Abstract
Background
Clinical differentiation of influenza from dengue and other febrile illnesses (OFI) is difficult, and available rapid diagnostic tests have limited sensitivity.
Methods
We conducted a retrospective study to compare clinical and laboratory findings between (i) influenza and dengue and (ii) influenza and OFI.
Results
Of 849 enrolled patients, the mean time between illness onset and hospital presentation was 1.7, 3.7, and 3 days for influenza, dengue, and OFI, respectively. Among pediatric patients (≤18 years) (445 influenza, 24 dengue, and 130 OFI), we identified absence of rashes, no leukopenia, and no marked thrombocytopenia (platelet counts <100 × 109 cells/L) as predictors to distinguish influenza from dengue, whereas rhinorrhea, malaise, sore throat, and mild thrombocytopenia (platelet counts 100-149 × 109/L) were predictors that differentiated influenza from OFI. Among adults (>18 years) (81 influenza, 124 dengue, and 45 OFI), no leukopenia and no marked thrombocytopenia distinguished influenza from dengue, while rhinorrhea and malaise differentiated influenza from OFI. A diagnostic algorithm developed to distinguish influenza from dengue using rash, leukopenia, and marked thrombocytopenia showed >90% sensitivity to identify influenza in pediatric patients.
Conclusions
This study identified simple clinical and laboratory parameters that can assist clinicians to distinguish influenza from dengue and OFI. These findings may help clinicians diagnose influenza and facilitate appropriate management of affected patients, particularly in resource-poor settings.
Electronic supplementary material
The online version of this article (doi:10.1186/s12879-014-0623-z) contains supplementary material, which is available to authorized users.
Keywords: Influenza, Dengue, Other febrile illnesses
Background
Influenza is one of the most common infectious diseases worldwide [1]. The spectrum of clinical manifestations ranges from mild-form nonspecific febrile illness, such as cough, sore throat, headache, rhinorrhea, malaise, and muscle ache, to respiratory failure and death [2]-[4]. Its symptoms and signs can be similar to other viral illnesses such as dengue, making the infections difficult to distinguish. The clinical manifestations of dengue illness vary greatly, ranging from a mild, flu-like, and self-limited febrile illness to severe dengue [5],[6]. Influenza and dengue overlap geographically in tropical and subtropical regions of the world [1],[7]. Concurrent influenza and dengue outbreaks have been reported [8]-[11]. The overlapping clinical features of influenza and dengue created clinical diagnosis and management challenges during simultaneous influenza and dengue outbreaks in Puerto Rico in 1977 [11]. Dengue epidemics have occurred in Taiwan for decades [12],[13]. An outbreak of 2009 pandemic H1N1 occurred in Taiwan after the first case was identified on May 20, 2009 [14]. The overlapping symptoms and signs of influenza and dengue created a confusing clinical situation and challenges in etiology identification. Although rapid diagnostic tests can help confirm an influenza diagnosis, their sensitivity ranges from 40-70% depending on the day of illness and specimen type [15]. Definitive diagnosis of dengue is made using serology tests, but these tests are not always readily available in most clinical laboratories [7],[16]. Our study aimed to identify clinical and laboratory features that distinguish influenza from dengue and other febrile illnesses (OFI) in dengue and non-dengue endemic settings. We applied decision tree analysis to our dataset to discriminate patients with influenza from those with dengue. Our findings may be valuable for clinicians working in crowded emergency rooms (ER) in countries with limited medical resources.
Methods
Ethics statement
The study was approved by the Institutional Review Board of Kaohsiung Chang Gung Memorial Hospital (KSCGMH) (Document no. 102-4695B). Informed consent was not required as the data were analyzed anonymously.
Study population, diagnosis and definition
We conducted a retrospective study of 849 febrile patients (ear temperature ≥38°C) who presented during 2008 and 2010 with possible dengue and influenza infections to the ER at KSCGMH, a 2,600-bed primary care and tertiary referral medical center in southern Taiwan. All patient medical records were reviewed, and clinical and laboratory data at the time of hospital presentation were extracted for analysis.
Patients with influenza infections were defined as those laboratory-positive for influenza with influenza-like illness. Influenza-like illness was defined according to World Health Organization (WHO) guidelines: fever, cough, or sore throat [17]. Laboratory diagnosis of influenza was made for patient's respiratory specimens (nasopharyngeal or pharyngeal swabs) positive for virus-specific ribonucleic acid by real-time reverse transcriptase-polymerase chain reaction (RT-PCR) (TAIGEN Bioscience Corporation, Taiwan) [18]. All PCRs were performed at the central testing laboratory of KSCGMH using the standard real-time RT-PCR influenza procedure described by the Centers for Disease Control and Prevention [19].
All dengue cases included in this study were confirmed by at least one of the following criteria: (i) positive dengue virus-specific real-time RT-PCR (QuantiTect SYBR Green RT-PCR kit; Qiagen, Hilden, Germany) [20], (ii) positive dengue virus-specific immunoglobulin (Ig) M antibody enzyme-linked immunosorbent assay of acute-phase serum, after excluding cross-reactions to Japanese encephalitis virus [21], (iii) a fourfold increase in dengue virus-specific IgG antibody in convalescent serum compared to the acute phase, and (iv) acute-phase serum positive for dengue virus-specific nonstructural glycoprotein-1 (NS1) antigen (Bio-Rad Laboratories, Marnes-la-Coquette, France) [22],[23]. Dengue diagnostic tests were performed by the Centers for Disease Control and Prevention in Taiwan. Dengue patients in this study were classified as dengue fever without warning signs, dengue fever with warning signs and severe dengue based on 2009 WHO case definitions [7]. Patients who did not meet the laboratory diagnosis criteria for influenza and dengue were classified as OFI in our analyses.
Leukopenia was defined as a peripheral white cell count <3.0 × 109 cells/L (reference value, 3.0-10.5 × 109 cells/L), mild thrombocytopenia as a peripheral platelet count of 149-100 × 109 cells/L, and marked thrombocytopenia as a peripheral platelet count <100 × 109 cells/L. Acute hepatitis referred to serum alanine aminotransferase (ALT) levels greater than 1,000 U/L (reference value, <40 U/L).
Statistical analysis
Patients were categorized as having (i) influenza, (ii) dengue, or (iii) OFI. To distinguish influenza from dengue or OFI in a dengue and non-dengue endemic settings, univariate analyses were performed to compare clinical and laboratory characteristics between (i) influenza and dengue, and (ii) influenza and OFI. Categorical variables were compared using Chi-square or Fisher exact tests, and continuous variables were compared using Student's t or Mann-Whitney U tests. A 2-tailed P <0.05 was considered statistically significant. Significant variables in the univariate analyses were entered into a multivariate logistic regression model to determine independent predictor(s) of (i) influenza versus dengue, and (ii) influenza versus OFI. The sensitivity and specificity of each significant variable was assessed. Classification and regression tree analysis was performed [24]. The variables found to be significant in multivariate logistic regression with sensitivity greater than 80% were used to establish diagnostic decision trees to distinguish between patients with influenza and those with dengue [24]. Diagnosis was achieved by stepwise binary splitting, where one variable was entered at each node and, depending on the answer, a branch of the tree containing another variable was followed. Splitting stopped at nodes with a minimum classification of dengue, maximum classification of influenza, or a small number of patients in the node. For each terminal node, patients were classified as having low or high probability for influenza infection. To investigate the impact of age on the clinical presentation of influenza, dengue, and OFI, stratified analyses were performed for pediatric (≤18 years) and adult (>18 years) patients to examine the differences in clinical and laboratory characteristics between (i) influenza and dengue, and (ii) influenza and OFI.
Results
Demographic and clinical features of the patients
Of 849 patients, 526 (315 men and 211 women; mean age, 13.6 ± 13.5 years) were diagnosed with influenza, 148 (83 men and 65 women; mean age, 45.1 ± 4.5 years) with dengue, and 175 (106 men and 69 women; mean age, 15.3 ± 15.9 years) with OFI. None of the patients had concurrent influenza and dengue infections. Demographic, clinical, and laboratory information are summarized in Tables 1 and 2.
Table 1.
Demographics, clinical features, and outcomes of patients with influenza, dengue, and other febrile illnesses
| Variable | Influenza (n = 526) | Dengue (n = 148) | Other febrile illnesses (n = 175) | P a | P b |
|---|---|---|---|---|---|
| Mean age (± SD), years | 13.6 ± 13.5 | 45.1 ± 4.5 | 15.3 ± 15.9 | <0.001 | 0.941 |
| Age group (n, [%]) | |||||
| ≤ 18 years | 445 (84.6) | 24 (16.2) | 130 (74.3) | - | - |
| >18 years | 81 (15.4) | 124 (83.8) | 45 (25.7) | - | - |
| Male gender (n, [%]) | 315 (59.9) | 83 (56.1) | 106 (60.6) | 0.406 | 0.873 |
| Underlying conditionc (n, [%]) | |||||
| Bronchial asthma | 14 (26.7) | 0 | 5 (2.9) | - | - |
| Diabetes mellitus | 10 (1.9) | 17 (11.5) | 2 (1.1) | - | - |
| Hypertension | 15 (2.8) | 30 (20.3) | 2 (1.1) | - | - |
| Influenza A virus subtype (n, [%]) | |||||
| Pandemic 2009 H1N1 | 447 (85) | - | - | - | - |
| Seasonal H3N2 | 79 (15) | - | - | - | - |
| Dengue disease severity (n, [%]) | |||||
| Dengue without warning signs | - | 64 (43.2) | - | - | - |
| Dengue with warning signs | - | 77 (52) | - | - | - |
| Severe dengue | - | 7 (4.7) | - | - | - |
| Dengue virus serotypes (n/N, [%]) | |||||
| Serotype I | - | 10/40 (25) | - | - | - |
| Serotype II | - | 17/40 (42.5) | - | - | - |
| Serotype III | - | 12/40 (30) | - | - | - |
| Serotype IV | - | 1/40 (2.5) | - | - | - |
| Mean interval from onset of symptoms to emergency room presentation, days (± SD) | 1.7 ± 1.2 | 3.7 ± 1.8 | 3.0 ± 2.1 | - | - |
| Mean fever duration, days (± SD) | 2.8 ± 1.6 | 4.0 ± 2.3 | ND | <0.001 | - |
| Use of oseltamivir >48 h (n/N, [%]) | 107/364 (29.4) | - | - | - | - |
| Fatalities (n, [%]) | 5 (0.9) | 0 | 0 | 0.234 | 0.196 |
n/N = number of patients/total number of patients with data available; ND = no data.
aInfluenza vs. dengue.
bInfluenza vs. other febrile illnesses.
cAn individual patient might have more than one underlying disease/condition.
Table 2.
Symptoms/signs and laboratory characteristics of patients with influenza, dengue, and other febrile illnesses
| Variable | Influenza (n = 526) | Dengue (n = 148) | Other febrile illnesses (n = 175) | P a | P b |
|---|---|---|---|---|---|
| Symptom/signc (n [%]) | |||||
| Rhinorrhea | 333 (63.3) | 0 | 73 (41.7) | <0.001 | <0.001 |
| Cough | 356 (67.6) | 38 (25.7) | 112 (64) | <0.001 | 0.396 |
| Sore throat | 207 (39.3) | 24 (16.2) | 43 (24.6) | <0.001 | <0.001 |
| Malaise | 183 (34.8) | 95 (64.2) | 21 (12) | <0.001 | <0.001 |
| Headache | 149 (28.3) | 62 (41.9) | 33 (18.9) | 0.001 | 0.015 |
| Vomiting/nausea | 116 (22.1) | 43 (29.1) | 37 (21.1) | 0.076 | 0.801 |
| Diarrhea | 57 (10.8) | 15 (10.1) | 26 (14.9) | 0.807 | 0.154 |
| Abdominal pain | 40 (7.6) | 31 (20.9) | 18 (10.3) | <0.001 | 0.265 |
| Joint pain | 26 (4.9) | 74 (50) | 10 (5.7) | <0.001 | 0.539 |
| Orbital pain | 0 | 17 (11.5) | 2 (1.1) | <0.001 | 0.062 |
| Rashes | 5 (0.9) | 88 (59.5) | 0 | <0.001 | 0.196 |
| Dyspnea | 41 (7.8) | 3 (2) | 1 (0.6) | 0.008 | <0.001 |
| Drowsiness | 13 (2.5) | 0 | 0 | 0.083 | 0.036 |
| Seizures | 8 (1.5) | 0 | 0 | 0.211 | 0.211 |
| Laboratory characteristics | |||||
| Leukopenia (WBC <3.0 × 109 cells/L) (n/N [%]) | 9/283 (3.2) | 82/146 (56) | 4/152 (2.6) | <0.001 | 0.749 |
| Platelet count 149-100 × 109 cells/L (n/N [%]) | 38/238 (15.9) | 27/146 (18.5) | 5/133 (3.7) | 0.522 | 0.003 |
| Platelet count <100 × 109 cells/L (n/N [%]) | 7/238 (2.9) | 109/146 (74.6) | 3/133 (2.3) | <0.001 | 0.696 |
WBC = white blood cell count; n/N = number of patients/total number of patients with data available.
aInfluenza vs. dengue.
bInfluenza vs. other febrile illnesses.
cAn individual patient might have more than one symptom/sign.
Of 526 (445 [84.6%] patients aged ≤18 years) influenza infections, 447 (85%) were 2009 pandemic H1N1 and 79 (15%) were seasonal H3N2. The mean interval between onset of illness and the patient's arrival at the ER was 1.7 ± 1.2 days, and the mean fever duration was 2.8 ± 1.6 days. Besides fever, the 3 most common symptoms were cough (67.6%), rhinorrhea (63.3%), and sore throat (39.3%). Mild thrombocytopenia was detected in 38 (15.9%) of the 238 patients with an available peripheral platelet count. Of 364 patients receiving oseltamivir, 107 (29.4%) started therapy >48 h after illness onset. The 3 most common influenza-associated complications were pneumonia (5.1%), meningoencephalitis (1.1%), and acute respiratory distress syndrome (ARDS) (0.9%) (Table 3). The mortality rate was 0.9%, comprised of 5 patients (2 pandemic 2009 H1N1 and 3 seasonal H3N2; 4 women and 1 man, median age of 24 years [range 2-72 years]). All fatal cases received oseltamivir therapy >48 h after illness onset. Pneumonia was detected in all 5 fatal cases, ARDS and meningoencephalitis each in 3 (60% each), intracranial hemorrhage in 2 (40%), and myocarditis and gastrointestinal bleeding each in 1 (20% each).
Table 3.
Complications of influenza and dengue infections
| Complication a | Influenza (n = 526) | Dengue (n = 148) | P |
|---|---|---|---|
| Pneumonia | 27b (5.1) | 1 (0.6) | 0.016 |
| ARDS | 5c (0.9) | 0 | 0.234 |
| Meningoencephalitis | 6d (1.1) | 0 | 0.192 |
| Intracranial bleeding | 2d (0.4) | 0 | 0.452 |
| Gastrointestinal bleeding | 2d (0.4) | 14 (9.5) | <0.001 |
| Myocarditis | 1d (0.2) | 0 | 0.596 |
| Acute hepatitis (ALT >1,000 U/L) | 2d (0.4) | 0 | 0.452 |
Data are number (%) of patients. ARDS = acute respiratory distress syndrome; ALT = alanine aminotransferase.
aAn individual patient might have more than one complication.
bOf 27 influenza patients with pneumonia, 15 cases were 2009 pandemic H1N1 and 12 cases were H3N2.
cOf 5 influenza patients with ARDS, 4 cases were 2009 pandemic H1N1 and 1 case was H3N2.
dPatient(s) with 2009 pandemic H1N1 infection.
Among 148 patients diagnosed with dengue infection (24 [16.2%] patients aged ≤18 years), 64 (43.2%) were dengue fever without warning signs, 77 (52%) were dengue fever with warning signs, and 7 (4.7%) were severe dengue, based on 2009 WHO case definitions [7]. An individual patient might have received more than one dengue diagnostic test. Of laboratory-positive dengue cases, 40 were confirmed by RT-PCR, 71 by IgM of acute phase serum, 47 by fourfold increase in IgG titer in paired acute and convalescent serum, and 78 by detection of NS1 antigen. Of 71 patients positive for IgM antibody, a fourfold rise in IgG titer in paired serum was found in 31 patients, detection of NS1 antigen in 29, positive RT-PCR and NS1 antigen in 6, fourfold increase in IgG titer in paired serum and positive NS1 antigen in 3, and fourfold rise in IgG titer in paired serum and positive RT-PCR in 2. The mean time lapse from onset of symptoms to ER presentation was 3.7 ± 1.8 days, and the mean fever duration was 4.0 ± 2.3 days. The 3 most common symptoms other than fever in dengue patients were malaise (64.2%), rashes (59.5%), and joint pain (50%). Marked thrombocytopenia and leukopenia were found in 109 (74.6%) and 82 (56%) patients, respectively, of 146 patients with complete blood counts. Forty patients had available RT-PCR data; of these, dengue virus serotype II was detected in 17 (42.5%) patients, serotype III in 12 (30%), serotype I in 10 (25%), and serotype IV in 1 (2.5%). Of 148 dengue patients, 14 (9.5%) experienced gastrointestinal bleeding (Table 3). All dengue patients recovered.
Table 4 describes the diagnosis of the 175 patients with OFI (130 [74.3%] patients aged ≤18 years). The 3 leading etiologies of OFI were acute pharyngitis (38.8%), acute bronchitis (25.7%), and bronchopneumonia (16%). The mean interval from onset of illness to ER presentation was 3.0 ± 2.1 days. All patients with OFI recovered.
Table 4.
Etiologies of 175 patients with other febrile illnesses
| Variable | |
|---|---|
| Acute pharyngitis | 68 (38.8) |
| Acute bronchitis | 45 (25.7) |
| Bronchopneumonia | 28 (16) |
| Acute tonsillitis | 16 (9.1) |
| Gastroenteritis | 11 (6.3) |
| Sinusitis | 2 (1.1) |
| Kawasaki | 1 (0.5) |
| Unknown | 4 (2.3) |
Data are number (%) of patients.
Clinical and laboratory characteristics distinguishing influenza from dengue
Comparisons between patients with influenza (n = 526) and dengue (n =148) are shown in Tables 1, 2, 3, 5, and 6. Patients with influenza were significantly younger, had shorter fever duration, and presented at the ER earlier than patients with dengue (Table 1). Rhinorrhea, cough, sore throat, and dyspnea were reported significantly more frequently in patients with influenza than those with dengue (Table 2). Significantly lower frequencies of malaise, headache, abdominal pain, joint pain, orbital pain, and rashes were noted in patients with influenza than in those with dengue (Table 2). Dengue patients had significantly higher incidences of leukopenia, marked thrombocytopenia, and gastrointestinal bleeding in addition to lower incidence of pneumonia (Tables 2 and 3). Multivariate analysis disclosed absence of rashes (odds ratio [OR], 131.336), no leukopenia (OR, 24.978), and no marked thrombocytopenia (OR, 105.973) as predictive factors that distinguished influenza from dengue (Table 6). The sensitivities of absence of rashes, without leukopenia, and no marked thrombocytopenia were 98.3%, 96.9%, and 97.9%, while the specificities were 60%, 55.9%, and 74.5%, respectively.
Table 5.
Age-specific symptoms/signs and laboratory features of patients with influenza, dengue, and other febrile illnesses
| Variable | Age ≤18 years | Age >18 years | ||
|---|---|---|---|---|
| Inf vs. Den (Inf, n = 445; Den, n = 24) | Inf vs. OFI (Inf, n = 445; OFI, n = 130) | Inf vs. Den (Inf, n = 81; Den, n = 124) | Inf vs. OFI (Inf, n = 81; OFI, n = 45) | |
| Symptom/signa (n [%]) | ||||
| Rhinorrhea | 297 (66.7) vs. 0*** | 297 (66.7) vs. 65 (50)** | 36 (44.4) vs. 0*** | 36 (44.4) vs. 8 (17.8)** |
| Cough | 311 (69.9) vs. 3 (12.5)*** | 311 (69.9) vs. 94 (72.3) | 45 (55.6) vs. 35 (28.2)*** | 45 (55.6) vs. 18 (40)* |
| Sore throat | 163 (36.6) vs. 4 (16.7) | 163 (36.6) vs. 27 (20.8)** | 44 (54.3) vs. 20 (16.1)*** | 44 (54.3) vs. 16 (35.6) |
| Malaise | 141 (31.6) vs. 14 (58.3)* | 141 (31.6) vs. 8 (6.2)*** | 42 (51.9) vs. 81 (65.3) | 42 (51.9) vs. 13 (28.9)* |
| Headache | 125 (28.1) vs. 11 (45.8) | 125 (28.1) vs. 21 (16.2)** | 24 (29.6) vs. 51 (41.1) | 24 (29.6) vs. 12 (26.7) |
| Vomiting/nausea | 106 (23.8) vs. 8 (33.3) | 106 (23.8) vs. 33 (25.4) | 10 (12.3) vs. 35 (28.2)** | 10 (12.3) vs. 4 (8.9) |
| Diarrhea | 54 (12.1) vs. 3 (12.5) | 54 (12.1) vs. 19 (14.6) | 3 (3.7) vs. 12 (9.7) | 3 (3.7) vs. 7 (15.6)* |
| Abdominal pain | 34 (7.6) vs. 4 (16.7) | 34 (7.6) vs. 14 (10.8) | 6 (7.4) vs. 27 (21.8)** | 6 (7.4) vs. 4 (8.9) |
| Joint pain | 18 (4) vs. 6 (25) | 18 (4) vs. 7 (5.4) | 8 (9.9) vs. 68 (54.8)*** | 8 (9.9) vs. 3 (6.7) |
| Orbital pain | 0 vs. 2 (8.3)** | 0 (0) vs. 2 (1.5) | 0 vs. 15 (12.1)** | 0 vs. 0 |
| Rashes | 5 (1.1) vs. 11(45.8)*** | 5 (1.1) vs. 0 | 0 vs. 77 (62.1)*** | 0 vs. 0 |
| Dyspnea | 24 (5.6) vs. 1 (4.1) | 24 (5.6) vs. 1 (0.8)* | 17 (3.7) vs. 2 (1.6)*** | 17 (3.7) vs. 0** |
| Drowsiness | 10 (2.2) vs. 0 | 10 (2.2) vs. 0 | 3 (3.7) vs. 0 | 3 (3.7) vs. 0 |
| Seizures | 6 (1.3) vs. 0 | 6 (1.3) vs. 0 | 2 (2.5) vs. 0 | 2 (2.5) vs. 0 |
| Laboratory characteristics | ||||
| Leukopenia (WBC <3.0 × 109 cells/L) (n/N [%]) | 6/231 (2.6) vs. 14/23 (60.8)*** | 6/231 (2.6) vs. 1/116 (0.8) | 3/52 (5.8) vs. 68/123 (55.3)*** | 3/52 (5.8) vs. 3/36 (8.3) |
| Platelet count 149-100 × 109 cells/L (n/N [%]) | 24/189 (12.6) vs. 6/23 (26) | 24/189 (12.6) vs. 2/106 (1.9)** | 14/49 (28.6) vs. 21/123 (17.1) | 14/49 (28.6) vs. 3/27 (11.1) |
| Platelet count <100 × 109 cells/L (n/N [%]) | 3/189 (1.6) vs. 12/23 (52.2)*** | 3/189 (1.6) vs. 2/106 (1.9) | 4/49 (8.2) vs. 97/123 (78.9)*** | 4/49 (8.2) vs. 1/27 (3.7) |
Inf = influenza; Den = dengue; OFI = other febrile illnesses; WBC = white blood count; n/N = number of patients/total number of patients with data available.
*P < 0.05; **P < 0.01; ***P < 0.001.
aAn individual patient might have more than one symptom/sign.
Table 6.
Sensitivity, specificity, and multivariate logistic regression for prediction of influenza versus dengue and influenza versus other febrile illnesses
| Influenza n/N (%) | Dengue n/N (%) | Other febrile illnesses n/N (%) | Adjusted odds ratio | 95% CI | P | Sensitivity% | Specificity% | |
|---|---|---|---|---|---|---|---|---|
| Influenza vs. dengue | ||||||||
| All ages | ||||||||
| No skin rashes | 234/238 (98.3) | 58/145 (40) | - | 131.336 | 35.416-487.039 | <0.001 | 98.3 | 60 |
| No leukopeniaa | 225/232 (96.9) | 64/145 (44.1) | - | 24.978 | 7.010-89.006 | <0.001 | 96.9 | 55.9 |
| No marked thrombocytopeniab | 233/238 (97.9) | 37/145 (25.5) | - | 105.973 | 31.687-354.419 | <0.001 | 97.9 | 74.5 |
| Age ≤18 years | ||||||||
| No skin rashes | 185/189 (97.9) | 12/23 (52.2) | - | 326.393 | 30.860-3452.098 | <0.001 | 97.9 | 52.2 |
| No leukopeniaa | 185/189 (97.9) | 9/23 (39.1) | - | 122.116 | 10.888-1369.665 | <0.001 | 97.9 | 60.9 |
| No marked thrombocytopeniab | 186/189 (98.4) | 11/23 (47.8) | - | 88.632 | 6.443-1219.174 | 0.001 | 98.4 | 52.2 |
| Age >18 years | ||||||||
| No leukopeniaa | 46/49 (93.9) | 55/122 (45.1) | - | 13.99 | 3.628-53.946 | <0.001 | 93.9 | 54.9 |
| No marked thrombocytopeniab | 45/49 (91.8) | 26/122 (21.3) | - | 34.096 | 10.622-109.445 | <0.001 | 91.8 | 78.7 |
| Influenza vs. other febrile illnesses | ||||||||
| All ages | ||||||||
| Rhinorrhea | 152/238 (63.9) | - | 57/137 (41.6) | 3.350 | 1.997-5.618 | <0.001 | 63.9 | 58.4 |
| Malaise | 105/238 (44.1) | - | 16/137 (11.7) | 6.050 | 3.207-11.414 | <0.001 | 44.1 | 88.3 |
| Sore throat | 105/238 (44.1) | - | 30/137 (21.9) | 3.407 | 1.960-5.922 | <0.001 | 44.1 | 78.1 |
| Dyspnea | 41/238 (17.3) | - | 1/137 (0.7) | 47.335 | 6.174-632.919 | <0.001 | 17.3 | 99.3 |
| Mild thrombocytopeniac | 38/238 (15.9) | - | 5/137 (3.6) | 3.779 | 1.309-10.908 | 0.014 | 15.9 | 96.4 |
| Age ≤18 years | ||||||||
| Rhinorrhea | 131/189 (69.3) | - | 53/106 (50) | 2.765 | 1.531-4.997 | 0.001 | 69.3 | 50 |
| Malaise | 76/189 (40.2) | - | 6/106 (5.7) | 11.129 | 4.455-27.802 | <0.001 | 40.2 | 94.3 |
| Sore throat | 83/189 (43.9) | - | 21/106 (19.8) | 3.575 | 1.892-6.753 | <0.001 | 43.9 | 80.2 |
| Dyspnea | 24/189 (12.7) | - | 1/106 (0.9) | 20.867 | 2.641-164.872 | 0.004 | 12.7 | 99 |
| Mild thrombocytopeniac | 24/189 (12.7) | - | 2/106 (1.9) | 7.138 | 1.509-33.769 | 0.013 | 12.7 | 98.1 |
| Age >18 years | - | |||||||
| Rhinorrhea | 36/81 (44.4) | - | 8/45 (17.8) | 4.726 | 1.654-13.5 | 0.004 | 44.4 | 82.2 |
| Malaise | 42/81 (51.9) | - | 13/45 (28.9) | 3.108 | 1.224-7.897 | 0.017 | 51.9 | 71.1 |
n/N = number of patients/total number of patients with data available; CI = confidence interval.
aLeukopenia is defined as white cell counts <3.0 × 109 cells/L.
bMarked thrombocytopenia is defined as platelet counts <100 × 109 cells/L.
cMild thrombocytopenia is defined as platelet counts 149-100 × 109 cells/L.
Symptoms and laboratory characteristics of influenza infection varied by patient age (Table 5). Pediatric influenza patients (≤18 years, n = 445) had significantly higher frequencies of rhinorrhea and cough; lower frequencies of malaise, orbital pain and rashes; and lower incidences of leukopenia and marked thrombocytopenia compared with dengue patients (n = 24) (Table 5). Multivariate analysis showed absence of rashes (OR, 326.393), no leukopenia (OR, 122.116) and no marked thrombocytopenia (OR, 88.632) to be independent predictors for distinguishing influenza from dengue in pediatric patients (Table 6). For individuals >18 years of age, patients with influenza (n = 81) had greater occurrence of rhinorrhea, cough, sore throat, and dyspnea; they also presented with lower frequencies of nausea and vomiting, abdominal pain, joint pain, orbital pain, and rashes as well as lower incidences of leukopenia and marked thrombocytopenia (Table 5) compared to patients with dengue (n = 124). Multivariate analysis indicated that no leukopenia (OR, 13.99) and no marked thrombocytopenia (OR, 34.096) distinguished influenza from dengue in adults (Table 6).
Clinical and laboratory characteristics distinguishing influenza from OFI
Comparisons between patients with influenza (n = 526) and OFI (n = 175) are summarized in Tables 1, 2, 5, and 6. Significant differences in clinical and laboratory features between influenza and OFI included the presence of rhinorrhea, sore throat, malaise, headache, dyspnea, drowsiness, and mild thrombocytopenia (Table 2). Multivariate analysis revealed that rhinorrhea (OR, 3.350), malaise (OR, 6.050), sore throat (OR, 3.407), dyspnea (OR, 47.335), and mild thrombocytopenia (OR, 3.779) were independent predictive factors that distinguished influenza from OFI (Table 6). The sensitivities of rhinorrhea, malaise, sore throat, dyspnea, and mild thrombocytopenia were 63.9%, 44.1%, 44.1%, 17.3%, and 15.9%, respectively.
As shown in Tables 5 and 6, significantly higher proportions of pediatric patients with influenza (n = 445) experienced rhinorrhea, sore throat, malaise, headache, dyspnea, and mild thrombocytopenia compared with OFI (n = 130); multivariate analysis showed rhinorrhea (OR, 2.765), malaise (OR, 11.129), sore throat (OR, 3.575), dyspnea (OR, 20.867), and mild thrombocytopenia (OR, 7.138) to be independent predictive factors that differentiated influenza from OFI in pediatric patients. Compared to adults with OFI (n = 45), adult patients with influenza (n = 81) had significantly higher frequencies of rhinorrhea, cough, malaise, diarrhea, and dyspnea; multivariate analysis revealed rhinorrhea (OR, 4.726) and malaise (OR, 3.108) to be predictive factors that distinguished influenza from OFI in adults.
Classification tree distinguishing influenza from dengue
Variables found to be significant in multivariate logistic regression were used to establish classification and regression trees. As shown in Figure 1, the initial splitting variable in the tree is a rash, followed by leukopenia and marked thrombocytopenia to distinguish influenza from dengue. The three nodes with low probability of influenza infection in pediatric patients were (i) presence of rashes, (ii) absence of rashes but with leukopenia and (iii) absence of rashes, without leukopenia, but with marked thrombocytopenia. There was a high probability of influenza infection among pediatric patients without rashes, no leukopenia and with no marked thrombocytopenia (94.2% of patients with influenza). Among adult patients, high probability of influenza was associated with no leukopenia and no marked thrombocytopenia (87.8% of patients with influenza) (Figure 2).
Figure 1.
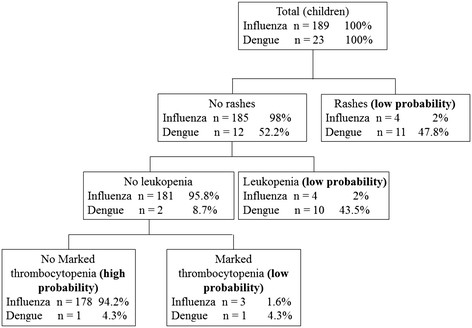
Diagnostic algorithm to discriminate between influenza and dengue in pediatric patients. Terminal nodes are marked as "low probability" or "high probability" for influenza infection.
Figure 2.
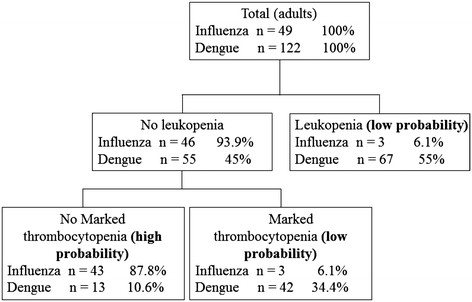
Diagnostic algorithm to discriminate between influenza and dengue in adult patients. Terminal nodes are marked as "low probability" or "high probability" for influenza infection.
Discussion
Our study investigated simple clinical and laboratory features to identify patients with influenza among children and adults with acute febrile illness in ER in dengue and non-dengue endemic areas. In our series, the mean time lapse between onset of illness and ER presentation was 1.7, 3.7, and 3 days for influenza, dengue, and OFI, respectively. This permits a detailed comparison of early clinical and laboratory characteristics between influenza and dengue or OFI.
The symptoms of influenza overlap substantially with dengue and OFI (Table 2). We found that symptoms resulting from respiratory tract infections such as cough, rhinorrhea, sore throat, and dyspnea were more prevalent in patients with influenza than with dengue. In contrast, non-respiratory tract symptoms including rashes, headache, abdominal pain, joint pain, and orbital pain were less common in the patients with influenza compared to patients with dengue. The influenza virus primarily infects epithelial cells of the respiratory tract and causes upper respiratory symptoms [25]. Damage to infected cells results in release of inflammatory mediators, leading to a systemic response (i.e., fever, headache, joint pain, malaise, and myalgia) with symptoms similar to other viral illnesses such as dengue [26],[27].
The overlapping geographic range of influenza and dengue as well as simultaneous dengue and influenza outbreaks [8]-[11], often in resource-limited countries, leads to diagnostic difficulties, as nonspecific symptoms are common to both infections. Early detection of influenza and dengue is especially important because influenza may be prevented through proper isolation and treated using antiviral agents [28],[29], whereas failure to make a timely dengue diagnosis with adequate fluid replacement can lead to severe dengue [30]. Our study demonstrated that the presence of leukopenia (white cell count <3.0 × 109 cells/L) and marked thrombocytopenia (platelet count <100 × 109 cells/L) are useful for differentiating dengue from influenza in both adults and pediatric patients. The absence of rash further discriminated influenza from dengue in pediatric patients. Rash associated with influenza is not a common manifestation, occurring in only 2-8% of patients, usually children [31],[32]. In contrast, the reported frequency of rash in dengue cases ranges from 46-68%, particularly in children less than 15 years who usually have a nonspecific febrile syndrome accompanied by rash [33],[34]. Mild leukopenia and relative lymphopenia are typical findings of influenza; thrombocytopenia may be present in complicated cases [4],[14],[25]. Notably, our study and others found leukopenia and thrombocytopenia to be common laboratory findings in dengue infection, with platelet counts below 20 × 109 per liter often observed in severe dengue [7],[30]. A study examining predictors of diagnosis in 1,962 febrile travelers returning from the tropics found the main predictors of dengue infection compared with other fevers, excluding malaria, to include skin rash, thrombocytopenia, and leukopenia [35]. This finding was consistent with our study findings.
Our data suggest that skin rash, leukopenia (white cell count <3.0 × 109 cells/L), and platelet counts <100 × 109 cells/L were useful to predict negative influenza results during influenza and dengue epidemics. However, it is unlikely that any single indicator will be useful in clinical practice because these symptoms and laboratory findings can be present in both infections [11]. In our series, we developed two simple and practical diagnostic algorithms using clinical and laboratory indicators to distinguish influenza from dengue in adult and pediatric patients, respectively. We found that the diagnostic algorithm correctly classified 94.2% of pediatric patients with influenza in the "high probability" group with only one misclassified dengue patient (Figure 1). Analysis of our data after excluding pediatric patients showed similar results, except that skin rash that was no longer associated with influenza infection in adult patients; however, the diagnostic tree (Figure 2) still correctly classified 87.8% of adult influenza cases as "high probability". These findings underscored that rash, leukopenia, and marked thrombocytopenia could help to establish a diagnostic algorithm to distinguish influenza from dengue patients during outbreaks of both diseases. Additional prospective studies are needed to validate this predictive model in other dengue-endemic regions and in populations with different ethnicities.
The wide range of influenza-associated symptoms often makes it difficult to distinguish from other febrile or respiratory illnesses [25]. A crowded ER can make it challenging for physicians to differentiate between influenza and OFI [36]. Early antiviral treatment (≤48 h) of influenza is especially important for patients with underlying risk factors to avoid otherwise preventable morbidity and mortality [37]. Our results demonstrate that rhinorrhea, malaise, sore throat, and dyspnea in addition to a slightly low platelet count (100-149 × 109 cells/L) in children, as well as rhinorrhea and malaise in adults were valuable predictors during the evaluation of the likelihood of influenza versus OFI in a non-dengue endemic setting. However, apart from rhinorrhea, which lacked adequate sensitivity and specificity, we found that all other variables were specific (>70%) but not sensitive enough in distinguishing influenza from OFI. This is not surprising as more than 60% of OFI cases included in this series were individuals with pharyngitis or bronchitis (Table 4), another viral infection commonly encountered in the ER [38]. The information of this study are most helpful for clinicians to facilitate diagnosis of influenza during periods of high influenza activity in non-dengue endemic setting.
In the present study, patients with influenza had a shorter fever duration than the dengue cases (mean 2.8 ± 1.6 vs. 4.0 ± 2.3 days). This finding is similar to a previous study that found a fever duration of <4 days for patients with influenza [25], while the average length of fever in dengue patients was approximately 5 days [39], coinciding with the disappearance of viremia.
Although influenza primarily causes upper respiratory tract infections, pulmonary and extra-pulmonary complications have also been reported [2],[40],[41]. Previous studies from the United States and Australia of critically ill patients with 2009 pandemic H1N1 infections found ARDS complication in 35.8% and 48.8% of cases and a 45% and 14.3% hospital mortality, respectively [40],[41]. ARDS was noted in 5 influenza cases in our study (Table 3); 3 were fatal. Remarkably, delayed oseltamivir therapy was found in all fatal cases in our series. The importance of a timely anti-viral therapy for severe influenza should therefore be emphasized.
Earlier studies describe neurological complications of influenza including aseptic meningitis, encephalopathy/encephalitis, Guillain-Barré syndrome, and transverse myelitis [42]-[44]. Neurological complications have been reported for patients with 2009 pandemic H1N1 infections [43]. Of 447 patients with 2009 pandemic H1N1 infection in this study, meningoencephalitis was found in 6 (1.3%), with presenting symptoms of altered mental status and seizures (Table 3). Other influenza-associated extrapulmonary complications among our patients included myocarditis, rhabdomyolysis, gastrointestinal bleeding, and intracranial hemorrhage (Table 3). Physicians should be aware of influenza-associated extrapulmonary complications when caring for influenza patients and manage them accordingly.
Gastrointestinal bleeding was the most common complication among dengue patients in this study. Previous studies have reported gastrointestinal bleeding to be a warning sign of severe dengue; timely management and intensive monitoring of patients with gastrointestinal bleeding is therefore necessary [45].
This study has several limitations. First, because it was conducted in a single medical center, disease severity may have been biased by referral patterns. Second, as a retrospective study, missing laboratory data was inevitable. Third, the decision to perform diagnostic tests for influenza and dengue infections was based on individual physicians' experience; therefore, patients included in our series may have been biased by individual physicians' personal judgments. In addition, our results were based on data from early stages of illness; future studies are necessary to validate these findings in different course of illness for better generalization of their utility.
Conclusions
This study demonstrates substantial overlap in clinical presentation between influenza and dengue as well as OFI. This study also identifies simple and useful clinical and laboratory data to enable identification and facilitate diagnosis of influenza in different clinical settings (dengue and non-dengue endemic areas). We provide two decision tree algorithms using simple clinical and laboratory data that can be easily implemented in resource-limited countries to differentiate patients with influenza from those with dengue. This information is especially important to clinicians in countries where medical resources are sparse and the burden of influenza and dengue is high.
Authors' contributions
SYH and IKL made major contributions to sample collection and wrote the manuscript. IKL conceived and designed the study, analysis of the data and preparation of the manuscript. SYH, SCH, CCC, TYC, WCH, JWL and LW collected clinical data. All authors read, commented on, and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Footnotes
Competing interests
The authors declare that they have no competing interests.
Contributor Information
Shi-Yu Huang, Email: sarawah@ms28.hinet.net.
Ing-Kit Lee, Email: leee@cgmh.org.tw.
Lin Wang, Email: ling6422@yahoo.com.tw.
Jien-Wei Liu, Email: jwliu@cgmh.org.tw.
Shih-Chiang Hung, Email: hsc0901@cgmh.org.tw.
Chien-Chih Chen, Email: pancreas133@gmail.com.
Tzu-Yao Chang, Email: ab4489@cloud.cgmh.org.tw.
Wen-Chi Huang, Email: heteyland@cloud.cgmh.org.tw.
References
- 1.Briand S, Mounts A, Chamberland M. Challenges of global surveillance during an influenza pandemic. Public Health. 2011;125(5):247–256. doi: 10.1016/j.puhe.2010.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rothberg MB, Haessler SD, Brown RB. Complications of viral influenza. Am J Med. 2008;121(4):258–264. doi: 10.1016/j.amjmed.2007.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murata Y, Walsh EE, Falsey AR. Pulmonary complications of interpandemic influenza A in hospitalized adult. J Infect Dis. 2007;195(7):1029–1037. doi: 10.1086/512160. [DOI] [PubMed] [Google Scholar]
- 4.Cate TR. Clinical manifestations and consequences of influenza. Am J Med. 1987;82(6A):15–19. doi: 10.1016/0002-9343(87)90555-9. [DOI] [PubMed] [Google Scholar]
- 5.Guzmán MG, Kourí G. Dengue: an update. Lancet Infect Dis. 2002;2(1):33–42. doi: 10.1016/S1473-3099(01)00171-2. [DOI] [PubMed] [Google Scholar]
- 6.Tsai CY, Lee IK, Lee CH, Yang KD, Liu JW. Comparisons of dengue illness classified based on the 1997 and 2009 World Health Organization dengue classification schemes. J Microbiol Immunol Infect. 2013;46(4):271–281. doi: 10.1016/j.jmii.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 7.Dengue Hemorrhagic Fever: Diagnosis, Treatment and Control. 2009, World Health Organization, Geneva
- 8.Silarug N, Foy HM, Kupradinon S, Rojanasuphot S, Nisalak A, Ponguswant Y. Epidemic of fever of unknown origin in rural Thailand caused by influenza A (H1N1) and dengue fever. Southeast Asian J Trop Med Public Health. 1990;21(1):61–67. [PubMed] [Google Scholar]
- 9.Hussain R, Al-Omar I, Memish ZA. The diagnostic challenge of pandemic H1N1 2009 virus in a dengue-endemic region: a case report of combined infection in Jeddah, Kingdom of Saudi Arabia. J Infect Public Health. 2012;5(2):199–202. doi: 10.1016/j.jiph.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 10.Lopez Rodriguez E, Tomashek KM, Gregory CJ, Munoz J, Hunsperger E, Lorenzi OD, Irizarry JG, Garcia-Gubern C. Co-infection with dengue virus and pandemic (H1N1) 2009 virus. Emerg Infect Dis. 2010;16(5):882–884. doi: 10.3201/eid1605.091920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morens DM, Rigau-Pérez JG, López-Correa RH, Moore CG, Ruiz-Tibén EE, Sather GE, Chiriboga J, Eliason DA, Casta-Velez A, Woodall JP. Dengue in Puerto Rico, 1977: public health response to characterize and control an epidemic of multiple serotypes. Am J Trop Med Hyg. 1986;35(1):197–211. doi: 10.4269/ajtmh.1986.35.197. [DOI] [PubMed] [Google Scholar]
- 12.Lee IK, Lee WH, Yang KD, Liu JW. Comparison of the effects of oral hydration and intravenous fluid replacement in adult patients with non-shock dengue hemorrhagic fever in Taiwan. Trans R Soc Trop Med Hyg. 2010;104(8):541–545. doi: 10.1016/j.trstmh.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 13.Lee IK, Liu JW, Yang KD. Clinical and laboratory characteristics and risk factors for fatality in elderly patients with dengue hemorrhagic fever. Am J Trop Med Hyg. 2008;79(2):149–153. [PubMed] [Google Scholar]
- 14.Lee IK, Liu JW, Wang L, Yang KD, Li CC, Eng HL. 2009 pandemic influenza A (H1N1): clinical and laboratory characteristics in pediatric and adult patients and in patients with pulmonary involvement. Influenza Other Respir Viruses. 2012;6(6):e152–161. doi: 10.1111/j.1750-2659.2012.00410.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheng CK, Cowling BJ, Chan KH, Fang VJ, Seto WH, Yung R, Uyeki TM, Houck PM, Peiris JS, Leung GM. Factors affecting QuickVue Influenza A + B rapid test performance in the community setting. Diagn Microbiol Infect Dis. 2009;65(1):35–41. doi: 10.1016/j.diagmicrobio.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 16.Shu PY, Huang JH. Current advances in dengue diagnosis. Clin Diagn Lab Immunol. 2004;11(4):642–650. doi: 10.1128/CDLI.11.4.642-650.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.World Health Organization: Clinical management of human infection with pandemic(H1N1)2009:revised guidance . Available at (Accessed November 2009)., [http://www.who.int/csr/resources/publications/swineflu/clinical_management/en/index.html]
- 18.Trademarks: QIAGEN®: QIAamp Viral RNA Mini Kit-for purification of viral RNA from cell-free body fluids . Available at (Accessed November 2007)., [http://www.qiagen.com/MyQIAcube]
- 19.World Health Organization: CDC protocol of real-time RT-PCR for influenza A(H1N1). Geneva: World Health Organization.,
- 20.Shu PY, Chang SF, Kuo YC, Yueh YY, Chien LJ, Sue CL, Lin TH, Huang JH. Development of group- and serotype-specific one-step SYBR Green I-based real-time reverse transcription-PCR assay for dengue virus. J Clin Microbiol. 2003;41(6):2408–2416. doi: 10.1128/JCM.41.6.2408-2416.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shu PY, Chen LK, Chang SF, Yueh YY, Chow L, Chien LJ, Chin C, Lin TH, Huang JH. Comparison of capture immunoglobulin M (IgM) and IgG enzyme-linked immunosorbent assay (ELISA) and nonstructural protein NS1 serotype-specific IgG ELISA for differentiation of primary and secondary dengue virus infections. Clin Diagnos Lab Immunol. 2003;10(4):622–630. doi: 10.1128/CDLI.10.4.622-630.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shu PY, Yang CF, Kao JF, Su CL, Chang SF, Lin CC, Yang WC, Shih H, Yang SY, Wu PF, Wu HS, Huang JH. Application of the dengue virus NS1 antigen rapid test for onsite detection of imported dengue cases at airports. Clin Vaccine Immunol. 2009;16(4):589e–91. doi: 10.1128/CVI.00475-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guidelines for dengue control. 2009, Centers for Disease Control, Department of Health, R.O.C (Taiwan)
- 24.Crichton NJ, Hinde JP, Marchini J. Models for diagnosing chest pain: is CART helpful? Stat Med. 1997;16(7):717–727. doi: 10.1002/(SICI)1097-0258(19970415)16:7<717::AID-SIM504>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 25.Eccles R. Understanding the symptoms of the common cold and influenza. Lancet Infect Dis. 2005;5(11):718–725. doi: 10.1016/S1473-3099(05)70270-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brydon EW, Morris SJ, Sweet C. Role of apoptosis and cytokines in influenza virus morbidity. FEMS Microbiol Rev. 2005;29(4):837–850. doi: 10.1016/j.femsre.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 27.Matsukura S, Kokubu F, Kubo H, Tomita T, Tokunaga H, Kadokura M, Yamamoto T, Kuroiwa Y, Ohno T, Suzaki H, Adachi M. Expression of RANTES by normal airway epithelial cells after influenza virus A infection. Am J Respir Cell Mol Biol. 1998;18(2):255–264. doi: 10.1165/ajrcmb.18.2.2822. [DOI] [PubMed] [Google Scholar]
- 28.Jeong I, Lee CH, Kim DK, Chung HS, Park SW. Mild form of 2009 H1N1 influenza infection detected by active surveillance: implications for infection control. Am J Infect Control. 2010;38(6):482–485. doi: 10.1016/j.ajic.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 29.Higuera Iglesias AL, Kudo K, Manabe T, Corcho Berdugo AE, Corrales Baeza A, Alfaro Ramos L, Guevara Gutiérrez R, Manjarrez Zavala ME, Takasaki J, Izumi S, Bautista E, Perez Padilla JR. Reducing occurrence and severity of pneumonia due to pandemic H1N1 2009 by early oseltamivir administration: a retrospective study in Mexico. PLoS One. 2011;6(7):e21838. doi: 10.1371/journal.pone.0021838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Simmons CP, Farrar JJ, Nguyen VV, Wills B. Dengue. N Engl J Med. 2012;366(15):1423–1432. doi: 10.1056/NEJMra1110265. [DOI] [PubMed] [Google Scholar]
- 31.Hope-Simpson RE, Higgins PG. A respiratory virus study in Great Britain: review and evaluation. Prog Med Virol. 1969;11:354–407. [PubMed] [Google Scholar]
- 32.Ryan-Poirier K. Influenza virus infection in children. Adv Pediatr Infect Dis. 1995;10:125–156. [PubMed] [Google Scholar]
- 33.Thomas EA, John M, Bhatia A. Cutaneous manifestations of dengue viral infection in Punjab (north India) Int J Dermatol. 2007;46(7):715–719. doi: 10.1111/j.1365-4632.2007.03298.x. [DOI] [PubMed] [Google Scholar]
- 34.Saleem K, Shaikh I. Skin lesions in hospitalized cases of dengue Fever. J Coll Physicians Surg Pak. 2008;18(10):608–611. doi: 10.2008/JCPSP.608611. [DOI] [PubMed] [Google Scholar]
- 35.Bottieau E, Clerinx J, Van den Enden E, Van Esbroeck M, Colebunders R, Van Gompel A. Fever after a stay in the tropics: diagnostic predictors of the leading tropical conditions. Medicine (Baltimore) 2007;86(1):18–25. doi: 10.1097/MD.0b013e3180305c48. [DOI] [PubMed] [Google Scholar]
- 36.Monmany J, Rabella N, Margall N, Domingo P, Gich I, Vazquez G. Unmasking influenza virus infection in patients attended to in the emergency department. Infection. 2004;32(2):89–97. doi: 10.1007/s15010-004-3088-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bautista E, Chotpitayasunondh T, Gao Z, Harper SA, Shaw M, Uyeki TM, Zaki SR, Hayden FG, Hui DS, Kettner JD, Kumar A, Lim M, Shindo N, Penn C, Nicholson KG. Clinical aspects of pandemic 2009 influenza A (H1N1) virus infection. N Engl J Med. 2010;362(18):1708–1719. doi: 10.1056/NEJMra1000449. [DOI] [PubMed] [Google Scholar]
- 38.Kumar R, Tripathi P, Tripathi S, Kanodia A, Pant S, Venkatesh V. Prevalence and clinical differentiation of dengue fever in children in northern India. Infection. 2008;36(5):444–449. doi: 10.1007/s15010-008-7172-6. [DOI] [PubMed] [Google Scholar]
- 39.Lee IK, Liu JW, Yang KD. Clinical characteristics and risk factors for concurrent bacteremia in adults with dengue hemorrhagic fever. Am J Trop Med Hyg. 2005;72(2):221–226. [PubMed] [Google Scholar]
- 40.Jain S, Kamimoto L, Bramley AM, Schmitz AM, Benoit SR, Louie J, Sugerman DE, Druckenmiller JK, Ritger KA, Chugh R, Jasuja S, Deutscher M, Chen S, Walker JD, Duchin JS, Lett S, Soliva S, Wells EV, Swerdlow D, Uyeki TM, Fiore AE, Olsen SJ, Fry AM, Bridges CB, Finelli L. Hospitalized patients with 2009 H1N1 influenza in the United States, April-June 2009. N Engl J Med. 2009;361(20):1935–1944. doi: 10.1056/NEJMoa0906695. [DOI] [PubMed] [Google Scholar]
- 41.Webb SA, Pettilä V, Seppelt I, Bellomo R, Bailey M, Cooper DJ, Cretikos M, Davies AR, Finfer S, Harrigan PW, Hart GK, Howe B, Iredell JR, McArthur C, Mitchell I, Morrison S, Nichol AD, Paterson DL, Peake S, Richards B, Stephens D, Turner A, Yung M. Critical care services and 2009 H1N1 influenza in Australia and New Zealand. N Engl J Med. 2009;361(20):1925–1934. doi: 10.1056/NEJMoa0908481. [DOI] [PubMed] [Google Scholar]
- 42.Hjalmarsson A, Blomqvist P, Brytting M, Linde A, Skoldenberg B. Encephalitis after influenza in sweden 1987-1998: a rare complication of a common infection. Eur Neuro. 2009;61(5):289–294. doi: 10.1159/000206854. [DOI] [PubMed] [Google Scholar]
- 43.Glaser CA, Winter K, DuBray K, Harriman K, Uyeki TM, Sejvar J, Gilliam S, Louie JK. A population-based study of neurologic manifestations of severe influenza A (H1N1) pdm09 in california. Clin Infect Dis. 2012;55(4):514–520. doi: 10.1093/cid/cis454. [DOI] [PubMed] [Google Scholar]
- 44.Sivadon-Tardy V, Orlikowski D, Porcher R, Sharshar T, Durand MC, Enouf V, Rozenberg F, Caudie C, Annane D, van der Werf S, Lebon P, Raphaël JC, Gaillard JL, Gault E. Guillain-barre syndrome and influenza virus infection. Clin Infect Dis. 2009;48(1):48–56. doi: 10.1086/594124. [DOI] [PubMed] [Google Scholar]
- 45.Lee IK, Liu JW, Yang KD. Fatal dengue hemorrhagic fever in adults: emphasizing the evolutionary pre-fatal clinical and laboratory manifestations. PLoS Negl Trop Dis. 2012;6(2):e1532. doi: 10.1371/journal.pntd.0001532. [DOI] [PMC free article] [PubMed] [Google Scholar]


