Abstract
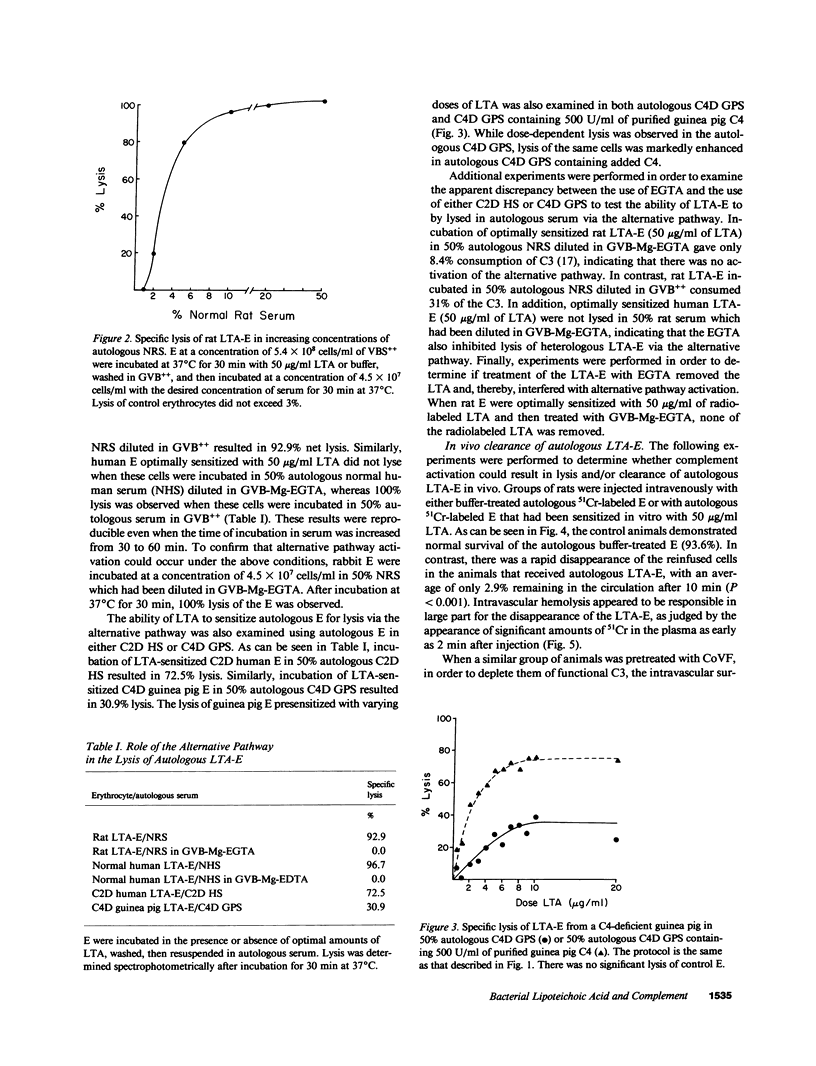
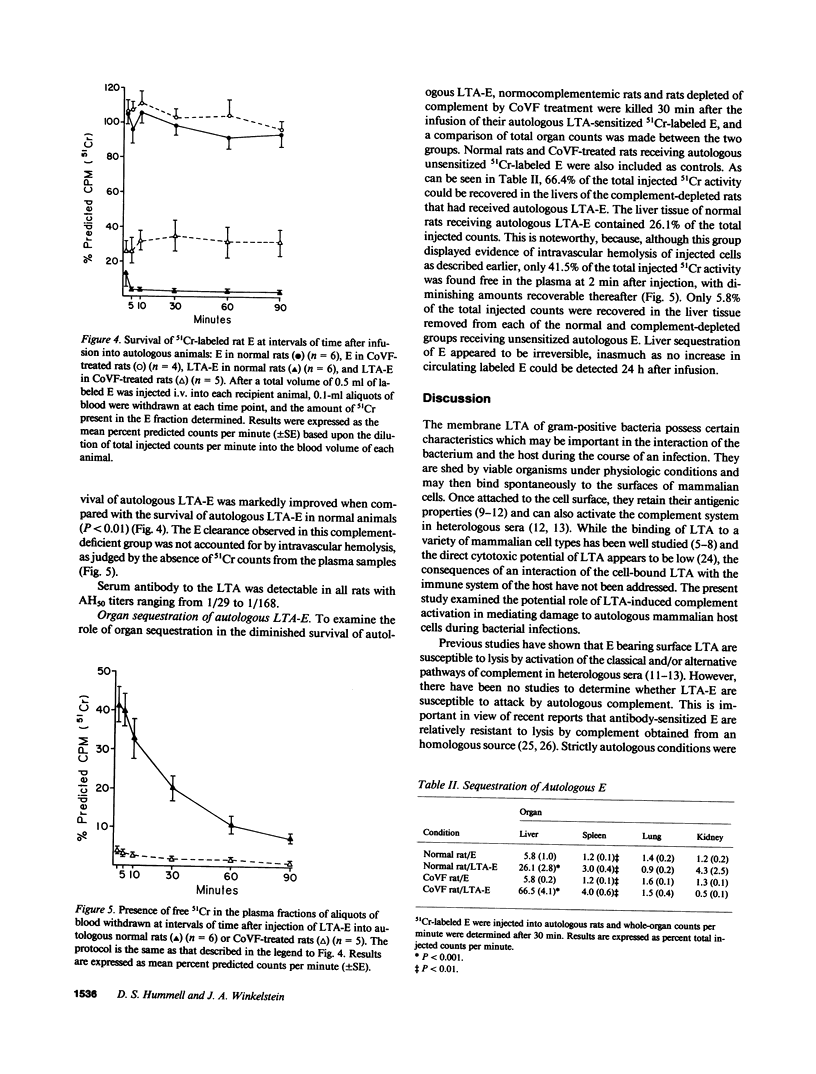
Lipoteichoic acids (LTA) released by gram-positive bacteria can spontaneously bind to mammalian cell surfaces. In the present study, erythrocytes (E) sensitized with pneumococcal LTA (LTA-E) were used as a model system to determine if LTA could render host cells susceptible to damage by autologous complement. Complement (C)-mediated lysis of LTA-E from normal rats and normal humans occurred when these cells were incubated in their respective autologous sera in vitro. In addition, when LTA-E from a C2-deficient human and from C4-deficient guinea pigs were incubated in their autologous sera, there was significant lysis in vitro, demonstrating a role for the alternative pathway. The in vivo survival of 51Cr-labeled autologous LTA-E was also studied. Only 2.9% of autologous LTA-E remained in the circulation of normal rats after 90 min. In contrast, 31.2% of autologous LTA-E remained in the circulation of rats depleted of C3. Intravascular hemolysis accounted for the clearance of LTA-E in the normal rats, whereas liver sequestration was responsible for clearance in the C3-depleted rats. These results demonstrate that LTA can render the host's cells susceptible to damage by its own complement system, establishing this as a possible mechanism of tissue damage in natural bacterial infections.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alkan M. L., Beachey E. H. Excretion of lipoteichoic acid by group A streptococci. Influence of penicillin on excretion and loss of ability to adhere to human oral mucosal cells. J Clin Invest. 1978 Mar;61(3):671–677. doi: 10.1172/JCI108979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beachey E. H., Chiang T. M., Ofek I., Kang A. H. Interaction of lipoteichoic acid of group A streptococci with human platelets. Infect Immun. 1977 May;16(2):649–654. doi: 10.1128/iai.16.2.649-654.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beachey E. H., Dale J. B., Grebe S., Ahmed A., Simpson W. A., Ofek I. Lymphocytes binding and T cell mitogenic properties of group A streptococcal lipoteichoic acid. J Immunol. 1979 Jan;122(1):189–195. [PubMed] [Google Scholar]
- Beachey E. H., Dale J. B., Simpson W. A., Evans J. D., Knox K. W., Ofek I., Wicken A. J. Erythrocyte binding properties of streptococcal lipoteichoic acids. Infect Immun. 1979 Mar;23(3):618–625. doi: 10.1128/iai.23.3.618-625.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briles E. B., Tomasz A. Pneumococcal Forssman antigen. A choline-containing lipoteichoic acid. J Biol Chem. 1973 Sep 25;248(18):6394–6397. [PubMed] [Google Scholar]
- Brown E. J., Joiner K. A., Frank M. M. Interaction of desialated guinea pig erythrocytes with the classical and alternative pathways of guinea pig complement in vivo and in vitro. J Clin Invest. 1983 Jun;71(6):1710–1719. doi: 10.1172/JCI110925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coonrod J. D., Jenkins S. D. Functional assay of the alternative complement pathway of rat serum. J Immunol Methods. 1979;31(3-4):291–301. doi: 10.1016/0022-1759(79)90142-x. [DOI] [PubMed] [Google Scholar]
- Ellman L., Green I., Frank M. Genetically controlled total deficiency of the fourth component of complement in the guinea pig. Science. 1970 Oct 2;170(3953):74–75. doi: 10.1126/science.170.3953.74. [DOI] [PubMed] [Google Scholar]
- HARRIS T. N., HARRIS S. Agglutination by human sera of erythrocytes incubated with streptococcal culture concentrates. J Bacteriol. 1953 Aug;66(2):159–165. doi: 10.1128/jb.66.2.159-165.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch R. L., Winkelstein J. A., Griffin D. E. The role of complement in viral infections. III. Activation of the classical and alternative complement pathways by Sindbis virus. J Immunol. 1980 May;124(5):2507–2510. [PubMed] [Google Scholar]
- Houle J. J., Hoffmann E. M. Evidence for restriction of the ability of complement to lyse homologous erythrocytes. J Immunol. 1984 Sep;133(3):1444–1452. [PubMed] [Google Scholar]
- Hummell D. S., Swift A. J., Tomasz A., Winkelstein J. A. Activation of the alternative complement pathway by pneumococcal lipoteichoic acid. Infect Immun. 1985 Feb;47(2):384–387. doi: 10.1128/iai.47.2.384-387.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyatt A. C., Altenburger K. M., Johnston R. B., Jr, Winkelstein J. A. Increased susceptibility to severe pyogenic infections in patients with an inherited deficiency of the second component of complement. J Pediatr. 1981 Mar;98(3):417–419. doi: 10.1016/s0022-3476(81)80708-1. [DOI] [PubMed] [Google Scholar]
- Leon O., Panos C. Cytotoxicity and inhibition of normal collagen synthesis in mouse fibroblasts by lipoteichoic acid from Streptococcus pyogenes type 12. Infect Immun. 1983 May;40(2):785–794. doi: 10.1128/iai.40.2.785-794.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NETER E. Bacterial hemagglutination and hemolysis. Bacteriol Rev. 1956 Sep;20(3):166–188. doi: 10.1128/br.20.3.166-188.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada H., Tanaka H., Okada N. Prevention of complement activation on the homologous cell membrane of nucleated cells as well as erythrocytes. Eur J Immunol. 1983 Apr;13(4):340–344. doi: 10.1002/eji.1830130413. [DOI] [PubMed] [Google Scholar]
- RANTZ L. A., RANDALL E., ZUCKERMAN A. Hemolysis and hemagglutination by normal and immune serums of erythrocytes treated with a nonspecies specific bacterial substance. J Infect Dis. 1956 Mar-Apr;98(2):211–222. doi: 10.1093/infdis/98.2.211. [DOI] [PubMed] [Google Scholar]
- RANTZ L. A., ZUCKERMAN A., RANDALL E. Hemolysis of red blood cells treated by bacterial filtrates in the presence of serum and complement. J Lab Clin Med. 1952 Mar;39(3):443–448. [PubMed] [Google Scholar]
- Shin H. S., Mayer M. M. The third component of the guinea pig complement system. II. Kinetic study of the reaction of EAC'4,2a with guinea pig C'3. Enzymatic nature of C'3 comsumption, multiphasic character of fixation, and hemolytic titration of C'3. Biochemistry. 1968 Aug;7(8):2997–3002. doi: 10.1021/bi00848a041. [DOI] [PubMed] [Google Scholar]
- Simpson W. A., Ofek I., Sarasohn C., Morrison J. C., Beachey E. H. Characteristics of the binding of streptococcal lipoteichoic acid to human oral epithelial cells. J Infect Dis. 1980 Apr;141(4):457–462. doi: 10.1093/infdis/141.4.457. [DOI] [PubMed] [Google Scholar]
- Smith M. R., Wood W. B., Jr Heat labile opsonins to pneumococcus. I. Participation of complement. J Exp Med. 1969 Dec 1;130(6):1209–1227. doi: 10.1084/jem.130.6.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasz A. Cellular metabolism in genetic transformation of pneumococci: requirement for protein synthesis during induction of competence. J Bacteriol. 1970 Mar;101(3):860–871. doi: 10.1128/jb.101.3.860-871.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utsui Y., Ohya S., Takenouchi Y., Tajima M., Sugawara S., Deguchi K., Suginaka H. Release of lipoteichoic acid from Staphylococcus aureus by treatment with cefmetazole and other beta-lactam antibiotics. J Antibiot (Tokyo) 1983 Oct;36(10):1380–1386. doi: 10.7164/antibiotics.36.1380. [DOI] [PubMed] [Google Scholar]
- Wicken A. J., Knox K. W. Lipoteichoic acids: a new class of bacterial antigen. Science. 1975 Mar 28;187(4182):1161–1167. doi: 10.1126/science.46620. [DOI] [PubMed] [Google Scholar]


