Abstract
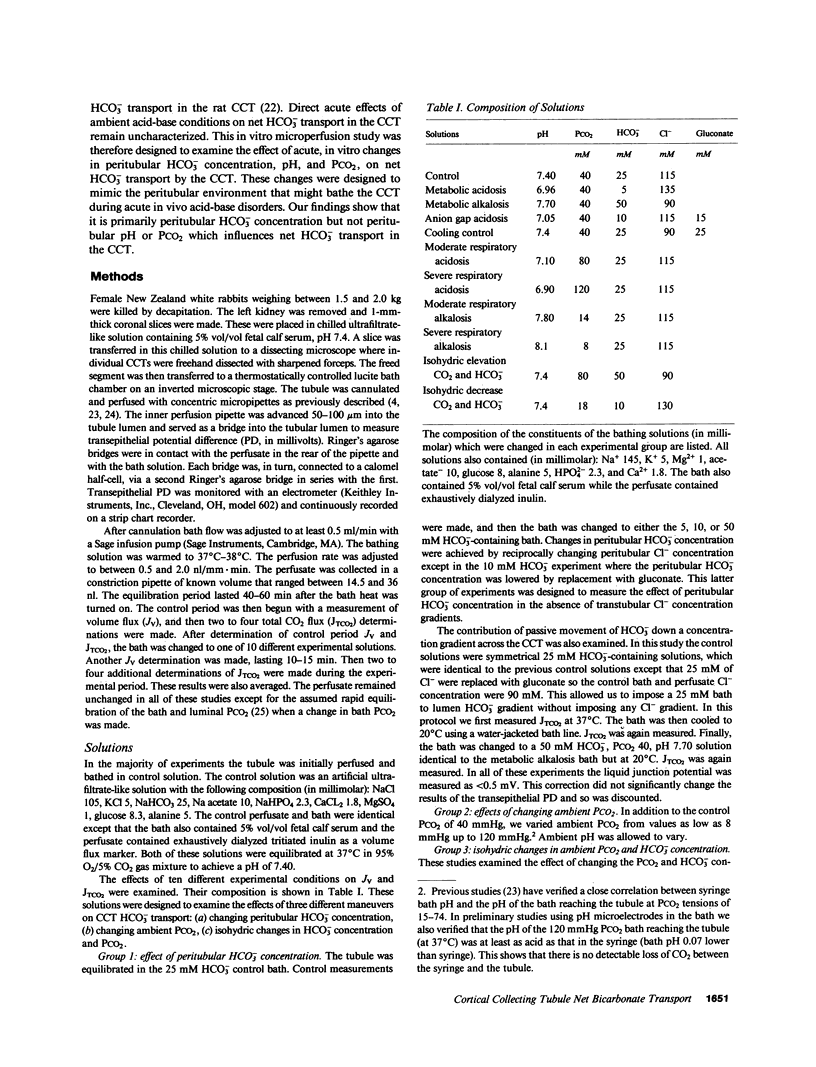
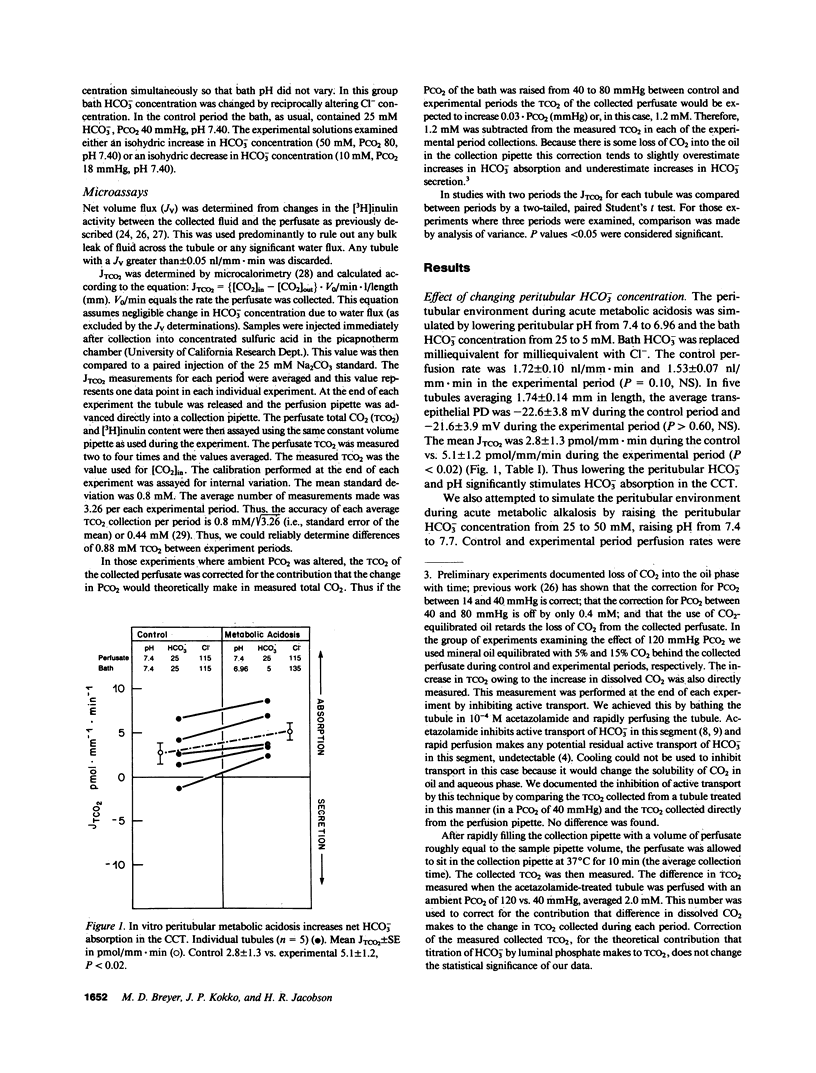
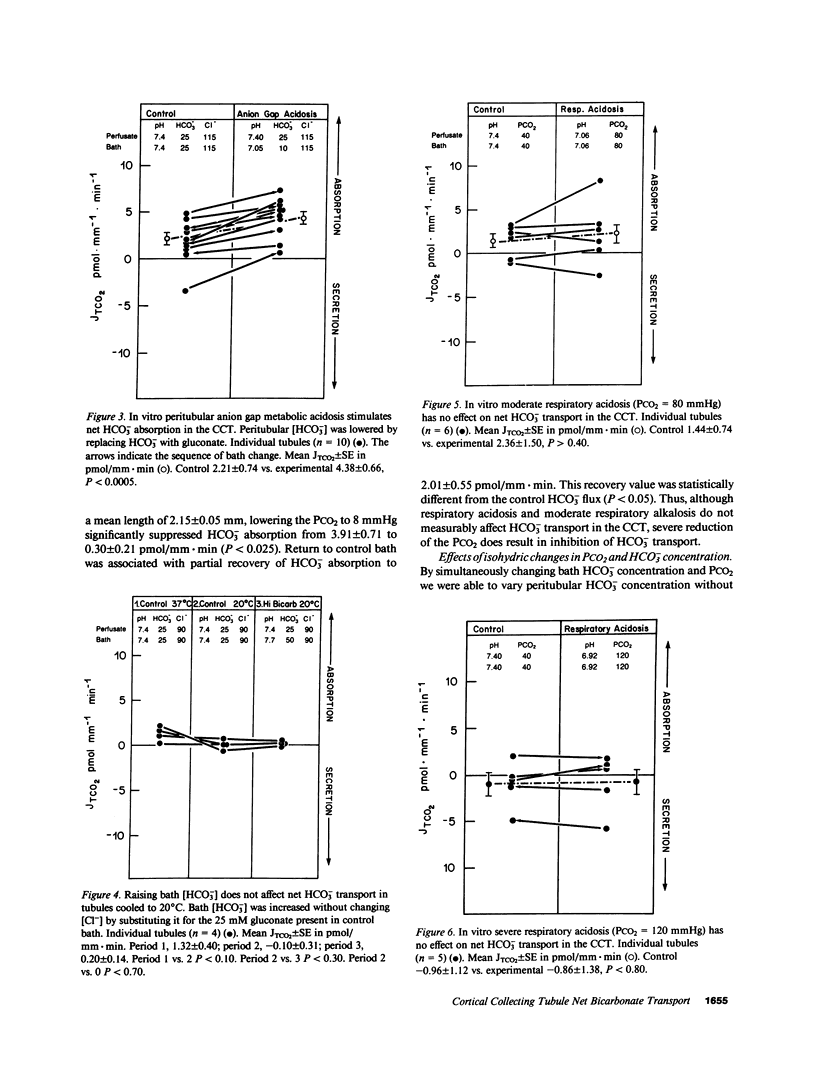
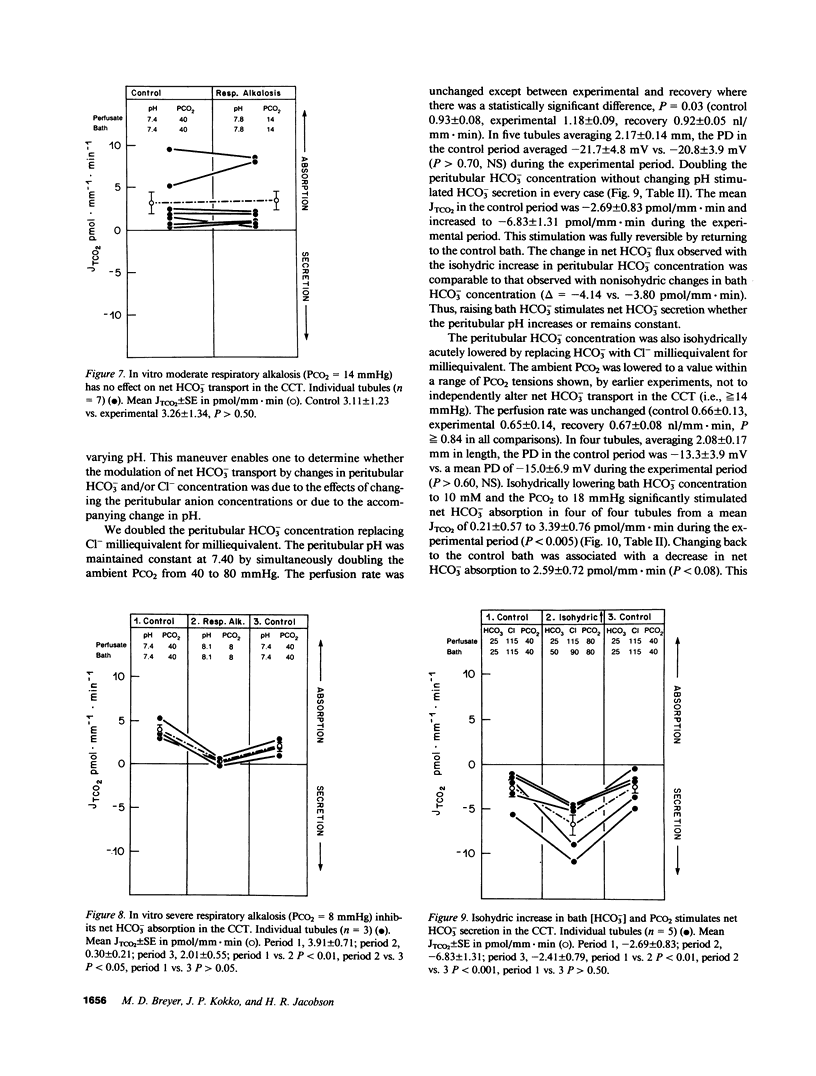
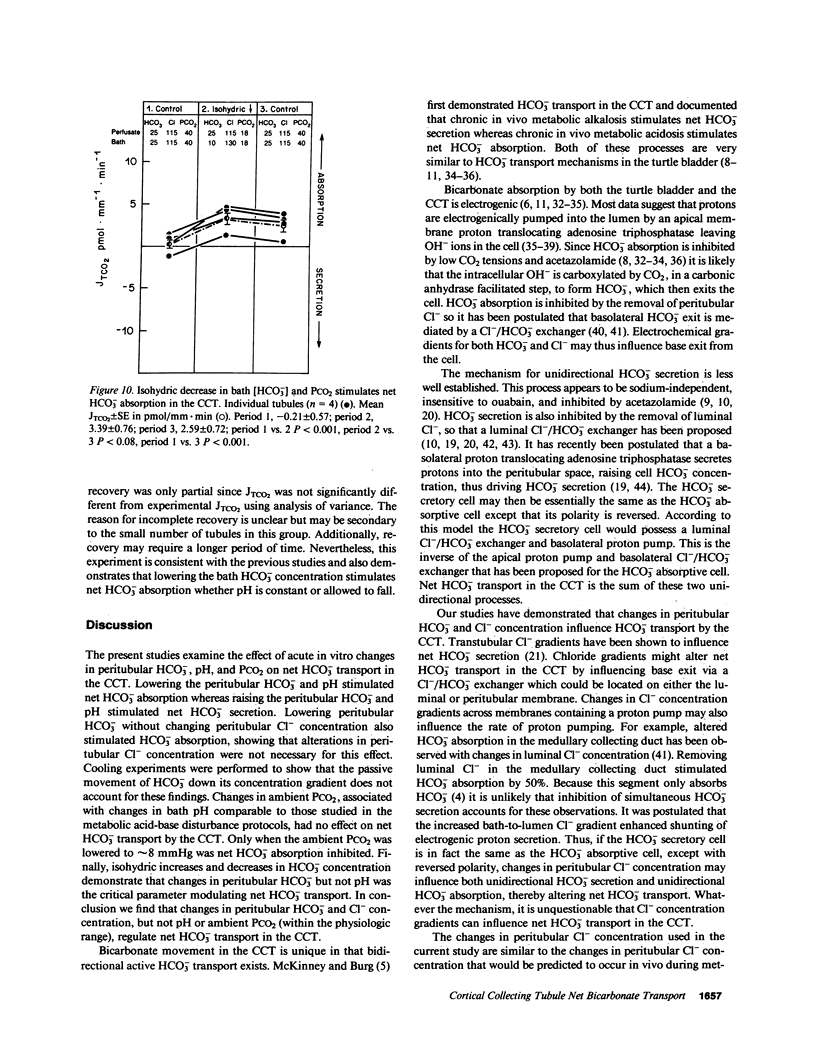
The effects of changes in peritubular pH, carbon dioxide tension (PCO2), and HCO3- concentration on net HCO3- transport was examined in in vitro perfused cortical collecting tubules (CCTs) from unpretreated New Zealand white rabbits. Lowering peritubular HCO3- concentration and pH by reciprocal replacement of HCO3- with Cl-, significantly stimulated net HCO3- absorption. Lowering peritubular HCO3- concentration and pH, by substitution of HCO3- with gluconate, while keeping Cl- concentration constant, also stimulated net HCO3- absorption. Raising peritubular HCO3- concentration and pH, by reciprocal replacement of Cl- with HCO3-, inhibited net HCO3- absorption (or stimulated net HCO3- secretion). When the tubule was cooled, raising peritubular HCO3- concentration had no effect on net HCO3- transport, suggesting these results are not due to the passive flux of HCO3- down its concentration gradient. The effect of changes in ambient PCO2 on net HCO3- transport were also studied. Increasing the ambient PCO2 from 40 mmHg to either 80 or 120 mmHg, allowing pH to fall, had no effect on net HCO3- transport. Similarly, lowering ambient PCO2 to 14 mmHg had no effect on net HCO3- transport. Simultaneously increasing peritubular HCO3- concentration and PCO2, without accompanying changes in peritubular pH, i.e., isohydric changes, stimulated net HCO3- secretion to the same degree as nonisohydric increases in peritubular HCO3- concentration. Likewise, isohydric lowering of peritubular HCO3- concentration and PCO2 stimulated net HCO3- absorption. We conclude that: acute changes in peritubular HCO3- concentration regulate acidification in the CCT and these effects are mediated by a transcellular process; acute changes in ambient PCO2 within the physiologic range have no effect on HCO3- transport in the in vitro perfused CCT; and acute in vitro regulation of CCT acidification is independent of peritubular pH.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Al-Awqati Q. H + transport in urinary epithelia. Am J Physiol. 1978 Aug;235(2):F77–F88. doi: 10.1152/ajprenal.1978.235.2.F77. [DOI] [PubMed] [Google Scholar]
- Al-awqati Q., Mueller A., Steinmetz P. R. Transport of H+ against electrochemical gradients in turtle urinary bladder. Am J Physiol. 1977 Dec;233(6):F502–F508. doi: 10.1152/ajprenal.1977.233.6.F502. [DOI] [PubMed] [Google Scholar]
- BARKER E. S., SINGER R. B., ELKINTON J. R., CLARK J. K. The renal response in man to acute experimental respiratory alkalosis and acidosis. J Clin Invest. 1957 Apr;36(4):515–529. doi: 10.1172/JCI103449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burg M., Grantham J., Abramow M., Orloff J. Preparation and study of fragments of single rabbit nephrons. Am J Physiol. 1966 Jun;210(6):1293–1298. doi: 10.1152/ajplegacy.1966.210.6.1293. [DOI] [PubMed] [Google Scholar]
- Cohen L. H., Steinmetz P. R. Control of active proton transport in turtle urinary bladder by cell pH. J Gen Physiol. 1980 Sep;76(3):381–393. doi: 10.1085/jgp.76.3.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen L. HCO3-Cl exchange transport in the adaptive response to alkalosis by turtle bladder. Am J Physiol. 1980 Aug;239(2):F167–F174. doi: 10.1152/ajprenal.1980.239.2.F167. [DOI] [PubMed] [Google Scholar]
- Dobyan D. C., Bulger R. E. Renal carbonic anhydrase. Am J Physiol. 1982 Oct;243(4):F311–F324. doi: 10.1152/ajprenal.1982.243.4.F311. [DOI] [PubMed] [Google Scholar]
- DuBose T. D., Jr, Pucacco L. R., Seldin D. W., Carter N. W. Direct determination of PCO2 in the rat renal cortex. J Clin Invest. 1978 Aug;62(2):338–348. doi: 10.1172/JCI109134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer J. L., Husted R. F., Steinmetz P. R. Chloride dependence of the HCO3 exit step in urinary acidification by the turtle bladder. Am J Physiol. 1983 Nov;245(5 Pt 1):F564–F568. doi: 10.1152/ajprenal.1983.245.5.F564. [DOI] [PubMed] [Google Scholar]
- Gluck S., Al-Awqati Q. An electrogenic proton-translocating adenosine triphosphatase from bovine kidney medulla. J Clin Invest. 1984 Jun;73(6):1704–1710. doi: 10.1172/JCI111378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluck S., Cannon C., Al-Awqati Q. Exocytosis regulates urinary acidification in turtle bladder by rapid insertion of H+ pumps into the luminal membrane. Proc Natl Acad Sci U S A. 1982 Jul;79(14):4327–4331. doi: 10.1073/pnas.79.14.4327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluck S., Kelly S., Al-Awqati Q. The proton translocating ATPase responsible for urinary acidification. J Biol Chem. 1982 Aug 25;257(16):9230–9233. [PubMed] [Google Scholar]
- Hogg R. J., Pucacco L. R., Carter N. W., Laptook A. R., Kokko J. P. In situ PCO2 in the renal cortex, liver, muscle, and brain of the New Zealand white rabbit. Am J Physiol. 1984 Sep;247(3 Pt 2):F491–F498. doi: 10.1152/ajprenal.1984.247.3.F491. [DOI] [PubMed] [Google Scholar]
- Holmberg C., Kokko J. P., Jacobson H. R. Determination of chloride and bicarbonate permeabilities in proximal convoluted tubules. Am J Physiol. 1981 Oct;241(4):F386–F394. doi: 10.1152/ajprenal.1981.241.4.F386. [DOI] [PubMed] [Google Scholar]
- Jacobson H. R. Effects of CO2 and acetazolamide on bicarbonate and fluid transport in rabbit proximal tubules. Am J Physiol. 1981 Jan;240(1):F54–F62. doi: 10.1152/ajprenal.1981.240.1.F54. [DOI] [PubMed] [Google Scholar]
- Jacobson H. R. Medullary collecting duct acidification. Effects of potassium, HCO3 concentration, and pCO2. J Clin Invest. 1984 Dec;74(6):2107–2114. doi: 10.1172/JCI111635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koeppen B. M., Helman S. I. Acidification of luminal fluid by the rabbit cortical collecting tubule perfused in vitro. Am J Physiol. 1982 May;242(5):F521–F531. doi: 10.1152/ajprenal.1982.242.5.F521. [DOI] [PubMed] [Google Scholar]
- LEMANN J., Jr, LENNON E. J., GOODMAN A. D., LITZOW J. R., RELMAN A. S. THE NET BALANCE OF ACID IN SUBJECTS GIVEN LARGE LOADS OF ACID OR ALKALI. J Clin Invest. 1965 Apr;44:507–517. doi: 10.1172/JCI105164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laski M. E., Kurtzman N. A. Characterization of acidification in the cortical and medullary collecting tubule of the rabbit. J Clin Invest. 1983 Dec;72(6):2050–2059. doi: 10.1172/JCI111170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laski M. E., Warnock D. G., Rector F. C., Jr Effects of chloride gradients on total CO2 flux in the rabbit cortical collecting tubule. Am J Physiol. 1983 Feb;244(2):F112–F121. doi: 10.1152/ajprenal.1983.244.2.F112. [DOI] [PubMed] [Google Scholar]
- LeFurgey A., Tisher C. C. Morphology of rabbit collecting duct. Am J Anat. 1979 May;155(1):111–124. doi: 10.1002/aja.1001550108. [DOI] [PubMed] [Google Scholar]
- Leslie B. R., Schwartz J. H., Steinmetz P. R. Coupling between Cl- absorption and HCO3- secretion in turtle urinary bladder. Am J Physiol. 1973 Sep;225(3):610–617. doi: 10.1152/ajplegacy.1973.225.3.610. [DOI] [PubMed] [Google Scholar]
- Lombard W. E., Kokko J. P., Jacobson H. R. Bicarbonate transport in cortical and outer medullary collecting tubules. Am J Physiol. 1983 Mar;244(3):F289–F296. doi: 10.1152/ajprenal.1983.244.3.F289. [DOI] [PubMed] [Google Scholar]
- Lucci M. S., Pucacco L. R., Carter N. W., DuBose T. D., Jr Direct evaluation of the permeability of the rat proximal convoluted tubule to CO2. Am J Physiol. 1982 May;242(5):F470–F476. doi: 10.1152/ajprenal.1982.242.5.F470. [DOI] [PubMed] [Google Scholar]
- McKinney T. D., Burg M. B. Bicarbonate absorption by rabbit cortical collecting tubules in vitro. Am J Physiol. 1978 Feb;234(2):F141–F145. doi: 10.1152/ajprenal.1978.234.2.F141. [DOI] [PubMed] [Google Scholar]
- McKinney T. D., Burg M. B. Bicarbonate secretion by rabbit cortical collecting tubules in vitro. J Clin Invest. 1978 Jun;61(6):1421–1427. doi: 10.1172/JCI109061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinney T. D., Burg M. B. Bicarbonate transport by rabbit cortical collecting tubules. Effect of acid and alkali loads in vivo on transport in vitro. J Clin Invest. 1977 Sep;60(3):766–768. doi: 10.1172/JCI108830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansom S. C., Weinman E. J., O'Neil R. G. Microelectrode assessment of chloride-conductive properties of cortical collecting duct. Am J Physiol. 1984 Aug;247(2 Pt 2):F291–F302. doi: 10.1152/ajprenal.1984.247.2.F291. [DOI] [PubMed] [Google Scholar]
- Sartorius O. W., Roemmelt J. C., Pitts R. F., Calhoon D., Miner P. THE RENAL REGULATION OF ACID-BASE BALANCE IN MAN. IV. THE NATURE OF THE RENAL COMPENSATIONS IN AMMONIUM CHLORIDE ACIDOSIS. J Clin Invest. 1949 May;28(3):423–439. doi: 10.1172/JCI102087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster V. L. Cyclic adenosine monophosphate-stimulated bicarbonate secretion in rabbit cortical collecting tubules. J Clin Invest. 1985 Jun;75(6):2056–2064. doi: 10.1172/JCI111925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz G. J., Al-Awqati Q. Carbon dioxide causes exocytosis of vesicles containing H+ pumps in isolated perfused proximal and collecting tubules. J Clin Invest. 1985 May;75(5):1638–1644. doi: 10.1172/JCI111871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz J. H., Bethencourt D., Rosen S. Specialized function of carbonic anhydrase-rich and granular cells of turtle bladder. Am J Physiol. 1982 Jun;242(6):F627–F633. doi: 10.1152/ajprenal.1982.242.6.F627. [DOI] [PubMed] [Google Scholar]
- Schwartz J. H. H+ current response to CO2 and carbonic anhydrase inhibition in turtle bladder. Am J Physiol. 1976 Aug;231(2):565–572. doi: 10.1152/ajplegacy.1976.231.2.565. [DOI] [PubMed] [Google Scholar]
- Steinmetz P. R. Cellular mechanisms of urinary acidification. Physiol Rev. 1974 Oct;54(4):890–956. doi: 10.1152/physrev.1974.54.4.890. [DOI] [PubMed] [Google Scholar]
- Stone D. K., Seldin D. W., Kokko J. P., Jacobson H. R. Anion dependence of rabbit medullary collecting duct acidification. J Clin Invest. 1983 May;71(5):1505–1508. doi: 10.1172/JCI110905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone D. K., Seldin D. W., Kokko J. P., Jacobson H. R. Mineralocorticoid modulation of rabbit medullary collecting duct acidification. A sodium-independent effect. J Clin Invest. 1983 Jul;72(1):77–83. doi: 10.1172/JCI110986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vurek G. G., Warnock D. G., Corsey R. Measurement of picomole amounts of carbon dioxide by calorimetry. Anal Chem. 1975 Apr;47(4):765–767. doi: 10.1021/ac60354a024. [DOI] [PubMed] [Google Scholar]



