Abstract
Heat stress, which strongly affects plant performance and often results in reduced vegetative growth and yields depression, has become an increasingly serious global problem. Populus euphratica Oliv. which has been considered as a tree model for the study of plant response to abiotic stresses, could be resistant to an extremely wide environmental temperature range (–40 °C to 45 °C). Previous study is mainly focused on its gene regulation upon drought and salt stress. However, little is known about gene regulation at the global transcriptome level upon heat stress. To understand the gene network controlling heat stress in P. euphratica, a transcriptome sequencing using Illumina Hiseq 2000 was performed to generate a 10 gigabases depth for each sample in the tissue of leaf. 119,573 unigeneswere generated with an average length of 474 bp. Approximately 49,605 (41.49%) unigenes exhibited significantly different expressions between two libraries. Among these unigenes, 11,165 (9.34%) were upregulated and 38,440 (32.15%) were down regulated. Heat shock proteins classified as molecular chaperones showed a significant percentage (1.13%) in the up regulated group. Heat responsive genes, such as polyubiquitins, were over expressed in heat treated sample. GO enrichment analysis revealed that the Go terms for differentially expressed unigenes were significantly enriched in hormone-mediated signal, biological process regulation and metabolic process regulation. Our data revealed a global transcriptome picture of P. euphratica in response to heat shock. The identified potential heat stress-related transcripts can be used to infer the gene regulation networks underlying the molecular mechanisms of heat response in P. euphratica.
Keywords: Heat shock, HSP, Polyubiquitin, Populus euphratica, Transcriptome.
INTRODUCTION
Previous reports have predicted that greenhouse gases, such as CO2, methane, chlorofluorocarbons, and nitrous oxides, gradually increase the global ambient temperature [1]. Heat stress is a serious global threat to plant growth and production because of high ambient temperatures [2]. When exposed to heat stress, severe cellular injury and even cell death may take place in a very short time; this adverse condition can be attributed to the disruption of cellular organization [3]. The changes at molecular level take place immediately after stress happened and result in altering gene expression and transcript accumulation. Thus, stress-related proteins are synthesized to induce stress tolerance [4]. Previous studies have established an important adaptive strategy, in which almost all of the organisms respond to heat shock by synthesizing heat shock proteins (HSPs) [1]. HSPs induce tolerance and improve physiological processes, including photosynthesis, assimilate partitioning, and membrane stability [1, 5].
Although HSP stress response strategy is conserved in prokaryotes and eukaryotes [6], only a few plant species or genotypes share abilities to cope with heat stress. High variations in heat stress-coping strategies among plants provide opportunities to study the mechanism of heat stress tolerancewithin and between species. In recent studies, deep sequencing technologies have been widely used [7, 8]. Using this method, scholars can capture expressed sequence tags (ESTs) and identify novel transcripts in a specific tissue at a particular point at a whole genome level without bias [9, 10].
Populus euphratica Oliv. is naturally distributed in semiarid areas and play important roles in maintaining local arid ecosystems [11-14]. As a tree species model widely used to elucidate abiotic resistance mechanisms [15-17], P. euphratica can tolerate temperatures as high as 45 °C. To obtain heat tolerance genes and investigate the mechanisms involved in heat stress response of this species, we presented a de novo assembly transcriptome of P. euphratica exposed to stress at 45 °C by using Solexa data. The acquired information may contribute to the development of strategies and facilitate improvement of heat tolerance of trees.
MATERIALS AND METHODS
Sample Preparation
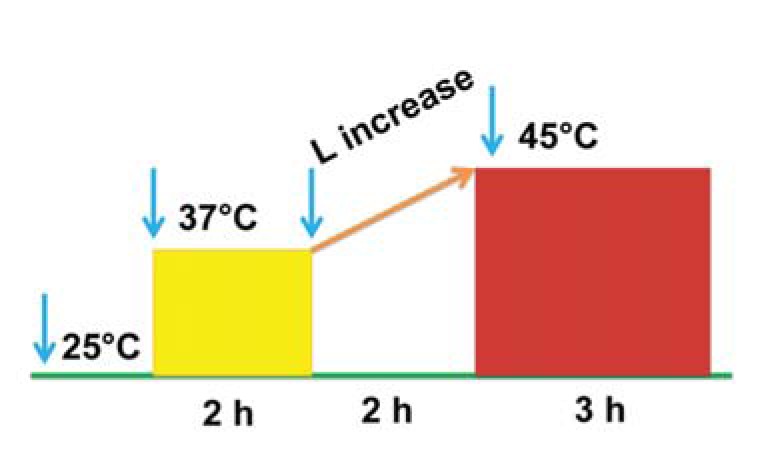
Plant materials were collected from two-year-old P. euphratica seedlings obtained from the Xinjiang Autonomous Region of China and planted in conditions as previously reported [13]. These potted plants were well irrigated at an interval of 3 d according to the evaporation rate two months before treatment. Heat treatment was designed as described in (Fig. 1). Plants were acclimated by heating the chamber at a constant, non-lethal temperature (37 °C, 2 h); the temperature was then increased linearly from 37 °C to 45 °C for 2 h and then stay at 45 °C for 3 h. At the end of the treatment, the leaves from four different plants were harvested, frozen immediately in liquid nitrogen, and stored at –80 °C for RNA extraction [13].
Fig. (1).
Designation of heat treatment. Shaded box represent the temperature designation for plants. After pretreatment at 37 °C for 2 h, linear increase of temperature was taken 2 h prior to the 45 °C treatment. The time for each stage were indicated under the line.
H2O2 Content Assay
The level of H2O2 was measured by monitoring the A415 of the titanium–peroxide complex according to the method reported by [7]. Absorbance values were calibrated to a standard curve generated with known concentrations of H2O2.
RNA Isolation and Illumina cDNA Library Construction
Total RNA was extracted using the CTAB [18]. After treated with RNAase free DNAase, the A260/A280 ratios were examined by NanoDrop 2000 and ratios ranging from 1.9 to 2.1 were selected [13]. The integrity of the RNA samples was examined using an Agilent 2100 Bioanalyzer and their RNA integrity number (RIN) values were >8.0 [13].
To construct the Illumina Hiseq 2000 libraries, 25 μg RNA sample with a concentration of ≥750 ng/μl was used for each cDNA library construction. Poly (A) mRNA was initially enriched with oligo(dT) and subsequently fragmented into small pieces of 200 bp to 700 bp by using divalent cations at an elevated temperature. Based on these cleaved RNA fragments, random hexamer-primer and reverse transcriptase (Invitrogen) were used to synthesize cDNA [13, 19]. Two paired-end cDNA libraries with an insert size of 200 bp were constructed and subsequently sequenced using Illumina Hiseq™ 2000 [13, 19].
De Novo Assembly and Assessment
Raw data generated from Solexa sequencing were preprocessed to remove non-sense sequences, including adapters, sequences with numerous unknown bases (>5%), and sequences with low-quality bases (>50% of the bases with a quality score ≤5), by using an in-house Perl script [19]. The preprocessed sequences were then assembled by using Trinity (Version: r2011-08-20) program [20]. Reads were first combined with certain overlap lengths to form fragments known as contigs,and then these contigs were further realigned to construct unigenes by Trinity. To fill the intra-scaffold gaps, we used the paired-end information to retrieve read pairs that contained one well-aligned read to the contigs and another read located in the gap region [21]; the collected reads were locally assembled. After the gap was closed, we constructed a non-redundant unigene set from the two assembled datasets by TGICL program [22]. To decide the sequential orientation of each unigene, we performed a set of sequential BLASTx alignment (E < 1e-5) against the non-redundant (NR) database of GenBank, Swiss-Prot protein database (http://www.expasy.ch/sprot), Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway database [23], and Clusters of Orthologous Groups of proteins (COG) database (http://www.ncbi.nlm.nih.gov/COG/). For unigenes that cannot be aligned to any of these databases, ESTScan was used to determine the sequence orientation [24].
Unigene Annotation and Function Classification
All assembled unique sequences were searched against the databases of NR, NT, Swiss-Prot, KEGG, and poplar transcripts version3 by BLASTn (E < 1e-5) to gain the most descriptive annotation. The protein with the highest sequence similarity was retrieved. Pathway mapping analysis was performed based on KEGG database alignment. According to Nr annotation, GO information was obtained using the Blast2GO server [25]. GO functional classification was performed using the WEGO software with plants categories defined by molecular function, cellular component, and biological process ontologies [26].
Gene Expression Analysis
The expression level of P. euphratica unigene was normalized according to the number of RPKM [27, 28]. After normalization was conducted, we determined the number of reads in each coding region in the control and treated libraries. The ratio of the reads in the two libraries was then calculated. The statistical significance of the differential expression value of each unigene was determined according to a previously described method [29]; the results of the statistical tests were corrected for multiple testing by using the Benjamini and Hochberg false FDR correction [30]. To eliminate the effect of the highly sensitive Solexa/Illumina sequencing method, we defined DE unigenes as those with an absolute value of log2Ratio ≥ 2, FDR < 0.001 as well as those expressed at ≥ 3 RPKM in ≥1 sample. The unigenes that failed to contain a minimum of three RPKM in at least one sample were removed [31].
To obtain the significantly enriched GO terms of DE unigenes on the genome background, we annotated all of the DE unigenes to the GO database by using a hypergeometric test [32]. The cutoff p value after correction was 0.05 on rigorous Bonferroni correction method. GO terms meeting these standards were defined as significantly enriched GO terms. The KEGG pathway enrichment analysis of DE unigenes was performed to determine the main biochemical pathways and signal transduction pathways involving DE unigenes. Corrected p ≤ 0.05 was used as a threshold to identify the overrepresented pathways.
Quantitative PCR Analysis
Quantitative PCR (qPCR) was conducted using a power SYBR Green PCR kit (ABI) in a MicroAmp™96-well plate with a StepOnePlus™ Real-time PCR System (ABI). The relative quantification value was calculated according to the 2-∆∆CT method [33]. Before qPCRanalyses, we illustrate the suitability of several reference gene including RPL17, TUB and 60S and finally chose PeActin (GenBank Accession Number: EF148840) as an internal control [34]. Reactions were prepared using a total volume of 20 µl containing 10 µl of 2× SYBR premix, 2 µl of cDNA template, and 0.5 µl of each specific primer to obtain a final concentration of 200 nM. The reactions were performed under the following conditions: 94 °C for 2 min; 45 cycles of 94 °C for 20 s; 60 °C for 35 s; and 68 °C for 1 min. The gene-specific primers used in the qPCR analysis are listed in (Table S1 (4.6MB, pdf) ). For PCR analyses, three independent samples were used which were different from those used for RNA-seq. Three technical replicates were performedfor each sample as shown in previous report [33].
RESULTS AND DISCUSSION
Solexa Sequencing Results
The total RNA obtained from approximately 30 leaves of four treated or non-treated plants was used to create biologically independent pools to capture heat-responsive genes. After the adapters, low-quality sequences, and ambiguous reads were removed [12], a total of 64.6 million and 68.9 million clean reads with a mean length of 90 bp were generated in the control group (CK) and heat shock-treated sample (HS4), respectively (Table 1). The raw data has been submitted to the NCBI Sequence Read Archive database with the accession number SRP029139. All of the trimmed reads were de novo assembled into contigs according to the Trinity method [20]. The average contig size was 135 and 134 bp in CK and HS4 libraries, respectively. Using paired-end information, we joined the contigs to obtain a total of 119,573 unigenes with an average length of 474 bp and a N50 of 548 bp (Table 1). Length distribution analysis results showed that 9.74% (11,643) of these unigenes were longer than 1,000 bp (Fig. S1 (4.6MB, pdf) ).
Table 1.
Overview of the sequencing and assembly.
| HS4 | CK | All | |
|---|---|---|---|
| Total Clean Reads | 64,611,114 | 68,888,892 | |
| Total Clean Nucleotides | 5,815,000,260 | 6,200,000,280 | |
| Q20 percentage | 96.30% | 96.20% | |
| N percentage | 0.01% | 0.01% | |
| GC percentage | 44.78% | 44.59% | |
| Contig | |||
| Total Number | 738,481 | 620,759 | |
| Total Length(nt) | 99,461,549 | 82,873,556 | |
| Mean Length(nt) | 135 | 134 | |
| N50 | 116 | 116 | |
| Unigene | |||
| Total Number | 130,421 | 109,624 | 119,573 |
| Total Length(nt) | 47,236,810 | 39,455,066 | 56,628,880 |
| Mean Length(nt) | 362 | 360 | 474 |
| N50 | 410 | 406 | 548 |
Functional Annotation of the All Unigenes
Using BLASTx or BLASTn with an e-value of <1.0E–5, we annotated all of the unigenes according to the public databases, including NR database, nucleotide sequence database, the poplar genome (v3), Swiss Prot database, COG and KEGG databases. Among the119,573 high quality unique sequences, 93,214 (78%) showed at least one significant match to an existing gene model in the BLAST search. The COG classification and functional classifications GO terms of all unigenes were shown in (Fig. S2 (4.6MB, pdf) ). In the biological process category of GO terms, the five largest groups included cellular process, metabolic process, stimulus response process, pigmentation process, and biological regulation process. In the molecular function category, the unigenes with binding and catalytic activities formed the largest groups (Fig. S3 (4.6MB, pdf) ).
Differential Expression Unigenes Identification
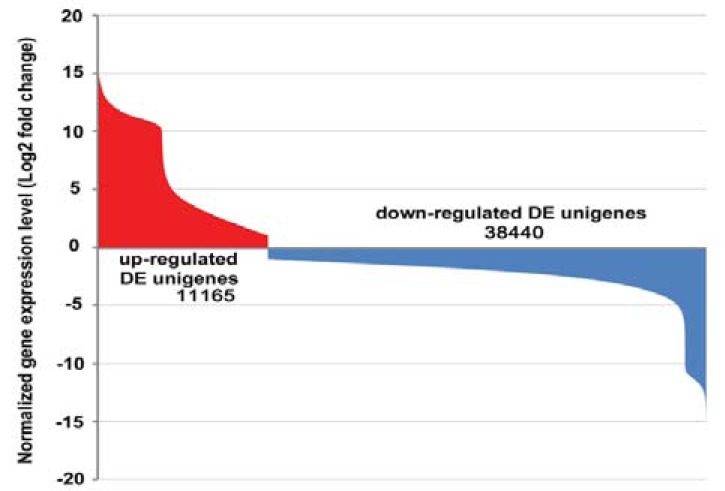
According to our applied criteria (FDR < 0.001, absolute value of log2Ratio ≥ 1, and RPKM ≥ 3 in ≥ 1 sample), approximately 41.49% (49,605 unigenes) of the total unigenes were identified as differentially expressed (DE) unigenes, in which 11,165 were up regulated and 38,440 were down regulated (Fig. 2). Among these DE unigenes, 43,827 had a gene model in poplar database (Table S2 (4.6MB, pdf) ).
Fig. (2).
Differential expression analyses of the putative unique transcripts. Transcripts that satisfied the conditions of “FDR < 0.001” and “|log2 fold-change (log2FC)| ≥1” were considered significantly differentially-expressed unigenes.
To identify DE unigenes accurately, we selected the top 100 most up regulated transcripts and the top 100 most down regulated transcripts with good annotation (FDR < 0.001, Table 2). Among the top 100 most upregulated unigenes, 98 transcripts could match a transcript in the genome database of P. trichocarpa. Those encoding HSPs (e.g., Unigene119328, Unigene105373, and Unigene119280), heat shock factors (HSFs, e.g., Unigene119554, Unigene103826, and Unigene100969), dehydration responsive element binding protein (DREB) transcription factors (e.g. Unigene 109904, Unigene118327, Unigene117802, and Unigene 118202), and ubiquitin/polyubiquitin (e.g., Unigene110702, Unigene118782, Unigene111816, Unigene117269, and Unigene117112) occupied 62% of the most up regulated interpretable transcripts in the HS4 sample (Table 2). This result indicates a concentrated function annotation. Furthermore, 49 chaperone proteins such as HSP and DnaJ were included in these 100 unigenes. The high percentage of HSPs and HSFs in the top 100 most up regulated gene list suggested that HSPs were the primary factors affecting the heat tolerance of P. euphratica, and also indicated the efficiency of our treatments.
Table 2.
Top 100 most upregulated unigenes of P. euphratica by heat shock stress treatment.
| Gene ID | Poplar v3 Model | Log2 (Fold Change) | Annotation |
|---|---|---|---|
| Unigene113351 | Potri.001G042700.1 | 12.52 | heat shock protein 70 cognate |
| Unigene119328 | Potri.008G062300.1 | 11.44 | 18.2 kDa class I heat shock protein |
| Unigene118411 | Potri.001G042700.1 | 11.41 | heat shock protein 70 cognate |
| Unigene100860 | Potri.004G187400.1 | 11.32 | low molecular weight heat shock protein |
| Unigene117613 | Potri.001G042700.1 | 11.28 | heat shock protein 70 cognate |
| Unigene119280 | Potri.010G195700.1 | 11.13 | 18.2 kDa class I heat shock protein |
| Unigene100276 | Potri.004G187400.1 | 11.12 | heat-shock protein |
| Unigene26446 | Potri.009G049800.1 | 11.12 | 17.5 kd heat shock protein GmHSP17.6L |
| Unigene117236 | Potri.009G049900.1 | 11.05 | Hsp20.1 protein |
| Unigene118998 | Potri.004G073600.1 | 11.04 | heat shock protein |
| Unigene110823 | Potri.010G195700.1 | 11.00 | 18.2 kDa class I heat shock protein |
| Unigene109379 | Potri.009G049900.1 | 10.92 | heat shock protein |
| Unigene100392 | Potri.009G147900.1 | 10.84 | 17.5 kDa class I heat shock protein |
| Unigene36975 | Potri.010G053400.1 | 10.78 | chloroplast small heat shock protein |
| Unigene119447 | Potri.006G223900.1 | 10.69 | heat shock protein 17.7 - garden pea |
| Unigene119010 | Potri.013G089200.1 | 10.61 | 22.0 kDa class IV heat shock protein |
| Unigene106116 | Potri.019G081200.1 | 10.59 | 17.5 kd heat shock protein GmHSP17.6L |
| Unigene106960 | Potri.003G167500.1 | 10.45 | Bcl-2-associated athanogene-like protein |
| Unigene119446 | Potri.003G109200.2 | 10.33 | LMW heat shock protein |
| Unigene115745 | Potri.012G022400.1 | 10.30 | heat shock protein |
| Unigene36846 | Potri.006G093500.1 | 10.15 | 18.2 kDa class I heat shock protein |
| Unigene116444 | Potri.003G071100.1 | 9.94 | 17.4 kDa class III heat shock protein |
| Unigene116523 | Potri.017G084000.1 | 9.93 | FtsH protease |
| Unigene115042 | Potri.004G187200.1 | 9.75 | cytosolic class I small heat shock protein type 2 |
| Unigene110702 | Potri.004G211600.1 | 9.68 | ubiquitin-like protein 5 |
| Unigene106090 | Potri.005G259900.1 | 9.61 | calmodulin-like protein |
| Unigene106270 | Potri.017G084000.1 | 9.32 | FtsH protease |
| Unigene117683 | Potri.017G084000.1 | 9.20 | FtsH protease; putative |
| Unigene115386 | Potri.003G071100.1 | 9.17 | 17.4 kDa class III heat shock protein |
| Unigene26606 | Potri.019G081200.1 | 9.11 | 17.5 kd heat shock protein Gmhsp17.6L |
| Unigene105373 | Potri.008G054000.1 | 8.98 | heat shock protein 70 |
| Unigene101586 | Potri.010G206600.1 | 8.89 | heat shock protein 70 |
| Unigene113828 | Potri.010G206600.1 | 8.84 | heat shock protein 70 |
| Unigene118352 | Potri.015G056900.1 | 8.79 | endopeptidaseClp (EC 3.4.21.-) ATP-binding chain SB100 |
| Unigene105915 | Potri.015G057000.1 | 8.59 | heat shock protein |
| Unigene106538 | Potri.010G206600.1 | 8.57 | heat shock protein 70 |
| Unigene118982 | Potri.002G166300.1 | 8.43 | BAG6; calmodulin binding / protein binding |
| Unigene107019 | Potri.015G004800.1 | 8.37 | Avr9/Cf-9 rapidly elicited protein 65 |
| Unigene119573 | Potri.002G166300.1 | 8.25 | BAG6; calmodulin binding / protein binding |
| Unigene111455 | Potri.010G088600.1 | 7.92 | heat shock protein 70 family protein |
| Unigene119411 | Potri.017G146600.1 | 7.80 | heat shock protein |
| Unigene117980 | Potri.009G039200.1 | 7.77 | 17.6 kDa class I heat shock protein;Hsp20.0 |
| Unigene34332 | Potri.012G021600.1 | 7.69 | Avr9/Cf-9 rapidly elicited protein 65 |
| Unigene117779 | Potri.011G057600.1 | 7.67 | dnaJ subfamily B member 5 |
| Unigene119554 | Potri.006G226800.3 | 7.56 | AtHSFA2 |
| Unigene118622 | Potri.013G018000.1 | 7.51 | heat shock protein 70 cognate |
| Unigene101449 | Potri.004G034400.2 | 7.50 | F-box family protein; late embryogenesis abundant protein |
| Unigene49867 | Potri.005G214800.2 | 7.31 | heat stress transcription factor A-6b |
| Unigene103826 | Potri.005G214800.2 | 7.18 | heat stress transcription factor A-6b |
| Unigene100924 | 7.10 | NADH dehydrogenase subunit 6 | |
| Unigene100969 | Potri.005G214800.2 | 7.01 | heat stress transcription factor A-6b |
| Unigene31571 | Potri.017G146600.1 | 7.01 | Heat shock protein 83 |
| Unigene118961 | Potri.004G213400.1 | 6.97 | Rubisco subunit binding-protein alpha subunit |
| Unigene118390 | Potri.005G183300.1 | 6.96 | calcium-binding protein |
| Unigene115732 | Potri.002G191600.1 | 6.89 | putative galactinol synthase |
| Unigene118782 | Potri.017G135600.3 | 6.88 | polyubiquitin |
| Unigene117269 | Potri.017G135600.1 | 6.88 | polyubiquitin |
| Unigene119548 | Potri.001G182100.1 | 6.80 | Probable pyridoxin biosynthesis PDX1-like protein 2 |
| Unigene26267 | Potri.013G018000.1 | 6.71 | heat shock protein 70 cognate |
| Unigene111816 | Potri.017G135600.1 | 6.52 | polyubiquitin |
| Unigene100739 | Potri.001G289700.1 | 6.52 | EGY3, ethylene-dependent gravitropism-deficient and yellow-green-like 3 |
| Unigene117112 | Potri.017G135600.1 | 6.45 | polyubiquitin |
| Unigene39330 | 6.43 | nad6;ubiquinone oxidoreductase chain 6;NADH dehydrogenase subunit 6 | |
| Unigene102654 | Potri.002G048200.1 | 6.42 | AtHSFA7A |
| Unigene102474 | Potri.004G034400.1 | 6.36 | F-box family protein; late embryogenesis abundant protein |
| Unigene119268 | Potri.001G289700.1 | 6.09 | EGY3, ethylene-dependent gravitropism-deficient and yellow-green-like 3 |
| Unigene103979 | Potri.001G303600.1 | 6.06 | phenylpropanoid:glucosyltransferase 1 |
| Unigene118877 | Potri.010G168300.1 | 6.04 | zinc finger (C3HC4-type RING finger) family protein |
| Unigene26854 | Potri.003G216100.1 | 6.04 | oxidoreductase/ transition metal ion binding protein |
| Unigene116435 | Potri.011G085100.2 | 6.03 | SGS domain-containing protein; calcyclin binding protein |
| Unigene110992 | Potri.008G073600.2 | 6.03 | Putative dehydration responsive element binding protein 2H |
| Unigene25167 | Potri.011G122500.1 | 6.01 | WD-40 repeat family protein |
| Unigene111646 | Potri.010G168300.1 | 5.98 | zinc finger (C3HC4-type RING finger) family protein |
| Unigene113318 | Potri.014G044300.2 | 5.87 | chaperonin 10 |
| Unigene110294 | Potri.016G003400.3 | 5.83 | HSP80 |
| Unigene4724 | Potri.011G085100.1 | 5.80 | SGS domain-containing protein; calcyclin binding protein |
| Unigene109904 | Potri.008G073600.2 | 5.75 | dehydration responsive element binding protein 2H |
| Unigene118305 | Potri.014G116800.3 | 5.73 | putative galactinol synthase |
| Unigene110176 | Potri.014G116800.3 | 5.72 | putative galactinol synthase |
| Unigene26341 | Potri.017G130700.2 | 5.70 | peroxisomal small heat shock protein |
| Unigene112432 | Potri.014G056600.1 | 5.69 | four F5 protein-related / 4F5 protein-related |
| Unigene118203 | Potri.010G183700.1 | 5.67 | dehydration responsive element binding protein |
| Unigene111511 | Potri.001G286700.1 | 5.66 | HSP80 |
| Unigene35342 | Potri.004G213400.1 | 5.63 | Rubisco subunit binding-protein alpha suunit |
| Unigene116440 | Potri.007G136700.1 | 5.56 | DNAJ chaperone C-terminal domain-containing protein |
| Unigene114084 | Potri.002G191600.1 | 5.54 | putative galactinol synthase |
| Unigene119368 | Potri.018G075200.1 | 5.52 | ethylene-responsive transcriptional coactivator |
| Unigene118327 | Potri.010G183700.1 | 5.47 | Dehydration responsive element binding protein |
| Unigene114471 | Potri.016G003400.2 | 5.46 | heat shock protein 90-2 |
| Unigene117802 | Potri.008G073600.2 | 5.41 | Dehydration responsive element binding protein |
| Unigene116777 | Potri.008G101600.1 | 5.41 | gibberellin 2-oxidase |
| Unigene25884 | Potri.001G381000.1 | 5.38 | 5-azacytidine resistance protein -related |
| Unigene107042 | Potri.014G184200.1 | 5.38 | Retrotransposon gag protein |
| Unigene34276 | Potri.016G120000.1 | 5.37 | DNAJ heat shock N-terminal domain-containing protein |
| Unigene113416 | Potri.006G073500.1 | 5.32 | low molecular weight heat-shock protein |
| Unigene109968 | Potri.014G116800.3 | 5.24 | putative galactinol synthase |
| Unigene44055 | Potri.009G099000.1 | 5.21 | glucosyltransferase |
| Unigene43602 | Potri.001G154200.1 | 5.18 | AP2/ERF domain-containing transcription factor |
| Unigene33231 | Potri.019G083800.1 | 5.17 | ubiquitin conjugating-like enzyme |
| Unigene118202 | Potri.010G183700.1 | 5.12 | dehydration responsive element binding protein 2H |
Distinct from those of up regulated unigenes, the unigenes with diverse functions were identified when the top 100 most downregulated analysis was performed. Some zinc finger proteins, cytochrome P450 genes, AP2/ERF-domain containing transcription factors, and protein kinases were listed in the top 100 most down regulated unigenes. The remaining clusters of unigenes primarily encoded abiotic stress-related transcription factors, general metabolism involving enzymes, and auxin-induced proteins (Table 3).
Table 3.
Top 100 most downregulated unigenes of P. euphratica by heat shock stress treatment.
| Gene ID | Poplar v3 Model | log2 (Fold Change) | Annotation |
|---|---|---|---|
| Unigene47078 | Potri.011G150100.1 | -7.21 | oxidoreductase, 2OG-Fe(II) oxygenase family protein |
| Unigene16607 | Potri.019G131600.2 | -6.10 | RPT2 |
| Unigene46509 | Potri.012G090000.1 | -5.89 | cytochrome P450 |
| Unigene35116 | Potri.016G071900.1 | -5.84 | aspartyl protease family protein |
| Unigene43437 | Potri.004G148400.1 | -5.67 | transducin family protein / WD-40 repeat family protein |
| Unigene118552 | Potri.007G121100.1 | -5.64 | DNA binding protein |
| Unigene27890 | Potri.008G061800.1 | -5.59 | protease inhibitor/seed storage/LTP family protein |
| Unigene51359 | Potri.004G162600.1 | -5.53 | salt tolerance-like protein |
| Unigene48152 | Potri.015G027700.1 | -5.52 | UDP-glucose:isoflavone 7-O-glucosyltransferase |
| Unigene15296 | Potri.001G118400.1 | -5.50 | cytochrome P450 |
| Unigene3678 | Potri.002G039100.1 | -5.42 | AP2/ERF domain-containing transcription factor |
| Unigene24729 | Potri.012G091500.2 | -5.41 | similar to Probable pectatelyase 22 precursor |
| Unigene26237 | Potri.012G083300.1 | -5.41 | alliinase family protein |
| Unigene41914 | Potri.002G144200.2 | -5.38 | GRAS family transcription factor |
| Unigene1323 | Potri.001G071000.1 | -5.34 | xyloglucanendotransglucosidase |
| Unigene42138 | Potri.011G147900.1 | -5.31 | zinc finger (C3HC4-type RING finger) family protein |
| Unigene25314 | Potri.006G232400.2 | -5.27 | 41 kD chloroplast nucleoid DNA binding protein (CND41) |
| Unigene47358 | Potri.014G020500.1 | -5.20 | cytochrome P450 |
| Unigene118351 | Potri.010G120100.1 | -5.17 | leucine-rich repeat receptor-like protein kinase |
| Unigene46286 | Potri.008G065000.1 | -5.15 | transferase family protein |
| Unigene15583 | Potri.004G152300.1 | -5.15 | polyol transporter |
| Unigene49328 | Potri.007G121100.1 | -5.14 | zinc finger (B-box type) family protein; |
| Unigene4718 | Potri.005G195600.1 | -5.13 | peroxidase 4 |
| Unigene23436 | Potri.016G071900.1 | -5.12 | aspartyl protease family protein |
| Unigene47056 | Potri.001G052300.1 | -5.09 | Similar to pectatelyase. |
| Unigene18168 | Potri.019G119300.2 | -5.00 | leucine-rich repeat family protein |
| Unigene22853 | Potri.015G068200.1 | -5.00 | Germin-like protein subfamily T member 2 |
| Unigene4523 | Potri.001G083900.1 | -4.99 | cytochrome P450 |
| Unigene19863 | Potri.001G079000.1 | -4.98 | Ferric reductase-like transmembrane component |
| Unigene110599 | Potri.004G049100.1 | -4.98 | HSL1; ATP binding protein serine/threonine kinase |
| Unigene29860 | Potri.001G124200.1 | -4.96 | glycerol 3-phosphate permease |
| Unigene41440 | Potri.010G042100.3 | -4.91 | polygalacturonase |
| Unigene17878 | Potri.002G085100.3 | -4.91 | nodulin family protein |
| Unigene33652 | Potri.016G038000.1 | -4.88 | type-a response regulator |
| Unigene104488 | Potri.016G083900.1 | -4.87 | MYB transcription factor |
| Unigene19269 | Potri.013G103800.1 | -4.86 | phenylcoumaranbenzylic ether reductase –like protein |
| Unigene47697 | Potri.016G083900.1 | -4.86 | MYB transcription factor |
| Unigene25745 | Potri.001G254100.2 | -4.85 | gibberellin induced protein |
| Unigene108845 | Potri.001G399600.8 | -4.82 | Protein SPA1-RELATED 3 |
| Unigene12317 | Potri.003G150700.1 | -4.79 | AP2/ERF domain-containing transcription factor |
| Unigene24847 | Potri.017G076800.1 | -4.78 | nitrate transporter NRT1-5 |
| Unigene114796 | Potri.T126700.1 | -4.72 | wall-associated receptor kinase-like 10-like, partial |
| Unigene35916 | Potri.001G348100.1 | -4.71 | glycosyltransferase UGT90A7 |
| Unigene109572 | Potri.017G134900.1 | -4.71 | similar to Putative receptor protein kinase TMK1 precursor |
| Unigene20711 | Potri.009G053800.1 | -4.69 | DiT1 (dicarboxylate transporter 1); oxoglutarate:malateantiporter |
| Unigene50973 | Potri.007G097500.2 | -4.68 | RING1; protein binding / ubiquitin-protein ligase/ zinc ion binding |
| Unigene41578 | Potri.005G117100.2 | -4.68 | zinc finger (B-box type) family protein |
| Unigene47294 | Potri.004G027300.1 | -4.66 | Very similar to receptor-like protein kinase |
| Unigene31087 | Potri.011G151200.2 | -4.66 | subtilase family protein |
| Unigene26479 | Potri.003G151000.1 | -4.65 | AP2/ERF domain-containing transcription factor |
| Unigene115429 | Potri.002G065300.1 | -4.63 | peroxidase |
| Unigene110741 | Potri.016G016800.1 | -4.62 | Anthocyanidin 3-O-glucosyltransferase 6 |
| Unigene42213 | Potri.009G169900.2 | -4.59 | 3-hydroxy-3-methylglutaryl coenzyme A reductase |
| Unigene115367 | Potri.004G057700.2 | -4.59 | cysteine protease |
| Unigene25400 | Potri.001G399600.8 | -4.59 | SPA4 (SPA1-RELATED 4); protein binding / signal transducer |
| Unigene30850 | Potri.002G248500.1 | -4.58 | basic helix-loop-helix (bHLH) family protein; |
| Unigene44683 | Potri.003G207200.2 | -4.57 | basic helix-loop-helix (bHLH) family protein; |
| Unigene24456 | Potri.015G068200.1 | -4.57 | germin-like protein |
| Unigene44617 | Potri.003G101800.1 | -4.56 | lipase |
| Unigene34224 | Potri.017G097800.1 | -4.56 | FERONIA receptor-like kinase |
| Unigene48675 | Potri.001G211000.7 | -4.56 | F-box family protein; |
| Unigene12711 | Potri.009G097300.2 | -4.54 | cyclase-like protein |
| Unigene25153 | Potri.011G110200.1 | -4.54 | SIEP1L protein |
| Unigene48872 | Potri.014G149700.1 | -4.53 | pectinesterase family protein |
| Unigene11709 | Potri.019G119300.2 | -4.53 | leucine-rich repeat family protein |
| Unigene115331 | Potri.014G094800.1 | -4.53 | similar to arabinogalactan-protein (AGP20); |
| Unigene103692 | Potri.012G031100.4 | -4.52 | SIGE (SIGMA FACTOR E); DNA binding / DNA-directed RNA polymerase |
| Unigene118975 | Potri.011G076400.1 | -4.51 | GDSL-motif lipase/hydrolase-like protein |
| Unigene34284 | Potri.008G166200.1 | -4.51 | AP2/ERF domain-containing transcription factor |
| Unigene26224 | Potri.001G161400.6 | -4.51 | plasma membrane proton ATPase |
| Unigene32382 | Potri.016G047500.1 | -4.50 | Hypothetical protein |
| Unigene51122 | Potri.001G211000.7 | -4.49 | F-box family protein; |
| Unigene14480 | Potri.009G113600.1 | -4.48 | polyol transporter |
| Unigene36234 | Potri.006G086100.1 | -4.47 | expansin |
| Unigene20944 | Potri.013G115800.1 | -4.47 | lectin protein kinase family protein |
| Unigene22167 | Potri.002G039100.1 | -4.47 | AP2/ERF domain-containing transcription factor |
| Unigene2790 | Potri.013G115800.1 | -4.47 | lectin protein kinase family protein |
| Unigene46536 | Potri.019G054800.3 | -4.47 | Similar to thioredoxin f precursor - garden pea. |
| Unigene102005 | Potri.010G236800.1 | -4.47 | GDSL-motif lipase/hydrolase family protein |
| Unigene111472 | Potri.004G084000.1 | -4.47 | leucine-rich repeat receptor-like protein kinase |
| Unigene20679 | Potri.014G037900.1 | -4.46 | cytochrome P450 |
| Unigene19398 | Potri.011G150400.1 | -4.45 | oxidoreductase, 2OG-Fe oxygenase family protein, expressed |
| Unigene105153 | Potri.006G047500.1 | -4.45 | bifunctionalmonodehydroascorbate reductase and carbonic anhydrase nectarin-3 |
| Unigene38315 | Potri.004G097000.1 | -4.44 | serine/threonine protein kinase family protein |
| Unigene27298 | Potri.008G152300.2 | -4.43 | with no lysine kinase |
| Unigene20610 | Potri.018G009200.1 | -4.43 | ATCNGC17 |
| Unigene47384 | Potri.014G060500.1 | -4.42 | GRAS family transcription factor |
| Unigene25829 | Potri.019G057500.4 | -4.41 | similar to Alpha-expansin 8 precursor;AtEXPA8 |
| Unigene4810 | Potri.010G046300.1 | -4.41 | low affinity inorganic phosphate transporter |
| Unigene3581 | Potri.008G152300.2 | -4.40 | with no lysine kinase |
| Unigene21605 | Potri.T079300.1 | -4.40 | cytochrome P450 |
| Unigene2029 | Potri.011G164200.1 | -4.40 | oxidoreductase, 2OG-Fe(II) oxygenase family protein |
| Unigene102830 | Potri.010G112200.6 | -4.40 | ATMPK15 |
| Unigene36053 | Potri.001G079600.1 | -4.39 | AP2/ERF domain-containing transcription factor |
| Unigene27223 | Potri.014G026700.1 | -4.38 | subtilisin-like protease preproenzyme |
| Unigene45648 | Potri.011G066900.4 | -4.37 | cysteine protease |
| Unigene15515 | Potri.001G210100.2 | -4.37 | protease inhibitor/seed storage/lipid transfer protein family protein |
| Unigene23053 | Potri.019G085400.1 | -4.37 | C2-H2 zinc finger protein |
| Unigene14649 | Potri.008G088300.2 | -4.36 | alpha-expansin 3 |
| Unigene110047 | Potri.012G087800.3 | -4.36 | ribitol dehydrogenase-like/short-chain dehydrogenase/reductase protein |
We further performed qPCR analysis to validate the reliability of our sequencing results. Ten members were randomly selected from the top 100 most up regulated and the top 100 most down regulated DE unigene list. The qPCR results indicated that all of the DE unigenes exhibited similar expression kinetics to the deep sequencing results (Table 4).
Table 4.
Quantitative RT-PCR for 10 selective DEGs.
| Gene ID | Poplar v3 Model | Annotation | 45°C Abundance |
|---|---|---|---|
| Unigene117613 | Potri.001G042700.1 | Heat shock protein 70 | 8.97±0.29 |
| Unigene117112 | Potri.017G135600.1 | Polyubiquitin UBQ14 | 8.64±0.68 |
| Unigene118203 | Potri.010G183700.1 | DRE-binding protein | 6.87±0.53 |
| Unigene117779 | Potri.011G057600.1 | DnaJ subfamily B member 5 | 7.69±0.56 |
| Unigene119554 | Potri.006G226800.3 | Heat shock transcription factor A2 | 7.32±1.39 |
| Unigene 100969 | Potri.005G214800.2 | Heat shock transcription factor A6B | 6.82±0.21 |
| Unigene 115732 | Potri.002G191600.1 | Galactinol synthase 4 | 6.21±0.17 |
| Unigene114471 | Potri.016G003400.2 | Heat shock protein 90-2 | 6.53±0.35 |
| Unigene 111646 | Potri.010G168300.1 | Zinc finger family protein | 5.59±0.89 |
| Unigene113416 | Potri.006G073500.1 | Low molecular weight heat shock protein | 5.23±0.10 |
All P. trichocarpa V3 gene models were obtain by blasting with a threthhold of 1e-5.qPCR was performed on 10 members randomly selected from top 100 upregulated unigenes of the HS4 DEG list.
KEGG Mapping and Gene Ontology Enrichment Analyses for DE Unigenes
Based on a comparison against the KEGG database, twenty-five pathways changed significantly (p ≤ 0.05) under heat shock stress, including the pathways involved in carbohydrate, energy, lipid, vitamin, hormone, and pyruvate metabolisms (Table 5). Plant pathogen interaction, N-glycan biosynthesis, and zeatin biosynthesis pathways were three of the top five most differentially expressed pathways that seem to play important roles in heat shock response.
Table 5.
Enrichment analysis for KEGG metabolic pathway of differentially expressed unigenes.
| Pathway ID | Pathway | DE Unigenes (13611) | Corrected p value | |
|---|---|---|---|---|
| Up | Down | |||
| ko04626 | Plant-pathogen interaction | 199 | 1065 | 1.48E-10 |
| ko00510 | N-Glycan biosynthesis | 1 | 129 | 3.45E-07 |
| ko04141 | Protein processing in endoplasmic reticulum | 105 | 280 | 6.58E-07 |
| ko00945 | Stilbenoid, diarylheptanoid and gingerol biosynthesis | 29 | 155 | 4.64E-05 |
| ko00908 | Zeatin biosynthesis | 29 | 98 | 1.21E-04 |
| ko03022 | Basal transcription factors | 12 | 115 | 1.21E-04 |
| ko00100 | Steroid biosynthesis | 9 | 57 | 8.00E-04 |
| ko00905 | Brassinosteroid biosynthesis | 2 | 31 | 8.78E-04 |
| ko00402 | Benzoxazinoid biosynthesis | 13 | 46 | 1.25E-03 |
| ko03040 | Spliceosome | 75 | 637 | 2.27E-03 |
| ko00944 | Flavone and flavonol biosynthesis | 4 | 63 | 2.43E-03 |
| ko00910 | Nitrogen metabolism | 11 | 103 | 2.60E-03 |
| ko00941 | Flavonoid biosynthesis | 48 | 142 | 5.21E-03 |
| ko00903 | Limonene and pinene degradation | 21 | 161 | 6.98E-03 |
| ko00511 | Other glycan degradation | 5 | 64 | 1.03E-02 |
| ko00966 | Glucosinolate biosynthesis | 8 | 51 | 1.56E-02 |
| ko02010 | ABC transporters | 11 | 142 | 2.19E-02 |
| ko00603 | Glycosphingolipid biosynthesis - globo series | 0 | 16 | 2.29E-02 |
| ko00040 | Pentose and glucuronate interconversions | 17 | 99 | 2.38E-02 |
| ko00531 | Glycosaminoglycan degradation | 3 | 39 | 2.68E-02 |
| ko00604 | Glycosphingolipid biosynthesis - ganglio series | 1 | 23 | 3.62E-02 |
| ko00600 | Sphingolipid metabolism | 4 | 69 | 3.75E-02 |
| ko00940 | Phenylpropanoid biosynthesis | 44 | 260 | 3.92E-02 |
| ko00450 | Selenocompound metabolism | 11 | 66 | 4.07E-02 |
| ko04712 | Circadian rhythm - plant | 13 | 157 | 4.33E-02 |
The cutoff p value after correction was 0.05 on Q value correction method.
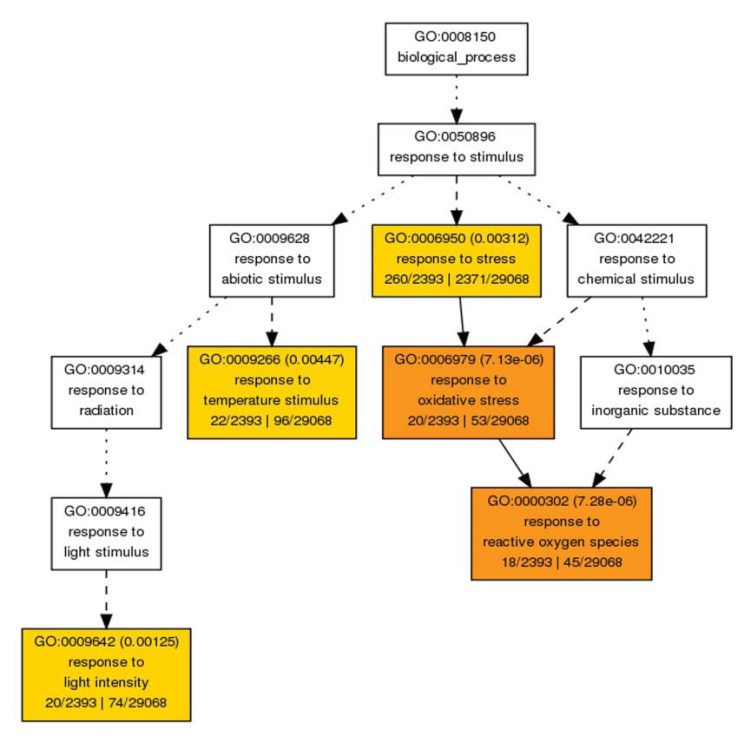
GO enrichment analysis showed biological processes related to biological regulation and hormone-mediated signaling were significantly enriched (p ≤ 0.05, Table 6). This result suggested that these processes are important in heat stress response. Unigenes related to endogenous stimulus and chemical stimulus responses were also enriched for DE unigenes, indicating a comprehensive change in gene expression in heat-treated P. euphratica. We also identified the enriched GO terms for upregulated DE unigenes based on genomic background. GO terms including stress response process, temperature stimulus response process, oxidative stress response process, and reactive oxygen species (ROS) response were enriched (Fig. 3). The increased transcripts in these systems may indicate their important roles in protection of P. euphratica under conditions of heat stress by removal of or response to ROS.
Table 6.
Enrichment analysis for GO terms of differentially expressed unigenes
| Gene Ontology Term | Cluster Frequency | Genome Frequency of Use | Corrected p-value |
|---|---|---|---|
| Biological Process | |||
| hormone-mediated signaling | 304 out of 10455 genes, 2.9% | 458 out of 19477 genes, 2.4% | 2.10E-05 |
| regulation of biological process | 1849 out of 10455 genes, 17.7% | 3221 out of 19477 genes, 16.5% | 0.00238 |
| biological regulation | 2185 out of 10455 genes, 20.9% | 3842 out of 19477 genes, 19.7% | 0.00652 |
| response to endogenous stimulus | 610 out of 10455 genes, 5.8% | 1014 out of 19477 genes, 5.2% | 0.01496 |
| regulation of cellular process | 753 out of 10455 genes, 7.2% | 1270 out of 19477 genes, 6.5% | 0.02336 |
| regulation of metabolic process | 1282 out of 10455 genes, 12.3% | 2220 out of 19477 genes, 11.4% | 0.03066 |
| response to chemical stimulus | 1133 out of 10455 genes, 10.8% | 1954 out of 19477 genes, 10.0% | 0.03993 |
| Molecular Function | |||
| oligosaccharyltransferase activity | 30 out of 12519 genes, 0.2% | 34 out of 23520 genes, 0.1% | 0.0092 |
| monooxygenase activity | 152 out of 12519 genes, 1.2% | 229 out of 23520 genes, 1.0% | 0.02015 |
The cutoff p value after correction was 0.05 on rigorous Bonferroni correction method.
Fig. (3).
Biological process network of over-representative GO terms of upregulated DE unigenes. Node filled color represented different p value. White nodes were not significant over-representative terms.
Heat Shock Proteins and Heat Shock Factors in Response to Heat Shock Stress
HSPs that function as molecular chaperones are usually induced by heat shock at any developmental stage of higher plants [35]. HSPs are considered as candidate genes providing protective traits in plants against abiotic stresses because HSPs function in stabilizing proteins, repairing protein structures, and assisting in protein refolding [36]. HSPs are classified into five families according to molecular size: Hsp100, Hsp90, Hsp70, Hsp60, and small HSPs (sHSP) [37, 38]. According to our dataset, numerous HSPs were significantly induced after heat shock stress treatment. Besides 49 HSPs presented in the top 100 most up regulated unigene list (Table 2), 93 members designated as heat shock-related genes were up regulated while 28 members were down regulated from the whole database (Table S3 (4.6MB, pdf) ). Among these unigenes, nine sequences encoding HSFs, which are known ascentral regulators of heat stress responsive genes [39], were up regulated. Eight sHSPs were up regulated, and none was down regulated, indicating a vital role played by sHSP in heat shock stress response. A highly conserved HSP gene named HSA32 has been reported in different plant species. However, only slightly increase of this gene has been found according our dataset. Close inspection of this gene revealed a higher abundance after 37 °C treatment, indicating its early response to heat in P. euphratica (data not shown). Heat shock cognate proteins (HSCs), DnaJ (HSP40) homologs and other HSPs were often classified as chaperone proteins. HSC70 is required to activate HSF1, which appears to control the early response of target genes in heat stress. DnaJ exhibits the same capability as HSP70 in reactivating proteins in bacteria and may respond to various environmental stresses independently as a molecular chaperone [40]. In our study, three sequences encoding HSC proteins were found and two of them were up regulated. In contrast to HSCs, DnaJ-like proteins comprise more down regulated members than up regulated ones, indicating that P. euphratica may initially utilize HSP70 and then DnaJs to resist heat stress. All these results indicated chaperone proteins function as master players for heat stress tolerance.
DREB Transcription Factors in Response to Heat Shock Stress
Some drought-responsive transcription factors, such as DREB, were overrepresented (FDR < 0.001) in the HS4 sample. Seven sequences with DREB annotation were identified in our dataset. Among these sequences, six were included in the top 100 most up regulated list. Based on Pfam database searches of predicted open reading frames and NCBI blast program, two genes, i.e., PeDREB2 (GenBank Accession No. EF137176.1; encoded by unigene 110992, unigene 109904 and unigene 117802) and PeDREB2L (GenBank Accession No. EF567422.1; encoded by unigene 118203, unigene 118327 and unigene 118202) were identified. DREB2-type transcription factors have been considered as important regulon not only in drought and salt stress signaling but also in heat stress response signaling in Arabidopsis [41, 42]. DREB2A can bind to HsfA3 promoter directly and function as a regulator of HsfA3 [43]. Here, we showed that PeDREB2 and PeDREB2L can be significantly induced by heat shock (Tables 2). Furthermore, two DREB2A-interacting protein genes that encode DRIP2 (unigene 52587 and unigene 38695), which were supposed to function negatively in response to abiotic stress, were dramatically down regulated in the HS4 sample (Table S2 (4.6MB, pdf) ). Considering the salt response function of PeDREB2 and drought response function of PeDREB2L we have reported [34, 41], we concluded that a complex cross-talk between different abiotic stress responses is involved in P. euphratica and that the DREB2-cellular signal transduction is crucial to the heat adaptation of this tree species. However, further studies should be conducted to determine whether these two factors or only one of them can participate in heat shock response.
Heat Stress Increases Intracellular Accumulation of H2O2
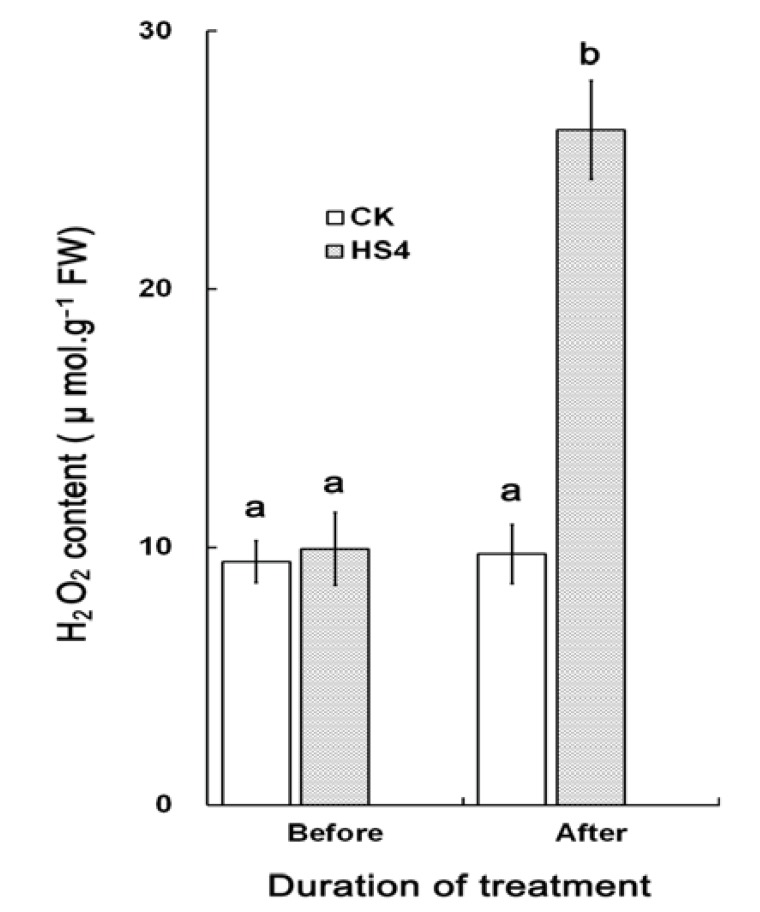
Many physiological damages occur when plants exposing to heat stress, e.g., causing ROS accumulation. DREB2A and HSFA2, which were highly induced after heat shock stress in our transcript profile, were also known to be involved in hydrogen peroxide (H2O2)-response signaling or strongly induced by H2O2 treatment [44]. HSPs, which encode cytosolic proteins for the detoxification of H2O2 and function as targets of HSFA2, were also overrepresented after heat shock stress. These results indicated that many H2O2-response genes may be implicated in heat shock stress. We further examined the H2O2 level before and after heat shock treatment. As shown in (Fig. 4), an increase intracellular accumulation of H2O2 has been investigated for heat stress treated plants, suggesting heat stress have induced oxidative production. Heat shock-induced H2O2 may facilitate transcripts accumulation of heat shock responsive genes in P. euphratica.
Fig. (4).
H2O2 content in P. euphratica leaves exposed to heat stress treatment. Each value is the means of three repeats.
We also investigated oxidative stress-responsive Ascorbate Peroxidases (APXs) that may be involved in heat stress response. Previously, a barley APX1 has been found to play roles in heat tolerance. A functional heat shock elements have been found in the 5’-promoter region of some Arabidopsis APX genes, indicating their potential role in heat stress response [37]. However, no APX gene has yet been investigated to be unregulated in our study. We supposed that some heat-responsive gene may be induced in different stages or in different tissues.
Calcium/calmodulin-mediated Signal Network Involved in Heat Stress
As a second universal messenger involved in abiotic stress responses in plants, calcium/calmodulin-mediated signal network functions as an important messenger in thermomemory associated signal transduction [45]. Calmodulin has been reported to be involved in heat shock signal transduction in wheat [46]. Calcium signals are perceived by calcium sensors such as calmodulins (CaMs) and calcium-dependent protein kinases (CDPK) that relay the signals into downstream targets in different stimuli [47].
As a regulatory protein involved in cellular calcium-dependent signaling pathways, CDPKs are monomeric proteins containing a CaM-like domain with four EF-hand motifs [48]. The CDPK family is a large family with various functions participating in different stress signal pathways. In the present study, four unigenes (Unigene24235, Unigene24346, Unigene20655, and Unigene113411) encoding different CDPK proteins were significantly up regulated after heat shock treatment, suggesting that CDPK might be an important component in heat shock response of P. euphratica. CaMs are small proteins that transmit the Ca2+ signal by interacting with target proteins and regulating their activity. Several CaMs, CMLs and dozens of CaM-binding protein genes were showed upregulated versus the control group. Particularly, some CaM-binding HSPs were found to be induced over 10-fold by heat shock treatment (Table 2). Thus, we propose that CaM might play important roles during the expression of HSPs through binding directly to cytoplasmic HSPs. To define the functional significance of these Ca2+ sensors, loss of-function and gain-of-function analyses for each of these genes under heat shock stress remains to be studies in the future.
Polyubiquitin in P. euphratica Heat Shock Response
Ubiquitin, a highly conserved protein of 76 amino acids present in eukaryotic cells, is involved in protein degradation via a multicatalytic proteasome complex [49, 50]. The ubiquitin system has been implicated in immune response, development, and programmed cell death [49]. As one of the two ubiquitin classes, polyubiquitins may have important functions in heat shock response in P. euphratica because of their overexpression (FDR < 0.001) in the top 100 up regulated gene list (Table 2). A yeast polybiquitin gene ubi4 is induced in response to environmental stresses, such as heat shock, starvation, and oxidative stress [50]. Ubi1 is another polybiquitin gene from an entophytic fungus that shows increased transcription at high temperatures; this result indicates a stress reaction of hyphal cells after heat shock treatment [49]. Although we cannot explain the mechanism of the overexpression of unigenes encoding polyubiquitin proteins in the heat shock-treated library precisely, the most probable explanation is that heat shock stress treatment induced damage to some proteins, which are targeted for proteolysis. In higher plants, the best-characterized outcome of ubiquitination is the process mediating target protein degradation via 26S proteasome, in which polyubiquitin is essential for the 26S proteasome recognition [51]. Evidence suggests that ubiquitination may have a critical function in regulating plant responses to abiotic stresses and has prompted researchers to conduct further studies to identify ubiquitin genes that mediate plant tolerance of abiotic stress. However, the mechanism by which polyubiquitin functions in response to adverse environmental conditions remains unknown. A recent study on the genome-wide transcriptome analyses of Populus simonii also established the crucial role that DREB played in heat response. However, few up-regulated polyubiquitin genes have been found in their study [52]. Basing on our sequencing results, we identified three polyubiquitin genes with full coding sequences against poplar genome database. Considering that all of these genes contain a common heat shock element sequence (CNNGAANNTTCNNG) in their upstream promoter region [53], we concluded that the up regulated polyubiquitin genes in P. euphratica play important roles in heat shock response.
CONCLUSION
In this work, we have analyzed global transcriptome profiling of P. euphratica upon heat shock stress.
Heat responsive genes, such as heat shock proteins, DREB transcription factors and polyubiquitins, were significantly up regulated in heat treated sample. Go terms for differentially expressed unigenes were significantly enriched in hormone-mediated signal, biological process regulation and metabolic process regulation. Our results provide a global picture of P. euphratica in response to heat shock stress at transcriptomic level by using Illumina Hiseq 2000. Detailed characterization of these heat shock responsive genes will be studied in future.
ACKNOWLEDGEMENTS
This work was supported by Fundamental Research funds for the Central Universities (YX2011-22 and DT2012-01), Beijing Higher Education Young Elite Teacher Project (YETP0754), National Natural Science Foundation of China (31100492 and 31370597), and Research Fund for the Doctoral Program of Higher Education of China (201100 14120003).
CONFLICT OF INTEREST
The author(s) confirm that this article content has no conflict of interest.
SUPPLEMENTARY MATERIALS
Supplementary material is available on the publisher’s web site along with the published article.
ABBREVIATIONS
- COG
= Clusters of Orthologous Group
- DE
= Differentially expressed
- DREB
= Dehydration responsive element binding protein
- EST
= Expressed sequence tag
- FDR
= False discovery rate
- GO
= Gene ontology
- H2O2
= Hydrogen peroxide
- HSP
= Heat shock protein
- HSF
= Heat shock factor
- KEGG
= Kyoto Encyclopedia of Genes and Genomes
- qPCR
= Quantitative real-time PCR
- RNA-seq
= RNA sequencing
- ROS
= Reactive oxygen species
- RPKM
= Reads per kilobase exon region per million mapped reads
REFERENCES
- 1.Wahid A, Gelani S, Ashraf M, Foolad M. Heat tolerance in plants an overview. Environ. Exp. Bot. 2007;61(3):199–223. [Google Scholar]
- 2.Knight CA, Rutledge CR, Cox ME, Acosta M, Hall SJ. Effect of superficial heat, deep heat, and active exercise warm-up on the extensibility of the plantar flexors. Phys. Ther. 2001;81(6):1206–1214. [PubMed] [Google Scholar]
- 3.Kesici M, Gulen H, Ergin S, Turhan E, Ahmet I, Koksal N. Heat-stress Tolerance of Some Strawberry (Fragaria× ananassa) Cultivars. Not. Bot. Horti Agrobot. Cluj. 2013;41(1):244–249. [Google Scholar]
- 4.Iba K. Acclimative response to temperature stress in higher plants approaches of gene engineering for temperature tolerance. Annu. Rev. Plant Biol. 2002;53:225–245. doi: 10.1146/annurev.arplant.53.100201.160729. [DOI] [PubMed] [Google Scholar]
- 5.Camejo D, Rodriguez P, Morales MA, Dell'Amico JM, Torrecillas A, Alarcon JJ. High temperature effects on photosynthetic activity of two tomato cultivars with different heat susceptibility. J. Plant Physiol. 2005;162(3):281–289. doi: 10.1016/j.jplph.2004.07.014. [DOI] [PubMed] [Google Scholar]
- 6.Baniwal SK, Bharti K, Chan KY, Fauth M, Ganguli A, Kotak S, Mishra SK, Nover L, Port M, Scharf KD, Tripp J, Weber C, Zielinski D, vonKoskull-Doring P. Heat stress response in plants a complex game with chaperones and more than twenty heat stress transcription factors. J.; Biosci. 2004;29(4):471–487. doi: 10.1007/BF02712120. [DOI] [PubMed] [Google Scholar]
- 7.Jiang Y, Zeng B, Zhao H, Zhang M, Xie S, Lai J. Genomeκwide Transcription Factor Gene Prediction and their Expressional TissueκSpecificities in MaizeF. J.; Integr. Plant Biol. 2012;54(9):616–630. doi: 10.1111/j.1744-7909.2012.01149.x. [DOI] [PubMed] [Google Scholar]
- 8.Wilhelm BT, Marguerat S, Watt S, Schubert F, Wood V, Goodhead I, Penkett CJ, Rogers J, Bahler J. Dynamic repertoire of a eukaryotic transcriptome surveyed at single-nucleotide resolution. Nature. 2008;453(7199):1239–1243. doi: 10.1038/nature07002. [DOI] [PubMed] [Google Scholar]
- 9.Wu J, Zhang Y, Zhang H, Huang H, Folta KM, Lu J. Whole genome wide expression profiles of Vitis amurensis grape responding to downy mildew by using Solexa sequencing technology. BMC Plant Biol. 2010;10:234. doi: 10.1186/1471-2229-10-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bausher M, Shatters R, Chaparro J, Dang P, Hunter W, Niedz R. An expressed sequence tag (EST) set from Citrus sinensis L Osbeck whole seedlings and the implications of further perennial source investigations. Plant Sci. 2003;165(2):415–422. [Google Scholar]
- 11.Browicz K. Chorology of Populus euphratica Olivier. Arbor. Kornickie. 1977;22:5–27. [Google Scholar]
- 12.Qiu Q, Ma T, Hu Q, Liu B, Wu Y, Zhou H, ang Q, Wang J, Liu J. Genome-scale transcriptome analysis of the desert poplar, Populus euphratica. Tree Physiol. 2011;tpr015 doi: 10.1093/treephys/tpr015. [DOI] [PubMed] [Google Scholar]
- 13.Chen J, Tian Q, Pang T, Jiang L, Wu R, Xia X, Yin W. Deep-sequencing transcriptome analysis of low temperature perception in a desert tree, Populus euphratica. BMC Genoics. 2014; 15(1):326. doi: 10.1186/1471-2164-15-326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang J, Xie P, Lascoux M, Meagher TR, Liu J. Rapidly Evolving Genes and Stress Adaptation of Two Desert Poplars, Populus euphratica and P pruinosa. PloS one. 2013;8(6):e66370. doi: 10.1371/journal.pone.0066370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tang S, Liang H, Yan D, Zhao Y, Han X, Carlson JE, Xia X, Yin W. Populus euphratica the transcriptomic response to drought stress. Plant Mol.Biol. 2013;83(6):539–557. doi: 10.1007/s11103-013-0107-3. [DOI] [PubMed] [Google Scholar]
- 16.Sun J, Wang MJ, Ding MQ, Deng SR, Liu MQ, Lu CF, Zhou XY, Shen X, Zheng XJ, Zhang ZK, Song J, Hu Z.M. Xu Y, Chen SL. H2O2 and cytosolic Ca2+ signals triggered by the PM H+-coupled transport system mediate K+/Na+ homeostasis in NaCl-stressed Populus euphratica cells. Plant Cell Environ. 2010;33(6):943–958. doi: 10.1111/j.1365-3040.2010.02118.x. [DOI] [PubMed] [Google Scholar]
- 17.Wu Y, Ding N, Zhao X, Zhao M, Chang Z, Liu J, Zhang L. Molecular characterization of PeSOS1: the putative Na+/H+ antiporter of Populus euphratica. Plant Mol. Biol. 2007;65:1–11. doi: 10.1007/s11103-007-9170-y. [DOI] [PubMed] [Google Scholar]
- 18.Chang S, Puryear J, Cairney J. A simple and efficient method for isolating RNA from pine trees. Plant Mol. Biol. Rep. 1993;11(2):13–116. [Google Scholar]
- 19.Zhao Z, Tan L, Dang C, Zhang H, Wu Q, An L. Deep-sequencing transcriptome analysis of chilling tolerance mechanisms of a subnival alpine plant, Chorispora bungeana. BMC plant Biol. 2012;12(1):222. doi: 10.1186/1471-2229-12-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grabherr MG, Haas BJ, Yassour M, Levin JZ, Thompson DA, Amit I, Adiconis X, Fan L, Raychowdhury R, Zeng Q, hen Z, Mauceli E, Hacohen N, Gnirke A, Rhind N, di Palma F, Birren BW, Nusbaum C, Lindblad-Toh K, Friedman N, Regev A. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat. Biotechnol. 2011;29(7):644–652. doi: 10.1038/nbt.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li R, Zhu H, Ruan J, Qian W, Fang X, Shi Z, Li Y, Li S, Shan G, Kristiansen K, Li S, Yang H, Wang J, Wang J. De novo assembly of human genomes with massively parallel short read sequencing. Genome Res. 2010;20(2):265–272. doi: 10.1101/gr.097261.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pertea G, Huang X, Liang F, Antonescu V, Sultana R, Karamycheva S, Lee Y, White J, Cheung F, Parvizi B, sai J, Quackenbush J. TIGR Gene Indices clustering tools (TGICL): a software system for fast clustering of large EST datasets. Bioinformatics. 2003;19(5):651–652. doi: 10.1093/bioinformatics/btg034. [DOI] [PubMed] [Google Scholar]
- 23.Kanehisa M, Araki M, Goto S, Hattori M, Hirakawa M, Itoh M, Katayama T, Kawashima S, Okuda S, Tokimatsu T, amanishi Y. KEGG for linking genomes to life and the environment. Nucleic Acids Res. 2008;36:D480–484. doi: 10.1093/nar/gkm882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iseli C, Jongeneel CV, Bucher P. ESTS can a program for detecting, evaluating, and reconstructing potential coding regions in EST sequences. Proc. Int. Conf. Intell. Syst. Mol. Biol. 1999:138–148. [PubMed] [Google Scholar]
- 25.Conesa A, Gotz S, Garcia-Gomez JM, Terol J, Talon M, Robles M. Blast2GO a universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics. 2005;21(18):3674–3676. doi: 10.1093/bioinformatics/bti610. [DOI] [PubMed] [Google Scholar]
- 26.Ye J, Fang L, Zheng H, Zhang Y, Chen J, Zhang Z, Wang J, Li S, Li R, Bolund L. WEGO a web tool for plotting GO annotations. Nucleic Acids Res. 2006;34:W293–297. doi: 10.1093/nar/gkl031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mortazavi A, Williams BA, McCue K, Schaeffer L, Wold B. Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat. Methods. 2008;5(7):621–628. doi: 10.1038/nmeth.1226. [DOI] [PubMed] [Google Scholar]
- 28.Pang T, Ye C-Y, Xia X, Yin W. De novo sequencing and transcriptome analysis of the desert shrub, Ammopiptanthus mongolicus, during cold acclimation using Illumina/Solexa. BMC Genomics. 2013;14(1):488. doi: 10.1186/1471-2164-14-488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Audic S, Claverie JM. The significance of digital gene expression profiles. Genome Res. 1997;7(10):986–995. doi: 10.1101/gr.7.10.986. [DOI] [PubMed] [Google Scholar]
- 30.Reiner A, Yekutieli D, Benjamini Y. Identifying differentially expressed genes using false discovery rate controlling procedures. Bionfo. 2003; 19(3):368–375. doi: 10.1093/bioinformatics/btf877. [DOI] [PubMed] [Google Scholar]
- 31.Liu C-F, Breidenbach A, Aschbacher-Smith L, Butler D, Wylie C. A Role for Hedgehog Signaling in the Differentiation of the Insertion Site of the Patellar Tendon in the Mouse. PloS one. 2013;8(6):e65411. doi: 10.1371/journal.pone.0065411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Valdes R, Ibarra N, Ruibal I, Beldarrain A, Noa E, Herrera N, Aleman R, Padilla S, Garcia J, érez M, Morales R, Chong E, Reyes B, Quiñones Y, Agraz A, Herrera L. Chromatographic removal combined with heat, acid and chaotropic inactivation of four model viruses. J. Biotechnol. 2002;96(3):251–258. doi: 10.1016/S0168-1656(02)00047-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen J, Xue B, Xia X, Yin W. A novel calcium-dependent protein kinase gene from Populus euphratica confers both drought and cold stress tolerance. Biochem. Biophys. Res. Commun. 2013;441(3):630–636. doi: 10.1016/j.bbrc.2013.10.103. [DOI] [PubMed] [Google Scholar]
- 34.Chen J, Xia X, Yin W. Expression profiling and functional characterization of a DREB2-type gene from Populus euphratica. Biochem. Biophys. Res. Commun. 2009;378(3):483–487. doi: 10.1016/j.bbrc.2008.11.071. [DOI] [PubMed] [Google Scholar]
- 35.Straus D, Walter W, Gross CA. DnaK, DnaJ, and GrpE heat shock proteins negatively regulate heat shock gene expression by controlling the synthesis and stability of sigma 32. Genes Dev. 1990;4(12A ):2202–2209. doi: 10.1101/gad.4.12a.2202. [DOI] [PubMed] [Google Scholar]
- 36.Torok Z, Goloubinoff P, Horvath I, Tsvetkova NM, Glatz A, Balogh G, Varvasovszki V, Los DA, Vierling E, Crowe JH, Vigh L. Synechocystis HSP17 is an amphitropic protein that stabilizes heat-stressed membranes and binds denatured proteins for subsequent chaperone-mediated refolding. Proc. Natl. Acad. Sci. USA. 2001;98(6):3098–3103. doi: 10.1073/pnas.051619498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hasanuzzaman M, Nahar K, Alam MM, Roychowdhury R, Fujita M. Physiological, biochemical, and molecular mechanisms of heat stress tolerance in plants. Int. J. Mol. Sci. 2013;14(5):9643–9684. doi: 10.3390/ijms14059643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morrow G, Tanguay RM. Small heat shock protein expression and functions during development. Int. J. Biochem. Cell Biol. 2012;44(10):1613–1621. doi: 10.1016/j.biocel.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 39.Krasensky J, Jonak C. Drought, salt, and temperature stress-induced metabolic rearrangements and regulatory networks. J. Exp. Bot. 2012;63(4):1593–1608. doi: 10.1093/jxb/err460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chang HC, Tang YC, Hayer-Hartl M, Hartl FU. Snap Shot molecular chaperones Part I. Cell. 2007;128(1):212. doi: 10.1016/j.cell.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 41.Chen H, Je J, Song C, Hwang JE, Lim CO. A Proximal Promoter Region of Arabidopsis DREB2C Confers Tissue-specific Expression under Heat Stress. J. Integr. Plant Biol. 2012;54(9):640–651. doi: 10.1111/j.1744-7909.2012.01137.x. [DOI] [PubMed] [Google Scholar]
- 42.Sakuma Y, Maruyama K, Qin F, Osakabe Y, Shinozaki K, Yamaguchi-Shinozaki K. Dual function of an Arabidopsis transcription factor DREB2A in water-stress-responsive and heat-stress-responsive gene expression. Proc. Natl. Acad. Sci. USA. 2006;103(49):18822–18827. doi: 10.1073/pnas.0605639103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schramm F, Larkindale J, Kiehlmann E, Ganguli A, Englich G, Vierling E, KoskullκDöring V. A cascade of transcription factor DREB2A and heat stress transcription factor HsfA3 regulates the heat stress response of Arabidopsis. lant J. . 2008; 53(2):264–274. doi: 10.1111/j.1365-313X.2007.03334.x. [DOI] [PubMed] [Google Scholar]
- 44.Shahnejat-Bushehri S, ueller-Roeber B, Balazadeh S. Arabidopsis NAC transcription factor JUNGBRUNNEN1 affects thermomemory-associated genes and enhances heat stress tolerance in primed and unprimed conditions. Plant Signal. Behav. 2012;7(12):1518–1521. doi: 10.4161/psb.22092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Song Y, Chen Q, Ci D, Zhang D. Transcriptome profiling reveals differential transcript abundance in response to chilling stress in Populus simonii. Plant Cell Rep. 2013;32(9):1407–1425. doi: 10.1007/s00299-013-1454-x. [DOI] [PubMed] [Google Scholar]
- 46.Liu HT, Li B, Shang ZL, Li XZ, Mu RL, Sun DY, Zhou RG. Calmodulin is involved in heat shock signal transduction in wheat. Plant Physiol. 2003;132(3):1186–1195. doi: 10.1104/pp.102.018564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sheen J. Ca2+-dependent protein kinases and stress signal transduction in plants. Science. 1996;274(5294):1900–1902. doi: 10.1126/science.274.5294.1900. [DOI] [PubMed] [Google Scholar]
- 48.Hrabak EM, Chan CW, Gribskov M, Harper JF, Choi JH, Halford N, Kudla J, Luan S, Nimmo HG, Sussman MR, Thomas M, Walker-Simmons K, Zhu JK, Harmon AC. The Arabidopsis CDPK-SnRK superfamily of protein kinases. Plant Physiol. 2003;132(2):666–680. doi: 10.1104/pp.102.011999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Loser K, Weltring K. Induction of a polyubiquitin gene (ubi1) by potato phytoalexins and heat shock in Gibberella pulicaris. Curr. Genet. 1998;34(5):404–409. doi: 10.1007/s002940050414. [DOI] [PubMed] [Google Scholar]
- 50.Cheng L, Watt R, Piper PW. Polyubiquitin gene expression contributes to oxidative stress resistance in respiratory yeast (Saccharomyces cerevisiae). Mol. Gen. Genet. 1994;243(3):358–362. doi: 10.1007/BF00301072. [DOI] [PubMed] [Google Scholar]
- 51.Wang F, Deng XW. Plant ubiquitin-proteasome pathway and its role in gibberellin signaling. Cell Res. 2011;21(9):1286–1294. doi: 10.1038/cr.2011.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Song Y, Chen Q, Ci D, Zhang D. Effects of high temperature on photosynthesis and related gene expression in poplar. BMC Plant Biol. 2014;14:111. doi: 10.1186/1471-2229-14-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Levy-Holtzman R, Schechter I. Schistosome extracts with heat shock factor activity revealed by the gel shift assay. Parasito. 1994;108 ( Pt 1):35–42. doi: 10.1017/s0031182000078495. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material is available on the publisher’s web site along with the published article.