8.41 (0.07) | 1,100 (300)a | n/a |
| Mannitol | 930 (320) | −8.34 (0.07) | 2,100 (700)a | n/a |
| MgSt | n/a | −8.25 (0.07)a | n/a | n/a |
aThe experimental time was 6 h
Since only β-NIF was formed, we could safely compare its nucleation rates under different excipients. We counted the number of crystals per unit area through microscope, as illustrated in the right column of Fig. 1. The results are shown in Table I. Note that these values are not the steady-state nucleation rate of surface crystals; we report only the density of surface nucleation averaged over the first 12 h. Given that the crystal growth rates are not significantly altered by contact with excipients (see below), our method provides a reasonable assessment of the effect of the contact with excipient particles on nucleation frequency.
Compared with control samples, MCC and lactose increased nucleation rate of NIF by two- to threefold, while mannitol increased the nucleation rate about 50 times. Due to the difficulty in removing excipient particles, the crystals under MgSt could not be counted clearly, and the corresponding nucleation rate could only be estimated. Based on the numerous crystals observed under polarized light microscopy, and the fact that samples were fully crystallized after 12 h of storage, the effect of MgSt on nucleation rate was deduced to be at least similar or even greater than mannitol. Therefore, all the excipients tested in this study increased nucleation rate of amorphous NIF, and the nucleation rate under different excipients was in the order of control ≤ MCC ≤ lactose < mannitol ≤ MgSt.
The effect of environmental moisture on the nucleation rate was evaluated by storing the amorphous NIF samples covered with dried excipients in a 50% RH chamber instead of a desiccator. In the presence of moisture, NIF crystallized faster and the crystals had a thicker and denser morphology on the surface (Fig. 2, right column). However, both Raman and XRPD confirmed that it was still the β polymorph.
Fig. 2.

Effect of contact with dried excipients on the surface crystal nucleation of amorphous NIF at 30°C, 50% RH. Storage time is 12 h for control and MCC, 6 h for Lac and Man. Left The appearance of whole NIF samples after excipient coverage. The samples are 15 mm in diameter. Right Photomicrographs of crystal nuclei at the center of NIF samples. For control samples, the morphology of a typical crystal after 24 h of storage was shown
As shown in Fig. 2 and Table I, more crystals were seen after storage at 50% RH compared with those observed in the desiccator, even with shorter storage time. Compared to desiccator results, moisture increased the nucleation rate of control samples and samples covered with MCC from five- to tenfold. Compared to control samples, the effect of MCC was similar in both conditions (two- to threefold). For the other excipients, samples covered with lactose and mannitol fully crystallized within 12 h at 50% RH, while samples under MgSt fully crystallized within 6 h (result not shown). For this reason, the number of crystals was counted after 6 h for those samples covered with lactose and mannitol (Table I). Although the result is not directly comparable to control samples, the increase in nucleation rate under lactose and mannitol was significant at 50% RH. The effect of MgSt on nucleation rate was again deduced to be at least similar or even greater than mannitol. Therefore, all of the dried excipients tested in this study picked up moisture from the environment and increased nucleation. At 50% RH, the nucleation rate under different excipients was in the order of control < MCC < lactose < mannitol ≤ MgSt.
Effect of Dried Excipients and Environmental Moisture on the Growth Rate of Nifedipine
We first measured the growth rate in the absence of environmental moisture. Using the bottom-illuminated inverted microscope sketched in Scheme 1, we could observe the boundaries of NIF surface crystals as circular patches under a layer of excipients. The radii of these patches increased linearly with time (Fig. 3). The total experimental time was about 12 h except for the MgSt coverage, where the growth front of the selected patches could not be seen clearly after 6 h due to fast nucleation. Similarly, multiple crystals were seen on the sample covered with mannitol after 12 h, which was consistent with our observation in the nucleation rate study. Raman microscopy was again used to confirm that the selected patches were the β polymorph.
Figure 3b shows the linear increase in the crystal radius vs. time for the central crystals in Fig. 3a. The slopes of the fitted lines represent the crystal growth rates. Although it was reported that the growth rate of NIF surface crystals decreased over time, no significant slowdown was observed in this study, partially due to the shorter experimental time which could be compared to the “early stage” studies reported previously (15). The growth rates of NIF surface crystals in contact with excipients were slightly faster than Control (Table I), with MgSt having the largest effect (approximately twofold increase in the growth rate). Note that the effect of dried excipients on growth rate was weaker than their effect on the nucleation rate (two- to threefold for MCC and lactose, about 50-fold for mannitol and MgSt). Therefore, although crystals with faster growth rate would more easily reach the size that could be seen and counted under the microscope during nucleation rate measurements, it seems unlikely that the small increase in the growth rate led to a significant change in the nucleation rate.
The effect of environmental moisture on the growth rate of NIF was measured only for the control samples (without excipients) and under dried MCC, due to the fast nucleation under other excipients and the longer equilibration needed for the humidity chamber. The log growth rate of NIF without excipients at 50% RH was determined to be −8.55(0.07) m/s, which was not significantly different from the samples stored in the desiccator (Table I). The growth rate of NIF under MCC is calculated from (1) before storage (a photo focusing on the selected nucleus under MCC) and (2) after storage (a “clear” photo of the grown crystal patch after the removal of MCC) (Fig. 4). In this clear photo, multiple crystals were seen on the growth front of the selected nucleus due to the increased nucleation rate, making it impossible to measure the diameter precisely. Therefore, the radius of the crystal from the center to the left was measured and used to calculate the growth rate. This procedure was applied for other selected crystals, and the log growth rate of NIF under MCC was determined to be −8.37(0.07) m/s, which was about 1.5-fold faster than the control samples. Although this difference is larger than the difference between the control samples and MCC in the desiccator, it is still less significant compared with its effect on the nucleation rate. Since at 50% RH, MCC only increased nucleation rate at two- to threefold compared to control samples, the 1.5-fold increase in growth rate could only partially explain the larger number of crystals observed under MCC. However, under the other excipients, since the nucleation rate increased more significantly at 50% RH, it seems very unlikely that growth rate changes affected the nucleation rate measurements.
Fig. 4.

Crystal growth of NIF at 30°C, 50% RH. For MCC, only the t 0 picture was taken with the presence of excipients
Effect of As-Received Excipients on the Crystallization of Amorphous Nifedipine
In its commercial form, MCC (Avicel® PH-101) contains about 5% (w/w) moisture, which is readily removed by drying (22). The weight loss upon drying of lactose, being a hydrate, is less than 1% (22). Previous experiments evaluated the effect of vacuum-dried excipients on the crystallization of amorphous NIF. After 12 h of dried excipient coverage inside a desiccator, the nucleation rate under different excipients was in the order of control ≤ MCC ≤ lactose < mannitol ≤ MgSt. However, in formulation practice, excipients are more likely to be mixed directly with the amorphous drug without further processing. To inspect whether drying the excipients prior to coverage will affect the nucleation rate, we used as-received MCC and lactose to cover the amorphous NIF samples and then stored the samples at 30°C in a desiccator. The experimental periods were set to 12–96 h consecutively without taking the samples out. Only the β polymorph was seen in all samples. The appearances of NIF samples after 72 h coverage of as-received excipients were recorded in Fig. 5. It is noteworthy that MCC significantly induced the nucleation of NIF, while the induction effect of lactose was less significant. The nucleation rate under as-received excipients was in order of control ≤ lactose < MCC, compared with control ≤ MCC ≤ lactose for dried excipients. Therefore, the free moisture contained inside commercial MCC significantly induced the crystallization of NIF, while drying MCC is essential for increasing its stability on amorphous NIF.
Fig. 5.

Effect of contact with as-received excipients on the surface crystal nucleation of amorphous NIF at 30°C for 72 h in a desiccator. Left The appearance of whole NIF samples after excipient coverage. The samples are 15 mm in diameter. Right Photomicrographs of crystal nuclei at the center of NIF samples
Effect of Excipients on the Crystallization of Amorphous Indomethacin
To assess the robustness of our method, we applied it to a different system, indomethacin. IMC glasses are known to crystallize more slowly than NIF glasses (6,23,24) and have slower surface diffusion (9,10). Two polymorphs can crystallize on the surface of IMC glasses with distinct morphologies: γ polymorph produces birefringent transparent patches, while α polymorph yields opaque patches of fine fibers (Fig. 6a) (6,25).
Fig. 6.
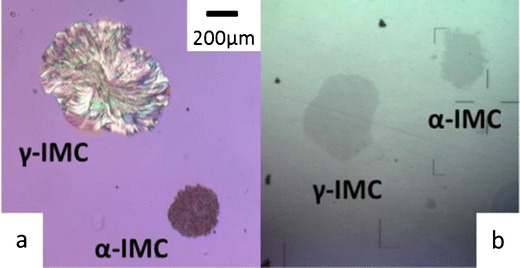
Photomicrographs of γ- and α-IMC surface crystal without excipients (a) or under a layer of MCC at 40°C for 10 days in a desiccator (b). a A reproduction from (25)
The bottom-illuminated inverted microscope was able to distinguish the morphology difference between the two polymorphs with the covering excipients (Fig. 6b). We observed the surface crystallization of IMC glasses in contact with excipient particles at 40°C using the same method as for evaluating NIF. Samples covered with as-received excipients were stored in the 40°C desiccator for 15 days before being examined under the microscope. Because of the slower crystallization of IMC, only cursory experiments were performed to test our method. Compared to the control samples, we observed no significant change in nucleation rate under tested excipients (MCC, lactose, mannitol, and MgSt) for both γ- and α-IMC.
We now comment on possible improvements of our method for evaluating the effect of contact with excipients on the crystallization of amorphous drugs. Knowledge of the growth rate of the amorphous drug is needed for designing the most accurate experiment for measuring the nucleation rate. Fast crystal growth requires that the experimental time be short after crystals are nucleated, to avoid the overlapping of crystals and ambiguity of counting nucleation sites. This situation was encountered in the measurement of nucleation rate of NIF glasses at elevated humidity. If the growth rate is fast, short observation intervals are necessary. If the growth rate is slow, a higher crystallization temperature may be used. The method described here also allows for flexibility in studying fast crystallizing (NIF) and slow crystallizing (IMC) drugs.
The mechanism of dried mannitol and MgSt significantly inducing the nucleation rate of β-NIF in desiccator deserves further investigation. The particle size of the excipients may also affect surface nucleation. In order to check the effect of particle size, we covered amorphous NIF samples with dried excipients following the same procedure as described in the “MATERIALS AND METHODS” part. Excess excipient was removed leaving those particles sticking to the surface. As shown in Fig. 7, MCC and mannitol have relatively larger particles, MgSt has small uniform particles, while lactose contains both large and fine particles. The overall coverage of MgSt is larger than other excipients, indicating a stronger adherence to amorphous NIF surface. However, since mannitol with larger particle size induced more nucleation than lactose, particle size itself may be insufficient to rationalize the effect of excipients. It could be hypothesized that for “inert” excipients, the variation in particle size may only lead to a changed physical disturbance on the surface (8), therefore the nucleation rate may not be significantly affected, such as in the cases of MCC and lactose. For excipients that promote nucleation, reduction of particle size or increase in overall coverage will significantly induced the nucleation rate, such as in the cases of mannitol and MgSt. Further studies are warranted to provide a rational basis for the interaction between excipients and amorphous NIF, and the effect of excipients on the stability of amorphous NIF.
Fig. 7.

Photomicrographs of excipient particles attached to the surface of amorphous NIF
CONCLUSION
In this study, we report a simple and effective method to evaluate the influence of common tablet excipients on the surface crystallization of amorphous drugs. In this procedure, the amorphous drugs NIF and IMC were covered with excipients, and light microscopy was used to observe the nucleation sites under the excipients and to measure the surface crystal growth rate and nucleation rate. The results demonstrated that contact with excipients can induce the surface crystallization of amorphous drugs. For NIF, vacuumed-dried MCC and lactose monohydrate increased the nucleation rate of the β polymorph from two to five times when samples were stored in a desiccator, while mannitol and magnesium stearate induced the nucleation rate 50 times. At 50% RH, vacuumed-dried excipients induced more nucleation than in the desiccator suggesting that moisture played an important role in the surface crystallization caused by the excipients. For example, the effect of as-received MCC, containing ∼5% residual moisture, on the nucleation rate was more significant than vacuum dried MCC. However, the effect of excipients on the crystal growth rate was not significant; suggesting that contact with excipients had a greater effect on the nucleation of nifedipine. For IMC, no significant change in the nucleation rate was observed under the excipients for two polymorphs of the drug, indicating the effect of excipients might be drug specific.
Acknowledgments
We thank Bristol-Myers Squibb Co. for supporting this work.
References
- 1.Yu L. Amorphous pharmaceutical solids: preparation, characterization and stabilization. Adv Drug Deliv Rev. 2001;48(1):27–42. doi: 10.1016/S0169-409X(01)00098-9. [DOI] [PubMed] [Google Scholar]
- 2.Hikima T, Adachi Y, Hanaya M, Oguni M. Determination of potentially homogeneous-nucleation-based crystallization in o-terphernyl and an interpretation of the nucleation-enhancement mechanism. Phys Rev B. 1995;52(6):3900–3908. doi: 10.1103/PhysRevB.52.3900. [DOI] [PubMed] [Google Scholar]
- 3.Hatase M, Hanaya M, Oguni M. Studies of homogeneous-nucleation-based crystal growth: significant role of phenyl ring in the structure formation. J Non-Cryst Solids. 2004;333(2):129–136. doi: 10.1016/j.jnoncrysol.2003.10.010. [DOI] [Google Scholar]
- 4.Sun Y, Xi HM, Chen S, Ediger MD, Yu L. Crystallization near glass transition: transition from diffusion-controlled to diffusionless crystal growth studied with seven polymorphs. J Phys Chem B. 2008;112(18):5594–5601. doi: 10.1021/jp7120577. [DOI] [PubMed] [Google Scholar]
- 5.Xi HM, Sun Y, Yu L. Diffusion-controlled and diffusionless crystal growth in liquid o-terphenyl near its glass transition temperature. J Chem Phys. 2009;130:094508. doi: 10.1063/1.3081047. [DOI] [PubMed] [Google Scholar]
- 6.Wu T, Yu L. Surface crystallization of indomethacin below T-g. Pharm Res. 2006;23(10):2350–2355. doi: 10.1007/s11095-006-9023-4. [DOI] [PubMed] [Google Scholar]
- 7.Zhu L, Wong L, Yu L. Surface-enhanced crystallization of amorphous nifedipine. Mol Pharm. 2008;5(6):921–926. doi: 10.1021/mp8000638. [DOI] [PubMed] [Google Scholar]
- 8.Sun Y, Zhu L, Kearns KL, Ediger MD, Yu L. Glasses crystallize rapidly at free surfaces by growing crystals upward. PNAS. 2011;108(15):5990–5995. doi: 10.1073/pnas.1017995108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhu L, Brian CW, Swallen SF, Straus PT, Ediger MD, Yu L. Surface self-diffusion of an organic glass. Phys Rev Lett. 2011;106(25):256103. doi: 10.1103/PhysRevLett.106.256103. [DOI] [PubMed] [Google Scholar]
- 10.Brian CW, Yu L. Surface self-diffusion of organic glasses. J Phys Chem A. 2013;117(50):13303–13309. doi: 10.1021/jp404944s. [DOI] [PubMed] [Google Scholar]
- 11.Wu T, Sun Y, Li N, de Villiers MM, Yu L. Inhibiting surface crystallization of amorphous indomethacin by nanocoating. Langmuir. 2007;23(9):5148–5153. doi: 10.1021/la070050i. [DOI] [PubMed] [Google Scholar]
- 12.Marsac PJ, Konno H, Taylor LS. A comparison of the physical stability of amorphous felodipine and nifedipine systems. Pharm Res. 2006;23(10):2306–2316. doi: 10.1007/s11095-006-9047-9. [DOI] [PubMed] [Google Scholar]
- 13.Ishida H, Wu T, Yu L. A sudden rise of crystal growth rate of nifedipine near t-g without and with polyvinylpyrrolidone. J Pharm Sci. 2007;96(5):1131–1138. doi: 10.1002/jps.20925. [DOI] [PubMed] [Google Scholar]
- 14.Kestur US, Lee H, Santiago D, Rinaldi C, Won Y-Y, Taylor LS. Effects of the molecular weight and concentration of polymer additives, and temperature on the melt crystallization kinetics of a small drug molecule. Cryst Growth Des. 2010;10(8):3585–3595. doi: 10.1021/cg1004853. [DOI] [Google Scholar]
- 15.Cai T, Zhu L, Yu L. Crystallization of organic glasses: effects of polymer additives on bulk and surface crystal growth in amorphous nifedipine. Pharm Res. 2011;28(10):2458–2466. doi: 10.1007/s11095-011-0472-z. [DOI] [PubMed] [Google Scholar]
- 16.Powell CT, Cai T, Hasebe M, Gunn EM, Gao P, Zhang G, et al. Low-concentration polymers inhibit and accelerate crystal growth in organic glasses in correlation with segmental mobility. J Phys Chem B. 2013;117(35):10334–10341. doi: 10.1021/jp406418n. [DOI] [PubMed] [Google Scholar]
- 17.Malan CEP, de Villiers MM, Lotter AP. Application of differential scanning calorimetry and high performance liquid chromatography to determine the effects of mixture composition and preparation during the evaluation of niclosamide-excipient compatibility. J Pharm Biomed Anal. 1997;15(4):549–557. doi: 10.1016/S0731-7085(96)01869-9. [DOI] [PubMed] [Google Scholar]
- 18.Narang AS, Desai D, Badawy S. Impact of excipient interactions on solid dosage form stability. Pharm Res. 2012;29(10):2660–2683. doi: 10.1007/s11095-012-0782-9. [DOI] [PubMed] [Google Scholar]
- 19.Chadha R, Bhandari S. Drug-excipient compatibility screening—role of thermoanalytical and spectroscopic techniques. J Pharm Biomed Anal. 2014;87:82–97. doi: 10.1016/j.jpba.2013.06.016. [DOI] [PubMed] [Google Scholar]
- 20.Simmons DL, Chen WS, Frechett M, Ranz RJ, Patel NK. Rotating compartmentalized disk for dissolution rate determinations. Can J Pharm Sci. 1972;7(2):62–65. [Google Scholar]
- 21.Gunn E, Guzei IA, Cai T, Yu L. Polymorphism of nifedipine: crystal structure and reversible transition of the metastable beta polymorph. Cryst Growth Des. 2012;12(4):2037–2043. doi: 10.1021/cg3000075. [DOI] [Google Scholar]
- 22.Kibbe AH. Handbook of pharmaceutical excipients. 3. London: American Pharmaceutical Association and Pharmaceutical Press; 2000. [Google Scholar]
- 23.Yoshioka M, Hancock BC, Zografi G. Crystallization of indomethacin from the amorphous state below and above its glass-transition temperature. J Pharm Sci. 1994;83(12):1700–1705. doi: 10.1002/jps.2600831211. [DOI] [PubMed] [Google Scholar]
- 24.Baird JA, Van Eerdenbrugh B, Taylor LS. A classification system to assess the crystallization tendency of organic molecules from undercooled melts. J Pharm Sci. 2010;99(9):3787–3806. doi: 10.1002/jps.22197. [DOI] [PubMed] [Google Scholar]
- 25.Stieger N, Aucamp M, Zhang SW, de Villiers MM. Hot-stage optical microscopy as an analytical tool to understand solid-state changes in pharmaceutical materials. Am Pharm Rev. 2012;15(2):32–36. [Google Scholar]


