Abstract
Incorporation of drug-loaded nanoparticles into swellable and respirable microparticles is a promising strategy to avoid rapid clearance from the lung and achieve sustained drug release. In this investigation, a copolymer of polyethylene glycol grafted onto phthaloyl chitosan (PEG-g-PHCs) was synthesized and then self-assembled with ciprofloxacin to form drug-loaded nanoparticles. The nanoparticles and free drug were encapsulated into respirable and swellable alginate micro hydrogel particles and assessed as a novel system for sustained pulmonary drug delivery. Particle size, morphology, dynamic swelling profile, and in vitro drug release were investigated. Results showed that drug-loaded nanoparticles with size of 218 nm were entrapped into 3.9-μm micro hydrogel particles. The dry nano-in-micro hydrogel particles exhibited a rapid initial swelling within 2 min and showed sustained drug release. Preliminary in vivo pharmacokinetic studies were performed with formulations delivered to rats by intratracheal insufflation. Ciprofloxacin concentrations in plasma and in lung tissue and lavage were measured up to 7 h. The swellable particles showed lower ciprofloxacin levels in plasma than the controlled group (a mixture of lactose with micronized ciprofloxacin), while swellable particles achieved higher concentrations in lung tissue and lavage, indicating the swellable particles could be used for controlling drug release and prolonging lung drug concentrations.
KEY WORDS: alveolar macrophage, antibiotics, cross-linking, hydrogel swelling, intratracheal insufflation
INTRODUCTION
Delivering antibiotics through the pulmonary route increases the local drug concentration in the lung, leading to improved local antibacterial effect in lung infections (1,2). In patients with lung infections, such as cystic fibrosis and pneumonia, the reduction of administration frequency (3), dose (4), and duration of inhalation treatment (5) will increase the patient’s compliance and adherence to the therapy (6). The administration frequency of inhalation aerosols could be reduced by prolonging the residence time of drug-releasing particles in the lung, essentially by using a sustained release formulation (7). However, there are few effective sustained release pulmonary formulations developed currently because of the efficient clearance of inhaled particles either by mucociliary escalator (8) or alveolar macrophage uptake (9).
For effective delivery of sustained release formulations into the lung, drug-loaded particles should have suitable aerodynamic properties, which are primarily determined either by the particle size, shape, or particle density. The preferred aerodynamic particle size range for deep lung delivery is around 0.5–5 μm (9). However, the conundrum is that particles within this size range are subject to rapid phagocytosis and are cleared by alveolar macrophages (10,11). To overcome the clearance due to alveolar macrophage uptake, two main approaches, larger particles with low density (12) and nanoparticles (13,14), have been explored. Low-density particles with larger micro size (i.e., greater than around 6 μm) have been shown to reduce the uptake rate by macrophages. However, there may be limitations of controlling the drug release from this type of particle system (15). Even though nanoparticles have shown promise in evading phagocytosis and mucociliary clearance, they may be exhaled easily following inhalation resulting in lower deposition in the airways (16,17). Additionally, it is critical that nanoparticles must not tend to aggregate together, forming microparticles which once again lead to rapid clearance by macrophages (15,18).
Thus, it would be extremely useful to develop an alternative pulmonary delivery vector for increasing the residence time of the drugs in the lung. Such a delivery rector should have efficient deposition in the deep lung and diminish alveolar macrophage uptake. Acknowledging the physiological barriers to successful pulmonary drug delivery of controlled release systems, our group has worked on a third approach for improving prolonged drug release in the lung. Specifically, we have developed a particle platform around swellable hydrogel particles intended for inhalation (9,19–21). The swellable particles have respirable aerodynamic size in dry state (i.e., during administration) but swell to larger geometric sizes after deposition in the hydrated wet respiratory tract, thus evading the macrophage uptake. Various cargos have been incorporated into these swellable particles, including the encapsulation of nanoparticles permitting controlled drug release (22).
In a recent formulation investigated in our laboratory, a copolymer of polyethylene glycol (PEG) grafted onto phthaloyl chitosan (PEG-g-PHCs) self-assembled nanoparticles was entrapped into Ca2+ cross-linked alginate to prepare respirable microparticles with semi-interpenetrating polymer networks (semi-IPN particles) for sustained pulmonary drug delivery. The preliminary in vitro evaluation of this hydrogel showed that it could be used as good potential carrier for sustained pulmonary drug delivery (15). Alginate is a natural, low-toxicity, and biocompatible polyanionic polymer composed of mannuronic acid (M) and guluronic acid (G) residues arranged linearly as consecutive G blocks (GGGGG), or consecutive M blocks (MMMMM), or even alternating G and M blocks (GMGMGM) (23). The anionic alginate interacts with cationic chitosan (24), which is biocompatible and biodegradable polysaccharide (25) and is capable to prolonged drug release (26). In biomedical applications, alginate has been extensively investigated due to its unique ability to form hydrogel via ionotropic cross-linking with divalent cations such as Ca2+ (27,28). However, it has been recently reported that calcium in alginate hydrogel stimulates an inflammatory response (29), which is undesirable in lungs for most therapeutic applications.
For biomedical applications of alginate, there has been debate about the immunogenicity of the polymer despite the extensive evaluations both in vitro and in vivo (23,30). One contention related to alginate immunogenicity is associated with the ratio of alginate blocks (31,32). Alginate consists of both M-block and G-block. It was reported that alginate with a high ratio of M-block to G-block was more immunogenic and thus triggered an increased release of cytokines than that with high ratio of G-block to M-block (33). In contrast, it has also been reported that no immunogenicity was observed when alginate with varied levels of M-block was investigated (34). Alginate immunogenicity may also be related to the impurities in different sources or batches of alginate used. Alginate is extracted from natural resources. Thus, impurities such as heavy metals, endotoxins, proteins, and polyphenolic compounds may be present in alginate that could potentially cause an immune response (23). But, no foreign body reaction was observed when alginate was processed via multistep purification technique in an animal model (30,35). Therefore, based on the literatures, it appears that in some cases, alginate polymer may exert immunogenic responses, but in others, alginate appears to be non-immunogenic.
In this manuscript, a nano-in-micro hydrogel particle formulation was developed for sustained pulmonary drug delivery, which takes the advantages of both nanoparticles and swellable and respirable hydrogel particles. Specifically, we have developed an alginate hydrogel which was free of Ca2+. PEG-g-PHCs was synthesized and then self-assembled into nanoparticles in combination with ciprofloxacin. The nanoparticle suspension was mixed with sodium alginate solution to form microparticle. In this microparticle, ciprofloxacin which was not incorporated into nanoparticles in the beginning would act as a cross-linker, similar to and replacing Ca2+, to form hydrogel with alginate. Subsequently, the hydrogel particles were spray-dried to dry swellable nano-in-micro hydrogel particles, which were further evaluated with in vitro and in vivo experiments in rats by intratracheal insufflation.
MATERIALS AND METHODS
Materials
Formulation Study
Chitosan (Cs) (MW: 4–5 × 105 Da with N-deacetylation (%) of about 76.4%), monomethoxy-poly(ethylene glycol) (m-PEG, Mn 5,000 Da), succinic anhydride, and 1-hydroxybenzotriazole (HOBt) were obtained from Aldrich (St. Louis, MO). 4-Dimethylaminopyridine (DMAP) and ciprofloxacin were purchased from Sigma (St. Louis, MO). 1-Ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDC·HCL) was provided by Fluka Chemical Corp. (Milwaukee, WI). Sodium alginate (low viscosity; 250 cps for a 2% solution at 25°C), triethylamine, and other chemicals were obtained from Sigma-Aldrich (St. Louis, MO). Phosphate-buffered saline (PBS, pH 7.4), absolute ethanol, and all other reagents were of analytical grade and used as received.
Cell Culture
Mouse macrophages cells, RAW 264.7 (8.8 × 105 cells/mL), and fetal bovine serum (FBS) were obtained from American Type Culture Collection, ATCC (Manassas, VA). Dulbecco’s modified Eagle’s medium (DMEM) was provided by Gibco (Grand Island, NY). Polystyrene (PS) (1 μm) particles (FluoSpheres fluorescent (505/515) and 112-μm PS particles were purchased from Invitrogen (Eugene, OR) and Bangs Laboratories, Inc. (Fishers, IN), respectively. Paraformaldehyde (PFA, 4%) solution was obtained from USB Corporation (Cleveland, Ohio).
Preliminary In Vivo Pharmacokinetic Studies
Lactose (Respitose® ML001) was obtained from DMV-Fonterra Excipients. Methanol and acetonitrile purchased from Fisher Scientific. Insufflator was provided by Penn-Century DP-4, Penn Century (Philadelphia, PA).
Methods
Preparation of PEG-g-PHCs
The copolymer of PEG grafted onto phthaloyl chitosan (PHCs) was synthesized by a modified method reported in details in our earlier studies (9,15) and described briefly as follows:
Firstly, PHCs was synthesized by reaction of 5 g of Cs with 22.5 g of phthalic anhydride in 150 mL of N,N'-dimethylformamide (DMF) at 130°C under dry nitrogen atmosphere for 10 h. The reaction mixture was left to reach room temperature, and then the PHCs was precipitated over ice water. The precipitated PHCs was filtered, washed with ethanol, and freeze dried. Secondly, m-PEG was converted into m-PEG-COOH through reaction with succinic anhydride. In brief, 10 g of m-PEG, 0.24 g of DMAP, 0.24 g of succinic anhydride, and 0.2 g of triethylamine were dissolved in 50 mL of dry dioxane. The reaction mixture was stirred at room temperature for 2 days under dry nitrogen atmosphere. The dioxane was evaporated and the residue (m-PEG-COOH) was taken up in CCl4, filtered, and precipitated by diethyl ether. Lastly, the PEG-g-PHCs copolymer was obtained by stirring of 3.8 g of m-PEG-COOH with 0.5 g of the dried PHCs in 15 mL DMF. Then, 0.3 g of the HOBt was added and the reaction mixture was stirred at room temperature until obtaining a clear solution. Afterwards, EDC·HCl (0.43 g) was added, and the reaction was continued overnight under stirring at room temperature. The obtained PEG-g-PHCs copolymer was purified by dialysis in distilled water, washed with ethanol, and freeze dried.
Preparation of Dry Swellable Nano-in-Micro Hydrogel Particles
The ciprofloxacin-loaded swellable hydrogel particles were obtained via spray drying of a combination of ciprofloxacin-loaded PEG-g-PHCs nanoparticles suspension and sodium alginate solution. Briefly, homogenous solutions of the ciprofloxacin-loaded PEG-g-PHCs nanoparticles (1% w/v) and sodium alginate (3% w/v) were prepared using 0.06% acetic acid and distilled water as solvents, respectively. The self-assembled PEG-g-PHCs nanoparticles were prepared by sonication of 1% w/v PEG-g-PHCs solution containing ciprofloxacin using a probe type sonicator (Misonix ultrasonic processor, S-4000, Misonix Inc, CT) at 60 W for 2 min. The sonication step was repeated twice and performed in an ice water bath. Then, 75 mL of ciprofloxacin-loaded PEG-g-PHCs nanoparticles suspension was added dropwise with stirring to 25 mL of 3% aqueous alginate solution. The mixture was completed with distilled water up to a final concentration of 1.5% w/v. Then, the homogenized polymer mixture was spray-dried with a 0.7-mm two-fluid pressurized atomizer at a feed rate of 25% (6 mL/min) in a Büchi Mini Spray Dryer B-290 (Büchi, Switzerland). The atomizing air flow rate was 500–600 NL/h. The inlet temperature was adjusted at 125°C, and the outlet temperature varied between 60°C and 65°C. The obtained hydrogel particle powder was collected and the spray drying yield (%) was calculated.
Determination of Particle Size
The size of the ciprofloxacin-loaded PEG-g-PHCs nanoparticles was estimated using dynamic light scattering (Wyatt Technology Corporation Dyna Pro-titan dynamic light scattering (DLS)) after sonication of a 1% PEG-g-PHCs solution containing ciprofloxacin for 2 min at a power of 60 W. The size of micro hydrogel particles was determined using laser diffraction (SYMPATEC, Sympatec Gmbt, System Partikl-Technik, Germany, He-Ne laser beam 5 mW max at 632.8 nm). The measurements were carried out in triplicates for the suspension of the micro hydrogel particles in acetone. Volume mean diameter (VMD, μm) was calculated from the particle size distribution curves for the micro hydrogel particles. Average aerodynamic diameter of the ciprofloxacin-loaded nano-in-micro hydrogel particles was also calculated using the following relationship (36):
| 1 |
where daer is the micro hydrogel particle aerodynamic diameter (μm); d is the geometric diameter (VMD, μm); and ρ is the micro hydrogel particles tapped density (g/cc).
Morphology of Micro Hydrogel Particles
The morphology of the prepared dry swellable ciprofloxacin-loaded nano-in-micro hydrogel particles was examined by scan electron microscope (SEM) (Hitachi S-800 field emission scanning electron microscope operated in secondary electron mode with a Robinson backscatter detector and with a Hitachi PCI system for digital image capture). Dry particles were mounted on aluminum stubs with double-sided conducting carbon tapes and coated with a 50/50 mixture of Au/Pd to minimize surface charging. The samples were scanned at an accelerating voltage of 20 kV.
Swelling Study of Micro Hydrogel Particles
The swelling pattern of the developed dry ciprofloxacin-loaded nano-in-micro hydrogel particles in PBS, pH 7.4, was studied by determining the increase in both VMD (μm) and the median diameter (X50, μm) of the particles with time using laser diffractometer (SYMPATEC, Sympatec Gmbt, System Partikl-Technik, Germany).
Investigation of Next-Generation Impactor (NGI)
Powder (10 (±1) mg), filled in size 3 Vcaps HPMC capsule, was dispersed through a commercial inhaler Handihaler® (Pfizer, Inc., USA; Boehringer Ingelheim, Inc., Germany) into a NGI (MSP Corp., MN) at a volumetric flow rate of 60 L min−1 actuated for 4 s. Drug content collected at each stage from the NGI apparatus was assessed via UV–VIS absorption spectroscopy at 280 nm. Emitted fraction (EF) was expressed as the total mass fraction of drug emitted from the inhaler. Fine particle fraction (FPF) was defined as the drug mass (<5 μm) deposited in the NGI divided by the emitted dose. Respirable fraction (RF) was defined as the drug mass (<5 μm) deposited in the NGI divided by drug mass recovered from the entire system.
In Vitro Release of Ciprofloxacin
The in vitro release pattern of the ciprofloxacin from the developed nano-in-micro hydrogel particles was determined by transferring a certain weight (10–30 mg) of particles to a vial containing 1.5 mL of PBS, pH 7.4. Samples were maintained at 37°C with shaking at 100 rpm. At predetermined intervals, 100-μL aliquot was withdrawn and analyzed at λmax 280 nm using a UV–VIS spectrophotometry. The withdrawn aliquots were replaced with the same volume of fresh buffer, to keep the volume of the release medium constant. The amount of ciprofloxacin released (μg) from the swellable particles was then calculated using a standard curve of ciprofloxacin in PBS, pH 7.4. Results were expressed as cumulative release (%) relative to the initially loaded weight of ciprofloxacin in particles. The data points represent average (with standard deviation) from three independent experiments.
Cytotoxicity Assay of Micro Hydrogel Particles
The effect of the developed swellable ciprofloxacin-loaded nano-in-micro hydrogel particles on the viability of RAW 264.7 cells was investigated. Cells were seeded in 96-well plates at 50,000 cells/well and incubated for 24 h at 37°C and 5% CO2. Fifty microliters of particle suspension (the total powder concentrations were 320, 800, and 1,600 μg/mL, respectively) were incubated with cells for 24 h. Control group was the cells grown without adding swellable particles, and the cytotoxicity of plain PEG-g-PHCs was also tested. The cell viability was estimated using a microtiter tetrazolium (MTT) cell proliferation assay kit provided by ATCC (Manassas, VA). After addition of the MTT reagent (10 μL), cells were incubated at 37°C and 5% CO2 for 4 h until the purple precipitate was visible. Afterwards, 100 μL of detergent reagent was added, and cells were left in the dark at room temperature for 2 h. Cell viability was determined through recording absorbance at 570 nm. Data represents average absorbance of triplicate samples (with standard deviation).
Preliminary In Vivo Pharmacokinetic Studies
Experimental Design
Male Sprague–Dawley rats weighing 350 ± 30 g were obtained from the Charles River and maintained in 12-h light/dark cycle. Rats were acclimated for 3 days before the experiment and were allowed free access to standard food and water. Temperature and relative humidity were maintained at 25°C and 50%, respectively. All animal procedures were approved by the Institutional Animal Care and Use Committee (IACUC) of The University of Texas at Austin. All experiments related to animals were performed in accordance with the American Association for Accreditation of Laboratory Animal Care.
Male Sprague–Dawley rats were randomly assigned to two groups, receiving formulation as follows: group 1, the powder mixture of micronized ciprofloxacin with lactose (ciprofloxacin concentration 30% w/w); group 2, dry swellable ciprofloxacin-loaded nano-in-micro hydrogel particle (ciprofloxacin concentration 30% w/w). The dosage of ciprofloxacin was around 15 mg/kg.
Briefly, prior to dosing, all rats were anesthetized by IP injection of ketamine (75 mg/kg)/xylazine (5 mg/kg) cocktail. The footpads were pinched firmly to test the lack of pedal reflex. Each animal was placed flat on its back, and the trachea was visualized with the help of a laryngoscope. The insufflator (Penn-Century DP-4, Penn Century, Philadelphia, PA) was inserted into the trachea. The dry powder in the chamber of insufflator was dispersed with the help of 2 mL of air from an empty syringe. After insufflation, each animal was held in an upright position for 1 min to ensure appropriate deposition of powder in the lung, then maintained at 30°C during the recovering period on the heating pad. The insufflator containing the powder was weighed before, after powder filling, and after administration, to know the exact dose insufflated.
Analysis of Ciprofloxacin Concentration in Plasma, Lavage, and Lung Tissue
For sampling after administration, a 0.4-mL blood sample was collected from the jugular vein. As for the lavage collection, animals were euthanized via CO2 inhalation and exsanguinated. The trachea was severed below the glottis between the thyroid glands and was immediately cannulated, and the lung was lavaged three times with 1 mL PBS (pH 7.4). The collected lavage was put in a 2-mL tube and kept in ice until analysis. Following lavage collection, the lung was removed, weighed, and placed into a 50-mL conical tube, snap frozen, and stored at −80°C until homogenization. For homogenization, lung tissues were thawed and 2 mL of PBS (pH 7.4) was added to each 50-mL tube. Lung was homogenized and the subsequent tissue slurry was analyzed for drug content.
The concentrations of the ciprofloxacin in the plasma, lavage, and lung tissue were measured by using a high-performance liquid chromatography (HPLC) system with a UV detector at room temperature. An aliquot of sample was injected into an HPLC column (Atlantis T3 Column, 4.6 × 250 mm, 5 μm, Waters Corporation, MA, USA). The mobile phase consisted of a mixture of 800 mL of 50 mL/L acetic acid, 110 mL of acetonitrile, and 90 mL of methanol per liter. The sample was detected under 280 nm with a flow rate of 1 mL/min at room temperature.
Statistical Analysis
The obtained data was analyzed and expressed as mean with standard deviation. The effects of various parameters were statistically analyzed by one-way ANOVA. Differences were considered significant at the level of p < 0.05.
RESULTS AND DISCUSSION
Preparation of the Swellable Ciprofloxacin-Loaded Nano-in-Micro Hydrogel Particles
One challenge for efficient pulmonary drug delivery is the lung physiological barriers (i.e., mucociliary escalator and alveolar macrophages), which should be considered thoroughly for the design of efficient pulmonary drug delivery systems. The preferred pulmonary delivery formulation should also possess controlled release profile along with minimal inactive excipients.
In order to overcome the challenges, a novel antibiotic hydrogel particle with high drug loading was formed for controlled pulmonary drug delivery. As well known, alginate can interact with Ca2+ to form hydrogel. However, Ca2+ can stimulate immune effects as mentioned previously. Instead of using Ca2+, this study utilized ciprofloxacin, an antibiotic drug, as the cross-linker interacting with alginate directly and thereafter forming a high ciprofloxacin-loaded hydrogel particle system. In this high drug loading formulation, one fraction of ciprofloxacin was incorporated into PEG-g-PHCs nanoparticles; one fraction of ciprofloxacin was incorporated as a cross-linking agent for alginate to form hydrogel, while the last fraction was physically entrapped in this hydrogel matrix (Scheme 1). Hence, ciprofloxacin initially exhibited a burst release and reached a quick Cmax due to free ciprofloxacin physically entrapped in the hydrogel matrix. Meanwhile, the other fractions of ciprofloxacin acting as the cross-linker, as well as the ciprofloxacin encapsulated in the nanoparticles, were sustained, which has the potential to control drug release in lungs.
Scheme 1.
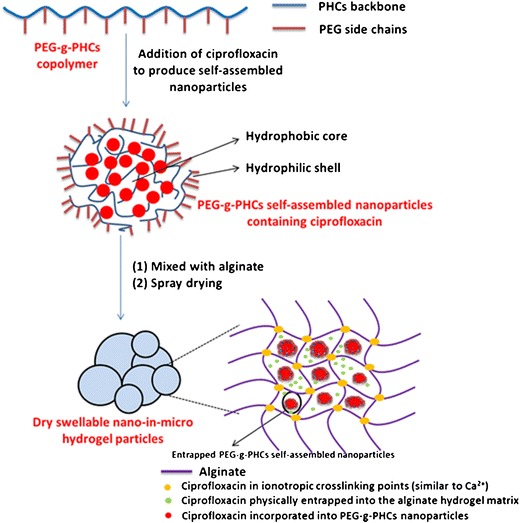
A schematic illustration for preparation of the dry swellable nano-in-micro hydrogel particles
The PEG-g-PHCs copolymer was synthesized through a modified method described in details in our previous study (9,15). As illustrated in Scheme 2, the copolymer synthesis was achieved through firstly a phthaloylation process of the free amino groups of Cs to produce PHCs. Fourier transform infrared (FTIR) spectrum of the PHCs (15) illustrated absorbance bands at 1,395 and 732 cm−1 which were assigned for the aromatic C=C and C–H bonds of phthaloyl groups, respectively. Secondly, m-PEG was converted to m-PEG-COOH using succinic anhydride. The modification of m-PEG was confirmed using EA and FTIR (15). Conjugation of m-PEG-COOH with PHCs was then carried out, and the obtained PEG-g-PHCs was also characterized using various analytical techniques (15).
Scheme 2.
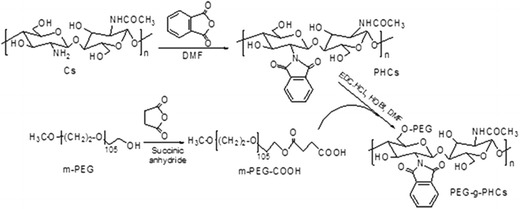
Synthesis of PEG-g-PHCs amphiphilic copolymer
The synthesized PEG-g-PHCs amphiphilic copolymer was utilized to prepare ciprofloxacin-free and ciprofloxacin-loaded self-assembled nanoparticles using sonication technique. Then, the resulting ciprofloxacin-loaded PEG-g-PHCs nanoparticles were incorporated into respirable micro hydrogel particles. These micro hydrogel particles were obtained via spray drying of a homogenous mixture of the ciprofloxacin-loaded PEG-g-PHCs nanoparticles and the aqueous alginate solution. The resulting nano-micro matrices were evaluated as carriers for sustained pulmonary delivery of ciprofloxacin (ciprofloxacin concentration 30% w/w) that combined the benefits of both nanoparticles and the micro hydrogel particles suggested in our previous studies (21).
Calcium, a divalent ion with two positive charges, interacts with alginate linear chains to form a cross-linked hydrogel (28). This mechanism of cross-linking has been studied and been reported elsewhere (23). Recently, we found ciprofloxacin, similar to calcium, can also act as a cross-linker with alginate to form a stable hydrogel. Although ciprofloxacin is zwitterionic, different from calcium which is divalent, the charges of ciprofloxacin could be well adjusted. Ciprofloxacin has three pKa values, pKa1 = 5.1, pKa2 = 6.4, and pKa3 = 9.0 (37). Therefore, at a pH lower than 5, the ciprofloxacin molecule has two positive charges, similar to ionic calcium. In our hydrogel formation experiment, ciprofloxacin was kept in a solution with pH less than 5.
Particle Size
The size of the prepared ciprofloxacin-loaded nanoparticles was found to be 218.6 ± 25.3 nm as determined by DLS. In the case of the prepared micro hydrogel particles, the VMDs of plain microparticles and ciprofloxacin-loaded nano-in-micro hydrogel particles were determined using laser diffraction and were found to be 2.1 ± 0.1 and 3.9 ± 0.1 μm, respectively. The developed particles showed relatively low-tapped densities (0.298 and 0.347 g/mL for plain microparticles and ciprofloxacin-loaded nano-in-micro hydrogel particles, respectively). The theoretical aerodynamic diameters (da) of both ciprofloxacin-free and ciprofloxacin-loaded particles were also calculated using the volume diameters and particle densities and were found to be 1.2 ± 0.7 and 2.3 ± 0.1 μm, respectively. Although the low da values of these prepared particles would lead to high particle respirability, the powder dispersion studies showed only modest aerosol performance of the pure powders. The powder dispersion performance of this dry powder, without added carrier particles, was assessed in vitro by a commercial inhaler Handihaler® via the NGI (MSP Corp., MN) at air flow rate of 60 ± 5% L/min. The fraction of powder deposited in each stage of NGI, capsule, device, adaptor, and throat was determined. It was found that respirable fraction (RF%) and fine particle fraction (FPF%) were 21.5% and 28.6%, respectively. The dispersibility of the pure powders was similar to currently marketed product performance values and was encouraging given the absence of any other carrier particle (i.e., lactose) which is well known to enhance the dispersibility of micronized powders (38).
In the control group, raw ciprofloxacin was jet-milled to achieve an X50 of 4.2 ± 0.5 μm. The X50 is the median diameter of the particles on a volume basis (as determined by laser diffraction). The jet milling procedure was optimized such that this particle size could match the particle size of the dried swellable hydrogel particles. The swellable particles with ciprofloxacin in dried form had an X50 of 4.6 ± 0.1 μm.
As a result of the similar particle size distribution of micronized ciprofloxacin and dried hydrogel particles, they represent well-matched treatment and control groups in terms of particle size. In addition, insufflation, the method of directly spraying the dispersed powders into the lower airways of the animals, enables a direct comparison of the groups. This method of administration diminishes deposition differences between the two groups because the insufflator is placed intratracheally which avoids upper airway filtering on the basis of aerodynamic particle sizes. Thus, the deposition site of the two kinds of particles in peripheral lung region after trachea insufflation should be similar. Therefore, after insufflation, differences in absorption and clearance of the two drug particles should be strongly related to particle size differences after wetting and swelling of the dried swellable particle, rather than the different deposition site of the two kinds of drug particles in the airways.
Surface Morphology
Figure 1 shows the scanning electron micrographs of the developed micro hydrogel particles encapsulating ciprofloxacin-free and ciprofloxacin-loaded PEG-g-PHCs nanoparticles. As apparent from Fig. 1a, the ciprofloxacin-free hydrogel particles were generally spherical with relatively smooth surfaces. However, for the ciprofloxacin-loaded particles, their surfaces were notably rougher (Fig. 1b). The differences in surface roughness may be attributed to the additional cross-linking that occurs between the ciprofloxacin (for drug-loaded particles) and the negatively charged sodium alginate chains during the spray drying process.
Fig. 1.
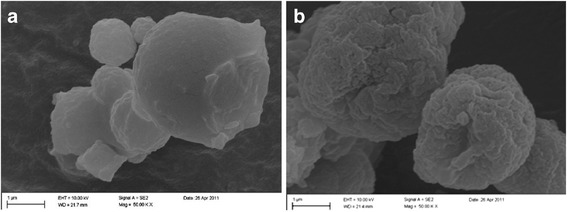
Scanning electron micrographs of a plain microparticles (no ciprofloxacin) and b ciprofloxacin-loaded nano-in-micro hydrogel articles
Dynamic Swelling Study
The swelling profile of the micro hydrogel particles incorporating ciprofloxacin-loaded PEG-g-PHCs self-assembled nanoparticles in PBS, pH 7.4, was shown in Fig. 2. The swelling profile of the developed hydrogel particles was obtained through determining the increase in the VMD (μm) of the particles at various time intervals using laser diffraction technique. As shown in Fig. 2, the prepared hydrogel particles showed a fast initial swelling within the first few minutes. For instance, the VMD of the developed particles has increased from about 3 μm when dry to 22 μm after 2 min of swelling. This swelling continued regularly with time to reach 41.9 μm at 14 min. The swelling can be attributed to the hydrophilic nature of the PEG side chains in the PEG-g-PHCs copolymer and the sodium alginate. As the swelling behavior demonstrated, the micro developed particles which had respirable aerodynamic sizes when dry showed large geometric sizes when swollen after a short period in simulated moist environment of the lung. We have studied this behavior in similar particles at shorter time scales and have also confirmed that particle swelling occurs slower than the calculated transport time of the particle in the high humidity of the airways. This enables the particles to remain small enough during their passage to the deep lung where they will be deposited. However, the swelling that occurs on the order of minutes, as shown in Fig. 2, enables the ciprofloxacin delivery systems to avoid macrophage uptake and at the same time confer sustained release of ciprofloxacin through a controlled polymeric architecture (9,15,20).
Fig. 2.
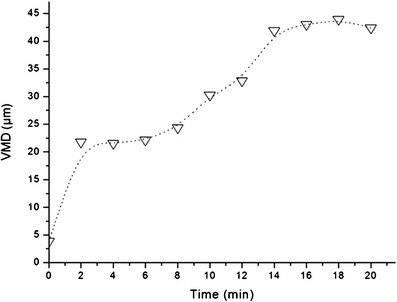
Dynamic swelling pattern of the swellable nano-in-micro hydrogel particles in PBS, pH 7.4
In Vitro Cumulative Release Study
The entrapment efficiency of ciprofloxacin in the developed swellable particles as quantified using UV–VIS spectrophotometry was found to be 30% w/w. The in vitro release profile of ciprofloxacin from the developed swellable particles was illustrated in Fig. 3. It was found that the investigated formulation showed a rapid initial release of ciprofloxacin (about 9%) within the first 5 h followed by a relatively slow release up to 144 h. The fast initial release may be due to the fast initial dynamic swelling of the investigated hydrogel particles as described in “In Vitro Cumulative Release Study” section. Several reasons are ascribed to the relatively slow and low release of the drug from the particles as observed in this in vitro assay. Firstly, a significant proportion of the ciprofloxacin present in the formulation will be bound to the alginate matrix and serves as a cross-linker. Therefore, displacement of the ciprofloxacin by smaller monovalent cations will be necessary to enable release. In addition, the conditions of the release media were not selected to mimic physiological environment (i.e., release was performed in PBS pH 7.4). It is anticipated that more extensive release could be achieved in in vivo studies.
Fig. 3.
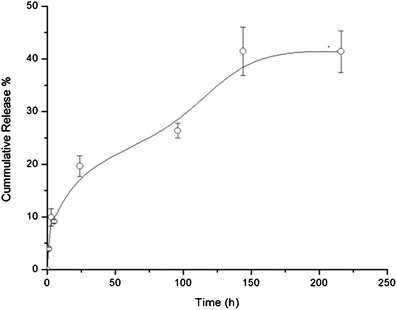
In vitro cumulative release of the ciprofloxacin from swellable nano-in-micro hydrogel particles in PBS, pH 7.4
Cytotoxicity Assay
The cytotoxicity of particles was determined using the MTT assay after the exposure of RAW 264.7 cells for 24 h to ciprofloxacin-loaded and plain PEG-g-PHCs at different concentrations (Fig. 4). From the figure, at powder concentrations (i.e., 320 μg/mL) of ciprofloxacin-loaded nano-in-micro hydrogel particles in which the ciprofloxacin and PEG-g-PHCs concentration were both around 106.6 μg/mL, the viability of the RAW 264.7 cells was reduced by 38.4% as compared to the control cells under the same experimental conditions. This reduction in cell viability might be primarily attributed to the drug (ciprofloxacin) rather than the excipients in particles since the viability of cells cultured with drug-free PEG-g-PHCs at 1,000 μg/mL was 93.6%.
Fig. 4.
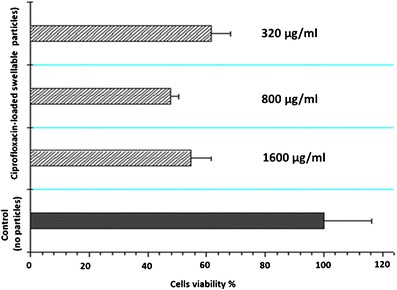
The effect of different concentrations (320, 800, and 1,600 μg/mL) of the developed swellable ciprofloxacin-loaded nano-in-micro hydrogel particles on the viability of RAW 264.7 macrophage cells. Cells were seeded at 50,000 cells/well and incubated with the particles for 24 h at 37°C and 5% CO2
Preliminary In Vivo Pharmacokinetic Studies
Plots of average ciprofloxacin plasma concentration versus time after administration of two dry powder formulations to male Sprague–Dawley rats were shown in Fig. 5. As apparent from the figure, there was a statistical difference in plasma concentrations of ciprofloxacin when comparing the swellable particle formulation with the powder mixture formulation at all time points (p < 0.05). The pharmacokinetic parameters were calculated by non-compartmental methods (39). For plasma, the areas under the curve (AUC0–7 h) were 2.8 and 11.6 μg h/mL for the group of swellable particles and the powder mixture group, respectively. For the Tmax, even no exact value of Tmax could be achieved due to the limitation of time points, an estimation within the range of 0.25 to 3 h was determined. Overall, the mixture formulation showed much higher ciprofloxacin absorption into the plasma compared to the swellable particles, providing evidence for a delayed release from the swellable particles.
Fig. 5.
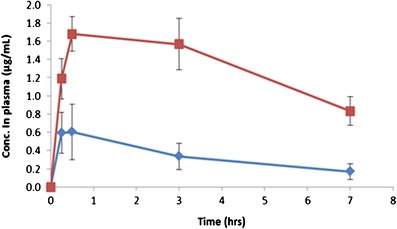
Time-course of concentration of ciprofloxacin in plasma ( ), swellable ciprofloxacin-loaded nano-in-micro hydrogel particles (
), swellable ciprofloxacin-loaded nano-in-micro hydrogel particles ( ), and powder mixture of micronized ciprofloxacin and lactose (n = 3–5). The dosage of ciprofloxacin was 15 mg/kg
), and powder mixture of micronized ciprofloxacin and lactose (n = 3–5). The dosage of ciprofloxacin was 15 mg/kg
In Fig. 6, the drug concentration measured in lung lavage fluids was not significantly different between the two formulation groups at the initial time point (t = 0.25 h). As expected, with time going on lavage fluid drug concentrations decreased for both formulations. However, most notably, ciprofloxacin decrease was observed in drug–lactose binary mixtures. This rapid decrease could be explained by different amount of dissolved drug available for absorption. Compared to swellable particles for controlled drug release, there would be more drugs dissolved from simply drug–lactose mixture group, ready for absorption into lung tissue and/or plasma. Additionally, micronized drug in powder mixture group was engulfed easily by macrophages. Subsequently, the engulfed ciprofloxacin would be transported along the respiratory surface to the mucociliary escalator or to lung interstitium and lymph nodes (40–42). The low retention of powder mixture group in the lung fluid was also confirmed by the AUC results. As calculated from Fig. 6, the ratio of AUC0–7 h in lavage for swellable particles and control group was 1,468.3 ± 377.5 and 592.6 ± 139.9 μg h/mL, respectively. These AUC data indicated that the swellable particles had significantly greater exposure to the lung fluid (p = 0.04) than the control group.
Fig. 6.
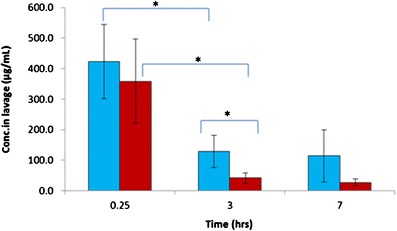
The concentration of ciprofloxacin in lung lavage ( ), swellable ciprofloxacin-loaded nano-in-micro hydrogel particles (
), swellable ciprofloxacin-loaded nano-in-micro hydrogel particles ( ), and powder mixture of micronized ciprofloxacin and lactose (n = 3–5, *p < 0.05). The dosage of ciprofloxacin was 15 mg/kg
), and powder mixture of micronized ciprofloxacin and lactose (n = 3–5, *p < 0.05). The dosage of ciprofloxacin was 15 mg/kg
In in vivo lung biodistribution studies, the drug concentrations in lung lavage are related to the unreleased drug. Therefore, in our studies, ciprofloxacin in lavage sample was regarded as unreleased drug from the dry swellable hydrogel particles. Based on this assumption, we determined that over 50% of drug was released from the swellable particles in vivo. Specifically, it was estimated that approximately 50% of the drug had been released after 0.25 h and approximately 80% at 3 h.
Ciprofloxacin concentrations were also determined in the lung tissue samples. Lung concentrations were found to be higher for the swellable particles group than in the physical mixture group during the experimental period shown (Fig. 7). The higher lung tissue concentrations from swellable particles could be attributed to two main factors. Firstly, the swellable particle formulation may extend the adhesion time between particles and lung epithelial cells, decreasing the particle clearance of mucociliary escalator in respiratory tract. Secondly, the release of the ciprofloxacin nanoparticles from the micro hydrogel particles can explain differences in the lung tissue levels. Nanoparticles may be more likely to be accumulated in the epithelial cells than free drug (as evidenced by the rapid plasma absorption of the free ciprofloxacin formulation). Further studies are required to determine the exact mechanisms of lung tissue distribution. Finally, the slightly different aerosol dispersion characteristics of the two treatment powders may also result in differences in deposition profiles (although this was minimized using the direct insufflation technique used in these studies) but may lead to small differences absorption and clearance from the lung.
Fig. 7.
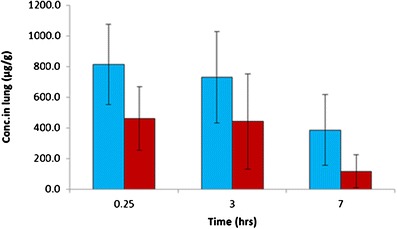
The concentration of ciprofloxacin in rat lung tissue ( ), swellable ciprofloxacin-loaded nano-in-micro hydrogel particles (
), swellable ciprofloxacin-loaded nano-in-micro hydrogel particles ( ), and powder mixture of micronized ciprofloxacin and lactose (n = 3–5). The dosage of ciprofloxacin was 15 mg/kg
), and powder mixture of micronized ciprofloxacin and lactose (n = 3–5). The dosage of ciprofloxacin was 15 mg/kg
CONCLUSION
In the present study, the self-assembled ciprofloxacin nanoparticles were encapsulated a dry swellable nano-in-micro hydrogel particles which were free of Ca2+. This formulation displayed suitable aerodynamic characteristics and sustained drug release profile. When delivered to rats, it enabled ciprofloxacin to achieve a low systemic exposure but maintained higher concentrations in the lung for more than 7 h. Further formulation optimization and studies focused at the cellular mechanisms of absorption and clearance are required.
ACKNOWLEDGMENTS
Research reported in this publication was supported by the National Heart, Lung, and Blood Institute, National Institute of Environmental Health Sciences, and National Institute of Biomedical Imaging and Bioengineering of the National Institutes of Health under award numbers of R21HL092812 and R03EB006892. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflict of Interest
The authors report no declarations of interest.
Footnotes
Ju Du and Ibrahim M. El-Sherbiny had contributed equally to this work.
Contributor Information
Ibrahim M. El-Sherbiny, Phone: +20 106 333 0913, Email: imelsherbiny@gmail.com
Hugh D. Smyth, Phone: 512-471-3383, Email: hugh.smyth@austin.utexas.edu
REFERENCES
- 1.Geller DE. Aerosol antibiotics in cystic fibrosis. Respir Care. 2009;54:658–70. doi: 10.4187/aarc0537. [DOI] [PubMed] [Google Scholar]
- 2.Pilcer G, Sebti T, Amighi K. Formulation and characterization of lipid-coated tobramycin particles for dry powder inhalation. Pharm Res. 2006;23:931–40. doi: 10.1007/s11095-006-9789-4. [DOI] [PubMed] [Google Scholar]
- 3.Weers JG, Bell J, Chan HK, Cipolla D, Dunbar C, Hickey AJ, et al. Pulmonary formulations: what remains to be done? J Aerosol Med Pulm Drug Deliv. 2010;23:S5–23. doi: 10.1089/jamp.2010.0838. [DOI] [PubMed] [Google Scholar]
- 4.Yang Y, Tsifansky MD, Wu CJ, Yang HI, Schmidt G, Yeo Y. Inhalable antibiotic delivery using a dry powder co-delivering recombinant deoxyribonuclease and ciprofloxacin for treatment of cystic fibrosis. Pharm Res. 2010;27:151–60. doi: 10.1007/s11095-009-9991-2. [DOI] [PubMed] [Google Scholar]
- 5.Stass H, Weimann B, Nagelschmitz J, Rolinck-Werninghaus C, Staab D. Tolerability and pharmacokinetic properties of ciprofloxacin dry powder for inhalation in patients with cystic fibrosis: a phase I, randomized, dose-escalation study. Clin Ther. 2013;35:1571–81. doi: 10.1016/j.clinthera.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 6.Ruge CA, Kirch J, Lehr CM. Pulmonary drug delivery: from generating aerosols to overcoming biological barriers—therapeutic possibilities and technological challenges. Lancet Respir Med. 2013;1:402–13. doi: 10.1016/S2213-2600(13)70072-9. [DOI] [PubMed] [Google Scholar]
- 7.Bailey MM, Berkland CJ. Nanoparticle formulations in pulmonary drug delivery. Med Res Rev. 2009;29:196–212. doi: 10.1002/med.20140. [DOI] [PubMed] [Google Scholar]
- 8.Houtmeyers E, Gosselink R, Gayan-Ramirez G, Decramer M. Regulation of mucociliary clearance in health and disease. Eur Respir J. 1999;13:1177–88. doi: 10.1034/j.1399-3003.1999.13e39.x. [DOI] [PubMed] [Google Scholar]
- 9.El-Sherbiny IM, McGill S, Smyth HD. Swellable microparticles as carriers for sustained pulmonary drug delivery. J Pharm Sci. 2010;99:2343–56. doi: 10.1002/jps.22003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wanakule P, Liu GW, Fleury AT, Roy K. Nano-inside-micro: disease-responsive microgels with encapsulated nanoparticles for intracellular drug delivery to the deep lung. J Control Release. 2012;162:429–37. doi: 10.1016/j.jconrel.2012.07.026. [DOI] [PubMed] [Google Scholar]
- 11.Du J, Du P, Smyth HD. Hydrogels for controlled pulmonary delivery. Ther Deliv. 2013;4:1293–305. doi: 10.4155/tde.13.90. [DOI] [PubMed] [Google Scholar]
- 12.Dellamary LA, Tarara TE, Smith DJ, Woelk CH, Adractas A, Costello ML, et al. Hollow porous particles in metered dose inhalers. Pharm Res. 2000;17:168–74. doi: 10.1023/A:1007513213292. [DOI] [PubMed] [Google Scholar]
- 13.Mansour HM, Rhee YS, Wu X. Nanomedicine in pulmonary delivery. Int J Nanomedicine. 2009;4:299–319. doi: 10.2147/IJN.S4937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rytting E, Nguyen J, Wang X, Kissel T. Biodegradable polymeric nanocarriers for pulmonary drug delivery. Expert Opin Drug Deliv. 2008;5:629–39. doi: 10.1517/17425247.5.6.629. [DOI] [PubMed] [Google Scholar]
- 15.El-Sherbiny IM, Smyth HD. Biodegradable nano-micro carrier systems for sustained pulmonary drug delivery: (I) self-assembled nanoparticles encapsulated in respirable/swellable semi-IPN microspheres. Int J Pharm. 2010;395:132–41. doi: 10.1016/j.ijpharm.2010.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Byron PR. Prediction of drug residence times in regions of the human respiratory tract following aerosol inhalation. J Pharm Sci. 1986;75:433–8. doi: 10.1002/jps.2600750502. [DOI] [PubMed] [Google Scholar]
- 17.Patton JS, Byron PR. Inhaling medicines: delivering drugs to the body through the lungs. Nat Rev Drug Discov. 2007;6:67–74. doi: 10.1038/nrd2153. [DOI] [PubMed] [Google Scholar]
- 18.Tsapis N, Bennett D, Jackson B, Weitz DA, Edwards DA. Trojan particles: large porous carriers of nanoparticles for drug delivery. Proc Natl Acad Sci U S A. 2002;99:12001–5. doi: 10.1073/pnas.182233999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Selvam P, El-Sherbiny IM, Smyth HD. Swellable hydrogel particles for controlled release pulmonary administration using propellant-driven metered dose inhalers. J Aerosol Med Pulm Drug Deliv. 2011;24:25–34. doi: 10.1089/jamp.2010.0830. [DOI] [PubMed] [Google Scholar]
- 20.El-Sherbiny IM, Smyth HD. Poly (ethylene glycol)–carboxymethyl chitosan-based pH-responsive hydrogels: photo-induced synthesis, characterization, swelling, and in vitro evaluation as potential drug carriers. Carbohydr Res. 2010;345:2004–12. doi: 10.1016/j.carres.2010.07.026. [DOI] [PubMed] [Google Scholar]
- 21.El-Sherbiny IM, Smyth HD. Controlled release pulmonary administration of curcumin using swellable biocompatible microparticles. Mol Pharm. 2011;9:269–80. doi: 10.1021/mp200351y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim DH, Martin DC. Sustained release of dexamethasone from hydrophilic matrices using PLGA nanoparticles for neural drug delivery. Biomaterials. 2006;27:3031–7. doi: 10.1016/j.biomaterials.2005.12.021. [DOI] [PubMed] [Google Scholar]
- 23.Lee KY, Mooney DJ. Alginate: properties and biomedical applications. Prog Polym Sci. 2012;37:106–26. doi: 10.1016/j.progpolymsci.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gåserød O, Smidsrød O, Skjåk-Bræk G. Microcapsules of alginate-chitosan—I: a quantitative study of the interaction between alginate and chitosan. Biomaterials. 1998;19:1815–25. doi: 10.1016/S0142-9612(98)00073-8. [DOI] [PubMed] [Google Scholar]
- 25.Sechriest VF, Miao YJ, Niyibizi C, Westerhausen-Larson A, Matthew HW, Evans CH, et al. GAG-augmented polysaccharide hydrogel: a novel biocompatible and biodegradable material to support chondrogenesis. J Biomed Mater Res. 2000;49:534–41. doi: 10.1002/(SICI)1097-4636(20000315)49:4<534::AID-JBM12>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 26.Bhattarai N, Gunn J, Zhang M. Chitosan-based hydrogels for controlled, localized drug delivery. Adv Drug Deliv Rev. 2010;62:83–99. doi: 10.1016/j.addr.2009.07.019. [DOI] [PubMed] [Google Scholar]
- 27.Augst AD, Kong HJ, Mooney DJ. Alginate hydrogels as biomaterials. Macromol Biosci. 2006;6:623–33. doi: 10.1002/mabi.200600069. [DOI] [PubMed] [Google Scholar]
- 28.Sikorski P, Mo F, Skjåk-Bræk G, Stokke BT. Evidence for egg-box-compatible interactions in calcium-alginate gels from fiber X-ray diffraction. Biomacromolecules. 2007;8:2098–103. doi: 10.1021/bm0701503. [DOI] [PubMed] [Google Scholar]
- 29.Chan G, Mooney DJ. Ca2+ released from calcium alginate gels can promote inflammatory responses in vitro and in vivo. Acta Biomater. 2013;9:9281–91. doi: 10.1016/j.actbio.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Orive G, Ponce S, Hernández RM, Gascón AR, Igartua M, Pedraz JL. Biocompatibility of microcapsules for cell immobilization elaborated with different type of alginates. Biomaterials. 2002;23:3825–31. doi: 10.1016/S0142-9612(02)00118-7. [DOI] [PubMed] [Google Scholar]
- 31.Soon-Shiong P, Otterlie M, Skjak-Braek G, Smidsrod O, Heintz R, Lanza RP, et al. An immunologic basis for the fibrotic reaction to implanted microcapsules. Transplant Proc. 1991;23:758–9. [PubMed] [Google Scholar]
- 32.Clayton HA, London NJ, Colloby PS, Bell PR, James RF. The effect of capsule composition on the biocompatibility of alginate-poly-1-lysine capsules. J Microencapsul. 1991;8:221–33. doi: 10.3109/02652049109071490. [DOI] [PubMed] [Google Scholar]
- 33.Otterlei M, Østgaard K, Skjåk-Bræk G, Smidsrød O, Soon-Shiong P, Espevik T. Induction of cytokine production from human monocytes stimulated with alginate. J Immunother. 1991;10:286–91. doi: 10.1097/00002371-199108000-00007. [DOI] [PubMed] [Google Scholar]
- 34.Zimmermann U, Klöck G, Federlin K, Hannig K, Kowalski M, Bretzel RG, et al. Production of mitogen‐contamination free alginates with variable ratios of mannuronic acid to guluronic acid by free flow electrophoresis. Electrophoresis. 1992;13:269–74. doi: 10.1002/elps.1150130156. [DOI] [PubMed] [Google Scholar]
- 35.Lee J, Lee KY. Local and sustained vascular endothelial growth factor delivery for angiogenesis using an injectable system. Pharm Res. 2009;26:1739–44. doi: 10.1007/s11095-009-9884-4. [DOI] [PubMed] [Google Scholar]
- 36.Edwards DA, Hanes J, Caponetti G, Hrkach J, Ben-Jebria A, Eskew ML, et al. Large porous particles for pulmonary drug delivery. Science. 1997;276:1868–71. doi: 10.1126/science.276.5320.1868. [DOI] [PubMed] [Google Scholar]
- 37.Lin CE, Deng Y, Jr, Liao WS, Sun SW, Lin WY, Chen CC. Electrophoretic behavior and pKa determination of quinolones with a piperazinyl substituent by capillary zone electrophoresis. J Chromatogr A. 2004;1051:283–90. doi: 10.1016/S0021-9673(04)01422-0. [DOI] [PubMed] [Google Scholar]
- 38.Smyth HD, Hickey AJ. Carriers in drug powder delivery. Am J Drug Deliv. 2005;3:117–32. doi: 10.2165/00137696-200503020-00004. [DOI] [Google Scholar]
- 39.Sung JC, Padilla DJ, Garcia-Contreras L, Verberkmoes JL, Durbin D, Peloquin CA, et al. Formulation and pharmacokinetics of self-assembled rifampicin nanoparticle systems for pulmonary delivery. Pharm Res. 2009;26:1847–55. doi: 10.1007/s11095-009-9894-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang YB, Watts AB, Peters JI, Liu S, Batra A, Williams RO., III In vitro and in vivo performance of dry powder inhalation formulations: comparison of particles prepared by thin film freezing and micronization. AAPS PharmSciTech. 2014 doi: 10.1208/s12249-014-0126-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang J, Wu L, Chan HK, Watanabe W. Formation, characterization, and fate of inhaled drug nanoparticles. Adv Drug Deliv Rev. 2011;63:441–55. doi: 10.1016/j.addr.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 42.Olsson B, Bondesson E, Borgstrom L, et al. Pulmonary drug metabolism, clearance, and absorption. In: Smyth HD, Hickey AJ, et al., editors. Controlled pulmonary drug delivery. New York: Springer; 2011. pp. 21–50. [Google Scholar]


