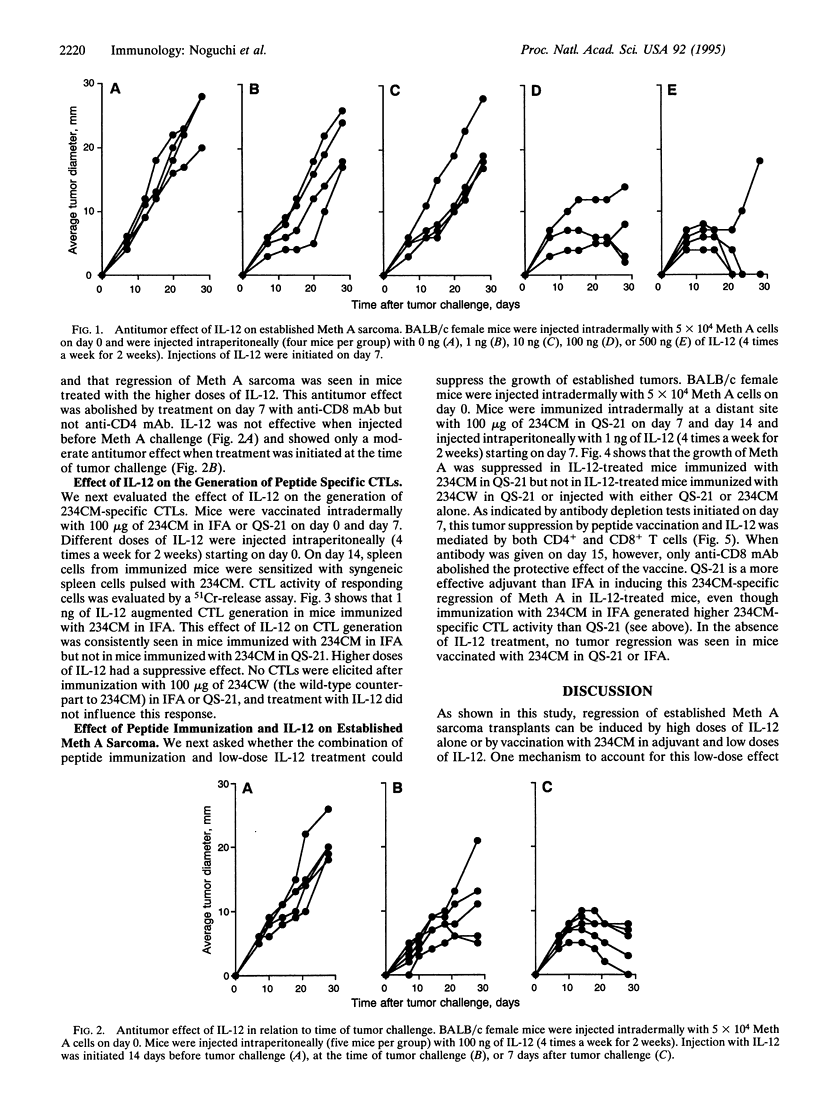
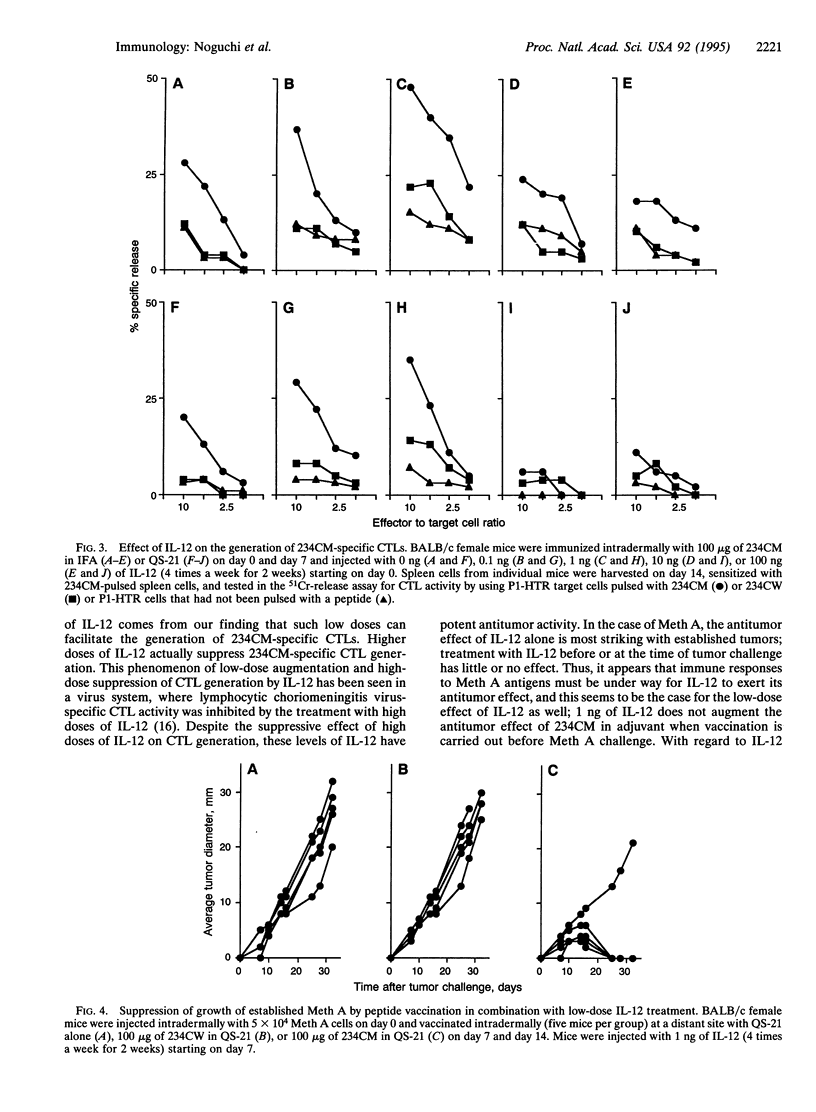
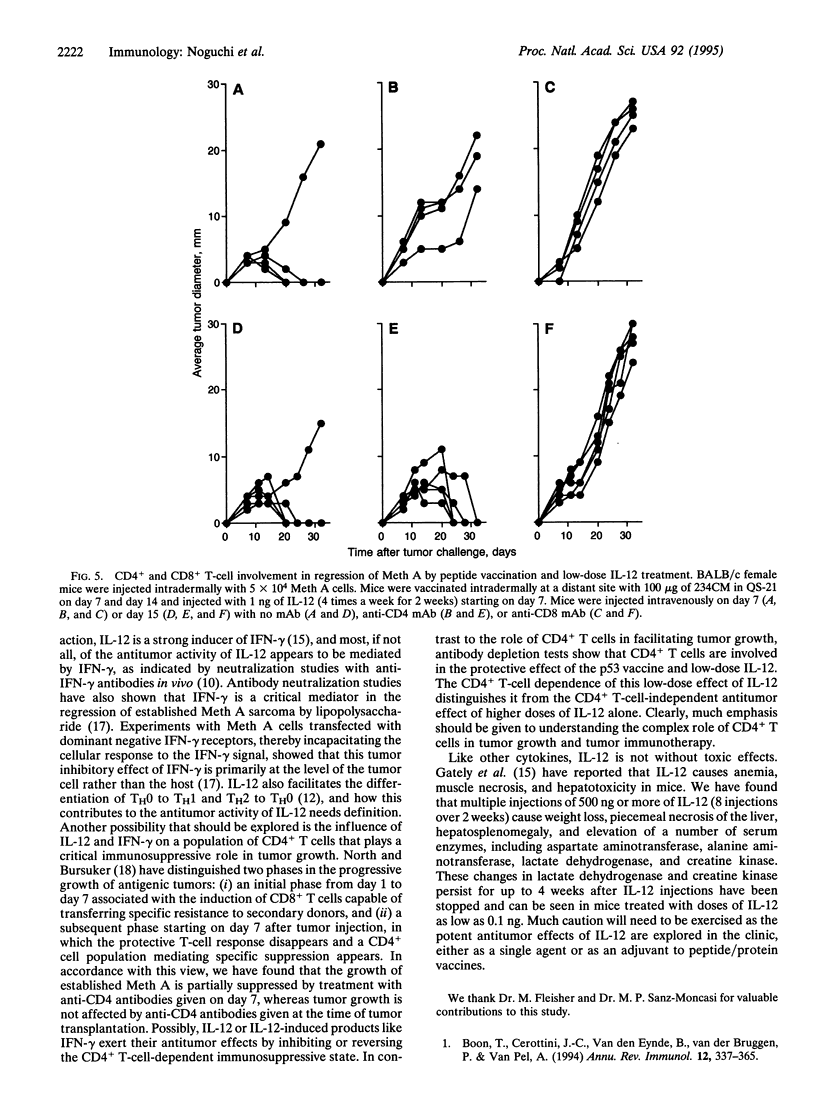
Abstract
BALB/c murine sarcoma Meth A is known to have three missense point mutations in p53. We previously reported that a nonamer peptide containing the codon 234 mutational product (designated 234CM) elicited 234CM-specific cytotoxic T cells and that immunization with 234CM in adjuvant before tumor challenge inhibited Meth A growth. Because interleukin 12 (IL-12) has been shown to have antitumor activity against established tumors and immuno-modulatory activities, we analyzed its effect on p53 peptide immunization and Meth A growth. Multiple injections of IL-12 alone (4 times a week for 2 weeks) caused regression of established Meth A sarcoma, and this effect was dose dependent. IL-12 treatment prior to Meth A challenge had little or no antitumor activity. To evaluate the effect of IL-12 on the generation of 234CM-specific cytotoxic T lymphocytes, spleen cells from BALB/c mice immunized with 234CM in adjuvant and injected with various doses of IL-12 were sensitized with 234CM in vitro. Multiple injections of 1 ng of IL-12 induced the highest cytotoxicity against target cells pulsed with 234CM. Higher doses of IL-12 suppressed 234CM-specific cytotoxic T-cell generation. Mice immunized with 234CM in QS-21 adjuvant and treated with 1 ng of IL-12 rejected established Meth A sarcoma. Mice comparably treated with 1 ng of IL-12 but immunized with 234CW peptide (the wild-type counterpart to 234CM) in QS-21 or with QS-21 alone showed progressive tumor growth.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boon T., Cerottini J. C., Van den Eynde B., van der Bruggen P., Van Pel A. Tumor antigens recognized by T lymphocytes. Annu Rev Immunol. 1994;12:337–365. doi: 10.1146/annurev.iy.12.040194.002005. [DOI] [PubMed] [Google Scholar]
- Brunda M. J., Luistro L., Warrier R. R., Wright R. B., Hubbard B. R., Murphy M., Wolf S. F., Gately M. K. Antitumor and antimetastatic activity of interleukin 12 against murine tumors. J Exp Med. 1993 Oct 1;178(4):1223–1230. doi: 10.1084/jem.178.4.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dighe A. S., Richards E., Old L. J., Schreiber R. D. Enhanced in vivo growth and resistance to rejection of tumor cells expressing dominant negative IFN gamma receptors. Immunity. 1994 Sep;1(6):447–456. doi: 10.1016/1074-7613(94)90087-6. [DOI] [PubMed] [Google Scholar]
- Gately M. K., Warrier R. R., Honasoge S., Carvajal D. M., Faherty D. A., Connaughton S. E., Anderson T. D., Sarmiento U., Hubbard B. R., Murphy M. Administration of recombinant IL-12 to normal mice enhances cytolytic lymphocyte activity and induces production of IFN-gamma in vivo. Int Immunol. 1994 Jan;6(1):157–167. doi: 10.1093/intimm/6.1.157. [DOI] [PubMed] [Google Scholar]
- Golumbek P. T., Lazenby A. J., Levitsky H. I., Jaffee L. M., Karasuyama H., Baker M., Pardoll D. M. Treatment of established renal cancer by tumor cells engineered to secrete interleukin-4. Science. 1991 Nov 1;254(5032):713–716. doi: 10.1126/science.1948050. [DOI] [PubMed] [Google Scholar]
- Hsieh C. S., Macatonia S. E., Tripp C. S., Wolf S. F., O'Garra A., Murphy K. M. Development of TH1 CD4+ T cells through IL-12 produced by Listeria-induced macrophages. Science. 1993 Apr 23;260(5107):547–549. doi: 10.1126/science.8097338. [DOI] [PubMed] [Google Scholar]
- Kensil C. R., Patel U., Lennick M., Marciani D. Separation and characterization of saponins with adjuvant activity from Quillaja saponaria Molina cortex. J Immunol. 1991 Jan 15;146(2):431–437. [PubMed] [Google Scholar]
- Kobayashi M., Fitz L., Ryan M., Hewick R. M., Clark S. C., Chan S., Loudon R., Sherman F., Perussia B., Trinchieri G. Identification and purification of natural killer cell stimulatory factor (NKSF), a cytokine with multiple biologic effects on human lymphocytes. J Exp Med. 1989 Sep 1;170(3):827–845. doi: 10.1084/jem.170.3.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandelboim O., Berke G., Fridkin M., Feldman M., Eisenstein M., Eisenbach L. CTL induction by a tumour-associated antigen octapeptide derived from a murine lung carcinoma. Nature. 1994 May 5;369(6475):67–71. doi: 10.1038/369067a0. [DOI] [PubMed] [Google Scholar]
- Manetti R., Parronchi P., Giudizi M. G., Piccinni M. P., Maggi E., Trinchieri G., Romagnani S. Natural killer cell stimulatory factor (interleukin 12 [IL-12]) induces T helper type 1 (Th1)-specific immune responses and inhibits the development of IL-4-producing Th cells. J Exp Med. 1993 Apr 1;177(4):1199–1204. doi: 10.1084/jem.177.4.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nastala C. L., Edington H. D., McKinney T. G., Tahara H., Nalesnik M. A., Brunda M. J., Gately M. K., Wolf S. F., Schreiber R. D., Storkus W. J. Recombinant IL-12 administration induces tumor regression in association with IFN-gamma production. J Immunol. 1994 Aug 15;153(4):1697–1706. [PubMed] [Google Scholar]
- Newman M. J., Wu J. Y., Gardner B. H., Munroe K. J., Leombruno D., Recchia J., Kensil C. R., Coughlin R. T. Saponin adjuvant induction of ovalbumin-specific CD8+ cytotoxic T lymphocyte responses. J Immunol. 1992 Apr 15;148(8):2357–2362. [PubMed] [Google Scholar]
- Noguchi Y., Chen Y. T., Old L. J. A mouse mutant p53 product recognized by CD4+ and CD8+ T cells. Proc Natl Acad Sci U S A. 1994 Apr 12;91(8):3171–3175. doi: 10.1073/pnas.91.8.3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North R. J., Bursuker I. Generation and decay of the immune response to a progressive fibrosarcoma. I. Ly-1+2- suppressor T cells down-regulate the generation of Ly-1-2+ effector T cells. J Exp Med. 1984 May 1;159(5):1295–1311. doi: 10.1084/jem.159.5.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orange J. S., Wolf S. F., Biron C. A. Effects of IL-12 on the response and susceptibility to experimental viral infections. J Immunol. 1994 Feb 1;152(3):1253–1264. [PubMed] [Google Scholar]
- Stern A. S., Podlaski F. J., Hulmes J. D., Pan Y. C., Quinn P. M., Wolitzky A. G., Familletti P. C., Stremlo D. L., Truitt T., Chizzonite R. Purification to homogeneity and partial characterization of cytotoxic lymphocyte maturation factor from human B-lymphoblastoid cells. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6808–6812. doi: 10.1073/pnas.87.17.6808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Pel A., De Plaen E., Boon T. Selection of highly transfectable variant from mouse mastocytoma P815. Somat Cell Mol Genet. 1985 Sep;11(5):467–475. doi: 10.1007/BF01534840. [DOI] [PubMed] [Google Scholar]



