Abstract
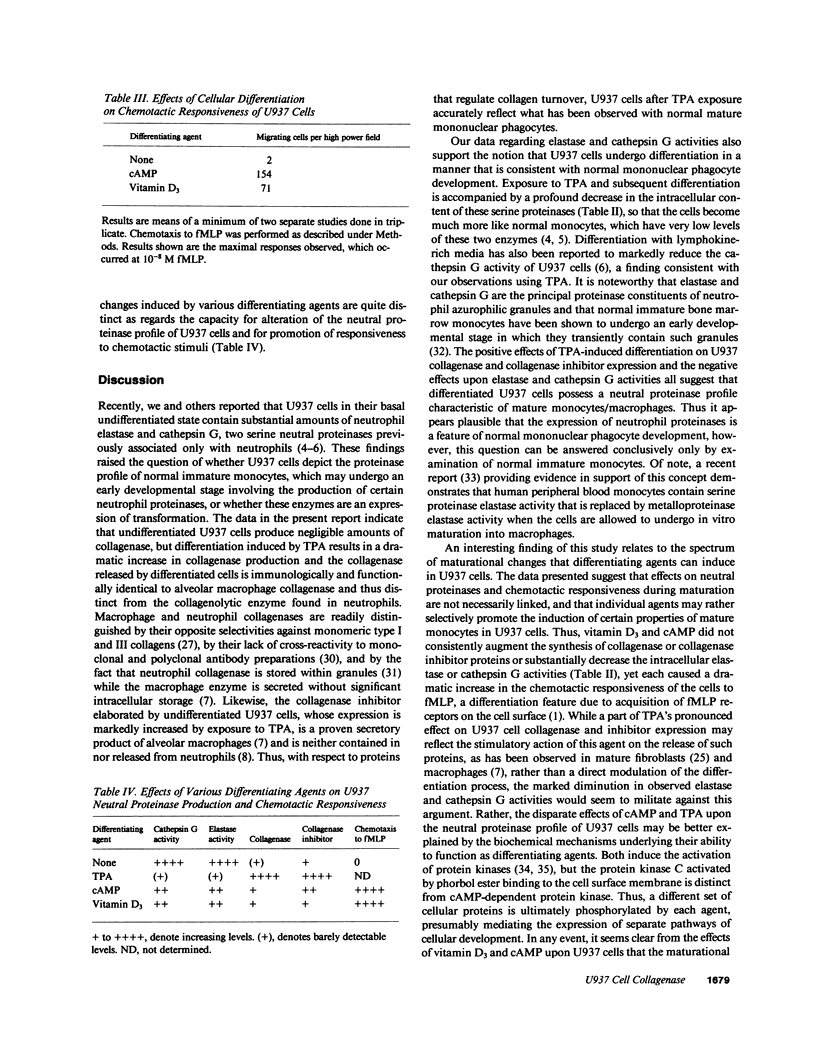
Human monocytic tumor cells of the U937 cell line contain substantial quantities of two neutrophil neutral proteinases, elastase and cathepsin G, raising the question of whether their presence reflects an expression of transformation or whether normal monocytes undergo a developmental stage in which they produce certain neutrophil proteinases. To address this issue, we examined U937 cells for production of collagenase, since human alveolar macrophages release fibroblast-like collagenase, an enzyme that is distinct from neutrophil collagenase. Using an immunoassay that utilized antibody to skin fibroblast collagenase, we found that U937 cells secreted barely detectable quantities of enzyme, 10-12 ng/10(6) cells per 24 h, under basal conditions. Upon incubation with 10 nM 12-o-tetradecanoyl-phorbol-13-acetate (TPA), however, collagenase release increased 200-fold, comparable to the amount secreted by phorbol-stimulated human fibroblasts. Metabolic labeling and immunoprecipitation confirmed the enhanced synthesis of U937 cell collagenase upon TPA exposure. This enzyme activity further resembled fibroblast collagenase and differed from neutrophil collagenase by exhibiting preferential cleavage of monomeric type III collagen relative to type I. As previously observed with human alveolar macrophages, U937 cells also released a protein identical to the collagenase inhibitor produced by human skin fibroblasts, a molecule not associated with neutrophils. Release of this inhibitor increased 10-fold with TPA exposure. In contrast to collagenase and collagense inhibitor, TPA-treated U937 cells contained only 10-15% as much elastase and cathepsin G activities as control cells. Thus, TPA-induced differentiation modified the presence of these enzymes in the direction of their content in normal monocytes. Since the neutral proteinase profile of undifferentiated U937 cells resembles that of neutrophils and changes markedly after cellular differentiation to one that is characteristic of monocytes, these data suggest that neutrophilic proteinases may be produced by normal monocytes during the early stages of their differentiation.
Full text
PDF

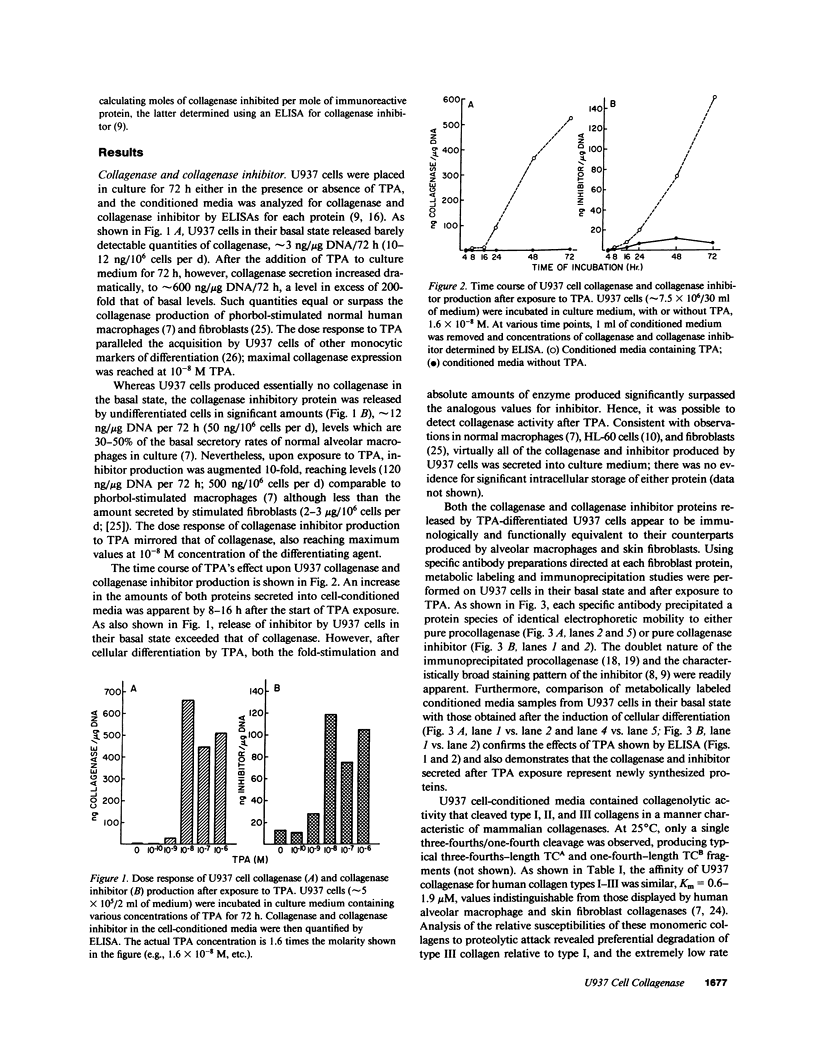
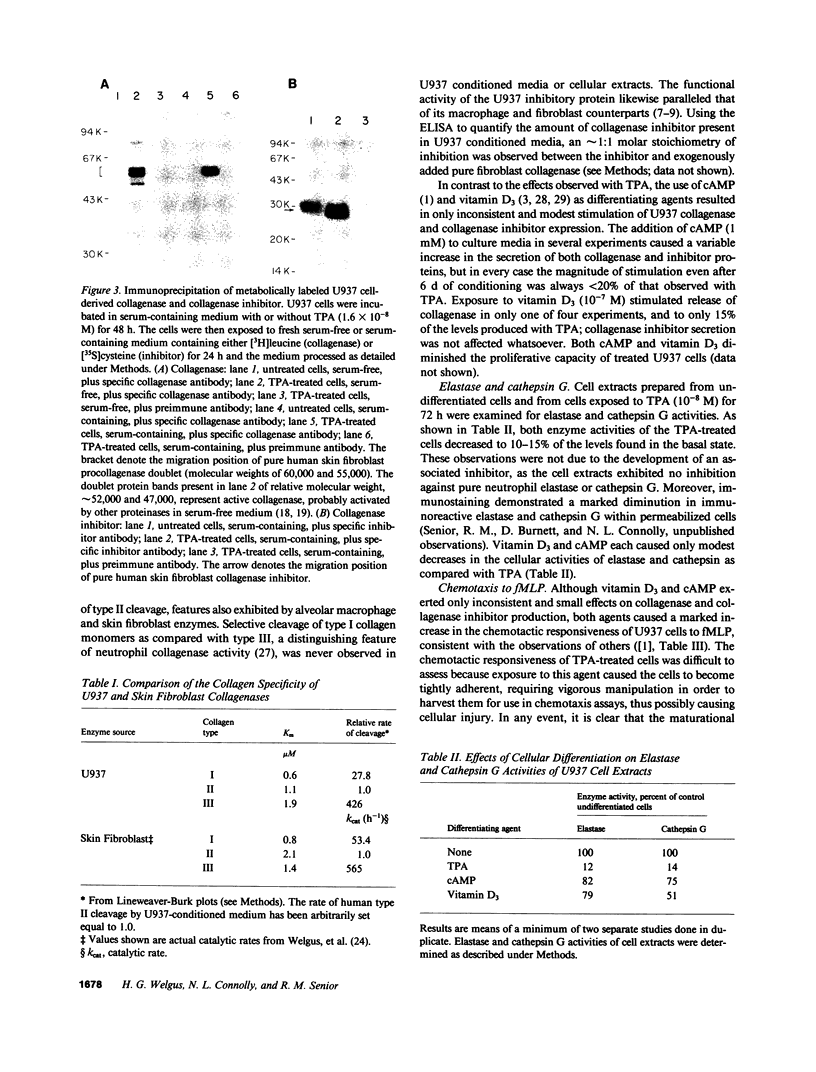


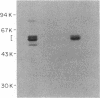
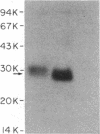
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amento E. P., Bhalla A. K., Kurnick J. T., Kradin R. L., Clemens T. L., Holick S. A., Holick M. F., Krane S. M. 1 alpha,25-dihydroxyvitamin D3 induces maturation of the human monocyte cell line U937, and, in association with a factor from human T lymphocytes, augments production of the monokine, mononuclear cell factor. J Clin Invest. 1984 Mar;73(3):731–739. doi: 10.1172/JCI111266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Shavit Z., Teitelbaum S. L., Stricklin G. P., Eisen A. Z., Kahn A. J., Welgus H. G. Differentiation of a human leukemia cell line and expression of collagenase inhibitor. Proc Natl Acad Sci U S A. 1985 Aug;82(16):5380–5384. doi: 10.1073/pnas.82.16.5380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baugh R. J., Travis J. Human leukocyte granule elastase: rapid isolation and characterization. Biochemistry. 1976 Feb 24;15(4):836–841. doi: 10.1021/bi00649a017. [DOI] [PubMed] [Google Scholar]
- Bielefeld D. R., Senior R. M., Yu S. Y. A new method for determination of elastolytic activity using (14C) labeled elastin and its application to leukocytic elastase. Biochem Biophys Res Commun. 1975 Dec 15;67(4):1553–1559. doi: 10.1016/0006-291x(75)90203-x. [DOI] [PubMed] [Google Scholar]
- Cabot M. C., Jaken S. Structural and chemical specificity of diacylglycerols for protein kinase C activation. Biochem Biophys Res Commun. 1984 Nov 30;125(1):163–169. doi: 10.1016/s0006-291x(84)80349-6. [DOI] [PubMed] [Google Scholar]
- Campbell E. J., White R. R., Senior R. M., Rodriguez R. J., Kuhn C. Receptor-mediated binding and internalization of leukocyte elastase by alveolar macrophages in vitro. J Clin Invest. 1979 Sep;64(3):824–833. doi: 10.1172/JCI109530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark S. D., Wilhelm S. M., Stricklin G. P., Welgus H. G. Coregulation of collagenase and collagenase inhibitor production by phorbol myristate acetate in human skin fibroblasts. Arch Biochem Biophys. 1985 Aug 15;241(1):36–44. doi: 10.1016/0003-9861(85)90358-3. [DOI] [PubMed] [Google Scholar]
- Cooper T. W., Bauer E. A., Eisen A. Z. Enzyme-linked immunosorbent assay for human skin collagenase. Coll Relat Res. 1983 May;3(3):205–215. doi: 10.1016/s0174-173x(83)80004-1. [DOI] [PubMed] [Google Scholar]
- DelMar E. G., Largman C., Brodrick J. W., Geokas M. C. A sensitive new substrate for chymotrypsin. Anal Biochem. 1979 Nov 1;99(2):316–320. doi: 10.1016/s0003-2697(79)80013-5. [DOI] [PubMed] [Google Scholar]
- GROSS J., LAPIERE C. M. Collagenolytic activity in amphibian tissues: a tissue culture assay. Proc Natl Acad Sci U S A. 1962 Jun 15;48:1014–1022. doi: 10.1073/pnas.48.6.1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasty K. A., Hibbs M. S., Kang A. H., Mainardi C. L. Heterogeneity among human collagenases demonstrated by monoclonal antibody that selectively recognizes and inhibits human neutrophil collagenase. J Exp Med. 1984 May 1;159(5):1455–1463. doi: 10.1084/jem.159.5.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz A. L., Hance A. J., Crystal R. G. Granulocyte collagenase: selective digestion of type I relative to type III collagen. Proc Natl Acad Sci U S A. 1977 Mar;74(3):897–901. doi: 10.1073/pnas.74.3.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalb V. F., Jr, Bernlohr R. W. A new spectrophotometric assay for protein in cell extracts. Anal Biochem. 1977 Oct;82(2):362–371. doi: 10.1016/0003-2697(77)90173-7. [DOI] [PubMed] [Google Scholar]
- Kay G. E., Lane B. C., Snyderman R. Induction of selective biological responses to chemoattractants in a human monocyte-like cell line. Infect Immun. 1983 Sep;41(3):1166–1174. doi: 10.1128/iai.41.3.1166-1174.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King J., Laemmli U. K. Polypeptides of the tail fibres of bacteriophage T4. J Mol Biol. 1971 Dec 28;62(3):465–477. doi: 10.1016/0022-2836(71)90148-3. [DOI] [PubMed] [Google Scholar]
- Louie J. S., Weiss J., Ryhänen L., Nies K. M., Rantala-Ryhänen S., Uitto J. The production of collagenase by adherent mononuclear cells cultured from human peripheral blood. Arthritis Rheum. 1984 Dec;27(12):1397–1404. doi: 10.1002/art.1780271210. [DOI] [PubMed] [Google Scholar]
- Martodam R. R., Baugh R. J., Twumasi D. Y., Liener I. E. A rapid procedure for the large scale purification of elastase and cathepsin G from human sputum. Prep Biochem. 1979;9(1):15–31. doi: 10.1080/00327487908061669. [DOI] [PubMed] [Google Scholar]
- Minta J. O., Pambrun L. In vitro induction of cytologic and functional differentiation of the immature human monocytelike cell line U-937 with phorbol myristate acetate. Am J Pathol. 1985 Apr;119(1):111–126. [PMC free article] [PubMed] [Google Scholar]
- Murphy G., Reynolds J. J., Bretz U., Baggiolini M. Collagenase is a component of the specific granules of human neutrophil leucocytes. Biochem J. 1977 Jan 15;162(1):195–197. doi: 10.1042/bj1620195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai Y., Lapiere C. M., Gross J. Tadpole collagenase. Preparation and purification. Biochemistry. 1966 Oct;5(10):3123–3130. doi: 10.1021/bi00874a007. [DOI] [PubMed] [Google Scholar]
- Nichols B. A., Bainton D. F., Farquhar M. G. Differentiation of monocytes. Origin, nature, and fate of their azurophil granules. J Cell Biol. 1971 Aug;50(2):498–515. doi: 10.1083/jcb.50.2.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson I., Gullberg U., Ivhed I., Nilsson K. Induction of differentiation of the human histiocytic lymphoma cell line U-937 by 1 alpha,25-dihydroxycholecalciferol. Cancer Res. 1983 Dec;43(12 Pt 1):5862–5867. [PubMed] [Google Scholar]
- Rigby W. F., Shen L., Ball E. D., Guyre P. M., Fanger M. W. Differentiation of a human monocytic cell line by 1,25-dihydroxyvitamin D3 (calcitriol): a morphologic, phenotypic, and functional analysis. Blood. 1984 Nov;64(5):1110–1115. [PubMed] [Google Scholar]
- Robert A., Grunberger G., Carpenter J. L., Dayer J. M., Orci L., Gorden P. The insulin receptor of a human monocyte-like cell line: characterization and function. Endocrinology. 1984 Jan;114(1):247–253. doi: 10.1210/endo-114-1-247. [DOI] [PubMed] [Google Scholar]
- Sandhaus R. A., McCarthy K. M., Musson R. A., Henson P. M. Elastolytic proteinases of the human macrophage. Chest. 1983 May;83(5 Suppl):60S–62S. doi: 10.1378/chest.83.5_supplement.60s. [DOI] [PubMed] [Google Scholar]
- Senior R. M., Campbell E. J. Cathepsin G in human mononuclear phagocytes: comparisons between monocytes and U937 monocyte-like cells. J Immunol. 1984 May;132(5):2547–2551. [PubMed] [Google Scholar]
- Senior R. M., Campbell E. J., Landis J. A., Cox F. R., Kuhn C., Koren H. S. Elastase of U-937 monocytelike cells. Comparisons with elastases derived from human monocytes and neutrophils and murine macrophagelike cells. J Clin Invest. 1982 Feb;69(2):384–393. doi: 10.1172/JCI110462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senior R. M., Griffin G. L., Mecham R. P. Chemotactic activity of elastin-derived peptides. J Clin Invest. 1980 Oct;66(4):859–862. doi: 10.1172/JCI109926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder R. A., Kaempfer C. E., Wintroub B. U. Chemistry of a human monocyte-derived cell line (U937): identification of the angiotensin I-converting activity as leukocyte cathepsin G. Blood. 1985 Jan;65(1):176–182. [PubMed] [Google Scholar]
- Stricklin G. P., Bauer E. A., Jeffrey J. J., Eisen A. Z. Human skin collagenase: isolation of precursor and active forms from both fibroblast and organ cultures. Biochemistry. 1977 Apr 19;16(8):1607–1615. doi: 10.1021/bi00627a013. [DOI] [PubMed] [Google Scholar]
- Stricklin G. P., Eisen A. Z., Bauer E. A., Jeffrey J. J. Human skin fibroblast collagenase: chemical properties of precursor and active forms. Biochemistry. 1978 Jun 13;17(12):2331–2337. doi: 10.1021/bi00605a012. [DOI] [PubMed] [Google Scholar]
- Stricklin G. P., Welgus H. G. Human skin fibroblast collagenase inhibitor. Purification and biochemical characterization. J Biol Chem. 1983 Oct 25;258(20):12252–12258. [PubMed] [Google Scholar]
- Vandenbark G. R., Kuhn L. J., Niedel J. E. Possible mechanism of phorbol diester-induced maturation of human promyelocytic leukemia cells. J Clin Invest. 1984 Feb;73(2):448–457. doi: 10.1172/JCI111231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl L. M., Wahl S. M., Mergenhagen S. E., Martin G. R. Collagenase production by endotoxin-activated macrophages. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3598–3601. doi: 10.1073/pnas.71.9.3598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welgus H. G., Campbell E. J., Bar-Shavit Z., Senior R. M., Teitelbaum S. L. Human alveolar macrophages produce a fibroblast-like collagenase and collagenase inhibitor. J Clin Invest. 1985 Jul;76(1):219–224. doi: 10.1172/JCI111949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welgus H. G., Jeffrey J. J., Eisen A. Z. The collagen substrate specificity of human skin fibroblast collagenase. J Biol Chem. 1981 Sep 25;256(18):9511–9515. [PubMed] [Google Scholar]
- Welgus H. G., Stricklin G. P. Human skin fibroblast collagenase inhibitor. Comparative studies in human connective tissues, serum, and amniotic fluid. J Biol Chem. 1983 Oct 25;258(20):12259–12264. [PubMed] [Google Scholar]
- Werb Z., Gordon S. Secretion of a specific collagenase by stimulated macrophages. J Exp Med. 1975 Aug 1;142(2):346–360. doi: 10.1084/jem.142.2.346. [DOI] [PMC free article] [PubMed] [Google Scholar]