Abstract
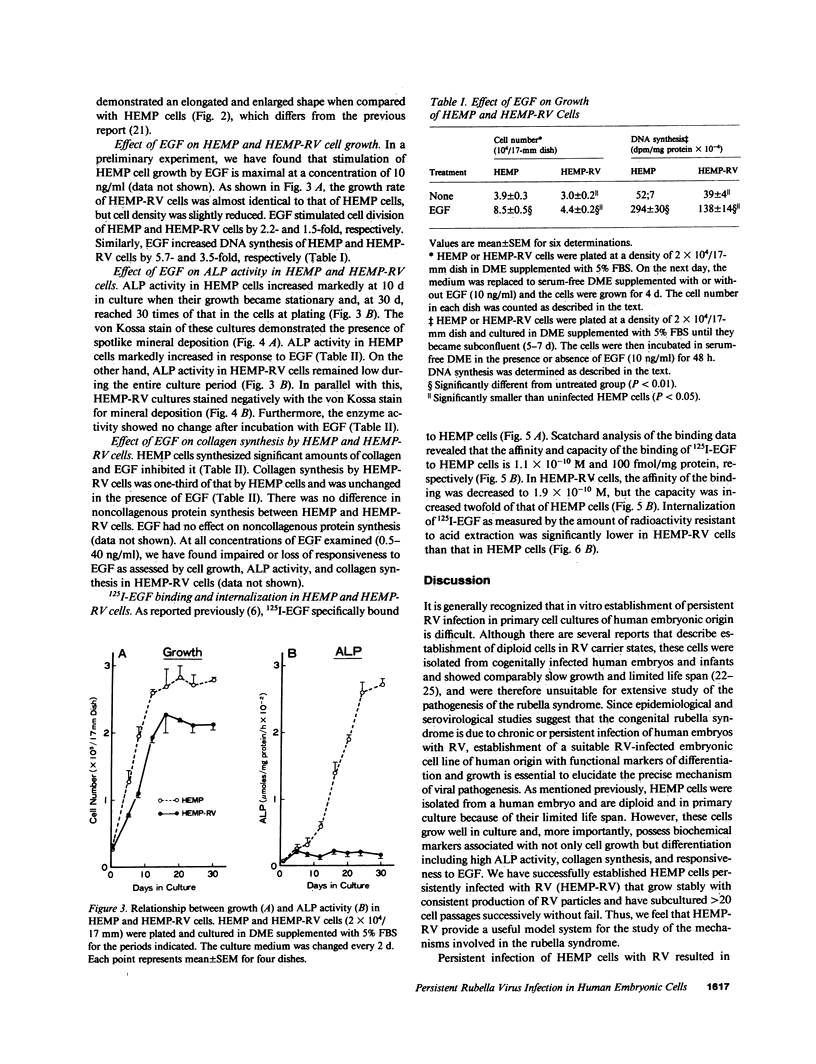
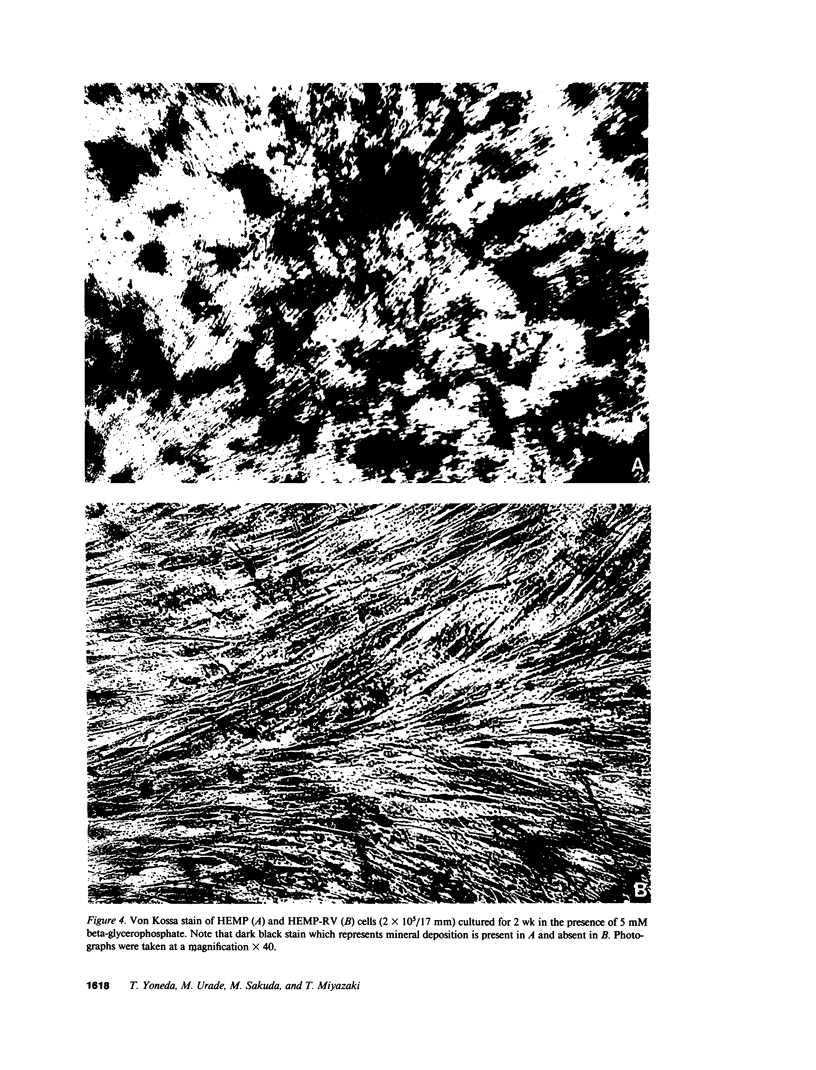
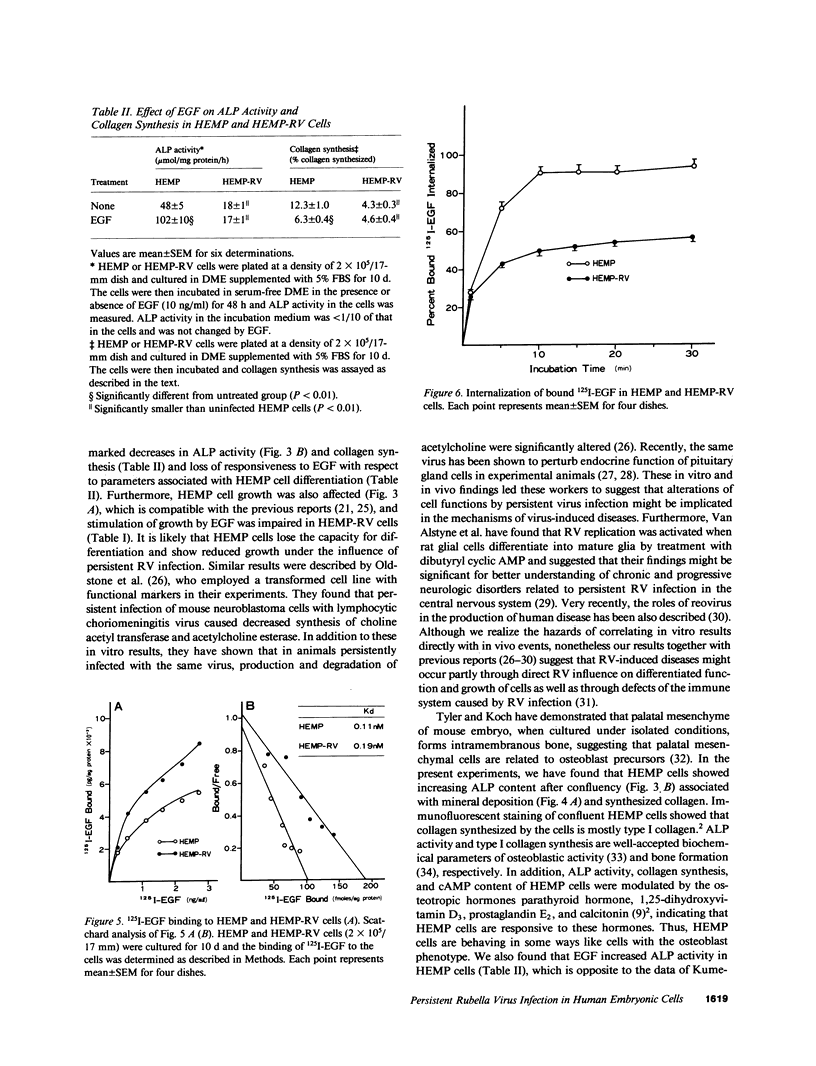
We previously demonstrated that human embryonic mesenchymal cells derived from the palate (HEMP cells) retain alkaline phosphatase (ALP) content and capacity for collagen synthesis after long-term culture, and their growth is markedly stimulated by epidermal growth factor (EGF). There was a dramatic decrease in ALP content and capacity to synthesize collagen in HEMP cells (HEMP-RV cells) persistently infected with rubella virus (RV). EGF increased ALP activity and decreased collagen synthesis in HEMP cells, whereas EGF showed no effect on these activities in HEMP-RV cells. Growth of HEMP-RV cells was slightly reduced compared with that of HEMP cells. EGF stimulated growth of HEMP cells and to a lesser extent of HEMP-RV cells. Binding of 125I-EGF to cell-surface receptors in HEMP-RV cells was, to our surprise, twice as much as that in HEMP cells. However, internalization of bound 125I-EGF in HEMP-RV cells was profoundly diminished. Thus, persistent RV infection causes not only changes in HEMP cell growth and differentiation but a decrease in or loss of HEMP cell responsiveness to EGF. The effects of persistent RV infection on palatal cell differentiation as well as growth may be responsible for the pathogenesis of congenital rubella. Furthermore, since HEMP cells appear to be closely related to osteoblasts, these results suggest a mechanism for RV-induced osseous abnormalities manifested in congenital rubella patients.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Becker Y. Antiviral drugs. Mode of action and chemotherapy of viral infections of man. Monogr Virol. 1976;11:1–130. [PubMed] [Google Scholar]
- Binderman I., Greene R. M., Pennypacker J. P. Calcification of differentiating skeletal mesenchyme in vitro. Science. 1979 Oct 12;206(4415):222–225. doi: 10.1126/science.482937. [DOI] [PubMed] [Google Scholar]
- Canalis E., Raisz L. G. Effect of epidermal growth factor on bone formation in vitro. Endocrinology. 1979 Apr;104(4):862–869. doi: 10.1210/endo-104-4-862. [DOI] [PubMed] [Google Scholar]
- Cotlier E., Fox J., Bohigian G., Beaty C., Du Pree A. Pathogenic effects of rubella virus on embryos and newborn rats. Nature. 1968 Jan 6;217(5123):38–40. doi: 10.1038/217038a0. [DOI] [PubMed] [Google Scholar]
- DULBECCO R., VOGT M. Plaque formation and isolation of pure lines with poliomyelitis viruses. J Exp Med. 1954 Feb;99(2):167–182. doi: 10.1084/jem.99.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hata R., Hori H., Nagai Y., Tanaka S., Kondo M., Hiramatsu M., Utsumi N., Kumegawa M. Selective inhibition of type I collagen synthesis in osteoblastic cells by epidermal growth factor. Endocrinology. 1984 Sep;115(3):867–876. doi: 10.1210/endo-115-3-867. [DOI] [PubMed] [Google Scholar]
- Heggie A. D. Growth inhibition of human embryonic and fetal rat bones in organ culture by rubella virus. Teratology. 1977 Feb;15(1):47–55. doi: 10.1002/tera.1420150107. [DOI] [PubMed] [Google Scholar]
- KAY H. E., PEPPERCORN M. E., PORTEFIELD J. S., MCCARTHY K., TAYLOR-ROBINSON C. H. CONGENITAL RUBELLA INFECTION OF A HUMAN EMBRYO. Br Med J. 1964 Jul 18;2(5402):166–167. doi: 10.1136/bmj.2.5402.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadowaki T., Kasuga M., Akanuma Y., Ezaki O., Takaku F. Decreased autophosphorylation of the insulin receptor-kinase in streptozotocin-diabetic rats. J Biol Chem. 1984 Nov 25;259(22):14208–14216. [PubMed] [Google Scholar]
- Kumegawa M., Hiramatsu M., Hatakeyama K., Yajima T., Kodama H., Osaki T., Kurisu K. Effects of epidermal growth factor on osteoblastic cells in vitro. Calcif Tissue Int. 1983 Jul;35(4-5):542–548. doi: 10.1007/BF02405091. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- London W. T., Fuccillo D. A., Anderson B., Sever J. L. Concentration of rubella virus antigen in chondrocytes of congenitally infected rabbits. Nature. 1970 Apr 11;226(5241):172–173. doi: 10.1038/226172a0. [DOI] [PubMed] [Google Scholar]
- Majeska R. J., Rodan G. A. Alkaline phosphatase inhibition by parathyroid hormone and isoproterenol in a clonal rat osteosarcoma cell line. Possible mediation by cyclic AMP. Calcif Tissue Int. 1982 Jan;34(1):59–66. doi: 10.1007/BF02411210. [DOI] [PubMed] [Google Scholar]
- Oldstone M. B., Holmstoen J., Welsh R. M., Jr Alterations of acetylcholine enzymes in neuroblastoma cells persistently infected with lymphocytic choriomeningitis virus. J Cell Physiol. 1977 Jun;91(3):459–472. doi: 10.1002/jcp.1040910316. [DOI] [PubMed] [Google Scholar]
- Oldstone M. B., Rodriguez M., Daughaday W. H., Lampert P. W. Viral perturbation of endocrine function: disordered cell function leads to disturbed homeostasis and disease. Nature. 1984 Jan 19;307(5948):278–281. doi: 10.1038/307278a0. [DOI] [PubMed] [Google Scholar]
- Oldstone M. B., Sinha Y. N., Blount P., Tishon A., Rodriguez M., von Wedel R., Lampert P. W. Virus-induced alterations in homeostasis: alteration in differentiated functions of infected cells in vivo. Science. 1982 Dec 10;218(4577):1125–1127. doi: 10.1126/science.7146898. [DOI] [PubMed] [Google Scholar]
- PLOTKIN S. A., BOUE A., BOUE J. G. THE IN VITRO GROWTH OF RUBELLA VIRUS IN HUMAN EMBRYO CELLS. Am J Epidemiol. 1965 Jan;81:71–85. doi: 10.1093/oxfordjournals.aje.a120499. [DOI] [PubMed] [Google Scholar]
- Peterkofsky B., Diegelmann R. Use of a mixture of proteinase-free collagenases for the specific assay of radioactive collagen in the presence of other proteins. Biochemistry. 1971 Mar 16;10(6):988–994. doi: 10.1021/bi00782a009. [DOI] [PubMed] [Google Scholar]
- Plotkin S. A., Vaheri A. Human fibroblasts infected with rubella virus produce a growth inhibitor. Science. 1967 May 5;156(3775):659–661. doi: 10.1126/science.156.3775.659. [DOI] [PubMed] [Google Scholar]
- RUDOLPH A. J., YOW M. D., PHILLIPS A., DESMOND M. M., BLATTNER R. J., MELNICK J. L. TRANSPLACENTAL RUBELLA INFECTION IN NEWLY BORN INFANTS. JAMA. 1965 Mar 8;191:843–845. doi: 10.1001/jama.1965.03080100061013. [DOI] [PubMed] [Google Scholar]
- Raisz L. G., Kream B. E. Regulation of bone formation (second of two parts). N Engl J Med. 1983 Jul 14;309(2):83–89. doi: 10.1056/NEJM198307143090206. [DOI] [PubMed] [Google Scholar]
- Raisz L. G., Maina D. M., Gworek S. C., Dietrich J. W., Canalis E. M. Hormonal control of bone collagen synthesis in vitro: inhibitory effect of 1-hydroxylated vitamin D metabolites. Endocrinology. 1978 Mar;102(3):731–735. doi: 10.1210/endo-102-3-731. [DOI] [PubMed] [Google Scholar]
- Rawls W. E., Melnick J. L. Rubella virus carrier cultures derived from congenitally infected infants. J Exp Med. 1966 May 1;123(5):795–816. doi: 10.1084/jem.123.5.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawls W. E. Viral persistence in congenital rubella. Prog Med Virol. 1974;18(0):273–288. [PubMed] [Google Scholar]
- Reed G. B., Jr Rubella bone lesions. J Pediatr. 1969 Feb;74(2):208–213. doi: 10.1016/s0022-3476(69)80068-5. [DOI] [PubMed] [Google Scholar]
- Rudolph A. J., Singleton E. B., Rosenberg H. S., Singer D. B., Phillips C. A. Osseous manifestations of the congenital rubella syndrome. Am J Dis Child. 1965 Oct;110(4):428–433. doi: 10.1001/archpedi.1965.02090030448012. [DOI] [PubMed] [Google Scholar]
- Sato Mmaeda N., Maeda N., Urade M., Kuribayashi M., Shirasuna K., Yoshida H., Miyazaki T. Persistent infection of primary human cell cultures with rubella variant carrying DNA Polymerase activity. Arch Virol. 1978;56(1-2):181–187. doi: 10.1007/BF01317294. [DOI] [PubMed] [Google Scholar]
- Sato M., Urade M., Maeda N., Miyazaki T., Watanabe M., Shibata T., Yamamoto N. Isolation and characterization of a new rubella variang with DNA polymerase activity. Arch Virol. 1978;56(1-2):89–103. doi: 10.1007/BF01317285. [DOI] [PubMed] [Google Scholar]
- Scott D. M., Kent G. N., Cohn D. V. Collagen synthesis in cultured osteoblast-like cells. Arch Biochem Biophys. 1980 May;201(2):384–391. doi: 10.1016/0003-9861(80)90526-3. [DOI] [PubMed] [Google Scholar]
- Sekeles E., Ornoy A. Osseous manifestations of gestational rubella in young human fetuses. Am J Obstet Gynecol. 1975 Jun 1;122(3):307–312. doi: 10.1016/0002-9378(75)90174-x. [DOI] [PubMed] [Google Scholar]
- Sharpe A. H., Fields B. N. Pathogenesis of viral infections. Basic concepts derived from the reovirus model. N Engl J Med. 1985 Feb 21;312(8):486–497. doi: 10.1056/NEJM198502213120806. [DOI] [PubMed] [Google Scholar]
- Singleton E. B., Rudolph A. J., Rosenberg H. S., Singer D. B. The roentgenographic manifestations of the rubella syndrome in newborn infants. Am J Roentgenol Radium Ther Nucl Med. 1966 May;97(1):82–91. doi: 10.2214/ajr.97.1.82. [DOI] [PubMed] [Google Scholar]
- Tyler M. S., Koch W. E. In vitro development of palatal tissues from embryonic mice. II. Tissue isolation and recombination studies. J Embryol Exp Morphol. 1977 Apr;38:19–36. [PubMed] [Google Scholar]
- Van Alstyne D., Paty D. W. The effect of dibutyryl cyclic AMP on restricted replication of rubella virus in rat glial cells in culture. Virology. 1983 Jan 15;124(1):173–180. doi: 10.1016/0042-6822(83)90301-x. [DOI] [PubMed] [Google Scholar]
- Yoneda T., Pratt R. M. Glucocorticoid receptors in palatal mesenchymal cells from the human embryo: relevance to human cleft palate formation. J Craniofac Genet Dev Biol. 1982;1(4):411–423. [PubMed] [Google Scholar]
- Yoneda T., Pratt R. M. Interaction between glucocorticoids and epidermal growth factor in vitro in the growth of palatal mesenchymal cells from the human embryo. Differentiation. 1981;19(3):194–198. doi: 10.1111/j.1432-0436.1981.tb01147.x. [DOI] [PubMed] [Google Scholar]
- Yoneda T., Pratt R. M. Mesenchymal cells from the human embryonic palate are highly responsive to epidermal growth factor. Science. 1981 Jul 31;213(4507):563–565. doi: 10.1126/science.7017936. [DOI] [PubMed] [Google Scholar]








