Abstract
Globally, the prevalence of obesity is increasing which subsequently increases the risk of the development of obesity-related chronic diseases. Low-grade chronic inflammation and dysregulated adipose tissue inflammatory mediator/adipokine secretion are well-established in obesity, and these factors increase the risk of developing inflammation-associated cancer. Breast cancer is of particular interest given that increased inflammation within the subcutaneous mammary adipose tissue depot can alter the local tissue inflammatory microenvironment such that it resembles that of obese visceral adipose tissue. Therefore, in obese women with breast cancer, increased inflammatory mediators both locally and systemically can perpetuate inflammation-associated pro-carcinogenic signaling pathways, thereby increasing disease severity. Herein, we discuss some of these inflammation-associated pro-carcinogenic mechanisms of the combined obese breast cancer phenotype and offer evidence that dietary long chain n-3 polyunsaturated fatty acids (PUFA) may have utility in mitigating the severity of obesity-associated inflammation and breast cancer.
Keywords: breast cancer, inflammation, obesity, adipokines, n-3 polyunsaturated fatty acids, leptin, adiponectin, aromatase, lipid rafts, eicosanoids
1. Introduction
Based on body mass index (BMI), globally 1.5 billion people are overweight (BMI ≥ 25.0 kg/m2), and 500 million of these individuals are classified as obese (BMI ≥ 30.0 kg/m2) [1]. The clinical consequence of obesity is that it acts as an independent risk factor for several other pathologies, including cancer [1,2]. In this context, obesity is associated with increased mortality in several types of cancer, including breast cancer (BC) [3]. It is estimated that obesity contributes to 50% of all BC cases in older women [4]. Furthermore, considering the prevalence of obesity in younger populations and the projected expansion of the obese population [1], the impact on BC incidence is likely to be exacerbated in the future. Obesity increases the risk of developing the most common BC subtype, estrogen receptor (ER)-positive and progesterone receptor (PR)-positive BC (i.e., hormone-sensitive form of the disease) [4,5,6,7]. Paradoxically the incidence of hormone sensitive BC increases with age, which coincides with increasing adiposity and decreasing circulating estrogen levels [8]. In fact, in postmenopausal women the majority of BC cases associated with obesity are ER-positive with a phenotype exhibiting larger and faster growing tumors that metastasize to axillary lymph nodes [4,5,9,10,11]. The positive association between BC development and obesity in postmenopausal women is well-established using multiple anthropometric indices of obesity including BMI, adiposity and waist:hip circumference ratio [4,5,9,10,11,12]. Interestingly, postmenopausal BC risk is increased by the degree of weight gain during adult life prior to menopause [13,14], thereby indicating that the effects of obesity during the premenopausal phase impact BC risk later in life. Conversely, in premenopausal women the link between BC risk and BMI as a measure of obesity status is more controversial [15,16,17]. Studies finding no association commonly normalize data to BMI alone, which is a poor discriminator of body fat and lean mass, and fails to account for visceral adiposity which is believed to be a more deleterious adipose depot compared to subcutaneous [18,19]. Premenopausal women with high BMI have been shown to develop significantly larger tumors and worse histopathological features including increased tumor vascularization and metastasis to axillary lymph nodes compared to healthy BMI BC patients [9]. Additionally, premenopausal obesity has been shown to increase the risk of developing triple negative BC (ER, PR and HER negative) [9,20] and hormone receptor-negative BC (ER and PR-negative) [21,22]. Collectively, these data indicate that independent of hormonal status, obesity increases overall BC risk, an effect that is, at least in part, attributed to inflammatory mechanisms and paracrine interactions (i.e., cross-talk) between cell types within the mammary tissue that promote tumorigenesis [4,23,24].
The connection between obesity and ER-positive BC in post-menopausal women is likely attributable to two main interrelated factors that will be a key focus of this review, including: (i) increased adipose tissue (AT) mass and the associated increase in inflammatory mediator production (both locally and systemically); and (ii) elevated AT aromatase activation, which is up-regulated by inflammatory mediators and drives aberrant estrogen production within the AT, thereby promoting BC tumorigenesis. The obesity-associated inflammatory mammary tumor microenvironment is complex and the resultant phenotype is underscored by autocrine and paracrine interactions between adipocytes, tumor infiltrating macrophages (TAM) and epithelial cells, which produce AT-derived inflammatory mediators, collectively referred to as adipokines, which will be discussed in more detail herein. The inflammatory mammary tumor microenvironment should not be confused with “inflammatory breast cancer” (IBC), a rare (1%–6% of all breast malignancies) aggressive BC subtype with higher grade metastatic hormone receptor negative tumors that has been reviewed elsewhere [25,26]. Moreover, we provide evidence that dietary long-chain (LC) n-3 polyunsaturated fatty acids (PUFA), particularly fish oil (marine)-derived eicosapentaenoic acid (EPA, 20:5n-3) and docosahexaenoic acid (DHA, 22:6n3), which have well established anti-inflammatory effects in obesity [27,28,29,30,31,32,33,34,35,36,37,38,39,40,41] and anti-carcinogenic effects in BC [42,43,44,45,46,47,48,49,50,51,52,53,54,55,56], may represent an effective complementary approach in the prevention and/or treatment of obesity-associated BC by attenuating inflammatory adipokine-mediated paracrine interactions within the mammary tumor microenvironment.
2. Obese Inflammatory Phenotype
AT is an endocrine organ that secretes greater than 50 recognized proteins including several cytokines and chemokines, both of which are included in the term adipokine (i.e., of AT origin) [57]. In the classic obese phenotype (reviewed elsewhere [2,58,59]), the tissue stress and remodeling that occurs in expanding visceral AT is associated with dysregulated adipokine secretion and a subsequent state of chronic, sub-clinical, low-grade, systemic inflammation [2,60]. The cellular source of these inflammatory mediators includes adipocytes and cells of the stromal vascular fraction (SVF) including endothelial cells, fibroblasts, macrophages and T cells [60]. These adipokines can influence whole-body metabolism, insulin sensitivity and inflammation through autocrine, paracrine and endocrine signaling. Most notably, in obesity both the local AT and circulating levels of inflammatory mediators, such as TNFα, IL-6, IL-1β, MCP-1, leptin and many others (reviewed by [58,59]), are elevated, while levels of adiponectin, an anti-inflammatory adipokine, are decreased [61]. Many of these same adipokines are up-regulated in obese BC and activate signaling pathways that drive inflammation-associated malignant transformation, and therefore, when present in the mammary tissue result in a more severe BC phenotype, as discussed in detail below.
3. n-3 Polyunsaturated Fatty Acids and Obesity
In obesity, marine source LC n-3 PUFA have been shown to modulate and improve several critical aspects of the obese phenotype, collectively reducing AT inflammation. Specifically, n-3 PUFA modulate the production of AT-derived adipokines by increasing anti-inflammatory adiponectin levels [27,28,29,30,31,32,33,34,35], while decreasing production of inflammatory mediators such as leptin [36,37,38,39] and cytokines including TNFα, IL-6 and MCP-1 [29,35,40,41]. Moreover, dietary n-3 PUFA have been found to reverse and/or improved obesity-associated hepatic steatosis and impairments in glucose metabolism and insulin sensitivity [27,28,29,35,62,63,64]. Collectively, these anti-inflammatory effects of n-3 PUFA alter the obesity-associated inflammatory microenvironment and improve the overall obese phenotype. One well-documented effect of n-3 PUFA is the suppression of inflammation by interfering with pro-inflammatory signaling cascades via peroxisome proliferator-activated receptor (PPAR)γ-dependent and independent mechanisms that involve up-regulation of adiponectin, in murine [65] and human adipocytes [66]. Additionally, PPARγ is involved in trans-repression of nuclear factor kappa-light-chain-enhancer of activated B cells (NFκB) transcriptional activity leading to decreased expression of NFκB responsive genes including several inflammatory cytokines (TNFα, IL-1β, IL-6 and MCP-1) [67]. In this connection, n-3 PUFA functioning as PPAR-receptor ligands also interfere with other transcription factors involved in inflammatory signal transduction pathways including AP-1, STAT-1 and NFAT [68].
n-3 PUFA can also perturb inflammatory signaling in obesity through PPARγ independent signaling mechanisms, most notably by acting as ligands for the G-protein coupled receptor 120 (GPR120) [69]. GPR120 has been shown to be partly responsible for the anti-inflammatory effects of DHA by using the adaptor β-arrestin2 to interfere with inflammatory mediator-stimulated NFκB activation in macrophages [69]. Additionally, EPA and DHA exert anti-inflammatory effects following their selective incorporation into the phospholipid fraction of cell membranes where they can act to decrease the signaling efficiency of protein complexes in lipid rafts [70], or serve as substrates for the synthesis of anti-inflammatory bioactive lipid mediators (i.e., eicosanoids) [71,72]. Taken together, n-3 PUFA may beneficially modulate obesity-associated pro-inflammatory paracrine interactions between the different cell types within AT. Overall, n-3 PUFA utilize multiple mechanisms to suppress inflammatory signaling, thereby modulating the obesity-associated inflammatory phenotype.
4. n-3 Polyunsaturated Fatty Acids and Breast Cancer
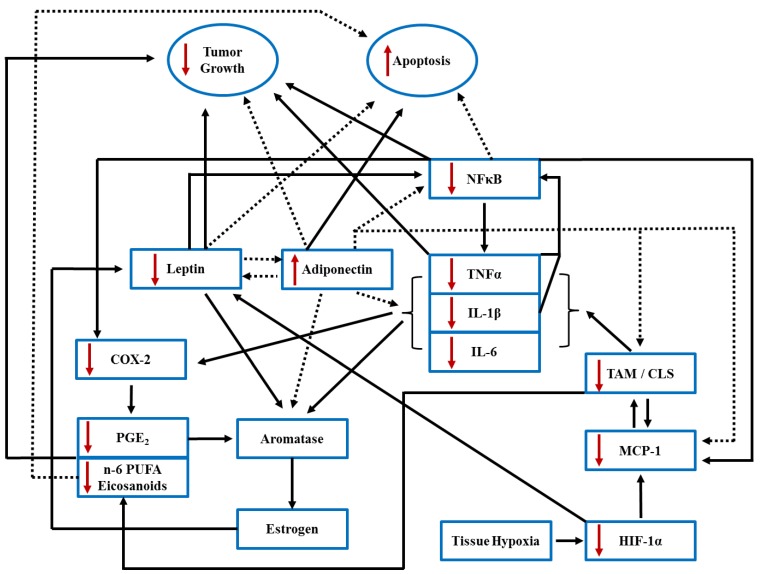
Marine-derived n-3 PUFA have well-established anti-tumorigenic effects in chemically induced, transgenic and xenograft rodent models of BC [73]. As a point of reference, amongst high LC n-3 PUFA consuming populations, the typical Japanese diet contains 1%–2% of daily energy as LC n-3 PUFA [74,75], whereas intake levels are higher amongst the Greenland Inuit who typically consume 2.4%–6.3% of daily energy as LC n-3 PUFA [76,77]. Although higher levels of n-3 PUFA intake can be achieved through supplementation, these physiologically relevant intake levels have been recapitulated in BC rodent dietary intervention studies which demonstrate a beneficial effect of n-3 PUFA on the BC phenotype [42,43,44,46,48,49,55,78]. In this connection, n-3 PUFA are recognized for their potential application in reducing obesity-associated inflammation and consequent tumorigenic risk [45]. In brief, LC n-3 PUFA are incorporated into mammary AT and tumor tissue [46,47], thereby increasing the levels of n-3 PUFA-derived lipid mediators at the expense of those derived from n-6 PUFA (i.e., arachidonic acid (AA, C20:4n-6)-derived eicosanoids) [42,56,78], altering adipokine secretion [54] and interrupting tumorigenic signaling pathways [79]. These chemoprotective effects of n-3 PUFA result in decreased cell proliferation and increased apoptosis, ultimately resulting in reduced BC tumor incidence, growth, multiplicity, and metastasis in rodent models of BC [43,44,46,48,49,50,51,52,53,55,79]. Further, in a model of obese postmenopausal BC, n-3 PUFA supplementation reduced mammary AT inflammation and markers of inflammatory M1 macrophage infiltration [80] which was associated with reduced tumor burden, indicating that the inflammatory microenvironment promotes tumorigenesis and that n-3 PUFA directly antagonize this process. Similar n-3 PUFA-mediated anti-tumorigenic effects have been reported in overweight humans wherein n-3 PUFA supplementation up-regulated the expression of several genes involved in cell cycle regulation [81]. These studies clearly demonstrate that n-3 PUFA can independently modulate responsiveness to cell proliferative and/or apoptotic signaling. This is further highlighted in Figure 1, which outlines the effects of n-3 PUFA on critical adipokine/inflammatory mediator levels that underlie the paracrine interactions within the obese mammary tumor microenvironment that ultimately impact proliferative and apoptotic signaling and will be discussed in this review.
Figure 1.
Summary of inflammatory mediator paracrine interactions produced within the obese mammary tissue tumor microenvironment highlighting the complex interactions mediated by adipocytes, macrophages and epithelial cells (main cellular sources of inflammatory mediators). Solid arrows denote stimulatory effects and dotted arrows denote inhibitory effects between inflammatory mediators. Red arrows indicate the effects of n-3 PUFA to increase or decrease inflammatory mediator levels, thereby subsequently up-regulating (adiponectin, n-3 PUFA-derived eicosanoids) or down-regulating (leptin, n-6 PUFA-derived eicosanoids, cytokines (TNFα, IL-1β, IL-6 and MCP-1) and macrophage tissue infiltration). TAM, tumor associated-macrophage; CLS, crown-like structure; HIF-1α, hypoxia induced factor-1α; COX-2, cyclooxygenase-2; PGE2, prostaglandin E2; TNFα, tumor necrosis factor-α; IL, interleukin; MCP-1, monocyte chemoattractant protein-1.
A recent meta-analysis that included 21 independent prospective cohort studies determined that marine source n-3 PUFA intake was associated with a 14% reduction in BC risk (RR = 0.86 for highest versus lowest category of intake (95% confidence interval 0.78–0.94)) [82]. The results were also sustained if the data was analyzed based on either reported dietary intake levels or tissue biomarker levels of n-3 PUFA, thereby removing concerns regarding intake compliance or accuracy in dietary recall data. In obese women, a decreased risk of BC was found to be significantly associated with both increased intake of n-3 PUFA and altered dietary fatty acid composition by increasing the ratio of n-3:n-6 PUFA intake, although this association was not significant in overweight or normal weight women [83]. These data suggest that obesity status may affect the association between n-3 PUFA intake and BC risk. To the best of our knowledge, this is the only case-control study that has specifically investigated the relationship between obesity, n-3 PUFA intake and BC risk. Interestingly, the chemoprotective effect of an increased ratio of n-3:n-6 PUFA intake has been reported elsewhere [84], and several case-control studies have reported an inverse association between BC risk and increased n-3 PUFA intake and/or increasing the n-3:n-6 PUFA ratio in breast AT [85,86,87,88]. Although obesity status was not independently assessed, some of these studies report a trend with more normal weight women in the control group and more overweight and obese women with BC [85,88]. In contrast, others have reported no association between AT n-3 PUFA levels and the development of BC; however, BMI was lower among these BC cases [89]. Taken together, while higher n-3 PUFA intake and tissue content is more often than not associated with a decreased risk of developing BC, controversy exists surrounding the associations between BC incidence, obesity status and n-3 PUFA intake, warranting case-control studies that investigate a relationship between all endpoints.
5. n-3 PUFA, Lipid Rafts and Breast Cancer
Extensive research has indicated that n-3 PUFA have a unique ability to broadly affect cell signaling. A ubiquitous mechanism by which n-3 PUFA can alter signal transduction is by modifying lipid rafts, which are heterogeneous, highly ordered membrane microdomains that facilitate several signaling events [90,91]. Lipid rafts laterally isolate their components from the bulk membrane and then are able to coalesce in response to stimuli to form signaling platforms [92,93], thereby playing an integral role in the propagation of multiple signaling events that are involved in tumor-promoting activities, including cell proliferation, survival, migration, and invasion [94]. The physical properties that facilitate the segregation of lipid raft domains from the bulk membrane is imparted by the enrichment of cholesterol, sphingolipids, and other phospholipids with saturated, long hydrocarbon chains within lipid rafts [95,96]. n-3 PUFA display low affinity for cholesterol due to their high degree of unsaturation [97,98]; therefore, enrichment of the membrane with n-3 PUFA can alter the composition and organization of raft domains. Altering the properties of lipid rafts can then have major effects on signaling events that are initiated or propagated by these integral domains.
An important property of lipid rafts is their size and number. Changes to the size of rafts can impose substantial changes on their function. It has been shown that rafts must be small and mobile for optimal activity [99]. Studies in BC cells, in addition to many other cell types, have demonstrated the ability of n-3 PUFA to alter the size of lipid rafts. Specifically, DHA was found to alter the size of lipid rafts in BC cells, resulting in lipid rafts of varying height [100]. The same study illustrated that DHA decreased the total amount of lipid rafts on the order of 20%–30%. This is particularly interesting because the levels of lipid rafts are elevated in some forms of cancer, including BC [101], and perturbing these domains can sensitize cells to apoptosis [102]. In addition to altering the size and number of lipid rafts, DHA was found to reduce cell surface levels of lipid rafts by enhancing their internalization [102]. These data implicate n-3 PUFA-induced changes in the physical properties of lipid rafts as a mechanism by which these fatty acids exert a chemoprotective effect.
The composition of raft domains is also central to their function as signaling platforms, and n-3 PUFA can substantially alter the contents of lipid rafts. The lipid composition of rafts endows the properties necessary for ordering and segregation. BC cells treated with a combination of EPA and DHA were demonstrated to have significantly reduced cholesterol, sphingomyelin, and diacylglycerol lipid raft content [103]. Another study demonstrated differential effects of EPA and DHA on the lipid composition of rafts [100]. EPA was shown to displace AA from raft domains, whereas DHA reduced cholesterol and sphingomyelin content. In addition to lipids, many proteins reside in lipid rafts and require localization to lipid rafts for signal transduction. Importantly, many of these proteins are established mediators of oncogenesis, and displacing these proteins can markedly reduce their signaling capacity. In MDA-MB-231 BC cells, several raft-associated proteins, including EGFR, Hsp90, Akt, and Src, are redistributed out of raft domains in response to DHA treatment [102], which induced increased BC cell apoptosis. Additionally, DHA was demonstrated to disrupt lipid rafts and reduce HER-2 signaling in mammary epithelial cells overexpressing HER-2 [104]. All of these proteins are involved in the regulation of cell survival and proliferation, and many are targets for cancer therapy. Another well-known therapeutic target for BC metastasis is the chemokine receptor, CXCR4. Treatment of BC cells with DHA or EPA caused redistribution of CXCR4 from lipid rafts to the cell surface [105], resulting in an overall reduction in cell migration. In addition to shifting proteins out of lipid rafts, n-3 PUFA can prompt the localization of some proteins into these domains. For example, CD95 (APO-1/FAS) is the transmembrane death receptor which activates the extrinsic apoptosis pathway and activation results in CD95 aggregation in the plasma membrane, followed closely by recruitment of Fas-associated death domain-containing protein (FADD) and caspase-8 to the CD95 receptor, forming the death-inducing signaling complex (DISC) [106]. EPA and DHA have been shown to induce translocation of CD95 into lipid rafts in MDA-MB-231 BC cells and this effect was accompanied by reduced cell growth [107]. Moreover, when DHA was used as a co-treatment it enhanced the chemotherapeutic effects of doxorubicin [107], an anti-neoplastic drug therapy, which has been shown to induce apoptosis and the movement of DISC to membrane rafts [108,109]. All of these data support the regulation of lipid rafts by n-3 PUFA as a mechanism by which they exert protective effects in BC and may also have utility as a complementary therapy in combination with pharmaceuticals, although further study is required.
6. Paracrine Interactions, Inflammatory Mediator Signaling and Breast Cancer
The majority of breast tissue is comprised of adipocytes, whereas epithelial cells account for only 10% of total breast cellular volume [110]. Mammary epithelial cells are embedded within the AT, which facilitates direct contact between epithelial cells and adjacent adipocytes and allows for direct functional interactions between AT and mammary tumor cells in a paracrine manner. Within the BC tumor microenvironment, these cellular interactions are further influenced by exposure to circulating AT adipokines [111,112]. Inflammation plays a role in the carcinogenic process and approximately 20% of all cancers originate in association with inflammation [113]. Given the chronic low grade-inflammatory state perpetuated in obesity [114,115], it is likely that obese AT-derived inflammatory mediator production could exacerbate inflammation-associated tumorigenic effects. Specifically, these mediators include n-6 PUFA-derived eicosanoids, and adipokines such as leptin and inflammatory cytokines (TNFα, IL-1β and IL-6) with a concomitant reduction in the anti-inflammatory adipokine, adiponectin (discussed below), which are produced in both visceral AT depots and surprisingly also within mammary AT depots, and collectively contribute to the development of a more severe BC phenotype via stimulating BC growth, invasion and metastasis. Typically in obesity-associated BC, inflammatory processes precede tumorigenesis. However, once developed, mammary tumor cells may also serve as a cellular source of inflammatory mediators and support the on-going inflammatory milieu within the mammary tumor microenvironment, thereby potentiating a feed-forward pro-tumorigenic mechanism facilitated by local inflammatory autocrine and paracrine interactions. In summary, autocrine and paracrine signaling between cell types within the mammary AT and tumor tissue are thought to play a central role in breast tumorigenesis [110,116], indicating that dysregulation of adipokines may underlie the association between obesity and BC.
One of the main cellular sources of these inflammatory mediators, apart from adipocytes, is macrophages that infiltrate obese AT and form crown-like structures (CLS) [117,118]. CLS are inflammatory lesions defined as adipocytes surrounded by an aggregation of macrophages that undergo necrosis and fuse to form a syncytium of lipid-containing giant multinucleated cells [119]. Obese mice have increased CLS formation in both visceral AT and mammary AT, which is associated with increased local inflammatory cytokine production (TNFα, IL-1β and IL-6), COX-2 induction and eicosanoid (PGE2) production, as well as increased aromatase gene expression, enzyme activity and subsequent estrogen synthesis [119,120,121,122]. Therefore, CLS formation represents a tissue localization wherein largely adipocyte-macrophage-mediated paracrine interactions promote both the development and persistence of an inflammatory AT microenvironment, which through further paracrine interactions signal to the mammary epithelial cells to promote BC growth and invasion [120,121]. Transformed mammary epithelial cells and/or the BC tumor itself can further serve as a cellular source for inflammatory mediator production and amplify the on-going local production of inflammatory mediators, and spread these signals to the surrounding non-involved mammary tissue. Moreover, in obesity, mammary AT, which is a subcutaneous AT depot, is transformed to mimic the inflammatory milieu that characterizes the obese visceral AT phenotype [120,121]. The obesity-associated mammary AT phenotypic switch is significant because evidence suggests that subcutaneous AT tends to be less inflammatory compared to visceral sources [118,123] and therefore, in obese mammary AT, a typically less inflammatory depot exhibits a more pronounced inflammatory phenotype that can drive tumorigenesis. In obesity, dietary n-3 PUFA supplementation has been shown to reduce AT CLS formation, reduce macrophage AT infiltration by reducing MCP-1 tissue expression and improve the inflammatory secretory profile, in part, by increasing adiponectin [27,28].
The mammary AT tumor microenvironment is complex. Increased local production of inflammatory adipokines underpin the paracrine signaling that regulates the cellular interactions between adipocytes, stromal epithelial cells and infiltrating macrophages, which ultimately drive and define mammary tumor development and the end-stage phenotype (tumor size, type and inflammatory status). Up-regulated paracrine interactions (i.e., cross-talk) in obesity-associated BC perpetuate the carcinogenic process by stimulating multiple, overlapping signaling pathways. These pathways converge to stimulate aromatase expression/activation that aberrantly produces local estrogen, promote cell proliferation and/or inhibit apoptosis and stimulate the production of additional inflammatory mediators within mammary tissue, all of which ultimately support tumorigenesis. The critical inflammatory mediators that are up-regulated in obesity-associated BC perpetuate the carcinogenic process and exhibit redundant effects by stimulating multiple and overlapping signaling pathways that converge to stimulate aromatase expression/activation, resulting in aberrant local estrogen production, which promotes cell proliferation and/or inhibits apoptosis, and stimulate the production of additional inflammatory mediators within mammary tissue, ultimately resulting in tumorigenesis. Since a large and diverse list of hormones, adipokines and lipid mediators are implicated in promoting obesity-associated mammary tumorigenesis, our review will focus on a critical subset that work in concert to promote estrogen production (via aromatase activation) and tumorigenesis, specifically eicosanoids, inflammatory cytokines, leptin and adiponectin; however, we recognize that other mediators play a role in this process such as insulin, insulin-like growth factors, resistin nampt/visfatin and cholesterol as reviewed elsewhere [124,125,126]. The specific mechanisms/pathways through which obesity-associated inflammatory adipokines exert pro-tumorigenic effects are discussed in detail below, and the complexity of these paracrine interactions are shown in Figure 1.
7. The Role of Estrogen and Aromatase Activation in BC
Circulating estrogen levels are higher in obese women compared to lean women, and increased circulating estrogen is associated with approximately a two-fold increased risk of postmenopausal BC [5,6,127,128,129]. Additionally, obesity, particularly abdominal adiposity, increases estradiol production and bioavailability due to a reduction in hepatic synthesis of sex hormone-binding globulin (SHBG) in postmenopausal women [6,12,127,129,130]. These hormonal changes are widely believed to play an underlying role in the increased risk of BC in obese postmenopausal women [6,12,127,131]. After menopause, the primary source of estrogen are extra-ovarian sites, primarily in the AT, and aberrant AT estrogen production is attributable to increased aromatase activity, which is present at higher levels in mammary tumors compared to normal mammary tissue [132,133]. Aromatase is the rate-limiting enzyme in the estrogen biosynthesis pathway [131] which catalyzes the peripheral conversion of androstenedione and testosterone to estrone and estradiol, respectively [110]. Downstream conversion of estrone to the biologically potent estradiol is catalyzed by 17β-hydroxysteroid dehydrogenase, which is also expressed within AT [131]. In obese individuals, aromatase expression is reported to be increased by two-fold compared to normal weight individuals [125]. Additionally, aromatase expression is four to five-fold higher within breast tumor tissue compared to non-involved tissues within the same breast [125]. Consequently, mammary tissue estrogen levels are reported to be 10–50 times higher compared to blood levels in postmenopausal healthy women, which has been shown to play a critical role in BC cell growth [134,135,136,137,138].
Typically, mammary tumors are located in regions of the breast with the highest aromatase expression and activity [139,140]. Furthermore, breast tissue aromatase expression is highest in the quadrant of the breast that contains the greatest proportion of adipose stromal cells, as there is little aromatase activity in mature adipocytes [141], and accordingly, aromatase expression is typically highest in the adipose stromal cells adjacent to the tumor mass [139,140,142]. Therefore, the ratio of stromal cells to adipocytes within mammary tissue may have a predictive value in potential tumor development. Moreover, mammary tumors are typically surrounded by a layer of proliferating cancer-associated fibroblasts (CAF) which have also been shown to express aromatase, thereby indicating that factors produced by the tumor may also stimulate aromatase expression in the surrounding CAF [143].
Aromatase expression and activity is strongly influenced by local inflammatory paracrine signaling within mammary tissue. For instance, malignant epithelial cells along with AT macrophages produce pro-inflammatory mediators, including the eicosanoid PGE2, which induce aromatase activity and stimulate estrogen production in pre-adipocytes [131,143,144]. The resultant inflammatory mammary tissue microenvironment is further propagated in the obese state, thereby creating a favorable tissue microenvironment to promote the progression of BC growth [120,121]. Further, in obese human BC tissue, aromatase expression is associated with increased tissue levels of COX-2 and PGE2 [121]. In BC tissue, COX-2 expression is induced by pro-inflammatory cytokines, notably TNFα, and the resultant increased PGE2 levels are associated with large tumor size and high proliferation rates [131,145], due to, in part, the induction of aromatase expression via activation of cAMP-PKA and PKC-mediated signaling cascades [141,146,147]. Conversely, PGE3, an n-3 PUFA-derived eicosanoid does not induce aromatase expression [148]. Aromatase expression is negatively regulated, in part, by AMP-activated protein kinase (AMPK), which also functions as a negative regulator of the Akt/mTOR signaling pathway that is frequently activated in BC [125]. Additionally, liver kinase B1 (LKB1) can function as a tumor suppressor and can regulate aromatase expression via directly phosphorylating and activating AMPK [149]. Therefore, LKB1 and AMPK both function as negative regulators of aromatase expression in BC. As many inflammatory and metabolic factors alter aromatase expression via effects on LKB1 and/or AMPK, this may provide a critical link between obesity, inflammation and aromatase expression in BC [125]. Leptin increases aromatase expression by decreasing LKB1 protein expression and phosphorylation, whereas adiponectin exerts the opposite effect by stimulating LKB1 and its activity, leading to decreased aromatase expression [150]. Additionally, PGE2 down-regulates the phosphorylation of AMPK and LKB1, thereby promoting aromatase expression [150]. Inflammatory cytokines such as IL-6, IL-1β and TNFα have also been shown to stimulate aromatase activity [151,152,153,154]. TNFα induces aromatase expression through two mechanisms, (i) stimulating the binding of c-fos and c-jun transcription factors to activating protein-1 (AP-1) binding site; and (ii) activation of NFκB and MAPK signaling pathways [152,153]. Obese ovariectomized rodents exhibit increased NFκB activation and inflammatory mediator production (TNF-α, IL-1β, COX-2), which is accompanied by elevated levels of aromatase expression and activity in both the mammary gland and visceral fat [120]. Collectively, these data demonstrate that inflammatory mediator signaling in the mammary tissue microenvironment is driven by autocrine/paracrine interactions that regulate critical aspects of the mammary tissue tumor phenotype including aromatase activation and local estrogen production. These inflammatory mediators can establish a positive feedback mechanism that stimulates cell proliferation and mammary tumor development.
8. Inflammatory and Chemopromotive Fatty Acid Derived Lipid Mediators: Differential Effects of n-6 versus n-3 PUFA
LC n-3 and n-6 PUFA are metabolized by the cyclooxygenase (COX), lipoxygenase (LOX) and cytochrome P450 pathways to produce eicosanoids [72]. Specifically, n-6 PUFA (such as AA) serves as a substrate for COX enzymes (producing two-series prostanoids such as prostaglandins (PG) and thromboxanes (TX)), LOX enzymes (producing four-series leukotrienes (LT) and hydroxyeicosatetraenoic acids (HETE), principally 5-, 12- and 15-HETE), or cytochrome P450 enzymes (producing primarily 20-HETE) [72,148,155]. The same enzymes metabolize n-3 PUFA to structurally different three-series prostanoids, five-series LT, and primarily 19- and 20-hydroxyeicosapentaenoic acids (HEPE) and 21- and 22-hydroxydocosahexaenoic acids (HDHE), as well as the unique lipid mediators E- and D-series resolvins and protectins [72,148,156]. Although both resolvins and protectins exert anti-inflammatory effects, resolvins can stimulate the resolution phase of inflammation to begin at an earlier point, thereby limiting the tissue exposure to inflammatory signaling [157], whereas protectins reduce inflammatory cytokine production [158]. Generally, n-6 PUFA-derived eicosanoids are pro-inflammatory and pro-carcinogenic, whereas n-3 PUFA-derived lipid mediators are less biologically active and functionally oppose the synthesis and activity n-6 PUFA-derived eicosanoids [72,148,159]. Excessive dietary intake of n-6 PUFA versus n-3 PUFA (five- to 20-fold greater amounts) results in a significantly greater proportion of eicosanoids generated from n-6 PUFA [72,160]. Importantly, the fatty acid profile of adipocyte, immune and tumor cell membrane phospholipids can be modified by increased intake of n-3 PUFA, thereby suppressing the biosynthesis of AA-derived eicosanoids in favor of EPA and DHA-derived lipid mediators [72,148,159,161].
AA has been shown to be preferentially taken up by MDA-MB-231 BC cells in comparison to EPA, especially in a pro-inflammatory microenvironment [162]. Also, COX-2 and 12-LOX enzymes are overexpressed in BC tumor tissue [131], thereby increasing production of AA-derived inflammatory eicosanoids, which have established pro-tumorigenic effects, and dominate the BC phenotype [131,163,164,165,166]. Specifically, PGE2, LTB4 and 5-, 12- and 15-HETE, have been shown to increase cell proliferation, down-regulate apoptotic pathways, and induce rapid growth and tumor metastasis, and these effects have been shown to be counteracted by n-3 PUFA [50,51,159,165,166,167,168,169]. Furthermore, n-6 PUFA-derived eicosanoid levels are elevated during obesity and have been shown to stimulate breast tumor growth, invasion, and metastasis [131], indicating that obesity perpetuates local inflammatory eicosanoid production.
EPA is the preferential substrate for LOX enzymes, and therefore, when present in comparable proportions, n-3 PUFA-derived eicosanoids will be produced at the expense of n-6 PUFA-derived eicosanoids [148]. Moreover, n-3 PUFA suppress COX-2 expression, which is associated with decreased mammary epithelial cell proliferation in MMTV-HER-2/neu transgenic mice [78]. Mammary tumor PGE2 and 12- and 15-HETE concentrations are dose-dependently reduced by increased dietary n-3 PUFA intake in female nude mice injected with MDA-MB-231 cells [42,170], which is associated with increased apoptotic activity and decreased breast tumor cell proliferation, growth and lung metastasis. Similar n-3 PUFA-mediated anti-tumorigenic effects have been associated with suppressed cell proliferation and decreased expression of Bcl-2 and other carcinogenic proteins including Ki-67, Her-2/neu and c-Myc [50,51,159,167,168,171]. Taken together, n-6 PUFA-derived eicosanoids provide a link between obesity-associated chronic inflammation and the development of BC, and are a potential target for dietary LC n-3 PUFA intervention to mitigate the pro-carcinogenic effects of inflammatory n-6 PUFA-derived eicosanoids within the mammary tumor microenvironment.
9. Role of Inflammatory Cytokines
Inflammatory cytokines (TNFα, IL-1β, IL-6 and MCP-1) also contribute to the local mammary tissue milieu through paracrine signaling and through an autocrine positive-feedback mechanism to further their own on-going production, in part, by activating the transcription factor NFκB. Interestingly, NFκB activation underlies many aspects of BC cell proliferation, invasion and metastasis [126,172,173]. Moreover, aberrant NFκB signaling is proposed to be one of the mechanisms through which chronic inflammation leads to cancer, as NFκB activation promotes tumorigenesis by inhibiting apoptosis (via activation of Bcl2, Bcl-xL, cFLIP and other genes) and increasing cell proliferation by regulating expression of cyclinD1, cyclinE, CDK2, and c-Myc [174]. Several inflammatory mediators are up-regulated by NFκB activation; specifically, inflammatory n-6 PUFA-derived eicosanoid production is stimulated by NFκB activation of COX-2 [175]. Additionally, within chronically inflamed rodent mammary tissue, NFκB increases production of TNFα and IL-1β [120] and similar findings are reported in the mammary tissue of both pre- and postmenopausal obese women [121]. IL-1β levels are increased in patients with invasive ductal carcinoma and ductal carcinomas in situ compared to benign mammary tissue levels [176,177], and are overexpressed in breast carcinomas, but undetectable in normal breast tissue [178]. IL-1β levels are positively correlated with the expression of angiogenic factor expression, tumor grade and the expression of AP-1 [176,177,178].
IL-1β and IL-6 have been shown to stimulate BC cell proliferation in an additive manner with estrogen [154], indicative of synergy between inflammatory mediators and hormones within the mammary tumor microenvironment. TNFα, another potent inflammatory cytokine, also promotes mammary tumor development [179] and has been shown to contribute to BC cell epithelial-mesenchymal transition (EMT) by increasing matrix metalloproteinase (MMP)-9 expression, thereby enhancing migration and invasive capacity [180,181,182]. Additionally, the high levels of IL-6 and TNFα found in obese rodent adipocyte-conditioned media and serum have been shown to promote cancer cell EMT [183]. Interestingly, the two main cellular sources of TNFα are tumor-associated macrophages (TAM) and the BC cells themselves [131], highlighting the role of inflammatory macrophages in BC.
Macrophage infiltration into mammary tumor sites (and subsequent development of CLS) is driven, in part, by chemotactic signaling. MCP-1, also referred to as CCL2, signals to increase macrophage infiltration into the inflamed mammary tissue, thereby increasing the number of deleterious TAM that accumulate in mammary tumor tissue [184,185]. The cellular sources of MCP-1 in primary breast tumor sites are tumor cells and the TAM themselves, which indicates a feed-forward mechanism wherein macrophage accumulation/tumor recruitment is perpetuated throughout the stages of tumor growth [184,185]. Overall, TAM form CLS within the mammary AT and act as the cellular source of several inflammatory mediators/cytokines that perpetuate the local inflammatory tissue microenvironment, which, through autocrine and paracrine interactions, further promotes BC development [119,120,121,122,131]. Collectively, this highlights the critical role that macrophages play in driving tumor-associated inflammatory paracrine interactions. MCP-1 tumor expression is associated with a more advanced course of tumor progression, wherein MCP-1 promotes angiogenesis by stimulating the production of angiogenic factors (such as IL-8 and VEGF) [184,185]. In obesity, circulating and AT levels of MCP-1 are increased. This chemokine provides the main chemoattractant signal that drives visceral AT macrophage infiltration and CLS formation, up-regulating local AT inflammatory mediator production and subsequently impairing glucose metabolism [58,59]. Macrophage recruitment is also stimulated by AT hypoxia. In obesity, adipocyte hypertrophy results in decreased oxygen diffusion, leading to localized tissue AT hypoxia, as evidenced by upregulation of the hypoxia master regulator, hypoxia induced factor (HIF-1α), which stimulates MCP-1 and subsequent macrophage chemotaxis [143,186,187].
Countering these effects in obesity, n-3 PUFA have been shown to improve the hypoxic AT microenvironment by reducing adipocyte size [27] and to decrease obesity-associated expression of HIF-1α [81,188]. Furthermore, n-3 PUFA reduce visceral AT MCP-1 levels [27,28,35,189,190], thereby reducing obesity-associated AT macrophage accumulation, CLS formation and AT inflammation [27,28]. We and others have shown that n-3 PUFA reduce inflammatory paracrine signaling between adipocytes and macrophages by decreasing NFκB activation and subsequent secretion of TNFα, MCP-1 and IL-6 [191,192]. Moreover, independent of cellular source, n-3 PUFA have been shown to inhibit NFκB activation by decreasing IκB phosphorylation and activation, thereby reducing production TNFα, IL-1β and IL-6 [29,35,40,41,67,191,193,194].
10. Role of Leptin
Leptin exerts pleiotropic effects apart from regulation of energy expenditure and food intake, including effects on immunity, inflammation, cell differentiation and proliferation [124,195], all of which have direct relevance to cancer. Classically, circulating leptin levels are proportional to the amount of body fat [196] and increased leptin levels are associated with increased risk of BC development and progression [197,198]. Leptin gene expression is detected in normal healthy breast epithelial tissue [110], consistent with its endogenous role in normal mammary gland development and lactation [199]; however, it is also capable of contributing to mammary tumorigenesis [200] and is expressed in the healthy tissue that surrounds malignant ductal lesions [201]. In primary tumors and various BC cell lines, both leptin and various isoforms of the leptin receptor (ObR) including the long signaling form ObR1 are overexpressed [197,202,203,204,205,206,207]. Recently, three single nucleotide polymorphisms in the leptin receptor gene (K109R, K656N and Q223R) were identified to be associated with increased BC risk, suggesting that tumor leptin receptor signaling can directly influence tumor growth and progression [208]. Interestingly, in BC patients high intra-tumor ObR gene expression was strongly correlated with decreased relapse-free survival [209], indicating that susceptibility to leptin signaling is strongly associated with BC disease prognosis.
Leptin signaling has been shown to exert autocrine and paracrine effects, ultimately promoting cell growth and proliferation via the activation of critical signaling pathways including those mediated by PKC, c-Jun N-terminal kinase (JNK), p38 MAPK, Janus Kinase2/Signal Transducer and Activator of Transcription 3 (JAK2/STAT3), PI3K/Akt/mTOR, Akt/GSK3 and MAPK/extracellular signal-related kinase 1/2 (ERK1/2) pathways [116,181,195,203,210,211,212,213]. Further, leptin increases the levels of cell cycle regulators in human MCF-7 BC cells by up-regulating the expression of cyclin dependent kinase 2 (cdk2) and cyclin D1, which advances cells from the G1 to S phase of the cell cycle [214], and induces cell proliferation in ZR-75-1 BC cells via up-regulation of cyclin D1 and c-Myc [215]. Therefore, overexpression and activation of leptin within the mammary tissue microenvironment can ultimately promote tumorigenesis. Similar to obese women, rodents with high leptin levels are more likely to develop mammary tumors [216,217]. Furthermore, in animal BC models leptin antagonism has produced successful outcomes. Specifically MMTV-TGF-α mice crossed with leptin deficient mice fail to develop tumors [218,219] and treatment with a leptin antagonist decreases the growth of murine triple-negative breast tumors [220] and 4T1 mammary cancer cell growth via reducing levels of VEGF, pSTAT3 and cyclin D1 [221].
An additional role of leptin in breast carcinogenesis is to potentiate estrogen signaling, as leptin has been shown to induce aromatase expression/activity and subsequent estrogen synthesis, thereby enhancing ERα activity in BC [210,222,223]. Leptin levels positively correlate with ER expression and BC tumor size [203,205]. Further, leptin can transactivate ERα via ERK1/2 signaling [210] and enhance ERα-dependent transcription by reducing ERα ubiquitination and degradation, even in the presence of an estrogen inhibitor [223], thereby potentiating the effects of estrogen on cell proliferation. Bi-directional influences of estrogen on leptin also exist, wherein estradiol induces leptin and ObR expression, in both AT and BC cell lines [203,224,225,226].
Another obesity-associated factor that can contribute to the increased local production of leptin and subsequent pro-tumorigenic effects is AT hypoxia. Leptin receptor expression can also be stimulated by tissue hypoxia [226] and, therefore, local tissue leptin expression is up-regulated by HIF-1α [227,228]. Conversely, n-3 PUFA have been shown to improve the hypoxic AT microenvironment by reducing adipocyte size [27] and to decrease obesity-associated expression of HIF-1α [81,188], thereby providing a mechanism through which local leptin signaling responsiveness could be attenuated.
Dietary n-3 PUFA have been shown to reduce leptin AT gene expression and/or circulating levels in obese rodents [36,37] and humans [38,39], an effect that was most prominent when combined with weight loss [39]. Decreased leptin signaling represents an additional mechanism through which n-3 PUFA attenuate the effects of leptin. In a rodent obesity model, n-3 PUFA supplementation was found to decrease leptin receptor gene expression [229], thereby decreasing leptin signaling. In this connection, leptin receptors have been shown to localize to lipid rafts and downstream proliferative effects of leptin mediated through p38 MAPK signaling is lipid raft dependent [230]. Thus, n-3 PUFA antagonism of lipid raft size and composition in BC [100,103], as already discussed herein, may also antagonize leptin-mediated proliferative signaling within the mammary tumor microenvironment. Therefore, these findings add to the complex interplay of autocrine and paracrine interactions that underlie the obesity-associated BC inflammatory phenotype, and suggest dietary n-3 PUFA as an intervention that may have utility in mitigating local mammary tissue leptin production and signaling to inhibit its pro-tumorigenic effects.
11. Role of Adiponectin
Adipocytes are the primary cellular source of adiponectin, which is secreted as a monomeric protein that can be oligomerized to form both low-molecular weight and high molecular weight complexes [231]. Additionally, cleavage reactions via the action of elastase, can generate globular oligomeric complexes [232] that bind with greater affinity to the adiponectin receptor 1 (AdipoR1), whereas the AdipoR2 preferentially binds full-length and multimeric adiponectin [233].
Within the context of the tumor microenvironment, adiponectin and leptin counter-regulate each other and exert opposing effects [126]. Decreased levels of adiponectin may explain, in part, the increased risk of BC in obesity. Circulating adiponectin levels are generally inversely correlated with BMI, adiposity and visceral fat mass [234,235] and the decreased adiponectin levels in obesity [236] correlate with increased BC risk [111,237,238]. Moreover, three recent independent meta-analyses of BC observational studies confirmed the correlation of higher circulating adiponectin levels with lower BC risk in postmenopausal women [239,240,241]. More specifically, an increase of 3 μg/mL of circulating adiponectin corresponded to a 5% reduction in BC risk [239]. In obese postmenopausal women, hypoadiponectinemia is associated with increased BC risk, and the disease has been shown to manifest with an aggressive metastatic phenotype [235,242]. In premenopausal women, however, adiponectin levels are not associated with BC risk (95% CI −0.164 to 0.204, p = 0.829) [239,240,241]. The local breast tumor tissue mRNA and protein expression of adiponectin is low, although its receptors are still expressed, indicating that adiponectin-mediated anti-tumorigenic signaling is possible in BC [111,243,244]. In animal studies, reduced production of adiponectin is associated with earlier tumor onset and accelerated tumor growth [245], and overexpression of adiponectin results in mice with reduced mammary tumor size and weight [246]. Studies using various BC cell lines demonstrate that the anti-proliferative effect of adiponectin is mediated through AdipoR1 and AdipoR2 signaling [246,247,248]. There is a negative correlation between AdipoR1 expression and tumor size, which suggests that the loss of AdipoR1 signaling favours tumor growth [249]. These data are indicative of a weak autocrine/paracrine activity of this hormone within the tumor microenvironment and a loss of the beneficial anti-tumor effects of adiponectin. Despite reduced tissue levels and blunted adiponectin signaling in obesity-associated BC, treatment strategies designed to stimulate adiponectin signaling might represent a novel therapeutic approach.
Adiponectin exerts an anti-proliferative effect in BC [246,247,250,251,252,253] by impacting several signaling pathways. Specifically, adiponectin has been shown to impact the glycogen synthase kinase-3β (GSK-3β)/β-catenin signaling pathway via inhibition of phosphorylation of Akt and GSK-3β and subsequent suppression of intracellular accumulation of β-catenin and its transcriptional activities, resulting in reduced cyclin-D1 expression [246,247,251]. Additionally, adiponectin has been shown to reduce BC cell proliferation by regulating the PTEN/PI3K/mTOR and MAPK pathways [212], specifically inactivating ERK1/2, stimulating AMPK activity and decreasing Akt phosphorylation, leading to reduced mTOR activity [4,251,252,253]. In MCF-7 BC cells, microarray analysis demonstrated that adiponectin represses expression of multiple important genes that regulate cell cycle (MAPK3 and ATM) and apoptosis (BAG1, BAG3, and TP53), as well as potential diagnostic/prognostic markers (ACADS, CYP19A1, DEGS1, and EVL) [250]. Adiponectin has also been shown to induce BC cell apoptosis [251,253] by down-regulating Bcl2 and up-regulating p53, Bax and p21 expression [4,248,251]; however, this outcome is dependent on the BC cell line utilized and duration of adiponectin incubation (reviewed [212]). Adiponectin also exerts anti-inflammatory effects by inhibiting the effects of leptin [126] and inhibiting TNFα production by macrophages and adipocytes [254]. Generally, independent of cell type, adiponectin signaling down-regulates the activation of NFκB and production of inflammatory cytokines (TNFα, IL-1β, IL-6 and MCP-1) [255]. Moreover, adiponectin reduces macrophage-mediated inflammation within the tumor microenvironment by suppressing IL-6 gene expression and antagonizing NFκB, JNK and p38 MAPK mediated signaling [255,256].
Increasing the production of adiponectin in obesity may be a beneficial strategy to mitigate inflammation. n-3 PUFA have been shown to up-regulate adiponectin secretion in both murine [65] and human adipocytes [66]. Furthermore, dietary n-3 PUFA improve the obesity-associated inflammatory secretory profile, in part, by increasing adiponectin levels [27,28,29,30,31,35,63]. In human clinical trials, dietary n-3 PUFA have been shown to increase adiponectin levels [32,33,34] in obese and overweight subjects, thereby demonstrating the potential utility of n-3 PUFA to stimulate the effects of this anti-inflammatory adipokine in obesity. Considering the anti-tumorigenic effects of adiponectin and the ability of n-3 PUFA to restore adiponectin function in obesity [27,28,29,30,31,32,33,34,35,63], further research initiatives should be undertaken to determine the utility of n-3 PUFA in mitigating obesity-associated BC inflammation and tumor production.
12. Conclusions
In obese women with BC, increased inflammatory adipokine production, both locally in the mammary AT depot and systemically, perpetuates inflammation-associated pro-tumorigenic signaling pathways, thereby increasing disease severity. A spectrum of inflammatory mediators/adipokines are produced by adipocytes, TAM, mammary epithelial cells and tumor cells, which collectively stimulate diverse and overlapping signaling pathways that converge to stimulate aromatase activity that aberrantly increases local estrogen production, up-regulates cell proliferation and down-regulates apoptosis. The complex nature of the obesity-associated BC inflammatory pathophysiology is not likely to be attenuated or prevented by targeting any individual inflammatory mediator and/or signaling pathway, which may explain why most drug therapies, in this context, are ineffective. Instead, a pan-anti-inflammatory approach is more likely to have success in mitigating obesity-associated mammary tissue inflammatory paracrine interactions and subsequent tumorigenesis, and in this context, n-3 PUFA may have utility. n-3 PUFA have been shown to concurrently target multiple aspects of the obese BC phenotype including reduction of macrophage AT infiltration and CLS formation and down-regulation of critical adipokine production. Collectively, increased n-3 PUFA intake could attenuate obesity-associated BC. Considering the current state of obesity world-wide, further studies in human at risk populations should be made a priority.
Acknowledgments
This work was supported by the Natural Sciences and Engineering Research Council of Canada (NSERC). Danyelle M. Liddle was supported by an Ontario Graduate Scholarship. Anna A. De Boer was supported by a NSERC graduate scholarship.
Author Contributions
All authors contributed to the writing and editing of this review. The final manuscript was approved by all authors.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Wang Y.C., McPherson K., Marsh T., Gortmaker S.L., Brown M. Health and economic burden of the projected obesity trends in the USA and the UK. Lancet. 2011;378:815–825. doi: 10.1016/S0140-6736(11)60814-3. [DOI] [PubMed] [Google Scholar]
- 2.Lumeng C.N., Saltiel A.R. Inflammatory links between obesity and metabolic disease. J. Clin. Investig. 2011;121:2111–2117. doi: 10.1172/JCI57132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Calle E.E., Rodriguez C., Walker-Thurmond K., Thun M.J. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N. Engl. J. Med. 2003;348:1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 4.Van Kruijsdijk R.C., van der Wall E., Visseren F.L. Obesity and cancer: The role of dysfunctional adipose tissue. Cancer Epidemiol. Biomark. Prev. 2009;18:2569–2578. doi: 10.1158/1055-9965.EPI-09-0372. [DOI] [PubMed] [Google Scholar]
- 5.Cleary M.P., Grossmann M.E. Minireview: Obesity and breast cancer: The estrogen connection. Endocrinology. 2009;150:2537–2542. doi: 10.1210/en.2009-0070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Key T.J., Appleby P.N., Reeves G.K., Roddam A., Dorgan J.F., Longcope C., Stanczyk F.Z., Stephenson H.E., Jr., Falk R.T., Miller R., et al. Body mass index, serum sex hormones, and breast cancer risk in postmenopausal women. J. Natl. Cancer Inst. 2003;95:1218–1226. doi: 10.1093/jnci/djg022. [DOI] [PubMed] [Google Scholar]
- 7.van den Brandt P.A., Spiegelman D., Yaun S.S., Adami H.O., Beeson L., Folsom A.R., Fraser G., Goldbohm R.A., Graham S., Kushi L., et al. Pooled analysis of prospective cohort studies on height, weight, and breast cancer risk. Am. J. Epidemiol. 2000;152:514–527. doi: 10.1093/aje/152.6.514. [DOI] [PubMed] [Google Scholar]
- 8.Li C.I., Daling J.R., Malone K.E. Incidence of invasive breast cancer by hormone receptor status from 1992 to 1998. J. Clin. Oncol. 2003;21:28–34. doi: 10.1200/JCO.2003.03.088. [DOI] [PubMed] [Google Scholar]
- 9.Biglia N., Peano E., Sgandurra P., Moggio G., Pecchio S., Maggiorotto F., Sismondi P. Body mass index (bmi) and breast cancer: Impact on tumor histopathologic features, cancer subtypes and recurrence rate in pre and postmenopausal women. Gynecol. Endocrinol. 2013;29:263–267. doi: 10.3109/09513590.2012.736559. [DOI] [PubMed] [Google Scholar]
- 10.Garrisi V.M., Tufaro A., Trerotoli P., Bongarzone I., Quaranta M., Ventrella V., Tommasi S., Giannelli G., Paradiso A. Body mass index and serum proteomic profile in breast cancer and healthy women: A prospective study. PLoS One. 2012;7:e49631. doi: 10.1371/journal.pone.0049631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kamineni A., Anderson M.L., White E., Taplin S.H., Porter P., Ballard-Barbash R., Malone K., Buist D.S. Body mass index, tumor characteristics, and prognosis following diagnosis of early-stage breast cancer in a mammographically screened population. Cancer Causes Control. 2013;24:305–312. doi: 10.1007/s10552-012-0115-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rinaldi S., Key T.J., Peeters P.H., Lahmann P.H., Lukanova A., Dossus L., Biessy C., Vineis P., Sacerdote C., Berrino F., et al. Anthropometric measures, endogenous sex steroids and breast cancer risk in postmenopausal women: A study within the epic cohort. Int. J. Cancer. 2006;118:2832–2839. doi: 10.1002/ijc.21730. [DOI] [PubMed] [Google Scholar]
- 13.John E.M., Phipps A.I., Sangaramoorthy M. Body size, modifying factors, and postmenopausal breast cancer risk in a multiethnic population: The San Francisco bay area breast cancer study. SpringerPlus. 2013;2:239. doi: 10.1186/2193-1801-2-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krishnan K., Bassett J.K., MacInnis R.J., English D.R., Hopper J.L., McLean C., Giles G.G., Baglietto L. Associations between weight in early adulthood, change in weight, and breast cancer risk in postmenopausal women. Cancer Epidemiol. Biomark. Prev. 2013;22:1409–1416. doi: 10.1158/1055-9965.EPI-13-0136. [DOI] [PubMed] [Google Scholar]
- 15.Cheraghi Z., Poorolajal J., Hashem T., Esmailnasab N., Doosti Irani A. Effect of body mass index on breast cancer during premenopausal and postmenopausal periods: A meta-analysis. PLoS One. 2012;7:e51446. doi: 10.1371/journal.pone.0051446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pierobon M., Frankenfeld C.L. Obesity as a risk factor for triple-negative breast cancers: A systematic review and meta-analysis. Breast Cancer Res. Treat. 2013;137:307–314. doi: 10.1007/s10549-012-2339-3. [DOI] [PubMed] [Google Scholar]
- 17.Amadou A., Ferrari P., Muwonge R., Moskal A., Biessy C., Romieu I., Hainaut P. Overweight, obesity and risk of premenopausal breast cancer according to ethnicity: A systematic review and dose-response meta-analysis. Obes. Rev. 2013;14:665–678. doi: 10.1111/obr.12028. [DOI] [PubMed] [Google Scholar]
- 18.Brown K.A. Impact of obesity on mammary gland inflammation and local estrogen production. J. Mammary Gland Biol. Neoplasia. 2014;19:183–189. doi: 10.1007/s10911-014-9321-0. [DOI] [PubMed] [Google Scholar]
- 19.Coutinho T., Goel K., Correa de Sa D., Carter R.E., Hodge D.O., Kragelund C., Kanaya A.M., Zeller M., Park J.S., Kober L., et al. Combining body mass index with measures of central obesity in the assessment of mortality in subjects with coronary disease: Role of “Normal weight central obesity”. J. Am. Coll. Cardiol. 2013;61:553–560. doi: 10.1016/j.jacc.2012.10.035. [DOI] [PubMed] [Google Scholar]
- 20.Kimura K., Tanaka S., Iwamoto M., Fujioka H., Takahashi Y., Satou N., Uchiyama K. Association between body mass index and breast cancer intrinsic subtypes in Japanese women. Exp. Ther. Med. 2012;4:391–396. doi: 10.3892/etm.2012.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Daling J.R., Malone K.E., Doody D.R., Johnson L.G., Gralow J.R., Porter P.L. Relation of body mass index to tumor markers and survival among young women with invasive ductal breast carcinoma. Cancer. 2001;92:720–729. doi: 10.1002/1097-0142(20010815)92:4<720::AID-CNCR1375>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 22.Fagherazzi G., Chabbert-Buffet N., Fabre A., Guillas G., Boutron-Ruault M.C., Mesrine S., Clavel-Chapelon F. Hip circumference is associated with the risk of premenopausal er-/pr- breast cancer. Int. J. Obes. 2012;36:431–439. doi: 10.1038/ijo.2011.66. [DOI] [PubMed] [Google Scholar]
- 23.Olefsky J.M., Glass C.K. Macrophages, inflammation, and insulin resistance. Annu. Rev. Physiol. 2010;72:219–246. doi: 10.1146/annurev-physiol-021909-135846. [DOI] [PubMed] [Google Scholar]
- 24.Pierce B.L., Ballard-Barbash R., Bernstein L., Baumgartner R.N., Neuhouser M.L., Wener M.H., Baumgartner K.B., Gilliland F.D., Sorensen B.E., McTiernan A., et al. Elevated biomarkers of inflammation are associated with reduced survival among breast cancer patients. J. Clin. Oncol. 2009;27:3437–3444. doi: 10.1200/JCO.2008.18.9068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Woodward W.A., Cristofanilli M. Inflammatory breast cancer. Semin. Radiat. Oncol. 2009;19:256–265. doi: 10.1016/j.semradonc.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 26.Cariati M., Bennett-Britton T.M., Pinder S.E., Purushotham A.D. “Inflammatory” Breast cancer. Surg. Oncol. 2005;14:133–143. doi: 10.1016/j.suronc.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 27.Rossmeisl M., Jilkova Z.M., Kuda O., Jelenik T., Medrikova D., Stankova B., Kristinsson B., Haraldsson G.G., Svensen H., Stoknes I., et al. Metabolic effects of n-3 pufa as phospholipids are superior to triglycerides in mice fed a high-fat diet: Possible role of endocannabinoids. PLoS One. 2012;7:e38834. doi: 10.1371/journal.pone.0038834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rossmeisl M., Medrikova D., van Schothorst E.M., Pavlisova J., Kuda O., Hensler M., Bardova K., Flachs P., Stankova B., Vecka M., et al. Omega-3 phospholipids from fish suppress hepatic steatosis by integrated inhibition of biosynthetic pathways in dietary obese mice. Biochim. Biophys. Acta. 2014;1841:267–278. doi: 10.1016/j.bbalip.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 29.Kalupahana N.S., Claycombe K., Newman S.J., Stewart T., Siriwardhana N., Matthan N., Lichtenstein A.H., Moustaid-Moussa N. Eicosapentaenoic acid prevents and reverses insulin resistance in high-fat diet-induced obese mice via modulation of adipose tissue inflammation. J. Nutr. 2010;140:1915–1922. doi: 10.3945/jn.110.125732. [DOI] [PubMed] [Google Scholar]
- 30.Flachs P., Mohamed-Ali V., Horakova O., Rossmeisl M., Hosseinzadeh-Attar M.J., Hensler M., Ruzickova J., Kopecky J. Polyunsaturated fatty acids of marine origin induce adiponectin in mice fed a high-fat diet. Diabetologia. 2006;49:394–397. doi: 10.1007/s00125-005-0053-y. [DOI] [PubMed] [Google Scholar]
- 31.Neschen S., Morino K., Rossbacher J.C., Pongratz R.L., Cline G.W., Sono S., Gillum M., Shulman G.I. Fish oil regulates adiponectin secretion by a peroxisome proliferator-activated receptor-gamma-dependent mechanism in mice. Diabetes. 2006;55:924–928. doi: 10.2337/diabetes.55.04.06.db05-0985. [DOI] [PubMed] [Google Scholar]
- 32.Itoh M., Suganami T., Satoh N., Tanimoto-Koyama K., Yuan X., Tanaka M., Kawano H., Yano T., Aoe S., Takeya M., et al. Increased adiponectin secretion by highly purified eicosapentaenoic acid in rodent models of obesity and human obese subjects. Arterioscler. Thromb. Vasc. Biol. 2007;27:1918–1925. doi: 10.1161/ATVBAHA.106.136853. [DOI] [PubMed] [Google Scholar]
- 33.Nomura S., Shouzu A., Omoto S., Inami N., Ueba T., Urase F., Maeda Y. Effects of eicosapentaenoic acid on endothelial cell-derived microparticles, angiopoietins and adiponectin in patients with type 2 diabetes. J. Atheroscler. Thromb. 2009;16:83–90. doi: 10.5551/jat.E091. [DOI] [PubMed] [Google Scholar]
- 34.Gammelmark A., Madsen T., Varming K., Lundbye-Christensen S., Schmidt E.B. Low-dose fish oil supplementation increases serum adiponectin without affecting inflammatory markers in overweight subjects. Nutr. Res. 2012;32:15–23. doi: 10.1016/j.nutres.2011.12.007. [DOI] [PubMed] [Google Scholar]
- 35.Todoric J., Loffler M., Huber J., Bilban M., Reimers M., Kadl A., Zeyda M., Waldhausl W., Stulnig T.M. Adipose tissue inflammation induced by high-fat diet in obese diabetic mice is prevented by n-3 polyunsaturated fatty acids. Diabetologia. 2006;49:2109–2119. doi: 10.1007/s00125-006-0300-x. [DOI] [PubMed] [Google Scholar]
- 36.Takahashi Y., Ide T. Dietary n-3 fatty acids affect mrna level of brown adipose tissue uncoupling protein 1, and white adipose tissue leptin and glucose transporter 4 in the rat. Br. J. Nutr. 2000;84:175–184. [PubMed] [Google Scholar]
- 37.Ruzickova J., Rossmeisl M., Prazak T., Flachs P., Sponarova J., Veck M., Tvrzicka E., Bryhn M., Kopecky J. Omega-3 pufa of marine origin limit diet-induced obesity in mice by reducing cellularity of adipose tissue. Lipids. 2004;39:1177–1185. doi: 10.1007/s11745-004-1345-9. [DOI] [PubMed] [Google Scholar]
- 38.Winnicki M., Somers V.K., Accurso V., Phillips B.G., Puato M., Palatini P., Pauletto P. Fish-rich diet, leptin, and body mass. Circulation. 2002;106:289–291. doi: 10.1161/01.CIR.0000025241.01418.4D. [DOI] [PubMed] [Google Scholar]
- 39.Mori T.A., Burke V., Puddey I.B., Shaw J.E., Beilin L.J. Effect of fish diets and weight loss on serum leptin concentration in overweight, treated-hypertensive subjects. J. Hypertens. 2004;22:1983–1990. doi: 10.1097/00004872-200410000-00022. [DOI] [PubMed] [Google Scholar]
- 40.Perez-Echarri N., Perez-Matute P., Marcos-Gomez B., Baena M.J., Marti A., Martinez J.A., Moreno-Aliaga M.J. Differential inflammatory status in rats susceptible or resistant to diet-induced obesity: Effects of epa ethyl ester treatment. Eur. J. Nutr. 2008;47:380–386. doi: 10.1007/s00394-008-0738-3. [DOI] [PubMed] [Google Scholar]
- 41.Puglisi M.J., Hasty A.H., Saraswathi V. The role of adipose tissue in mediating the beneficial effects of dietary fish oil. J. Nutr. Biochem. 2011;22:101–108. doi: 10.1016/j.jnutbio.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Connolly J.M., Gilhooly E.M., Rose D.P. Effects of reduced dietary linoleic acid intake, alone or combined with an algal source of docosahexaenoic acid, on mda-mb-231 breast cancer cell growth and apoptosis in nude mice. Nutr. Cancer. 1999;35:44–49. doi: 10.1207/S1532791444-49. [DOI] [PubMed] [Google Scholar]
- 43.Hardman W.E., Munoz J., Jr., Cameron I.L. Role of lipid peroxidation and antioxidant enzymes in omega 3 fatty acids induced suppression of breast cancer xenograft growth in mice. Cancer Cell Int. 2002;2:10. doi: 10.1186/1475-2867-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hardman W.E., Sun L., Short N., Cameron I.L. Dietary omega-3 fatty acids and ionizing irradiation on human breast cancer xenograft growth and angiogenesis. Cancer Cell Int. 2005;5:12. doi: 10.1186/1475-2867-5-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Howe L.R., Subbaramaiah K., Hudis C.A., Dannenberg A.J. Molecular pathways: Adipose inflammation as a mediator of obesity-associated cancer. Clin. Cancer Res. 2013;19:6074–6083. doi: 10.1158/1078-0432.CCR-12-2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Leslie M.A., Abdelmagid S.A., Perez K., Muller W.J., Ma D.W. Mammary tumour development is dose-dependently inhibited by n-3 polyunsaturated fatty acids in the mmtv-neu(ndl)-yd5 transgenic mouse model. Lipids Health Dis. 2014;13:96. doi: 10.1186/1476-511X-13-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ma D.W., Ngo V., Huot P.S., Kang J.X. n-3 polyunsaturated fatty acids endogenously synthesized in fat-1 mice are enriched in the mammary gland. Lipids. 2006;41:35–39. doi: 10.1007/s11745-006-5067-9. [DOI] [PubMed] [Google Scholar]
- 48.MacLennan M.B., Clarke S.E., Perez K., Wood G.A., Muller W.J., Kang J.X., Ma D.W. Mammary tumor development is directly inhibited by lifelong n-3 polyunsaturated fatty acids. J. Nutr. Biochem. 2013;24:388–395. doi: 10.1016/j.jnutbio.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 49.Mandal C.C., Ghosh-Choudhury T., Yoneda T., Choudhury G.G., Ghosh-Choudhury N. Fish oil prevents breast cancer cell metastasis to bone. Biochem. Biophys. Res. Commun. 2010;402:602–607. doi: 10.1016/j.bbrc.2010.10.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Manna S., Chakraborty T., Ghosh B., Chatterjee M., Panda A., Srivastava S., Rana A., Chatterjee M. Dietary fish oil associated with increased apoptosis and modulated expression of bax and bcl-2 during 7,12-dimethylbenz(alpha)anthracene-induced mammary carcinogenesis in rats. Prostaglandins Leukot. Essent. Fatty Acids. 2008;79:5–14. doi: 10.1016/j.plefa.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 51.Manna S., Janarthan M., Ghosh B., Rana B., Rana A., Chatterjee M. Fish oil regulates cell proliferation, protect DNA damages and decrease her-2/neu and c-myc protein expression in rat mammary carcinogenesis. Clin. Nutr. 2010;29:531–537. doi: 10.1016/j.clnu.2009.12.012. [DOI] [PubMed] [Google Scholar]
- 52.Menendez J.A., Lupu R., Colomer R. Exogenous supplementation with omega-3 polyunsaturated fatty acid docosahexaenoic acid (dha; 22:6n-3) synergistically enhances taxane cytotoxicity and downregulates her-2/neu (c-erbb-2) oncogene expression in human breast cancer cells. Eur. J. Cancer Prev. 2005;14:263–270. doi: 10.1097/00008469-200506000-00011. [DOI] [PubMed] [Google Scholar]
- 53.Menendez J.A., Vazquez-Martin A., Ropero S., Colomer R., Lupu R. Her2 (erbb-2)-targeted effects of the omega-3 polyunsaturated fatty acid, alpha-linolenic acid (ala; 18:3n-3), in breast cancer cells: The “Fat features” Of the “Mediterranean diet” As an “Anti-her2 cocktail”. Clin. Transl. Oncol. 2006;8:812–820. doi: 10.1007/s12094-006-0137-2. [DOI] [PubMed] [Google Scholar]
- 54.Mizuno N.K., Rogozina O.P., Seppanen C.M., Liao D.J., Cleary M.P., Grossmann M.E. Combination of intermittent calorie restriction and eicosapentaenoic acid for inhibition of mammary tumors. Cancer Prev. Res. 2013;6:540–547. doi: 10.1158/1940-6207.CAPR-13-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yee L.D., Young D.C., Rosol T.J., Vanbuskirk A.M., Clinton S.K. Dietary (n-3) polyunsaturated fatty acids inhibit her-2/neu-induced breast cancer in mice independently of the ppargamma ligand rosiglitazone. J. Nutr. 2005;135:983–988. doi: 10.1093/jn/135.5.983. [DOI] [PubMed] [Google Scholar]
- 56.Zou Z., Bellenger S., Massey K.A., Nicolaou A., Geissler A., Bidu C., Bonnotte B., Pierre A.S., Minville-Walz M., Rialland M., et al. Inhibition of the her2 pathway by n-3 polyunsaturated fatty acids prevents breast cancer in fat-1 transgenic mice. J. Lipid Res. 2013;54:3453–3463. doi: 10.1194/jlr.M042754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kershaw E.E., Flier J.S. Adipose tissue as an endocrine organ. J. Clin. Endocrinol. Metab. 2004;89:2548–2556. doi: 10.1210/jc.2004-0395. [DOI] [PubMed] [Google Scholar]
- 58.Trayhurn P., Wood I.S. Adipokines: Inflammation and the pleiotropic role of white adipose tissue. Br. J. Nutr. 2004;92:347–355. doi: 10.1079/BJN20041213. [DOI] [PubMed] [Google Scholar]
- 59.Balistreri C.R., Caruso C., Candore G. The role of adipose tissue and adipokines in obesity-related inflammatory diseases. Mediat. Inflamm. 2010;2010:802078. doi: 10.1155/2010/802078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Surmi B.K., Hasty A.H. Macrophage infiltration into adipose tissue: Initiation, propagation and remodeling. Future Lipidol. 2008;3:545–556. doi: 10.2217/17460875.3.5.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yu J.G., Javorschi S., Hevener A.L., Kruszynska Y.T., Norman R.A., Sinha M., Olefsky J.M. The effect of thiazolidinediones on plasma adiponectin levels in normal, obese, and type 2 diabetic subjects. Diabetes. 2002;51:2968–2974. doi: 10.2337/diabetes.51.10.2968. [DOI] [PubMed] [Google Scholar]
- 62.Peyron-Caso E., Fluteau-Nadler S., Kabir M., Guerre-Millo M., Quignard-Boulange A., Slama G., Rizkalla S.W. Regulation of glucose transport and transporter 4 (glut-4) in muscle and adipocytes of sucrose-fed rats: Effects of n-3 poly- and monounsaturated fatty acids. Horm. Metab. Res. 2002;34:360–366. doi: 10.1055/s-2002-33467. [DOI] [PubMed] [Google Scholar]
- 63.Tishinsky J.M., de Boer A.A., Dyck D.J., Robinson L.E. Modulation of visceral fat adipokine secretion by dietary fatty acids and ensuing changes in skeletal muscle inflammation. Appl. Physiol. Nutr. Metab. 2014;39:28–37. doi: 10.1139/apnm-2013-0135. [DOI] [PubMed] [Google Scholar]
- 64.Gonzalez-Periz A., Horrillo R., Ferre N., Gronert K., Dong B., Moran-Salvador E., Titos E., Martinez-Clemente M., Lopez-Parra M., Arroyo V., et al. Obesity-induced insulin resistance and hepatic steatosis are alleviated by omega-3 fatty acids: A role for resolvins and protectins. FASEB J. 2009;23:1946–1957. doi: 10.1096/fj.08-125674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Oster R.T., Tishinsky J.M., Yuan Z., Robinson L.E. Docosahexaenoic acid increases cellular adiponectin mRNA and secreted adiponectin protein, as well as ppargamma mrna, in 3t3-l1 adipocytes. Appl. Physiol. Nutr. Metab. 2010;35:783–789. doi: 10.1139/H10-076. [DOI] [PubMed] [Google Scholar]
- 66.Tishinsky J.M., Ma D.W., Robinson L.E. Eicosapentaenoic acid and rosiglitazone increase adiponectin in an additive and ppargamma-dependent manner in human adipocytes. Obesity. 2011;19:262–268. doi: 10.1038/oby.2010.186. [DOI] [PubMed] [Google Scholar]
- 67.Glass C.K., Saijo K. Nuclear receptor transrepression pathways that regulate inflammation in macrophages and t cells. Nat. Rev. Immunol. 2010;10:365–376. doi: 10.1038/nri2748. [DOI] [PubMed] [Google Scholar]
- 68.Szanto A., Nagy L. The many faces of ppargamma: Anti-inflammatory by any means? Immunobiology. 2008;213:789–803. doi: 10.1016/j.imbio.2008.07.015. [DOI] [PubMed] [Google Scholar]
- 69.Oh D.Y., Talukdar S., Bae E.J., Imamura T., Morinaga H., Fan W., Li P., Lu W.J., Watkins S.M., Olefsky J.M. Gpr120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell. 2010;142:687–698. doi: 10.1016/j.cell.2010.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Stulnig T.M., Huber J., Leitinger N., Imre E.M., Angelisova P., Nowotny P., Waldhausl W. Polyunsaturated eicosapentaenoic acid displaces proteins from membrane rafts by altering raft lipid composition. J. Biol. Chem. 2001;276:37335–37340. doi: 10.1074/jbc.M106193200. [DOI] [PubMed] [Google Scholar]
- 71.Calder P.C. Feeding the immune system. Proc. Nutr. Soc. 2013;72:299–309. doi: 10.1017/S0029665113001286. [DOI] [PubMed] [Google Scholar]
- 72.Calder P.C. n-3 fatty acids, inflammation and immunity: New mechanisms to explain old actions. Proc. Nutr. Soc. 2013;72:326–336. doi: 10.1017/S0029665113001031. [DOI] [PubMed] [Google Scholar]
- 73.Liu J., Ma D.W.L. The role of n-3 polyunsaturated fatty acids in the prevention and treatment of breast cancer. Nutrients. 2014 doi: 10.3390/nu6115184. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Conquer J.A., Holub B.J. Effect of supplementation with different doses of dha on the levels of circulating dha as non-esterified fatty acid in subjects of asian indian background. J. Lipid Res. 1998;39:286–292. [PubMed] [Google Scholar]
- 75.Kris-Etherton P.M., Taylor D.S., Yu-Poth S., Huth P., Moriarty K., Fishell V., Hargrove R.L., Zhao G., Etherton T.D. Polyunsaturated fatty acids in the food chain in the United States. Am. J. Clin. Nutr. 2000;71:179S–188S. doi: 10.1093/ajcn/71.1.179S. [DOI] [PubMed] [Google Scholar]
- 76.Damsgaard C.T., Frokiaer H., Lauritzen L. The effects of fish oil and high or low linoleic acid intake on fatty acid composition of human peripheral blood mononuclear cells. Br. J. Nutr. 2008;99:147–154. doi: 10.1017/S0007114507791900. [DOI] [PubMed] [Google Scholar]
- 77.Feskens E.J., Kromhout D. Epidemiologic studies on eskimos and fish intake. Ann. N. Y. Acad. Sci. 1993;683:9–15. doi: 10.1111/j.1749-6632.1993.tb35688.x. [DOI] [PubMed] [Google Scholar]
- 78.Yee L.D., Agarwal D., Rosol T.J., Lehman A., Tian M., Hatton J., Heestand J., Belury M.A., Clinton S.K. The inhibition of early stages of her-2/neu-mediated mammary carcinogenesis by dietary n-3 pufas. Mol. Nutr. Food Res. 2013;57:320–327. doi: 10.1002/mnfr.201200445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chen Z., Zhang Y., Jia C., Wang Y., Lai P., Zhou X., Wang Y., Song Q., Lin J., Ren Z., et al. Mtorc1/2 targeted by n-3 polyunsaturated fatty acids in the prevention of mammary tumorigenesis and tumor progression. Oncogene. 2014;33:4548–4557. doi: 10.1038/onc.2013.402. [DOI] [PubMed] [Google Scholar]
- 80.Chung H., Lee Y.S., Mayoral R., Oh D.Y., Siu J.T., Webster N.J., Sears D.D., Olefsky J.M., Ellies L.G. Omega-3 fatty acids reduce obesity-induced tumor progression independent of gpr120 in a mouse model of postmenopausal breast cancer. Oncogene. 2014 doi: 10.1038/onc.2014.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bouwens M., van de Rest O., Dellschaft N., Bromhaar M.G., de Groot L.C., Geleijnse J.M., Muller M., Afman L.A. Fish-oil supplementation induces antiinflammatory gene expression profiles in human blood mononuclear cells. Am. J. Clin. Nutr. 2009;90:415–424. doi: 10.3945/ajcn.2009.27680. [DOI] [PubMed] [Google Scholar]
- 82.Zheng J.S., Hu X.J., Zhao Y.M., Yang J., Li D. Intake of fish and marine n-3 polyunsaturated fatty acids and risk of breast cancer: Meta-analysis of data from 21 independent prospective cohort studies. BMJ. 2013;346:f3706. doi: 10.1136/bmj.f3706. [DOI] [PubMed] [Google Scholar]
- 83.Chajes V., Torres-Mejia G., Biessy C., Ortega-Olvera C., Angeles-Llerenas A., Ferrari P., Lazcano-Ponce E., Romieu I. Omega-3 and omega-6 polyunsaturated fatty acid intakes and the risk of breast cancer in mexican women: Impact of obesity status. Cancer Epidemiol. Biomark. Prev. 2012;21:319–326. doi: 10.1158/1055-9965.EPI-11-0896. [DOI] [PubMed] [Google Scholar]
- 84.Goodstine S.L., Zheng T., Holford T.R., Ward B.A., Carter D., Owens P.H., Mayne S.T. Dietary (n-3)/(n-6) fatty acid ratio: Possible relationship to premenopausal but not postmenopausal breast cancer risk in U.S. Women. J. Nutr. 2003;133:1409–1414. doi: 10.1093/jn/133.5.1409. [DOI] [PubMed] [Google Scholar]
- 85.Maillard V., Bougnoux P., Ferrari P., Jourdan M.L., Pinault M., Lavillonniere F., Body G., le Floch O., Chajes V. n-3 and n-6 fatty acids in breast adipose tissue and relative risk of breast cancer in a case-control study in tours, France. Int. J. Cancer. 2002;98:78–83. doi: 10.1002/ijc.10130. [DOI] [PubMed] [Google Scholar]
- 86.Bagga D., Anders K.H., Wang H.J., Glaspy J.A. Long-chain n-3-to-n-6 polyunsaturated fatty acid ratios in breast adipose tissue from women with and without breast cancer. Nutr. Cancer. 2002;42:180–185. doi: 10.1207/S15327914NC422_5. [DOI] [PubMed] [Google Scholar]
- 87.Klein V., Chajes V., Germain E., Schulgen G., Pinault M., Malvy D., Lefrancq T., Fignon A., le Floch O., Lhuillery C., et al. Low alpha-linolenic acid content of adipose breast tissue is associated with an increased risk of breast cancer. Eur. J. Cancer. 2000;36:335–340. doi: 10.1016/S0959-8049(99)00254-3. [DOI] [PubMed] [Google Scholar]
- 88.Kim J., Lim S.Y., Shin A., Sung M.K., Ro J., Kang H.S., Lee K.S., Kim S.W., Lee E.S. Fatty fish and fish omega-3 fatty acid intakes decrease the breast cancer risk: A case-control study. BMC Cancer. 2009;9:216. doi: 10.1186/1471-2407-9-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Witt P.M., Christensen J.H., Schmidt E.B., Dethlefsen C., Tjonneland A., Overvad K., Ewertz M. Marine n-3 polyunsaturated fatty acids in adipose tissue and breast cancer risk: A case-cohort study from Denmark. Cancer Causes Control. 2009;20:1715–1721. doi: 10.1007/s10552-009-9423-y. [DOI] [PubMed] [Google Scholar]
- 90.Pike L.J. Lipid rafts: Heterogeneity on the high seas. Biochem. J. 2004;378:281–292. doi: 10.1042/BJ20031672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Staubach S., Hanisch F.G. Lipid rafts: Signaling and sorting platforms of cells and their roles in cancer. Expert Rev. Proteomics. 2011;8:263–277. doi: 10.1586/epr.11.2. [DOI] [PubMed] [Google Scholar]
- 92.Hofman E.G., Ruonala M.O., Bader A.N., van den Heuvel D., Voortman J., Roovers R.C., Verkleij A.J., Gerritsen H.C., van Bergen En Henegouwen P.M. Egf induces coalescence of different lipid rafts. J. Cell Sci. 2008;121:2519–2528. doi: 10.1242/jcs.028753. [DOI] [PubMed] [Google Scholar]
- 93.Lingwood D., Simons K. Lipid rafts as a membrane-organizing principle. Science. 2010;327:46–50. doi: 10.1126/science.1174621. [DOI] [PubMed] [Google Scholar]
- 94.Patra S.K. Dissecting lipid raft facilitated cell signaling pathways in cancer. Biochim. Biophys. Acta. 2008;1785:182–206. doi: 10.1016/j.bbcan.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 95.Pike L.J., Han X., Chung K.N., Gross R.W. Lipid rafts are enriched in arachidonic acid and plasmenylethanolamine and their composition is independent of caveolin-1 expression: A quantitative electrospray ionization/mass spectrometric analysis. Biochemistry. 2002;41:2075–2088. doi: 10.1021/bi0156557. [DOI] [PubMed] [Google Scholar]
- 96.Simons K., Sampaio J.L. Membrane organization and lipid rafts. Cold Spring Harb. Perspect. Biol. 2011;3:a004697. doi: 10.1101/cshperspect.a004697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Brzustowicz M.R., Cherezov V., Caffrey M., Stillwell W., Wassall S.R. Molecular organization of cholesterol in polyunsaturated membranes: Microdomain formation. Biophys. J. 2002;82:285–298. doi: 10.1016/S0006-3495(02)75394-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wassall S.R., Brzustowicz M.R., Shaikh S.R., Cherezov V., Caffrey M., Stillwell W. Order from disorder, corralling cholesterol with chaotic lipids. The role of polyunsaturated lipids in membrane raft formation. Chem. Phys. Lipids. 2004;132:79–88. doi: 10.1016/j.chemphyslip.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 99.Nicolau D.V., Jr., Burrage K., Parton R.G., Hancock J.F. Identifying optimal lipid raft characteristics required to promote nanoscale protein-protein interactions on the plasma membrane. Mol. Cell. Biol. 2006;26:313–323. doi: 10.1128/MCB.26.1.313-323.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Corsetto P.A., Cremona A., Montorfano G., Jovenitti I.E., Orsini F., Arosio P., Rizzo A.M. Chemical-physical changes in cell membrane microdomains of breast cancer cells after omega-3 pufa incorporation. Cell Biochem. Biophys. 2012;64:45–59. doi: 10.1007/s12013-012-9365-y. [DOI] [PubMed] [Google Scholar]
- 101.Li H., Sugimura K., Kaji Y., Kitamura Y., Fujii M., Hara I., Tachibana M. Conventional MRI capabilities in the diagnosis of prostate cancer in the transition zone. AJR Am. J. Roentgenol. 2006;186:729–742. doi: 10.2214/AJR.04.0775. [DOI] [PubMed] [Google Scholar]
- 102.Lee E.J., Yun U.J., Koo K.H., Sung J.Y., Shim J., Ye S.K., Hong K.M., Kim Y.N. Down-regulation of lipid raft-associated onco-proteins via cholesterol-dependent lipid raft internalization in docosahexaenoic acid-induced apoptosis. Biochim. Biophys. Acta. 2014;1841:190–203. doi: 10.1016/j.bbalip.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 103.Schley P.D., Brindley D.N., Field C.J. (n-3) pufa alter raft lipid composition and decrease epidermal growth factor receptor levels in lipid rafts of human breast cancer cells. J. Nutr. 2007;137:548–553. doi: 10.1093/jn/137.3.548. [DOI] [PubMed] [Google Scholar]
- 104.Ravacci G.R., Brentani M.M., Tortelli T., Jr., Torrinhas R.S., Saldanha T., Torres E.A., Waitzberg D.L. Lipid raft disruption by docosahexaenoic acid induces apoptosis in transformed human mammary luminal epithelial cells harboring her-2 overexpression. J. Nutr. Biochem. 2013;24:505–515. doi: 10.1016/j.jnutbio.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 105.Altenburg J.D., Siddiqui R.A. Omega-3 polyunsaturated fatty acids down-modulate cxcr4 expression and function in mda-mb-231 breast cancer cells. Mol. Cancer Res. 2009;7:1013–1020. doi: 10.1158/1541-7786.MCR-08-0385. [DOI] [PubMed] [Google Scholar]
- 106.Fulda S., Strauss G., Meyer E., Debatin K.M. Functional cd95 ligand and cd95 death-inducing signaling complex in activation-induced cell death and doxorubicin-induced apoptosis in leukemic T cells. Blood. 2000;95:301–308. [PubMed] [Google Scholar]
- 107.Ewaschuk J.B., Newell M., Field C.J. Docosahexanoic acid improves chemotherapy efficacy by inducing cd95 translocation to lipid rafts in er(-) breast cancer cells. Lipids. 2012;47:1019–1030. doi: 10.1007/s11745-012-3717-7. [DOI] [PubMed] [Google Scholar]
- 108.Bollinger C.R., Teichgraber V., Gulbins E. Ceramide-enriched membrane domains. Biochim. Biophys. Acta. 2005;1746:284–294. doi: 10.1016/j.bbamcr.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 109.Kim H.S., Lee Y.S., Kim D.K. Doxorubicin exerts cytotoxic effects through cell cycle arrest and fas-mediated cell death. Pharmacology. 2009;84:300–309. doi: 10.1159/000245937. [DOI] [PubMed] [Google Scholar]
- 110.Lorincz A.M., Sukumar S. Molecular links between obesity and breast cancer. Endocr. Relat. Cancer. 2006;13:279–292. doi: 10.1677/erc.1.00729. [DOI] [PubMed] [Google Scholar]
- 111.Korner A., Pazaitou-Panayiotou K., Kelesidis T., Kelesidis I., Williams C.J., Kaprara A., Bullen J., Neuwirth A., Tseleni S., Mitsiades N., et al. Total and high-molecular-weight adiponectin in breast cancer: In vitro and in vivo studies. J. Clin. Endocrinol. Metab. 2007;92:1041–1048. doi: 10.1210/jc.2006-1858. [DOI] [PubMed] [Google Scholar]
- 112.Iyengar P., Combs T.P., Shah S.J., Gouon-Evans V., Pollard J.W., Albanese C., Flanagan L., Tenniswood M.P., Guha C., Lisanti M.P., et al. Adipocyte-secreted factors synergistically promote mammary tumorigenesis through induction of anti-apoptotic transcriptional programs and proto-oncogene stabilization. Oncogene. 2003;22:6408–6423. doi: 10.1038/sj.onc.1206737. [DOI] [PubMed] [Google Scholar]
- 113.Grivennikov S.I., Karin M. Inflammatory cytokines in cancer: Tumour necrosis factor and interleukin 6 take the stage. Ann. Rheum. Dis. 2011;70(Suppl. 1):i104–i108. doi: 10.1136/ard.2010.140145. [DOI] [PubMed] [Google Scholar]
- 114.Horng T., Hotamisligil G.S. Linking the inflammasome to obesity-related disease. Nat. Med. 2011;17:164–165. doi: 10.1038/nm0211-164. [DOI] [PubMed] [Google Scholar]
- 115.Hotamisligil G.S. Inflammation and metabolic disorders. Nature. 2006;444:860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 116.Dalamaga M., Diakopoulos K.N., Mantzoros C.S. The role of adiponectin in cancer: A review of current evidence. Endocr. Rev. 2012;33:547–594. doi: 10.1210/er.2011-1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Fain J.N. Release of inflammatory mediators by human adipose tissue is enhanced in obesity and primarily by the nonfat cells: A review. Mediat. Inflamm. 2010;2010:513948. doi: 10.1155/2010/513948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Fain J.N., Madan A.K., Hiler M.L., Cheema P., Bahouth S.W. Comparison of the release of adipokines by adipose tissue, adipose tissue matrix, and adipocytes from visceral and subcutaneous abdominal adipose tissues of obese humans. Endocrinology. 2004;145:2273–2282. doi: 10.1210/en.2003-1336. [DOI] [PubMed] [Google Scholar]
- 119.Cinti S., Mitchell G., Barbatelli G., Murano I., Ceresi E., Faloia E., Wang S., Fortier M., Greenberg A.S., Obin M.S. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J. Lipid Res. 2005;46:2347–2355. doi: 10.1194/jlr.M500294-JLR200. [DOI] [PubMed] [Google Scholar]
- 120.Subbaramaiah K., Howe L.R., Bhardwaj P., Du B., Gravaghi C., Yantiss R.K., Zhou X.K., Blaho V.A., Hla T., Yang P., et al. Obesity is associated with inflammation and elevated aromatase expression in the mouse mammary gland. Cancer Prev. Res. 2011;4:329–346. doi: 10.1158/1940-6207.CAPR-10-0381. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 121.Morris P.G., Hudis C.A., Giri D., Morrow M., Falcone D.J., Zhou X.K., Du B., Brogi E., Crawford C.B., Kopelovich L., et al. Inflammation and increased aromatase expression occur in the breast tissue of obese women with breast cancer. Cancer Prev. Res. 2011;4:1021–1029. doi: 10.1158/1940-6207.CAPR-11-0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Bhardwaj P., Du B., Zhou X.K., Sue E., Harbus M.D., Falcone D.J., Giri D., Hudis C.A., Kopelovich L., Subbaramaiah K., et al. Caloric restriction reverses obesity-induced mammary gland inflammation in mice. Cancer Prev. Res. 2013;6:282–289. doi: 10.1158/1940-6207.CAPR-12-0467. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 123.Ibrahim M.M. Subcutaneous and visceral adipose tissue: Structural and functional differences. Obes. Rev. 2010;11:11–18. doi: 10.1111/j.1467-789X.2009.00623.x. [DOI] [PubMed] [Google Scholar]
- 124.Dalamaga M. Obesity, insulin resistance, adipocytokines and breast cancer: New biomarkers and attractive therapeutic targets. World J. Exp. Med. 2013;3:34–42. doi: 10.5493/wjem.v3.i3.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ferguson R.D., Gallagher E.J., Scheinman E.J., Damouni R., LeRoith D. The epidemiology and molecular mechanisms linking obesity, diabetes, and cancer. Vitam. Horm. 2013;93:51–98. doi: 10.1016/B978-0-12-416673-8.00010-1. [DOI] [PubMed] [Google Scholar]
- 126.Rose D.P., Vona-Davis L. Biochemical and molecular mechanisms for the association between obesity, chronic inflammation, and breast cancer. BioFactors. 2014;40:1–12. doi: 10.1002/biof.1109. [DOI] [PubMed] [Google Scholar]
- 127.Baglietto L., English D.R., Hopper J.L., MacInnis R.J., Morris H.A., Tilley W.D., Krishnan K., Giles G.G. Circulating steroid hormone concentrations in postmenopausal women in relation to body size and composition. Breast Cancer Res. Treat. 2009;115:171–179. doi: 10.1007/s10549-008-0069-3. [DOI] [PubMed] [Google Scholar]
- 128.Yager J.D., Davidson N.E. Estrogen carcinogenesis in breast cancer. N. Engl. J. Med. 2006;354:270–282. doi: 10.1056/NEJMra050776. [DOI] [PubMed] [Google Scholar]
- 129.Key T., Appleby P., Barnes I., Reeves G., Endogenous H., Breast Cancer Collaborative G. Endogenous sex hormones and breast cancer in postmenopausal women: Reanalysis of nine prospective studies. J. Natl. Cancer Inst. 2002;94:606–616. doi: 10.1093/jnci/94.8.606. [DOI] [PubMed] [Google Scholar]
- 130.Haffner S.M., Katz M.S., Dunn J.F. Increased upper body and overall adiposity is associated with decreased sex hormone binding globulin in postmenopausal women. Int. J. Obes. 1991;15:471–478. [PubMed] [Google Scholar]
- 131.Vona-Davis L., Rose D.P. The obesity-inflammation-eicosanoid axis in breast cancer. J. Mammary Gland Biol. Neoplasia. 2013;18:291–307. doi: 10.1007/s10911-013-9299-z. [DOI] [PubMed] [Google Scholar]
- 132.Suzuki T., Miki Y., Ohuchi N., Sasano H. Intratumoral estrogen production in breast carcinoma: Significance of aromatase. Breast Cancer. 2008;15:270–277. doi: 10.1007/s12282-008-0062-z. [DOI] [PubMed] [Google Scholar]
- 133.Irahara N., Miyoshi Y., Taguchi T., Tamaki Y., Noguchi S. Quantitative analysis of aromatase mRNA expression derived from various promoters (i.4, i.3, pii and i.7) and its association with expression of TNF-alpha, il-6 and cox-2 mRNAs in human breast cancer. Int. J. Cancer. 2006;118:1915–1921. doi: 10.1002/ijc.21562. [DOI] [PubMed] [Google Scholar]
- 134.Brodie A., Lu Q., Nakamura J. Aromatase in the normal breast and breast cancer. J. Steroid Biochem. Mol. Biol. 1997;61:281–286. doi: 10.1016/S0960-0760(97)80024-6. [DOI] [PubMed] [Google Scholar]
- 135.Macaulay V.M., Nicholls J.E., Gledhill J., Rowlands M.G., Dowsett M., Ashworth A. Biological effects of stable overexpression of aromatase in human hormone-dependent breast cancer cells. Br. J. Cancer. 1994;69:77–83. doi: 10.1038/bjc.1994.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Simpson E.R., Mahendroo M.S., Nichols J.E., Bulun S.E. Aromatase gene expression in adipose tissue: Relationship to breast cancer. Int. J. Fertil. Menopausal Stud. 1994;39(Suppl. 2):S75–S83. [PubMed] [Google Scholar]
- 137.Pasqualini J.R., Chetrite G.S. Recent insight on the control of enzymes involved in estrogen formation and transformation in human breast cancer. J. Steroid Biochem. Mol. Biol. 2005;93:221–236. doi: 10.1016/j.jsbmb.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 138.Simpson E.R., Ackerman G.E., Smith M.E., Mendelson C.R. Estrogen formation in stromal cells of adipose tissue of women: Induction by glucocorticosteroids. Proc. Natl. Acad. Sci. USA. 1981;78:5690–5694. doi: 10.1073/pnas.78.9.5690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.O’Neill J.S., Elton R.A., Miller W.R. Aromatase activity in adipose tissue from breast quadrants: A link with tumour site. Br. Med. J. 1988;296:741–743. doi: 10.1136/bmj.296.6624.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Bulun S.E., Price T.M., Aitken J., Mahendroo M.S., Simpson E.R. A link between breast cancer and local estrogen biosynthesis suggested by quantification of breast adipose tissue aromatase cytochrome p450 transcripts using competitive polymerase chain reaction after reverse transcription. J. Clin. Endocrinol. Metab. 1993;77:1622–1628. doi: 10.1210/jcem.77.6.8117355. [DOI] [PubMed] [Google Scholar]
- 141.Bulun S.E., Chen D., Moy I., Brooks D.C., Zhao H. Aromatase, breast cancer and obesity: A complex interaction. Trends Endocrinol. Metab. 2012;23:83–89. doi: 10.1016/j.tem.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Bulun S.E., Simpson E.R. Regulation of aromatase expression in human tissues. Breast Cancer Res. Treat. 1994;30:19–29. doi: 10.1007/BF00682738. [DOI] [PubMed] [Google Scholar]
- 143.Simpson E.R., Brown K.A. Obesity and breast cancer: Role of inflammation and aromatase. J. Mol. Endocrinol. 2013;51:T51–T59. doi: 10.1530/JME-13-0217. [DOI] [PubMed] [Google Scholar]
- 144.Subbaramaiah K., Morris P.G., Zhou X.K., Morrow M., Du B., Giri D., Kopelovich L., Hudis C.A., Dannenberg A.J. Increased levels of cox-2 and prostaglandin e2 contribute to elevated aromatase expression in inflamed breast tissue of obese women. Cancer Discov. 2012;2:356–365. doi: 10.1158/2159-8290.CD-11-0241. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 145.Pender-Cudlip M.C., Krag K.J., Martini D., Yu J., Guidi A., Skinner S.S., Zhang Y., Qu X., He C., Xu Y., et al. Delta-6-desaturase activity and arachidonic acid synthesis are increased in human breast cancer tissue. Cancer Sci. 2013;104:760–764. doi: 10.1111/cas.12129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Chen D., Reierstad S., Lin Z., Lu M., Brooks C., Li N., Innes J., Bulun S.E. Prostaglandin e(2) induces breast cancer related aromatase promoters via activation of p38 and c-jun nh(2)-terminal kinase in adipose fibroblasts. Cancer Res. 2007;67:8914–8922. doi: 10.1158/0008-5472.CAN-06-4751. [DOI] [PubMed] [Google Scholar]
- 147.Zhao Y., Agarwal V.R., Mendelson C.R., Simpson E.R. Estrogen biosynthesis proximal to a breast tumor is stimulated by pge2 via cyclic amp, leading to activation of promoter II of the cyp19 (aromatase) gene. Endocrinology. 1996;137:5739–5742. doi: 10.1210/endo.137.12.8940410. [DOI] [PubMed] [Google Scholar]
- 148.Larsson S.C., Kumlin M., Ingelman-Sundberg M., Wolk A. Dietary long-chain n-3 fatty acids for the prevention of cancer: A review of potential mechanisms. Am. J. Clin. Nutr. 2004;79:935–945. doi: 10.1093/ajcn/79.6.935. [DOI] [PubMed] [Google Scholar]
- 149.Shackelford D.B., Shaw R.J. The lkb1-ampk pathway: Metabolism and growth control in tumour suppression. Nat. Rev. Cancer. 2009;9:563–575. doi: 10.1038/nrc2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Brown K.A., McInnes K.J., Hunger N.I., Oakhill J.S., Steinberg G.R., Simpson E.R. Subcellular localization of cyclic amp-responsive element binding protein-regulated transcription coactivator 2 provides a link between obesity and breast cancer in postmenopausal women. Cancer Res. 2009;69:5392–5399. doi: 10.1158/0008-5472.CAN-09-0108. [DOI] [PubMed] [Google Scholar]
- 151.Purohit A., Newman S.P., Reed M.J. The role of cytokines in regulating estrogen synthesis: Implications for the etiology of breast cancer. Breast Cancer Res. 2002;4:65–69. doi: 10.1186/bcr425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Zhao Y., Nichols J.E., Valdez R., Mendelson C.R., Simpson E.R. Tumor necrosis factor-alpha stimulates aromatase gene expression in human adipose stromal cells through use of an activating protein-1 binding site upstream of promoter 1.4. Mol. Endocrinol. 1996;10:1350–1357. doi: 10.1210/mend.10.11.8923461. [DOI] [PubMed] [Google Scholar]
- 153.To S.Q., Knower K.C., Clyne C.D. Nfkappab and mapk signalling pathways mediate tnfalpha-induced early growth response gene transcription leading to aromatase expression. Biochem. Biophys. Res. Commun. 2013;433:96–101. doi: 10.1016/j.bbrc.2013.02.058. [DOI] [PubMed] [Google Scholar]
- 154.Honma S., Shimodaira K., Shimizu Y., Tsuchiya N., Saito H., Yanaihara T., Okai T. The influence of inflammatory cytokines on estrogen production and cell proliferation in human breast cancer cells. Endocr. J. 2002;49:371–377. doi: 10.1507/endocrj.49.371. [DOI] [PubMed] [Google Scholar]
- 155.Panigrahy D., Kaipainen A., Greene E.R., Huang S. Cytochrome p450-derived eicosanoids: The neglected pathway in cancer. Cancer Metastasis Rev. 2010;29:723–735. doi: 10.1007/s10555-010-9264-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Arnold C., Konkel A., Fischer R., Schunck W.H. Cytochrome p450-dependent metabolism of omega-6 and omega-3 long-chain polyunsaturated fatty acids. Pharmacol. Rep. 2010;62:536–547. doi: 10.1016/S1734-1140(10)70311-X. [DOI] [PubMed] [Google Scholar]
- 157.Bannenberg G., Arita M., Serhan C.N. Endogenous receptor agonists: Resolving inflammation. Sci. World J. 2007;7:1440–1462. doi: 10.1100/tsw.2007.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Bannenberg G.L., Chiang N., Ariel A., Arita M., Tjonahen E., Gotlinger K.H., Hong S., Serhan C.N. Molecular circuits of resolution: Formation and actions of resolvins and protectins. J. Immunol. 2005;174:4345–4355. doi: 10.4049/jimmunol.174.7.4345. [DOI] [PubMed] [Google Scholar]
- 159.Rose D.P., Connolly J.M. Omega-3 fatty acids as cancer chemopreventive agents. Pharmacol. Ther. 1999;83:217–244. doi: 10.1016/S0163-7258(99)00026-1. [DOI] [PubMed] [Google Scholar]
- 160.Calder P.C. The role of marine omega-3 (n-3) fatty acids in inflammatory processes, atherosclerosis and plaque stability. Mol. Nutr. Food Res. 2012;56:1073–1080. doi: 10.1002/mnfr.201100710. [DOI] [PubMed] [Google Scholar]
- 161.Wei N., Wang B., Zhang Q.Y., Mi M.T., Zhu J.D., Yu X.P., Yuan J.L., Chen K., Wang J., Chang H. Effects of different dietary fatty acids on the fatty acid compositions and the expression of lipid metabolic-related genes in mammary tumor tissues of rats. Nutr. Cancer. 2008;60:810–825. doi: 10.1080/01635580802192858. [DOI] [PubMed] [Google Scholar]
- 162.Kaur B., Jorgensen A., Duttaroy A.K. Fatty acid uptake by breast cancer cells (mda-mb-231): Effects of insulin, leptin, adiponectin, and tnfalpha. Prostaglandins Leukot. Essent. Fatty Acids. 2009;80:93–99. doi: 10.1016/j.plefa.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 163.Liu X.H., Connolly J.M., Rose D.P. The 12-lipoxygenase gene-transfected mcf-7 human breast cancer cell line exhibits estrogen-independent, but estrogen and omega-6 fatty acid-stimulated proliferation in vitro, and enhanced growth in athymic nude mice. Cancer Lett. 1996;109:223–230. doi: 10.1016/S0304-3835(96)04462-X. [DOI] [PubMed] [Google Scholar]
- 164.Connolly J.M., Rose D.P. Enhanced angiogenesis and growth of 12-lipoxygenase gene-transfected mcf-7 human breast cancer cells in athymic nude mice. Cancer Lett. 1998;132:107–112. doi: 10.1016/S0304-3835(98)00171-2. [DOI] [PubMed] [Google Scholar]
- 165.Avis I., Hong S.H., Martinez A., Moody T., Choi Y.H., Trepel J., Das R., Jett M., Mulshine J.L. Five-lipoxygenase inhibitors can mediate apoptosis in human breast cancer cell lines through complex eicosanoid interactions. FASEB J. 2001;15:2007–2009. doi: 10.1096/fj.00-0866fje. [DOI] [PubMed] [Google Scholar]
- 166.Tong W.G., Ding X.Z., Adrian T.E. The mechanisms of lipoxygenase inhibitor-induced apoptosis in human breast cancer cells. Biochem. Biophys. Res. Commun. 2002;296:942–948. doi: 10.1016/S0006-291X(02)02014-4. [DOI] [PubMed] [Google Scholar]
- 167.Rose D.P., Connolly J.M. Effects of fatty acids and inhibitors of eicosanoid synthesis on the growth of a human breast cancer cell line in culture. Cancer Res. 1990;50:7139–7144. [PubMed] [Google Scholar]
- 168.Navarro-Tito N., Soto-Guzman A., Castro-Sanchez L., Martinez-Orozco R., Salazar E.P. Oleic acid promotes migration on mda-mb-231 breast cancer cells through an arachidonic acid-dependent pathway. Int. J. Biochem. Cell Biol. 2010;42:306–317. doi: 10.1016/j.biocel.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 169.Hu N., Li Y., Zhao Y., Wang Q., You J.C., Zhang X.D., Ye L.H. A novel positive feedback loop involving fasn/p-erk1/2/5-lox/ltb4/fasn sustains high growth of breast cancer cells. Acta Pharmacol. Sin. 2011;32:921–929. doi: 10.1038/aps.2011.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Rose D.P., Connolly J.M., Rayburn J., Coleman M. Influence of diets containing eicosapentaenoic or docosahexaenoic acid on growth and metastasis of breast cancer cells in nude mice. J. Natl. Cancer Inst. 1995;87:587–592. doi: 10.1093/jnci/87.8.587. [DOI] [PubMed] [Google Scholar]
- 171.Erickson K.L., Hubbard N.E. Fatty acids and breast cancer: The role of stem cells. Prostaglandins Leukot. Essent. Fatty Acids. 2010;82:237–241. doi: 10.1016/j.plefa.2010.02.019. [DOI] [PubMed] [Google Scholar]
- 172.Sethi S., Sarkar F.H., Ahmed Q., Bandyopadhyay S., Nahleh Z.A., Semaan A., Sakr W., Munkarah A., Ali-Fehmi R. Molecular markers of epithelial-to-mesenchymal transition are associated with tumor aggressiveness in breast carcinoma. Transl. Oncol. 2011;4:222–226. doi: 10.1593/tlo.10244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Zhou Y., Yau C., Gray J.W., Chew K., Dairkee S.H., Moore D.H., Eppenberger U., Eppenberger-Castori S., Benz C.C. Enhanced NF kappa b and ap-1 transcriptional activity associated with antiestrogen resistant breast cancer. BMC Cancer. 2007;7:59. doi: 10.1186/1471-2407-7-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Naugler W.E., Karin M. NF-kappab and cancer-identifying targets and mechanisms. Curr. Opin. Genet. Dev. 2008;18:19–26. doi: 10.1016/j.gde.2008.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Calder P.C. Lipids for intravenous nutrition in hospitalised adult patients: A multiple choice of options. Proc. Nutr. Soc. 2013;72:263–276. doi: 10.1017/S0029665113001250. [DOI] [PubMed] [Google Scholar]
- 176.Kurtzman S.H., Anderson K.H., Wang Y., Miller L.J., Renna M., Stankus M., Lindquist R.R., Barrows G., Kreutzer D.L. Cytokines in human breast cancer: Il-1alpha and il-1beta expression. Oncol. Rep. 1999;6:65–70. doi: 10.3892/or.6.1.65. [DOI] [PubMed] [Google Scholar]
- 177.Pantschenko A.G., Pushkar I., Anderson K.H., Wang Y., Miller L.J., Kurtzman S.H., Barrows G., Kreutzer D.L. The interleukin-1 family of cytokines and receptors in human breast cancer: Implications for tumor progression. Int. J. Oncol. 2003;23:269–284. [PubMed] [Google Scholar]
- 178.Chavey C., Bibeau F., Gourgou-Bourgade S., Burlinchon S., Boissiere F., Laune D., Roques S., Lazennec G. Oestrogen receptor negative breast cancers exhibit high cytokine content. Breast Cancer Res. 2007;9:R15. doi: 10.1186/bcr1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Candido J., Hagemann T. Cancer-related inflammation. J. Clin. Immunol. 2013;33(Suppl. 1):S79–S84. doi: 10.1007/s10875-012-9847-0. [DOI] [PubMed] [Google Scholar]
- 180.Kim S., Choi J.H., Kim J.B., Nam S.J., Yang J.H., Kim J.H., Lee J.E. Berberine suppresses TNF-alpha-induced mmp-9 and cell invasion through inhibition of ap-1 activity in mda-mb-231 human breast cancer cells. Molecules. 2008;13:2975–2985. doi: 10.3390/molecules13122975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Wu Y., Deng J., Rychahou P.G., Qiu S., Evers B.M., Zhou B.P. Stabilization of snail by NF-kappab is required for inflammation-induced cell migration and invasion. Cancer Cell. 2009;15:416–428. doi: 10.1016/j.ccr.2009.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Cho S.G., Li D., Stafford L.J., Luo J., Rodriguez-Villanueva M., Wang Y., Liu M. Kiss1 suppresses tnfalpha-induced breast cancer cell invasion via an inhibition of rhoa-mediated NF-kappab activation. J. Cell Biochem. 2009;107:1139–1149. doi: 10.1002/jcb.22216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Kushiro K., Nunez N.P. Ob/ob serum promotes a mesenchymal cell phenotype in b16bl6 melanoma cells. Clin. Exp. Metastasis. 2011;28:877–886. doi: 10.1007/s10585-011-9418-4. [DOI] [PubMed] [Google Scholar]
- 184.Soria G., Ben-Baruch A. The inflammatory chemokines ccl2 and ccl5 in breast cancer. Cancer Lett. 2008;267:271–285. doi: 10.1016/j.canlet.2008.03.018. [DOI] [PubMed] [Google Scholar]
- 185.Ueno T., Toi M., Saji H., Muta M., Bando H., Kuroi K., Koike M., Inadera H., Matsushima K. Significance of macrophage chemoattractant protein-1 in macrophage recruitment, angiogenesis, and survival in human breast cancer. Clin. Cancer Res. 2000;6:3282–3289. [PubMed] [Google Scholar]
- 186.Ye J., Gao Z., Yin J., He Q. Hypoxia is a potential risk factor for chronic inflammation and adiponectin reduction in adipose tissue of ob/ob and dietary obese mice. Am. J. Physiol. Endocrinol. Metab. 2007;293:E1118–E1128. doi: 10.1152/ajpendo.00435.2007. [DOI] [PubMed] [Google Scholar]
- 187.Cancello R., Henegar C., Viguerie N., Taleb S., Poitou C., Rouault C., Coupaye M., Pelloux V., Hugol D., Bouillot J.L., et al. Reduction of macrophage infiltration and chemoattractant gene expression changes in white adipose tissue of morbidly obese subjects after surgery-induced weight loss. Diabetes. 2005;54:2277–2286. doi: 10.2337/diabetes.54.8.2277. [DOI] [PubMed] [Google Scholar]
- 188.Itariu B.K., Zeyda M., Hochbrugger E.E., Neuhofer A., Prager G., Schindler K., Bohdjalian A., Mascher D., Vangala S., Schranz M., et al. Long-chain n-3 pufas reduce adipose tissue and systemic inflammation in severely obese nondiabetic patients: A randomized controlled trial. Am. J. Clin. Nutr. 2012;96:1137–1149. doi: 10.3945/ajcn.112.037432. [DOI] [PubMed] [Google Scholar]
- 189.Awada M., Meynier A., Soulage C.O., Hadji L., Geloen A., Viau M., Ribourg L., Benoit B., Debard C., Guichardant M., et al. n-3 pufa added to high-fat diets affect differently adiposity and inflammation when carried by phospholipids or triacylglycerols in mice. Nutr. Metab. 2013;10:23. doi: 10.1186/1743-7075-10-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Titos E., Rius B., Gonzalez-Periz A., Lopez-Vicario C., Moran-Salvador E., Martinez-Clemente M., Arroyo V., Claria J. Resolvin d1 and its precursor docosahexaenoic acid promote resolution of adipose tissue inflammation by eliciting macrophage polarization toward an m2-like phenotype. J. Immunol. 2011;187:5408–5418. doi: 10.4049/jimmunol.1100225. [DOI] [PubMed] [Google Scholar]
- 191.Oliver E., McGillicuddy F.C., Harford K.A., Reynolds C.M., Phillips C.M., Ferguson J.F., Roche H.M. Docosahexaenoic acid attenuates macrophage-induced inflammation and improves insulin sensitivity in adipocytes-specific differential effects between lc n-3 pufa. J. Nutr. Biochem. 2012;23:1192–1200. doi: 10.1016/j.jnutbio.2011.06.014. [DOI] [PubMed] [Google Scholar]
- 192.De Boer A.A., Monk J.M., Robinson L.E. Docosahexaenoic acid decreases pro-inflammatory mediators in an in vitro murine adipocyte macrophage co-culture model. PLoS One. 2014;9:e85037. doi: 10.1371/journal.pone.0085037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Novak T.E., Babcock T.A., Jho D.H., Helton W.S., Espat N.J. Nf-kappa b inhibition by omega-3 fatty acids modulates lps-stimulated macrophage tnf-alpha transcription. Am. J. Physiol. Lung Cell. Mol. Physiol. 2003;284:L84–L89. doi: 10.1152/ajplung.00077.2002. [DOI] [PubMed] [Google Scholar]
- 194.Weldon S.M., Mullen A.C., Loscher C.E., Hurley L.A., Roche H.M. Docosahexaenoic acid induces an anti-inflammatory profile in lipopolysaccharide-stimulated human thp-1 macrophages more effectively than eicosapentaenoic acid. J. Nutr. Biochem. 2007;18:250–258. doi: 10.1016/j.jnutbio.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 195.Moon H.S., Dalamaga M., Kim S.Y., Polyzos S.A., Hamnvik O.P., Magkos F., Paruthi J., Mantzoros C.S. Leptin’s role in lipodystrophic and nonlipodystrophic insulin-resistant and diabetic individuals. Endocr. Rev. 2013;34:377–412. doi: 10.1210/er.2012-1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Widjaja A., Stratton I.M., Horn R., Holman R.R., Turner R., Brabant G. Ukpds 20: Plasma leptin, obesity, and plasma insulin in type 2 diabetic subjects. J. Clin. Endocrinol. Metab. 1997;82:654–657. doi: 10.1210/jcem.82.2.3744. [DOI] [PubMed] [Google Scholar]
- 197.Jarde T., Caldefie-Chezet F., Damez M., Mishellany F., Penault-Llorca F., Guillot J., Vasson M.P. Leptin and leptin receptor involvement in cancer development: A study on human primary breast carcinoma. Oncol. Rep. 2008;19:905–911. [PubMed] [Google Scholar]
- 198.Revillion F., Charlier M., Lhotellier V., Hornez L., Giard S., Baranzelli M.C., Djiane J., Peyrat J.P. Messenger RNA expression of leptin and leptin receptors and their prognostic value in 322 human primary breast cancers. Clin. Cancer Res. 2006;12:2088–2094. doi: 10.1158/1078-0432.CCR-05-1904. [DOI] [PubMed] [Google Scholar]
- 199.Neville M.C., McFadden T.B., Forsyth I. Hormonal regulation of mammary differentiation and milk secretion. J. Mammary Gland Biol. Neoplasia. 2002;7:49–66. doi: 10.1023/A:1015770423167. [DOI] [PubMed] [Google Scholar]
- 200.Garofalo C., Surmacz E. Leptin and cancer. J. Cell. Physiol. 2006;207:12–22. doi: 10.1002/jcp.20472. [DOI] [PubMed] [Google Scholar]
- 201.Caldefie-Chezet F., Damez M., de Latour M., Konska G., Mishellani F., Fusillier C., Guerry M., Penault-Llorca F., Guillot J., Vasson M.P. Leptin: A proliferative factor for breast cancer? Study on human ductal carcinoma. Biochem. Biophys. Res. Commun. 2005;334:737–741. doi: 10.1016/j.bbrc.2005.06.077. [DOI] [PubMed] [Google Scholar]
- 202.Dieudonne M.N., Machinal-Quelin F., Serazin-Leroy V., Leneveu M.C., Pecquery R., Giudicelli Y. Leptin mediates a proliferative response in human mcf7 breast cancer cells. Biochem. Biophys. Res. Commun. 2002;293:622–628. doi: 10.1016/S0006-291X(02)00205-X. [DOI] [PubMed] [Google Scholar]
- 203.Garofalo C., Koda M., Cascio S., Sulkowska M., Kanczuga-Koda L., Golaszewska J., Russo A., Sulkowski S., Surmacz E. Increased expression of leptin and the leptin receptor as a marker of breast cancer progression: Possible role of obesity-related stimuli. Clin. Cancer Res. 2006;12:1447–1453. doi: 10.1158/1078-0432.CCR-05-1913. [DOI] [PubMed] [Google Scholar]
- 204.Hu X., Juneja S.C., Maihle N.J., Cleary M.P. Leptin—A growth factor in normal and malignant breast cells and for normal mammary gland development. J. Natl. Cancer Inst. 2002;94:1704–1711. doi: 10.1093/jnci/94.22.1704. [DOI] [PubMed] [Google Scholar]
- 205.Ishikawa M., Kitayama J., Nagawa H. Enhanced expression of leptin and leptin receptor (ob-r) in human breast cancer. Clin. Cancer Res. 2004;10:4325–4331. doi: 10.1158/1078-0432.CCR-03-0749. [DOI] [PubMed] [Google Scholar]
- 206.Laud K., Gourdou I., Pessemesse L., Peyrat J.P., Djiane J. Identification of leptin receptors in human breast cancer: Functional activity in the t47-d breast cancer cell line. Mol. Cell. Endocrinol. 2002;188:219–226. doi: 10.1016/S0303-7207(01)00678-5. [DOI] [PubMed] [Google Scholar]
- 207.Yin N., Wang D., Zhang H., Yi X., Sun X., Shi B., Wu H., Wu G., Wang X., Shang Y. Molecular mechanisms involved in the growth stimulation of breast cancer cells by leptin. Cancer Res. 2004;64:5870–5875. doi: 10.1158/0008-5472.CAN-04-0655. [DOI] [PubMed] [Google Scholar]
- 208.Nyante S.J., Gammon M.D., Kaufman J.S., Bensen J.T., Lin D.Y., Barnholtz-Sloan J.S., Hu Y., He Q., Luo J., Millikan R.C. Common genetic variation in adiponectin, leptin, and leptin receptor and association with breast cancer subtypes. Breast Cancer Res. Treat. 2011;129:593–606. doi: 10.1007/s10549-011-1517-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Miyoshi Y., Funahashi T., Tanaka S., Taguchi T., Tamaki Y., Shimomura I., Noguchi S. High expression of leptin receptor mRNA in breast cancer tissue predicts poor prognosis for patients with high, but not low, serum leptin levels. Int. J. Cancer. 2006;118:1414–1419. doi: 10.1002/ijc.21543. [DOI] [PubMed] [Google Scholar]
- 210.Catalano S., Mauro L., Marsico S., Giordano C., Rizza P., Rago V., Montanaro D., Maggiolini M., Panno M.L., Ando S. Leptin induces, via erk1/erk2 signal, functional activation of estrogen receptor alpha in mcf-7 cells. J. Biol. Chem. 2004;279:19908–19915. doi: 10.1074/jbc.M313191200. [DOI] [PubMed] [Google Scholar]
- 211.Grossmann M.E., Cleary M.P. The balance between leptin and adiponectin in the control of carcinogenesis—Focus on mammary tumorigenesis. Biochimie. 2012;94:2164–2171. doi: 10.1016/j.biochi.2012.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Jarde T., Perrier S., Vasson M.P., Caldefie-Chezet F. Molecular mechanisms of leptin and adiponectin in breast cancer. Eur. J. Cancer. 2011;47:33–43. doi: 10.1016/j.ejca.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 213.Ray A. Adipokine leptin in obesity-related pathology of breast cancer. J. Biosci. 2012;37:289–294. doi: 10.1007/s12038-012-9191-9. [DOI] [PubMed] [Google Scholar]
- 214.Okumura M., Yamamoto M., Sakuma H., Kojima T., Maruyama T., Jamali M., Cooper D.R., Yasuda K. Leptin and high glucose stimulate cell proliferation in mcf-7 human breast cancer cells: Reciprocal involvement of pkc-alpha and ppar expression. Biochim. Biophys. Acta. 2002;1592:107–116. doi: 10.1016/s0167-4889(02)00276-8. [DOI] [PubMed] [Google Scholar]
- 215.Chen C., Chang Y.C., Liu C.L., Chang K.J., Guo I.C. Leptin-induced growth of human zr-75-1 breast cancer cells is associated with up-regulation of cyclin d1 and c-myc and down-regulation of tumor suppressor p53 and p21waf1/cip1. Breast Cancer Res. Treat. 2006;98:121–132. doi: 10.1007/s10549-005-9139-y. [DOI] [PubMed] [Google Scholar]
- 216.Seilkop S.K. The effect of body weight on tumor incidence and carcinogenicity testing in b6c3f1 mice and f344 rats. Fundam. Appl. Toxicol. 1995;24:247–259. doi: 10.1006/faat.1995.1028. [DOI] [PubMed] [Google Scholar]
- 217.Klurfeld D.M., Lloyd L.M., Welch C.B., Davis M.J., Tulp O.L., Kritchevsky D. Reduction of enhanced mammary carcinogenesis in la/n-cp (corpulent) rats by energy restriction. Proc. Soc. Exp. Biol. Med. 1991;196:381–384. doi: 10.3181/00379727-196-43202. [DOI] [PubMed] [Google Scholar]
- 218.Cleary M.P., Juneja S.C., Phillips F.C., Hu X., Grande J.P., Maihle N.J. Leptin receptor-deficient mmtv-tgf-alpha/lepr(db)lepr(db) female mice do not develop oncogene-induced mammary tumors. Exp. Biol. Med. 2004;229:182–193. doi: 10.1177/153537020422900207. [DOI] [PubMed] [Google Scholar]
- 219.Cleary M.P., Phillips F.C., Getzin S.C., Jacobson T.L., Jacobson M.K., Christensen T.A., Juneja S.C., Grande J.P., Maihle N.J. Genetically obese mmtv-tgf-alpha/lep(ob)lep(ob) female mice do not develop mammary tumors. Breast Cancer Res. Treat. 2003;77:205–215. doi: 10.1023/A:1021891825399. [DOI] [PubMed] [Google Scholar]
- 220.Otvos L., Jr., Kovalszky I., Riolfi M., Ferla R., Olah J., Sztodola A., Nama K., Molino A., Piubello Q., Wade J.D., et al. Efficacy of a leptin receptor antagonist peptide in a mouse model of triple-negative breast cancer. Eur. J. Cancer. 2011;47:1578–1584. doi: 10.1016/j.ejca.2011.01.018. [DOI] [PubMed] [Google Scholar]
- 221.Gonzalez R.R., Cherfils S., Escobar M., Yoo J.H., Carino C., Styer A.K., Sullivan B.T., Sakamoto H., Olawaiye A., Serikawa T., et al. Leptin signaling promotes the growth of mammary tumors and increases the expression of vascular endothelial growth factor (vegf) and its receptor type two (vegf-r2) J. Biol. Chem. 2006;281:26320–26328. doi: 10.1074/jbc.M601991200. [DOI] [PubMed] [Google Scholar]
- 222.Catalano S., Marsico S., Giordano C., Mauro L., Rizza P., Panno M.L., Ando S. Leptin enhances, via ap-1, expression of aromatase in the mcf-7 cell line. J. Biol. Chem. 2003;278:28668–28676. doi: 10.1074/jbc.M301695200. [DOI] [PubMed] [Google Scholar]
- 223.Garofalo C., Sisci D., Surmacz E. Leptin interferes with the effects of the antiestrogen ici 182,780 in mcf-7 breast cancer cells. Clin. Cancer Res. 2004;10:6466–6475. doi: 10.1158/1078-0432.CCR-04-0203. [DOI] [PubMed] [Google Scholar]
- 224.Machinal-Quelin F., Dieudonne M.N., Pecquery R., Leneveu M.C., Giudicelli Y. Direct in vitro effects of androgens and estrogens on ob gene expression and leptin secretion in human adipose tissue. Endocrine. 2002;18:179–184. doi: 10.1385/ENDO:18:2:179. [DOI] [PubMed] [Google Scholar]
- 225.O’Neil J.S., Burow M.E., Green A.E., McLachlan J.A., Henson M.C. Effects of estrogen on leptin gene promoter activation in mcf-7 breast cancer and jeg-3 choriocarcinoma cells: Selective regulation via estrogen receptors alpha and beta. Mol. Cell. Endocrinol. 2001;176:67–75. doi: 10.1016/S0303-7207(01)00473-7. [DOI] [PubMed] [Google Scholar]
- 226.Liu X., Wu Y.M., Xu L., Tang C., Zhong Y.B. [Influence of hypoxia on leptin and leptin receptor gene expression of c57bl/6j mice] Zhonghua Jie He He Hu Xi Za Zhi. 2005;28:173–175. [PubMed] [Google Scholar]
- 227.Ambrosini G., Nath A.K., Sierra-Honigmann M.R., Flores-Riveros J. Transcriptional activation of the human leptin gene in response to hypoxia. Involvement of hypoxia-inducible factor 1. J. Biol. Chem. 2002;277:34601–34609. doi: 10.1074/jbc.M205172200. [DOI] [PubMed] [Google Scholar]
- 228.Grosfeld A., Andre J., Hauguel-De Mouzon S., Berra E., Pouyssegur J., Guerre-Millo M. Hypoxia-inducible factor 1 transactivates the human leptin gene promoter. J. Biol. Chem. 2002;277:42953–42957. doi: 10.1074/jbc.M206775200. [DOI] [PubMed] [Google Scholar]
- 229.Fan C., Liu X., Shen W., Deckelbaum R.J., Qi K. The regulation of leptin, leptin receptor and pro-opiomelanocortin expression by n-3 pufas in diet-induced obese mice is not related to the methylation of their promoters. Nutr. Metab. 2011;8:31. doi: 10.1186/1743-7075-8-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Zeidan A., Javadov S., Chakrabarti S., Karmazyn M. Leptin-induced cardiomyocyte hypertrophy involves selective caveolae and rhoa/rock-dependent p38 mapk translocation to nuclei. Cardiovasc. Res. 2008;77:64–72. doi: 10.1093/cvr/cvm020. [DOI] [PubMed] [Google Scholar]
- 231.Kadowaki T., Yamauchi T. Adiponectin and adiponectin receptors. Endocr. Rev. 2005;26:439–451. doi: 10.1210/er.2005-0005. [DOI] [PubMed] [Google Scholar]
- 232.Waki H., Yamauchi T., Kamon J., Kita S., Ito Y., Hada Y., Uchida S., Tsuchida A., Takekawa S., Kadowaki T. Generation of globular fragment of adiponectin by leukocyte elastase secreted by monocytic cell line thp-1. Endocrinology. 2005;146:790–796. doi: 10.1210/en.2004-1096. [DOI] [PubMed] [Google Scholar]
- 233.Yamauchi T., Kamon J., Ito Y., Tsuchida A., Yokomizo T., Kita S., Sugiyama T., Miyagishi M., Hara K., Tsunoda M., et al. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature. 2003;423:762–769. doi: 10.1038/nature01705. [DOI] [PubMed] [Google Scholar]
- 234.Ryan A.S., Berman D.M., Nicklas B.J., Sinha M., Gingerich R.L., Meneilly G.S., Egan J.M., Elahi D. Plasma adiponectin and leptin levels, body composition, and glucose utilization in adult women with wide ranges of age and obesity. Diabetes Care. 2003;26:2383–2388. doi: 10.2337/diacare.26.8.2383. [DOI] [PubMed] [Google Scholar]
- 235.Vona-Davis L., Rose D.P. Adipokines as endocrine, paracrine, and autocrine factors in breast cancer risk and progression. Endocr. Relat. Cancer. 2007;14:189–206. doi: 10.1677/ERC-06-0068. [DOI] [PubMed] [Google Scholar]
- 236.Kern P.A., di Gregorio G.B., Lu T., Rassouli N., Ranganathan G. Adiponectin expression from human adipose tissue: Relation to obesity, insulin resistance, and tumor necrosis factor-alpha expression. Diabetes. 2003;52:1779–1785. doi: 10.2337/diabetes.52.7.1779. [DOI] [PubMed] [Google Scholar]
- 237.Miyoshi Y., Funahashi T., Kihara S., Taguchi T., Tamaki Y., Matsuzawa Y., Noguchi S. Association of serum adiponectin levels with breast cancer risk. Clin. Cancer Res. 2003;9:5699–5704. [PubMed] [Google Scholar]
- 238.Mantzoros C., Petridou E., Dessypris N., Chavelas C., Dalamaga M., Alexe D.M., Papadiamantis Y., Markopoulos C., Spanos E., Chrousos G., et al. Adiponectin and breast cancer risk. J. Clin. Endocrinol. Metab. 2004;89:1102–1107. doi: 10.1210/jc.2003-031804. [DOI] [PubMed] [Google Scholar]
- 239.Macis D., Guerrieri-Gonzaga A., Gandini S. Circulating adiponectin and breast cancer risk: A systematic review and meta-analysis. Int. J. Epidemiol. 2014;43:1226–1236. doi: 10.1093/ije/dyu088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Ye J., Jia J., Dong S., Zhang C., Yu S., Li L., Mao C., Wang D., Chen J., Yuan G. Circulating adiponectin levels and the risk of breast cancer: A meta-analysis. Eur. J. Cancer Prev. 2014;23:158–165. doi: 10.1097/CEJ.0b013e328364f293. [DOI] [PubMed] [Google Scholar]
- 241.Liu L.Y., Wang M., Ma Z.B., Yu L.X., Zhang Q., Gao D.Z., Wang F., Yu Z.G. The role of adiponectin in breast cancer: A meta-analysis. PLoS One. 2013;8:e73183. doi: 10.1371/journal.pone.0073183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Grossmann M.E., Ray A., Nkhata K.J., Malakhov D.A., Rogozina O.P., Dogan S., Cleary M.P. Obesity and breast cancer: Status of leptin and adiponectin in pathological processes. Cancer Metastasis Rev. 2010;29:641–653. doi: 10.1007/s10555-010-9252-1. [DOI] [PubMed] [Google Scholar]
- 243.Takahata C., Miyoshi Y., Irahara N., Taguchi T., Tamaki Y., Noguchi S. Demonstration of adiponectin receptors 1 and 2 mRNA expression in human breast cancer cells. Cancer Lett. 2007;250:229–236. doi: 10.1016/j.canlet.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 244.Jarde T., Caldefie-Chezet F., Damez M., Mishellany F., Perrone D., Penault-Llorca F., Guillot J., Vasson M.P. Adiponectin and leptin expression in primary ductal breast cancer and in adjacent healthy epithelial and myoepithelial tissue. Histopathology. 2008;53:484–487. doi: 10.1111/j.1365-2559.2008.03121.x. [DOI] [PubMed] [Google Scholar]
- 245.Lam J.B., Chow K.H., Xu A., Lam K.S., Liu J., Wong N.S., Moon R.T., Shepherd P.R., Cooper G.J., Wang Y. Adiponectin haploinsufficiency promotes mammary tumor development in mmtv-pyvt mice by modulation of phosphatase and tensin homolog activities. PLoS One. 2009;4:e4968. doi: 10.1371/journal.pone.0004968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Wang Y., Lam J.B., Lam K.S., Liu J., Lam M.C., Hoo R.L., Wu D., Cooper G.J., Xu A. Adiponectin modulates the glycogen synthase kinase-3beta/beta-catenin signaling pathway and attenuates mammary tumorigenesis of mda-mb-231 cells in nude mice. Cancer Res. 2006;66:11462–11470. doi: 10.1158/0008-5472.CAN-06-1969. [DOI] [PubMed] [Google Scholar]
- 247.Nakayama S., Miyoshi Y., Ishihara H., Noguchi S. Growth-inhibitory effect of adiponectin via adiponectin receptor 1 on human breast cancer cells through inhibition of s-phase entry without inducing apoptosis. Breast Cancer Res. Treat. 2008;112:405–410. doi: 10.1007/s10549-007-9874-3. [DOI] [PubMed] [Google Scholar]
- 248.Dos Santos E., Benaitreau D., Dieudonne M.N., Leneveu M.C., Serazin V., Giudicelli Y., Pecquery R. Adiponectin mediates an antiproliferative response in human mda-mb 231 breast cancer cells. Oncol. Rep. 2008;20:971–977. [PubMed] [Google Scholar]
- 249.Pfeiler G., Hudelist G., Wulfing P., Mattsson B., Konigsberg R., Kubista E., Singer C.F. Impact of adipor1 expression on breast cancer development. Gynecol. Oncol. 2010;117:134–138. doi: 10.1016/j.ygyno.2009.12.018. [DOI] [PubMed] [Google Scholar]
- 250.Jarde T., Caldefie-Chezet F., Goncalves-Mendes N., Mishellany F., Buechler C., Penault-Llorca F., Vasson M.P. Involvement of adiponectin and leptin in breast cancer: Clinical and in vitro studies. Endocr. Relat. Cancer. 2009;16:1197–1210. doi: 10.1677/ERC-09-0043. [DOI] [PubMed] [Google Scholar]
- 251.Dieudonne M.N., Bussiere M., dos Santos E., Leneveu M.C., Giudicelli Y., Pecquery R. Adiponectin mediates antiproliferative and apoptotic responses in human mcf7 breast cancer cells. Biochem. Biophys. Res. Commun. 2006;345:271–279. doi: 10.1016/j.bbrc.2006.04.076. [DOI] [PubMed] [Google Scholar]
- 252.Taliaferro-Smith L., Nagalingam A., Zhong D., Zhou W., Saxena N.K., Sharma D. Lkb1 is required for adiponectin-mediated modulation of ampk-s6k axis and inhibition of migration and invasion of breast cancer cells. Oncogene. 2009;28:2621–2633. doi: 10.1038/onc.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Nkhata K.J., Ray A., Schuster T.F., Grossmann M.E., Cleary M.P. Effects of adiponectin and leptin co-treatment on human breast cancer cell growth. Oncol. Rep. 2009;21:1611–1619. doi: 10.3892/or_00000395. [DOI] [PubMed] [Google Scholar]
- 254.Villarreal-Molina M.T., Antuna-Puente B. Adiponectin: Anti-inflammatory and cardioprotective effects. Biochimie. 2012;94:2143–2149. doi: 10.1016/j.biochi.2012.06.030. [DOI] [PubMed] [Google Scholar]
- 255.Ouchi N., Walsh K. Adiponectin as an anti-inflammatory factor. Clin. Chim. Acta. 2007;380:24–30. doi: 10.1016/j.cca.2007.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Folco E.J., Rocha V.Z., Lopez-Ilasaca M., Libby P. Adiponectin inhibits pro-inflammatory signaling in human macrophages independent of interleukin-10. J. Biol. Chem. 2009;284:25569–25575. doi: 10.1074/jbc.M109.019786. [DOI] [PMC free article] [PubMed] [Google Scholar]