Abstract
Background and Purpose
Epidemiological studies show strong associations between kidney dysfunction and risk of ischaemic stroke, the mechanisms of which are incompletely understood. We investigated whether these associations may reflect shared heritability due to a common polygenic basis and whether this differed for ischaemic stroke subtypes.
Methods
Polygenic models were derived using GWAS meta-analysis results for three kidney traits: estimated glomerular filtration rate using serum creatinine (eGFRcrea: N=73,998), eGFR using cystatin C (eGFRcys: N=22,937) and urinary albumin to creatinine ratio (UACR: N=31,580). For each, SNPs passing ten P-value thresholds were used to form profile scores in 4,561 ischaemic stroke cases and 7,094 controls from the UK, Germany and Australia. Scores were tested for association with ischaemic stroke and its three aetiological subtypes: large artery atherosclerosis (LAA), cardioembolism (CE) and small vessel disease (SVD).
Results
Polygenic scores correlating with higher eGFRcrea were associated with reduced risk of LAA, with five scores reaching P<0.05 (peak P=0.004) and all showing the epidemiologically expected direction of effect. A similar pattern was observed for polygenic scores reflecting higher UACR, of which three associated with LAA (peak P=0.01) and all showed the expected directional association. One UACR-based score also associated with SVD (P=0.03). The global pattern of results was unlikely to have occurred by chance (P=0.02).
Conclusions
This study suggests possible polygenic correlation between renal dysfunction and ischaemic stroke. The shared genetic components may be specific to stroke subtypes, particularly large artery atherosclerotic stroke. Further study of the genetic relationships between these disorders appears merited.
Keywords: stroke, kidney, genetic epidemiology
Introduction
Epidemiological evidence supports an association between kidney dysfunction and risk of cardiovascular diseases, including stroke. In fact, the majority of individuals with chronic kidney disease (CKD) die of a cardiovascular cause before developing end-stage renal disease 1. In relation to stroke, kidney dysfunction is associated with multiple outcomes including incident stroke 2, recurrent stroke 3, and mortality following stroke 4. These relationships seem related more to ischaemic, rather than haemorrhagic stroke 5, 6.
The mechanisms for these phenotypic correlations are not completely understood. Sharing of established cardiovascular risk factors – such as age, gender, blood pressure, cholesterol, smoking and diabetes – can explain some, but not all excess stroke risk in patients with CKD 3. Sharing of pathophysiological correlates of vascular disease including carotid atherosclerosis 7, arterial stiffness 8, and cerebral white matter hyperintensity (WMH) lesions 9 also appear to explain an additional component, but not all of the excess risk.
Kidney traits and ischaemic stroke both have substantial heritable components, with about 30–50% of observed variation in glomerular filtration rate (eGFR) 10, 15–45% of variation in albuminuria 11, and 30–40% of variation in ischaemic stroke risk 12 attributable to genetic effects. Thus, associations between kidney dysfunction and ischaemic stroke may partly reflect a shared genetic component.
In recent years, genome-wide association studies (GWAS) have identified a number of single nucleotide polymorphisms (SNPs) associated with kidney traits and aetiological subtypes of ischaemic stroke. However, these variants explain only a minority (1–2%) of population variation in their respective traits. This “missing heritability” partly reflects a genetic architecture comprising numerous risk variants with effects too small to show significant association in available sample collections. However numerous SNPs aggregated into polygenic scores may show stronger association and explain more trait variation 13.
The polygenic basis of complex traits also hampers attempts to demonstrate pleiotropy for individual SNPs, since effect sizes are typically small for both traits. Using the largest available datasets, a recent study assessed individual SNPs for joint contribution to CKD and cardiovascular disease 14. Significant cross-trait association was observed for SNPs at only one locus (SH2B3), suggesting that if genetic overlap between CKD and cardiovascular disease exists, the effect sizes of pleiotropic variants are likely too small to permit their individual detection.
Building on this earlier work, we hypothesised that phenotypic correlations between kidney dysfunction and ischaemic stroke may result from polygenic overlap; that is, sharing of a genetic component consisting of numerous, small-effect SNPs. This hypothesis was tested by deriving polygenic scores using GWAS results for kidney traits and testing their performance in stroke GWAS datasets. These analyses used GWAS meta-analysis results from the CKDGen Consortium and individual-level genotype data from three ischaemic stroke case-control collections.
Methods
Data sources and study samples
For the derivation stage we used genome-wide association meta-analysis results for three established kidney function traits: 1) estimated glomerular filtration rate (eGFR) based on serum creatinine (eGFRcrea: N=73,998) 15; 2) eGFR based on Cystatin C (eGFRcys: N=22,937) 15 and; 3) urinary albumin to creatinine ratio (UACR: N=31,580) 16. Higher GFR describes better kidney function, while elevated UACR suggests kidney disease. Details of individual studies are provided in the Data Supplement (Tables I–II).
Testing of polygenic scores were performed using individual-level genotype data for ischaemic stroke cases and controls from the Wellcome Trust Case Control Consortium 2 (WTCCC2) Ischaemic Stroke Study 17 and the Australian Stroke Genetics Collaborative (ASGC) 18. Three major aetiological subtypes of ischaemic stroke were determined: 1) large artery atherosclerosis (LAA); 2) cardioembolism (CE) and; 3) small vessel disease (SVD). Stroke subtyping was performed using the TOAST system 19. All studies were approved by appropriate ethics committees, and participants provided written informed consent.
Genotyping, imputation and quality control
For ischaemic stroke (IS) studies, genotyping was performed using Illumina arrays followed by quality control and imputation to the HapMap Phase II CEU reference. Principal components analysis (PCA) was performed using Eigenstrat 20, based on 95,016 approximately independent, directly genotyped SNPs. Principal component covariates of ancestry were calculated following three iterations of PCA with outlier removal.
SNP enrichment assessment, polygenic scoring and association analyses
Using GWAS meta-analysis results for the three kidney traits, sets of SNPs passing 10 graded P-values (Pthreshold=0.0001, 0.001, 0.01, 0.05, 0.1, 0.2, 0.3, 0.4, 0.5, and 1) were extracted. SNP sets were pruned by removing correlated SNPs (r2>0.2) within 1Mb, preferentially retaining the most significantly associated SNP 21. Pruned SNP sets were used to form polygenic scores for ischaemic stroke cases and controls as the sum of reference alleles for each SNP weighted by the summary regression coefficient for the relevant kidney trait. Polygenic scores were tested for association with ischaemic stroke and its subtypes using mixed effects logistic regression adjusted for three ancestry principal components, incorporating study site as a random effect. The proportion of stroke case-control variation explained by polygenic scores (R2) was estimated as previously described for mixed effects logistic models 22. Polygenic score tests were one-sided, since a priori, we sought to identify effects consistent with established epidemiological evidence. For eGFRcrea and eGFRcys, the pre-specified effect direction was negative, since higher eGFR correlates with reduced stroke risk 2. For UACR a positive effect was pre-specified 23. The study-wise significance threshold was derived using the method proposed by Galwey 24 (Data Supplement). A flowchart describing the polygenic analysis is in the Online Data Supplement (Figure I).
At a significance threshold of 0.05, we had 98% power to detect polygenic scores explaining ≥0.2% of variance in case/control status for any validation subtype, 81–82% power to detect scores explaining ≥0.1% of variance (varying by subtype) and 51–53% power for scores explaining ≥0.05% of variance 13. At a significance threshold of 0.001, the corresponding power estimates were 76–78%, 32–34% and 10–11%, respectively.
Lookup for individual SNPs associated with kidney or stroke traits
As a secondary analysis, we conducted targeted, cross-trait analyses for individual SNPs previously associated with kidney traits or ischaemic stroke subtypes. These analyses are described in the Online Data Supplement.
Results
SNP Enrichment
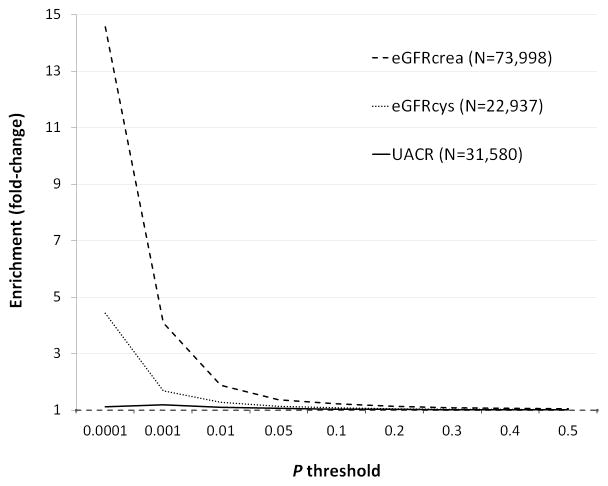
SNP set enrichment across P-value thresholds for the three kidney traits is shown in Figure 1. Enrichment was strongest for eGFR based on serum creatinine (eGFRcrea). Modest SNP enrichment was also observed for eGFR based on Cystatin C (eGFRcys). Results for urinary albumin to creatinine ratio (UACR) showed less marked evidence for enrichment.
Figure 1.
Observed SNP enrichment across P-value thresholds in GWAS results for kidney traits. N denotes total sample size. Enrichment reflects the fold-change increase in SNPs reaching each threshold, relative to the value expected by chance.
Polygenic scoring results
A total of 4,561 ischaemic stroke cases and 7,094 controls were used for polygenic score association testing (Table 1). Based on observed correlation among traits (Online Data Supplement), the adjusted study-wise significance threshold was α=0.001.
Table 1.
Ischaemic stroke samples used for polygenic score testing
| Study | IS | LAA | CE | SVD | Controls |
|---|---|---|---|---|---|
| ASGC | 1,071 | 375 | 226 | 287 | 1,212 |
| WTCCC2-Munich | 1,140 | 338 | 322 | 104 | 775† |
| WTCCC2-UK | 2,350 | 494 | 450 | 471 | 5,107 |
|
| |||||
| Total | 4,561 | 1,207 | 998 | 862 | 7,094 |
LAA, large artery atherosclerosis; CE, cardioembolism; SVD, small vessel disease.
WTCCC2-Munich controls were selected from the German KORA study, which also contributed to kidney trait meta-analyses.
Two scores derived from eGFRcrea showed nominal association with broad IS, reaching P=0.02 and P=0.05 (for Pthreshold=0.001 and 0.01, respectively, see Table 2). Five eGFRcrea-based scores showed nominal association with LAA, with peak association (P=0.004) observed for Pthreshold=0.05. This score explained an estimated 0.26% of LAA case-control variation (Data Supplement, Table III). For both IS and LAA, scores for all ten P-value thresholds demonstrated the expected negative direction of effect, with genetic scores indicative of higher eGFRcrea correlating with reduced (negative) stroke risk. No eGFRcrea-based score showed association with either cardioembolism (CE) or small vessel disease (SVD) and effect directions were also inconsistent within these subtypes.
Table 2.
Association of eGFRcrea-based polygenic scores with ischaemic stroke
| Ischaemic stroke | LAA* | CE* | SVD* | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Pthreshold† | NSNPs╪ | P|| | Direction | P|| | Direction§ | P | Direction | P | Direction |
| 0.0001 | 280 | 0.13 | − | 0.29 | − | 0.69 | + | 0.20 | − |
| 0.001 | 1,193 | 0.02 | − | 0.03 | − | 0.31 | − | 0.06 | − |
| 0.01 | 6,769 | 0.05 | − | 0.07 | − | 0.36 | − | 0.26 | − |
| 0.05 | 24,621 | 0.07 | − | 0.004 | − | 0.32 | − | 0.57 | + |
| 0.1 | 42,861 | 0.18 | − | 0.04 | − | 0.37 | − | 0.59 | + |
| 0.2 | 74,252 | 0.16 | − | 0.06 | − | 0.36 | − | 0.41 | − |
| 0.3 | 101,719 | 0.21 | − | 0.07 | − | 0.33 | − | 0.41 | − |
| 0.4 | 125,887 | 0.16 | − | 0.06 | − | 0.24 | − | 0.33 | − |
| 0.5 | 147,541 | 0.14 | − | 0.04 | − | 0.28 | − | 0.35 | − |
| 1 | 220,045 | 0.10 | − | 0.04 | − | 0.21 | − | 0.27 | − |
LAA, large artery atherosclerosis; CE, cardioembolism; SVD, small vessel disease.
Threshold for selecting SNPs into the score.
Number of SNPs forming the score.
Indicates whether a score predicting higher values of the kidney trait predicts increased (+) or decreased (−) stroke risk.
Results reaching P<0.05 are underlined and in bold font.
Polygenic scores derived from eGFRcys showed no association with broad ischaemic stroke or any of its subtypes (all P>0.05, see Data Supplement, Table IV). Further, effect directions were inconsistent across tests within each stroke type.
For UACR, one score (Pthreshold=0.01) showed nominal association with broad IS (P=0.04) (Table 3). Three scores were also nominally associated with LAA (peak P=0.01 at Pthreshold=0.001, R2=0.11%) and one score showed nominal association with SVD (P=0.03 at Pthreshold=0.0001, R2=0.14%, see Data Supplement Table III). For the three traits (IS, LAA and SVD), all effects were in the expected direction, with scores indicative of higher UACR (microalbumuria) correlating with positive stroke risk.
Table 3.
Association of UACR-based polygenic scores with ischaemic stroke
| Ischaemic stroke | LAA | CE | SVD | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Pthreshold | NSNPs | P | Direction | P | Direction | P | Direction | P | Direction |
| 0.0001 | 89 | 0.29 | + | 0.08 | + | 0.84 | − | 0.03 | + |
| 0.001 | 722 | 0.27 | + | 0.01 | + | 0.88 | − | 0.47 | + |
| 0.01 | 5,541 | 0.04 | + | 0.03 | + | 0.73 | − | 0.12 | + |
| 0.05 | 22,476 | 0.15 | + | 0.04 | + | 0.57 | − | 0.24 | + |
| 0.1 | 40,818 | 0.38 | + | 0.11 | + | 0.65 | − | 0.19 | + |
| 0.2 | 72,518 | 0.35 | + | 0.07 | + | 0.54 | − | 0.24 | + |
| 0.3 | 99,798 | 0.28 | + | 0.09 | + | 0.36 | + | 0.25 | + |
| 0.4 | 124,037 | 0.26 | + | 0.09 | + | 0.40 | + | 0.21 | + |
| 0.5 | 145,753 | 0.27 | + | 0.07 | + | 0.39 | + | 0.18 | + |
| 1 | 218,171 | 0.33 | + | 0.08 | + | 0.40 | + | 0.23 | + |
None of the polygenic associations quite passed the study-wide significance threshold of 0.001. However within each of the five trait combinations showing nominal association (eGFRcrea-IS, eGFRcrea-LAA, UACR-IS, UACR-LAA, UACR-SVD), the direction of effect across all ten tests was in accordance with prior evidence. We conducted simulations to empirically estimate the probability of this pattern occurring by chance for any given kidney-stroke trait combination (Online Data Supplement). For eGFRcrea, the probability of a set of ten tests showing a negative effect was P=0.080, based on 10,000 simulations. For eGFRcys and UACR, the corresponding one-sided probabilities were 0.067 and 0.069, respectively. Thus, the observed pattern was unlikely to have arisen by chance in any given set of ten tests.
If we consider all kidney-stroke combinations where such consistency was observed, the results appear even less likely to have occurred by chance. Excluding results for broad IS, we conducted three sets of approximately independent stroke subtype tests for each of the three kidney traits. Among these nine sets of tests, we observed three in which consistent effects in the expected direction were uniformly observed (eGFRcrea-LAA, UACR-LAA, and UACR-SVD). The probability of this occurring by chance is approximately 0.023 (Online Data Supplement), or about one in every 43 studies such as ours. This suggests our nominal associations likely to represent true polygenic correlations of small effect.
Lookup for individually associated SNPs
Of 40 SNPs previously associated with kidney traits, one (rs653178) was significantly associated (one-sided P=20×10−4) with large artery atherosclerotic stroke (LAA) 25 after multiple testing adjustment. Of seven stroke-associated SNPs, none were significantly associated with kidney traits (Tables V–VI, Data Supplement).
Discussion
This study suggests that reported epidemiological associations between renal disease and stroke may be partly explained by shared genetic factors, and that this association may differ for ischaemic stroke subtypes, in this study being most marked for large artery stroke. However, the causal genetic variants are likely of very small individual effect, detectable in the current study only when aggregated into highly polygenic scores and even then, achieving only nominal significance. Independent replication will be necessary to confirm the validity of these results.
An important factor affecting the significance of our results was power for individual stroke subtypes. For nominally significant associations, the kidney trait profile scores explained from 0.1 to 0.26% of case-control variation for different stroke subtypes. Power analyses indicated larger samples would be necessary to identify the observed effects at more stringent significance levels.
Although the proportion of stroke subtype variance explained by kidney-based scores was low, this does not mean the true genetic overlap is small. Profile scoring combines errors in effect estimates across all SNPs in the score, which usually produces estimates of explained variance markedly lower than true values 26.
We observed the strongest polygenic correlations between eGFR defined based on serum creatinine and large artery atherosclerotic stroke (LAA). Using eGFRcrea as the derivation trait, nominal significance of polygenic scores with LAA was sustained across nearly the full range of P-value derivation thresholds. If these results reflect a true genetic correlation, the pattern of results is consistent with a complex genetic model involving numerous small-effect variants influencing diverse biological processes 13.
The primary pathophysiological mechanism for LAA is atherosclerosis of the large cerebral arteries 19, a surrogate marker of which is carotid intima media thickness (cIMT). Various epidemiological studies have reported inverse associations between eGFR and cIMT. The majority show that this association can be explained by traditional cardiovascular risk factors including age, smoking, hypertension, obesity, diabetes and dyslipidaemia 27, 28. Thus, polygenic correlations between eGFR and LAA – if confirmed – may reflect genetic variants influencing atherosclerosis or its heritable risk factors.
We observed no co-trait association for eGFR scores derived from cystatin C at P<0.05. The lack of similar results between eGFRcrea and eGFRcys may reflect the smaller sample size for the latter; the larger discovery set for eGFRcrea will increase polygenic score precision 13. Greater discriminatory power of eGFRcrea-based scores was also supported by stronger SNP enrichment across P-value thresholds (Figure 1). Previous GWAS meta-analyses have also identified considerably more SNPs associated with eGFRcrea than eGFRcys. Given that both are measures of GFR and have similar heritability, the different results may largely reflect differences in sample size and power to identify variants of modest effect.
Polygenic scores derived from microalbuminuria (UACR) showed nominal associations with LAA across various P-value thresholds. This is consistent with epidemiological associations between UACR, cIMT and cardiovascular disease, although the pathophysiological basis of these relationships is less clear. In contrast to GFR, microalbuminuria seems not to reflect generalised atherosclerosis 23, but may represent another common pathophysiologic process such as endothelial dysfunction or low-grade inflammation 29.
We observed no evidence for polygenic overlap between renal function and cardioembolic stroke (CE), in spite of epidemiological associations between CKD and atrial fibrillation (AF), the major CE risk factor. However, increased AF prevalence has mainly been shown in patients with advanced kidney disease 30, 31. Furthermore, factors associated with AF in the general population seem not to be associated with AF in CKD 32, suggesting pathophysiological differences.
Our results suggested possible polygenic overlap between microalbuminuria and small vessel disease (SVD). These results are consistent with epidemiological associations between renal function and cerebral small vessel disease 9, which have been interpreted as suggesting a systemic generalised microvascular disease underlies both pathologies 33. Given the epidemiological evidence, more significant genetic associations might have been expected. Our modest results may reflect two factors. Firstly, accuracy of diagnosis of small vessel stroke is greatly improved by the routine use of MRI, which was only used for about 50% of stroke patients in the current study. Secondly pathological and imaging data suggest that small vessel disease is phenotypically heterogeneous, incorporating two distinct subtypes. One is characterised by single larger lacunar infarcts and thought to primarily relate to atherosclerosis; the other is characterised by multiple small lacunar infarcts and leukoaraiosis, and is related to a diffuse small vessel arteriopathy 34, 35. The latter subtype has been particularly associated with microalbuminuria. If renal disease is genetically correlated with only one SVD subtype, the heterogeneity of broadly defined SVD will have reduced our ability to detect any genetic overlap. The study of SVD samples with finer-scale phenotyping will likely provide better insights into genetic pleiotropy for SVD.
We observed largely negative evidence for cross-trait association for individual SNPs strongly associated with either kidney function or stroke. Only one SNP previously associated with eGFR was associated with both large artery and small vessel stroke; this SNP has been recently associated with broad ischaemic stroke 36 and was also the only variant showing cross-trait association in the previous CKDGen analysis 14.
An important limitation of this study was modest sample sizes for stroke subtypes. Sample size is a challenge for genetic studies of stroke, reflecting the technical nature of case ascertainment and the presence of multiple aetiological types. These are among the largest current IS samples with GWAS but our results should be validated in larger, well-phenotyped stroke GWAS datasets as they become available.
Summary
This study suggests a potential polygenic basis for epidemiological associations between renal dysfunction and ischaemic stroke. The effects of the putative shared genetic components appear small and potentially specific to distinct stroke types.
Supplementary Material
Acknowledgments
Sources of Funding
EGH is supported by the Australian Heart Foundation and National Stroke Foundation (100071). MT is funded by a UK Stroke Association project grant. HSM is supported by an NIHR Senior Investigator award. HSM and SB’s work in this area is supported by the Cambridge University Hospital Trust NIHR Biomedical Research centre. PMR holds NIHR and Wellcome Trust Senior Investigator Awards and is funded by the NIHR Biomedical Research Centre, Oxford. CS, HSM and SB have received research funding from the Wellcome Trust. CS has received funding from the UK Binks Trust. JC, MdA, CSF, WHLK BM and ST have received research funding from the US National Institutes of Health. PH and JT have received research funding from Genome Quebec, Canada. JT has received funding from the Canadian Institutes for Health Research. CL and RJS have received research funding from the Australian National Health and Medical Research Council.
Footnotes
Disclosures
None
References
- 1.Menon V, Sarnak MJ. The epidemiology of chronic kidney disease stages 1 to 4 and cardiovascular disease: A high-risk combination. Am J Kidney Dis. 2005;45:223–232. doi: 10.1053/j.ajkd.2004.09.022. [DOI] [PubMed] [Google Scholar]
- 2.Lee M, Saver JL, Chang KH, Liao HW, Chang SC, Ovbiagele B. Low glomerular filtration rate and risk of stroke: Meta-analysis. BMJ. 2010;341:c4249. doi: 10.1136/bmj.c4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ovbiagele B, Bath PM, Cotton D, Sha N, Diener HC. Low glomerular filtration rate, recurrent stroke risk, and effect of renin-angiotensin system modulation. Stroke. 2013;44:3223–3225. doi: 10.1161/STROKEAHA.113.002463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Famakin B, Weiss P, Hertzberg V, McClellan W, Presley R, Krompf K, et al. Hypoalbuminemia predicts acute stroke mortality: Paul coverdell georgia stroke registry. J Stroke Cerebrovasc Dis. 2010;19:17–22. doi: 10.1016/j.jstrokecerebrovasdis.2009.01.015. [DOI] [PubMed] [Google Scholar]
- 5.Guo Y, Wang H, Zhao X, Zhang Y, Zhang D, Ma J, et al. Sequential changes in renal function and the risk of stroke and death in patients with atrial fibrillation. Int J Cardiol. 2013;168:4678–4684. doi: 10.1016/j.ijcard.2013.07.179. [DOI] [PubMed] [Google Scholar]
- 6.Zheng L, Sun Z, Zhang X, Li J, Hu D, Sun Y. The association between glomerular filtration rate and stroke in hypertensive patients in rural areas of china. J Hypertens. 2012;30:901–907. doi: 10.1097/HJH.0b013e328352abc0. [DOI] [PubMed] [Google Scholar]
- 7.Kastarinen H, Ukkola O, Kesaniemi YA. Glomerular filtration rate is related to carotid intima-media thickness in middle-aged adults. Nephrol Dial Transplant. 2009;24:2767–2772. doi: 10.1093/ndt/gfp172. [DOI] [PubMed] [Google Scholar]
- 8.Elias MF, Davey A, Dore GA, Gillespie A, Abhayaratna WP, Robbins MA. Deterioration in renal function is associated with increased arterial stiffness. Am J Hypertens. 2014;27:207–214. doi: 10.1093/ajh/hpt179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ikram MA, Vernooij MW, Hofman A, Niessen WJ, van der Lugt A, Breteler MM. Kidney function is related to cerebral small vessel disease. Stroke. 2008;39:55–61. doi: 10.1161/STROKEAHA.107.493494. [DOI] [PubMed] [Google Scholar]
- 10.Raggi P, Su S, Karohl C, Veledar E, Rojas-Campos E, Vaccarino V. Heritability of renal function and inflammatory markers in adult male twins. Am J Nephrol. 2010;32:317–323. doi: 10.1159/000319449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fox CS, Yang Q, Guo CY, Cupples LA, Wilson PW, Levy D, et al. Genome-wide linkage analysis to urinary microalbuminuria in a community-based sample: The framingham heart study. Kidney Int. 2005;67:70–74. doi: 10.1111/j.1523-1755.2005.00056.x. [DOI] [PubMed] [Google Scholar]
- 12.Bevan S, Traylor M, Adib-Samii P, Malik R, Paul NL, Jackson C, et al. Genetic heritability of ischemic stroke and the contribution of previously reported candidate gene and genomewide associations. Stroke. 2012;43:3161–3167. doi: 10.1161/STROKEAHA.112.665760. [DOI] [PubMed] [Google Scholar]
- 13.Dudbridge F. Power and predictive accuracy of polygenic risk scores. PLoS Genet. 2013;9:e1003348. doi: 10.1371/journal.pgen.1003348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Olden M, Teumer A, Bochud M, Pattaro C, Kottgen A, Turner ST, et al. Overlap between common genetic polymorphisms underpinning kidney traits and cardiovascular disease phenotypes: The ckdgen consortium. Am J Kidney Dis. 2013;61:889–898. doi: 10.1053/j.ajkd.2012.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pattaro C, Kottgen A, Teumer A, Garnaas M, Boger CA, Fuchsberger C, et al. Genome-wide association and functional follow-up reveals new loci for kidney function. PLoS Genet. 2012;8:e1002584. doi: 10.1371/journal.pgen.1002584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boger CA, Chen MH, Tin A, Olden M, Kottgen A, de Boer IH, et al. Cubn is a gene locus for albuminuria. J Am Soc Nephrol. 2011;22:555–570. doi: 10.1681/ASN.2010060598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bellenguez C, Bevan S, Gschwendtner A, Spencer CC, Burgess AI, Pirinen M, et al. Genome-wide association study identifies a variant in hdac9 associated with large vessel ischemic stroke. Nat Genet. 2012;44:328–333. doi: 10.1038/ng.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holliday EG, Maguire JM, Evans TJ, Koblar SA, Jannes J, Sturm JW, et al. Common variants at 6p21.1 are associated with large artery atherosclerotic stroke. Nat Genet. 2012;44:1147–1151. doi: 10.1038/ng.2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Adams HP, Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. Toast. Trial of org 10172 in acute stroke treatment. Stroke. 1993;24:35–41. doi: 10.1161/01.str.24.1.35. [DOI] [PubMed] [Google Scholar]
- 20.Price AL, Patterson NJ, Plenge RM, Weinblatt ME, Shadick NA, Reich D. Principal components analysis corrects for stratification in genome-wide association studies. Nat Genet. 2006;38:904–909. doi: 10.1038/ng1847. [DOI] [PubMed] [Google Scholar]
- 21.Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, et al. Plink: A tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81:559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Snijders TAB, Bosker RJ. Multilevel analysis. Sage Publications Ltd; 2012. pp. 305–306. [Google Scholar]
- 23.Matsushita K, van der Velde M, Astor BC, Woodward M, Levey AS, de Jong PE, et al. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: A collaborative meta-analysis. Lancet. 2010;375:2073–2081. doi: 10.1016/S0140-6736(10)60674-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Galwey NW. A new measure of the effective number of tests, a practical tool for comparing families of non-independent significance tests. Genet Epidemiol. 2009;33:559–568. doi: 10.1002/gepi.20408. [DOI] [PubMed] [Google Scholar]
- 25.Traylor M, Farrall M, Holliday EG, Sudlow C, Hopewell JC, Cheng YC, et al. Genetic risk factors for ischaemic stroke and its subtypes (the Metastroke collaboration): A meta-analysis of genome-wide association studies. Lancet Neurol. 2012;11:951–962. doi: 10.1016/S1474-4422(12)70234-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wray NR, Yang J, Hayes BJ, Price AL, Goddard ME, Visscher PM. Pitfalls of predicting complex traits from snps. Nature reviews. Genetics. 2013;14:507–515. doi: 10.1038/nrg3457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bobbert T, Mai K, Fischer-Rosinsky A, Osterhoff M, Pfeiffer AF, Spranger J. Relation between physiological variation of renal function and carotid intima media thickness in non-diabetic individuals. J Atheroscler Thromb. 2010;17:242–248. doi: 10.5551/jat.3020. [DOI] [PubMed] [Google Scholar]
- 28.Takahashi W, Tsukamoto Y, Ohnuki T, Takizawa S, Kawada S, Takagi S. Is mild renal dysfunction a risk factor for carotid atherosclerosis in apparently healthy adults? Intern Med. 2011;50:2285–2289. doi: 10.2169/internalmedicine.50.5725. [DOI] [PubMed] [Google Scholar]
- 29.Stehouwer CD, Smulders YM. Microalbuminuria and risk for cardiovascular disease: Analysis of potential mechanisms. J Am Soc Nephrol. 2006;17:2106–2111. doi: 10.1681/ASN.2005121288. [DOI] [PubMed] [Google Scholar]
- 30.Nimmo C, Wright M, Goldsmith D. Management of atrial fibrillation in chronic kidney disease: Double trouble. Am Heart J. 2013;166:230–239. doi: 10.1016/j.ahj.2013.05.010. [DOI] [PubMed] [Google Scholar]
- 31.Bansal N, Fan D, Hsu CY, Ordonez JD, Marcus GM, Go AS. Incident atrial fibrillation and risk of end-stage renal disease in adults with chronic kidney disease. Circulation. 2013;127:569–574. doi: 10.1161/CIRCULATIONAHA.112.123992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Soliman EZ, Prineas RJ, Go AS, Xie D, Lash JP, Rahman M, et al. Chronic kidney disease and prevalent atrial fibrillation: The chronic renal insufficiency cohort (cric) Am Heart J. 2010;159:1102–1107. doi: 10.1016/j.ahj.2010.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Knopman DS. Invited commentary: Albuminuria and microvascular disease of the brain--a shared pathophysiology. Am J Epidemiol. 2010;171:287–289. doi: 10.1093/aje/kwp429. author reply 290–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Boiten J, Lodder J, Kessels F. Two clinically distinct lacunar infarct entities? A hypothesis. Stroke. 1993;24:652–656. doi: 10.1161/01.str.24.5.652. [DOI] [PubMed] [Google Scholar]
- 35.Fisher CM. Lacunar strokes and infarcts: A review. Neurology. 1982;32:871–876. doi: 10.1212/wnl.32.8.871. [DOI] [PubMed] [Google Scholar]
- 36.Kilarski LL, Achterberg S, Devan WJ, Traylor M, Malik R, Lindgren A, et al. Meta-analysis in more than 17,900 cases of ischemic stroke reveals a novel association at 12q24.12. Neurology. 2014;83:678–685. doi: 10.1212/WNL.0000000000000707. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.