Abstract
Nanomedicine refers to biomedical and pharmaceutical applications of nanosized cargos of drugs/vaccine/DNA therapeutics including nanoparticles, nanoclusters, and nanospheres. Such particles have unique characteristics related to their size, surface, drug loading, and targeting potential. They are widely used to combat disease by controlled delivery of bioactive(s) or for diagnosis of life-threatening problems in their very early stage. The bioactive agent can be combined with a diagnostic agent in a nanodevice for theragnostic applications. However, the formulation scientist faces numerous challenges related to their development, scale-up feasibilities, regulatory aspects, and commercialization. This article reviews recent progress in the method of development of nanoparticles with a focus on polymeric and lipid nanoparticles, their scale-up techniques, and challenges in their commercialization.
KEY WORDS: lipid nanoparticles, nanomedicine, polymeric nanoparticles, scale-up production
INTRODUCTION
“Nanomedicine” is the application of purposely designed nanoconstructs for better therapeutic and diagnostic outcomes, which cannot be otherwise achieved using micro or macro-systems. Nanoconstructs may be in the form of nanocrystals, nanoparticles (polymeric or lipid or metallic), and any self-assemblage system of nanosized carrier for drug, vaccine, or genetic material (1–4). Last three decades have witnessed exponential progress in the drug delivery research using such nanoconstructs. The production of nanoparticles is a challenging task in terms of reproducibility of size and polydispersity. The method of nanoparticle production depends on many factors including intention of synthesis, i.e., application, material used for preparation, nature of bioactive to be loaded, etc. The suitable selection of materials and appropriate method of production of nanodevices is required because the in vitro and in vivo performances of the systems depend on the material characteristics as well as the production method. The preparation methods that require the use of organic solvents and the removal of residual solvents from the final product can often be tedious. The regulatory guidelines require the manufacturers to ensure the purity and safety of the final nanoparticle-based formulations. The present article provides an overview of nanoparticle production methods, scale-up issues highlighting industrial applicability, and challenges associated in their successful application in clinical nanomedicine.
BIOMEDICAL APPLICATIONS OF NANOPARTICLES
Nanotechnology has tremendously revolutionized the pharmaceutical and biomedical research. Controlled drug/gene delivery, vaccine delivery, and cell-based diagnosis are featured in the applications of nanoparticles (5,6). Polymer, lipid, polymer-lipid grafts, metallic nanoparticles, carbon nanotubes, etc. have been widely explored in the new generation “nanomedicine.” These nanosystems are synthesized in the laboratory using different techniques which vary significantly from case to case. Therefore, it becomes necessary to understand the method of production of these nanosystems and the scale-up issues thereof for the various systems designed to perform specific biomedical applications (Fig. 1). Table I summarizes notable features and limitations of various methods of nanoparticle production.
Fig. 1.
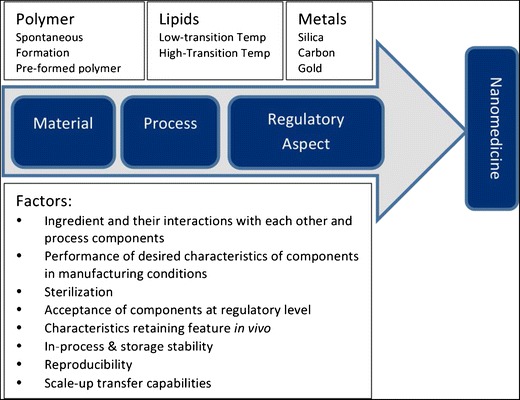
Schematic representation of material and process parameters, and regulatory aspects in the development of a nanomedicine
Table I.
Comparative Feature of Various Methods of Polymer and Lipid Nanoparticle Production
| Material | Method | Remarks | Limitations |
|---|---|---|---|
| Polymer | Nanoprecipitation | Small particle size, low cost | Difficulties in containing and controlling particle growth, applicability to lipophilic drugs only |
| Salting out | High efficiency, easy scaled up, useful for thermolabile drugs | Limitation of application to lipophilic drugs and the extensive nanoparticle washing | |
| Supercritical fluid technology (SCF) | Narrow particle size distribution, mild operating temperatures and absence of residual solvent | Poor solvent power of CO2, the cost and necessity of voluminous usage of the CO2 | |
| Extrusion | Simple, inexpensive, scaled up, solvent free, fast and continuous process, low cost | Temperature and downstream processing | |
| Microfluidizer | Precise control of the size | A relatively high number of cycles are required | |
| Ionic gelation method | Spontaneous formation of small size particle, scale-up feasibility | Size depends upon cross-linking between the polymers | |
| Lipid | High-pressure homogenization | Scale-up feasibility and no use of organic solvent | Larger particles and broader size distribution of lipid nanoparticles in case of cold process, while hot process is not suitable for thermolabile drugs Energy intensive process |
| Micro-emulsion | Scale-up feasibility, low mechanical energy input | High concentration of surfactant and co-surfactant is used, low nanoparticle concentration | |
| Solvent Emulsification evaporation | Avoidance of thermal stress, scalability, continuous process | Use of organic solvent, loss of loaded biomolecule during process | |
| Solvent emulsification-diffusion | Low particle size and very low polydispersity | Organic solvent is used | |
| Membrane contact | Simple apparatus, controllable nanoparticle size scaling-up abilities | Aqueous phase and lipid phase temperatures are controllable features | |
| Ultrasonication | For preformed microparticles, reduced shear stress | Broader particle size distribution, chances of potential metal contamination to probe particles, physical instability like particle growth upon storage | |
| Multiple-emulsion | Suitable for hydrophilic drug | Large particle size |
METHOD OF PRODUCTION OF NANOPARTICLES
Several methods are reported in the literature for the production of nanoparticles depending upon the type of material such as polymer, lipid, and metal. Figure 2 represents the summary of such methods which have been discussed in the following section.
Fig. 2.
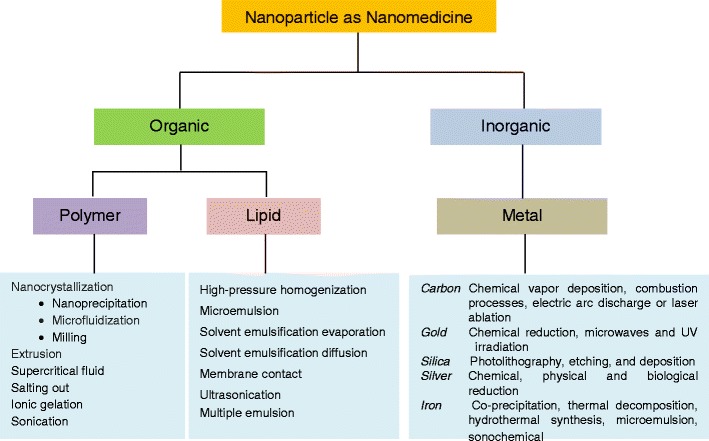
Scheme showing method of nanoparticle production as nanomedicine with various materials
Polymeric Nanoparticles
Nanocrystallization
Nanocrystallization directly converts poorly water-soluble drugs into nanoparticles with altered physical properties (7). Several formulations developed by nanocrystallization method are available in the market including aprepitant, fenofibrate, and sirolimus. The nanoparticle may be crystalline or amorphous depending upon the production technology and processing of the material. It is possible to develop nanocrystals of desired shape and size by controlling the process parameters. The drug nanocrystals are developed by three approaches, i.e., precipitation, homogenization methods (such as microfluidizer technology), and milling.
Nanoprecipitation
Nanoprecipitation is a simple technique which is generally adopted for preformed polymers. Briefly, the polymer is dissolved in a water-miscible organic solvent and the solvent is then diffused in the aqueous medium in the presence of a surfactant, leading to the instantaneous formation of a colloidal suspension (8). This is also known as solvent displacement technique. The method is useful for the preparation of nanocapsules, which is a reservoir type of nanosystem. A high drug payload can be achieved using this technique in nanocapsules; however, its usefulness is limited to water-miscible solvents (better for lipophilic drug encapsulation) and it cannot be adopted for water-immiscible solvents (inefficient to encapsulate hydrophilic drugs). The assembly and stability of drug nanoparticles depend on super saturation and precipitation. The controlling factors for nanoprecipitation method are nucleation and growth kinetics, which decide the final particle size and the size distribution (9). Similarly, the phase of the drug in nanoparticle form (i.e., crystalline or amorphous) has major impact on stability and bioavailability in vivo.
Microfluidizer Technology
Microfluidizer technology is fairly latest and one of the most emerging technologies adopted for producing nanoparticles (Microfluidizer®, Microfluidics Inc.). A frontal collision occurs between the two fluids under high pressure conditions (up to 1,700 bar) (10). During collision of the fluids, many changes take place simultaneously such as shear forces and cavitation forces (11). To stabilize developed nanoparticles, surfactants are added in the solution. In order to get the desired size of nanoparticles, several cycles (may range from 50 to even 100) are required which is the limitation of this method. Microfluidic-based liposomes preparation is recently reported in the literature (12). This technology offers a number of advantages over the more traditional methods of preparation of liposomes such as extrusion and sonication. Microfluidic hydrodynamic focusing has been used to synthesize nanoparticles and vesicles of various lipids (12–15).
Milling Method
Milling is a traditional process of nanocrystals production. NanoCrystals® technology employs generally a bead or a pearl mill in order to get drug nanoparticles. One can develop ultra-fine suspensions of drugs using a ball mill (7). The principle of size reduction is generation of shear forces of impact by the milling media, which leads to nanoparticles (16). The factors affecting size and physical stability of nanoparticles include milling media, dispersion medium, and the stabilizer. However, the chances of product contamination with erosion of the milling material, a comparatively long milling time in case of crystalline drugs and scale-up limitation, are some of the unfavorable features of this technique.
Extrusion
Extrusion is a simple method of nanoparticle production. Converting hydrophobic drugs directly into nanoparticles is a scalable and less costly method. Manual extrusion using gas-tight syringes and polycarbonate membranes is a common technique. However, it may develop heterogeneity especially when one uses pore sizes less than 100 nm. This happens due to variability of manual pressure applied each time (17). Recently, Guo et al., reported a nanoporous membrane extrusion method for nanoparticle production of hydrophobic drugs (18). This method induces precipitation of drug-loaded nanoparticles at the exits of nanopores. Recently, Khinast et al. reported a novel one-step process for converting a liquid stabilized nano-suspension into a solid formulation via hot-melt extrusion combined with an internal devolatilization process (19). In this process, the polymer is fed into the extruder and allowed to melt. Subsequently, a stable nano-suspension is added to this via side-feeding devices. The water is then removed by devolatilization and the polymer solidified at the outlet.
Supercritical Fluid Technology
One of the most promising techniques of nanoparticle production is supercritical fluid (SCF) technology. Mild temperature conditions and avoidance of organic solvent in the production of polymeric nanoparticles are clear benefits of this technique over the others (20). Pressure and temperature are simultaneously higher for an SCF than those at the critical point. SCF can be used for various purposes in the nanoparticle production as follows: [A] solvent—rapid expansion from supercritical solutions process; [B] swelling and plasticizing agent—gas saturated solutions process; [C] an antisolvent—gas antisolvent or supercritical antisolvent process or aerosol solvent extraction system process and solution-enhanced dispersion by SCF process; [D] a solvent for polymerization in dispersed media. The formulation scientist gets advantages like particle de-aggregation, improved solubility and dissolution rate, controlled release, and drug absorption enhancement with this method (21–23). In order to get desired properties of nanoparticles such as size and size distribution, morphology, inner core structure, and minimal residual solvent concentration, this method is the most suitable. Figure 3 shows a schematic presentation of SCF technology used for nanoparticle production. The remarkable feature of SCF technique is that particles with smooth surfaces, small particle size and distribution, and free flowing can be obtained. However, the poor solvent power of CO2, the cost, and use of large amount of the CO2 are few limitations as well (21).
Fig. 3.
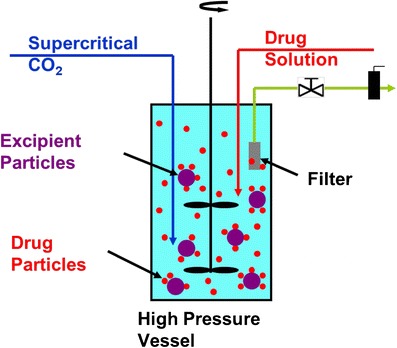
Supercritical fluid technology for producing nanoparticles in which both the drug and the polymer (example: microcrystalline cellulose) are processed together to achieve nanoparticle stability and de-aggregation
Salting Out
“Salting out” is a modified method of emulsification diffusion. Both the drug and polymer are dissolved in a water-miscible solvent, followed by emulsification into an aqueous gel having the salting-out agent and a colloidal stabilizer (24,25). Salting-out agent can be an electrolyte (magnesium chloride, calcium chloride, or magnesium acetate) or a non-electrolyte (sucrose). In addition, a colloidal stabilizer such as polyvinylpyrrolidone or hydroxyethylcellulose can be used to achieve the stability of the dispersion phase of the emulsion. A significantly large volume of aqueous medium is added to this oil/water (o/w) emulsion to enhance the diffusion of solvent into the aqueous phase, and hence as a result, nanoparticles are produced. To remove the solvent and salting-out agent, cross-flow filtration is then performed. A similar method is also adopted in the preparation of lipid nanoparticles. It is extremely important to carefully select the salting-out agent, since it can affect encapsulation efficiency of the drug. The advantages of this technique are high efficiency, easy scale-up, useful for thermolabile substances, and minimal stress to the protein encapsulated. However, this method has a limitation of its applicability mostly to lipophilic drugs and the requirement of extensive washing.
Ionic Gelation Technique
Some of the biodegradable hydrophilic polymers such as chitosan, gelatin, and sodium alginate are cross-linked to generate nanoparticles. The method involves the use of a mixture of two aqueous phases having counter ionic materials to be cross-linked. For example, chitosan, a cationic polymer, is cross-linked with a poly anion such as sodium tripolyphosphate (26). In this method, the positively charged amino group of chitosan interacts with negative charged tripolyphosphate. Nanosize coacervates are formed as a result of electrostatic interaction. This is followed by ionic gelation which involves the transition of the material from liquid to gel due to ionic interaction conditions at room temperature.
Sonication Method
Polymeric nanodispersion is created simply by dissolving the polymer in the organic phase and by nanoemulsification using an appropriate method of sonication (27). Then, the solvent is slowly removed from the polymer containing nanodroplets to generate nanoparticles.
Lipid Nanoparticles
High-Pressure Homogenization
High-pressure homogenization can be classified into two parts: hot homogenization and cold homogenization. Hot homogenization is carried out at temperature above the melting point of the lipid and is applied to lipophilic drugs (28–30). Drug-loaded lipid (in melted form) and aqueous phase are mixed by a high-shear mixer, keeping both at the same temperature. It is important to note that the final form of the nanoparticle largely depends upon quality of pre-emulsion; which is often a nanoemulsion. The dispersed phase in the nanoemulsion congeals upon cooling down to room temperature forming the lipid nanoparticles. However; this method is not suitable for the thermolabile drugs. Other limitation is that the drug distribution and loss into the aqueous phase take place in the homogenization process. An alternative approach of lipid nanoparticle production is cold homogenization. This is a type of high-pressure milling of suspension in which lipid with the drug is melted and solidified quickly to get lipid microparticles. The developed microparticles are subsequently processed for high-speed stirring in cold aqueous surfactant followed by homogenization below room temperature to convert them into nanoparticles. If we compare both the methods, it can be inferred that hot high-pressure homogenization is suitable for high lipid concentrations and produces very narrow particle size distributions (31). However, cold homogenization process yields large particles with broad size distribution. This method also limits thermal exposure to the drug and offers negligible degradation of temperature-sensitive biomolecules including drugs. Factors affecting size distribution of lipid nanoparticles in both hot and cold homogenization include temperature, type of homogenizer, pressure, and homogenization cycles.
Microemulsion
Gasco and coworkers developed lipid nanoparticles using microemulsion technique (32). Briefly, lipid nanoparticles can be obtained from hot microemulsion consisted of low melting lipids, an emulsifier, co-surfactant, and water. Further, this microemulsion is added into excess cold water under stirring which results in the precipitation of lipid phase forming nanoparticles. Subsequently, the excess water is removed by a suitable method like ultrafiltration or lyophilization. The remarkable feature of this method is that nanoparticles can be produced without energy inputs and hence is suitable for loading thermolabile drugs in lipid nanoparticles. Factors like composition, temperature gradient, and pH of the medium significantly affect the product quality. The limitations are requirement of removal of excess water from lipid nanoparticles and high concentration of surfactant and co-surfactant. The later one is highly important from regulatory prospects and should be kept in mind when designing this protocol for nanoparticle production.
Solvent Emulsification Evaporation
Lipid nanoparticle dispersions can be obtained by solvent emulsification evaporation method (33). In this method, lipid and lipophilic drugs are dissolved in organic solvent (immiscible to water). The organic phase is then emulsified in an aqueous phase resulting into o/w emulsion. Organic solvent is then removed under pressure to obtain lipid nanoparticles due to precipitation of lipid into aqueous phase. The size of these nanoparticles is controlled by the type and concentration of the lipid, surfactant, and co-surfactant in the organic phase. The amount of lipid content (up to 5% by weight) produces a particle size of about 30–100 nm range while high lipid content increases the particle size probably because of high viscosity of the dispersed phase with a decrease in the homogenization efficiency. This method totally avoids thermal stress and hence is suitable for thermolabile drugs.
Solvent Emulsification-Diffusion
This is an improved version of the “solvent emulsification evaporation” method as described earlier. The lipid nanoparticle dispersion is prepared from the emulsion containing solvents (partially water-miscible) with low toxicity (34). Similar to above-described method, drug dissolved in the organic solvent precipitates out instantly as a result of diffusion of the organic solvent. Using this approach, nanoparticles below 100 nm with low polydispersity can be produced. Use of a surfactant is a factor in optimization of desired size. For example, non-ionic surfactants produce larger particles than ionic surfactants. It is suggested that a combination of two or more surfactants better control the particle size (34). This is because such combination has a synergistic effect on the emulsion stability in terms of coalescence rate. Combination of surfactants produces mixed films at the interface with high surfactant coverage vis a vis sufficient viscosity for the desired stability.
Membrane Contact
Membrane contact method is used for large-scale production of lipid nanoparticles (35). Briefly, melted lipid is pressed through a porous membrane allowing formation of nanosized droplets. Inside the membrane module, the aqueous phase is circulated to sweep the droplet away from the pore outlets. These swiped droplets are cooled enough to get solidified. Factors like temperature of both the aqueous phase and the lipid phase, cross-flow velocity of aqueous phase, lipid phase pressure, and membrane pore size determine the particle size of lipid nanoparticles. The advantageous features of this method include the use of simple apparatus, controllable particle size by careful selection of process parameters, and scaling-up feasibilities.
Ultrasonication
Ultrasonication can be opted for the preparation of lipid nanoparticles from preformed microparticles (36). The size of nanoparticles can be controlled by varying the frequency and intensity of ultrasonication. Furthermore, a combination of high-speed stirring and ultrasonication at higher temperature can also be explored. However, the broader particle size distribution is a major limitation along with potential metal contamination arising from the sonication probe.
Multiple-Emulsion
Multiple emulsion method based on solvent emulsification-evaporation has also been introduced for the incorporation of hydrophilic drug in lipid matrix of the nanoparticles (37). However, the particle size is in the micrometer range to designate the particles as “lipospheres” in place of nanoparticles. A stabilizer is further added to encapsulate a hydrophilic drug into the internal aqueous phase. This minimizes partitioning of the drug from internal to external phase when solvent evaporates from multiple emulsion.
Metallic Nanoparticles
Metallic nanoparticles are useful for numerous drug delivery applications alone or in combination with polymers (38,39). The later one serves as thermal release triggers upon irradiation with infrared light or get excited upon applying suitable magnetic field. Silica, carbon, and gold nanoparticles are extremely important as they have been developed for drug targeting and controlled release of therapeutically active biomolecules. Porous silica or silicon dioxide is a popular form of silica to develop nanoparticles. Several methods have been adopted to produce silica nanoparticles such as photolithography, etching, and deposition techniques. These nanoparticles possess porous architectures such as calcified nanopores, platinum-containing nanopores, porous nanoparticles, and nanoneedles. The advantage of such porous structure is controlled delivery of loaded drug/biomacromolecules with near zero-order drug release kinetics. Carbon-based nanostructures have been recently investigated in drug delivery (40). Two nanostructures that have been widely explored are nanotubes and fullerenes. Both are hollow, carbon-based, and cage-like architectures. Most common carbon nanostructures are single-wall nanotubes, multiwall nanotubes, and C60 fullerenes which have common configurations. The unique characteristics of these systems such as size, geometry, and surface characteristics make them attractive carrier for drug delivery. It can be prepared by chemical vapor deposition, combustion processes, electric arc discharge, or laser ablation. Surface-functionalized carbon nanotubes have been reported for intracellular gene/DNA delivery and vaccine delivery as well. Fullerenes have also shown drug targeting potential (40). Site-specific intracellular targeting of mitochondria is reported using fullerene nanostructures. Recently, gold nanoparticles have been identified as a promising delivery system as they are inert, non-toxic, and biocompatible (4). They can be fabricated easily with a wide range of core sizes (1 to 150 nm) with low poly dispersibility index. Advantageous feature of gold nanoparticles is ease of surface functionalization and high surface area-to-volume ratio of nanoparticles, which provides dense loading of targeting ligand on surface at the low core diameter size. By proper selection of stabilizing agent, which prevents aggregation, one can get gold nanoparticles with varying particles size.
QUALITY CONTROL FOR NANOPARTICLES
The size, shape, morphology, size distribution, targetability, and functionality of developed nanoparticles are the key parameters for their effective biomedical applications. Such desired characteristics should be reproducible and scalable. A desired reproducible drug release profile from nanoparticles is required to further establish batch to batch uniformity and quality performance by in vitro in vivo correlation (IVIVC) performance. At present, very little data is available to establish such critically important parameters of quality control of nanomedicine. Even, till date, no officially established drug release method is available for their evaluation. Mostly, conventional official dissolution methods (established for solid dosage forms) such as paddle or basket method are generally used to perform the same. Dialysis method is also used in various variants (reverse dialysis, rotating dialysis, double dialysis, etc.) to estimate drug release of nanoparticles; however, these methods need validation. Since, nanomedicines are specialized nanounits which have been developed to deliver the bioactive very precisely to their biological targets; an effective way is needed to generate IVIVC for them to ensure their quality production. Despite significant advances in health science and pharmaceutical industry, selection of the method and material is affected by market demands too. One of the important aspects for scale-up production of nanoparticles is the cost of the finished product and also consumable market (41,42). For more details, readers are suggested to go through an article written by Hock et al. (43).
CHALLENGES IN SCALE-UP PRODUCTION OF NANOMEDICINE
All the methods described above can be classified in bottom-up (i.e., starting from a dissolved molecule to a precipitate) and top-down processes (i.e., starting from a macro-size drug powder to be reduced to smaller one). The later one has been adopted by pharmaceutical industry at large. However, bottom-up approach is less popular at industrial level as this approach needs removal of the traces of the remaining solvent which is a difficult process (7). There are several components associated with scale-up of a nanomedicine product from bench to the market. For examples, nature of material and its generally regarded as safe (GRAS) status, toxicological features associated with size and shape of nanoparticle (44), in vivo biodegradability of nanocarriers, and balancing of multicomponent system at large scale are a few of them. One has to be careful before selection of materials, solvent, procedure of nanoparticle development, cost, and acceptability of finished product both by clinicians and patients. During scale-up of laboratory method, sometimes the desired features of nanoparticles are lost. For example, in a study of scale up of nanoparticle prepared using emulsion method, it was observed that increase in impeller speed and agitation time, particles size was decreased although entrapment efficiency was not altered (45). Selection of nanoparticle production method is also important to save time during pilot batch production from scale-up point of view. In a comparative study of ibuprofen-loaded nanoparticles, it was found that nanoprecipitation method took less time (about 2 h) than emulsion-based method (about 3 h) for nanoparticle production (25). After optimization of therapeutic need, market demand, research and development, production steps, scale-up feasibilities, clinical trials, and regulatory issues, a nanomedicine product reaches to market. Some of the commercially available nanomedicine products for the therapeutic purposes are Doxil® (Bridgewater, USA), Daunoxome (Gilead) Abraxane® (Abraxis Bioscience), Ambisome® (Gilead), Estrasorb (Novavax) Emend (Elan), MegaceES (Elan), Tricor (Elan), and Triglide (SkyPharma). In addition, nanoparticles-based formulations currently available for in vivo imaging include Resovist (Schering), Feridex (Advanced Magnetics), and Gastromark (Advanced Magnetics). The nanomedicine products no doubt are superior in therapeutic performances than conventional drug delivery systems and hence are highly demanded. The overall market for the nanomedicine product in the year 2012 was about 12 billion dollar (46).
CONCLUSION
Numerous methods have been developed in order to produce nanoparticles of desired characteristics. Emerging methods such as membrane extrusion, supercritical fluid technology, and microfluidizer technology have scale-up capabilities, and few products of these technologies are in the market. However, application of these methods for developing targeted and surface functionalized nanoparticles at large scale is still debatable. On the basis of presently available literature, it seems promising that nanomedicine will translate many products from laboratory to market in future for various kind of therapeutics and clinical purposes.
ACKNOWLEDGMENTS
The authors are thankful to National Corn Growers Association (NCGA) for their research grant support to SP and post-doctoral fellowship to RP.
Abbreviations
- NME
Nanoporous membrane extrusion
- SCF
Supercritical fluid
REFERENCES
- 1.Hughes GA. Nanostructure-mediated drug delivery. Nanomedicine. 2005;1:22–30. doi: 10.1016/j.nano.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 2.Ladj R, Bitar A, Eissa MM, Fessi H, Mugnier Y, Le Dantec R, et al. Polymer encapsulation of inorganic nanoparticles for biomedical applications. Int J Pharm. 2013;458(1):230–41. doi: 10.1016/j.ijpharm.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 3.Tang L, Cheng J. Nonporous silica nanoparticles for nanomedicine application. Nano Today. 2013;8:290–12. doi: 10.1016/j.nantod.2013.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khan MS, Vishakante GD, Siddaramaiah H. Gold nanoparticles: a paradigm shift in biomedical applications. Adv Colloid Interface Sci. 2013;199–200:44–58. doi: 10.1016/j.cis.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 5.Brannon-Peppas L, Blanchette JO. Nanoparticle and targeted systems for cancer therapy. Adv Drug Deliv Rev. 2004;56:1649–59. doi: 10.1016/j.addr.2004.02.014. [DOI] [PubMed] [Google Scholar]
- 6.Langer R. Drug delivery and targeting. Nature. 1998;392:5–10. [PubMed] [Google Scholar]
- 7.Junghanns JAH, Müller RH. Nanocrystal technology, drug delivery and clinical applications. Int J Nanomedicine. 2008;3:295–310. doi: 10.2147/ijn.s595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Petros RA, DeSimone JM. Strategies in the design of nanoparticles for therapeutic applications. Nat Rev Drug Discov. 2010;9:615–27. doi: 10.1038/nrd2591. [DOI] [PubMed] [Google Scholar]
- 9.Merisko-Liversidge E, Liversidge GG, Cooper ER. Nanosizing: a formulation approach for poorly-water-soluble compounds. Eur J Pharm Sci. 2003;18:113–20. doi: 10.1016/S0928-0987(02)00251-8. [DOI] [PubMed] [Google Scholar]
- 10.Bruno RP, McIlwrick R. Microfluidizer processor technology for high performance particle size reduction, mixing and dispersion. Eur J Pharm Biopharm. 1999;56:29–36. [Google Scholar]
- 11.Tunick MH, Van Hecken DL, Cooke PH, et al. Transmission electron microscopy of mozzarella cheeses made from microfluidized milk. J Agric Food Chem. 2002;50:99–103. doi: 10.1021/jf010633c. [DOI] [PubMed] [Google Scholar]
- 12.Mijajlovic M, Wright D, Zivkovic V, Bi JX, Biggs MJ. Microfluidic hydrodynamic focusing based synthesis of POPC liposomes for model biological systems. Colloids Surf B: Biointerfaces. 2013;104:276–81. doi: 10.1016/j.colsurfb.2012.12.020. [DOI] [PubMed] [Google Scholar]
- 13.Hung LH, Lee AP. Microfluidic devices for the synthesis of nanoparticles and biomaterials. J of Med Biol Eng. 2007;27:1–6. [Google Scholar]
- 14.Park J, Saffari A, Kumar S, Gunther A, Kumacheva E, Clarke DR, et al. Microfluidic synthesis of polymer and inorganic particulate materials. Ann Rev Mat Res. 2010;40:415–43. doi: 10.1146/annurev-matsci-070909-104514. [DOI] [Google Scholar]
- 15.Napoli M, Atzberger P, Pennathur S. Experimental study of the separation behavior of nanoparticles in micro- and nanochannels. Microfluid Nanofluid. 2011;10:69–80. doi: 10.1007/s10404-010-0647-7. [DOI] [Google Scholar]
- 16.Xing T, Sunarso J, Yang W, Yin Y, Glushenkov AM, Li LH, et al. Ball milling: a green mechanochemical approach for synthesis of nitrogen doped carbon nanoparticles. Nanoscale. 2013;5:7970–6. doi: 10.1039/c3nr02328a. [DOI] [PubMed] [Google Scholar]
- 17.Aydin E, Planell JA, Hasirci V. Hydroxyapatite nanorod-reinforced biodegradable poly(Llactic acid) composites for bone plate applications. J Mater Sci Mater Med. 2011;22:2413–27. doi: 10.1007/s10856-011-4435-z. [DOI] [PubMed] [Google Scholar]
- 18.Guo P, Hsu TM, Zhao Y, Martin CR, Zare RN. Preparing amorphous hydrophobic drug nanoparticles by nanoporous membrane extrusion. Nanomedicine (Lond) 2013;8:333–41. doi: 10.2217/nnm.12.119. [DOI] [PubMed] [Google Scholar]
- 19.Khinast J, Baumgartner R, Roblegg E. Nano-extrusion: a one-step process for manufacturing of solid nanoparticle formulations directly from the liquid phase. AAPS PharmSciTech. 2013;14:601–4. doi: 10.1208/s12249-013-9946-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Elizondo E, Veciana J, Ventosa N. Nanostructuring molecular materials as particles and vesicles for drug delivery, using compressed and supercritical fluids. Nanomedicine (Lond) 2012;7:1391–408. doi: 10.2217/nnm.12.110. [DOI] [PubMed] [Google Scholar]
- 21.Sheth P, Sandhu H, Singhal D, Malick W, Shah N, Kislalioglu MS. Nanoparticles in the pharmaceutical industry and the use of supercritical fluid technologies for nanoparticle production. Curr Drug Deliv. 2012;9:269–84. doi: 10.2174/156720112800389052. [DOI] [PubMed] [Google Scholar]
- 22.Byrappa K, Ohara S, Adschiri T. Nanoparticles synthesis using supercritical fluid technology—towards biomedical applications. Adv Drug Deliv Rev. 2008;60:299–327. doi: 10.1016/j.addr.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 23.Sun YP, Meziani MJ, Pathak P, Qu L. Polymeric nanoparticles from rapid expansion of supercritical fluid solution. Chemistry. 2005;11:1366–73. doi: 10.1002/chem.200400422. [DOI] [PubMed] [Google Scholar]
- 24.Mendoza-Muñoz N, Quintanar-Guerrero D, Allémann E. The impact of the salting out technique on the preparation of colloidal particulate systems for pharmaceutical applications. Recent Pat Drug Deliv Formul. 2012;3:236–49. doi: 10.2174/187221112802652688. [DOI] [PubMed] [Google Scholar]
- 25.Galindo-Rodríguez SA, Puel F, Briançon S, Allémann E, Doelker E, Fessi H. Comparative scale-up of three methods for producing ibuprofen-loaded nanoparticles. Eur J Pharm Sci. 2005;25(4):357–67. doi: 10.1016/j.ejps.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 26.Fàbregas A, Miñarro M, García-Montoya E, Pérez-Lozano P, Carrillo C, Sarrate R, et al. Impact of physical parameters on particle size and reaction yield when using the ionic gelation method to obtain cationic polymeric chitosan-tripolyphosphate nanoparticles. Int J Pharm. 2013;446:199–204. doi: 10.1016/j.ijpharm.2013.02.015. [DOI] [PubMed] [Google Scholar]
- 27.Fuchs S, Winter G, Coester C. Ultrasonic resonator technology as a new quality control method evaluating gelatin nanoparticles. J Microencapsul. 2010;27:242–52. doi: 10.3109/02652040903079534. [DOI] [PubMed] [Google Scholar]
- 28.Corrias F, Lai F. New methods for lipid nanoparticles preparation. Recent Pat Drug Deliv Formul. 2011;5:201–13. doi: 10.2174/187221111797200597. [DOI] [PubMed] [Google Scholar]
- 29.Rai S, Paliwal R, Gupta PN, Khatri K, Goyal AK, Vaidya B, et al. Solid lipid nanoparticles (SLNs) as a rising tool in drug delivery science: one step up in nanotechnology. Curr Nanosci. 2008;4:30–44. doi: 10.2174/157341308783591816. [DOI] [Google Scholar]
- 30.Lamprecht A, Ubrich N, Hombreiro Pérez M, Lehr C, Hoffman M, Maincent P. Biodegradable mono dispersed nanoparticles prepared by pressure homogenization emulsification. Int J Pharm. 1999;184:97–105. doi: 10.1016/S0378-5173(99)00107-6. [DOI] [PubMed] [Google Scholar]
- 31.Hou D, Xie C, Huang K, Zhu C. The production and characteristics of solid lipid nanoparticles (SLNs) Biomaterials. 2003;24:1781–5. doi: 10.1016/S0142-9612(02)00578-1. [DOI] [PubMed] [Google Scholar]
- 32.Gasco MR. Method for producing solid lipid microspheres having a narrow size distribution. United States patent, US 188837. 1993. 5.
- 33.Xie S, Wang S, Zhao B, Han C, Wang M, Zhou W. Effect of PLGA as a polymeric emulsifier on preparation of hydrophilic protein-loaded solid lipid nanoparticles. Colloids Surf B: Biointerfaces. 2008;67:199–204. doi: 10.1016/j.colsurfb.2008.08.018. [DOI] [PubMed] [Google Scholar]
- 34.Trotta M, Debernardi F, Caputo O. Preparation of solid lipid nanoparticles by a solvent emulsification-diffusion technique. Int J Pharm. 2003;257:153–60. doi: 10.1016/S0378-5173(03)00135-2. [DOI] [PubMed] [Google Scholar]
- 35.Charcosset C, El-Harati A, Fessi H. Preparation of solid lipid nanoparticles using a membrane contactor. J Control Release. 2005;108:112–20. doi: 10.1016/j.jconrel.2005.07.023. [DOI] [PubMed] [Google Scholar]
- 36.Bose S, Michniak-Kohn B. Preparation and characterization of lipid based nanosystems for topical delivery of quercetin. Eur J Pharm Sci. 2012;48:442–52. doi: 10.1016/j.ejps.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 37.Lamprecht A, Ubrich N, Hombreiro Pérez M, Lehr C, Hoffman M, Maincent P. Influences of process parameters on nanoparticle preparation performed by a double emulsion pressure homogenization technique. Int J Pharm. 2000;196:177–82. doi: 10.1016/S0378-5173(99)00422-6. [DOI] [PubMed] [Google Scholar]
- 38.Ghosh P, Han G, De M, Kim CK, Rotello VM. Gold nanoparticles in delivery applications. Adv Drug Deliv Rev. 2008;60:1307–15. doi: 10.1016/j.addr.2008.03.016. [DOI] [PubMed] [Google Scholar]
- 39.Hu H, Nie L, Feng S, Suo J. Preparation, characterization and in vitro release study of gallic acid loaded silica nanoparticles for controlled release. Pharmazie. 2013;68:401–5. [PubMed] [Google Scholar]
- 40.Bianco A, Kostarelos K, Prato M. Applications of carbon nanotubes in drug delivery. Curr Opin Chem Biol. 2005;9:674–9. doi: 10.1016/j.cbpa.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 41.Burnett K, Tyshenko MG. A comparison of human capital levels and the future prospect of the nanotechnology industry in early sector investors and recent emerging markets. Intl J of Nanotech. 2010;7:187–208. doi: 10.1504/IJNT.2010.031310. [DOI] [Google Scholar]
- 42.Vladisavljevic GT, Khalid N, Neves MA, Kuroiwa T, Nakajima M, Uemura K, et al. Industrial lab-on-a-chip: design, applications and scale-up for drug discovery and delivery. Adv Drug Deliv Rev. 2013;65(11–12):1626–63. doi: 10.1016/j.addr.2013.07.017. [DOI] [PubMed] [Google Scholar]
- 43.Hock SC, Ying YM, Wah CL. A review of the current scientific and regulatory status of nanomedicines and the challenges ahead. PDA J Pharm Sci Technol. 2011;65:177–95. [PubMed] [Google Scholar]
- 44.Buzea C, Pacheco II, Robbie K. Nanomaterials and nanoparticles: sources and toxicity. Biointerphases. 2007;2(4):MR17–MR7. doi: 10.1116/1.2815690. [DOI] [PubMed] [Google Scholar]
- 45.Colombo A, Briançon S, Lieto J, Fessi H. Project, design, and use of a pilot plant for nanocapsule production. Drug Dev Ind Pharm. 2001;27(10):1063–72. doi: 10.1081/DDC-100108369. [DOI] [PubMed] [Google Scholar]
- 46.Wagner V, Dullaart A, Bock A-K, Zweck A. The emerging nanomedicine landscape. Nat Biotechnol. 2006;24(10):1211–8. doi: 10.1038/nbt1006-1211. [DOI] [PubMed] [Google Scholar]


