Abstract
Background
To avert the differentiation of allergen-specific Th2 cells in atopic individuals is a major goal in the prevention and therapy of IgE-mediated allergy. We aimed to compare different toll-like receptor (TLR) agonists regarding their effects on antigen-presenting cells and the differentiation of naïve T cells from allergic patients.
Methods
Monocytes and monocyte-derived dendritic cells (mdDC) from allergic patients were stimulated with Pam3CSK4 (TLR1/2 ligand), FSL-1 (TLR2/6 ligand), monophosphoryl lipid (MPL)-A, lipopolysaccharide (LPS, both TLR4 ligands), and flagellin (TLR5 ligand). Allergen uptake and upregulation of CD40, CD80, CD83, CD86, CD58, CCR7 and PD-L1 were analyzed by flow cytometry. Functional maturation of mdDC was tested in mixed leukocyte reactions, and the synthesis of proinflammatory cytokines, IL-10 and members of the IL-12 family was assessed. TLR-ligand-activated mdDC were used to stimulate naïve CD4+ T cells, and cytokine responses were assessed in supernatants and intracellularly.
Results
All TLR ligands except flagellin enhanced allergen uptake. All TLR ligands induced functional maturation of mdDC with differential expression of surface molecules and cytokines and promoted the differentiation of IFN-γ-producing T cells. LPS-matured mdDC exclusively induced Th1-like responses, whereas mdDC stimulated with the other TLR ligands induced both Th1- and Th0-like cells. Pam3CSK4 and flagellin additionally induced Th2-like cells. Th1-like responses were associated with higher expression levels of co-stimulatory molecules, PD-L1, IL-6, TNF-α, and IL-12p70. None of the TLR-ligand-stimulated mdDC induced IL-10- or IL-17-producing T cells.
Conclusion
Different TLR ligands differently influence T-cell responses due to varying activation of the three signals relevant for T-cell activation, that is, antigen presentation, co-stimulation and cytokine milieu.
Keywords: cytokines, dendritic cells, innate immune system, T-cell differentiation, toll-like receptor
IgE-mediated hypersensitivity results from an overwhelming Th2 response leading to the production of IgE antibodies (Abs), which then mediate allergic symptoms. In contrast, the response to allergens in nonallergic individuals is characterized by Th0/Th1 responses and regulatory T (Treg) cells (1, 2). Hence, the restoration of a balanced allergenspecific T-cell response in atopic individuals is a major goal in the prevention and treatment of allergic diseases. Indeed, successful allergen-specific immunotherapy (SIT) has been associated with the modulation of allergen-specific Th2 toward Th0/Th1 and Treg cells (3). The differentiation of naïve T cells into effector cells requires three signals. Antigen-specific activation of the T-cell receptor represents signal 1 and is mediated by antigen-derived peptides loaded to MHC class II molecules. Co-stimulatory molecules expressed on the surface of professional antigen-presenting cells (APC), for example, dendritic cells (DC), represent signal 2. Finally, APC-derived cytokines (signal 3) play a decisive role in skewing CD4+ T-cell responses toward a particular effector subset. For example, the presence of members of the IL-12 family and/or IL-18 promotes the induction of Th1 cells, whereas IL-10 promotes the differentiation of Treg cells (4–6).
Stimulating toll-like receptors (TLR) that recognize pathogen-associated molecular patterns is one possibility to promote Th1 responses (7). Therefore, TLR ligands have been considered as immunomodulators in SIT. In this respect, MPL-A, a nontoxic derivative of LPS targeting surface-expressed TLR4, and immunostimulatory DNA sequences of bacterial origin targeting intracellular TLR9 have been clinically evaluated (8–11). Both TLR ligands have been demonstrated to induce Th1-like cytokines in PBMC from allergic individuals (12, 13). Today, allergy vaccines containing MPL-A are routinely used for SIT of adults and children (14, 15) whereas the use of immunostimulatory DNA for allergy treatment is currently not pursued. Besides TLR4, TLR1, TLR2, TLR5, and TLR6 are located on the surface of various types of professional APC, namely myeloid DC, monocytes, and Langerhans cells (16). Monocyte-derived (md)DC from allergic donors have been shown to express high levels of TLR2, TLR4, TLR5 and TLR6 and to respond to TLR activation like mdDC from nonallergic individuals (16). However, the effects of mdDC stimulated with different TLR ligands on T-cell differentiation have not been compared yet.
To address this issue, mdDC from allergic patients were stimulated with Pam3CSK4 (TLR1/2), FSL-1 (TLR2/6), lipopolysaccharide (LPS), monophosphoryl lipid (MPL)-A (both TLR4 ligands), and flagellin from Salmonella typhimurium (TLR5). Allergen uptake was studied by employing fluorescence-labeled Bet v 1, the major birch pollen allergen. The expression of the maturation marker CD83, co-stimulatory molecules (CD40, CD80, CD86), and the homing factor CCR7 was analyzed. We also included the analysis of CD58 expression levels because CD2-CD58 interaction has been shown to induce regulatory T cells (17). Furthermore, the expression of the negative regulatory molecule programmed death ligand 1 (PD-L1) was assessed as PD-L1 expression has been linked to the ability to promote Th1-like responses (18). TLR-ligand-induced levels of proinflammatory cytokines (IL-1β, IL-6, TNF-α), different members of the IL-12 family (IL-12p70, IL-23, IL-27), and IL-10 were determined. Finally, the polarizing capacity of TLR-ligand-activated mdDC was tested in co-cultures with naïve CD4+ T cells.
Materials and methods
Reagents
FSL-1, Pam3CSK4, MPL-A, LPS (source: E. coli 0111:B4), and flagellin from S. typhimurium were purchased from InvivoGen, San Diego, CA, USA. Recombinant Bet v 1.0101 (referred to as Bet v 1) was purchased from Biomay AG, Vienna, Austria. Endotoxin levels were below 1 EU/mg for each reagent. Labeling of Bet v 1 with pHrodo succinimidyl ester (Invitrogen, Carlsbad, CA, USA) was performed as described previously (19).
Allergic individuals
Heparinized blood was obtained from individuals allergic to birch pollen. All patients suffered from rhinoconjunctivitis during spring, and none of them had allergic asthma and atopic dermatitis. Moreover, allergy was documented by positive skin prick reactions and allergen-specific IgE levels of >3.5 kU/L (ImmunoCAP; Thermo Fisher Scientific, Uppsala, Sweden). Nonallergic individuals had neither allergic symptoms nor specific IgE levels. The study had been approved by the local medical ethics committee, and informed written consent was obtained from all individuals.
Allergen uptake
Monocytes (2 × 105) isolated by anti-CD14-coated magnetic beads (Miltenyi Biotec, Bergisch-Gladbach, Germany) were incubated with pHrodo-labeled Bet v 1 (3 μg/ml) in the presence or absence of TLR ligands. After 14 h, cells were counter-stained with fluorescence-labeled mAb specific to CD14 (BD Biosciences, San Jose, CA, USA), HLA-DR (BioLegend, San Diego, CA, USA), or the respective isotype controls and analyzed by flow cytometry.
Monocyte-derived dendritic cells
Monocyte-derived dendritic cells (mdDC) were generated from isolated monocytes by incubation with IL-4 and GM-CSF as described (20). Immature mdDC (1 × 104/well) were either left untreated or stimulated with Pam3CSK4, FSL-1, LPS, MPL-A, or flagellin in 96 well plates (Nunclon; Nunc, Copenhagen, Denmark). After 48 h, cells were incubated with 20% human AB serum in PBS containing 0.1% BSA and 0.1% NaN3 for 20 min at 4°C. Thereafter, cells were stained with fluorescence-labeled mAb specific to CD80, CD83, CD86, CD40, HLA-DR (all from BD Biosciences), and CCR7 (BioLegend). Anti-CD58 and anti-PD-L1 mAb were detected with a PE-conjugated anti-mouse IgG Ab (BioLegend). Respective isotype controls were done in parallel. Flow cytometry was performed with a FACS Canto (BD Biosciences) and evaluated by flowjo software v10 (Treestar Inc., Ashland, OR, USA). Cytokine levels were determined in supernatants (SN) harvested after 48 h using the Luminex System 100 (Luminex, Austin, TX, USA).
For mixed leukocyte reactions, mdDC were stimulated with TLR ligands for 48 h and irradiated (60 Gy), and allogeneic PBMC (1 × 105) were added in UltraCulture medium (Lonza, Basel, Switzerland) supplemented with 2 mM L-glutamine and 2 × 10−5M 2-mercaptoethanol for 6 days. Proliferation was assessed by the addition of [3H]-thymidine (New England Nuclear Corp GmbH, Vienna, Austria; 0.5 μCi/well) during the last 16 h of culture. All samples were analyzed in triplicate.
Stimulation of naïve CD4+ T cells
Naïve CD4+ T cells were purified from PBMC using the Naïve CD4+ T cell Isolation Kit II (Miltenyi Biotec), resulting in >90% CD4+CD45RA+ cells. CD4+CD45RA+ T cells (1 × 105) were mixed with irradiated autologous mdDC (1 × 104) that had been stimulated with TLR ligands for 24 h. After 6 days, SN was collected and proliferation was assessed by the addition of [3H]-thymidine for 16 h. Cytokine levels in SN were analyzed using the Luminex System 100. For intracellular cytokine detection, naïve CD4+ T cells (1 × 105) were mixed with irradiated, allogeneic mdDC (1 × 104) prestimulated with TLR ligands for 16 h. After 6 days, these T cells were re-stimulated with freshly isolated TLR-ligand-activated mdDC and, after another 6 days, activated with PMA (1 ng/ml), ionomycin (1.25 μM), and brefeldin A (10 μg/ml) for 5 h. Cells were stained with fixable viability dye eFluor® 506 (eBioscience Inc., San Diego, CA, USA), then fixed, permeabilized and stained with fluorescence-labeled mAb specific to IL-4 (BioLegend), CD4, IL-13 (both from BD Biosciences), IL-10, and IFN-γ (both from eBioscience Inc.) or the respective isotype controls as described (21).
Statistics
Statistical data analysis was performed using ibm spss Statistics 20 (SPSS, Chicago, IL, USA). Data were not normally distributed according to the Shapiro–Wilk test. Differences according to the Wilcoxon signed rank test were considered statistically significant for values of P < 0.05.
Results
Effects of TLR stimulation on allergen uptake by antigen-presenting cells
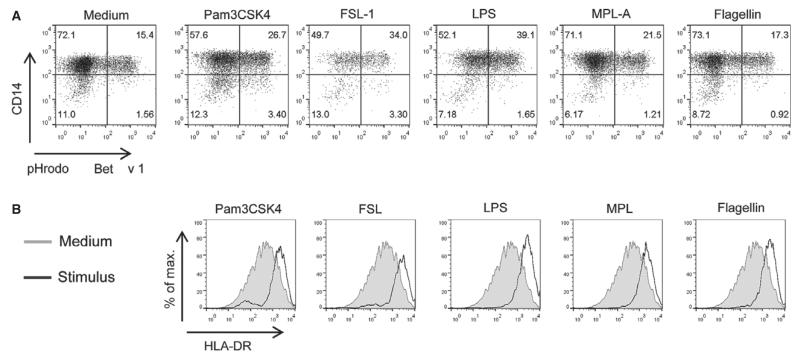
We first investigated the effects of TLR ligands on allergen uptake as a prerequisite for the loading of MHC class II molecules with allergen-derived peptides. Because TLR-ligand-matured mdDC showed the expected lower capacity to internalize antigens than immature mdDC (data not shown), purified CD14+ cells were incubated with Bet v 1 in the absence or presence of Pam3CSK4 (100 nM), FSL-1 (100 nM), LPS (50 nM), MPL-A (50 nM), or flagellin (3 nM). These concentrations had been determined to give highest levels of mdDC maturation, that is, highest upregulation of CD83, for each TLR ligand in pilot experiments (data not shown), and were used throughout the study. Monocytes responded to LPS, FSL-1, or Pam3CSK4 with a clearly enhanced uptake of the major birch pollen allergen (Fig. 1A). MPL-A only slightly increased internalization of Bet v 1, whereas flagellin had no effect on allergen uptake. All TLR ligands similarly induced activation of monocytes as demonstrated by HLA-DR upregulation (Fig. 1B).
Figure 1. Effects of TLR ligands on allergen uptake.
(A) Purified CD14+ cells were incubated with pHrodo-labeled Bet v 1 (3 μg/ml) without/with Pam3CSK4 (100 nM), FSL-1 (100 nM), LPS (50 nM), MPL-A (50 nM), or flagellin (3 nM). (B) HLA-DR expression on monocytes. One of four separate experiments with similar results is shown.
Phenotypic and functional maturation of mdDC in response to different TLR ligands
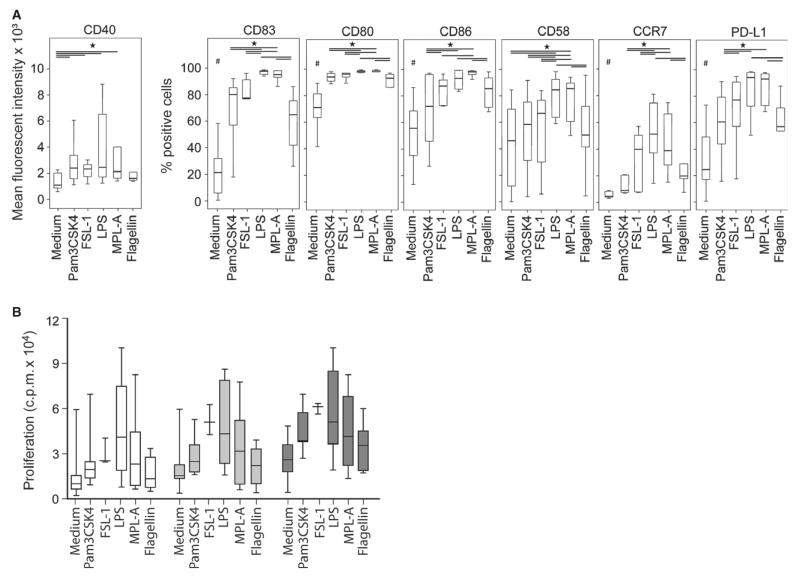
Monocyte-derived dendritic cells from five allergic individuals were stimulated with Pam3CSK4, FSL-1, LPS, MPL-A, or flagellin. Compared to medium alone, all TLR ligands significantly enhanced the expression of CD80, CD83, CD86, CCR7, and PD-L1 (P < 0.05, Fig. 2A). Pam3CSK4, FSL-1, LPS, and MPL-A significantly upregulated the expression of CD40, whereas only TLR4 ligands significantly enhanced CD58. TLR4 ligands induced a significantly higher expression of all surface molecules than TLR2 ligands and flagellin. TLR2 ligands and flagellin had similar effects. FSL-1 (TLR2/6) induced significantly higher expression of CD86 and PDL1 than Pam3CSK4 (TLR1/2).
Figure 2. Maturation of mdDC by different TLR ligands.
(A) Immature mdDC (n = 5) were incubated without/with Pam3CSK4 (100 nM), FSL-1 (100 nM), LPS (50 nM), MPL-A (50 nM), or flagellin (3 nM) for 48 h. Surface markers were assessed by flow cytometry, *P < 0.05, Wilcoxon signed rank test, #all stimuli significantly differed from medium (P < 0.05). (B) Proliferation (counts per minute, cpm) of PBMC to increasing numbers of prestimulated allogeneic mdDC (white box plots = 2500 mdDC, light gray = 5000 and dark gray = 10 000 mdDC).
To test their functional capacity, TLR-ligand-activated mdDC were co-cultured with allogeneic PBMC. Consistent with the expression of co-stimulatory molecules, mdDC treated by TLR4 ligands and FSL-1 induced the strongest proliferative responses, followed by Pam3CSK4 and flagellin (Fig. 2B).
Cytokine responses of mdDC in response to different TLR ligands
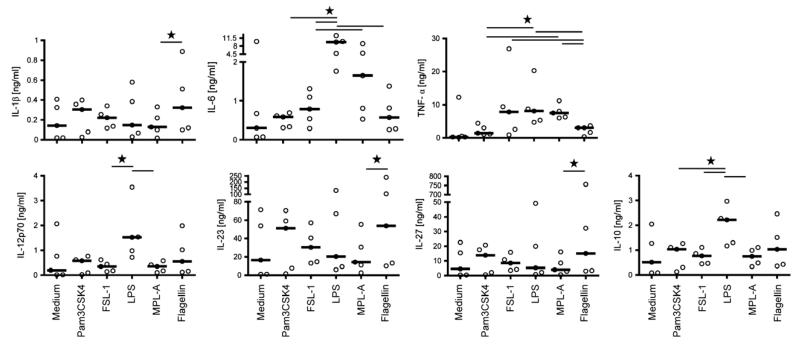
We analyzed the production of proinflammatory cytokines (IL-1β, IL-6, and TNF-α) and different members of the IL-12 family (IL-12p70, IL-23, and IL-27) as well as IL-10 by mdDC stimulated with the different TLR ligands. Distinct response patterns to the different TLR ligands were found (Fig. 3). TLR4 ligands induced the production of IL-6, TNF-α, IL-12p70, and IL-10 but not of IL-1β, IL-23, and IL-27. In all experiments, LPS was a more potent inducer of cytokine synthesis than MPL-A. TLR2 ligands clearly enhanced the synthesis of IL-1β, IL-6, TNF-α, IL-12p70, IL-23, IL-27, and IL-10. Except for IL-6 and TNF-α, Pam3CSK4 was the more potent stimulus than FSL-1. Similar to TLR2 ligands, flagellin induced all cytokines under investigation.
Figure 3. Cytokine responses of mdDC to different TLR ligands.
Immature mdDC (n = 5) were incubated without/with Pam3CSK4 (100 nM), FSL-1 (100 nM), LPS (50 nM), MPL-A (50 nM), or flagellin (3 nM) for 48 h, and secreted cytokines were determined in SN. Bold lines indicate median values. *P < 0.05, Wilcoxon signed rank test.
Effects of TLR-ligand-activated mdDC on naïve CD4+ T cells
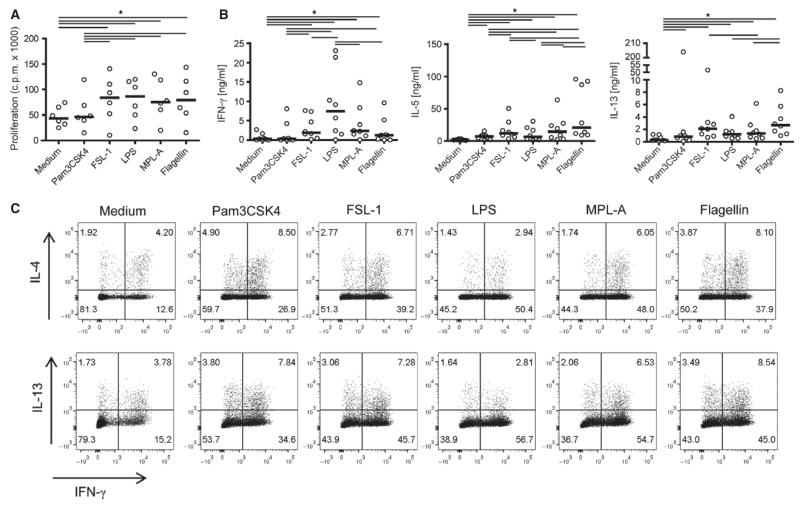
TLR-ligand-preactivated mdDC from eight allergic donors were incubated with naïve T cells, and proliferative and cytokine responses were assessed after 6 days (Fig. 4A,B). MdDC matured with all TLR ligands except Pam3CSK4 induced significant T-cell proliferation. As expected, LPS-matured mdDC promoted strong IFN-γ synthesis and low levels of IL-5 and IL-13, characteristic for a Th1-like response. In contrast, T-cell responses to mdDC matured with MPL-A and FSL-1 were characterized by significantly lower levels of IFN-γ and significantly higher levels of Th2 cytokines. Pam3CSK4-treated mdDC induced low levels of Th2 cytokines and negligible amounts of IFN-γ, whereas mdDC treated with flagellin promoted the synthesis of IFN-γ and the highest levels of both IL-5 and IL-13. Similar results were obtained with naïve T cells and mdDC derived from three nonallergic individuals (data not shown). IL-4, IL-10, and IL-17 were not detectable in any of the cultures.
Figure 4. T-cell-polarizing activity of TLR-ligand-activated mdDC.
(A) Proliferative and (B) cytokine responses of naïve CD4+ T cells incubated with TLR-ligand-stimulated mdDC for 6 days. Bold lines represent median values. (C) Intracellular cytokine staining of naïve CD4+ T cells primed with TLR-ligand-activated mdDC. One representative experiment of four with similar results is shown. cpm, counts per minute; *P < 0.05, Wilcoxon signed rank test.
To detect IL-4 and IL-10 production as well as possible differences in the Th-cell subsets differentiated by different TLR-ligand-activated mdDC, we intracellularly stained for these cytokines, IL-13 and IFN-γ in T cells from four allergic patients. IL-4+IFN-γ− and IL-13+IFN-γ− cells were defined as Th2-like, IL-4+IFN-γ+, and IL-13+IFN-γ+ cells as Th0-like and IL-4−IFN-γ+ and IL-13−IFN-γ+ cells as Th1-like cells, respectively. Figure 4C shows that mdDC matured with all TLR ligands induced Th1-like cells though to a varying degree (LPS>MPL-A>FSL-1>flagellin>Pam3CSK4). LPS-matured mdDC exclusively promoted Th1-like responses, whereas mdDC stimulated with MPL-A, Pam3CSK4, FSL-1, or flagellin also primed Th0-like cells. The strongest induction of Th0-like cells was observed in response to Pam3CSK4 and flagellin. These TLR ligands and to a lower extent also FSL-1 induced Th2-like cells. IL-10 could not be detected in any of the cultures (data not shown).
Discussion
To avert the development of allergen-specific Th2 responses in atopic individuals is an important strategy in the prevention and cure of IgE-mediated allergic disorders. Here, we assessed the T-cell-polarizing capacity of ligands for TLR1/2, TLR2/6, TLR4, and TLR5, which are expressed on the surface of myeloid DC, macrophages, and Langerhans cells located in the skin, that is, at the site of vaccine injection. We deliberately used the concentration of each TLR ligand that induced highest levels of mdDC maturation as fully matured DC are a prerequisite for the successful induction of Th1 responses in vivo (22). Under these conditions, all TLR ligands instructed mdDC to induce IFN-γ-producing T cells (23). However, analyses at the single cell level revealed clear differences in the obtained T-cell subsets: LPS-treated mdDC promoted solely Th1 cells while mdDC activated by MPL-A, FSL-1, Pam3CSK4, and flagellin also induced Th0-like cells that concomitantly produced Th2 cytokines and IFN-γ. Only mdDC matured with flagellin, Pam3CSK4 and to a lower extent with FSL-1 additionally induced Th2 cells. Priming of Th2 responses accorded with a less pronounced upregulation of CD40, CD80, CD86, CD58, and PD-L1, a limited production of IL-6 and TNF-α, and an enhanced synthesis of IL-1β, IL-23, and IL-27 by mdDC. Recently, it has been reported that TLR4-induced IL-1β and not IL-27 significantly enhanced the production of IL-13 in PBMC (24). Along these lines, our data provide evidence that IL-1β may also be an important cytokine for the polarization of naïve T cells toward Th2 responses. It has been observed previously that Pam3CSK4 instructs human DC to induce Th2 responses (25), and there is evidence for a Th2-promoting capacity of flagellin in mice (26, 27). In addition, patients with allergic asthma showed significantly higher titers of flagellin-specific antibodies compared with healthy controls (26). Furthermore, human keratinocytes have been shown to produce thymic stromal lymphopoietin (TSLP), a Th2-promoting cytokine, in response to flagellin (28). These studies already suggested a role for flagellin as risk factor for asthma and atopic dermatitis. Our study is first to demonstrate the induction of human Th2 cells by flagellin. Notably, flagellin also promoted Th2 polarization in naïve T cells derived from three nonallergic individuals (data not shown), indicating that the Th2-promoting capacity of flagellin is not restricted to the atopic status. Moreover, we noted that FSL-1 targeting TLR2/6 elicited effects distinct from Pam3CSK4 targeting TLR1/2. Pam3CSK4 more potently induced IL-10, IL-12p70, IL-23, and IL-27 than FSL-1. On the other hand, FSL-1 induced a significantly higher expression of CD86 and PD-L1, higher proliferative responses of allogeneic PBMC as well as a more pronounced production of IL-6 and TNF-α than Pam3CSK4. Together, these effects translated into a stronger induction of Th1-like cells by FSL-1 than by Pam3CSK4. Hence, we provide first evidence that stimulation of distinct TLR2 heterodimers has divergent effects on innate APC and subsequent differentiation of T cells from allergic patients.
Besides co-stimulatory molecules and cytokines, the mode of activation of the T-cell receptor influences T-cell polarization. A higher density of MHC class II complexes loaded with antigen-derived peptides on the surface of APC has been demonstrated to promote Th1-like responses whereas a lower density supported Th2-like responses (29). We found that LPS and FSL-1 strongly enhanced allergen uptake whereas Pam3CSK4 and MPL-A showed weaker effects. Flagellin only marginally affected allergen uptake of APC. Thus, the Th1-polarizing activity of the different TLR ligands accorded with enhanced endocytosis of allergen except for MPL-A. In addition to signal 2 and 3, also signal 1 for T-cell activation seemed to be differently regulated by TLR ligands targeting distinct TLR2-heterodimers, as FSL-1 and Pam3CSK4 showed diverse effects on the uptake of allergen.
Collectively, we found that agonists of TLR4 and TLR5 and the heterodimers TLR1/2 and TLR2/6 exert divergent T-cell-polarizing activities that result from differential effects on APC. In all cases, TLR4 ligands were significantly more effective than TLR2 and TLR5 ligands. Regarding TLR4 and TLR2 ligands, this observation accords with Agrawal et al. (25) who have studied mdDC from nonallergic individuals. Divergent modes of activation of human DC have been reported for TLR2 and TLR4 ligands (30). In contrast to TLR2 ligands, TLR4 agonists induced the chemokine IFN-inducible protein (IP)-10 and IL-12p70, which are both associated with Th1-like responses. We also found marked differences between TLR4 and TLR2 ligands. Whereas TLR4 ligands were stronger inducers of IL-6, TNF-α, IL-12p70, and IL-10 synthesis by mdDC, solely TLR2 ligands induced the production of IL-1β, IL-23, and IL-27. In our experimental setup, we could not detect IL-10-producing T cells in response to TLR-ligand-activated mdDC which may be referred to the use of fully matured mdDC.
Our in vitro analyses in allergic patients attest that different TLR ligands should be considered in the design of improved allergy vaccines. Possibly, TLR ligands that promote the differentiation of IL-4+IFN-γ+ T cells may have the advantage of not exclusively promoting potentially harmful Th1-like immune responses. The assessment of regulatory effects is still technically challenging. For definitive statements on the suitability of TLR ligands as adjuvants, the different aspects of their effects need to be addressed in humans in vivo.
Acknowledgments
The work was supported by the Austrian Science Fund, project FWF-SFB-F4610, the Christian Doppler Research Association and Biomay AG, Vienna, Austria.
Abbreviations
- APC
antigen-presenting cell
- DC
dendritic cell
- LPS
lipopolysaccharide
- mdDC
monocyte-derived dendritic cells
- MLR
mixed leukocyte reaction
- MPL
monophosphoryl lipid
- PD-L1
programmed death ligand 1
- SIT
allergen-specific immunotherapy
- SN
supernatant
- TCC
T-cell clone
- Th
T helper
- TLR
toll-like receptor
Footnotes
Conflicts of interest: The authors declare no competing financial interests.
References
- 1.Ebner C, Schenk S, Najafian N, Siemann U, Steiner R, Fischer GW, et al. Nonallergic individuals recognize the same T cell epitopes of Bet v 1, the major birch pollen allergen, as atopic patients. J Immunol. 1995;154:1932–1940. [PubMed] [Google Scholar]
- 2.Akdis M, Verhagen J, Taylor A, Karamloo F, Karagiannidis C, Crameri R, et al. Immune responses in healthy and allergic individuals are characterized by a fine balance between allergen-specific T regulatory 1 and T helper 2 cells. J Exp Med. 2004;199:1567–1575. doi: 10.1084/jem.20032058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Larche M, Akdis CA, Valenta R. Immuno-logical mechanisms of allergen-specific immunotherapy. Nat Rev Immunol. 2006;6:761–771. doi: 10.1038/nri1934. [DOI] [PubMed] [Google Scholar]
- 4.Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol. 2003;3:133–146. doi: 10.1038/nri1001. [DOI] [PubMed] [Google Scholar]
- 5.Brombacher F, Kastelein RA, Alber G. Novel IL-12 family members shed light on the orchestration of Th1 responses. Trends Immunol. 2003;24:207–212. doi: 10.1016/S1471-4906(03)00067-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smits HH, van Beelen AJ, Hessle C, Westland R, de Jong E, Soeteman E, et al. Commensal Gram-negative bacteria prime human dendritic cells for enhanced IL-23 and IL-27 expression and enhanced Th1 development. Eur J Immunol. 2004;34:1371–1380. doi: 10.1002/eji.200324815. [DOI] [PubMed] [Google Scholar]
- 7.Medzhitov R. Approaching the asymptote: 20 years later. Immunity. 2009;30:766–775. doi: 10.1016/j.immuni.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 8.Drachenberg KJ, Wheeler AW, Stuebner P, Horak F. A well-tolerated grass pollen-specific allergy vaccine containing a novel adjuvant, monophosphoryl lipid A, reduces allergic symptoms after only four preseasonal injections. Allergy. 2001;56:498–505. doi: 10.1034/j.1398-9995.2001.056006498.x. [DOI] [PubMed] [Google Scholar]
- 9.Drachenberg KJ, Heinzkill M, Urban E, Woroniecki SR. Efficacy and tolerability of short-term specific immunotherapy with pollen allergoids adjuvanted by monophosphoryl lipid A (MPL) for children and adolescents. Allergol Immunopathol. 2003;31:270–277. doi: 10.1016/s0301-0546(03)79195-2. [DOI] [PubMed] [Google Scholar]
- 10.Pfaar O, Barth C, Jaschke C, Hormann K, Klimek L. Sublingual allergen-specific immunotherapy adjuvanted with monophosphoryl lipid A: a phase I/IIa study. Int Arch Allergy Immunol. 2010;154:336–344. doi: 10.1159/000321826. [DOI] [PubMed] [Google Scholar]
- 11.Creticos PS, Schroeder JT, Hamilton RG, Balcer-Whaley SL, Khattignavong AP, Lindblad R, et al. Immunotherapy with a ragweed-toll-like receptor 9 agonist vaccine for allergic rhinitis. N Engl J Med. 2006;355:1445–1455. doi: 10.1056/NEJMoa052916. [DOI] [PubMed] [Google Scholar]
- 12.Puggioni F, Durham SR, Francis JN. Monophosphoryl lipid A (MPL) promotes allergen-induced immune deviation in favour of Th1 responses. Allergy. 2005;60:678–684. doi: 10.1111/j.1398-9995.2005.00762.x. [DOI] [PubMed] [Google Scholar]
- 13.Bohle B, Jahn-Schmid B, Maurer D, Kraft D, Ebner C. Oligodeoxynucleotides containing CpG motifs induce IL-12, IL-18 and IFN-gamma production in cells from allergic individuals and inhibit IgE synthesis in vitro. Eur J Immunol. 1999;29:2344–2353. doi: 10.1002/(SICI)1521-4141(199907)29:07<2344::AID-IMMU2344>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 14.Rosewich M, Schulze J, Eickmeier O, Telles T, Rose MA, Schubert R, et al. Tolerance induction after specific immunotherapy with pollen allergoids adjuvanted by monophosphoryl lipid A in children. Clin Exp Immunol. 2010;160:403–410. doi: 10.1111/j.1365-2249.2010.04106.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Musarra A, Bignardi D, Troise C, Passalacqua G. Long-lasting effect of a monophosphoryl lipid-adjuvanted immunotherapy to parietaria. A controlled field study. Eur Ann Allergy Clin Immunol. 2010;42:115–119. [PubMed] [Google Scholar]
- 16.Terhorst D, Kalali BN, Weidinger S, Illig T, Novak N, Ring J, et al. Monocyte-derived dendritic cells from highly atopic individuals are not impaired in their pro-inflammatory response to toll-like receptor ligands. Clin Exp Allergy. 2007;37:381–390. doi: 10.1111/j.1365-2222.2006.02639.x. [DOI] [PubMed] [Google Scholar]
- 17.Wakkach A, Cottrez F, Groux H. Differentiation of regulatory T cells 1 is induced by CD2 costimulation. J Immunol. 2001;167:3107–3113. doi: 10.4049/jimmunol.167.6.3107. [DOI] [PubMed] [Google Scholar]
- 18.Tseng SY, Otsuji M, Gorski K, Huang X, Slansky JE, Pai SI, et al. B7-DC, a new dendritic cell molecule with potent costimulatory properties for T cells. J Exp Med. 2001;193:839–846. doi: 10.1084/jem.193.7.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kitzmuller C, Nagl B, Deifl S, Walterskirchen C, Jahn-Schmid B, Zlabinger GJ, et al. Human blood basophils do not act as antigen-presenting cells for the major birch pollen allergen Bet v 1. Allergy. 2012;67:593–600. doi: 10.1111/j.1398-9995.2011.02764.x. [DOI] [PubMed] [Google Scholar]
- 20.Mutschlechner S, Egger M, Briza P, Wallner M, Lackner P, Karle A, et al. Naturally processed T cell-activating peptides of the major birch pollen allergen. J Allergy Clin Immunol. 2010;125:711–718. doi: 10.1016/j.jaci.2009.10.052. [DOI] [PubMed] [Google Scholar]
- 21.Gerstmayr M, Ilk N, Jahn-Schmid B, Sleytr UB, Bohle B. Natural self-assembly of allergen-S-layer fusion proteins is no prerequisite for reduced allergenicity and T cell stimulatory capacity. Int Arch Allergy Immunol. 2009;149:231–238. doi: 10.1159/000199718. [DOI] [PubMed] [Google Scholar]
- 22.Rutella S, Danese S, Leone G. Tolerogenic dendritic cells: cytokine modulation comes of age. Blood. 2006;108:1435–1440. doi: 10.1182/blood-2006-03-006403. [DOI] [PubMed] [Google Scholar]
- 23.Vicente-Suarez I, Brayer J, Villagra A, Cheng F, Sotomayor EM. TLR5 ligation by flagellin converts tolerogenic dendritic cells into activating antigen-presenting cells that preferentially induce T-helper 1 responses. Immunol Lett. 2009;125:114–118. doi: 10.1016/j.imlet.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kucuksezer UC, Palomares O, Ruckert B, Jartti T, Puhakka T, Nandy A, et al. Triggering of specific Toll-like receptors and proinflammatory cytokines breaks allergen-specific T-cell tolerance in human tonsils and peripheral blood. J Allergy Clin Immunol. 2013;131:875–885. doi: 10.1016/j.jaci.2012.10.051. [DOI] [PubMed] [Google Scholar]
- 25.Agrawal S, Agrawal A, Doughty B, Gerwitz A, Blenis J, Van Dyke T, et al. Cutting edge: different Toll-like receptor agonists instruct dendritic cells to induce distinct Th responses via differential modulation of extracellular signal-regulated kinase-mitogen-activated protein kinase and c-Fos. J Immunol. 2003;171:4984–4989. doi: 10.4049/jimmunol.171.10.4984. [DOI] [PubMed] [Google Scholar]
- 26.Wilson RH, Maruoka S, Whitehead GS, Foley JF, Flake GP, Sever ML, et al. The Toll-like receptor 5 ligand flagellin promotes asthma by priming allergic responses to indoor allergens. Nat Med. 2012;18:1705–1710. doi: 10.1038/nm.2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Didierlaurent A, Ferrero I, Otten LA, Dubois B, Reinhardt M, Carlsen H, et al. Flagellin promotes myeloid differentiation factor 88-dependent development of Th2-type response. J Immunol. 2004;172:6922–6930. doi: 10.4049/jimmunol.172.11.6922. [DOI] [PubMed] [Google Scholar]
- 28.Le TA, Takai T, Vu AT, Kinoshita H, Chen X, Ikeda S, et al. Flagellin induces the expression of thymic stromal lymphopoietin in human keratinocytes via toll-like receptor 5. Int Arch Allergy Immunol. 2011;155:31–37. doi: 10.1159/000318679. [DOI] [PubMed] [Google Scholar]
- 29.Constant S, Pfeiffer C, Woodard A, Pasqualini T, Bottomly K. Extent of T cell receptor ligation can determine the functional differentiation of naive CD4+ T cells. J Exp Med. 1995;182:1591–1596. doi: 10.1084/jem.182.5.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Re F, Strominger JL. Toll-like receptor 2 (TLR2) and TLR4 differentially activate human dendritic cells. J Biol Chem. 2001;276:37692–37699. doi: 10.1074/jbc.M105927200. [DOI] [PubMed] [Google Scholar]