Abstract
Cationic liposomes have long been used as non-viral vectors for small interfering RNA (siRNA) delivery but are associated with high toxicity, less transfection efficiency, and in vivo instability. In this investigation, we have developed siRNA targeted to RRM1 that is responsible for development of resistance to gemcitabine in cancer cells. Effect of different lipid compositions has been evaluated on formation of stable and less toxic lipoplexes. Optimized cationic lipoplex (D2CH) system was comprised of dioleoyl-trimethylammoniumpropane (DOTAP), dioleoyl-sn-glycero-3-phosphoethanolamine (DOPE), hydrogenated soya phosphocholine (HSPC), cholesterol, and methoxy(polyethyleneglycol)2000–1,2-distearoyl-sn-glycero-3-phosphoethanolamine (mPEG2000–DSPE). D2CH lipoplexes have shown particle size (147.5 ± 2.89 nm) and zeta potential (12.26 ± 0.54 mV) characteristics essential for their in vivo use. In vitro cytotoxicity study has shown low toxicity of developed lipoplexes as compared with lipofectamine-2000 up to N/P ratio as high as 7.5. Cell uptake studies and gene expression studies have confirmed intracellular availability of siRNA. In addition, developed lipoplexes also showed ~3 times less hemolytic potential as compared with DOTAP/DOPE lipoplexes at lipid concentration of 5 mg/mL. Lipoplexes also maintained particle size less than 200 nm on exposure to high electrolyte concentration and showed >70% siRNA retention in presence of serum showing siRNA protection conferred by lipoplexes. Furthermore, in vivo acute toxicity studies in mice showed that formulation was non-toxic up to a dosage of 0.75 mg of siRNA/kg as lipoplexes and 300 mg lipid/kg as blank liposomes indicating tolerability of lipoplexes at a dose much higher than required for therapeutic use. Promising results of this study warrant further investigation of developed siRNA lipoplexes for cancer treatment.
KEY WORDS: cancer, gene expression, lipoplex, siRNA, toxicity
INTRODUCTION
Cancer of the lung tissue is a leading cause of mortality accounting for more than 1.5 million deaths worldwide every year (1). It is characterized by uncontrolled proliferation and metastasis of lung tissue cells. Smoking and tobacco use are the main causes for lung cancer development (2–4). Amongst the two main lung cancer types, non-small cell lung cancer (NSCLC) and small cell lung cancer , NSCLC is responsible for almost 80% of lung cancer.
Though primary resort for NSCLC treatment is to remove the cancerous tissue by surgery, it alone is not sufficient. Chemotherapy is of prime importance to eliminate remnants of cancer tissue after surgery. Chemotherapy is often associated with development of resistance causing less effectiveness of chemotherapeutic agents. Gemcitabine is an important drug for patients with various cancers including non-small cell lung cancer. Gemcitabine resistance has been attributed to various factors mainly deoxycytidine kinase deficiency, increased ribonucleotide reductase subunit 1 (RRM1) gene activity, and increased deoxycytidine monophosphate deaminase (5). Studies on RRM1 expression have shown improved survival of patients treated with gemcitabine-based therapy at low expression levels of RRM1 (6). So, small interfering RNA (siRNA) targeting RRM1 can be of potential use repressing the RRM1 gene activity by mRNA knock-down.
In past decade, siRNA delivery has come up as an advanced alternative for the treatment of various diseases, but its widespread use is hindered by various shortcomings associated with it (7). Main barriers for the delivery of siRNA are its negative charge and hydrophilicity which hinder its easy diffusion through negatively charged cell membranes and degradation of siRNA by nucleases during blood circulation and after endocytosis ruling out the possibility of use of naked siRNA for better transfection of target cells. Viral vector-based siRNA delivery has been developed for effective delivery of siRNA inside the cells overcoming the barriers related to the use of naked siRNA (8,9). But this method itself is not devoid of disadvantages. The main obstacle for use of viral vectors is their immunogenicity which may lead to severe adverse effect and even death. With the advent of nanotechnology and novel drug delivery systems, we are now available with various nanoformulations like lipoplexes, polyplexes, calcium phosphate–siRNA nanocomposites, dendrimers, etc. (7,10,11), which can be useful for such purposes without significant adverse effects or toxicities. Cationic lipid-based siRNA delivery has been widely studied and explored. Lipoplexes have come up as a potential carrier system for gene delivery after the inception of liposomal drug delivery. They can provide a delivery system with good biological characteristics along with effective transfection approaching viral vectors. Major demerits associated with their use are their cytotoxicity.
In present investigation, development of cationic lipoplexes made using cationic lipid, dioleoyl-trimethylammoniumpropane (DOTAP), and various supporting lipids, i.e., dioleoyl-sn-glycero-3-phosphoethanolamine (DOPE), hydrogenated soya phosphocholine (HSPC), dimyristoyl-sn-glycero-3-phosphoglycerol (DMPG), cholesterol, methoxy(polyethyleneglycol)–1,2-distearoyl-sn-glycero-3-phosphoethanolamine (mPEG–DSPE) has been proposed. Effect of various lipids on formulation feasibility of lipoplexes from the intravenous use standpoint were studied by evaluating their siRNA complexation efficiency, particle size, zeta potential, cytotoxicity, cellular uptake, stability in presence of serum and electrolytes, hemolytic potential, transfection efficiency, and stability on storage.
MATERIALS AND METHODS
Materials
siRNA targeted to human RRM1 mRNA was purchased from Eurofins MWG Operons Ltd, Germany. Carboxyfluorescein-labelled negative control siRNA (FAM-NC-siRNA) and negative control siRNA (NC-siRNA) were obtained as gift samples from GenePharma, Shanghai, China. DOTAP, DOPE, HSPC, DMPG, and methoxy(polyethyleneglycol)2000–1,2-distearoyl-sn-glycero-3-phosphoethanolamine (mPEG2000–DSPE) were purchased from the Lipoid Ltd., Ludwigshafen, Germany. A549 and H1299 lung cancer cell lines were procured from National Centre for Cell Science, Pune, India. Cholesterol (Chol), diethylpyrocarbonate (DEPC), Dulbecco’s modified eagle medium (DMEM), antibiotic solution (penicillin G, streptomycin, and amphotericin B solution), fetal bovine serum (FBS), l-glutamine and 3-(4,5-dimethylthiazol-2-ly)-2,5-diphenyl-tetrazolium bromide (MTT), and 4′,6-diamidino-2-phenylindole were purchased from Himedia, Mumbai, India. Analytical grade solvents and chemicals were used for all purposes.
Preparation of Lipoplexes
siRNA lipoplexes were prepared by incubating preformed liposomes composed of cationic lipid, DOTAP, and other helper lipids with siRNA. Thin-film hydration method was employed for preparation of liposomes. Lipids were taken in different molar ratios and dissolved in sufficient quantity of chloroform in a 50-mL round-bottom flask. Organic solvent was evaporated under −600 mmHg vacuum and 45°C temperature at 100 rpm speed to produce thin film of lipids using rotary flask evaporator (IKA RV-10, USA). Nitrogen was purged gently to remove any remaining traces of chloroform. Thin lipid film obtained was then hydrated with nuclease-free water at 60°C for 1 h. Particle size of obtained liposomes was reduced by successive passages through 1, 0.4, 0.2, and 0.1 μ polycarbonate membranes (Whatman, USA) using a high-pressure extruder (Avestin, USA). Size-reduced liposomes were stored at 2–8°C until further use.
Prepared cationic liposomes were incubated with siRNA at different N/P (quaternary nitrogen of DOTAP/phosphate of siRNA) ratios ranging from 0 to 2.0 for 30 min at 37°C temperature to get lipoplexes. Lipid components of different liposomes used to complex with siRNA are shown in Table I. Lipoplexes were separated from uncomplexed siRNA by ultracentrifugation at 70,000×g for 4 h at 4°C.
Table I.
Liposomal Formulations with Their Composition
| Formulationa | Composition |
|---|---|
| D liposomes | DOTAP |
| D2 liposomes | DOTAP/DOPE |
| D2H liposomes | DOTAP/DOPE/HSPC |
| D2C liposomes | DOTAP/DOPE/Chol |
| D2CH liposomes | DOTAP/DOPE/HSPC/Chol |
| D2CHP liposomes | DOTAP/DOPE/HSPC/Chol/DMPG |
DOTAP dioleoyl-trimethylammoniumpropane, DOPE dioleoyl-sn-glycero-3-phosphoethanolamine, HSPC hydrogenated soya phosphocholine, Chol cholesterol, DMPG dimyristoyl-sn-glycero-3-phosphoglycerol
aAll formulations contained 5 mol% mPEG2000–DSPE on total lipid basis
siRNA Complexation Efficiency of Liposomes
Efficiency of preformed cationic liposomes to complex with siRNA was assessed by gel electrophoresis method. Lipoplexes were mixed with 2 μL of 6× DNA gel loading buffer (Fermentas Life Sciences, USA) and run on 2% agarose gel preloaded with 0.5 μg/mL of ethidium bromide for 20 min at 100 V potential in TBE buffer (10.80 g/L Tris base, 5.5 g/L boric acid, and 0.58 g/L EDTA). Post-run, gel was visualized by UV on GelDoc System (Bio-Rad Lab., USA) to quantify the uncomplexed siRNA using Image Lab™ Software (Bio-Rad Lab., USA). siRNA complexation efficiency of liposomes was determined using following equation:
| 1 |
where RNAtotal and RNAfree are the total amount of siRNA taken in preparation and free siRNA, respectively.
Particle Size and Zeta Potential
Dynamic light scattering technique was used to determine liposomes’ particle size. Liposomes were diluted with DEPC-treated nuclease-free water before analysis. Particle size and zeta potential of liposomes were determined on Malvern Zetasizer Nano ZS (Malvern Instruments, Malvern, UK) at 25°C.
In Vitro Cytotoxicity Assay
MTT assay was performed to evaluate the cytotoxicity of lipoplexes. A549 and H1299 cells at a density of 5,000 cells/well were seeded on a 96-well plate and incubated for 24 h at 37°C in humidified air with 5% CO2. Cells were then incubated separately with D, D2, D2H, D2C, and D2CH lipoplexes at varying N/P ratio ranging from 2.5 to 12.5 for 6 h at 100 nM of NC-siRNA concentration. After treatment, cells were supplemented with fresh DMEM containing 10% FBS and 1% antibiotics and again incubated for a 48-h period. Then after, cells were incubated with 20 μL of 5 mg/mL MTT solution for 4 h. The culture media in each well was then removed and replaced with 200 μL of a dimethyl sulfoxide. Cell viability in each well was determined by detection of formazan product produced by living cells by reduction of MTT at 570 nm by enzyme-linked immunosorbent assay plate reader (Bio-Rad, USA). Phosphate buffered saline-treated cells were considered as negative control, and cell viability for each formulation treatment was expressed as percentage relative to that of negative control. Comparison of developed formulations was also done with commercial transfection agent lipofectamine-2000 (Invitrogen, USA) used as a positive control.
Hemolysis Study
Hemolysis study as described by Oku and Namba (12) was used with some modifications. Blood samples were collected by retro-orbital puncture from Sprague–Dawley Rats and heparinized. Heparinized blood was centrifuged at 3,000 rpm to pelletize RBCs. RBC pellet was washed thrice with normal saline to remove plasma proteins. Washed pellet was suspended in normal saline to give 2% v/v dispersion of RBCs. Different lipoplexes at lipid concentrations of 0.01 to 5 nM were evaluated for their hemolytic potential. Treatment with 0.05% Triton-X100 and normal saline were used as positive and negative control, respectively. RBC suspensions with various treatments were incubated at 37°C for 30 min. Post-incubation, RBCs were pelleted at 3,000 rpm for 5 min, and haeme released in the supernatant was determined using UV absorption at 541 nm against normal saline. Percentage hemolysis was calculated using following equation:
| 2 |
Where AS and APC were absorbance obtained from samples and positive control, respectively.
Serum Stability Study
Ability of liposomes to protect siRNA from serum nucleases was evaluated in presence of serum. Naked siRNA and D2CH lipoplexes were incubated with 50 μL of FBS keeping constant siRNA concentration of 2.6 μg for all samples, and final volume was kept so as to give serum concentration of 50%v/v. All samples were incubated at 37°C. After incubation, samples were mixed properly with 100 μL of phenol/chloroform (1:1 v/v) and centrifuged at 14,000 rpm for 10 min at 4°C. The 25 μL of the aqueous layer was sampled and treated with microliter 6× DNA loading buffer. Treated samples were run on agarose gel as described earlier, and concentration of intact siRNA was quantified.
Electrolyte-Induced Flocculation Study
Lipoplexes were studied for electrolyte-induced flocculation to study the effect of electrolytes on stability of lipoplexes. Lipoplexes were mixed with sodium chloride solution (1–5% w/v) and incubated at 37°C for 1 h. Lipoplexes were used at 100 nM concentration of NC-siRNA. Post-incubation, particle size of lipoplexes was determined, and changes in particle size were noted.
Cell Uptake Study
Cellular uptake of different siRNA carrier systems was evaluated using FAM-labelled negative control siRNA (FAM-NC-siRNA). Cell uptake of naked siRNA, D2CH lipoplexes, and lipofectamine-2000 complexed siRNA in A549 and H1299 cells was assessed quantitatively in A549 and H1299 lung carcinoma cells using flow cytometry. Cells at a density of 50,000 cells/well were seeded on a 24-well plate. Cells were incubated at 37°C for 24 h and then treated with D2CH lipoplexes containing FAM-NC-siRNA at a concentration of 100 nM for 6 h. Post-incubation, cells were washed three times with cold PBS (pH 7.4) and examined for fluorescence by fluorescence activated cell sorter (FACS-BD-Aria III, BD, USA). Comparison of cellular uptake of developed liposomal formulations was done with naked FAM-NC-siRNA (negative control) and lipofectamine-2000-complexed FAM-NC-siRNA (positive control).
Gene Expression Study
RRM1 mRNA knockdown by siRNA was quantified by carrying out transfection study using developed liposomes. Real-time polymerase chain reaction (RT-PCR) was used to quantify mRNA expressed in A549 and H1299 cells transfected with different siRNA formulations. A549 cells and H1299 cells at a density of 50,000 cells/well were seeded on a 24-well plate and incubated at 37°C until 80% confluency was achieved. Cells were then treated with lipoplexes at three different siRNA concentrations—5 nM, 500 pM, and 50 pM. Transfection with lipofectamine-2000-complexed siRNA was used as a positive control. After incubation for 48 h, total RNA was isolated using TRIzol reagent (Invitrogen, USA), and cDNA was constructed by RNA to cDNA conversion kit (Invitrogen, USA), and mRNA was estimated by Step One RT-PCR (Applied Biosciences, USA) using SYBR Green Master mix (Applied Biosciences, USA) forward and reverse primers for RRM1 and housekeeping gene GAPDH (for RRM1: 5′-TGAGCAGCGCCTGGACCTAA-3′, 5′-GCATCGCAGCTAGTGGCTGA-3′, respectively, and for GAPDH: 5′-TCGCTCTCTGCTCCTCCTGTT-3′, 5′-TGACTCCGACCTTCACCTTCC-3′, respectively) and 2 ng of cDNA in a total reaction volume of 20 μL. Expression level of mNRA was quantified against the reference gene GAPDH.
Lyophilization
To bestow lipoplexes with physical stability, lyophilization was performed. Three different lyoprotectants namely lactose, sucrose, and mannitol, at different concentrations (25, 50, and 75 mg/mL) were used to optimize the lyophilization process. Lyoprotectant was dissolved in lipoplex dispersion appropriately diluted with nuclease-free DEPC-treated water and filled into 2 mL glass vials (Schott, USA). Vials were half stoppered with grey bromobutyl slotted rubber stoppers (Helvoet, Belgium) and kept on the shelf of the lyophilizer (Virtis-Advantage plus, USA). Lipoplexes were frozen to −40°C and dried under vacuum for 44 h to obtain lyophilized cake in the vial.
Residual Water Content
The residual water content of lyophilized lipoplexes was determined by Karl Fischer titration (13). Commercially available pyridine-free reagent was used for analysis. The reagent was standardized with addition and determination of known quantity of water (250 mg). Firstly, 40 mL of methanol was added into the titration vessel and titrated with the reagent to remove traces of water present in methanol. Following this, samples were dissolved in methanol, and water content of the sample was determined.
Cryo-transmission Electron Microscopy (Cryo-TEM)
Morphology and lamellarity of developed lipoplexes were visualized on cryo-transmission electron microscope (TECNAI G2 Spirit Bio TWIN, FEI-Netherlands) at operational conditions of 0.27 nm resolution and ×750,000 magnification. Transition of grid from hydrophobic to hydrophilic nature was carried out using glow discharge prior to microscopy. Grid was then evenly covered with optimized liposomal dispersion and frozen in liquid ethane at −180°C. Cryo-frozen grid prepared so was transferred to cryo-holder maintained at −175°C using liquid nitrogen storage box. After inserting cryo-holder inside the microscope, imaging of the sample was carried out.
Stability Study
Comparative stability studies were carried out for D2CH lipoplexes at accelerated storage conditions (25°C ± 2°C, 60% relative humidity (RH) ± 5%RH) for 6 months and at long-term storage conditions (5°C ± 3°C) up to 6 months. Liposomal formulations were filled into type-1 tubular glass vials, purged with nitrogen, sealed, and stored at the above-mentioned condition. At each sampling time, a different vial was removed and visually observed for discoloration. Vial contents were further examined for siRNA complexation efficiency, particle size, zeta potential, and residual water content.
In Vivo Acute Toxicity Study
Animal studies were approved by Institutional Animal Ethics Committee. Fixed dose procedure of Organization for Economic Co-operation and Development (OECD) was employed for determination of acute toxicity of developed lipoplexes (14). Swiss Albino female mice were used for toxicity assessment. All animal cares were observed as per the guidelines of OECD and CPCSEA (Committee for the Purpose of Control and Supervision on Experiments on Animals, India). Formulations sterilized by filtration through 0.2 μ polyethersulfone filter (Pall, India) were injected through tail vein of restrained mice. Study was carried out in two phases, sighting study and main test (Table II). Sighting study was performed using a single animal at single dose to determine starting dose for the main test. No sign of toxicity or death within next 24 h after dosing was used as an indication for shifting to higher dose. The highest dose from the sighting study at which no signs of toxicity was observed was selected for the main test. The doses selected for sighting study and main test are as shown in Table II. For each dose level in main study, five animals were used. Each animal was observed for signs of toxicity like tremors, convulsions, salivation, diarrhea, lethargy, sleep, coma, weight loss, and death as well at least once every 30 min, 4 h, and periodically during 24 h thereafter for 14 days.
Table II.
Dosing Protocol for In Vivo Toxicity Study
| Sr. no. | Formulation | Dose (mg/kg) | ||
|---|---|---|---|---|
| Sighting study | ||||
| 1. | D2CH lipoplexes | 0.5 mg/kg of siRNA | 0.75 mg/kg of siRNA | − |
| 2. | D2CH liposomes (placebo) | 50 mg/kg of lipids | 100 mg/kg of lipids | 150 mg/kg of lipids |
| Main test | ||||
| 1. | D2CH lipoplexes | 0.75 mg/kg of siRNA | − | − |
| 2. | D2CH liposomes (placebo) | 150 mg/kg of lipids | 200 mg/kg of lipids | 300 mg/kg of lipids |
siRNA small interfering RNA
Statistical Analysis
Triplicate analyses were performed for all characterization tests unless otherwise specified, and data are presented herein as mean ± standard deviation. ANOVA analysis was performed using GraphPad Prism (ver. 5, USA) for statistical evaluation of data. Statistical difference was considered at p values < 0.05.
RESULTS
Preparation of Lipoplexes
siRNA lipoplexes were prepared by incubating preformed size-reduced liposomes made of DOTAP, DOPE, and other helper lipids with siRNA. Effects of lipid composition of liposomes on siRNA complexation and other characteristics of lipoplexes were evaluated. Table III shows effects of various formulation parameters on siRNA complexation.
Table III.
Effect of Various Lipids on siRNA Complexation
| Formulation | Lipids | Lipid molar ratio | N/P | % siRNA complexeda | Remarks |
|---|---|---|---|---|---|
| D lipoplexes | DOTAP | 1 | 0.5 to 2 | − | Loosely bound complex formed at N/P = 1.25 and above |
| D2 lipoplexes | DOTAP/DOPE | 1:1 | 0.5 to 2 | − | Loosely bound complex formed at N/P = 1.25 and above. |
| D2H lipoplexes | DOTAP/DOPE/HSPC | 1:1:1.3 | 1.0 | 52.2 ± 1.68 | N/HSPC = 0.30 gave complete complexation at N/P = 2.0. |
| 1:1:1.3 | 1.5 | 60.5 ± 1.45 | |||
| 1:1:1.3 | 2.0 | 69.3 ± 0.98 | |||
| 1:1:2.6 | 1.0 | 55.5 ± 2.63 | |||
| 1:1:2.6 | 1.5 | 63.3 ± 2.40 | |||
| 1:1:2.6 | 2.0 | 73.3 ± 1.86 | |||
| 1:1:3.3 | 1.0 | 70.3 ± 1.56 | |||
| 1:1:3.3 | 1.5 | 85.2 ± 2.23 | |||
| 1:1:3.3 | 2.0 | 98.4 ± 1.53 | |||
| D2C Lipoplexes | DOTAP/DOPE/cholesterol | 1:1:1.3 | 1.0 | 40.2 ± 0.68 | At N/Chol = 0.38, siRNA complexation occurred at N/P = 2. |
| 1:1:1.3 | 1.5 | 52.3 ± 1.48 | |||
| 1:1:1.3 | 2.0 | 90.5 ± 1.23 | |||
| 1:1:2.6 | 1.0 | 43.2 ± 1.02 | |||
| 1:1:2.6 | 1.5 | 55.3 ± 1.63 | |||
| 1:1:2.6 | 2.0 | 97.5 ± 1.24 | |||
| 1:1:3.9 | Improper liposome formation | ||||
| D2CH lipoplexes | DOTAP/DOPE/HSPC/cholesterol (N/HSPC = 0.30) | 1:1:3.3:1.3 | 1.0 | 45.3 ± 1.65 | Complete complexation was achieved using combination of DOTAP, DOPE, HSPC and cholesterol (1:1:3.3:2.6) at N/P = 2.0. |
| 1:1:3.3:1.3 | 1.5 | 58.3 ± 1.06 | |||
| 1:1:3.3:1.3 | 2.0 | 87.5 ± 1.26 | |||
| 1:1:3.3:2.6 | 1.0 | 55.3 ± 1.09 | |||
| 1:1:3.3:2.6 | 1.5 | 85.6 ± 0.79 | |||
| 1:1:3.3:2.6 | 2.0 | 98.2 ± 1.57 | |||
| 1:1:3.3:3.9 | 1.0 | 33.3 ± 2.04 | |||
| 1:1:3.3:3.9 | 1.5 | 49.7 ± 1.63 | |||
| 1:1:3.3:3.9 | 2.0 | 63.2 ± 1.96 | |||
| D2CHP lipoplexes | DOTAP/DOPE/HSPC/cholesterol/DMPG (N/PC = 0.30), (N/Chol = 0.38) | 1:1:3.3:2.6:0 | 2.0 | 98.2 ± 0.86 | DMPG at all levels led to decreased siRNA complexation efficiency |
| 1:1:3.3:2.6:0.3 | 2.0 | 90.4 ± 1.21 | |||
| 1:1:3.3:2.6:0.5 | 2.0 | 74.5 ± 1.66 | |||
| 1:1:3.3:2.6:0.8 | 2.0 | 56.4 ± 2.13 | |||
| 1:1:3.3:2.6:1.0 | 2.0 | 37.5 ± 1.65 | |||
siRNA small interfering RNA, DOTAP dioleoyl-trimethylammoniumpropane, DOPE dioleoyl-sn-glycero-3-phosphoethanolamine, HSPC hydrogenated soya phosphocholine, Chol cholesterol, DMPG dimyristoyl-sn-glycero-3-phosphoglycerol, N/P represents ratio of quaternary nitrogen of DOTAP to phosphate of siRNA, N/PC represents molar ratio of DOTAP and HSPC, N/Chol represents molar ratio of DOTAP and cholesterol
aValues are represented as mean ± SD, n = 3
D and D2 Lipoplexes
Gel electrophoretic analysis showed considerable amounts of free siRNA below N/P ratio of 1.0. Complexation took place between lipid and siRNA above N/P of 1.25 in both types of liposomes. As shown in Fig. 1a, complex was formed at N/P of 1.25 and very soon at N/P of 1.5 complexation was very low. However, by repeating the same formulation, complexation was found to occur at 1.5 showing non-consistent complexation pattern. Again free siRNA was seen at N/P of 1.75 and 2.0. In case of D2 lipoplexes (Fig. 1b), with increasing N/P ratio, siRNA complexation was found to increase consistently upto N/P of 1.0, which on further increase showed inconsistent complexation efficiency.
Fig. 1.

Gel electrophoresis of D and D2 lipoplexes; a D lipoplexes: 1 = naked siRNA, 2 = N/P-0.5, 3 = N/P-0.75, 4 = N/P-1.0, 5 = N/P-1.25, 6 = N/P-1.5, 7 = N/P-1.75, 8 = N/P-2.0. b D2 lipoplexes: 1 = naked siRNA, 2 = N/P-0.5, 3 = N/P-0.75, 4 = N/P-1.0, 5 = N/P-1.25, 6 = N/P-1.5, 7 = N/P-1.75, 8 = N/P-2.0
D2H Lipoplexes
HSPC was incorporated at different molar ratios with DOTAP, viz, N/HSPC ratio of 0.25, 0.38, and 0.77. Figure 2 shows the effect of different HSPC levels on lipoplex formation at different N/P ratios. More than 95% complexation was achieved at higher concentration of HSPC (N/HSPC = 0.30). Complexation capability of liposomes was increased with increasing amounts of HSPC.
Fig. 2.
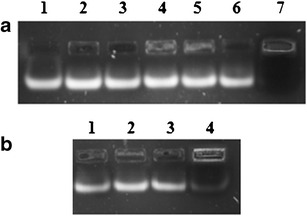
Gel electrophoresis of D2H lipoplexes. a 1 = naked siRNA, 2 = N/P-1.0 and N/HSPC-0.77, 3 = N/P-1.5 and N/HSPC-0.77, 4 = N/P-2.0 and N/HSPC-0.77, 5 = N/P-1.0 and N/HSPC-0.25, 6 = N/P-1.5 and N/HSPC-0.25, 7 = N/P-2.0 and N/HSPC-0.25. b 1 = naked siRNA, 2 = N/P-1.0 and N/HSPC-0.38, 3 = N/P-1.5 and N/HSPC-0.38, 4 = N/P-2.0 and N:HSPC-0.38
D2C Lipoplexes
Three levels of cholesterol were used i.e., N/Chol = 0.77, 0.38, and 0.25. Figure 3 summarizes the effect of addition of cholesterol in D2C liposomes with respect to siRNA complexation. Complete incorporation of siRNA was detected at N/Chol ratio of 0.38. However, at lower concentration, 90.5% complexation was observed. At higher concentration (N/Chol = 0.25), cholesterol incorporation in liposomes led to improper liposome formation. Hence, further increment in cholesterol amount, more than enough to provide N/Chol ratio of 0.38 in D2C liposomes, was restricted.
Fig. 3.

Gel electrophoresis of D2C lipoplexes. a Free siRNA detection at N/Chol ratio of 0.77, 1 = naked siRNA, 2 = N/P-1.0, 3 = N/P-1.5, 4 = N/P-2.0. b Free siRNA detection at N/Chol ratio of 0.38, 1 = naked siRNA, 2 = N/P-0.5, 3 = N/P-0.75, 4 = N/P-1.0, 5 = N/P-1.25, 6 = N/P-1.5, 7 = N/P-1.75, 8 = N/P-2.0
D2CH Lipoplexes
Effect of both HSPC and cholesterol on siRNA complexation was evaluated. Cholesterol was incorporated keeping N/HSPC ratio of 0.30, and siRNA complexation was measured. Three concentrations of cholesterol were used, i.e., N/Chol = 0.77, 0.38, and 0.25. Figure 4 shows gel-electrophoresis of D2CH lipoplexes at different N:Chol ratios. At DOTAP/DOPE/HSPC/Chol ratio of 1:1:3.3:2.6 and N/Chol of 0.38, complete siRNA complexation (98.2%) was achieved. At all cholesterol levels, liposomes were formed in contrast to D2C liposomes where higher cholesterol level (N/Chol = 0.25) led to improper liposomes. Figure 4 also shows that lipoplexes formed by incubating pre-liposomes made up of DOTAP, DOPE, HSPC, and cholesterol completely inhibited the electrophoretic mobility of siRNA at N/P ratio of 2.0.
Fig. 4.

Gel electrophoresis of D2CH lipoplexes. 1 = naked siRNA, 2 = N/P-0.5, 3 = N/P-0.75, 4 = N/P-1.0, 5 = N/P-1.25, 6 = N/P-1.5, 7 = N/P-1.75, 8 = N/P-2.0
D2CHP Lipoplexes
DMPG was incorporated in liposome formation at four levels, i.e., N/DMPG = 3.33, 2.0, 1.25, and 1.0, and siRNA complexation was determined (Fig. 5). Decreased siRNA complexation from ~98% complexation to ~50% complexation was observed as the level of DMPG was increased.
Fig. 5.

Gel electrophoresis of D2CHP lipoplexes. 1 = naked siRNA, 2 = naked siRNA + naked DMPG, 3 = N/P-2.0 without DMPG, 4 = N/P-2.0 and N/DMPG-3.33, 5 = N/P-2.0 and N/DMPG-2, 6 = N/P-2.0 and N/DMPG-1.25, 7 = N/P-2.0 and N/DMPG-1.0
Particle Size and Zeta Potential
Particle size of the lipoplexes was mainly dependent on the rigidity of preformed cationic liposomes. Table IV describes change in particle size and zeta potential of developed cationic liposomes. siRNA complexation drastically increased the particle size (p < 0.05) of the D, D2, and D2H lipoplexes. D2C and D2CH lipoplexes showed comparatively less size increase on siRNA complexation. Zeta potential of lipoplexes was found to be decreased after siRNA complexation.
Table IV.
Effect of siRNA Complexation on Particle Size and Zeta Potential
| Sr. no. | Lipoplexesa | Lipid molar ratio | Complexation efficiency (%) | Particle sizeb (nm) | Change in mean particle size (%) | Zeta potentialb (mV) | Change in mean zeta potential (%) | ||
|---|---|---|---|---|---|---|---|---|---|
| Before | After | Before | After | ||||||
| 1 | D | 1 | Loosely bound complex | 85.6 ± 2.6 | 504.6 ± 7.5 | 489.5 | 38.63 ± 0.35 | 15.84 ± 0.64 | 59.00 |
| 2 | D2 | 1:1 | 87.3 ± 5.9 | 593.1 ± 6.9 | 579.4 | 37.48 ± 0.79 | 16.24 ± 0.76 | 56.67 | |
| 3 | D2H | 1:1:3.3 | 98.4 ± 2.79 | 101.4 ± 6.3 | 261.5 ± 7.4 | 157.9 | 34.42 ± 1.36 | 13.39 ± 0.87 | 61.10 |
| 4 | D2C | 1:1:2.6 | 97.5 ± 3.60 | 91.7 ± 2.5 | 174.3 ± 6.7 | 90.1 | 35.91 ± 0.68 | 13.52 ± 0.68 | 62.35 |
| 5 | D2CH | 1:1:3.3:2.6 | 98.2 ± 1.89 | 104.5 ± 4.6 | 145.9 ± 8.7 | 39.6 | 34.83 ± 0.98 | 12.90 ± 0.68 | 62.96 |
aAll formulations are prepared at N/P ratio of 2.0 with 5 mol% of mPEG2000–DSPE
bValues are represented as mean ± SD, n = 3
In Vitro Cytotoxicity Assay
For the in vitro assessment of cytotoxicity of developed lipoplexes, A549 and H1299 cells were treated with lipoplexes prepared using 100 nM NC-siRNA at increasing N/P ratios (2.5, 5.0, 7.5, 10.0, and 12.5). Graphical representation of cytotoxicity of various lipoplex formulations is shown in Fig. 6. Cytotoxicity of D and D2 lipoplexes was higher than all other formulations while D2CH lipoplexes showed highest viability. D2H and D2C lipoplexes showed intermediate toxicity. In both cell lines, D2CH lipoplexes showed least toxicity up to N/P ratio of 10.0. D2CH lipoplexes reduced viability to 85.6 ± 2.1% at N/P ratio of 2.5, while lipofectamine-2000 reduced viability to 79.4 ± 1.7% following 48 h of incubation. At 12.5 N/P ratio, D2CH lipoplexes showed 79.8 ± 1.4% cell viability. Based on cytotoxicity studies, D2CH lipoplexes were evaluated further for other in vitro and in vivo characterization.
Fig. 6.

Cytotoxicity of different lipoplexes on A549 and H1299 cells
Hemolysis Study
Blank D2 and D2CH liposomes and PEGylated and non-PEGylated D2CH lipoplexes were evaluated for their hemolytic potential. Results of hemolysis study are presented graphically in Fig. 7a. Blank D2 liposomes showed highest hemolysis ranging from 4.16 ± 1.14% at 0.01 mM lipid concentration to 27.6 ± 1.3% at 5 mM lipid concentration. Relative hemolysis increased from 2.85 ± 0.35% to 19.20 ± 1.20% for blank D2CH liposomes, from 2.70 ± 0.30% to 14.60 ± 0.80% for non-PEGylated D2CH lipoplexes and from 2.00 ± 0.10% to 9.15 ± 0.25% for PEGylated D2CH lipoplexes, while concentrations of formulations were changed from 0.01 to 5 mM on lipid basis.
Fig. 7.
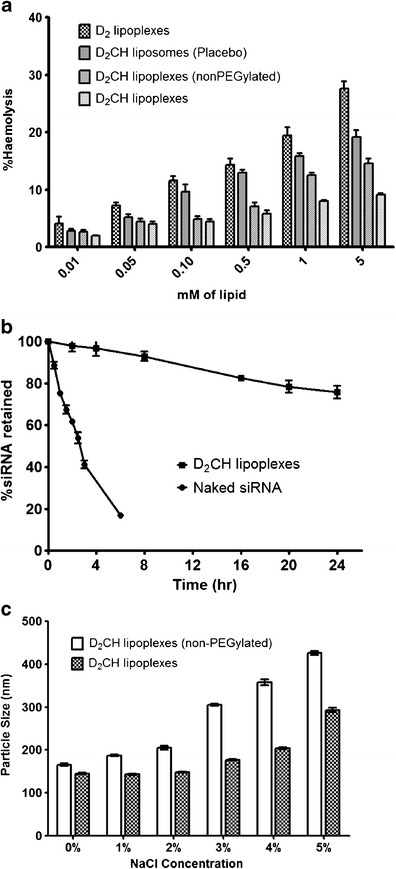
In vitro characterization of D2CH lipoplexes. a Hemolytic potential of lipoplexes, b serum stability of naked siRNA and D2CH lipoplexes: Plot represents siRNA retained at different time points after treatment with serum; c electrolyte induced flocculation of different formulations
Serum Stability Study
Gel electrophoresis was performed to quantify siRNA retained in the liposomes on exposure to serum conditions. siRNA was extracted using phenol:chloroform (1:1 v/v) and was run on gel. From the plot of siRNA retained at different time points (Fig. 7b), it can be seen that more than 75% of siRNA was retained in lipoplexes up to 24 h which was significantly higher than naked siRNA, degradation of which started within 30 min showing around 12% degradation, and only 16.90 ± 1.00% was left intact after 6 h of serum exposure.
Electrolyte-Induced Flocculation Study
Figure 7c depicts increase in particle size of PEGylated and non-PEGylated D2CH lipoplexes with increasing concentration of sodium chloride. Particle size was significantly affected at all concentrations of sodium chloride in case of non-PEGylated lipoplexes causing drastic increase in particle size. Up to 4% electrolyte concentration, D2CH lipoplexes maintained their particle size below or around 200 nm, while in the case of non-PEGylated D2CH lipoplexes, 3% and higher concentrations led to drastic change in particle size leading to more than 400 nm at 5% concentration.
Cell Uptake Study
Quantitative estimation of lipoplex uptake in A459 and H1299 cells was carried out using flow cytometric analysis using either naked FAM-NC-siRNA or its lipoplexes. Mean fluorescence intensity inside cells was determined, and cellular uptake of each lipoplex formulation was measured. As shown in the Fig. 8, fluorescent intensity inside cells after treatment with various siRNA formulations can be ordered as follows:
in A549 cells: naked siRNA < lipofectamine-2000 < D2CH lipoplexes
in H1299 cells: naked siRNA < D2CH lipoplexes < lipofectamine-2000
Fig. 8.

Cellular uptake and gene expression study of D2CH lipoplexes in comparison to naked siRNA and lipofectamine-2000. a1 uptake of D2CH lipoplexes in A549 cells; a2 gene expression in D2CH-treated A549 cells. b1 uptake of D2CH lipoplexes in H1299 cells; b2 gene expression in D2CH-treated H1299 cells. Number sign indicates a statistically significant difference with p < 0.05. ns indicates a statistically insignificant difference with p > 0.05. Asterisk indicates a statistically significant difference from naked siRNA group with p < 0.05
D2CH lipoplexes showed high cellular uptake with mean fluorescent intensity of 83.83 ± 1.01% in A549 cells and 67.07 ± 0.86% in HT1299 cells which was five to six fold higher than that obtained with naked FAM-NC-siRNA. The difference existed between the cellular uptakes of D2CH lipoplexes in both cell types. It was either higher than lipofectamine-2000 (in A549 cells) (p < 0.05) or comparable to lipofectamine-2000 (in H1299 cells) (p > 0.05). D2C lipoplexes showed intermediate cellular uptake in both cell lines.
Gene Expression Study
RT-PCR was used to quantify RRM1 mRNA knock-down. Amplification threshold of 0.81 and 0.31 for GAPDH and RRM1, respectively, was set, and high-resolution melt curves were generated to study the quality of amplified mRNA. High-intensity sharp peaks for both mRNA were obtained showing melt temperature of 82.89°C for RRM1 mRNA and 83.34°C for GAPDH mRNA. Figure 8 shows gene expression of A549 and H1299 cells treated with different formulations.
As it can be seen, D2CH lipoplexes showed higher (p < 0.05) mRNA knock-down than naked siRNA, and also, activity comparable to lipofectamine-2000 was achieved at 5 nM concentrations in A549 cells. At 5 nM concentration D2CH lipoplexes showed 27.15 ± 1.85% gene expression while naked siRNA exhibited 83.50 ± 2.5% gene expression in A549 cells. Similar trend was observed in H1299 cells, showing gene expression of 28.25 ± 1.05% for D2CH lipoplexes and 85.9 ± 2.5% for naked siRNA. There was no significant difference (p > 0.05) between lipoplexes and lipofectamine-2000 at all concentrations. As the concentration of siRNA was lowered from 5 nM, i.e., 500 and 50 pM, mRNA knockdown was markedly decreased with both lipoplexes and lipofectamine-2000. Naked form demonstrated very poor gene silencing at lower concentrations, i.e., 96.45 ± 3.35% and 97.6 ± 5.60% gene expression at 500 and 50 pM, respectively, in A549 cells while 96.55 ± 2.65% and 101.15 ± 2.05% gene expression at 500 and 50 pM in H1299 cells.
Cryo-transmission Electron Microscopy (Cryo-TEM)
Cryo-TEM micrograph of developed D2CH lipoplexes is shown in Fig. 9. From the image, spherical shape and unilamellar nature of developed lipoplexes are apparent. Additionally, the size of the particle and thickness of lamella were also determined. Particle size of the liposomes was observed to be between 100 and 150 nm which was in concordance with the particle size results obtained by dynamic light scattering. The thickness of the lamella was found to be 5–10 nm.
Fig. 9.

TEM micrograph of D2CH lipoplexes
Lyophilization
Lyophilized formulations were tested for physical appearance, complexation efficiency, particle size, and zeta potential. Results of the analyses are shown in Table V. No degradation of siRNA was observed on reconstitution. All the sugars led to poor cake integrity at 25 mg/mL concentration, while stable cake was formed at 50 and 75 mg/mL concentrations. Lactose and mannitol led to increase in particle size to more than 200 nm, while sucrose maintained particle size below 200 nm, with optimum results at 50 mg/mL. Zeta potential of each lyophilized formulation was found to be non-significantly affected. Water content of freeze-dried lipoplexes was characterized by Karl Fischer titration method. All lyophilized samples were found to contain less than 4% w/w water content. At 75 mg/mL sugar level, more than 3% residual water was found, while at lower sugar levels <2% w/w residual water was found in lyophilized cakes.
Table V.
Effect of Lyophilization with Various Lyoprotectants on siRNA Lipoplexes
| Lyoprotectants | Concentration (mg/mL) | Before lyophilization | After lyophilization | Residual water contenta (%w/w) | ||
|---|---|---|---|---|---|---|
| Particle sizea (nm) | Zeta potentiala (mV) | Particle sizea (nm) | Zeta potentiala (mV) | |||
| Lactose | 25 | 147.5 ± 2.89 | 12.26 ± 0.54 | 300.5 ± 7.7 | 11.32 ± 1.20 | 1.13 ± 0.05 |
| Sucrose | 176.3 ± 6.9 | 11.84 ± 0.65 | 1.43 ± 0.09 | |||
| Mannitol | 246.2 ± 10.3 | 12.47 ± 0.84 | 1.61 ± 0.03 | |||
| Lactose | 50 | 254.3 ± 7.3 | 11.52 ± 0.45 | 1.17 ± 0.04 | ||
| Sucrose | 152.8 ± 8.6 | 12.03 ± 0.68 | 1.89 ± 0.01 | |||
| Mannitol | 220.4 ± 9.5 | 12.26 ± 0.94 | 1.78 ± 0.09 | |||
| Lactose | 75 | 259.3 ± 7.3 | 14.32 ± 1.10 | 3.27 ± 0.12 | ||
| Sucrose | 154.3 ± 6.7 | 12.64 ± 0.99 | 3.65 ± 0.08 | |||
| Mannitol | 213.2 ± 9.9 | 11.59 ± 0.75 | 3.01 ± 0.05 | |||
aValues are represented as mean ± SD, n = 3
Stability Study
For evaluating stability of lyophilized formulation, ICH guidelines for the products to be stored at refrigerated conditions were followed. Lyophilized cakes stored in air-tight vials were exposed to two stability conditions, accelerated storage condition (25°C ± 2°C, 60% RH ± 5% RH), and long-term storage condition (5°C ± 3°C). Results of the analyses at different time points are shown in Table VI. At all conditions and sampling periods, lipoplexes were observed as white cakes. At both accelerated and refrigerated conditions, siRNA complexation efficiencies were found to be within range of 95–105% of initial. Residual water content showed significant rise at accelerated conditions while refrigerated conditions maintained water content close to initial level even after 3 months with a rise to ~2.5% after 6 months. Particle size was found to increase over the storage period with least increase occurring at refrigeration conditions. There was no significant change in zeta potential value at both conditions.
Table VI.
Stability Testing Data of D2CH Lipoplexes
| Sampling time (month) | % siRNA complexation efficiencya | Water contenta (%w/w) | Particle sizea (nm) | Polydispersity index | Zeta potentiala (mV) |
|---|---|---|---|---|---|
| Initial | 98.20 ± 3.34 | 1.89 ± 0.10 | 147.5 ± 2.13 | 0.164 | 12.26 ± 0.65 |
| Accelerated storage conditions (25°C ± 2°C, 60% RH ± 5% RH) | |||||
| 1 | 97.83 ± 3.10 | 1.96 ± 0.17 | 148.5 ± 2.04 | 0.120 | 12.36 ± 0.72 |
| 2 | 97.73 ± 2.52 | 2.51 ± 0.34 | 156.2 ± 2.82 | 0.204 | 13.48 ± 1.23 |
| 3 | 96.51 ± 1.95 | 3.09 ± 0.24 | 174.7 ± 3.79 | 0.147 | 12.39 ± 1.21 |
| 6 | 96.03 ± 0.97 | 3.79 ± 0.19 | 169.3 ± 3.20 | 0.103 | 13.37 ± 1.10 |
| Long-term storage conditions (5°C ± 3°C) | |||||
| 1 | 97.36 ± 1.69 | 1.86 ± 0.18 | 148.9 ± 2.02 | 0.173 | 12.73 ± 1.20 |
| 2 | 98.01 ± 2.30 | 1.91 ± 0.33 | 158.0 ± 3.71 | 0.242 | 12.08 ± 1.73 |
| 3 | 96.32 ± 2.75 | 2.14 ± 0.20 | 163.3 ± 1.98 | 0.121 | 13.83 ± 1.16 |
| 6 | 96.06 ± 3.02 | 2.52 ± 0.31 | 162.4 ± 2.90 | 0.239 | 13.73 ± 1.43 |
siRNA small interfering RNA, RH relative humidity
aValues are represented as mean ± SD, n = 3
In Vivo Acute Toxicity Study
OECD guideline 420 for determination of acute toxicity was used to assess safety profile of developed lipoplex formulation. Based on the sighting study, maximum dose of each formulation was selected as a starting dose of the main test. Lipoplex formulation at a dose of 0.75 mg/kg of RRM1 siRNA was selected as a starting dose for main test, and lipoplexes were injected in five extra animals. Dose in main study was restricted to 0.75 mg/kg on siRNA basis, as this much dosing of siRNA would be sufficient to achieve therapeutic effects. For the placebo lipoplexes, dosing sequence for the main test was started at 150 mg/kg on lipid basis which was escalated further to determine the MTD of the carrier. The results suggested no signs of toxicity or mortality at a dose as high as 300 mg/kg on lipid basis. And at this much amount of lipid level, lipoplexes would be sufficient to provide siRNA higher than that required for therapeutic efficacy proving the safety of developed lipoplexes at therapeutic doses. And hence, the dose escalation was not done further to determine the maximum tolerated dose.
DISCUSSION
The preformed cationic liposomes were used to complex with negatively charged siRNA. Various lipids were used to prepare liposomes. DOTAP was the key lipid for preparation of lipoplexes as the positively charged quaternary nitrogen of DOTAP complexes with negatively charged phosphate of siRNA through ionic interaction. Other helper lipids like DOPE, a fusogenic lipid; HSPC, a neutral lipid; DMPG, a negatively charged lipid; mPEG–DSPE, a PEGylated lipid; and cholesterol, a neutral pentacyclic lipid were also included in formulation to impart specific characteristics to formed lipoplexes, i.e., fusion and cytoplasmic release after endocytosis, stable lipid bilayer formation, to neutralize excess surface charge of DOTAP, to impart long circulation feature, and to lend rigidity to lipoplexes, respectively. Gel retardation pattern of siRNA was affected mainly by N/P ratios used to formulate lipoplexes. Quaternary nitrogen (N) is responsible for positive charge of DOTAP. P in the N/P ratio denotes phosphate groups of nucleic acid base of siRNA which interact with quaternary nitrogen of DOTAP. Apart from N/P ratio, incorporation of other lipids is also crucial for siRNA complexation. Incorporation of HSPC and cholesterol in formulation of different lipoplexes was studied thoroughly by evaluating the effect of N/HSPC and N/Chol ratios on siRNA complexation efficiency.
Results obtained suggest the inability of D and D2 liposomes for complete complexation of siRNA. Such liposomes due to high surface charge density show multiple layer interaction with siRNA. A layer of siRNA would have covered the surface of the liposomes, but the surface charge being incompletely neutralized for D and D2 liposomes, more siRNA would be attracted towards the liposomes forming second layer of siRNA which would cause increase in particle size, as well as loose complexation of siRNA. Loose complexation was confirmed from gel electrophoresis of lipoplexes which exhibited undefined pattern for siRNA complexation at higher N/P ratios (1.25 to 1.75), i.e., complete retardation at N/P of 1.25 and intense siRNA band at 1.5. Such lose complexes may also release siRNA before reaching target site or before entering inside the target cells and certainly can lead to toxicity or diminished therapeutic activity. Owing to higher rigidity (Tg = 55°C) and bilayer forming capability, HSPC was incorporated in lipoplex formulation (15). Strong complexation observed between D2H liposomes and siRNA might be due to separation of cationic charge on the surfaces of liposomes due to placing of HSPC molecules in between the DOTAP molecules. This would provide easy interaction between siRNA and cationic DOTAP and hence better complexation strength. Cholesterol was incorporated in D2 liposomes for its membrane rigidizing capacity (16,17). From the results of complexation efficiency of various D2C liposomes, it was found that incorporation of cholesterol led to an increment in siRNA complexation as seen in Table III. This in turn can be ascribed to, as in the case of D2H lipoplexes, distribution of the cationic charge over liposome surface providing better complexation. At higher cholesterol level (DOTAP/DOPE/Chol ratio of 1:1:3.9), improper liposome formation was observed. Furthermore, the effect of both HSPC and cholesterol was evaluated on complexation efficiency of lipoplexes. There was no problem in liposome formation at higher cholesterol concentration as seen with D2C liposomes. This can be ascribed to higher accommodation of added cholesterol by the membrane forming lipid HSPC. Additionally, as described earlier, incorporation of HSPC and cholesterol would help in better distribution of charge on surface in contrast to D and D2 liposomes where surface charge density would be higher causing high concentration of siRNA to be complexed on surface of liposomes leading to loose interaction through multiple layer formation. Due to incorporation of cationic DOTAP in complex formation, all lipoplex formulations showed strong positive zeta potentials. This cationic charge, as described earlier, can lead to cytotoxicity (18). Therefore, an effort was made to neutralize the surface charge by incorporating a negatively charged lipid–DMPG. However, incorporation of DMPG, at all levels studied, showed reduced siRNA complexation which can be explained by hindered interaction between the DOTAP and siRNA due to repulsion by negatively charged DMPG head groups (19). Therefore, this strategy was not carried further.
From the optimization results of each level of formulation development, conclusions derived were: Loose complexes were obtained with D and D2 liposomes, and hence these formulations were not considered optimal formulations for siRNA delivery. D2H liposomes were able to form tight complex with siRNA, but there was significant increase in particle size of liposomes. D2C and D2CH liposomes formed very stable tightly bound complex with siRNA. Furthermore, particle size was also below 200 nm after complexation. In case of the D2CH liposomes, particle size after complexation was found to be below 150 nm, and hence, this formulation with internal lipid ratio of 1:1:3.3:2.6 was considered optimum.
Incubation of preformed liposomes with siRNA led to increase in the particle size which is imputed mainly to surface complexation of siRNA with preformed liposomes. Ionic interaction of siRNA in multiple layers with D and D2 liposomes can lead to increase in particle size by aforementioned mechanism. Incorporation of HSPC and cholesterol, by allowing efficient charge separation, formed stable complexes with sirNA. However, there existed a difference between the D2H lipoplexes and D2C lipoplexes, i.e., in particle size before and after complexation. In case of D2H liposomes, the positioning of the HSPC would not be able to effectively separate cationic charge, and hence, it gave size increase on siRNA complexation due to higher surface charge density (though lower than that of D and D2 liposomes) as described earlier. In contrast, in case of D2C liposomes, where cholesterol, due to its better interdigitaiton capability, would have separated the charges more effectively due to greater monomer separation in cholesterol modified bilayer (20,21). The tendency of liposomes to complex with siRNA in a monolayer fashion can be ordered in decreasing order as: D2CH>D2H~D2C>D2>D. D2CH lipoplexes exhibited least particle size increase (from 104.5 ± 4.6 nm to only 145.9 ± 8.7) as compared with others. D2CH liposomes, by allowing only monolayer interaction with siRNA, would cause minimal particle size increase on siRNA complexation. Particle size of D2CH lipoplexes observed with Cryo-TEM concorded well with dynamic light scattering analysis results. Furthermore, it confirmed unilamellarity and spherical nature of developed lipoplexes. The particle size observed for optimized D2CH lipoplexes allows for their passive targeting through enhanced permeation and retention in tumor tissue (22,23). Zeta potential was found to be decreased after siRNA complexation which confirms the surface interaction between positively charged liposomes and negatively charged siRNA. Furthermore, apart from particle size, zeta potential measurement serves as a tool for predicting interaction of nanoformulation with cell surfaces (24,25). Positive zeta potential of developed lipoplexes would heighten the chances for lipoplexes to interact with negatively charged cell membrane and hence to transfect the cells.
In cytotoxicity study, among all the formulations examined, D2CH lipoplexes showed lowest cytotoxicity. This concludes lesser toxic potential and hence better safety profile of developed lipoplexes than lipofecatmine-2000-based lipoplexes. D and D2 lipoplexes showed higher toxicity which can be explained by DOTAP-mediated cationic surface charge and DOPE-mediated pore formation and membrane destabilization which would have disrupted cell membrane (18). Heightened cytotoxicity of lipofectamine-2000 can also be explained similarly. In contrast, uniform surface distribution of DOTAP in DOPE, HSPC, and/or cholesterol matrix of D2H, D2C, and D2CH lipoplexes would reduce high charge exposure of cell surfaces which would have been the reason for lower cytotoxicity of these lipoplexes. As it can be perceived, combined effects of HSPC and cholesterol would be the reason for higher viability with D2CH lipoplexes as compared with D2H and D2C lipoplexes.
Lipoplexes were prepared using cationic liposomes, and hence, they are highly toxic to red blood cells causing hemolysis when injected intravenously. The same toxicity applies for normal cells as well. Hemolytic potential of various formulations developed can be ordered as: blank D2 liposomes>blank D2CH liposomes>non-PEGylated D2CH lipoplexes>D2CH lipoplexes. Hemolysis was due to the positively charged DOTAP which causes leaching of hemoglobin from erythrocytes by pore formation in erythrocyte membrane (18). Furthermore, fusogenic lipid–DOPE promotes pore formation effect of DOTAP causing enhanced hemolysis. Lipoplexes exhibited less hemolysis as compared with blank liposomes. This can be explained by charge neutralization by complexation of DOTAP to negatively charged siRNA molecules. This is in agreement with previous study by van der Woude et al. (18). Reduced toxicity of non-PEGylated D2CH liposomes as compared with D2 liposomes can also be due to increased interaction of D2 liposomes owing to higher surface charge density in contrast to D2CH liposomes containing HSPC and cholesterol which reduce the surface charge density. PEGylation of liposomes showed substantially reduced hemolysis which can be attributed to the diminished interaction of DOTAP and DOPE to surface of erythrocytes due to coating of lipoplexes surface by PEG chains.
siRNA was complexed with cationic liposomes which will hold siRNA on the outer surfaces of lamella. So serum stability study was performed to evaluate whether liposomes were capable of protecting the siRNA from degradation by serum nucleases (26) and whether the PEGylation was efficient enough to prevent binding of liposomes with serum proteins which would ultimately contribute to their RES uptake. Results showed that D2CH lipoplexes were able to protect siRNA form serum-induced degradation efficiently as compared with naked siRNA. The loss of siRNA from lipoplexes can be due to loss of surface complexed siRNA into the media or degradation of surface-localized siRNA due to direct exposure to serum nucleases. Lipoplexes are charge based complexes between the negatively charged siRNA and cationic lipids. Therefore, they may get affected by electrolytes causing aggregation of lipoplexes when injected systemically. Electrolyte-induced flocculation test also established the effect of PEGylation in preventing such interactions between electrolytes present in blood circulation with prepared lipoplexes (27). Results show inferior stability of non-PEGylated D2CH liposomes which might be due to the presence of positive groups on the liposomal surface, i.e., free ammonium groups of DOTAP, which provide ease of interaction between electrolyte and liposomal surface. Incorporation of 5 mol% of mPEG2000–DSPE did protect lipoplexes from such particle size increase by hindering direct interaction of lipoplex surfaces with electrolytes by providing a steric barrier around lipoplexes.
From the results of cell uptake studies, it can be concluded that all the lipoplexes were able to enhance siRNA translocation into the cells as compared to free siRNA. Naked siRNA was not able to penetrate negatively charged cell surface due to repulsion by net negative charge and poor diffusion through membrane due to hydrophilicity. Lipoplexes can enhance cellular uptake by cationic lipids used in the formulation which promote the interaction of lipoplexes with negative cell surfaces, consequently leading to endocytosis or fusion (28). DOPE present in the lipoplexes would have promoted the fusion of lipoplexes with cell membrane leading to release of siRNA inside the cells (29). Various reports suggest efficient delivery of siRNA using DOTAP and DOPE combination (30–32), but the combination alone would not be able to transfect cells efficiently due to loose complexation and lack of rigidity in lipid bilayer structure. However, incorporation of HSPC and cholesterol would provide sufficient strength to lipoplexes in terms of rigidity, as well as complexation as described earlier which will help lipoplexes retain siRNA and transfect cells efficiently.
Results of gene expression study were in concordance with that of cell uptake studies concluding minimal transfection and hence highest gene expression with naked siRNA and higher intracellular delivery of siRNA by all lipoplex formulations. More-so-ever, siRNA (naked or lipoplexes) endocytosed would also be degraded by the endolysozomal pH and enzymes (33). Higher gene silencing was observed with all lipid-based formulations concluding endosomal escape mechanism playing role in transfection by lipoplex formulation. Cationic lipid, DOTMA, used in lipofectamine-2000, enhanced the interaction of lipoplexes with cell surface and subsequent endosomal uptake. Similarly, D2CH lipoplexes enhanced the cellular uptake by DOTAP-mediated endosomal uptake. After internalization into endosomes, interaction between lipoplexes and endosomal membrane that cause anionic phospholipids to diffuse into lipoplex membrane forming neutral ion-pairs with cationic lipids would be the reason for the fusion process, as well as displacement and release of siRNA in cytosol up on fusion (19,34,35). DOPE present in all lipoplex formulations would have enhanced cytoplasmic availability of siRNA by causing direct fusion of lipoplexes with cell membrane and also fusion-mediated intracellular release of siRNA after endosomal uptake due to its hexagonal phases that form especially at acidic pH due to ethanolamine headgroups (36). Conclusively, surface charge density of D2CH lipoplexes was lower enough to reduce their cytotoxicity but sufficient enough for higher cellular uptake and efficient inhibition of gene expression.
Lyoprotectants function to provide physical stability to lipoplexes on lyophilization by preventing detrimental effects of freezing on lipoplexes (37). They help lipoplexes retain the particle size and siRNA complexation characteristics during freezing by preventing aggregation and providing physical structure to the lyophilized cake for easy dispersion on reconstitution. Additionally, ice formation during freezing stage can rupture the morphology of prepared lipoplexes. Lyoprotectants reduce such effects by reducing freezing point of water and reducing formation of crystals of ice which can damage lipoplexes (38). From the results of the effects of lyoprotectants on lipoplexes, lyoprotection capacity of all three sugars can be represented in decreasing order as sucrose>mannitol>lactose. Lactose and mannitol at all concentrations failed to maintain particle size below 200 nm depicting their inability to efficiently protect lipoplexes. Sucrose, though led to increase in particle size from initial, maintained the particle size below 200 nm. Efficient protection was observed at 50 mg/mL sucrose concentration with no further improvement at higher concentration. At the same concentration, only 1.89% residual water content was observed in lyophilized cake. From the results, it was apparent that sucrose at 50 mg/mL concentration was the best suitable lyoprotectant for freeze-drying of lipoplexes. Physical stability of liposomes is one of the biggest obstacles in formulation of a commercially viable product (39). Lipoplexes should be stable for maximum time period in terms of siRNA complexation efficiency to be pharmaceutically acceptable. Hence, siRNA leakage, particle size growth, change in zeta-potential, chemical stability of siRNA, and water content were determined for D2CH lipoplexes at regular intervals at accelerated and long-term storage conditions. Results of the stability studies concluded that developed D2CH lipoplexes were more stable at refrigerated conditions.
In vivo acute toxicity study of D2CH lipoplexes was performed in Swiss Albino female mice following OECD guidelines. Results of the in vivo toxicity studies of D2CH lipoplexes and blank D2CH liposomes showed no toxicity of lipoplexes at doses used for treatment purpose. Maximum tolerated dose of the formulation was considered to be higher than the highest dose used for main test, i.e., 0.75 mg/kg of RRM1 siRNA as lipoplexes and 300 mg/kg of blank liposomes on lipid basis. This warrants out better safety profile of developed D2CH lipoplexes for in vivo use.
CONCLUSION
Form the present investigation, a new cationic lipoplex formulation has been proposed for siRNA delivery which provides low cytotoxicity up to N/P of 7.5 with comparable to higher cellular uptake and gene silencing activity with respect to lipofectamine-2000 which is an established lipid combination used for in vitro transfection purposes. This concludes that developed lipoplexes will provide similar transfection with low cytotoxicity (i.e., at N/P of 2) as compared with lipofectamine-2000, in other words, higher transfection (i.e., at N/P of 7.5) at cytotocxicity profile equivalent to lipofectamine-2000. In addition, other positive attributes of developed lipoplexes lies in their low hemolytic potential as compared with DOTAP/DOPE-based formulation and stability in presence of high electrolyte concentration. Stability studies indicated that the storage of such developed lipoplexes should be done at refrigerated conditions. From the results of in vivo toxicity studies, the lipoplexes were well tolerated at a dose way higher than required for therapeutic use. These promising in vitro and in vivo results suggest further development and clinical evaluation of proposed formulation for cancer treatment.
ACKNOWLEDGMENTS
The authors acknowledge the TIFAC CORE in NDDS, Government of India, New Delhi, for providing the research facilities to the team and Department of Biotechnology (DBT-SBIRI), New Delhi, India for financial assistance. The authors also acknowledge Dr. Vikram Sarabhai Science Block, DBT-ILSPARE, Faculty of Science, M. S. University, Vadodara for providing facility to carry out cell uptake studies.
Conflict of Interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript.
REFERENCES
- 1.Lung cancer incidence and mortality worldwide in 2008 GLOBACON 2008 (IARC), Section of Cancer Information. 2008.
- 2.Horn L, Pao W, Johnson DH. Neoplasms of the lung. In: Longo D, Fauci A, Kasper D, Hauser S, Jameson J, Loscalzo J, editors. Harrison’s principles of internal medicine. 18. United States: McGraw-Hill; 2011. [Google Scholar]
- 3.Thun MJ, Hannan LM, Adams-Campbell LL, Boffetta P, Buring JE, Feskanich D, et al. Lung cancer occurrence in never-smokers: an analysis of 13 cohorts and 22 cancer registry studies. PLoS Med. 2008;5(9):e185. doi: 10.1371/journal.pmed.0050185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tsao AS. Tumors of the lungs. In: Porter RS, Kaplan JL, editors. The merck manual—fot the healthcare professionals. US: MERCK PUBLISHING GROUP; 2010. [Google Scholar]
- 5.Rha SY, Jeung HC, Choi YH, Yang WI, Yoo JH, Kim BS, et al. An association between RRM1 haplotype and gemcitabine-induced neutropenia in breast cancer patients. Oncologist. 2007;12(6):622–30. doi: 10.1634/theoncologist.12-6-622. [DOI] [PubMed] [Google Scholar]
- 6.Rosell R, Danenberg KD, Alberola V, Bepler G, Sanchez JJ, Camps C, et al. Ribonucleotide reductase messenger RNA expression and survival in gemcitabine/cisplatin-treated advanced non-small cell lung cancer patients. Clin Cancer Res Off J Am Assoc Cancer Res. 2004;10(4):1318–25. doi: 10.1158/1078-0432.CCR-03-0156. [DOI] [PubMed] [Google Scholar]
- 7.Khatri N, Rathi M, Baradia D, Trehan S, Misra A. In vivo delivery aspects of miRNA, shRNA and siRNA. Crit Rev Ther Drug Carrier Syst. 2012;29(6):487–527. doi: 10.1615/CritRevTherDrugCarrierSyst.v29.i6.20. [DOI] [PubMed] [Google Scholar]
- 8.Mah C, Byrne BJ, Flotte TR. Virus-based gene delivery systems. Clin Pharmacokinet. 2002;41(12):901–11. doi: 10.2165/00003088-200241120-00001. [DOI] [PubMed] [Google Scholar]
- 9.Strayer DS. Viral gene delivery. Expert Opin Investig Drugs. 1999;8(12):2159–72. doi: 10.1517/13543784.8.12.2159. [DOI] [PubMed] [Google Scholar]
- 10.Khatri NI, Rathi MN, Kolte AA, Kore GG, Lalan MS, Trehan S, et al. Patents review in siRNA delivery for pulmonary disorders. Recent Patents Drug Deliv Formul. 2012;6(1):45–65. doi: 10.2174/187221112799219116. [DOI] [PubMed] [Google Scholar]
- 11.Khatri N, Baradia D, Vhora I, Rathi M, Misra A. cRGD grafted liposomes containing inorganic nano-precipitate complexed siRNA for intracellular delivery in cancer cells. J Control Release. 2014;182:45–57. doi: 10.1016/j.jconrel.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 12.Oku N, Namba Y. Glucuronate-modified, long-circulating liposomes for the delivery of anticancer agents. Methods Enzymol. 2005;391:145–62. doi: 10.1016/S0076-6879(05)91008-2. [DOI] [PubMed] [Google Scholar]
- 13.van Winden ECA, Crommelin DJA. Long term stability of freeze-dried, lyoprotected doxorubicin liposomes. Eur J Pharm Biopharm. 1997;43(3):295–307. doi: 10.1016/S0939-6411(97)00058-1. [DOI] [Google Scholar]
- 14.OECD guideline for testing of chemicals, OECD Test Guideline 420: acute oral toxicity—fixed dose procedure. OECD Publishing, Organization for Economic Co-operation and Development; 2008.
- 15.Drummond DC, Meyer O, Hong K, Kirpotin DB, Papahadjopoulos D. Optimizing liposomes for delivery of chemotherapeutic agents to solid tumors. Pharmacol Rev. 1999;51(4):691–744. [PubMed] [Google Scholar]
- 16.Sułkowski WW, Pentak D, Nowak K, Sułkowska A. The influence of temperature, cholesterol content and pH on liposome stability. J Mol Struct. 2005;744–747:737–47. doi: 10.1016/j.molstruc.2004.11.075. [DOI] [Google Scholar]
- 17.De Gier J, Mandersloot JG, Van Deenen LLM. Lipid composition and permeability of liposomes. Biochim Biophys Acta Biomembr. 1968;150(4):666–75. doi: 10.1016/0005-2736(68)90056-4. [DOI] [PubMed] [Google Scholar]
- 18.van der Woude I, Visser HW, ter Beest MB, Wagenaar A, Ruiters MH, Engberts JB, et al. Parameters influencing the introduction of plasmid DNA into cells by the use of synthetic amphiphiles as a carrier system. Biochim Biophys Acta. 1995;1240(1):34–40. doi: 10.1016/0005-2736(95)00161-1. [DOI] [PubMed] [Google Scholar]
- 19.Zelphati O, Szoka FC. Mechanism of oligonucleotide release from cationic liposomes. Proc Natl Acad Sci. 1996;93(21):11493–8. doi: 10.1073/pnas.93.21.11493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bhattacharya S, Haldar S. The effects of cholesterol inclusion on the vesicular membranes of cationic lipids. Biochim Biophys Acta. 1996;1283(1):21–30. doi: 10.1016/0005-2736(96)00064-8. [DOI] [PubMed] [Google Scholar]
- 21.Bhattacharya S, Haldar S. Interactions between cholesterol and lipids in bilayer membranes. Role of lipid headgroup and hydrocarbon chain-backbone linkage. Biochim Biophys Acta. 2000;1467(1):39–53. doi: 10.1016/S0005-2736(00)00196-6. [DOI] [PubMed] [Google Scholar]
- 22.Fang J, Nakamura H, Maeda H. The EPR effect: unique features of tumor blood vessels for drug delivery, factors involved, and limitations and augmentation of the effect. Adv Drug Deliv Rev. 2011;63(3):136–51. doi: 10.1016/j.addr.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 23.Maeda H, Wu J, Sawa T, Matsumura Y, Hori K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: a review. J Control Release Off J Control Release Soc. 2000;65(1–2):271–84. doi: 10.1016/S0168-3659(99)00248-5. [DOI] [PubMed] [Google Scholar]
- 24.Zhang Y, Yang M, Park JH, Singelyn J, Ma H, Sailor MJ, et al. A surface-charge study on cellular-uptake behavior of F3-peptide-conjugated iron oxide nanoparticles. Small (Weinheim Bergstrasse Germany) 2009;5(17):1990–6. doi: 10.1002/smll.200900520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang Y, Yang M, Portney NG, Cui D, Budak G, Ozbay E, et al. Zeta potential: a surface electrical characteristic to probe the interaction of nanoparticles with normal and cancer human breast epithelial cells. Biomed Microdevices. 2008;10(2):321–8. doi: 10.1007/s10544-007-9139-2. [DOI] [PubMed] [Google Scholar]
- 26.Haupenthal J, Baehr C, Kiermayer S, Zeuzem S, Piiper A. Inhibition of RNAse A family enzymes prevents degradation and loss of silencing activity of siRNAs in serum. Biochem Pharmacol. 2006;71(5):702–10. doi: 10.1016/j.bcp.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 27.Subramanian N, Murthy RS. Use of electrolyte induced flocculation technique for an in vitro steric stability study of steric stabilized liposome formulations. Die Pharm. 2004;59(1):74–6. [PubMed] [Google Scholar]
- 28.Lu JJ, Langer R, Chen J. A novel mechanism is involved in cationic lipid-mediated functional siRNA delivery. Mol Pharm. 2009;6(3):763–71. doi: 10.1021/mp900023v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Takahashi T, Kono K, Itoh T, Emi N, Takagishi T. Synthesis of novel cationic lipids having polyamidoamine dendrons and their transfection activity. Bioconjug Chem. 2003;14(4):764–73. doi: 10.1021/bc025663f. [DOI] [PubMed] [Google Scholar]
- 30.Martino S, di Girolamo I, Tiribuzi R, Angelo F, Datti A, et al. Efficient siRNA delivery by the cationic liposome DOTAP in human hematopoietic stem cells differentiating into dendritic cells. J Biomed Biotechnol. 2009;2009. doi: 10.1155/2009/410260. [DOI] [PMC free article] [PubMed]
- 31.Terp MC, Bauer F, Sugimoto Y, Yu B, Brueggemeier RW, Lee LJ, et al. Differential efficacy of DOTAP enantiomers for siRNA delivery in vitro. Int J Pharm. 2012;430(1–2):328–34. doi: 10.1016/j.ijpharm.2012.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ma Z, Li J, He F, Wilson A, Pitt B, Li S. Cationic lipids enhance siRNA-mediated interferon response in mice. Biochem Biophys Res Commun. 2005;330(3):755–9. doi: 10.1016/j.bbrc.2005.03.041. [DOI] [PubMed] [Google Scholar]
- 33.Dominska M, Dykxhoorn DM. Breaking down the barriers: siRNA delivery and endosome escape. J Cell Sci. 2010;123(8):1183–9. doi: 10.1242/jcs.066399. [DOI] [PubMed] [Google Scholar]
- 34.Xu Y, Szoka FC., Jr Mechanism of DNA release from cationic liposome/DNA complexes used in cell transfection. Biochemistry. 1996;35(18):5616–23. doi: 10.1021/bi9602019. [DOI] [PubMed] [Google Scholar]
- 35.Zhou X, Huang L. DNA transfection mediated by cationic liposomes containing lipopolylysine: characterization and mechanism of action. Biochim Biophys Acta (BBA) Biomembr. 1994;1189(2):195–203. doi: 10.1016/0005-2736(94)90066-3. [DOI] [PubMed] [Google Scholar]
- 36.Zuhorn IS, Bakowsky U, Polushkin E, Visser WH, Stuart MC, Engberts JB, et al. Nonbilayer phase of lipoplex-membrane mixture determines endosomal escape of genetic cargo and transfection efficiency. Mol Ther J Am Soc Gene Ther. 2005;11(5):801–10. doi: 10.1016/j.ymthe.2004.12.018. [DOI] [PubMed] [Google Scholar]
- 37.Chen C, Han D, Cai C, Tang X. An overview of liposome lyophilization and its future potential. J Control Release Off J Control Release Soc. 2010;142(3):299–311. doi: 10.1016/j.jconrel.2009.10.024. [DOI] [PubMed] [Google Scholar]
- 38.Lewis DA, Alpar HO. Erhythrocytes as microvesicles. In: Danbrow M, editor. Microcapsules and nanoparticles in medicine and pharmacy. FL: CRC Press; 1991. pp. 299–314. [Google Scholar]
- 39.Fildes F. Liposomes: the industrial view point. in: liposomes from physical structure to therapeutic applications. New York: Elsevier Biomedical Press; 1998. [Google Scholar]


