Abstract
Background
The incidence of autoimmune diseases such as systemic lupus erythematosus, rheumatoid arthritis, and primary biliary cirrhosis has increased significantly in China. Information about the susceptibility or potential of autoimmune diseases in the general population is lacking.
Objective
To explore the prevalence of antinuclear antibody (ANA) and its specificities in the general population in China.
Methods
Twenty thousand nine hundred seventy sera samples were taken from the physical examination center in Baoding, China. Indirect immunofluorescence and line immunoassays were used to detect ANA and its specificities, respectively.
Results
Samples from females had a higher prevalence of ANA than samples from males (χ2 = 278.55; P < 0.01). For both sexes, the prevalence of ANA positively correlated with age and there were significant differences among different age groups at 10-year intervals, except the 80 years group (P < 0.05). One thousand two hundred forty-three ANA-positive samples were further analyzed with line immunoassays. There was a significant difference among age groups and between sex groups in terms of the specific autoantibodies (P < 0.01). The autoantibodies with the top-3 positive frequencies were anti-Ro-52, anti-M2, and anti-SSA.
Conclusions
There was a high prevalence of ANA positivity in the general Chinese population that seemed to be influenced by sex and age and correlated with specific autoantibodies.
Key words: antinuclear antibody, autoimmune diseases, Chinese, prevalence
Introduction
The incidence of autoimmune diseases (AIDs) such as systemic lupus erythematosus (SLE), rheumatoid arthritis, and primary biliary cirrhosis (PBC) has been increasing in China.1,2 Until now, the antinuclear antibody (ANA) test, wherein antibodies are detected by indirect immunofluorescence assay on HEp-2 cells, is commonly used as an initial screening method. The presence of ANA is nonspecific and can be associated with many nonautoimmune factors, such as carcinoma, infection, pharmaceuticals, and environmental factors. As a result, an ANA positive frequency in healthy individuals >20% has been reported.3 On the other hand, ANA may exist several years before an AID can be diagnosed,4 with higher titer of ANA being correlated closely with a higher risk of the onset of AIDs during adolescence and adulthood.5–8 The epidemiologic characteristics of AID in different countries and districts varies.9 To our knowledge, information about the ANA prevalence and the susceptibility or potential of AIDs in the general population of China is lacking. We aimed to evaluate the ANA prevalence in a wide range of the general Chinese population (aged 2–88 years).
Materials and Methods
Study population
To assess the ANA positive rate among the general population of China, we conducted a cross-sectional study. Twenty thousand nine hundred seventy sera were taken from the physical examination center in Baoding, Hebei, China, from July 2011 to September 2013 (Figure 1). The sample size and male to female ratio conform to the standard of the sixth National Census of China in 2010–2011 (http://www.stats.gov.cn/tjsj/pcsj/rkpc/6rp/indexch.htm). A total of 6800 children (including 3300 girls and 3200 boys) aged from 2 to 18 years were recruited from 3 public kindergartens, 5 middle schools, and 2 high schools. The other participants consisted of 14,170 individuals (7220 women and 6950 men) obtained by random sampling. Participants with established AID were excluded from this study. Informed consent was obtained from all participants. The study complied with the World Medical Association Declaration of Helsinki and was approved by the Ethics Committee of the First Center Hospital of Bao Ding. All data were dealt with in an anonymous way.
Figure 1.
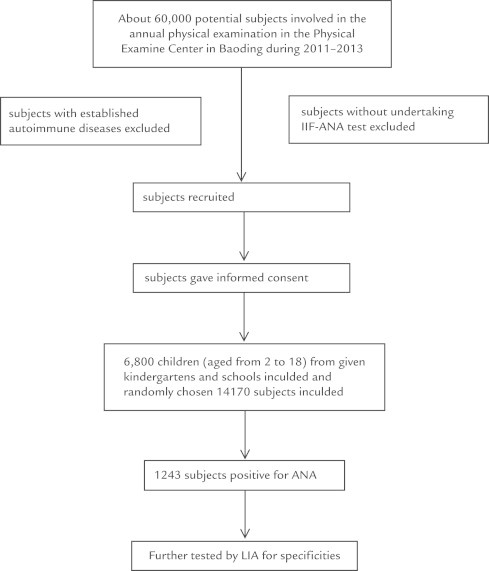
Flowchart for the involvement of potential participants in the study. ANA = antinuclear antibodies; IIF = indirect immunofluorescence; LIA = line immunoassay.
Antibody assays
Sera were tested by indirect fluorescence on HEp-2 cells according to the manufacturer’s instructions (Euroimmun AG, Lubeck, Germany). Titer ≥1:320 was considered to be positive. As a result, 243 positive samples were further tested by line immunoassay (LIA) (Euroimmun AG) for 15 specific autoantibodies (ie, anti-nRNP, anti-Sm, anti-SSA, anti-Ro52, anti-SSB, anti-Jo-1, anti-Scl-70, anti-CNEPB, anti-dsDNA, anti-His, anti-PCNA, anti-Nuk, anti-Rib, anti-M2, and anti-PMScl-70). The sera for LIA test were diluted 1:100. EUROBlotMaster (Euroimmun AG) and EUROLineScan (Euroimmun AG) were used to complete the operation and for test result interpretation, respectively.
Statistical analysis
SPSS for Windows version 17.0 (IBM-SPSS, Inc, Armonk, New York) was used for statistical calculations. Nonparametric analysis with the Wilcoxon signed-rank test was used for matched pairs. Categorical variables were compared using χ2 test as appropriate. P < 0.05 was considered to be statistically significant.
Results
The age and sex distribution of 20,970 participants are summarized in Table I. The ages ranged from 2 to 88 years, with mean age 32 (19.7) years for both sexes.
Table I.
Distribution of antinuclear antibody positive frequency in male and female participants at different ages.
| Age group, y | Positive/case numbers |
χ2 | P | |
|---|---|---|---|---|
| Male | Female | |||
| 2–<10 | 21/1300 | 33/1200 | 25.96 | <0.001 |
| 10–<20 | 55/2200 | 176/2150 | 12.74 | <0.001 |
| 20–<30 | 39/1700 | 143/1680 | 64.12 | <0.001 |
| 30–<40 | 48/1800 | 109/1840 | 23.40 | <0.001 |
| 40–<50 | 49/1500 | 105/1480 | 22.27 | <0.001 |
| 50–<60 | 43/1000 | 138/970 | 58.15 | <0.001 |
| 60–<70 | 43/600 | 95/550 | 27.75 | <0.001 |
| 70–<80 | 30/350 | 79/400 | 18.78 | <0.001 |
| 80+ | 12/100 | 25/150 | 1.04 | 0.365 |
| Total | 340/10,550 | 903/10,420 | 278.55 | <0.001 |
The prevalence of ANA in male and female participants by age groups are shown in Table I. The overall prevalence of ANA was 5.92% and correlated positively with age. There were significant differences among each age group except age older than 80 years, as shown in Table I (P < 0.01). In the female group, there are 2 sharp peaks in ANA positivity at the 20-year and 40-year age groups.
In 1243 ANA-positive sera tested by LIA, 44.2% were positive for at least 1 of 15 specific ANA antibodies. In terms of the effect of age and sex on ANA positivity, there were significant differences among the 3 groups (ie, ≤20 years, 21–49 years, and ≥50 years; χ2 = 275.04; P < 0.01), and between male and female (χ2 = 236.47; P < 0.01), as shown in Table I and Table II, respectively. The autoantibodies with the top-3 positive frequency were anti-Ro-52 (19%), anti-M2 (17.8%), and anti-SSA (14.3%); whereas anti-Scl-70, anti-Jo-1, and anti-Sm were the less frequently detected antibodies among the autoantibodies detected (Table III).
Table II.
Numbers of antinuclear antibody (ANA)-specific antibodies in positive sera, by age group.
| ANA-specific antibody | Positive numbers |
χ2 | P | ||
|---|---|---|---|---|---|
| ≤20 y | 21–49 y | ≥50 y | |||
| Anti-nRNP | 13 | 14 | 25 | 5.12 | 0.08 |
| Anti-Sm | 6 | 8 | 17 | 5.35 | 0.07 |
| Anti-SSA | 21 | 40 | 67 | 28.24 | <0.01 |
| Anti-Ro52 | 25 | 43 | 98 | 55.96 | <0.01 |
| Anti-SSB | 6 | 10 | 19 | 7.60 | 0.02 |
| Anti-Jo-1 | 0 | 3 | 14 | 14.63 | <0.01 |
| Anti-Scl-70 | 0 | 3 | 3 | -- | -- |
| Anti-CB | 5 | 22 | 38 | 22.84 | <0.01 |
| Anti-dsDNA | 8 | 11 | 22 | 7.95 | 0.02 |
| Anti-his | 6 | 16 | 35 | 22.84 | <0.01 |
| Anti-PCNA | 16 | 0 | 8 | 2.67 | 0.10 |
| Anti-Nuk | 0 | 6 | 11 | 0.89 | 0.35 |
| Anti-Rib | 2 | 8 | 19 | 15.38 | <0.01 |
| Anti-M2 | 25 | 17 | 112 | 109.90 | <0.01 |
| Anti-PM | 6 | 8 | 24 | 12.95 | <0.01 |
| Total | 139 | 209 | 512 | 275.04 | <0.01 |
Table III.
Numbers of antinuclear antibody (ANA)-specific antibodies in positive sera, by sex.
| ANA-specific antibody | Positive numbers |
Frequency (%) | χ2 | P value | |
|---|---|---|---|---|---|
| Male | Female | ||||
| Anti-nRNP | 16 | 37 | 6.0 | 8.32 | <0.01 |
| Anti-Sm | 10 | 24 | 4.0 | 0.67 | 0.41 |
| Anti-SSA | 30 | 93 | 14.3 | 32.27 | <0.01 |
| Anti-Ro52 | 40 | 123 | 19.0 | 42.26 | <0.01 |
| Anti-SSB | 11 | 24 | 4.1 | 4.83 | 0.03 |
| Anti-Jo-1 | 6 | 13 | 2.2 | 2.58 | 0.11 |
| Anti-Scl-70 | 0 | 6 | 0.7 | --- | --- |
| Anti-CB | 5 | 60 | 7.6 | 46.54 | <0.01 |
| Anti-dsDNA | 8 | 33 | 4.8 | 15.24 | <0.01 |
| Anti-his | 16 | 41 | 6.6 | 10.97 | <0.01 |
| Anti-PCNA | 5 | 19 | 2.8 | 8.17 | <0.01 |
| Anti-Nuk | 8 | 10 | 2.1 | 0.222 | 0.637 |
| Anti-Rib | 5 | 24 | 3.4 | 12.45 | <0.01 |
| Anti-M2 | 32 | 121 | 17.8 | 51.77 | <0.01 |
| Anti-PM | 13 | 27 | 4.7 | 4.90 | 0.03 |
| Total | 205 | 655 | 100 | 236.47 | <0.01 |
Discussion
This is the first research into the prevalence of ANA in the general Chinese population. Satoh et al9 hold that the ANA prevalence in the US population of individuals aged 12 years and older is 13.8%, and based on National Health and Nutrition Examination Survey 1999-2004 data, more than 32 million people in the United States are positive for ANA. Other studies indicate that Japanese show a 9.5% prevalence at a 1:100 cutoff level dilution,10 whereas for Indians it is 12.3%.11 Anti-Ro-52/SSA was the most detected antibody in both of those studies.10,11 In the present and another relevant study,12 Chinese showed a relatively moderate frequency of 13.98% at 1:100 titers. Based on that comparison among different nations, we hold the opinion that ANA positive frequency in adults differs by geography. Sex and age had been assumed to be the factors that influenced ANA positivity. In our study, ANA prevalence correlated positively with age on the whole, with higher prevalence present in the 20- to 30-year and 40- to 50-year groups. The latter peak is in line with the single peak at age 40 to 49 years described in a US population9 that was characterized by the physiologic stage of puberty and menopause, implying that estrogen may play an important role. As such, some similarities of genetic deposition and hormone factors may have an influence on disease manifestations.13 Between the sexes, our results indicate that the female to male ratio in ANA positive cohorts is almost 4:1, except in the 80 years group.
Our study also showed that ANA positivity in girls (8.2%) was higher than in boys (2.5%). This indicated that females have a higher ANA titer than males even at a relatively immature stage. Because ANA-IIF is a primary screening test for adolescent SLE,14 this method maybe used routinely to screen for ANA-positivity in younger people. Hayashi et al10 reported that it is important to emphasize the ANA titer as a prognostic marker in certain AIDs, and a 1:160 or 1:320 dilution titer is recommended. Although ANA may be influenced by nonimmune factors, several articles show that the positive frequency is no more than 5% at a dilution titer of 1:160 in the general population.10,15 In our study, at the cutoff value of 1:320 titer, we found a similar result to previous studies in that older people were prone to higher frequency of ANA positivity than younger people (Figure 2).
Figure 2.
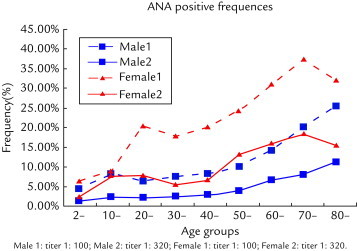
Frequency of the presence of antinuclear antibodies (ANA), by age group and sex.
Consistent with the findings in a Japanese population,4,10 we found anti-Ro52/SSA was the most prevalent autoantibody in the general population. Ro-52/SSA is a useful serologic marker for SLE and Sjogren syndrome, myositis, systemic sclerosis, and PBC.15 Anti-Ro/SSA and antichromatin had the highest predictive value for SLE diagnosis.16,17 In our study the positive rate of anti-M2 reached 12.7%. The prevalence of patients with asymptomatic PBC in an Asian population derived from previous studies18,19 seemed to be larger than that inferred from our study. Coincident to the data from Japanese residents,10 our results showed that anti-Scl-70, anti-Jo-1, anti-Nuk, and anti-Sm were the least-detected antibodies.
Certain limitations exist in our study. It is a pilot study on a large-scale population without comprehensive consideration of other factors such as occupation, genetic deposition, and biochemical factors.
Conclusions
ANA positive frequency is high in the general Chinese population and differs in different sex and age groups.
Conflicts of Interest
The authors have indicated that they have no conflicts of interest regarding the content of this article.
Acknowledgments
Ya-Ping Guo and Hong-Xing Li provided the idea for the study. Chun-Guang Wang, Xin Liu, Yi-Qian Huang, De-Li Guo, Xing-Zhuo Jing, Song Yang, and Jin-Mei Liu designed the study and analyzed the data. Chun-Guang Wang, Chun-Guang Yuan, Meng-Si Han, and Hong-Xing Li were responsible for writing the manuscript and revising it critically for important intellectual content. All authors read and approved the final manuscript.
References
- 1.Feldman C.H., Hiraki L.T., Liu J. Epidemiology and sociodemographics of systemic lupus erythematosus and lupus nephritis among US adults with Medicaid coverage, 2000-2004. Arthritis Rheum. 2013;65:753–763. doi: 10.1002/art.37795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baldursdottir T.R., Bergmann O.M., Jonasson J.G. The epidemiology and natural history of primary biliary cirrhosis: a nationwide population-based study. Eur J Gastroenterol Hepatol. 2012;24:824–830. doi: 10.1097/MEG.0b013e328353753d. [DOI] [PubMed] [Google Scholar]
- 3.Wananukul S., Voramethkul W., Kaewopas Y. Prevalence of positive antinuclear antibodies in healthy children. Asian Pac J Allergy Immunol. 2005;23:153–157. [PubMed] [Google Scholar]
- 4.Eriksson C., Kokkonen H., Johansson M. Autoantibodies predate the onset of systemic lupus erythematosus in northern Sweden. Arthritis Res Ther. 2011;13:30–35. doi: 10.1186/ar3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shin J.I., Kim K.H., Chun J.K. Prevalence and patterns of anti-nuclear antibodies in Korean children with juvenile idiopathic arthritis according to ILAR criteria. Scand J Rheumatol. 2008;37:348–351. doi: 10.1080/03009740801998762. [DOI] [PubMed] [Google Scholar]
- 6.Chou I.J., Kuo C.F., See L.C. Antinuclear antibody status and risk of death in children and adolescents. Scand J Rheumatol. 2011;40:472–477. doi: 10.3109/03009742.2011.593546. [DOI] [PubMed] [Google Scholar]
- 7.Ishikawa M., Konta T., Hao Z. Relationship between antinuclear antibody and microalbuminuria in the general population: the Takahata study. Clin Exp Nephrol. 2008;12:200–206. doi: 10.1007/s10157-008-0030-0. [DOI] [PubMed] [Google Scholar]
- 8.Wijeyesinghe U., Russell A.S. Outcome of high titer antinuclear antibody positivity in individuals without connective tissue disease: a 10-year follow-up. Clin Rheumatol. 2008;27:1399–1402. doi: 10.1007/s10067-008-0932-y. [DOI] [PubMed] [Google Scholar]
- 9.Satoh M., Chan E.K., Ho L.A. Prevalence and sociodemographic correlates of antinuclear antibodies in the United States. Arthritis Rheum. 2012;64:2319–2327. doi: 10.1002/art.34380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hayashi N., Koshiba M., Nishimura K. Prevalence of disease-specific antinuclear antibodies in general population: estimates from annual physical examinations of residents of a small town over a 5-year period. Mod Rheumatol. 2008;18:153–160. doi: 10.1007/s10165-008-0028-1. [DOI] [PubMed] [Google Scholar]
- 11.Minz R.W., Kumar Y., Anand S. Antinuclear antibody positive autoimmune disorders in North India: an appraisal. Rheumatol Int. 2012;32:2883–2888. doi: 10.1007/s00296-011-2134-1. [DOI] [PubMed] [Google Scholar]
- 12.Shapira Y., Poratkatz B.S., Gilburd B. Geographical differences in autoantibodies and anti-infectious agents antibodies among healthy adults. Clin Rev Allergy Immunol. 2012;42:154–163. doi: 10.1007/s12016-010-8241-z. [DOI] [PubMed] [Google Scholar]
- 13.Cacciapaglia F., Arcarese L., Rigon A. Antinuclear antibodies prevalence in Filipinos migrated to Italy. Eur Rev Med Pharmacol Sci. 2008;12:267–270. [PubMed] [Google Scholar]
- 14.Dipti T.R., Azam M.S., Sattar M.H. Detection of anti-nuclear antibody by immunofluorescence assay and enzyme immunoassay inchildhood systemic lupus erythematosus: experience from Banglade. Int J Rheum Dis. 2012;15:121–125. doi: 10.1111/j.1756-185X.2011.01694.x. [DOI] [PubMed] [Google Scholar]
- 15.Wang K.Y., Yang Y.H., Chuang Y.H. The initial manifestations and final diagnosis of patients with high and low titers of antinuclear antibodies after 6 months of follow-up. J Microbiol Immunol Infect. 2011;44:222–228. doi: 10.1016/j.jmii.2011.01.019. [DOI] [PubMed] [Google Scholar]
- 16.Feki S., Frikha F., Ben Hadj Hmida Y. Prevalence and diagnostic value of antinuclear antibodies without identified antigenic target: A retrospective study of 90 patients. Rev Med Interne. 2012;30:45–49. doi: 10.1016/j.revmed.2012.04.017. [DOI] [PubMed] [Google Scholar]
- 17.Yoshimi R., Ueda A., Ozato K. Clinical and pathological roles of Ro/SSA autoantibody system. Clin Dev Immunol. 2012;12:606–615. doi: 10.1155/2012/606195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu H., Liu Y., Wang L. Prevalence of primary biliary cirrhosis in adults referring hospital for annual health check-up in Southern China. BMC Gastroenterol. 2010;10:100–109. doi: 10.1186/1471-230X-10-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Osio-Salido E., Manapat-Reyes H. Epidemiology of systemic lupus erythematosus in Asia.Lupus. 2010;19:1365–1373. doi: 10.1177/0961203310374305. [DOI] [PubMed] [Google Scholar]


