Abstract
Gelatin capsules are a widely used dosage form both for pharmaceutical drug products as well as dietary supplements. Gelatin in the presence of certain compounds, mainly aldehydes, or in high humidity and high temperature conditions can cross-link. Cross-linking involves covalent bonding of the amine group of a lysine side chain of one gelatin molecule to a similar amine group on another molecule. The covalent bonding is, for practical purposes, irreversible. Cross-linking results in the formation of a pellicle on the internal or external surface of the gelatin capsule shell that prevents the capsule fill from being released. In vitro dissolution testing of cross-linked gelatin capsules can result in slower release of the drug or no release at all. The data obtained by the Gelatin Capsule Working Group, created in the early 90s to investigate noncompliance of gelatin capsules, was used to establish the type and amounts of enzymes that can be added to the dissolution medium in the case of test failure to the presence of cross-linking in the gelatin. The two-tier dissolution testing was included in the US Pharmacopeia and it recommends the addition of pepsin (pH below 6.8) or pancreatin (pH above 6.8) to the medium depending on its pH. Pepsin shows good protease activity up to pH 4 and pancreatin above pH 6 leaving a gap where neither one has good activity. Possible proteolytic enzymes that could be used for the pH range 4–6.8 could be papain or bromelain.
KEY WORDS: cross linking, gelatin capsules, two-tier dissolution testing
INTRODUCTION
Gelatin capsules are a widely used dosage form because it is simple to formulate them for drugs in powdered or granular form and because they provide an easy-to-swallow container that effectively masks odors and the bitter taste of drugs. With the advent of pellet technology that enabled modified drug release, capsules provided a useful vehicle into which multiparticulates could be filled without risk of modifying the release characteristics associated with other processing methods such as tablet compression. Since the early 1980s, technology has been available to permit accurate dosing and sealing of liquids into hard gelatin capsules. Current technologies allow the encapsulation of lipid solutions, suspensions, or semisolid formulations (1–3). In the presence of certain compounds such as aldehydes or when exposed to high humidity and temperature, gelatin can cross-link rendering it insoluble in aqueous solvents. The presence of cross-linking will alter the in vitro dissolution behavior of the gelatin capsules; the capsule will not open and release its contents into the dissolution medium (1–7). This failure may not reflect a possible failure to dissolve in the body. USP general chapter <711 > dissolution (8) allows the addition of proteolytic enzymes in the dissolution medium where gelatin capsules or gelatin-coated tablets experience dissolution failure due to the presence of cross-linking. The general chapter recommends the use of pepsin or pancreatin depending on the pH of the dissolution medium. A discussion on the issues associated with the dissolution conditions for cross-linked gelatin capsules as well as suggestions to overcome them will be presented in this paper.
GELATIN
Gelatin is obtained from the partial hydrolysis of collagen, which is the most abundant animal protein in nature. Collagen is an insoluble, highly ordered fibrous protein and is the primary fibrous component of bone, skin, and connective tissue. The majority of pharmaceutical gelatin is produced from bovine bone, bovine hide, and porcine skin. Gelatin is graded primarily on the strength of the gel it forms and, depending on the process used and the tissue source, noticeable differences in strength are apparent among suppliers and even between lots from the same supplier. Consequently, controlling the strength of the gel from batch to batch, measured as bloom strength, is key to obtain a consistently performing product. Gelatin manufacturers commonly blend different sublots of gelatin to meet bloom requirements. Bloom strength is a measure of the ability of a given weight of gelatin to set up in water under controlled conditions and is a function of the molecular weight of the gelatin molecules, the concentration of the gelatin in the gel, and the pH of the gel. Bloom strength increases when the gelatin concentration in the gel increases, when the average molecular weight of the gelatin increases, and when the pH of the gel approaches neutrality. In addition, as bloom strength increases, the cost of gelatin increases and gel dissolution rate decreases (1,9).
GELATIN CAPSULES
Gelatin capsule shells are prepared from a molten gel mass that is composed of gelatin and a plasticizer dissolved in an aqueous vehicle. For hard gelatin capsules (hardgels) shells, water acts as both the plasticizer and the vehicle. For soft gelatin capsules (softgel) shells, small polyhydroxy compounds such as glycerol, sorbitol, and maltitol typically are used as plasticizers. Although many parameters affect the physical and chemical properties of the shell, the ratio of polymer to plasticizer primarily determines the rigidity, brittleness, and dissolution performance of the shell. Other minor components added to the gel mass may include colorants, flavors, stabilizers, buffers, and opacifiers.
Softgels have a thicker shell and typically exhibit a higher degree of elasticity because of the added plasticizer. They have slightly longer rupture time when compared with hardgels. By comparison, hardgel capsules have a thinner and more rigid shell than do softgel capsules.
Capsules can also be characterized by the chemical properties of the fill material (hydrophobic-based vs. hydrophilic-based fill materials) or by the physical properties of the fill material (solution vs. dispersion vs. solid) (1,9,10).
CROSS-LINKING IN GELATIN
The factors that can affect the properties of the gelatin capsule shell include moisture exchange between the shell and the fill material, which can potentially create brittleness in the gelatin shell, and chemical interactions between the fill material and gelatin or between the gelatin and the environment during storage, which can result in gelatin cross-linking. Cross-linking involves strong chemical linkages beyond simple hydrogen and ionic bonding between gelatin chains (5,6,11). One of the strongest and most common types of cross-linking involves the covalent bonding of the amine group of a lysine side chain of one gelatin molecule to a similar amine group on another molecule. This reaction generally is catalyzed by trace amounts of reactive aldehydes. Formaldehyde, glutaraldehyde, glyoxal, and reducing sugars are the most common catalysts. The covalent bonding produced with this type of cross-linking is, for all practical purposes, irreversible, and dissolution of the shell must involve the breaking of other bonds, e.g., by enzyme-mediated breaking of peptide bonds in protein chains. It has been proposed that chemically modified gelatin—by adding succinic acid groups to the lysine side chains—may prevent or at least diminish aldehyde-mediated cross-linking. Another, weaker, type of cross-linking is complexation of free carboxylic acid groups on two different gelatin molecules with trivalent metal ions, such as Fe3+ and Al3+. These cations may be found in some of the dyes used as colorants or as low levels of contaminants in excipients. Higher bloom gelatin, which is normally associated with higher quality, facilitates efficient cross-linking because fewer links are needed to join greater lengths of gelatin chains.
Common causes of cross-linking include:
Aldehydes present in active pharmaceutical ingredients, excipients, packaging materials, or degradants formed in situ during storage
High humidity
Substances that facilitate a cross-linking reaction
Substances that promote decomposition of stabilizer in corn starch (hexamethylenetetramine) resulting in the formation of ammonia and formaldehyde
Rayon coilers that contain an aldehyde functional group (furfural)
Polyethylene glycols that may auto-oxidize to form aldehydes
UV light, especially in the presence of high heat and humidity
Heat, which can catalyze aldehyde formation
Cross-linking is going to result in the formation of a pellicle on the internal or external surface of the gelatin capsule shell. A pellicle is a thin, water insoluble clear membrane of cross-linked protein on the inner or outer surface of the capsule that prevents the capsule fill from being released. Cross-linking is evidenced by the observation of a thin membrane or gelatinous mass during dissolution testing because the pellicle itself may be difficult to observe (1) (see Figs. 1 and 2).
Fig. 1.
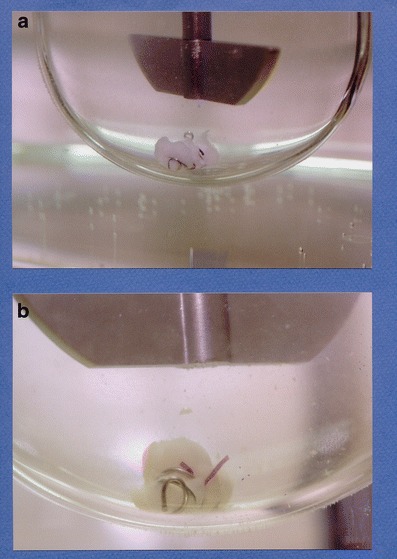
Cross-linked gelatin capsule with sinker in USP dissolution apparatus 2 (Copyright 2014 Vivian Gray, used by permission)
Fig. 2.
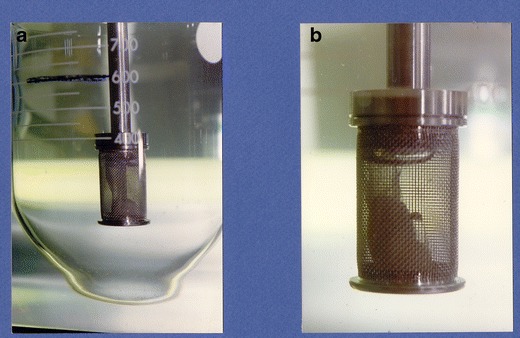
Cross-linked gelatin capsule in USP apparatus 1 (Copyright 2014 Vivian Gray, used by permission)
In vitro dissolution testing of cross-linked capsules can result in slower release of the drug or no release at all (10,12). Once the cross-linking process starts, it does not stop even if the cause is removed. On rare occasions, if there are seam defects in liquid-filled capsules, the capsule can rupture at the seam even in the presence of cross-linking in the gelatin, resulting in an early release of the capsule fill in the dissolution medium. The degree of cross-linking is not uniform within one capsule or among different capsules. As consequence, dissolution results will have higher variability when gelatin capsules are cross-linked (1,5,6,9,10).
Non-gelatin capsules do not show the phenomenon of cross-linking. Pharmaceutical capsules can also be made with iota and kappa carrageenans, modified corn, potato, and pea starches, and modified celluloses, such as hypromellose (HPMC). They possess the required gelling characteristics similar to gelatin and they do not show cross-linking. This type of capsules exhibit a slightly longer dissolution time when compared to gelatin capsules (9,13).
DISSOLUTION TESTING OF CROSS-LINKED GELATIN CAPSULES
In the early 90s, a Gelatin Capsule Working Group was formed to study noncompliance of gelatin capsule products during in vitro dissolution tests and potential changes in bioavailability. A full investigation was conducted in a joint effort between Center for Drug Evaluation and Research (CDER)/FDA, representatives of pharmaceutical industry trade associations, gelatin capsules manufacturers, and the United States Pharmacopeia and academia. This working group developed a protocol using differentially stressed hard and soft gelatin capsules to determine the relationship between in vitro and in vivo performance.
Hard and soft gelatin capsules were carefully stressed to the point where they failed the USP dissolution test official at that time but passed after the addition of enzyme. The sample capsules were stressed further to the point where they failed both the initial test as well as the test with enzyme. Dissolution of stressed and unstressed capsules was compared during in vivo and in vitro testing. Capsules were tested both in vitro and in vivo to determine the proper level of enzyme that would discriminate between bioequivalent and bioinequivalent products. Hard gelatin capsules were cross-linked by contact with lactose spiked with known amounts of formaldehyde, and then the lactose was removed and replaced by acetaminophen. Cross-linked soft gelatin capsules were obtained by being filled with acetaminophen dissolved in polyethylene glycol and spiked with small amounts of formaldehyde. Bioequivalence studies of unstressed, moderately stressed, and overstressed hard and soft gelatin capsules were conducted. The results indicated that moderately stressed capsules were bioequivalent to unstressed capsules, whereas overstressed capsules failed to demonstrate bioequivalence (4,14).
In vitro dissolution testing was conducted to determine the proper amounts of enzyme that would allow bioequivalent capsules to pass both tiers of the dissolution test, while bioinequivalent capsules would fail. Results indicated that moderately stressed capsules, which are bioequivalent to unstressed capsules, did not meet the dissolution test specifications official at that time but did pass the test upon the addition of enzymes to the medium. Overstressed capsules, which failed to demonstrate bioequivalence, failed to meet the dissolution test specifications with and without enzymes in the medium (4,14). The results of these studies were used to establish the amount of enzymes that could be added to the dissolution medium in the case of test failure due to the presence of cross-linking in the gelatin. The two-tier dissolution testing was included in the First Supplement of USP 24 (15): pepsin is added to acidic medium or water to achieve an activity of 750,000 units (activity determined by spectrophotometry using hemoglobin) or less per liter. Pancreatin USP is added to a medium at or above pH 6.8 to achieve a protease activity of not more than 1,750 units of protease activity per liter.
CHALLENGES IN THE USE OF ENZYMES
Failure in the Dissolution Testing
The current text in the USP general chapter <711 > dissolution (8) allows the addition of enzymes to the dissolution medium when hard or soft gelatin capsules and gelatin-coated tablets do not conform to the dissolution specification. The text does not explicitly mention that it is the failure due to the presence of cross-linking in the gelatin. If the gelatin capsule is rupturing and the contents are being released into the dissolution medium but the capsule is failing the dissolution test, the addition of enzymes to the dissolution medium probably is not going to improve the results.
The dissolution failure by cross-linking should be properly documented and justified. Cross-linking in the gelatin can be evidenced by (a) visual observations (see Figs. 1 and 2), the presence of a thin membrane around the capsule or the capsule is transformed in a gelatinous mass with no rupture; (b) instrumental techniques such as carbon 13-nuclear magnetic resonance spectroscopy, Fourier transform near-infrared, Fourier transform infrared (16,17), magnetic resonance imaging, etc. (16); (c) by transferring the capsule contents to a non-cross-linked capsule, when possible (18).
Pepsin
Current USP text recommends the addition of pepsin to the dissolution medium when the medium is water or is a medium with a pH less than 6.8. Pepsin shows a good proteolytic activity up to pH 4 and almost no protease activity above pH 5.5 (see Fig. 3) (19). Pepsin is not a suitable enzyme for two-tier dissolution testing when the dissolution medium has pH above 4.
Fig. 3.

The pH stability and pH activity of pepsin (19)
The amount of pepsin to be added to the dissolution medium was defined based on the data generated by the Gelatin Capsule Working Group (see “Dissolution Testing of Cross-linked Gelatin Capsules” above). This amount was defined based on the determination of the protease activity of pepsin using a spectrophotometric procedure with hemoglobin described under Purified Pepsin in the Reagent Specifications section of USP (20). There are other methods that can be used to determine the activity of pepsin. Up to now, there is no information available about the equivalence of the pepsin activity determined by different methods. For dissolution applications, it is recommended to determine the activity of pepsin before use employing the spectrophotometric procedure described in USP.
Pepsin is a very stable enzyme, retaining its activity in solution for 1 year when stored at 4°C, indicating that autohydrolysis is negligible (21).
Pancreatin
Pancreatin is an enzyme complex containing enzymes with various substrate specificities, including trypsin, α-chymotrypsin, carboxypeptidase, lipase, and amylase. Pancreatin is widely used due to its wide substrate specificity and the ability to hydrolyze proteins, fats, and polysaccharides. Pancreatin shows a good proteolytic activity around the pH range of 6–8, depending on its source (pig or bovine; see Fig. 4) (22).
Fig. 4.
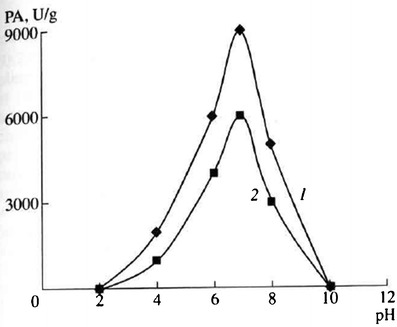
Dependency of protease activities (PA) of pancreatin complex from pig (1) and bovine (2) on medium pH (15)
For dissolution applications with cross-linked gelatin capsules, it is the protease activity that should be considered. The protease activity of pancreatin is determined by an assay that measures the casein digestive power of the enzyme as stated in the USP monograph for pancreatin (23).
Possible Enzymes for pH 4–6.8
As pepsin has good activity to pH 4 and pancreatin above pH 6.8, suitable protease enzymes should be used for the pH range 4–6.8. Possible candidates are papain and bromelain. These enzymes are not found in the human body. Therefore, their application would only be to digest the cross-linked gelatin during the dissolution test and not to mimic any in vivo behavior. There are other in vivo factors (presence of bile salts, gastric motility, etc.) that can overcome the effect of cross-linking in gelatin capsules but are not easy to reproduce in routine dissolution testing.
Papain
Papain is a protease isolated from the latex of the leaves and green fruit of the papaya tree (24). It is almost completely soluble in water (25,26). The optimal pH for proteolysis by papain lies in the range of pH 4 to 7 (27,28). The protease activity of papain can be determined by casein digestive power as stated in the USP monograph for papain (29).
Bromelain
Bromelain is the collective name for proteolytic enzymes found in various members of the family Bromeliaceae. The one commercially widely available is the bromelain from pineapple. It is readily soluble in water. The optimum pH range is about 4.5–7.5 (30). Its protease activity can be determined by the casein digestive power test in a procedure similar to the one used for papain.
Dissolution Media Containing Surfactants
Although useful solubilizing agents, surfactants should be used cautiously because they can interact with the gelatin in the capsule shell hindering disintegration or dissolution. They can also inhibit the enzymes used in the dissolution medium when the gelatin capsules show cross-linking (9,18,31,32).
Although it is frequently used to improve the solubility of poorly soluble drugs in dissolution, sodium lauryl sulfate should be considered only in the absence of other alternatives because it readily denatures enzymes that are used to overcome cross-linking in dissolution tests (1,18,31,32).
If the dissolution medium contains surfactants, a pretreatment of the cross-linked gelatin capsules with the medium containing the enzyme but not the surfactant may be appropriate (18,31). This pretreatment consists of adding the enzyme to the specified medium without the surfactant, and running the agitation for a short period of time, in most cases not more than 15 min. After this period, a solution containing the surfactant is added to the dissolution vessel in such a way that the final concentration of the surfactant in the vessel will be the one specified in the method and the test is carried for the remaining period of time. The pretreatment time is included in the total run of the dissolution method, if the total time of the test is 45 min and a 15-min pretreatment is done; after the addition of the surfactant to the medium, the test is going to run for an additional 30 min, resulting in the total of 45 min stated in the method. Some examples of this pretreatment can be found in the FDA-recommend Dissolution methods database available at http://www.accessdata.fda.gov/scripts/cder/dissolution (see Fig. 5).
Fig. 5.
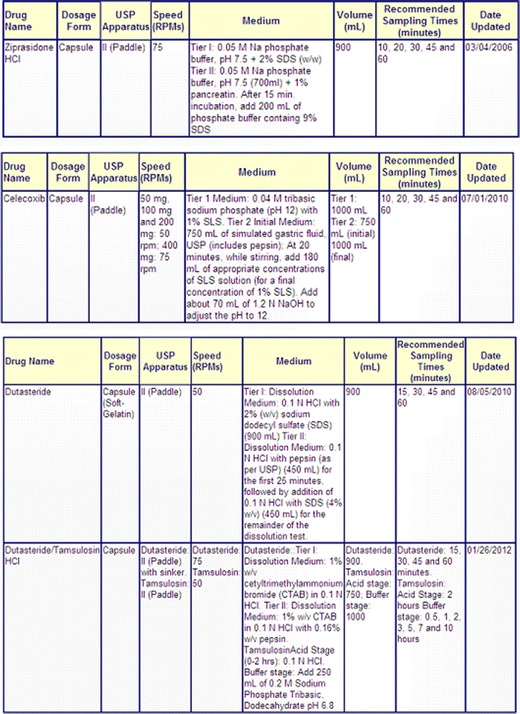
Examples of dissolution method with pretreatment step when the dissolution medium contains surfactant (from http://www.accessdata.fda.gov/scripts/cder/dissolution/index.cfm accessed on Jan 24, 2014)
Using ziprasidone hydrochloride capsules as an example, the dissolution medium is 1,000 mL of 0.05 M sodium phosphate buffer, pH 7.5, and 2% (w/w) sodium dodecyl sulfate (synonym of sodium lauryl sulfate). In the presence of cross-linking, the samples are going to be submitted to a 15-min pretreatment where the medium is 700 mL of 0.05 M sodium phosphate buffer containing 1% of pancreatin. After 15 min, 200 mL of 0.05 M sodium phosphate buffer containing 9% of sodium dodecyl sulfate is going to be added to the vessel and the test is going to be run for the remaining time of the test.
The stepwise addition of enzyme and surfactant enable both agents to take effect individually and sequentially in the dissolution medium. The enzyme digests the cross-linked capsule shell at the beginning, whereas the addition of surfactant afterwards increases drug solubility and wettability. The pretreatment can be used even when the surfactant is a component of the formulation. Once the cross-linked capsule shell ruptures and dissolution starts, the surfactant inside the formulation will work as expected (18).
DEVELOPING AND VALIDATING DISSOLUTION METHODS
During the development of the dissolution procedure for any dosage form containing gelatin, it is recommended to evaluate and define the procedure to be used in the event that the gelatin will present cross-linking. In some cases, depending on the formulation and packaging system, the cross-linking of the gelatin will happen only during the accelerated stability studies.
In addition to the general method parameters usually validated (filters, sinkers, deaeration, specificity, linearity, accuracy, precision, etc.) (33), the following items should be evaluated and defined.
Forced Cross-Linking
To obtain the samples necessary for all the tests and evaluations, forced cross-linking of the capsules can be promoted by:
Selection and Activity Determination of the Enzyme
The enzyme to be used should have optimal activity in the pH of the dissolution medium used.
The method for protease activity determination of the enzyme needs to be validated in the conditions of use at the lab. Also, the frequency for reevaluation of enzyme activity should be established. Care must be taken in using the information on the label of the enzyme as it is just an indication of the purity of the material. When using the information in the certificate of analysis of the batch of the enzyme to be used, attention must be paid to the method used by the supplier to determine the protease activity of the enzyme as there is no direct conversion from units of activity among different methods.
Pretreatment Step
If the dissolution medium contains surfactants, the details of the pretreatment of the cross-linked capsules with medium containing enzyme but no surfactant need to be established: selection of the enzyme, composition and volume of pretreatment medium, contact time, and concentration of surfactant in the solution to be added to the medium containing enzyme in order to achieve the final concentration specified in the original procedure.
CONCLUDING REMARKS
Since the release of the conclusions of the Gelatin Working Group (4,14) and the inclusion of the two-tier dissolution testing for cross-linked gelatin products in the US Pharmacopeia (15), additional findings and data are showing that a revision of the instructions for the procedure is needed in the compendia. This revision includes better justification of the failure in the dissolution testing, inclusion of enzymes with good protease activity in the pH range from 4 to 6.8, and pretreatment when the dissolution medium contains surfactants. A few years ago, the US Pharmacopeia created an expert panel with members representing enzyme, gelatin capsule, excipient manufacturers, and pharmaceutical and dietary supplement companies. The panel was charged with the task of updating the two-tier dissolution testing in the USP general chapter <711 > dissolution. The revision will be published in a future issue of Pharmacopeial Forum (www.usppf.com) for a period for public comments.
References
- 1.Marques MRC, Cole E, Kruep D, Gray V, Murachanian D, Brown WE, et al. Liquid-filled gelatin capsules. Pharm Forum. 2009;35(4). www.usppf.com.
- 2.Podczeck F, Jones BE. Pharmaceutical capsules. London: Pharmaceutical Press; 2004. [Google Scholar]
- 3.Hu J, Kyad A, Ku V, Zhou P, Cauchon N. A comparison of dissolution testing on lipid soft gelatin capsules using USP apparatus 2 and apparatus 4. Dissol Technol. 2005;12(2):6–9. doi: 10.14227/DT120205P6. [DOI] [Google Scholar]
- 4.Meyer MC, Strughn AB, Mhatre RM, Hussain A, Sha VP, Bottom CB, et al. The effect of gelatin cross-linking on the bioequivalence of hard and soft gelatin acetaminophen capsules. Pharm Res. 2000;17(8):962–966. doi: 10.1023/A:1007579221726. [DOI] [PubMed] [Google Scholar]
- 5.Chang CK, Alvarez-Nunez FA, Rinella JV, Jr, Magnusson LE, Sued K. Roller compaction, granulation and capsule product dissolution of drug formulations containing a lactose or mannitol filler, starch, and talc. AAPS PharmSciTech. 2008;9(2):597–604. doi: 10.1208/s12249-008-9088-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elmeshad AN, Darwish MK. Stability studies of the effect of crosslinking on hydrochlorothiazide release. Drug Discov Ther. 2009;3(3):136–142. [PubMed] [Google Scholar]
- 7.St. Clair MJ, Purdie J, Hu Y, McGeough P. The effect of cross-linking on the in vitro disintegration of hard gelatin capsules. J Pharm Pharmacol. 2010;62(10):1235–1236. [Google Scholar]
- 8.USP. USP 36 – NF 31. <711 > Dissolution. Rockville: US Pharmacopeial Convention; 2013. p. 307.
- 9.<1094 > Capsules—dissolution testing and related quality attributes. Pharm Forum. 2013;39(3). www.usppf.com.
- 10.Cole ET, Cade D, Benameur H. Challenges and opportunities in the encapsulation of liquid and semi-solid formulations into capsules for oral administration. Adv Drug Deliv Rev. 2008;60:747–756. doi: 10.1016/j.addr.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 11.Rose PI. Gelatin. In: The theory of the photographic process. 4th ed. New York: Macmillan Publ. Co.; 1977. p. 51–67.
- 12.Kalantzi L, Page R, Nicolaides E, Digenis G, Reppas C. In vitro methods can forecast the effects of intragastric residence on dosage form performance. Eur J Pharm Sci. 2008;33:445–451. doi: 10.1016/j.ejps.2008.01.014. [DOI] [PubMed] [Google Scholar]
- 13.Jones BE. How gelatin and hypromellose capsules differ in product release during dissolution testing. Tablets & Capsules. 2010; January: 16–19.
- 14.Aikman M, et al. Collaborative development of two-tier dissolution testing for gelatin capsules and gelatin-coated tablets using enzyme-containing media. Pharm Forum. 1998;24(5):704–7050. [Google Scholar]
- 15.USP. USP 24 – NF 19, First supplement. <711 > Dissolution. Rockville: US Pharmacopeial Convention; 2000. p. 2696
- 16.Singh S, Rao KVR, Venugopal K, Manikandan R. Alteration in dissolution characteristics of gelatin-containing formulations. Pharm Technol. 2002;26:36–58. [Google Scholar]
- 17.Tengroth C, Gasslander U, Anderson FO, Jacobsson SP. Cross-linking of gelatin capsules with formaldehyde and other aldehydes: an FTIR spectroscopy study. Pharm Dev Technol. 2005;10:405–412. doi: 10.1081/PDT-65693. [DOI] [PubMed] [Google Scholar]
- 18.Song X, Cui Y, Xie M. Gelatin capsule shell cross-linking. Tier II dissolution method development in the presence of sodium lauryl sulfate. Pharm Technol. 2011;35(5):62–68. [Google Scholar]
- 19.Piper DW, Fenton BH. pH stability and activity curves of pepsin with special reference to their clinical importance. Gut. 1965;6:506–508. doi: 10.1136/gut.6.5.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.USP. USP 36 – NF 31. Purified pepsin. Rockville: US Pharmacopeial Convention; 2013. p. 1181.
- 21.Kristo K, Pintye-Hodi K. Effects of pharmaceutical processing on pepsin activity during the formulation of solid dosage forms. Pharm Dev Technol. 2013;18(1):17–21. doi: 10.3109/10837450.2012.717946. [DOI] [PubMed] [Google Scholar]
- 22.Berdutina AV, Neklyudov AD, Ivankin AI, Karpo BS, Mitaleva SI. Comparison of proteolytic activities of the enzyme complex from mammalian pancreas and pancreatin. Appl Biochem Microbiol. 2000;36:363–367. doi: 10.1007/BF02738043. [DOI] [PubMed] [Google Scholar]
- 23.USP. USP 36 – NF 31. Pancreatin. Rockville: US Pharmacopeial Convention; 2013. p. 4672.
- 24.Azarkan M, El Moussaoui A, van Wuytswinkel D, Dehon G, Looze Y. Fractionation and purification of the enzymes stored in the latex of Carica papaya. J Chromatogr B Analyt Technol Biomed Life Sci. 2003;790(1–2):229–238. doi: 10.1016/S1570-0232(03)00084-9. [DOI] [PubMed] [Google Scholar]
- 25.Arnon R. Papain. In: Perlmann G, Lorand L, editors. Method in enzymology. New York: Academic; 1970. pp. 226–245. [Google Scholar]
- 26.Kimmel JR, Smith EL. The properties of papain. In: Nord FF, editor. Advances in enzymology and related areas of molecular biology. New York: Interscience Publ; 1957. pp. 267–333. [Google Scholar]
- 27.Lineweaver H, Schwimmer S. Some properties of crystalline papain. Enzymologia. 1941;10:81–86. [Google Scholar]
- 28.Hoover SR, Kokes ELC. Effect of pH upon proteolysis by papain. J Biol Chem. 1947;167(1):199–207. [PubMed] [Google Scholar]
- 29.USP. USP 36 – NF 31. Papain. Rockville: US Pharmacopeial Convention; 2013. p. 4186.
- 30.Corzo CA, Waliszewski KN, Welti-Chanes J. Pineapple fruit bromelain affinity to different protein substrates. Food Chem. 2012;133(3):631–635. doi: 10.1016/j.foodchem.2011.05.119. [DOI] [Google Scholar]
- 31.Lu X, Xiao B, Lo L, Bolgar MS, Lloyd DK. Development of a two-step tier-2 dissolution method for blinded overencapsulated erlotinib tablets using UV fiber optic detection. J Pharm Biomed Anal. 2011;56:23–29. doi: 10.1016/j.jpba.2011.04.026. [DOI] [PubMed] [Google Scholar]
- 32.Pennings FH, Kwee BLS, Vromans H. Influence of enzymes and surfactants on the disintegration behavior of cross-linked hard gelatin capsules during dissolution. Drug Dev Ind Pharm. 2006;32:33–37. doi: 10.1080/03639040500387955. [DOI] [PubMed] [Google Scholar]
- 33.USP. USP 36 – NF 31. The dissolution procedure: development and validation <1092>, Rockville: US Pharmacopeial Convention; 2013. p. 735.
- 34.Gallery J, Han JH, Abraham C. Pepsin and pancreatin performance in the dissolution of crosslinked gelatin capsules from pH 1 to 8. Pharm Forum. 2004;30(3):1084–1089. www.usppf.com.
- 35.Bottom CB, Clark M, Carstensen JT. Dissolution testing of soft shell capsules—acetaminophen and nifedipine. J Pharm Sci. 1997;86(9):1057–1061. doi: 10.1021/js960263k. [DOI] [PubMed] [Google Scholar]


