Abstract
A combination of positron emission tomography (PET) with 18F-labeled fluoro-2-deoxyglucose (18F-FDG) and computed tomography (18F-FDG-PET/CT) has increasingly become a widely used imaging modality for the diagnosis and management of head and neck cancer. On the basis of both recent literature and our professional experience, we present a set of principles with pictorial illustrations and clinical applications of FDG-PET/CT in the evaluation and management planning of squamous cell carcinoma of the oral cavity and oropharynx. We feel that this paper will be of interest and will aid the learning of oral and maxillofacial radiology trainees and practitioners.
Keywords: Positron-Emission Tomography; Tomography, X-Ray Computed; Head and Neck Neoplasms
Biophysics
The principle of positron emission tomography (PET) is that radiation emitted from a radiopharmaceutical injected intravenously into a patient is registered by external detectors positioned at different orientations. The isotope distributes within different tissues according to the carrier molecule (isotopic labeling) and emits a positron (a positively charged electron). This positron in turn interacts with a free electron and an annihilation reaction occurs, resulting in the production of two 511-KeV photons emitted at almost 180° to each other. Then, the distribution of radioactivity is estimated by (filtered) backprojection methods. The directionality of the annihilation photons (two 511-keV annihilation photons emitted in opposite directions) provides a mechanism for localizing the origin of the photons and hence, the radioactive decay process that resulted in their emission (Fig. 1).
Fig. 1.
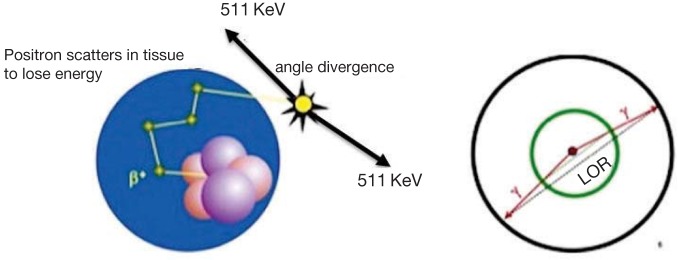
Annihilation coincidence detection (ACD). When a positron is emitted by a nuclear transformation, it scatters through matter losing energy and annihilates with an electron, resulting in two 511-keV photons that are emitted in nearly opposite directions (left). When two interactions are simultaneously detected within a ring of detectors surrounding the patient (right), it is assumed that annihilation occurred on the line connecting the interactions (line of response, LOR). ACD acts as a collimator for the positron emission tomography (PET) scanner (electronic collimation) by determining the path of the detected photons.
A PET scanner consists of several rings of detectors around the patient. The detector crystals are often made of bismuth germanate. Electronically coupled opposing detectors simultaneously identify the pair of γ photons by using coincidence detection circuits that measure annihilation events within 10-20 ns. The annihilation reaction is thus known to occur along the line joining the two detectors. Raw PET scan data consist of a number of these coincidence lines, which are recognized as projections. These data are processed using backprojection and iterative reconstruction algorithms to form a number of contiguous axial slices. The PET system's computer then reconstructs the transverse images from the projection data, as does the computer of the CT scanner. Modern multislice PET scanners permit a simultaneous acquisition of up to 45 slices over an axial distance of 16 cm. The spatial resolution of the PET system is limited to 5 mm. PET images are often fused with contrast-enhanced computed tomography (CT) images to facilitate anatomic localization of the radiopharmaceutical.1
There are many cyclotron-generated positron-emitting radiopharmaceuticals (e.g., 11C, 13N, 15O, 18F, and 68Ga). To date, most clinical applications of PET scans have employed 18F-labeled fluoro-2-deoxyglucose (18F-FDG). 18FFDG is a glucose analog radiopharmaceutical that has a half-life of 110 min and is commonly used for studying brain and heart glucose metabolism and for detecting cancer metastases. However, the radiopharmaceuticals 11C, 13N, and 15O are primarily used in direct metabolic studies as they have significant physiologic potential because they can replace atoms in molecules that are essential for metabolism. The utility of PET is based not only on its sensitivity but also on the fact that the most commonly used radiopharmaceuticals are isotopes of elements that occur naturally in organic molecules.2
Standardized uptake value (SUV) is often used in PET imaging for a semiquantitative analysis. SUV is usually calculated as a ratio of (1) the mean region of interest (ROI) activity in megabecquerels per milliliter and (2) the injected activity in megabecquerels, divided by the body weight in grams.
Clinical Applications
Head and neck squamous cell carcinoma (HNSCC) accounts for approximately 3% of all new cancer diagnoses in the United States with more than 40,000 new cases diagnosed every year.3 Early diagnosis and accurate staging can strongly influence the prognosis. The use of traditional tomographic imaging modalities (CT and magnetic resonance imaging (MRI)) has greatly improved staging and monitoring for HNSCC. However, small metastases and early recurrent disease can still be underestimated.4
Hybrid PET/CT imaging allows fusing of the anatomic data of CT with the functional information of PET, offering improved localization of metabolic abnormalities and thus, more accurate detection of malignant lesions in the head and neck. In the post-therapy setting, the PET/CT technology is helpful in differentiating a recurrent or residual tumor from post-therapy inflammatory changes. Receiver operating characteristic (ROC) curve analyses demonstrate that PET/CT is significantly superior to PET or CT alone for the detection of malignancy in the head and neck.5,6,7 Further, the false-positive (FP) values associated with PET imaging can be significantly reduced in favor of the positive predictive values (PPV) by the integration of CT. Moreover, the CT component of fusion imaging is used for the attenuation correction of the PET data, which shortens the overall acquisition time by almost 20 min. Sensitivity and specificity rates for the detection of nodal metastasis in HNSCC were 100% and 87.5% for PET/CT, respectively.8
FDG-PET/CT in HNSCC
HNSCCs are staged according to the tumor node metastasis (TNM) classification system. Assessment of the tumor stage does not pertain only to the size of the primary lesion, but also includes the depth of invasion, the involvement of the surrounding structures, and midline crossing. The greatest clinical concern is the determination of mandibular bone invasion in oral cancers to determine the necessity and extent of mandibular resection. CT and MRI could be suboptimal in detecting bone invasion as a result of artifacts generated by dental amalgams and implants. Therefore, PET/CT imaging has the potential to delineate mandibular involvement (Fig. 2).9
Fig. 2.
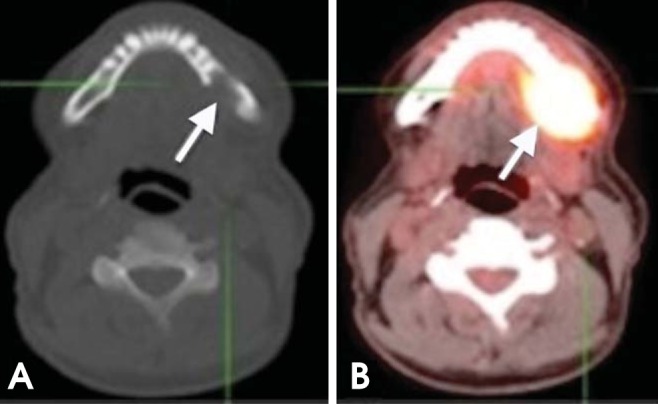
A 64-year-old man with T4 squamous cell carcinoma (SCC) of the lingual gingiva of the left posterior mandible. (A) Axial plain computed tomography (CT) image shows bone destruction of the left posterior mandible (arrow). (B) Axial PET/CT scan shows intense fluoro-2-deoxyglucose (FDG) uptake in the same area (arrow).
More than 50% of HNSCC patients have regional lymph node metastasis on their first clinical presentation. Nodal metastasis currently stands as the most important prognostic factor, negatively affecting the 5-year survival rate by 40%-50%.10,11 Recent studies show that PET/CT imaging is superior to CT and MRI in the detection of the metastatic disease of cervical lymph nodes (Figs. 3 and 4). However, the management of negative neck (N0 neck) is still controversial, although most oncologic surgeons favor routine elective neck dissection with or without postoperative radiotherapy for cancer sites with a high propensity of nodal metastasis (e.g., tongue and oropharynx). That is attributed to the relatively high rate of occult metastasis (micrometastasis) and extracapsular infiltration in HNSCC patients. Currently, the evidence in the literature is insufficient to substantiate the role of PET/CT in the preclusion of neck dissection.12,13,14
Fig. 3.
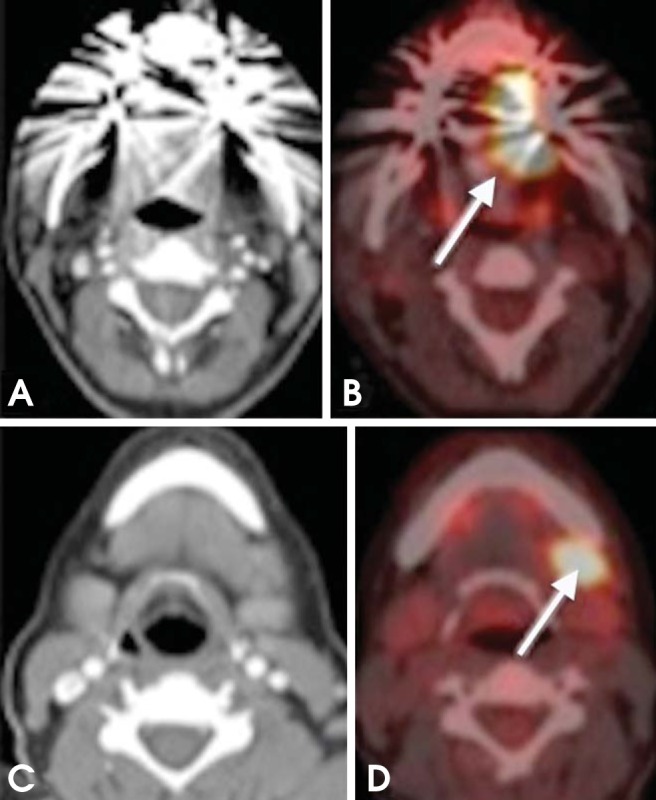
A 56-year-old man diagnosed with SCC of the lateral border of the tongue (left side). (A, B) Axial CT and PET/CT images reveal increased FDG uptake in the lateral border of the tongue (arrow). (C, D) Axial CT and PET/CT images at a lower level show increased uptake in a left level IB lymph node (arrow).
Fig. 4.
False-negative CT overlooks nodal disease. A 57-year-old man diagnosed with Hodgkin's lymphoma. (A) Non-enhanced CT scan showed normal study. The lymph nodes are normal according to radiographic criteria. However, the fused PET/CT images (B) show many pathologic nodes surrounding the paraspinal musculature.
Incidence of distant metastasis at the initial presentation of HNSCC is quite low. However, a majority of distant metastases occur in the lungs (Fig. 5). In general, PET/CT is more sensitive and specific than other tomographic imaging modalities (CT and MRI) in the early detection of distant and skeletal metastases, and second primaries.15 However, studies show that PET/CT is as good as bone scintigraphy in detecting bone metastases.16
Fig. 5.

Metastatic head and neck SCC (HNSCC). A 61-year-old male with T3 SCC of the right tosillar fossa. Whole-body PET shows a focus of increased uptake (arrow) in the sternum, which proved to be a distant metastasis. The CT component is still needed to adequately assess lesion location. PET/CT is useful for detecting distant metastasis in patients undergoing restaging or surveillance.
The current literature suggests that PET/CT can detect unknown primary tumors in up to 50% of HNSCC patients.17,18 Recent studies show a large number of false-negative results for the depiction of unknown primary tumors, overshadowing the role of PET/CT in this respect. Evaluation of patients with unknown primaries typically consists of a comprehensive physical examination, endoscopy of the aerodigestive tract, contrast-enhanced CT and/or MRI with gadolinium, and tonsillectomies. However, PET/CT should be part of the diagnostic workup of patients with unknown primary tumors (Fig. 6).
Fig. 6.
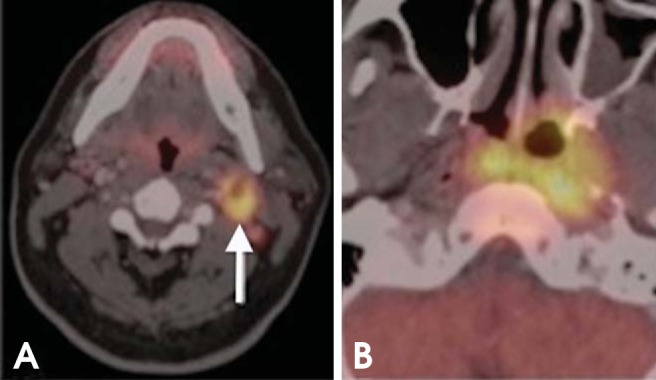
Unknown primary tumor. A 54-year-old patient presented with an enlarging left neck mass. (A) CT shows a level IIA lymph node (arrow); fine-needle aspiration biopsy showed metastatic squamous cell carcinoma. CT chest and panendoscopy failed to disclose the primary tumor. (B) PET/CT shows the patient's primary tumor in the nasopharynx.
There are conflicting reports on the usefulness of SUVs as a measure of aggressiveness, thereby acting as a prognostic factor. Although the prediction of the disease outcome based on SUVs is usually hampered by multiple confounding factors, there is general acceptance among most researchers that tumors with SUVs greater than 9 lead to poor prognosis.19,20,21
There has been a growing interest in the use of PET/CT-guided intensity-modulated radiotherapy (PET/CT-guided IMRT) for tumor contouring, allowing for accurate delineation of the target volume and sparing of normal tissues for effective radiation therapy, because of its ability to provide both anatomic and functional information. Despite its high accuracy, PET/CT complements, but does not replace, CT and MRI evaluation.
Clinical examination and tomographic imaging modalities are often insufficient to detect early or subtle changes in a treated neck, as postsurgical and postradiation inflammatory changes can complicate the identification of recurrent or residual disease.22,23 PET/CT can detect and differentiate radiation and surgical changes from residual or recurrent tumors because cancer cells retain more FDG for longer periods of time than inflammatory tissues. Recent studies have shown that PET/CT had a sensitivity of more than 90% for localization of recurrent disease.24,25
Patients with primary HNSCC are at a high risk of developing additional primary cancers in the aerodigestive tract as a result of field cancerization phenomena. Second primary lesions occur either around the same time as the first primary tumor (synchronous tumor) or after a short interval (metachronous tumor) (Figs. 7 and 8). Panendoscopy at regular intervals following treatment has not been shown to be effective in detecting second primaries; as a result, treatment guidelines tabulated by the American Head and Neck Society do not recommend routine post-treatment panendoscopy. PET/CT has proven useful in the detection of second primary tumors in patients previously treated for HNSCC and being watched for recurrence.26 However, some debate still exists on the optimal timing of the first surveillance scan. Early scanning with PET/CT has low sensitivity and specificity because there may be residual viable tumor cells that are stunned from radiotherapy; moreover, FDG uptake in the early post-treatment period may reflect post-operative or post-radiotherapy inflammation. Therefore, a considerable amount of recent data suggests that initial PET/CT surveillance as early as 8 weeks after completion of therapy may yield highly sensitive and specific information regarding the presence of residual neoplasm, distant metastases, or a second primary tumor (sensitivity, 90.9%; specificity, 93.3%) (Fig. 9).16,27
Fig. 7.
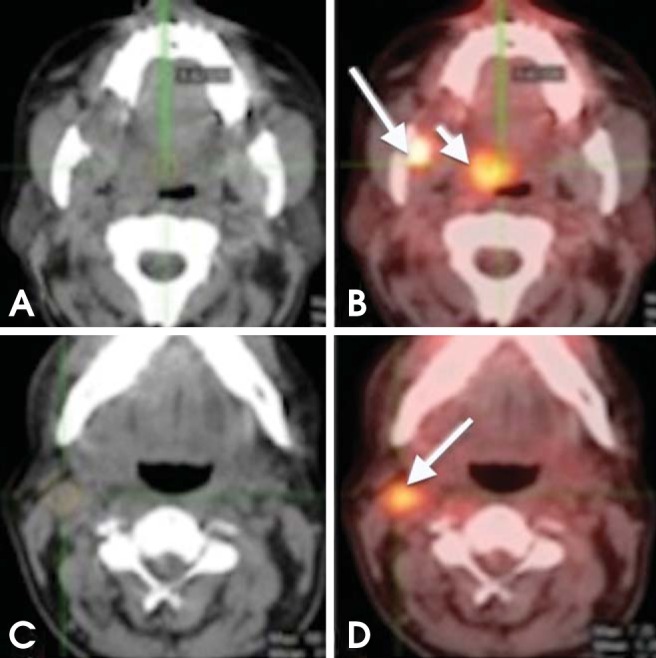
Synchronous tumor. A 65-year-old man recently diagnosed with SCC of the right mandibular retromolar trigon. (A, B) Axial CT and PET/CT images show intense FDG uptake in the retromolar trigon; another focal area of increased uptake can be seen in the right soft palate (short arrow); further biopsy confirmed a tumor (metachronous). (C, D) Axial CT and PET/CT images show increased FDG uptake in a right level IIA lymph node (arrow).
Fig. 8.
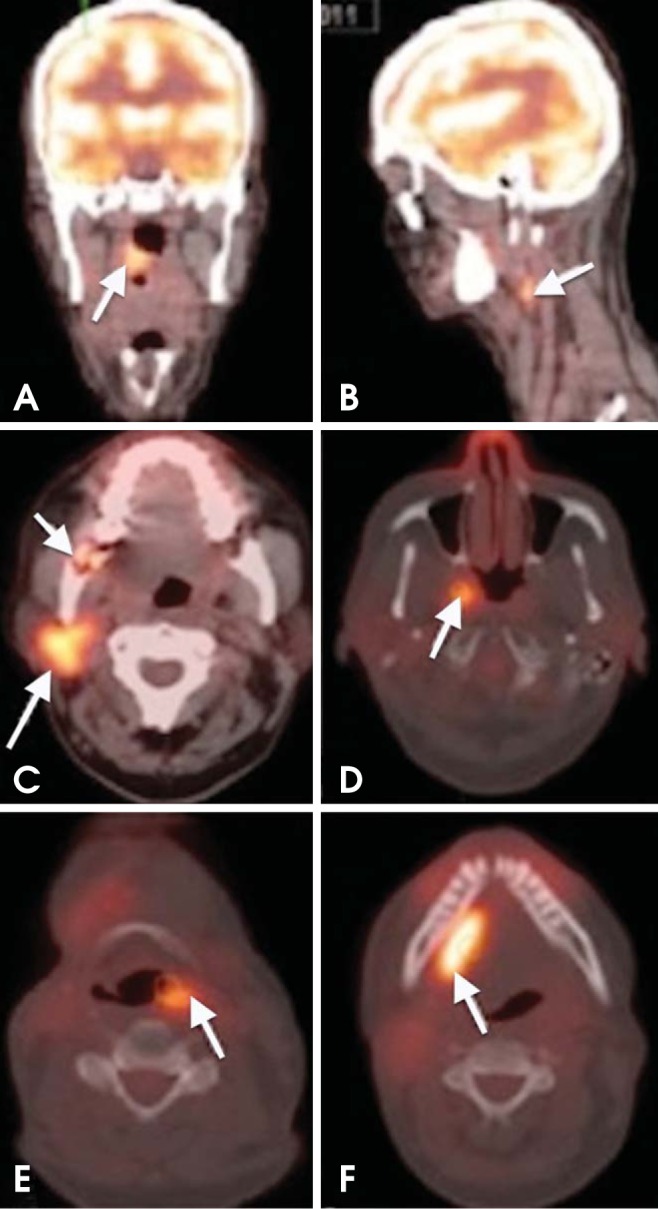
Metachronous tumors. A 64-year-old woman diagnosed with SCC of the right tonsillar fossa. (A, B) Axial PET/CT images show increased FDG uptake in the right tonsillar fossa and ipsilateral level IIA level nodal metastasis. (C, D) Surveillance PET/CT scan performed 10 months after chemoradiation demonstrates new tumors in the right parotid gland and right lateral wall of the nasopharynx. The right retromolar trigon shows bone destruction with slightly increased metabolic activity of maximum standardized uptake value (SUVmax) of 5.68. This area represented radiation-induced osteomyelitis (short arrow in C). (E, F) The PET/CT scan performed 9 months later reveals additional metachronous tumors in the left piriform fossa and right floor of the mouth.
Fig. 9.
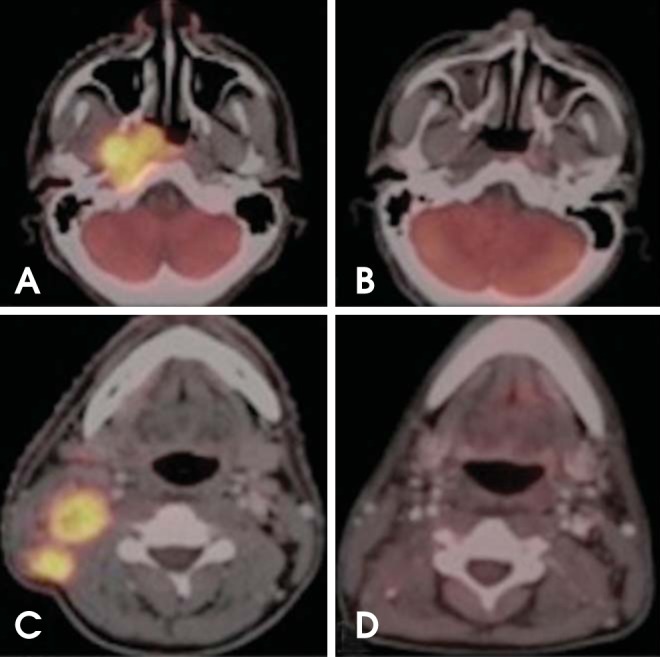
Monitoring therapy response. Nasopharyngeal carcinoma with ipsilateral level IIB nodal metastasis. (A, C) Axial PET/CT images show increased FDG uptake in the right nasopharynx and ipsilateral level IIB lymph nodes. (B, D) Six months after initiation of chemoradiation, complete anatomic and metabolic response to treatment.
Limitations and Pitfalls
Despite the superior accuracy of FDG-PET/CT, there are certain situations where a misinterpretation of FDG uptake may be unavoidable.
Physiologic FDG uptake
Anatomic structures such as Waldeyer's ring, the salivary glands, and the muscles of mastication have inherently high FDG uptake (Figs. 10 and 11). Variable FDG avidity in normal structures may make it more difficult to distinguish pathologic activity from physiologic activity.28 However, the superior localization of FDG uptake with combined PET/CT can help avoid misinterpretation between a tumor and physiologic uptake.29
Fig. 10.
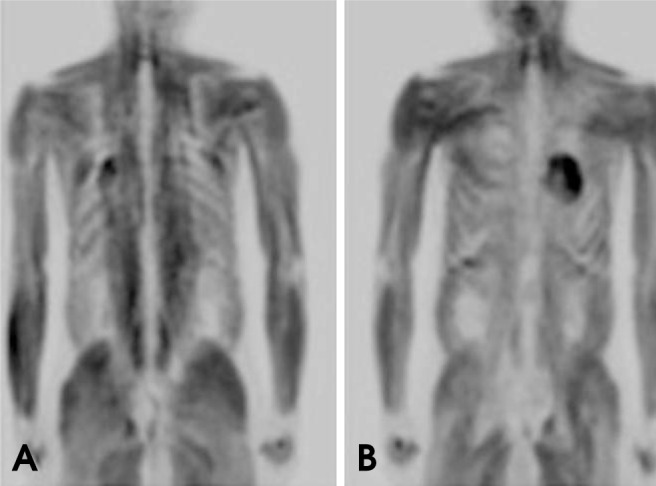
Coronal PET images show diffuse whole-body muscle uptake of FDG. This condition can be caused by insulin, recent food intake, and strenuous exercise that involves many muscle groups.
Fig. 11.
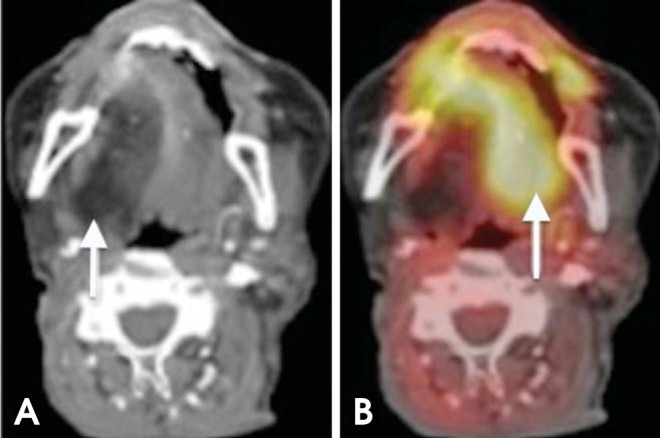
XII nerve dysfunction. (A) Axial CT image shows fatty degeneration of the right half of the tongue. (B) PET/CT shows increased FDG avidity in the left (normal) half of the tongue (paradoxical finding).
Inflammatory changes
Inflammatory and infectious conditions such as those induced by cancer therapy or foreign bodies, granulomatous processes, and odontogenic abscesses are associated with increased FDG uptake (Fig. 12). This is due to diffuse FDG accumulation by macrophages in inflammatory and neoplastic lesions.30 However, the timing of tumor surveillance is critical.
Fig. 12.

A 51-year-old man with a 3-year history of nasopharyngeal carcinoma. The patient presented with new mucosal swelling in the left retromolar trigon; recurrence was suspected. PET-CT image shows increased metabolism in the corresponding area (arrow); however, further clinical examination and biopsy revealed a dental abscess.
Metallic artifacts
Metallic artifacts caused by dental fillings, bridges, implants, or reconstructive hardware can severely degrade the interpretability and hence, the diagnostic localization of CT images. However, on FDG-PET/CT images, metallic artifacts do not negatively affect the identification of primary HNSCC, because tumors usually have sufficiently high FDG uptake to not be obscured by metallic artifacts.
Low FDG uptake
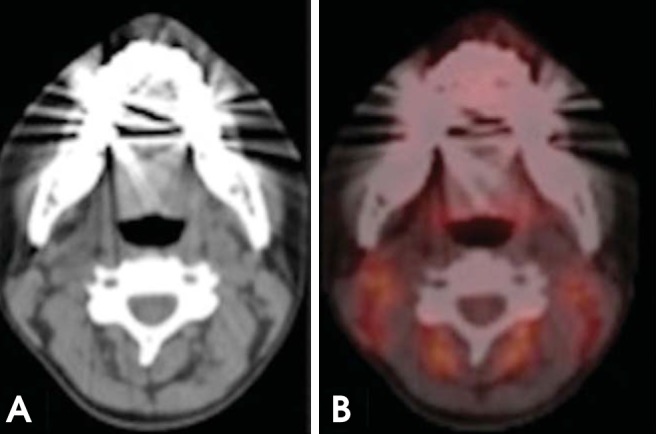
Salivary gland tumors (e.g., mucoepidermoid and adenoid cystic carcinoma), cystic lesions, and necrotic lesions have inherently low FDG uptake.31 PET/CT examination of such conditions may yield false-negative findings (Fig. 13).
Fig. 13.
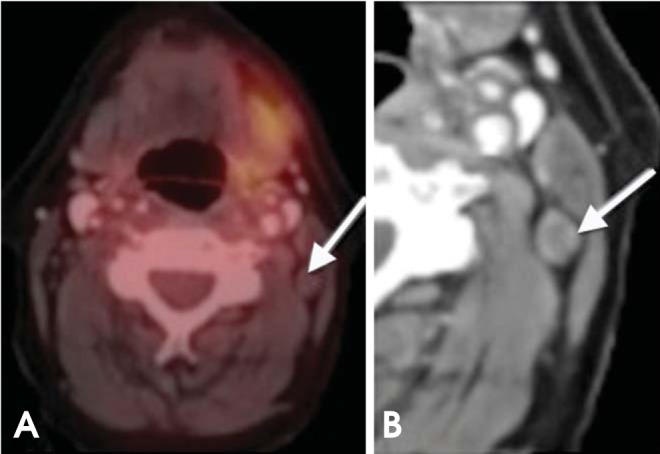
False-negative PET/CT overlooks nodal metastasis. (A) On axial PET/CT, the lymph node (arrow) has low FDG uptake belying nodal disease. (B) On axial contrast-enhanced CT, the central necrosis and rounded configuration are clues to the nodal metastasis; confirmed with further biopsies. A PET/CT examination of such conditions may yield false-negative findings (metastatic thyroid carcinoma).
Scanner resolution
FDG-PET/CT imaging can underestimate tumors that are smaller than two times the spatial resolution of the scanner. Thus, the sensitivity of the PET scanners currently used decreases with lesions smaller than 1 cm. In the future, improved scanner resolution may help overcome this limitation.28
The role of FDG-PET/CT in HNSCC can be summarized as follows:
Whole-body PET/CT is the modality of choice for staging patients with T3 and T4 disease; however, it is not very necessary for the T1 and T2 stages.
PET/CT is a supplement to, but not a substitute for, panendoscopy and tissue sampling.
PET/CT is not sufficiently accurate to preclude neck dissection in patients with negative neck (N0).
PET/CT is superior to conventional imaging modalities for radiation treatment planning, allowing for improved tumor coverage and sparing of normal tissues.
PET/CT is useful for monitoring treatment response in HNSCC.
PET/CT is useful for restaging, including nodal disease, distant metastasis, and recurrence
Initial surveillance PET/CT should be performed at least 8 weeks after the conclusion of therapy.
Acknowledgments
We would like to express our sincere gratitude to Dr. Richard H. Wiggins III, Professor of Radiology, University of Utah Health Sciences Center, for the provision of Figs. 6 and 10.
References
- 1.Chandra R. Nuclear medicine physics: the basics. 6th ed. Philadelphia: Lippincott Williams & Wilkins; 2004. [Google Scholar]
- 2.Blodgett TM, Fukui MB, Snyderman CH, Branstetter BF, 4th, McCook BM, Townsend DW, et al. Combined PET-CT in the head and neck: part 1. Physiologic, altered physiologic, and artifactual FDG uptake. Radiographics. 2005;25:897–912. doi: 10.1148/rg.254035156. [DOI] [PubMed] [Google Scholar]
- 3.Jemal A, Siegel R, Ward E, Murray T, Xu J, Smigal C, et al. Cancer statistics, 2006. CA Cancer J Clin. 2006;56:106–130. doi: 10.3322/canjclin.56.2.106. [DOI] [PubMed] [Google Scholar]
- 4.Forastiere A, Koch W, Trotti A, Sidransky D. Head and neck cancer. N Engl J Med. 2001;345:1890–1900. doi: 10.1056/NEJMra001375. [DOI] [PubMed] [Google Scholar]
- 5.Antoch G, Vogt FM, Freudenberg LS, Nazaradeh F, Goehde SC, Barkhausen J, et al. Whole-body dual-modality PET/CT and whole-body MRI for tumor staging in oncology. JAMA. 2003;290:3199–3206. doi: 10.1001/jama.290.24.3199. [DOI] [PubMed] [Google Scholar]
- 6.Ha PK, Hdeib A, Goldenberg D, Jacene H, Patel P, Koch W, et al. The role of positron emission tomography and computed tomography fusion in the management of early-stage and advanced-stage primary head and neck squamous cell carcinoma. Arch Otolaryngol Head Neck Surg. 2006;132:12–16. doi: 10.1001/archotol.132.1.12. [DOI] [PubMed] [Google Scholar]
- 7.Suzuki H, Hasegawa Y, Terada A, Ogawa T, Hyodo I, Suzuki M, et al. Limitations of FDG-PET and FDG-PET with computed tomography for detecting synchronous cancer in pharyngeal cancer. Arch Otolaryngol Head Neck Surg. 2008;134:1191–1195. doi: 10.1001/archotol.134.11.1191. [DOI] [PubMed] [Google Scholar]
- 8.Branstetter BF, 4th, Blodgett TM, Zimmer LA, Snyderman CH, Johnson JT, Raman S, et al. Head and neck malignancy: Is PET/CT more accurate than PET or CT alone? Radiology. 2005;235:580–586. doi: 10.1148/radiol.2352040134. [DOI] [PubMed] [Google Scholar]
- 9.Yau YY, Samman N, Yeung RW. Positron emission tomography/computed tomography true fusion imaging in clinical head and neck oncology: early experience. J Oral Maxillofac Surg. 2005;63:479–486. doi: 10.1016/j.joms.2004.07.022. [DOI] [PubMed] [Google Scholar]
- 10.Layland MK, Sessions DG, Lenox J. The influence of lymph node metastasis in the treatment of squamous cell carcinoma of the oral cavity, oropharynx, larynx, and hypopharynx: N0 versus N+ Laryngoscope. 2005;115:629–639. doi: 10.1097/01.mlg.0000161338.54515.b1. [DOI] [PubMed] [Google Scholar]
- 11.McHam SA, Adelstein DJ, Rybicki LA, Lavertu P, Esclamado RM, Wood BG, et al. Who merits a neck dissection after definitive chemoradiotherapy for N2-N3 squamous cell head and neck cancer? Head Neck. 2003;25:791–798. doi: 10.1002/hed.10293. [DOI] [PubMed] [Google Scholar]
- 12.Brouwer J, Senft A, de Bree R, Comans EF, Golding RP, Castelijns JA, et al. Screening for distant metastases in patients with head and neck cancer: is there a role for (18)FDG-PET? Oral Oncol. 2006;42:275–280. doi: 10.1016/j.oraloncology.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 13.Jones J, Farag I, Hain SF, McGurk M. Positron emission tomography (PET) in the management of oro-pharyngeal cancer. Eur J Surg Oncol. 2005;31:170–176. doi: 10.1016/j.ejso.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 14.Ng SH, Yen TC, Liao CT, Chang JT, Chan SC, Ko SF, et al. 18F-FDG PET and CT/MRI in oral cavity squamous cell carcinoma: a prospective study of 124 patients with histologic correlation. J Nucl Med. 2005;46:1136–1143. [PubMed] [Google Scholar]
- 15.Krabbe CA, Dijkstra PU, Pruim J, van der Laan BF, van der Wal JE, Gravendeel JP, et al. FDG PET in oral and oropharyngeal cancer. Value for confirmation of N0 neck and detection of occult metastases. Oral Oncol. 2008;44:31–36. doi: 10.1016/j.oraloncology.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 16.Kim MR, Roh JL, Kim JS, Choi SH, Nam SY, Kim SY. 18F-fluorodeoxyglucose-positron emission tomography and bone scintigraphy for detecting bone metastases in patients with malignancies of the upper aerodigestive tract. Oral Oncol. 2008;44:148–152. doi: 10.1016/j.oraloncology.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 17.Kwee TC, Kwee RM. Combined FDG-PET/CT for the detection of unknown primary tumors: systematic review and metaanalysis. Eur Radiol. 2009;19:731–744. doi: 10.1007/s00330-008-1194-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Freudenberg LS, Fischer M, Antoch G, Jentzen W, Gutzeit A, Rosenbaum SJ, et al. Dual modality of 18F-fluorodeoxyglucose-positron emission tomography/computed tomography in patients with cervical carcinoma of unknown primary. Med Princ Pract. 2005;14:155–160. doi: 10.1159/000084632. [DOI] [PubMed] [Google Scholar]
- 19.Torizuka T, Tanizaki Y, Kanno T, Futatsubashi M, Naitou K, Ueda Y, et al. Prognostic value of 18F-FDG PET in patients with head and neck squamous cell cancer. AJR Am J Roentgenol. 2009;192:W156–W160. doi: 10.2214/AJR.08.1429. [DOI] [PubMed] [Google Scholar]
- 20.Machtay M, Natwa M, Andrel J, Hyslop T, Anne PR, Lavarino J, et al. Pretreatment FDG-PET standardized uptake value as a prognostic factor for outcome in head and neck cancer. Head Neck. 2009;31:195–201. doi: 10.1002/hed.20942. [DOI] [PubMed] [Google Scholar]
- 21.Allal AS, Slosman DO, Kebdani T, Allaoua M, Lehmann W, Dulguerov P. Prediction of outcome in head-and-neck cancer patients using the standardized uptake value of 2-[18F]fluoro-2-deoxy-D-glucose. Int J Radiat Oncol Biol Phys. 2004;59:1295–1300. doi: 10.1016/j.ijrobp.2003.12.039. [DOI] [PubMed] [Google Scholar]
- 22.Yao M, Smith RB, Graham MM, Hoffman HT, Tan H, Funk GF, et al. The role of FDG PET in management of neck metastasis from head-and-neck cancer after definitive radiation treatment. Int J Radiat Oncol Biol Phys. 2005;63:991–999. doi: 10.1016/j.ijrobp.2005.03.066. [DOI] [PubMed] [Google Scholar]
- 23.de Visscher AV, Manni JJ. Routine long-term follow-up in patients treated with curative intent for squamous cell carcinoma of the larynx, pharynx, and oral cavity. Does it make sense? Arch Otolaryngol Head Neck Surg. 1994;120:934–939. doi: 10.1001/archotol.1994.01880330022005. [DOI] [PubMed] [Google Scholar]
- 24.Porceddu SV, Jarmolowski E, Hicks RJ, Ware R, Weih L, Rischin D, et al. Utility of positron emission tomography for the detection of disease in residual neck nodes after (chemo) radiotherapy in head and neck cancer. Head Neck. 2005;27:175–181. doi: 10.1002/hed.20130. [DOI] [PubMed] [Google Scholar]
- 25.Zimmer LA, Snyderman C, Fukui MB, Blodgett T, McCook B, Townsend DW, et al. The use of combined PET/CT for localizing recurrent head and neck cancer: the Pittsburgh experience. Ear Nose Throat J. 2005;84:104, 106, 108–110. [PubMed] [Google Scholar]
- 26.Goerres GW, Schmid DT, Grätz KW, von Schulthess GK, Eyrich GK. Impact of whole body positron emission tomography on initial staging and therapy in patients with squamous cell carcinoma of the oral cavity. Oral Oncol. 2003;39:547–551. doi: 10.1016/s1368-8375(03)00016-2. [DOI] [PubMed] [Google Scholar]
- 27.Ryan WR, Fee WE, Jr, Le QT, Pinto HA. Positron emission tomography for surveillance of head and neck cancer. Laryngoscope. 2005;115:645–650. doi: 10.1097/01.mlg.0000161345.23128.d4. [DOI] [PubMed] [Google Scholar]
- 28.Fukui MB, Blodgett TM, Snyderman CH, Johnson JJ, Myers EN, Townsend DW, et al. Combined PET-CT in the head and neck. Part 2. Diagnostic uses and pitfalls of oncologic imaging. Radiographics. 2005;25:913–930. doi: 10.1148/rg.254045136. [DOI] [PubMed] [Google Scholar]
- 29.Kubota R, Yamada S, Kubota K, Ishiwata K, Tamahashi N, Ido T. Intratumoral distribution of fluorine-18-fluorodeoxyglucose in vivo: high accumulation in macrophages and granulation tissues studied by microautoradiography. J Nucl Med. 1992;33:1972–1980. [PubMed] [Google Scholar]
- 30.Veeramachaneni R, Gulick J, Halldorsson A, Van T, Zhang P, Herrera G. Benign myoepithelioma of the lung: a case report and review of the literature. Arch Pathol Lab Med. 2001;125:1494–1496. doi: 10.5858/2001-125-1494-BMOTL. [DOI] [PubMed] [Google Scholar]


