Abstract
The objective of the present study was to develop fast dissolving oral film of the antipsychotic drug, flupentixol dihydrochloride, to enhance its bioavailability, optimize its therapeutic effect when used to treat depression with anxiety, and increase the convenience and compliance by the mentally ill, developmentally disable, elderly, and pediatric patients. Six formulae were prepared with different concentrations of water-soluble polymers vis. hydroxypropyl methylcellulose (HPMC E5) and carboxymethyl cellulose (CMC) by solvent casting technique. The prepared films were subjected to characterization for folding endurance, weight variations, thickness, disintegration time, drug release pattern, and drug content. Physical compatibility between the drug and excipients was guaranteed in the selected formulation (2% HPMC) by means of differential scanning calorimetry analysis and Fourier-transform infrared spectroscopy. This formulation revealed high stability after testing according to the International Conference on Harmonisation guidelines. In vivo studies based on single phase parallel design were carried out for the optimized formulation in healthy human volunteers. The concentration of flupentixol dihydrochloride in plasma samples was analyzed by a developed validated LC-MS/MS assay method and the pharmacokinetic parameters of the established formulation were compared with the commercially available oral tablets. Faster rate of absorption of flupentixol could be obtained from the oral film formulation and the relative bioavailability was found to be 151.06% compared to the marketed product.
KEY WORDS: fast dissolving, flupentixol dihydrochloride, LC-MS/MS analysis, oral film, pharmacokinetics
INTRODUCTION
The field of fast disintegrating or dissolving oral dosage forms has become a rapidly growing area in the pharmaceutical industry. Fast dissolving oral delivery systems are solid dosage forms, which dissolve within 1 min when placed in the mouth without drinking or chewing (1).
Fast dissolving oral films are the most advanced form of oral solid dosage forms. Mouth dissolving films consist of a very thin oral strip, which is simply placed on the patient’s tongue or any oral mucosal tissue and instantly wet by saliva. The film rapidly hydrates and adheres onto the site of application. It then rapidly disintegrates and dissolves to release the medication for oromucosal absorption or will maintain the quick-dissolving aspects which allow for gastrointestinal absorption to be achieved when swallowed (2).
Fast dissolving films offer fast, accurate dosing in a safe, efficacious format that is convenient and portable, without the need for water or measuring devices (3). Oral thin films (OTFs) are typically the size of a postage stamp and disintegrate on a patient’s tongue in a matter of seconds for the rapid release of one or more active pharmaceutical ingredients (APIs) (4).
One or combination of the following process can be used to manufacture the mouth dissolving films: solvent casting, semisolid casting, hot melt extrusion, solid dispersion extrusion, and rolling (5). A variety of polymers are available for preparation of oral film. The polymers can be used alone or in combination to obtain the desired strip properties. The film obtained should be tough enough so that there is no any damage while handling or during transportation. The robustness of the strip depends on the type and amount of the polymer in the formulation (6).
Flupentixol dihydrochloride is chemically (Z)-4-[3-[2-(trifluoromethyl)-9H-thioxanthen-9-ylidene]propyl]-1-piperazineethanol dihydrochloride. It is an antipsychotic medication used to treat schizophrenia and or depression in dose range of 0.5–3 mg/tablet. A higher dose is given to schizophrenic patient while lower dose for depressed patients. This medication is different than other ones used for treatment of depression in that the patient will feel the effects of it in 2 to 3 days. Absorption of flupenthixol is fairly slow and incomplete, with a bioavailability in humans of about 55% (7). It is probable that flupenthixol undergoes first-pass metabolism, either in the gut wall or the liver (8). The time to reach peak concentrations for commercially available tablet formulations ranged from 3 to 8 h and the elimination half-life (t½ β) is about 35 h (9). This work investigated the possibility of developing flupentixol dihydrochloride fast dissolving film allowing fast and reproducible drug dissolution in the oral cavity; thus, pre-gastric absorption can avoid the first-pass metabolism and support its indication for the management of depression with anxiety (10).
MATERIALS AND METHODS
Flupentixol dihydrochloride was obtained as a gift sample from Delta Pharma Company, Cairo (Egypt). Hydroxypropyl methylcellulose (HPMC E5) and carboxymethyl cellulose (CMC) were from Dow Wolff Cellulosics, Bomlitz (Germany). Acetonitrile (HPLC grade) was from Sigma-Aldrich Chemical Co. (USA). Ammonium acetate, disodium hydrogen phosphate, and potassium dihydrogen phosphate were of analytical grade. Aspartame was from Parchem-fine & specialty chemicals (USA) and mannitol from Roquette (France). Glycerol was obtained as a gift from Cid Company (Cairo, Egypt). All other chemicals or solvents were of analytical grade.
Preparation of Fast Dissolving Films
The two film-forming polymers HPMC E5 and CMC were employed in concentrations of 1, 2, and 4% w/v to prepare fast dissolving films (FDF) and glycerol was used as plasticizer in the range of 6–10% v/v. Aspartame and mannitol were used as sweetening agents to mask the bitter taste of the drug.
Oral fast dissolving films were prepared by the solvent casting method. Aqueous solution was prepared by dissolving film-forming polymer and plasticizer in specific proportion in distilled water maintained at 70°C and was stirred for 3 h with a magnetic stirrer. The obtained dispersion was then cooled down to 40°C. Flupentixol, mannitol, and aspartame were then added in the required quantity as given in Table I. The dispersion was stirred for 1 h and cooled down to room temperature. The dispersion was used after at least 24 h of rest to remove all the air bubbles entrapped.
Table I.
Composition of Fast Dissolving Films
| Formulation | F1 | F2 | F3 | F4 | F5 | F6 |
|---|---|---|---|---|---|---|
| Flupentixol dihydrochloride, g | 0.03 | 0.03 | 0.03 | 0.03 | 0.03 | 0.03 |
| HPMC E5, g | 1 | 2 | 4 | – | – | – |
| CMC, g | – | – | – | 1 | 2 | 4 |
| Mannitol, g | 0.04 | 0.04 | 0.04 | 0.04 | 0.04 | 0.04 |
| Aspartame, g | 0.04 | 0.04 | 0.04 | 0.04 | 0.04 | 0.04 |
| Glycerol, mL | 10 | 8 | 6 | 10 | 8 | 6 |
| Water, mL to | 100 | 100 | 100 | 100 | 100 | 100 |
Ten milliliters of the solution was cast onto each glass Petri dish (5.0 cm, internal diameter) and then kept for drying at 75°C for first 30 min and at 45°C for the next 24 h. The resultant film was cut into the dimension of 2.5 × 2.5 cm2 in size in which 1 mg of flupentixol dihydrochloride was included (11).
Films with air bubbles, cuts, or imperfections were excluded from the study. Selected films were subjected for different evaluation parameters as described by Choudhary et al. (12).
Evaluation Parameters of Fast Dissolving Film
Film Forming Capacity
It is the ability of film formers to form desired films. It is categorized according to strip forming capacity such as very poor, poor, average, good, very good, and excellent (13).
Appearance of Films
Appearances of films were evaluated by visual observation such as transparent or opaque (14).
Weight Variation
All films were evaluated for their weight variation. Weight variation was evaluated by using electronic balance (Shimadzu corp., Japan, type AX200).
Folding Endurance
The folding endurance of the films was determined by repeatedly folding one film at the same place till it broke or folded up to 300 times, which is considered satisfactory to reveal good film properties. This test was done on all the films for five times (15).
Surface pH
The films were allowed to swell in closed Petri dish at room temperature for 30 min in 1 mL of distilled water. Solution was placed under digital pH meter, to determine the surface pH (16).
Thickness Uniformity of the Films
The thickness of each film was measured using vernier caliper (thickness tester) at different positions of the film and the average was calculated (1).
Drug Content
Film (size of 2 × 2 cm2) was taken from different areas of the film and placed in a 10-mL volumetric flask; 10 mL of phosphate buffer pH 6.8 was added and kept aside till the film dissolves completely; from this solution, 1 mL was pipetted out and diluted to 10 mL with phosphate buffer pH 6.8. The solution was analyzed by UV–Visible spectrophotometer at 229 nm (17).
In Vitro Disintegration Time
Disintegration time provides an indication about the disintegration characteristics of the film. The required size of the film (2.5 × 2.5 cm2) was put in 900 mL phosphate buffer pH 6.8 at 37 ± 0.5°C. Time taken by film to break and dissolve was measured as in vitro disintegration time. All studies were performed in triplicate for each batch (18).
In Vitro Dissolution Studies
In vitro dissolution study for all the formulations was performed for 5 min in USP basket apparatus. The dissolution medium consisted of 900 mL of phosphate buffer pH 6.8 was kept at 37 ± 0.5°C and baskets were rotated at 100 rpm. The samples (5 mL) were withdrawn every 1 min and the same volume of fresh medium was replenished. Samples were then analyzed for the drug content by a validated HPLC method using UV–Vis detector at 229 nm (19) (Agilent 1100 series, 20 μL loop; manual injector; a UV–visible wavelength detector; column (Zorbax XDB-C18 column (4.6 mm ID × 150 mm and 5.0 μm particle diameter), USA), using acetonitrile/ammonium acetate (60:40) at pH 3 as mobile phase delivered at flow rate of 1 mL/min.
Differential Scanning Calorimetry
Differential scanning calorimetry (DSC) experiments were performed with differential scanning calorimeter (model TA-50 WSI, Shimadzu, Japan) calibrated with indium. The DSC thermograms of pure flupentixol dihydrochloride, HPMC E5, and the drug-loaded film were recorded. The samples were heated from 20 to 300°C at a heating rate of 10°C/min in an inert nitrogen atmosphere.
Fourier Transform Infrared Spectroscopy Studies
Various drug–polymers interactions have been investigated mainly using Fourier-transform infrared spectroscopy (FTIR) (FTIR 1615; PerkinElmer, USA). IR spectra of pure flupentixol dihydrochloride, HPMC E5, and their physical mixture were recorded in the range 400–4,000 cm−1 at a resolution of 2 cm−1 using a KBr background.
Accelerated Stability Studies of the Optimized Formulation (F2)
The stability studies were carried out as per ICH Q1A (R2) guidelines (20) for the optimized formulation (F2). The studies were carried out at 40 ± 2°C and 75 ± 5% relative humidity (RH) where films were wrapped in aluminum package to protect from light (21) and placed in a stability chamber for 3 months. The effects of temperature and humidity on the physical characteristics of the film after the period of 3 months were evaluated. The studied parameters were disintegration time, weight variation, folding endurance, surface pH, drug content, and dissolution rate. A validated stability indicating HPLC assay was carried out using acetonitrile/ammonium acetate (60:40) at pH 3 as mobile phase delivered at 1 mL/min using C18 Hi-Q Sil column (4.6 × 250 mm, 5 μm packing diameter). The injected volume was 20 μL and detected by UV detector at 229 nm.
In vivo Pharmacokinetic Study
Study Design and Subjects
The study was carried out to compare the pharmacokinetics of flupentixol from the prepared film formulation (F2) to the commercially available conventional tablet formulation following administration of single doses of 1 mg each, using a blind, two-treatment parallel design. The study was based on one single phase parallel design because flupentixol has long terminal half-life.
Six healthy male volunteers participated in this study after signing informed written consent and they were fully informed with all the data concerning the drug. The volunteers were randomly assigned to two groups of equal size. Their ages ranged from 23 to 34 years and body weight from 60 to 75 kg. The study was approved by the Faculty of Pharmacy, Cairo University Ethics Committee, and the protocol complies with the declarations of Helsinki (22) for humans. All subjects were instructed to abstain from taking medicines and smoking for 1 week before the beginning of the studies to the end of the test. All subjects fasted for at least 10 h before the study day.
Three volunteers received the film formulation (F2) (1 mg) (a specimen of the film was placed in the buccal cavity by the volunteer, directly on the tongue) and the other three volunteers received the conventional commercial tablet (half tablet equivalent to 1 mg was swallowed with 200 mL of water). Food and drink (other than water, which was allowed after 2 h) were not allowed until 4 h after dosing and then a standard breakfast, lunch, and dinner were given to all volunteers according to a time schedule.
Each group was supervised by a physician who was also responsible for their safety and collection of samples during the trial. Adverse events were reported and evaluated. Venous blood samples (5 mL) were collected into small stoppered heparinized tubes at the following time intervals: 0 (pre-dose), 0.5, 1, 1.5, 2, 4, 6, 8, 24, 48, and 72 h.
Plasma was obtained by centrifugation at 5,000 rpm for 10 min (Hettich EBA 21 Centrifuge, Germany). The collected plasma was transferred by pipetting into 5 mL pre-labeled polypropylene screw cap tubes and was stored immediately at −80°C till drug analysis.
Assay of Flupentixol in Human Plasma
Chromatographic Conditions
An Agilent 1100 series (Wilmington, DE, USA) consisting of 20 μL loop, vacuum degasser, a binary pump, and auto-injector was used for sample separation and delivery. Chromatography was carried out using SunFire column C18 (3.5 μm 4.6 × 50 mm) with a flow rate of 1.0 mL/min. The pump is LC-20AD, Shimadzu, Japan. The auto sampler is SIL-20A, Shimadzu, Japan, and the degasser is DGU-20AS, Shimadzu, Japan. The mobile phase consists of 70:30 acetonitrile to deionized water and 0.1% formic acid.
Mass Spectrometric Conditions
The analysis was carried out utilizing API 3200 QTRAP® LC-MS/MS (Applied Biosystems, Canada) equipped with nitrogen generator (Model NM30LA, PEAK Scientific Instruments, Australia).
The mass spectrometer was operated in a positive ion multiple reaction monitoring (MRM) mode. The ion source parameters were set as follows: collision gas = 6 psi, curtain gas = 40 psi, ion spray voltage = 5,500 V, temperature = 400°C. The declustering potential, collision energy, and other compound parameters for flupentixol and IS were optimized individually.
Sample Preparation
For extraction procedure, 6 mL of tertiary butyl methyl ether and 100 μL of torsemide as internal standard were added to 1 mL of plasma. The mixture was vortexed for 30 s and then centrifuged for 10 min at 5,000 rpm then the organic layer was separated and evaporated to dryness at 45°C and finally reconstituted with 250 μL of mobile phase. Sample (0.05 mL) was injected to LC-MS/MS.
Method Validation
Analytical method validation was performed in accordance to the recommendations published by the FDA (23).
Specificity was ascertained by analyzing six blank human plasma samples to determine the interference with the drug. Matrix effects were evaluated by comparing the peak areas of the drug in extracted samples of blank plasma from six different drug-free volunteers spiked with known concentrations with the corresponding peak areas obtained by direct injection of standard solutions. Matrix effects for the IS were also investigated.
The calibration curve was constructed. The lowest standard on the calibration curve should be accepted as the limit of quantification if the analyte response at the lowest limit of quantitation at least five times the response compared to blank response. The drug peak (response) should be identifiable, discrete, and reproducible with a precision of 20% and accuracy of 80–120%. The limit of detection (LOD) was the concentration with signal-to-noise ratio of 3.
Pharmacokinetic and Statistical Analysis
Pharmacokinetic analysis was performed by means of a non-compartmental approach using WinNonlin® 5.3 software. The maximum drug concentration (Cmax, ng/mL) and the time to reach Cmax (Tmax, h) were obtained from the individual plasma concentration–time curves. The area under the curve from zero to infinity (AUC0–∞, ng.h/mL) was calculated by computing AUC0–72 using the trapezoidal rule method and adding the value where, Cp* is the last measured concentration and k is the elimination rate constant.
Results are expressed as mean values of three volunteers. A one-way analysis of variance (ANOVA) was performed for the transformed data derived from the pharmacokinetic parameters; Cmax, Tmax, and AUC0–∞ using WinNonlin® 5.3 software, in order to investigate the statistical significance between the two formulations. P value <0.05 was considered statistically significant.
The extent of absorption from the film formulation (F2) relative to the marketed one (conventional oral tablets) was calculated as the relative bioavailability by using the formula given below:
RESULTS AND DISCUSSION
Preparation and Evaluation of Flupentixol Fast Dissolving Films
Cellulose derivatives are known for their good film-forming properties and have an excellent acceptability (24). Cellulose derivatives namely HPMC E5 and CMC were used as main film formers. Various trials were done to formulate the FDF. Films of HPMC E5 and CMC were transparent in appearance with excellent film forming capacity. Films which were prepared at 1% w/v concentration for both polymers were very thin, brittle, and easily broken which causes problems during manufacturing, storage, and transportation.
Films with 2 and 4% w/v concentration for both polymers were clear, transparent, and of acceptable mechanical properties.
Glycerol was used as plasticizer in the prepared oral films due to its small molecular weight which allows it to be more readily inserted between the polymer chains and consequently exert more influence on the mechanical properties than the larger molecules (25).
All the strips were found to contain an almost uniform quantity of the drug, as per content uniformity studies indicating reproducibility of the technique. Drug content in the films was evaluated and the values were ranged from 97.0 ± 1.75% to 102.7 ± 0.5 for the different formulations. In this study, film thickness was measured by using vernier calipers. As all the formulations contained different amounts of polymer, hence the thickness was varied in the range of 0.08 ± 0.05 to 0.27 ± 0.22 mm. The weight of the different formulations was ranged from 7.3 ± 1.50 to 49.0 ± 0.90 mg.
Folding endurance measures the ability of the film to withstand rupture. The higher the folding endurance, the lower will be chances of the film to rupture easily. Folding endurance was measured manually; it ranged from 130 ± 0.1 to 350 ± 0.9. The folding endurance was increased as the concentration of the polymer increased. In vitro disintegration study showed that some batches of fast dissolving films disintegrate in less than 30 s. In vitro disintegration time was found to increase with increase in the amount of polymer used in the formulations. F1 formulation provided minimum disintegration time (15 ± 0.05 s) compared to other preparations. Disintegration time ranged from 15 ± 0.05 to 120.0 ± 0.82 s. The surface pH of the films ranged from 5.7 ± 0.2 and 6.9 ± 0.1. Surface pH of films was found to be around neutral pH, indicating that there will not be any kind of irritation to the mucosal lining of the oral cavity (26). All results are shown in Table II.
Table II.
Evaluation Parameters of Flupentixol Fast Dissolving Films
| Formulation | Film forming capacity | Appearance | Weight (mg) | Thickness (mm) | Surface pH | Disintegration time (s) | Drug content (%) | Folding endurance |
|---|---|---|---|---|---|---|---|---|
| F1 | Excellent | Transparent | 7.3 ± 1.50 | 0.08 ± 0.05 | 5.9 ± 0.1 | 15.0 ± 0.05 | 99.3 ± 1.09 | 130 ± 0.1 |
| F2 | Excellent | Transparent | 8.4 ± 0.01 | 0.11 ± 0.14 | 5.7 ± 0.2 | 24.0 ± 1.25 | 102.7 ± 0.50 | 170 ± 1.0 |
| F3 | Excellent | Transparent | 9.0 ± 0.11 | 0.23 ± 0.02 | 6.9 ± 0.1 | 40.3 ± 2.21 | 101.0. ± 1.63 | 220 ± 1.5 |
| F4 | Good | Transparent | 16.0 ± 0.25 | 0.09 ± 0.11 | 6 ± 0.1 | 50.3 ± 0.56 | 99.3 ± 1.11 | 190 ± 2.6 |
| F5 | Good | Transparent | 25.0 ± 0.55 | 0.18 ± 0.06 | 6.8 ± 0.1 | 66.0 ± 0.91 | 98.0 ± 1.13 | 270 ± 0.7 |
| F6 | Good | Transparent | 49.0 ± 0.90 | 0.27 ± 0.22 | 6.7 ± 0.2 | 120.0 ± 0.82 | 97.0 ± 1.75 | 350 ± 0.9 |
Results are expressed in terms of mean ± SD (n = 3)
In Vitro Release of Flupentixol Dihydrochloride from Fast Dissolving Films
The in vitro drug release profile from the films of formulae F1 to F3 compared to marketed product in phosphate buffer pH 6.8 was shown in Fig. 1. The in vitro drug release profile from the films of formulae F4 to F6 compared to marketed product in phosphate buffer pH 6.8 was shown in Fig. 2. Drug release rate was decreased by increasing the amount of HPMC E5 and CMC in the formulation. After 5 min, more than 95% of drug was released from batches F1, F2, F3, and F4, while 85% was released from F5 and 60% was released from F6. These results indicated that the components of HPMC E5 films promote the dissolution rate of flupentixol than from CMC films and this may be attributed to the lower viscosity of HPMC (4–6 cps) than that of CMC (25–50 cps), regarding that about 6.9% was released from the marketed product during the first 5 min of dissolution.
Fig. 1.
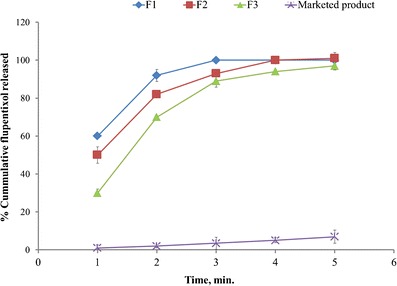
In vitro release of flupentixol dihydrochloride from fast dissolving films of HPMC E5 compared to marketed product
Fig. 2.
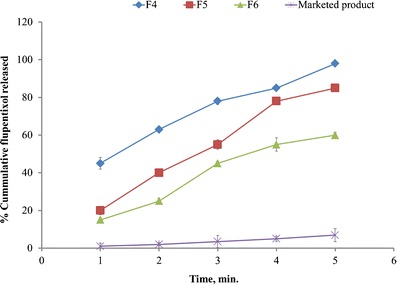
In vitro release of flupentixol dihydrochloride from fast dissolving films of CMC compared to marketed product
Differential Scanning Calorimetry
Differential scanning calorimeter allows the fast evaluation of possible incompatibilities because it shows changes in the appearance, shift of melting endotherms, exotherms, and/or variations in the corresponding enthalpies of reaction.
DSC thermograms were obtained for pure flupentixol, HPMC E5, and the drug-loaded film. The pure powdered flupentixol showed a melting endotherm at 236.30°C. The DSC thermogram of the film showed a melting peak of the drug at 230.21°C. There was no significant change in the melting point of drug when prepared in the form of film. The evaluation of the thermograms obtained from DSC revealed no interaction between the drug and the excipients in the film, including HPMC. The results of DSC study are given in Fig. 3.
Fig. 3.
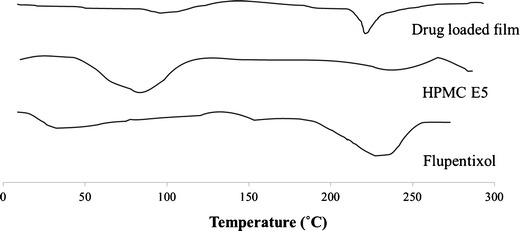
Differential scanning calorimetry thermograms of flupentixol, HPMC E5, and drug-loaded film
Fourier Transform Infrared Spectroscopy Studies
The main absorbance band of HPMC is at 1,045 cm−1 (C–O stretch vibration), but several smaller peaks are found between 1,200 and 1,500 cm−1. Flupentixol dihydrochloride exhibited its major bands at 1,119, 1,320, 1,160, 1,081, 1,253, 1,287, 2,430, 2,978, and 3,309 cm−1. The charts of drug–HPMC physical mixture revealed that the drug was compatible with HPMC as shown in Fig. 4.
Fig. 4.
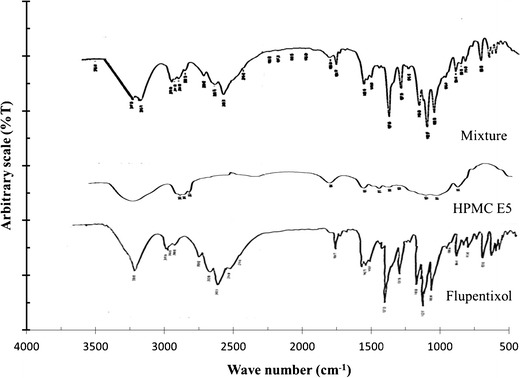
FTIR spectra of flupentixol, HPMC E5, and physical mixture of flupentixol dihydrochloride and HPMC
Stability of Flupentixol Films
The stability study for fast dissolving film was carried out according to the ICH guidelines at 40 ± 2°C and 75 ± 5% RH for 3 months by storing the aluminum-packed samples in a stability chamber. No appreciable change in physical characteristics, weight, thickness, surface pH, disintegration time, and drug content was observed after the evaluation for 3 months. The results are presented in Table III. There is also no significant change in the dissolution rate after storage for 3 months. In stability indicating HPLC assay, the excipients did not interfere with the drug retention time. These findings suggest that flupentixol films are stable at high temperatures and at the proposed shelf life.
Table III.
Accelerated Stability Studies of Oral Film Formulation (F2) After Storage at 40 ± 2°C, 75 ± 5% RH for 3 months
| Time (months) | Thickness (mm) | Weight (mg) | Folding endurance | Drug content (%) | Disintegration time (s) | Surface pH |
|---|---|---|---|---|---|---|
| Initial | 0.11 ± 0.14 | 8.4 ± 0.01 | 170 ± 1.0 | 102.7 ± 0.50 | 24.0 ± 1.25 | 5.7 ± 0.2 |
| 1 | 0.12 ± 0.17 | 8.3 ± 0.03 | 167 ± 1.1 | 99.90 ± 0.59 | 25.0 ± 0.95 | 5.6 ± 0.6 |
| 3 | 0.12 ± 0.15 | 8.0 ± 0.03 | 165 ± 1.2 | 99.95 ± 0.77 | 26.0 ± 1.34 | 5.6 ± 0.7 |
Bioanalytical Method Validation
Flupentixol is weak base; therefore, the mass spectrometer was functioned in the positive ion multiple reaction monitoring mode in the LC-MS/MS analysis. ESI spectra revealed higher signals for m/z 435.2.
The structurally related compound with similar chromatographic behaviors, mass spectrometric behaviors, and extraction characteristics, torsemide, was chosen as IS for flupentixol.
The chromatographic conditions were investigated to adjust sensitivity, speed, and peak shape. Acetonitrile was chosen as the organic solvent. It was also found that the presence of 0.1% of formic acid in the mobile phase could improve the sensitivity by supporting the ionization of the analytes. The lower limit of quantitation (LLOQ) was 0.01 ng/mL for flupentixol. The coefficient of determination (r2) during the validation (n = 6) was >0.99. The calibration range was from 0.01 to 2 ng/mL.
The mean extraction recovery of flupentixol from matrix plasma was more than 60%.
The intra-day accuracy ranged between 98.1 and 101.9% with a precision of 1.76–4.87%, the inter-day accuracy between 98.2 and 101.7% with a precision of 1.55–4.92%. A good stability of the analytes in plasma at room temperature and 4°C for 72 h was demonstrated. The plasma samples were also stable after three freeze/thaw cycles.
Pharmacokinetic Parameters of Flupentixol Dihydrochloride in Healthy Human Volunteers
The above-mentioned in vitro evaluations suggested film formula F2, for in vivo evaluation. The use of parallel designs avoids the problem of long washouts (27) for drugs having long terminal half-life like flupentixol. The plasma concentration of flupentixol dihydrochloride was plotted versus time as illustrated in Fig. 5. The peak plasma concentration (Cmax), the time to reach the maximum peak (tmax), and the extent of absorption (AUC0−∞) were calculated.
Fig. 5.
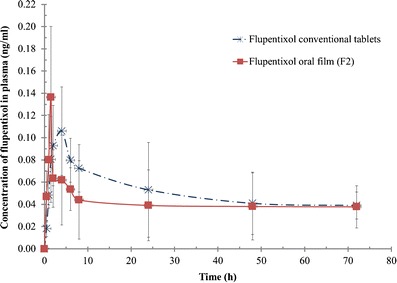
Plasma concentrations of flupentixol after oral administration of 1 mg of fast dissolving film and conventional tablet to healthy volunteers
The estimates of the mean pharmacokinetic parameters obtained by non-compartmental fitting of the concentration-time data of flupentixol dihydrochloride following oral administration of 1 mg fast dissolving film (F2) and 1 mg of the marketed product (half tablet) are presented in Table IV.
Table IV.
Mean Pharmacokinetic Parameters of Flupentixol Dihydrochloride After Oral Administration of 1 mg Fast Dissolving Film (F2) and 1 mg of the Marketed Tablets to Healthy Human Volunteers Under Fasted Conditions
| Formulation | C max (ng/mL) | T max (h) (median) | AUC0−∞ (ng.h/mL) |
|---|---|---|---|
| Oral film (F2) | 0.144 ± 0.046 | 1.5 | 11.73 ± 6.72 |
| Marketed tablets | 0.115 ± 0.016 | 4.0 | 7.76 ± 2.94 |
The mean Cmax values for F2 and the marketed product were 0.144 ± 0.046 and 0.115 ± 0.016 ng/mL, respectively. The median values of Tmax were 1.5 and 4.0 h, respectively.
Statistically significant difference (P = 0.044) between the two treatments for Tmax was attained. The oral film formulation (F2) showed higher Cmax and lesser Tmax values than those of the commercially available tablets, indicating an unambiguous difference in the rate of absorption between the two formulations.
The increase in the extent of absorption of flupentixol from the prepared oral film formulation (F2) is also distinguished from AUC0−∞ values for both treatments.
The calculated AUC0−∞ values were 11.73 ± 6.72 and 7.76 ± 2.94 ng.h/mL for F2 and marketed product correspondingly. Compared with the commercially available tablets, the relative bioavailability was found to be 151.06%. This increase in the extent of drug absorption from oral film formulation could be attributable to the fact that the buccal route of administration bypasses the gastrointestinal tract and the hepatic first pass effect (28).
CONCLUSIONS
The fast dissolving oral thin films containing flupentixol dihydrochloride were prepared for the first time. The film prepared using 2% HPMC revealed excellent uniformity and stability of flupentixol and rapidly disintegrated in water.
The in vivo evaluation in healthy human volunteers revealed that rapid and improved absorption of flupentixol could be obtained from the oral films indicated by lower values of Tmax with 1.51-fold increase in bioavailability than that obtained after administration of the marketed tablet. These findings support its indication for the management of depression with anxiety and ensure patient satisfaction and compliance.
References
- 1.Cilurzo F, Cupone IE, Minghetti P, Selmin F, Montanari L. Fast dissolving films made of maltodextrins. Eur J Pharm Biopharm. 2008;70(3):895–900. doi: 10.1016/j.ejpb.2008.06.032. [DOI] [PubMed] [Google Scholar]
- 2.Bala R, Pawar P, Khanna S, Arora S. Orally dissolving strips: a new approach to oral drug delivery system. Int J Pharm Investig. 2013;3(2):67–76. doi: 10.4103/2230-973X.114897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saha S, Tomaro-Duchesneau C, Daoud JT, Tabrizian M, Prakash S. Novel probiotic dissolvable carboxymethyl cellulose films as oral health biotherapeutics: in vitro preparation and characterization. Expert Opin Drug Deliv. 2013 doi: 10.1517/17425247.2013.799135. [DOI] [PubMed] [Google Scholar]
- 4.Legendre JY, Schnitzler I, Li QY, Hausen C, Huart M, Luengo GS, et al. Formulation, characterization, and efficacy of an adenosine-containing dissolvable film for a localized anti-wrinkle effect. J Cosmet Sci. 2007;58(2):147–155. [PubMed] [Google Scholar]
- 5.Lam JK, Xu Y, Worsley A, Wong IC. Oral transmucosal drug delivery for pediatric use. Adv Drug Deliv Rev. 2013 doi: 10.1016/j.addr.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 6.Garsuch V, Breitkreutz J. Comparative investigations on different polymers for the preparation of fast-dissolving oral films. J Pharm Pharmacol. 2010;62(4):539–545. doi: 10.1211/jpp.62.04.0018. [DOI] [PubMed] [Google Scholar]
- 7.Jorgensen A. Pharmacokinetic studies in volunteers of intravenous and oral cis (Z)-flupentixol and intramuscular cis (Z)-flupentixol decanoate in Viscoleo. Eur J Clin Pharmacol. 1980;18(4):355–360. doi: 10.1007/BF00561395. [DOI] [PubMed] [Google Scholar]
- 8.Covell NH, McEvoy JP, Schooler NR, Stroup TS, Jackson CT, Rojas IA, et al. Effectiveness of switching from long-acting injectable fluphenazine or haloperidol decanoate to long-acting injectable risperidone microspheres: an open-label, randomized controlled trial. J Clin Psychiatry. 2012;73(5):669–675. doi: 10.4088/JCP.11m07074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Product Monograph http://www.lundbeck.com/upload/ca/en/files/pdf/product_monograph/fluanxo_mkt_pm_ctrl148918_18oct2011_eng.pdf. Accessed on 18 Jan 2014.
- 10.Young JP, Hughes WC, Lader MH. A controlled comparison of flupenthixol and amitriptyline in depressed outpatients. Br Med J. 1976;1(6018):1116–1118. doi: 10.1136/bmj.1.6018.1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kunte S, Tandale P. Fast dissolving strips: a novel approach for the delivery of verapamil. J Pharm Bioallied Sci. 2010;2(4):325–328. doi: 10.4103/0975-7406.72133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Choudhary DR, Patel VA, Chhalotiya UK, Patel HV, Kundawala AJ. Development and characterization of pharmacokinetic parameters of fast-dissolving films containing levocetirizine. Sci Pharm. 2012;80(3):779–787. doi: 10.3797/scipharm.1205-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dixit RP, Puthli SP. Oral strip technology: overview and future potential. J Control Release. 2009;139(2):94–107. doi: 10.1016/j.jconrel.2009.06.014. [DOI] [PubMed] [Google Scholar]
- 14.Mashru RC, Sutariya VB, Sankalia MG, Parikh PP. Development and evaluation of fast-dissolving film of salbutamol sulphate. Drug Dev Ind Pharm. 2005;31(1):25–34. doi: 10.1081/DDC-43947. [DOI] [PubMed] [Google Scholar]
- 15.Sushma M, Prasanna YR, Sundaresan C, Vandana K, Kumar NV, Chowdary VH. Transmucosal delivery of metformin—a comprehensive study. Curr Drug Deliv. 2014;11(2):172–178. doi: 10.2174/15672018113109990045. [DOI] [PubMed] [Google Scholar]
- 16.El-Kamel AH, Ashri LY, Alsarra IA. Micromatricial metronidazole benzoate film as a local mucoadhesive delivery system for treatment of periodontal diseases. AAPS Pharm Sci Tech. 2007;8(3):E75. doi: 10.1208/pt0803075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Acharjya SK, Panda P, Mallick P, Kumar KR, Narendra A, Sravani Y, et al. Spectrophotometric methods for simultaneous estimation of flupentixol dihydrochloride and melitracen hydrochloride in combined tablet dosage form. J Chem Pharm Res. 2010;2(3):158–171. [Google Scholar]
- 18.Gohel MC, Parikh RK, Aghara PY, Nagori SA, Delvadia RR, Dabhi MR. Application of simplex lattice design and desirability function for the formulation development of mouth dissolving film of salbutamol sulphate. Curr Drug Deliv. 2009;6(5):486–494. doi: 10.2174/156720109789941696. [DOI] [PubMed] [Google Scholar]
- 19.Walter S, Bauer S, Roots I, Brockmoller J. Quantification of the antipsychotics flupentixol and haloperidol in human serum by high-performance liquid chromatography with ultraviolet detection. J Chromatogr B Biomed Sci Appl. 1998;720(1–2):231–237. doi: 10.1016/S0378-4347(98)00432-0. [DOI] [PubMed] [Google Scholar]
- 20.Website: http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Quality/Q1A_R2/Step4/Q1A_R2__Guideline.pdf. Accessed on 13 Jan 2014.
- 21.Enever RP, Li Wan Po A, Shotton E. Flupenthixol dihydrochloride decomposition in aqueous solution. J Pharm Sci. 1979;68(2):169–171. doi: 10.1002/jps.2600680212. [DOI] [PubMed] [Google Scholar]
- 22.Declaration of Helsinki as amended by the 52nd World Medical Assembly (WMA) Edinburgh, Scotland: World Medical Association 2000.
- 23.USFDA, 2001, http://www.fda.gov/downloads/Drugs/Guidances/ucm070107pdf, Accessed on 20 Jan 2014.
- 24.Patel JG, Modi AD. Formulation, optimization and evaluation of levocetirizine dihyrochloride oral thin strip. J Pharm Bioallied Sci 2012; (Suppl 1):S35-6 [DOI] [PMC free article] [PubMed]
- 25.Bourtoom T. Plasticizer effect on the properties of biodegradable blend film from rice starch-chitosan Songklanakarin. J Sci Technol. 2008;30(Suppl. 1):149–165. [Google Scholar]
- 26.Koland M, Sandeep V, Charyulu N. Fast dissolving sublingual films of ondansetron hydrochloride: effect of additives on in vitro drug release and mucosal permeation. J Young Pharm. 2010;3:216–222. doi: 10.4103/0975-1483.66790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Midha KK, Hubbard JW, Rawson MJ, McKay G, Schwede R. The roles of stereochemistry and partial areas in a parallel design study to assess the bioequivalence of two formulations of hydroxychloroquine: a drug with a very long half life. Eur J Pharm Sci. 1996;4(5):283–292. doi: 10.1016/0928-0987(96)00178-9. [DOI] [Google Scholar]
- 28.Morales JO, McConville JT. Manufacture and characterization of mucoadhesive buccal films. Eur J Pharm Biopharm. 2011;77(2):187–199. doi: 10.1016/j.ejpb.2010.11.023. [DOI] [PubMed] [Google Scholar]


