Abstract
HTLV-I is a transforming human retrovirus that is an etiologic agent of adult T cell leukemia/lymphoma. To investigate the effects of this virus on T cell functions, two OKT3+, OKT4+, OKT8- cytotoxic clones (8.7 and 8.8) specific for allogeneic cells bearing DPw2, a class II histocompatibility antigen, were studied before and after infection with HTLV-I. The clones retained cytotoxic function for up to 70 d after exposure to HTLV-I, even without subsequent antigenic stimulation, but then lost their cytotoxic activity. Prior to infection with HTLV-I, clone 8.8 also lysed OKT3 hybridoma cells; after infection, cytotoxic activity against these OKT3-antibody bearing cells was lost in parallel with the loss of activity against DPw2-bearing target cells. In addition, expression of T3 surface antigen by HTLV-I-infected 8.8 cells was decreased at a time when they lost their cytotoxic activity, possibly contributing to the loss of cytotoxic function. Finally, clone 8.8 could provide help for nonspecific IgG production by autologous B cells when stimulated with irradiated DPw2-bearing non-T cells. After infection with HTLV-I, this helper function became independent of DPw2-stimulation and persisted even when the cytotoxic activity was lost. An OKT4+ T cell clone thus could simultaneously manifest both cytotoxic and helper T cell activities, and these activities were differentially affected after HTLV-I infection.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernards R., Schrier P. I., Houweling A., Bos J. L., van der Eb A. J., Zijlstra M., Melief C. J. Tumorigenicity of cells transformed by adenovirus type 12 by evasion of T-cell immunity. 1983 Oct 27-Nov 2Nature. 305(5937):776–779. doi: 10.1038/305776a0. [DOI] [PubMed] [Google Scholar]
- Biddison W. E., Rao P. E., Talle M. A., Goldstein G., Shaw S. Possible involvement of the T4 molecule in T cell recognition of class II HLA antigens. Evidence from studies of CTL-target cell binding. J Exp Med. 1984 Mar 1;159(3):783–797. doi: 10.1084/jem.159.3.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britt W. J., Chesebro B. H-2D control of recovery from Friend virus leukemia: H-2D region influences the kinetics of the T lymphocyte response to Friend virus. J Exp Med. 1983 Jun 1;157(6):1736–1745. doi: 10.1084/jem.157.6.1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunn P. A., Jr, Schechter G. P., Jaffe E., Blayney D., Young R. C., Matthews M. J., Blattner W., Broder S., Robert-Guroff M., Gallo R. C. Clinical course of retrovirus-associated adult T-cell lymphoma in the United States. N Engl J Med. 1983 Aug 4;309(5):257–264. doi: 10.1056/NEJM198308043090501. [DOI] [PubMed] [Google Scholar]
- Clarke M. F., Gelmann E. P., Reitz M. S., Jr Homology of human T-cell leukaemia virus envelope gene with class I HLA gene. Nature. 1983 Sep 1;305(5929):60–62. doi: 10.1038/305060a0. [DOI] [PubMed] [Google Scholar]
- Depper J. M., Leonard W. J., Krönke M., Waldmann T. A., Greene W. C. Augmented T cell growth factor receptor expression in HTLV-1-infected human leukemic T cells. J Immunol. 1984 Oct;133(4):1691–1695. [PubMed] [Google Scholar]
- Hoffman R. W., Bluestone J. A., Leo O., Shaw S. Lysis of anti-T3-bearing murine hybridoma cells by human allospecific cytotoxic T cell clones and inhibition of that lysis by anti-T3 and anti-LFA-1 antibodies. J Immunol. 1985 Jul;135(1):5–8. [PubMed] [Google Scholar]
- Kannagi M., Sugamura K., Kinoshita K., Uchino H., Hinuma Y. Specific cytolysis of fresh tumor cells by an autologous killer T cell line derived from an adult T cell leukemia/lymphoma patient. J Immunol. 1984 Aug;133(2):1037–1041. [PubMed] [Google Scholar]
- Kannagi M., Sugamura K., Sato H., Okochi K., Uchino H., Hinuma Y. Establishment of human cytotoxic T cell lines specific for human adult T cell leukemia virus-bearing cells. J Immunol. 1983 Jun;130(6):2942–2946. [PubMed] [Google Scholar]
- Klein G. Immune and non-immune control of neoplastic development: contrasting effects of host and tumor evolution. Cancer. 1980 May 15;45(10):2486–2499. doi: 10.1002/1097-0142(19800515)45:10<2486::aid-cncr2820451005>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Krönke M., Leonard W. J., Depper J. M., Greene W. C. Deregulation of interleukin-2 receptor gene expression in HTLV-I-induced adult T-cell leukemia. Science. 1985 Jun 7;228(4704):1215–1217. doi: 10.1126/science.2988127. [DOI] [PubMed] [Google Scholar]
- Leonard W. J., Depper J. M., Uchiyama T., Smith K. A., Waldmann T. A., Greene W. C. A monoclonal antibody that appears to recognize the receptor for human T-cell growth factor; partial characterization of the receptor. Nature. 1982 Nov 18;300(5889):267–269. doi: 10.1038/300267a0. [DOI] [PubMed] [Google Scholar]
- Mann D. L., Popovic M., Sarin P., Murray C., Reitz M. S., Strong D. M., Haynes B. F., Gallo R. C., Blattner W. A. Cell lines producing human T-cell lymphoma virus show altered HLA expression. Nature. 1983 Sep 1;305(5929):58–60. doi: 10.1038/305058a0. [DOI] [PubMed] [Google Scholar]
- Manzari V., Wong-Staal F., Franchini G., Colombini S., Gelmann E. P., Oroszlan S., Staal S., Gallo R. C. Human T-cell leukemia-lymphoma virus (HTLV): cloning of an integrated defective provirus and flanking cellular sequences. Proc Natl Acad Sci U S A. 1983 Mar;80(6):1574–1578. doi: 10.1073/pnas.80.6.1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
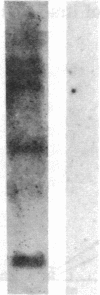
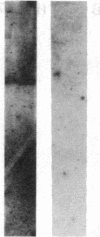
- Meuer S. C., Acuto O., Hussey R. E., Hodgdon J. C., Fitzgerald K. A., Schlossman S. F., Reinherz E. L. Evidence for the T3-associated 90K heterodimer as the T-cell antigen receptor. Nature. 1983 Jun 30;303(5920):808–810. doi: 10.1038/303808a0. [DOI] [PubMed] [Google Scholar]
- Mitsuya H., Guo H. G., Cossman J., Megson M., Reitz M. S., Jr, Broder S. Functional properties of antigen-specific T cells infected by human T-cell leukemia-lymphoma virus (HTLV-I). Science. 1984 Sep 28;225(4669):1484–1486. doi: 10.1126/science.6206569. [DOI] [PubMed] [Google Scholar]
- Mitsuya H., Guo H. G., Megson M., Trainor C., Reitz M. S., Jr, Broder S. Transformation and cytopathogenic effect in an immune human T-cell clone infected by HTLV-I. Science. 1984 Mar 23;223(4642):1293–1296. doi: 10.1126/science.6322299. [DOI] [PubMed] [Google Scholar]
- Mitsuya H., Matis L. A., Megson M., Bunn P. A., Murray C., Mann D. L., Gallo R. C., Broder S. Generation of an HLA-restricted cytotoxic T cell line reactive against cultured tumor cells from a patient infected with human T cell leukemia/lymphoma virus. J Exp Med. 1983 Sep 1;158(3):994–999. doi: 10.1084/jem.158.3.994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyoshi I., Kubonishi I., Yoshimoto S., Akagi T., Ohtsuki Y., Shiraishi Y., Nagata K., Hinuma Y. Type C virus particles in a cord T-cell line derived by co-cultivating normal human cord leukocytes and human leukaemic T cells. Nature. 1981 Dec 24;294(5843):770–771. doi: 10.1038/294770a0. [DOI] [PubMed] [Google Scholar]
- Morimoto C., Matsuyama T., Oshige C., Tanaka H., Hercend T., Reinherz E. L., Schlossman S. F. Functional and phenotypic studies of Japanese adult T cell leukemia cells. J Clin Invest. 1985 Mar;75(3):836–843. doi: 10.1172/JCI111780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poiesz B. J., Ruscetti F. W., Gazdar A. F., Bunn P. A., Minna J. D., Gallo R. C. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7415–7419. doi: 10.1073/pnas.77.12.7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popovic M., Flomenberg N., Volkman D. J., Mann D., Fauci A. S., Dupont B., Gallo R. C. Alteration of T-cell functions by infection with HTLV-I or HTLV-II. Science. 1984 Oct 26;226(4673):459–462. doi: 10.1126/science.6093248. [DOI] [PubMed] [Google Scholar]
- Popovic M., Sarin P. S., Robert-Gurroff M., Kalyanaraman V. S., Mann D., Minowada J., Gallo R. C. Isolation and transmission of human retrovirus (human t-cell leukemia virus). Science. 1983 Feb 18;219(4586):856–859. doi: 10.1126/science.6600519. [DOI] [PubMed] [Google Scholar]
- Robert-Guroff M., Nakao Y., Notake K., Ito Y., Sliski A., Gallo R. C. Natural antibodies to human retrovirus HTLV in a cluster of Japanese patients with adult T cell leukemia. Science. 1982 Feb 19;215(4535):975–978. doi: 10.1126/science.6760397. [DOI] [PubMed] [Google Scholar]
- Robert-Guroff M., Ruscetti F. W., Posner L. E., Poiesz B. J., Gallo R. C. Detection of the human T cell lymphoma virus p19 in cells of some patients with cutaneous T cell lymphoma and leukemia using a monoclonal antibody. J Exp Med. 1981 Dec 1;154(6):1957–1964. doi: 10.1084/jem.154.6.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salahuddin S. Z., Markham P. D., Lindner S. G., Gootenberg J., Popovic M., Hemmi H., Sarin P. S., Gallo R. C. Lymphokine production by cultured human T cells transformed by human T-cell leukemia-lymphoma virus-I. Science. 1984 Feb 17;223(4637):703–707. doi: 10.1126/science.6320367. [DOI] [PubMed] [Google Scholar]
- Schrier P. I., Bernards R., Vaessen R. T., Houweling A., van der Eb A. J. Expression of class I major histocompatibility antigens switched off by highly oncogenic adenovirus 12 in transformed rat cells. 1983 Oct 27-Nov 2Nature. 305(5937):771–775. doi: 10.1038/305771a0. [DOI] [PubMed] [Google Scholar]
- Shaw S., Goldstein G., Springer T. A., Biddison W. E. Susceptibility of cytotoxic T lymphocyte (CTL) clones to inhibition by anti-T3 and anti-T4 (but not anti-LFA-1) monoclonal antibodies varies with the "avidity" of CTL-target interaction. J Immunol. 1985 May;134(5):3019–3026. [PubMed] [Google Scholar]
- Sodroski J. G., Rosen C. A., Haseltine W. A. Trans-acting transcriptional activation of the long terminal repeat of human T lymphotropic viruses in infected cells. Science. 1984 Jul 27;225(4660):381–385. doi: 10.1126/science.6330891. [DOI] [PubMed] [Google Scholar]
- Tosato G., Magrath I. T., Blaese R. M. T cell-mediated immunoregulation of Epstein Barr virus- (EBV) induced B lymphocyte activation in EBV-seropositive and EBV-seronegative individuals. J Immunol. 1982 Feb;128(2):575–579. [PubMed] [Google Scholar]
- Tsuda H., Takatsuki K. Specific decrease in T3 antigen density in adult T-cell leukaemia cells: I. Flow microfluorometric analysis. Br J Cancer. 1984 Dec;50(6):843–845. doi: 10.1038/bjc.1984.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchiyama T., Sagawa K., Takatsuki K., Uchino H. Effect of adult T-cell leukemia cells on pokeweed mitogen-induced normal B-cell differentiation. Clin Immunol Immunopathol. 1978 May;10(1):24–34. doi: 10.1016/0090-1229(78)90005-3. [DOI] [PubMed] [Google Scholar]
- Volkman D. J., Popovic M., Gallo R. C., Fauci A. S. Human T cell leukemia/lymphoma virus-infected antigen-specific T cell clones: indiscriminant helper function and lymphokine production. J Immunol. 1985 Jun;134(6):4237–4243. [PubMed] [Google Scholar]
- Waldmann T. A., Greene W. C., Sarin P. S., Saxinger C., Blayney D. W., Blattner W. A., Goldman C. K., Bongiovanni K., Sharrow S., Depper J. M. Functional and phenotypic comparison of human T cell leukemia/lymphoma virus positive adult T cell leukemia with human T cell leukemia/lymphoma virus negative Sézary leukemia, and their distinction using anti-Tac. Monoclonal antibody identifying the human receptor for T cell growth factor. J Clin Invest. 1984 Jun;73(6):1711–1718. doi: 10.1172/JCI111379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss A., Stobo J. D. Requirement for the coexpression of T3 and the T cell antigen receptor on a malignant human T cell line. J Exp Med. 1984 Nov 1;160(5):1284–1299. doi: 10.1084/jem.160.5.1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong-Staal F., Hahn B., Manzari V., Colombini S., Franchini G., Gelmann E. P., Gallo R. C. A survey of human leukaemias for sequences of a human retrovirus. Nature. 1983 Apr 14;302(5909):626–628. doi: 10.1038/302626a0. [DOI] [PubMed] [Google Scholar]
- Yamada Y. Phenotypic and functional analysis of leukemic cells from 16 patients with adult T-cell leukemia/lymphoma. Blood. 1983 Jan;61(1):192–199. [PubMed] [Google Scholar]
- Yarchoan R., Schneider H. S., Wray B. B., Nelson D. L. Specific anti-influenza virus antibody production in vitro by lymphocytes from a subset of patients with hypogammaglobulinemia. J Clin Invest. 1983 Jun;71(6):1720–1727. doi: 10.1172/JCI110926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida M., Miyoshi I., Hinuma Y. Isolation and characterization of retrovirus from cell lines of human adult T-cell leukemia and its implication in the disease. Proc Natl Acad Sci U S A. 1982 Mar;79(6):2031–2035. doi: 10.1073/pnas.79.6.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]