Abstract
Objective
Enterococcus faecalis (E. faecalis) is the most frequently isolated strain in failed endodontic therapy cases since it is resistant to calcium hydroxide (CH). Whether a combination of CH and chlorhexidine (CHX) is more effective than CH alone against E. faecalis is a matter of controversy. Thus, the aim of this study was to conduct a systematic review and meta-analysis of the literature.
Material and Methods
A comprehensive search in PubMed, EMbase, EBSCOhost, The Cochrane Library, SciELO, and BBO databases, Clinical trials registers, Open Grey, and conference proceedings from the earliest available date to February 1, 2013 was carried out and the relevant articles were identified by two independent reviewers. Backward and forward search was performed and then inclusion and exclusion criteria were applied. The included studies were divided into "comparisons" according to the depth of sampling and dressing period of each medicament. Meta-analysis was performed using Stata software 10.0. The level of significance was set at 0.05.
Results
Eighty-five studies were retrieved from databases and backward/forward searches. Fortyfive studies were considered as relevant (5 in vivo, 18 in vitro, 18 ex vivo, and 4 review articles). Nine studies were included for meta-analysis. Inter-observer agreement (Cohen kappa) was 0.93. The included studies were divided into 21 comparisons for meta-analysis. Chi-square test showed the comparisons were heterogeneous (p<0.001). Random effect model demonstrated no significant difference between CH/CHX mixture and CH alone in their effect on E. faecalis (p=0.115).
Conclusions
According to the evidence available now, mixing CH with CHX does not significantly increase the antimicrobial activity of CH against E. faecalis. It appears that mixing CH with CHX does not improve its ex vivo antibacterial property as an intracanal medicament against E. faecalis. Further in vivo studies are necessary to confirm and correlate the findings of this study with the clinical outcomes.
Keywords: Products with antimicrobial action, Calcium hydroxide, Chlorhexidine, Enterococcus faecalis, Meta-analysis
INTRODUCTION
Microbial invasion of the root canal system has an important role in initiating and sustaining periapical disease41. The aim of root canal therapy is to eliminate bacteria and their by-products from the root canal system74. Although chemomechanical cleaning and shaping of the canal is effective in reducing bacterial counts, microorganisms may persist in the anatomical complexities of root canal system and increase the risk of treatment failure12,109. Therefore, intracanal medication is advocated to further reduce bacteria in the root canal system and increase the success of root canal treatment11.
Calcium hydroxide (CH) is the most commonly used intracanal medicament in endodontics93. It dissociates into calcium and hydroxyl ions in an aqueous solution. The antimicrobial property of CH is attributed to the release of hydroxyl ions and provides a highly alkaline environment with a pH value of approximately 12.593,101. Most of the microorganisms in infected root canals are unable to survive in the alkaline environment37. However, CH is not equally effective against all the bacteria found in the root canal70.
Chlorhexidine gluconate (CHX) can be used in endodontics as an irrigant and intracanal medicament due to its biocompatibility, substantivity and wide antimicrobial activity17,18. The antimicrobial property of CHX is attributed to its cationic molecule, which is adsorbed to the negatively charged inner cell membrane, resulting in the leakage of intracellular components. It is an effective agent against gram-positive and gram-negative bacteria39. Importantly, it is effective against microorganisms resistant to CH90.
Enterococcus faecalis (E. faecalis) is a gram-positive facultative anaerobic bacteria species. It is one of the most CH-resistant microorganisms of the root canal system100. Although it comprises a small proportion of the root canal fora in initial endodontic infections, environmental changes can be advantageous to E. faecalis, resulting in persistent infections99. Some resistance factors of this bacterial species are deep dentinal penetration ability33, high pH tolerance19, surviving in food deprivation condition100, and surviving without any support from other microbial species70.
Many studies have attempted to compare antibacterial effect of CH alone or in combination with CHX. Some studies have shown an increased antibacterial effect when CHX is added to CH8,14,15,20,80, while other studies have failed to show any benefts in incorporating CHX4,55,85,98. It seems that the usefulness of mixing CH with CHX remains unclear and controversial61. Therefore, the aim of this systematic review and meta-analysis was to determine whether adding CHX to CH can improve the efficacy of CH against E. faecalis in dentinal tubules or not.
MATERIAL AND METHODS
Review question
The following well-defned review question was developed by using the Population, Intervention, Comparison, and Outcome (PICO) framework: Does CH/CHX mixture (I), compared to CH alone (C), result in higher antimicrobial efficacy (0) against E. faecalis (P) in infected dentin? Therefore, the key words for search strategy were "Enterococcus faecalis" and "E. faecalis" as Population, "chlorhexidine" as Intervention, "calcium hydroxide" as Comparison, and "antimicrobial" and "antibacterial" as Outcome.
Search strategy
A comprehensive search of the literature was performed in Medline (PubMed), EMbase, EBSCOhost, The Cochrane Library SciELO, and BBO databases from the earliest available date to February 1, 2013 by an expert researcher in health and medical sciences (HN). Also, unpublished data, abstracts, and gray literature were sought through clinical trials registries (Australian New Zealand Clinical Trials Registry, Brazilian Clinical Trials Registry, Iranian Registry of Clinical Trials, United States National Institutes of Health, ClinicalTrials.gov, World Health Organization International Clinical Trials Registry Platform, and European Union EU Clinical Trials Registry), Open Grey, and conference proceedings.
The key words were organized according to the PICO model and were ["Enterococcus faecalis" OR "E. faecalis"] AND chlorhexidine AND "calcium hydroxide" AND [antimicrobial OR antibacterial]. No limitations were implemented by country of origin, language or date. The identified studies were combined using the bibliographic software EndNote X4 (Thomson Reuters, Carlsbad, CA, USA).
Study selection and data extraction
Two independent reviewers (MS, AS) screened the titles and abstracts of all the identified studies to determine relevant studies which met predetermined inclusion criteria. If there were insufficient data to make a clear decision, the full text was considered. Backward and forward searches from the relevant studies were also conducted, and the references of relevant studies were checked as backward search. Also, a forward search was undertaken on the titles of the relevant studies. Articles that had cited these studies were also identified through http://www.scholar.google.com to identify potentially relevant subsequent primary research.
These two independent reviewers assessed the full texts of relevant studies based on inclusion and exclusion criteria, which were proposed by three professionals related to each part of the study: two endodontists (MS, AS), an epidemiologist (MM), and a microbiologist (HS).
Inclusion criteria were as follows:
In vivo or ex vivo study using dentin block model microbiological assessment
Human or bovine dentin
CH in combination with CHX in the CH/CHX group
CH in combination with distilled water or saline in the CH-alone group
E. faecalis as a strain for microbiological assessment
Dressing period of at least 1 day
Quantitative results provided
Exclusion criteria were as follows:
Review article
In vitro study
Vehicles other than distilled water or saline for CH-alone group
CH or CHX as medicament in other materials
Any intervention except medicament dressing for bacterial elimination
Qualitative results or invalid means and standard deviations (SD) reported
Any disagreements on study inclusion and exclusion criteria were discussed and resolved by consulting a third reviewer.
Data extraction, synthesis and analysis
The included studies were reviewed and divided into "comparisons" according to dressing periods and depths of sampling. Sample size, microbiologic unit, depth of sampling, type and concentration of CHX, significance, and means (SD) were recorded for each comparison individually. A microbiological unit for two included studies8,98 was optical density (OD) and for other included studies it was the colony forming unit (CFU). In order to identify the measurement scale, the results of these two studies were transformed from OD to CFU according to microbiological equation (OD of 0.5 corresponding to ~5×108CFU/mL)36. The results of six included studies4,8,15,20,55,98 were converted to the logarithm of CFU in order to achieve identical data for meta-analysis. Since SD had not been reported in some studies15,55,98, it was estimated and used for further analysis by using formula of t-test and application of means, sample size, and p value of each study.
Statistical analysis was performed using Stata software version 10.0 for Windows (Stata Corp LP, College Station, Texas, USA). The level of significance was set at 0.05. After checking the heterogeneity of comparisons using Chi-square analysis, Random-effect meta-analysis model was used to estimate the combined effect. The results of these comparisons were represented by Forest plot. The potential risk of publication bias was evaluated using Begg's and Egger's tests.
Although the antibacterial effect of medicaments was evaluated with different depths of sampling ranges (from 0.05 mm to 0.45 mm) in the included studies, subgroup analysis was performed based on the depth of sampling. Therefore, the comparisons were divided into surface (depth of sampling ≤0.2 mm) and deep (depth of sampling >0.2 mm) dentin groups.
RESULTS
The results of the search strategy are presented in Figure 1. Figure 2 presents a flow chart of the systematic review process. The fnal results of the search in Medline (via PubMed), EMbase, EBSCOhost, The Cochrane Library, SciELO, and BBO were 77, 65, 17, 3, 4, and 7 studies, respectively. After the primary review, 44 studies2-8,14-16,20,22,26-30,35,40,50,52,55-57,62-64,67,68,71,72,80,85,89,95,97,98,102,104,105,107,113-115 were considered relevant, and 40 studies1,9,10,13,19,24,25,31,34,38,42-44,46-49,51,53,54,58-60,65,66,69,75-79,81,83,86,94,103,106,110-112 were irrelevant. Inter-observer agreement (Cohen kappa) was 0.93. One additional study82 was considered relevant by backward and forward search. Thus, 45 studies were considered as irrelevant (5 in vivo, 18 in vitro, 18 ex vivo, and 4 review articles). By implementation of inclusion and exclusion criteria, nine studies4,8,14,15,20,55,80,85,98 were included and 36 studies2,3,5-7,16,22,26-30,35,40,50,52,56,57,62-64,67,68,71,72,82,89,95,97,102,104,105,107,113-115 were excluded (Figure 3).
Figure 1.
Search strategy through PubMed, EMbase, EBSCOhost, The Cochrane Library, SciELO, and BBO
| Entry | Results | |||||
|---|---|---|---|---|---|---|
| PubMed | EMbase | EBSCO | Chochrane | SciELO | BBO | |
| #1: "Enterococcus faecalis" OR "E. faecalis" | 11298 | 18879 | 2938 | 194 | 218 | 197 |
| #2: Chlorhexidine | 7664 | 14568 | 2167 | 2222 | 192 | 438 |
| #3: "Calcium hydroxide" | 4259 | 4599 | 1540 | 296 | 163 | 703 |
| #4: antibacterial OR antimicrobial | 1286445 | 2250318 | 80454 | 7143 | 2775 | 447 |
| #5: #1 AND #2 AND #3 AND #4 | 77 | 65 | 17 | 3 | 4 | 7 |
Figure 2.
Flow chart of the search strategy
Figure 3.
Excluded studies with reasons for exclusion
| Studies | Exclusion criteria |
|---|---|
| Estrela, et al.16 (2001) | 2 & 6 |
| Basrani, et al.7 (2002) | 5 |
| Gomes, et al.27 (2003) | 3 |
| Haenni, et al.35 (2003) | 2 |
| Lin, et al.52 (2003) | 2 |
| Zehnder, et al.114 (2003) | 6 |
| Siren, et al.95 (2004) | 6 |
| Zerella, et al.115 (2005) | 5 |
| Onçag, et al.68 (2006) | 2 |
| Oztan, et al.71 (2006) | 2 & 3 & 4 |
| Gomes, et al.28 (2006) | 2 |
| Ballal, et al.6 (2007) | 2 |
| Wang Kou; Siguas Meneses113 (2007) | 4 & 5 |
| Souza-Filho, et al.97 (2008) | 2 |
| Vianna, et al.109 (2008) | 2 & 5 |
| Gomes, et al.26 (2009) | 2 |
| Ravishanker; Rao82 (2009) | 2 |
| Aguiar3 (2009) | 5 |
| Turk, et al.102 (2009) | 2 |
| Valera, et al.105 (2009) | 5 |
| Jhamb, et al.40 (2010) | 2 |
| Mohammadi62 (2010) | 1 |
| Gondim29 (2010) | 3 & 5 |
| Oliveira, et al.67 (2010) | 2 & 3 |
| Maekawa56 (2010) | 5 |
| Valera, et al.104 (2010) | 5 |
| Mohammadi; Dummer63 (2011) | 1 |
| Silveira, et al.89 (2011) | 2 |
| Gondim, et al.30 (2012) | 2 & 3 |
| Lima, et al.50 (2012) | 3 |
| Fedorowicz, et al.22 (2012) | 1 |
| Maekawa, et al.57 (2013) | 5 |
| Pacios, et al.72 (2012) | 2 |
| Adl, et al.2 (2012) | 2 |
| Mohammadi; Shalavi64 (2012) | 1 |
| Atila-Pektaş, et al.5 (2013) | 4 |
1=Review article, 2=In vitro study, 3=Vehicles other than distilled water or saline for CH-alone group, 4=CH or CHX as medicament in other materials, 5=Any intervention except medicament dressing for bacterial elimination, 6=Qualitative results or invalid means and standard deviations (SD) reported
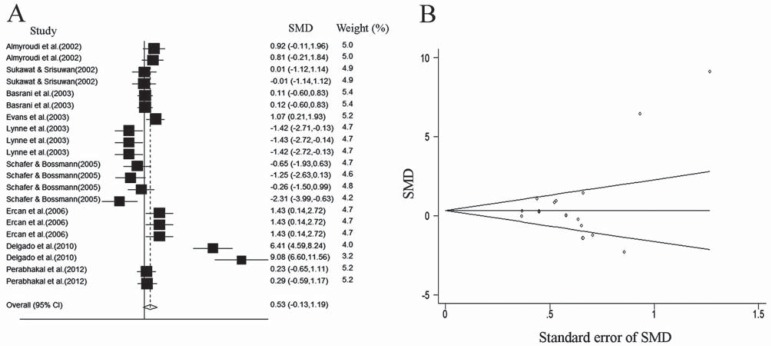
Twenty-one comparisons from the nine included studies were extracted (Table 1). Eight comparisons showed no significant differences in antibacterial effects between CH and CH/CHX mixture; ten comparisons showed significant differences in favor of CH/CHX mixture; and 3 comparisons showed significant differences in favor of CH alone against E. faecalis. The 21 comparisons were heterogeneous (Cochran Q Test of Homogeneity (X2)=144.22, df=20, p<0.001). Therefore, random effect method for combining comparison estimates was used and an overall estimate was produced. There were no significant differences in antibacterial effects between CH/CHX mixture and CH alone against E. faecalis (p=0.115) (Figure 4A).
Table 1.
Comparisons within 9 included studies
| Reference | Sample size | Microbiologic unit | Depth (mm) | Dressing period (day) | Sig. | CHX type & concentration | Mean (SD) | |
|---|---|---|---|---|---|---|---|---|
| CH | CH/CHX | |||||||
| Almyroudi, et al.4 (2002) | 16 | CFU | 0.1 | 14 | 0 | 1% Gel | 2.70(2.90) | 0.70(0.97) |
| Almyroudi, et al.4 (2002) | 16 | CFU | 0.35 | 14 | 0 | 1% Gel | 1.57(1.85) | 0.40(0.85) |
| Sukawat; Srisuwan98 (2002) | 12 | OD | 0.2 | 7 | 0 | 0.2% Sol | 9.44(0.76*) | 9.43(0.76*) |
| Sukawat; Srisuwan98 (2002) | 12 | OD | 0.35 | 7 | 0 | 0.2% Sol | 9.70(3.80*) | 9.75(3.80*) |
| Basrani, et al.8 (2003) | 30 | OD | 0.1 | 7 | 1 | 0.2% Gel | 8.76(7.78*) | 7.90(7.60*) |
| Basrani, et al.8 (2003) | 30 | OD | 0.2 | 7 | 1 | 0.2% Gel | 8.83(7.60*) | 7.95(7.48*) |
| Evans, et al.20 (2003) | 24 | CFU | 0.45 | 7 | 1 | 2% Sol | 3.02(1.50) | 1.36(1.61) |
| Lynne, et al.55 (2003) | 12 | CFU | 0.29 | 1 | 2 | 0.12% Sol | 5.25(0.35*) | 5.75(0.35*) |
| Lynne, et al.55 (2003) | 12 | CFU | 0.35 | 1 | 2 | 0.12% Sol | 5.17(0.26*) | 5.54(0.26*) |
| Lynne, et al.55 (2003) | 12 | CFU | 0.42 | 1 | 2 | 0.12% Sol | 5.20(0.31*) | 5.64(0.31*) |
| Schafer; Bossmann85 (2005) | 10 | CFU | 0.05 | 3 | 0 | 2% Sol | 2.14(0.26) | 2.57(0.04) |
| Schafer; Bossmann85 (2005) | 10 | CFU | 0.1 | 3 | 0 | 2% Sol | 1.85(0.41) | 1.93(0.16) |
| Schafer; Bossmann85 (2005) | 10 | CFU | 0.15 | 3 | 0 | 2% Sol | 1.43(0.18) | 1.52(0.08) |
| Schafer; Bossmann85 (2005) | 10 | CFU | 0.2 | 3 | 0 | 2% Sol | 1.06(0.31) | 1.36(0.14) |
| Ercan, et al.15 (2006) | 12 | CFU | 0.4 | 7 | 1 | 2% Sol | 7.90(0.42*) | 7.30(0.42*) |
| Ercan, et al.15 (2006) | 12 | CFU | 0.4 | 15 | 1 | 2% Sol | 7.90(0.63*) | 7.00(0.63*) |
| Ercan, et al.15 (2006) | 12 | CFU | 0.4 | 30 | 1 | 2% Sol | 7.90(0.63*) | 7.00(0.63*) |
| Delgado, et al.14 (2010) | 30 | CFU | 0.1 | 14 | 1 | 2% Gel | 4.01(0.42) | 0.50(0.35) |
| Delgado, et al.14 (2010) | 30 | CFU | 0.2 | 14 | 1 | 2% Gel | 3.69(0.47) | 0.77(0.44) |
| Perabhakal, et al.80 (2012) | 20 | CFU | 0.16 | 1 | 1 | 0.5% Sol | 2.40(1.68) | 2.05(1.35) |
| Perabhakal, et al.80 (2012) | 20 | CFU | 0.16 | 7 | 1 | 0.5% Sol | 2.28(1.62) | 1.87(1.16) |
Sig.=Significance, CHX=Chlorhexidine, CH=Calcium Hydroxide, Sol=Solution, CFU=Colony Forming Unit, OD=Optical Density,
=estimated standard deviation, Sig. 0= no significant difference between CH and CH/CHX, Sig. 1 = in favor of CH/ CHX, Sig. 2= in favor of CH. SD= standard deviation.
Figure 4.
A: Forest plot for antibacterial effect of medicaments on E. faecalis. The box, its size and the horizontal line show the point of estimation, statistical weight and 95% confidence interval of each comparison, respectively. The diamond at the bottom of the figure illustrates the combined effect on the random effect model; B: Begg's Funnel Plot with 95% confidence limit for antibacterial effect of medicaments on E. faecalis. The plot shows a low risk of publication bias among the included articles. SMD: Standardized Mean Differentiation
The estimated ranks of correlation coefficients of Begg's and Egger's tests were 0.21 (p=0.809) and 0.23 (p=0.215), respectively, which means that there is no evidence for considerable publication bias in this study (Figure 4B).
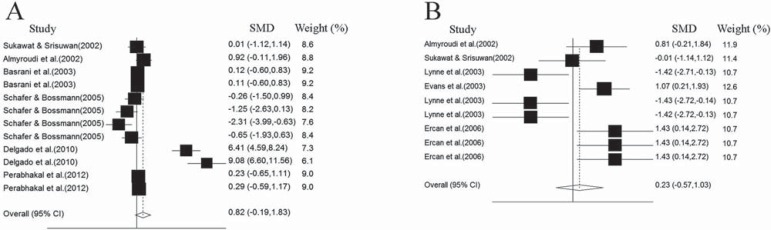
In addition, subgroup analysis showed no significant differences in antibacterial effects between CH/CHX mixture and CH alone against E. faecalis in the surface (p=0.11) and deep (p=0.57) dentin (Figures 5A and 5B).
Figure 5.
A and B: Forest plots for antibacterial effect of medicaments against E. faecalis in surface and deep dentin respectively. SMD: Standardized Mean Differentiation
DISCUSSION
The beneft of mixing CH with CHX to improve the antibacterial property of CH as an intracanal medicament in elimination of E. faecalis remains a matter of controversy. The possible reasons for this controversy are the differences in the methods and materials used, including microbiological assessments (i.e. agar diffusion method, dentin block model etc.), concentrations and physical forms of CHX (i.e. gel, solution), time periods of experiments, strains and concentrations of E. faecalis, methods of bacterial inoculation, methods used for placing the medicaments, and depths of sampling.
Various methods have been used in order to defne the antimicrobial effects of intracanal medicaments, such as dentin powder model, dentin block model, agar diffusion method, and broth dilution method. Agar diffusion is an in vitro model which has been the most commonly used technique91. However, it has some critical disadvantages, including "carryover" effect, unknown reactions between agar plate ingredients and the antimicrobial agent, absence of a true correlation between the results of agar diffusion method and the in vivo environment, the buffering capacity of the agar plate compromising the capacity of antimicrobial agent, and absence of differentiation between bactericidal and bacteriostatic agents33,91. Therefore, agar diffusion method was considered as an exclusion criterion. Dentin powder is an ex vivo model that has also some disadvantages, including partial loss of microanatomical structure of the tooth and the difficulty to create microbial biofilm33, therefore, it was also set as an exclusion criterion. Dentin block model, another ex vivo model, is the most standard method, and a statistical comparison is somehow feasible34. Penetration into dentinal tubules is the most important resistance mechanism of E. faecalis against antibacterial agents in endodontic 1=Review article, 2=In vitro study, 3=Vehicles other than distilled water or saline for CH-alone group, 4=CH or CHX as medicament in other materials, 5=Any intervention except medicament dressing for bacterial elimination, 6=Qualitative results or invalid means and standard deviations (SD) reported treatment32,92. This model provides reconstruction of the microanatomy of dentin, especially dentinal tubules. Dentin block model also simulates the chemical environment of the root canal and the ability of biofilm development33, therefore, it was set as an inclusion criterion.
In general, three types of vehicles are used for preparing CH: aqueous, viscous, and oil21. The first group promotes a high degree of solubility when the paste remains in direct contact with tissues and tissue fuids21. The two other types result in the lower solubility and diffusion of the paste within the tissues21. In addition, some aqueous vehicles such as camphorated monochlorophenol have antibacterial effect on microorganisms, therefore, vehicles other than distilled water or saline solution for CH-alone group was set as exclusion criteria.
Since the evaluation of antibacterial effect of CH as an intracanal dressing was the aim of this meta-analysis, the use of antimicrobial irrigants in addition to the CH intracanal medication were considered confounding factors. Some relevant articles have presented this confounding factor as chemomechanical preparation of the canal before CH dressing and after microbial suspension inoculation into the canal3,29,56,57,104,105,108,113,115. In addition, one study has applied chemomechanical preparation after CH dressing7, leading to the exclusion of these studies.
The time needed for CH to optimally disinfect the root canal system is still unknown and might be related to root canal exudate, the microorganism type, microorganism location in the root canal system, the smear layer, and the degree of susceptibility to the medication28. Although Shuping, et al.88 (2000) reported that use of CH in the canals for 1 week resulted in a 92.5% reduction, evidence shows that CHX has antibacterial activity against E. faecalis after 1 day27,34,45. In addition, two studies showed that CH can be effective against enterococci after 24 hours84,96. Therefore, at least one day of dressing period was set as an inclusion criterion. Furthermore, the main dentinal structure of human and bovine teeth is not significantly different32. Therefore, the results of studies using both of them were used in the meta-analysis.
Evans, et al.20 (2003) evaluated the antibacterial effect of CH/CHX mixture with two different depths of sampling, but they reported one mean and SD. Therefore, this study was considered as one comparison and was included in the meta-analysis. Another included study4 was divided into nine comparisons according to different dressing periods and depths of sampling, but seven comparisons were excluded because of invalid means and standard deviations.
Qualitative data are not suitable for metaanalysis. Despite meeting all the inclusion criteria, two relevant studies95,114 were excluded because they did not provide quantitative data. The results of four included studies8,14,55,98 were illustrated in charts. Therefore, quantitative data were extracted from the illustrated charts using Adobe Photoshop software 5.0 (Adobe Systems, Mountain View, CA, USA) for Windows. To achieve more reliable data, a 300% zoom was used.
The greatest difference in the antibacterial activity of CH/CHX and CH groups has been reported in the study performed by Delgado, et al.14 (2010). This might be due to longer dressing period, sample size, type and concentration of CHX compared with other studies.
In the present study, Cochran Q Test of Homogeneity showed that the 21 comparisons were heterogeneous. This might be due to differences in subjects (human or bovine dentin), method of medicament placement, dressing period, depth of sampling, and type and concentration of CHX.
Meta-analysis is a research tool designed to analyze and combine the inconsistent results of controversial subjects, particularly with those of randomized clinical trials. However, this method has been applied to in vitro studies23,73,87,107. Since there were no clinical trials on the subject of this systematic review, in vitro studies had to be selected. Therefore, only ex vivo dentin block model studies were selected, which have the greatest similarity to clinical conditions. This model, in comparison to other microbiological assessment models, is of high methodological quality and can simulate the clinical situation in the best way possible. On the other hand, the effectiveness of the medicament in vivo can be reduced by a variety of factors. These include problems in delivery, low overall volume, poor/incomplete penetration in the main root canal system, poor penetration into dentin, short contact time, or inactivation of the activity of the antibacterial agent by one or more of the chemical compounds present in the necrotic root canal.
The results of the present meta-analysis showed that CHX does not increase the antibacterial effect of CH. This may be due to deprotonation of CHX at high pH, which reduces its solubility and alters its interaction with bacterial surfaces as a result of the altered charge of the molecule64.
In conclusion it appears that mixing CH with CHX does not improve its ex vivo antibacterial property as an intracanal medicament against E. faecalis. Further in vivo studies are necessary to confirm and correlate the findings of this study with the clinical outcomes.
ACKNOWLEDGMENTS
This study was supported by Isfahan University of Medical Sciences, Iran (grant no. 291230). The authors declare no conflict of interests.
References
- 1.Abdullah M, Ng YL, Gulabivala K, Moles DR, Spratt DA. Susceptibilties of two Enterococcus faecalis phenotypes to root canal medications. J Endod. 2005;31:30–36. doi: 10.1097/01.don.0000136205.80807.5a. [DOI] [PubMed] [Google Scholar]
- 2.Adl A, Shojaee NS, Motamedifar M. A comparison between the antimicrobial effects of triple antibiotic paste and calcium hydroxide against Enterococcus faecalis. Iran Endod J. 2012;7:149–155. [PMC free article] [PubMed] [Google Scholar]
- 3.Aguiar APS. Ação in vitro do extrato glicólico de gengibre e medicamentos sobre Candida albicans, Enterococcus faecalis, Escherichia coli e sua endotoxina em canais radiculares [Masters Degree] São José dos Campos: Univ. Estadual Paulista - UNESP; 2009. Available from: http://www.athena.biblioteca.unesp.br/exlibris/bd/bsj/33004145070P8/2009/aguiar_aps_me_sjc.pdf. [Google Scholar]
- 4.Almyroudi A, Mackenzie D, McHugh S, Saunders WP. The effectiveness of various disinfectants used as endodontic intracanal medications: an in vitro study. J Endod. 2002;28:163–167. doi: 10.1097/00004770-200203000-00005. [DOI] [PubMed] [Google Scholar]
- 5.Atila-Pektas B, Yurdakul P, Gülmez D, Görduysus O. Antimicrobial effects of root canal medicaments against Enterococcus faecalis and Streptococcus mutans. Int Endod J. 2013;46:413–418. doi: 10.1111/iej.12004. [DOI] [PubMed] [Google Scholar]
- 6.Ballal V, Kundabala M, Acharya S, Ballal M. Antimicrobial action of calcium hydroxide, chlorhexidine and their combination on endodontic pathogens. Aust Dent J. 2007;52:118–121. doi: 10.1111/j.1834-7819.2007.tb00475.x. [DOI] [PubMed] [Google Scholar]
- 7.Basrani B, Santos JM, Tjäderhane L, Grad H, Gorduysus O, Huang J, et al. Substantive antimicrobial activity in chlorhexidine-treated human root dentin. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002;94:240–245. doi: 10.1067/moe.2002.124002. [DOI] [PubMed] [Google Scholar]
- 8.Basrani B, Tjäderhane L, Santos JM, Pascon E, Grad H, Lawrence HP, et al. Efficacy of chlorhexidine- and calcium hydroxide-containing medicaments against Enterococcus faecalis in vitro. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003;96:618–624. doi: 10.1016/s1079-2104(03)00166-5. [DOI] [PubMed] [Google Scholar]
- 9.Bhardwaj A, Ballal S, Velmurugan N. Comparative evaluation of the antimicrobial activity of natural extracts of Morinda citrifolia, papain and aloe vera (all in gel formulation), 2% chlorhexidine gel and calcium hydroxide, against Enterococcus faecalis: an in vitro study. J Conserv Dent. 2012;15:293–297. doi: 10.4103/0972-0707.97964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bozza FL, Molgatini SL, Pérez SB, Tejerina DP, Pérez Tito RI, Kaplan AE. Antimicrobial effect in vitro of chlorhexidine and calcium hydroxide impregnated gutta-percha points. Acta Odontol Latinoam. 2005;18:51–56. [PubMed] [Google Scholar]
- 11.Byström A, Claesson R, Sundqvist G. The antibacterial effect of camphorated paramonochlorophenol, camphorated phenol and calcium hydroxide in the treatment of infected root canals. Dent Traumatol. 1985;1:170–175. doi: 10.1111/j.1600-9657.1985.tb00652.x. [DOI] [PubMed] [Google Scholar]
- 12.Byström A, Sundqvist G. Bacteriologic evaluation of the efficacy of mechanical root canal instrumentation in endodontic therapy. Scand J Dent Res. 1981;89:321–328. doi: 10.1111/j.1600-0722.1981.tb01689.x. [DOI] [PubMed] [Google Scholar]
- 13.Cook J, Nandakumar R, Fouad AF. Molecular- and culture-based comparison of the effects of antimicrobial agents on bacterial survival in infected dentinal tubules. J Endod. 2007;33:690–692. doi: 10.1016/j.joen.2007.01.022. [DOI] [PubMed] [Google Scholar]
- 14.Delgado RJ, Gasparoto TH, Sipert CR, Pinheiro CR, Moraes IG, Garcia RB, et al. Antimicrobial effects of calcium hydroxide and chlorhexidine on Enterococcus faecalis. J Endod. 2010;36:1389–1393. doi: 10.1016/j.joen.2010.04.013. [DOI] [PubMed] [Google Scholar]
- 15.Ercan E, Dalli M, Dülgergil CT. In vitro assessment of the effectiveness of chlorhexidine gel and calcium hydroxide paste with chlorhexidine against Enterococcus faecalis and Candida albicans. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;102:e27–e31. doi: 10.1016/j.tripleo.2006.02.022. [DOI] [PubMed] [Google Scholar]
- 16.Estrela C, Bammann LL, Pimenta FC, Pécora JD. Control of microorganisms in vitro by calcium hydroxide pastes. Int Endod J. 2001;34:341–345. doi: 10.1046/j.1365-2591.2001.00368.x. [DOI] [PubMed] [Google Scholar]
- 17.Estrela CR, Estrela C, Reis C, Bammann LL, Pécora JD. Control of microorganisms in vitro by endodontic irrigants. Braz Dent J. 2003;14:187–192. doi: 10.1590/s0103-64402003000300009. [DOI] [PubMed] [Google Scholar]
- 18.Evanov C, Liewehr F, Buxton TB, Joyce AP. Antibacterial efficacy of calcium hydroxide and chlorhexidine gluconate irrigants at 37 degrees C and 46 degrees C. J Endod. 2004;30:653–657. doi: 10.1097/01.don.0000121620.11272.22. [DOI] [PubMed] [Google Scholar]
- 19.Evans M, Davies J, Sundqvist G, Figdor D. Mechanisms involved in the resistance of Enterococcus faecalis to calcium hydroxide. Int Endod J. 2002;35:221–228. doi: 10.1046/j.1365-2591.2002.00504.x. [DOI] [PubMed] [Google Scholar]
- 20.Evans MD, Baumgartner JC, Khemaleelakul SU, Xia T. Efficacy of calcium hydroxide: chlorhexidine paste as an intracanal medication in bovine dentin. J Endod. 2003;29:338–339. doi: 10.1097/00004770-200305000-00005. [DOI] [PubMed] [Google Scholar]
- 21.Fava LR, Saunders WP. Calcium hydroxide pastes: classification and clinical indications. Int Endod J. 1999;32:257–282. doi: 10.1046/j.1365-2591.1999.00232.x. [DOI] [PubMed] [Google Scholar]
- 22.Fedorowicz Z, Nasser M, Sequeira-Byron P, Souza RF, Carter B, Heft M. Irrigants for non-surgical root canal treatment in mature permanent teeth. Cochrane Database Syst Rev. 2012;9: doi: 10.1002/14651858.CD008948.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Finnema KJ, Özcan M, Post WJ, Ren Y, Dijkstra PU. In-vitro orthodontic bond strength testing: a systematic review and meta-analysis. Am J Orthod Dentofac Orthop. 2010;137:615-22.e3. doi: 10.1016/j.ajodo.2009.12.021. [DOI] [PubMed] [Google Scholar]
- 24.Fouad AF, Barry J. The effect of antibiotics and endodontic antimicrobials on the polymerase chain reaction. J Endod. 2005;31:510–513. doi: 10.1097/01.don.0000152899.54247.4e. [DOI] [PubMed] [Google Scholar]
- 25.Gomes BP, Ferraz CC, Garrido FD, Rosalen PL, Zaia AA, Teixeira FB, et al. Microbial susceptibility to calcium hydroxide pastes and their vehicles. J Endod. 2002;28:758–761. doi: 10.1097/00004770-200211000-00003. [DOI] [PubMed] [Google Scholar]
- 26.Gomes BP, Montagner F, Berber VB, Zaia AA, Ferraz CC, Almeida JF, et al. Antimicrobial action of intracanal medicaments on the external root surface. J Dent. 2009;37:76–81. doi: 10.1016/j.jdent.2008.09.009. [DOI] [PubMed] [Google Scholar]
- 27.Gomes BP, Souza SF, Ferraz CC, Teixeira FB, Zaia AA, Valdrighi L, et al. Effectiveness of 2% chlorhexidine gel and calcium hydroxide against Enterococcus faecalis in bovine root dentine in vitro. Int Endod J. 2003;36:267–275. doi: 10.1046/j.1365-2591.2003.00634.x. [DOI] [PubMed] [Google Scholar]
- 28.Gomes BP, Vianna ME, Sena NT, Zaia AA, Ferraz CC, Souza FJ., Filho In vitro evaluation of the antimicrobial activity of calcium hydroxide combined with chlorhexidine gel used as intracanal medicament. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;102:544–550. doi: 10.1016/j.tripleo.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 29.Gondim JO. Efeito antibacteriano e sucesso clínico/radiográfico da pasta de hidróxido de cálcio associada à clorexidina como medicação intracanal no tratamento de dentes decíduos humanos com polpa necrótica [PhD] Araraquara: Univ. Estadual Paulista - UNESP; 2010. Available from: http://www.athena.biblioteca.unesp.br/exlibris/bd/boa/33004030010P2/2010/gondim_jo_dr_arafo.pdf. [Google Scholar]
- 30.Gondim JO, Avaca-Crusca JS, Valentini SR, Zanelli CF, Spolidorio DM, Giro EM. Effect of a calcium hydroxide/chlorhexidine paste as intracanal dressing in human primary teeth with necrotic pulp against Porphyromonas gingivalis and Enterococcus faecalis. Int J Paediatr Dent. 2012;22:116–124. doi: 10.1111/j.1365-263X.2011.01174.x. [DOI] [PubMed] [Google Scholar]
- 31.Gurgel-Filho ED, Vivacqua-Gomes N, Gomes BP, Ferraz CC, Zaia AA, Souza-Filho FJ. In vitro evaluation of the effectiveness of the chemomechanical preparation against Enterococcus faecalis after single- or multiple-visit root canal treatment. Braz Oral Res. 2007;21:308–313. doi: 10.1590/s1806-83242007000400005. [DOI] [PubMed] [Google Scholar]
- 32.Haapasalo M, Ørstavik D. In vitro infection and of dentinal tubules. J Dent Res. 1987;66:1375–1379. doi: 10.1177/00220345870660081801. [DOI] [PubMed] [Google Scholar]
- 33.Haapasalo M, Qian W. Irrigants and intracanal medicaments. In: Ingle JI, Bakland LK, Baumgartner JC, editors. Endodontics. 6th ed. Hamilton: BC Decker; 2008. pp. 996–996. [Google Scholar]
- 34.Haapasalo H, Sirén E, Waltimo T, Ørstavik D, Haapasalo MP. Inactivation of local root canal medicaments by dentine: an in vitro study. Int Endod J. 2000;33:126–131. doi: 10.1046/j.1365-2591.2000.00291.x. [DOI] [PubMed] [Google Scholar]
- 35.Haenni S, Schmidlin PR, Mueller B, Sener B, Zehnder M. Chemical and antimicrobial properties of calcium hydroxide mixed with irrigating solutions. Int Endod J. 2003;36:100–105. doi: 10.1046/j.1365-2591.2003.00629.x. [DOI] [PubMed] [Google Scholar]
- 36.Heikens E, Bonten MJM, Willems RJ. Enterococcal surface protein Esp is important for biofilm formation of Enterococcus faecium E1162. J Bacteriol. 2007;189:8233–8240. doi: 10.1128/JB.01205-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Heithersay GS. Calcium hydroxide in the treatment of pulpless teeth with associated pathology. Int Endod J. 1975;8:74–93. doi: 10.1111/j.1365-2591.1975.tb01000.x. [DOI] [PubMed] [Google Scholar]
- 38.Heling I, Steinberg D, Kenig S, Gavrilovich I, Sela M, Friedman M. Efficacy of a sustained release device containing chlorhexidine and Ca (OH)2 in preventing secondary infection of dentinal tubules. Int Endod J. 1992;25:20–24. doi: 10.1111/j.1365-2591.1992.tb00944.x. [DOI] [PubMed] [Google Scholar]
- 39.Jeansonne MJ, White RR. A comparison of 2.0% chlorhexidine gluconate and 5.25% sodium hypochlorite as antimicrobial endodontic irrigants. J Endod. 1994;20:276–278. doi: 10.1016/s0099-2399(06)80815-0. [DOI] [PubMed] [Google Scholar]
- 40.Jhamb S, Nikhil V, Singh V. An in vitro study of antibacterial effect of calcium hydroxide and chlorhexidine on Enterococcus faecalis. Indian J Dent Res. 2010;21:512–514. doi: 10.4103/0970-9290.74222. [DOI] [PubMed] [Google Scholar]
- 41.Kakehashi S, Stanley HR, Fitzgerald RJ. The effects of surgical exposures of dental pulps in germ-free and conventional laboratory rats. Oral Surg Oral Med Oral Pathol. 1965;20:340–349. doi: 10.1016/0030-4220(65)90166-0. [DOI] [PubMed] [Google Scholar]
- 42.Kandaswamy D, Venkateshbabu N, Gogulnath D, Kindo AJ. Dentinal tubule disinfection with 2% chlorhexidine gel, propolis, morinda citrifolia juice, 2% povidone iodine, and calcium hydroxide. Int Endod J. 2010;43:419–423. doi: 10.1111/j.1365-2591.2010.01696.x. [DOI] [PubMed] [Google Scholar]
- 43.Kayaoglu G, Erten H, Ørstavik D. Possible role of the adhesin ace and collagen adherence in conveying resistance to disinfectants on Enterococcus faecalis. Oral Microbiol Immunol. 2008;23:449–454. doi: 10.1111/j.1399-302X.2008.00446.x. [DOI] [PubMed] [Google Scholar]
- 44.Kayaoglu G, Ömürlü H, Akca G, Gürel M, Gencay Ö, Sorkun K, et al. Antibacterial activity of Propolis versus conventional endodontic disinfectants against Enterococcus faecalis in infected dentinal tubules. J Endod. 2011;37:376–381. doi: 10.1016/j.joen.2010.11.024. [DOI] [PubMed] [Google Scholar]
- 45.Komorowski R, Grad H, Yu Wu X, Friedman S. Antimicrobial substantivity of chlorhexidine-treated bovine root dentin. J Endod. 2000;26:315–317. doi: 10.1097/00004770-200006000-00001. [DOI] [PubMed] [Google Scholar]
- 46.Krithikadatta J, Indira R, Dorothykalyani AL. Disinfection of dentinal tubules with 2% chlorhexidine, 2% metronidazole, bioactive glass when compared with calcium hydroxide as intracanal medicaments. J Endod. 2007;33:1473–1476. doi: 10.1016/j.joen.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 47.Lee JK, Park YJ, Kum KY, Han S, Chang SW, Kaufman B, et al. Antimicrobial efficacy of a human ß-defensin-3 peptide using an Enterococcus faecalis dentine infection model. Int Endod J. 2013;46:406–412. doi: 10.1111/iej.12002. [DOI] [PubMed] [Google Scholar]
- 48.Lee Y, Han SH, Hong SH, Lee JK, Ji H, Kum KY. Antimicrobial efficacy of a polymeric chlorhexidine release device using in vitro model of Enterococcus faecalis dentinal tubule infection. J Endod. 2008;34:855–858. doi: 10.1016/j.joen.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 49.Lenet BJ, Komorowski R, Wu XY, Huang J, Grad H, Lawrence HP, et al. Antimicrobial substantivity of bovine root dentin exposed to different chlorhexidine delivery vehicles. J Endod. 2000;26:652–655. doi: 10.1097/00004770-200011000-00005. [DOI] [PubMed] [Google Scholar]
- 50.Lima RK, Guerreiro-Tanomaru JM, Faria-Júnior NB, Tanomaru-Filho M. Effectiveness of calcium hydroxide-based intracanal medicaments against Enterococcus faecalis. Int Endod J. 2012;45:311–316. doi: 10.1111/j.1365-2591.2011.01976.x. [DOI] [PubMed] [Google Scholar]
- 51.Lin S, Levin L, Weiss EI, Peled M, Fuss Z. In vitro antibacterial efficacy of a new chlorhexidine slow-release device. Quintessence Int. 2006;37:391–394. [PubMed] [Google Scholar]
- 52.Lin YH, Mickel AK, Chogle S. Effectiveness of selected materials against Enterococcus faecalis: part 3. The antibacterial effect of calcium hydroxide and chlorhexidine on Enterococcus faecalis. J Endod. 2003;29:565–566. doi: 10.1097/00004770-200309000-00006. [DOI] [PubMed] [Google Scholar]
- 53.Lucena JM, Decker EM, Walter C, Boeira LS, Löst C, Weiger R. Antimicrobial effectiveness of intracanal medicaments on Enterococcus faecalis: chlorhexidine versus octenidine. Int Endod J. 2012;46:53–61. doi: 10.1111/j.1365-2591.2012.02093.x. [DOI] [PubMed] [Google Scholar]
- 54.Lui JN, Sae-Lim V, Song KP, Chen NN. In vitro antimicrobial effect of chlorhexidine-impregnated gutta percha points on Enterococcus faecalis. Int Endod J. 2004;37:105–113. doi: 10.1111/j.0143-2885.2004.00734.x. [DOI] [PubMed] [Google Scholar]
- 55.Lynne RE, Liewehr FR, West LA, Patton WR, Buxton TB, McPherson JC. In vitro antimicrobial activity of various medication preparations on E. faecalis in root canal dentin. J Endod. 2003;29:187–190. doi: 10.1097/00004770-200303000-00006. [DOI] [PubMed] [Google Scholar]
- 56.Maekawa LE. Avaliação dos extratos de própolis e de gengibre como medicação intracanal sobre microrganismos e endotoxinas em canais radiculares [PhD] São José dos Campos: Univ Estadual Paulista - UNESP; 2010. Available from: http://www.athena.biblioteca.unesp.br/exlibris/bd/bsj/33004145070P8/2010/maekawa_le_dr_sjc.pdf. [Google Scholar]
- 57.Maekawa LE, Valera MC, Oliveira LD, Carvalho CAT, Camargo CH, Jorge AO. Effect of Zingiber officinale and propolis on microorganisms and endotoxins in root canals. J Appl Oral Sci. 2013;21:25–31. doi: 10.1590/1678-7757201302129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Maia EM, Filho, Maia CCR, Bastos ACSC, Novais TMG. In vitro antimicrobial effect of different endodontic materials and propolis on Enterococcus faecalis. RGO. 2008;56:21–25. [Google Scholar]
- 59.Mattigati S, Ratnakar P, Moturi S, Varma S, Rairam S. Antimicrobial effect of conventional root canal medicaments vs propolis against Enterococcus faecalis, Staphylococcus aureus and Candida albicans. J Contemp Dent Prac. 2012;13:305–309. doi: 10.5005/jp-journals-10024-1142. [DOI] [PubMed] [Google Scholar]
- 60.Menezes M, Valera M, Jorge A, Koga-Ito C, Camargo C, Mancini M. In vitro evaluation of the effectiveness of irrigants and intracanal medicaments on microorganisms within root canals. Int Endod J. 2004;37:311–319. doi: 10.1111/j.0143-2885.2004.00799.x. [DOI] [PubMed] [Google Scholar]
- 61.Metzger Z, Basrani B, Goodis H. Instruments, materials, and devices. In: Hargreaves KM, Cohen S, editors. Pathways of the pulp. 10th ed. St. Louis: Mosby; 2011. pp. 283–348. [Google Scholar]
- 62.Mohammadi Z. Chemomechanical strategies to manage endodontic infections. Dent Today. 2010;29:91-2, 4, 6 passim. quiz 9. [PubMed] [Google Scholar]
- 63.Mohammadi Z, Dummer PM. Properties and applications of calcium hydroxide in endodontics and dental traumatology. Int Endod J. 2011;44:697–730. doi: 10.1111/j.1365-2591.2011.01886.x. [DOI] [PubMed] [Google Scholar]
- 64.Mohammadi Z, Shalavi S. Is chlorhexidine an ideal vehicle for calcium hydroxide? A microbiologic review. Iran Endod J. 2012;7:115–122. [PMC free article] [PubMed] [Google Scholar]
- 65.Neelakantan P, Sanjeev K, Subbarao CV. Duration-dependent susceptibility of endodontic pathogens to calcium hydroxide and chlorhexidene gel used as intracanal medicament: an in vitro evaluation. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;104:e138–e141. doi: 10.1016/j.tripleo.2007.04.035. [DOI] [PubMed] [Google Scholar]
- 66.Noetzel J, Nonhoff J, Bitter K, Wagner J, Neumann K, Kielbassa AM. Efficacy of calcium hydroxide, Er:YAG laser or gaseous ozone against Enterococcus faecalis in root canals. Am J Dent. 2009;22:14–18. [PubMed] [Google Scholar]
- 67.Oliveira JC, Alves FR, Uzeda M, Rôças IN, Siqueira JF., Jr Influence of serum and necrotic soft tissue on the antimicrobial effects of intracanal medicaments. Braz Dent J. 2010;21:295–300. doi: 10.1590/s0103-64402010000400001. [DOI] [PubMed] [Google Scholar]
- 68.Onçag O, Cogulu D, Uzel A. Efficacy of various intracanal medicaments against Enterococcus faecalis in primary teeth: an in vivo study. J Clin Pediatr Dent. 2006;30:233–237. [PubMed] [Google Scholar]
- 69.Onçag O, Cogulu D, Uzel A, Sorkun K. Efficacy of propolis as an intracanal medicament against Enterococcus faecalis. Gen Dent. 2006;54:319–322. [PubMed] [Google Scholar]
- 70.Ørstavik D, Haapasalo M. Disinfection by endodontic irrigants and dressings of experimentally infected dentinal tubules. Dent Traumatol. 1990;6:142–149. doi: 10.1111/j.1600-9657.1990.tb00409.x. [DOI] [PubMed] [Google Scholar]
- 71.Oztan MD, Kiyan M, Gerçeker D. Antimicrobial effect, in vitro, of gutta-percha points containing root canal medications against yeasts and Enterococcus faecalis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;102:410–416. doi: 10.1016/j.tripleo.2005.10.073. [DOI] [PubMed] [Google Scholar]
- 72.Pacios MG, Silva C, López ME, Cecilia M. Antibacterial action of calcium hydroxide vehicles and calcium hydroxide pastes. J Invest Clin Dent. 2012;3:264–270. doi: 10.1111/j.2041-1626.2012.00147.x. [DOI] [PubMed] [Google Scholar]
- 73.Papia E, Larsson C, du Toit M, Vult von Steyern P. Bonding between oxide ceramics and adhesive cement systems: a systematic review. J Biomed Mater Res B Appl Biomater. 2014;102:395–413. doi: 10.1002/jbm.b.33013. [DOI] [PubMed] [Google Scholar]
- 74.Peters L, Wesselink P, Moorer W. The fate and the role of bacteria left in root dentinal tubules. Int Endod J. 1995;28:95–99. doi: 10.1111/j.1365-2591.1995.tb00166.x. [DOI] [PubMed] [Google Scholar]
- 75.Piovesani JF, Semenoff-Segundo A, Pedro FL, Borges ÁH, Neves ANP, Mamede I, Neto, et al. Capacidade antimicrobiana de diferentes medicações intracanais sobre o Enterococcus faecalis. Dent Press Endod. 2012;2:53–58. [Google Scholar]
- 76.Podbielski A, Boeckh C, Haller B. Growth inhibitory activity of gutta-percha points containing root canal medications on common endodontic bacterial pathogens as determined by an optimized quantitative in vitro assay. J Endod. 2000;26:398–403. doi: 10.1097/00004770-200007000-00005. [DOI] [PubMed] [Google Scholar]
- 77.Podbielski A, Spahr A, Haller B. Additive antimicrobial activity of calcium hydroxide and chlorhexidine on common endodontic bacterial pathogens. J Endod. 2003;29:340–345. doi: 10.1097/00004770-200305000-00006. [DOI] [PubMed] [Google Scholar]
- 78.Portenier I, Haapasalo H, Rye A, Waltimo T, Ørstavik D, Haapasalo M. Inactivation of root canal medicaments by dentine, hydroxylapatite and bovine serum albumin. Int Endod J. 2001;34:184–188. doi: 10.1046/j.1365-2591.2001.00366.x. [DOI] [PubMed] [Google Scholar]
- 79.Portenier I, Waltimo T, Ørstavik D, Haapasalo M. The susceptibility of starved, stationary phase, and growing cells of Enterococcus faecalis to endodontic medicaments. J Endod. 2005;31:380–386. doi: 10.1097/01.don.0000145421.84121.c8. [DOI] [PubMed] [Google Scholar]
- 80.Prabhakar A, Hadakar SG, Raju OS. Comparative evaluation of pH and antibacterial effect of various calcium hydroxide combinations on E. faecalis and its effect on root strength: an in vitro study. Contemp Clin Dent. 2012;3:42–47. doi: 10.4103/0976-237X.94545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Queiroz AM, Nelson-Filho P, Silva LA, Assed S, Silva RA, Ito IY. Antibacterial activity of root canal filling materials for primary teeth: zinc oxide and eugenol cement, Calen paste thickened with zinc oxide, Sealapex and EndoREZ. Braz Dent J. 2009;20:290–296. doi: 10.1590/s0103-64402009000400005. [DOI] [PubMed] [Google Scholar]
- 82.Ravishanker P, Rao CS. Antimicrobial efficacy of four calcium hydroxide formulations and chlorhexidine gel using agar diffusion model [online] Internet J Dent Sci. 2009;8(1) Available from: http://ispub.com/IJDS/8/1/7932. [Google Scholar]
- 83.Roach RP, Hatton JF, Gillespie MJ. Prevention of the ingress of a known virulent bacterium into the root canal system by intracanal medications. J Endod. 2001;27:657–660. doi: 10.1097/00004770-200111000-00001. [DOI] [PubMed] [Google Scholar]
- 84.Safavi KE, Spngberg LSW, Langeland K. Root canal dentinal tubule disinfection. J Endod. 1990;16:207–210. doi: 10.1016/s0099-2399(06)81670-5. [DOI] [PubMed] [Google Scholar]
- 85.Schäfer E, Bössmann K. Antimicrobial efficacy of chlorhexidine and two calcium hydroxide formulations against Enterococcus faecalis. J Endod. 2005;31:53–56. doi: 10.1097/01.don.0000134209.28874.1c. [DOI] [PubMed] [Google Scholar]
- 86.Schirrmeister JF, Liebenow AL, Braun G, Wittmer A, Hellwig E, Al-Ahmad A. Detection and eradication of microorganisms in root-filled teeth associated with periradicular lesions: an in vivo study. J Endod. 2007;33:536–540. doi: 10.1016/j.joen.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 87.Shahravan A, Haghdoost AA, Adl A, Rahimi H, Shadifar F. Effect of smear layer on sealing ability of canal obturation: a systematic review and meta-analysis. J Endod. 2007;33:96–105. doi: 10.1016/j.joen.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 88.Shuping GB, Orstavik D, Sigurdsson A, Trope M. Reduction of intracanal bacteria using nickel-titanium rotary instrumentation and various medications. J Endod. 2000;26:751–755. doi: 10.1097/00004770-200012000-00022. [DOI] [PubMed] [Google Scholar]
- 89.Silveira CF, Cunha RS, Fontana CE, Martin AS, Gomes BP, Motta RH, et al. Assessment of the antibacterial activity of calcium hydroxide combined with chlorhexidine paste and other intracanal medications against bacterial pathogens. Eur J Dent. 2011;5:1–7. [PMC free article] [PubMed] [Google Scholar]
- 90.Siqueira JF, Jr, Batista MM, Fraga RC, Uzeda M. Antibacterial effects of endodontic irrigants on black-pigmented gram-negative anaerobes and facultative bacteria. J Endod. 1998;24:414–416. doi: 10.1016/S0099-2399(98)80023-X. [DOI] [PubMed] [Google Scholar]
- 91.Siqueira JF, Jr, de Uzeda M. Intracanal medicaments: evaluation of the antibacterial effects of chlorhexidine, metronidazole, and calcium hydroxide associated with three vehicles. J Endod. 1997;23:167–169. doi: 10.1016/S0099-2399(97)80268-3. [DOI] [PubMed] [Google Scholar]
- 92.Siqueira JF, Jr, de Uzeda M, Fonseca ME. A scanning electron microscopic evaluation of in vitro dentinal tubules penetration by selected anaerobic bacteria. J Endod. 1996;22:308–310. doi: 10.1016/S0099-2399(96)80265-2. [DOI] [PubMed] [Google Scholar]
- 93.Siqueira JF, Jr, Lopes H. Mechanisms of antimicrobial activity of calcium hydroxide: a critical review. Int Endod J. 1999;32:361–369. doi: 10.1046/j.1365-2591.1999.00275.x. [DOI] [PubMed] [Google Scholar]
- 94.Siqueira JF, Jr, Paiva SS, Rôças IN. Reduction in the cultivable bacterial populations in infected root canals by a chlorhexidine-based antimicrobial protocol. J Endod. 2007;33:541–547. doi: 10.1016/j.joen.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 95.Siren EK, Haapasalo MP, Waltimo TM, Orstavik D. In vitro antibacterial effect of calcium hydroxide combined with chlorhexidine or iodine potassium iodide on Enterococcus faecalis. Eur J Oral Sci. 2004;112:326–331. doi: 10.1111/j.1600-0722.2004.00144.x. [DOI] [PubMed] [Google Scholar]
- 96.Sjögren U, Figdor D, Spångberg L, Sundqvist G. The antimicrobial effect of calcium hydroxide as a short-term intracanal dressing. Int Endod J. 2007;24:119–125. doi: 10.1111/j.1365-2591.1991.tb00117.x. [DOI] [PubMed] [Google Scholar]
- 97.Souza-Filho FJ, Soares AJ, Vianna ME, Zaia AA, Ferraz CC, Gomes BP. Antimicrobial effect and pH of chlorhexidine gel and calcium hydroxide alone and associated with other materials. Braz Dent J. 2008;19:28–33. doi: 10.1590/s0103-64402008000100005. [DOI] [PubMed] [Google Scholar]
- 98.Sukawat C, Srisuwan T. A comparison of the antimicrobial efficacy of three calcium hydroxide formulations on human dentin infected with Enterococcus faecalis. J Endod. 2002;28:102–104. doi: 10.1097/00004770-200202000-00013. [DOI] [PubMed] [Google Scholar]
- 99.Sundqvist G. Ecology of the root canal flora. J Endod. 1992;18:427–430. doi: 10.1016/S0099-2399(06)80842-3. [DOI] [PubMed] [Google Scholar]
- 100.Sundqvist G, Figdor D, Persson S, Sjögren U. Microbiologic analysis of teeth with failed endodontic treatment and the outcome of conservative re-treatment. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;85:86–93. doi: 10.1016/s1079-2104(98)90404-8. [DOI] [PubMed] [Google Scholar]
- 101.Tronstad L, Andreasen JO, Hasselgren G, Kristerson L, Riis I. pH changes in dental tissues after root canal filling with calcium hydroxide. J Endod. 1981;7:17–21. doi: 10.1016/S0099-2399(81)80262-2. [DOI] [PubMed] [Google Scholar]
- 102.Turk BT, Sen BH, Ozturk T. In vitro antimicrobial activity of calcium hydroxide mixed with different vehicles against Enterococcus faecalis and Candida albicans. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;108:297–301. doi: 10.1016/j.tripleo.2009.03.029. [DOI] [PubMed] [Google Scholar]
- 103.Vaghela DJ, Kandaswamy D, Venkateshbabu N, Jamini N, Ganesh A. Disinfection of dentinal tubules with two different formulations of calcium hydroxide as compared to 2% chlorhexidine: as intracanal medicaments against Enterococcus faecalis and Candida albicans: an in vitro study. J Conserv Dent. 2011;14:182–186. doi: 10.4103/0972-0707.82625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Valera MC, Salvia AC, Maekawa LE, Camargo SE, Carvalho CA, Camargo CH, et al. Antimicrobial analysis of chlorhexidine gel and intracanal medicaments against microorganisms inoculated in root canals. Minerva Stomatol. 2010;59:415–421. [PubMed] [Google Scholar]
- 105.Valera MC, Silva KC, Maekawa LE, Carvalho CA, Koga-Ito CY, Camargo CH, et al. Antimicrobial activity of sodium hypochlorite associated with intracanal medication for Candida albicans and Enterococcus faecalis inoculated in root canals. J Appl Oral Sci. 2009;17:555–559. doi: 10.1590/S1678-77572009000600003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Van der Waal SV, Jiang LM, de Soet JJ, van der Sluis LW, Wesselink PR, Crielaard W. Sodium chloride and potassium sorbate: a synergistic combination against Enterococcus faecalis biofilms: an in vitro study. Eur J Oral Sci. 2012;120:452–457. doi: 10.1111/j.1600-0722.2012.00982.x. [DOI] [PubMed] [Google Scholar]
- 107.Vanajasan PP, Dhakshinamoorthy M, Rao CS. Factors affecting the bond strength of self-etch adhesives: a meta-analysis of literature. J Conserv Dent. 2011;14:62–67. doi: 10.4103/0972-0707.80746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Vianna ME, Gomes BP. Efficacy of sodium hypochlorite combined with chlorhexidine against Enterococcus faecalis in vitro. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;107:585–589. doi: 10.1016/j.tripleo.2008.10.023. [DOI] [PubMed] [Google Scholar]
- 109.Vianna ME, Horz HP, Conrads G, Feres M, Gomes BP. Comparative analysis of endodontic pathogens using checkerboard hybridization in relation to culture. Oral Microbiol Immunol. 2008;23:282–290. doi: 10.1111/j.1399-302X.2007.00425.x. [DOI] [PubMed] [Google Scholar]
- 110.Vidya S. Old and novel intracanal medicaments against Candida albicans. Aust Dent J. 2007;52:257. doi: 10.1111/j.1834-7819.2007.tb00498.x. [DOI] [PubMed] [Google Scholar]
- 111.Vivacqua-Gomes N, Gurgel-Filho ED, Gomes BP, Ferraz CC, Zaia AA, Souza-Filho FJ. Recovery of Enterococcus faecalis after single- or multiple-visit root canal treatments carried out in infected teeth ex vivo. Int Endod J. 2005;38:697–704. doi: 10.1111/j.1365-2591.2005.00992.x. [DOI] [PubMed] [Google Scholar]
- 112.Wang CS, Arnold RR, Trope M, Teixeira FB. Clinical efficiency of 2% chlorhexidine gel in reducing intracanal bacteria. J Endod. 2007;33:1283–1289. doi: 10.1016/j.joen.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 113.Wang Kou LV, Siguas Meneses DM. Comparative study of the antibacterial effects of the association of chlorhexidine 2% solution, calcium hydroxide, points of calcium hydroxide and points of chlorhexidine against Enterococcus faecalis. Kiru. 2007;4:14–16. [Google Scholar]
- 114.Zehnder M, Grawehr M, Hasselgren G, Waltimo T. Tissue-dissolution capacity and dentin-disinfecting potential of calcium hydroxide mixed with irrigating solutions. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003;96:608–613. doi: 10.1016/s1079-2104(03)00157-4. [DOI] [PubMed] [Google Scholar]
- 115.Zerella JA, Fouad AF, Spångberg LS. Effectiveness of a calcium hydroxide and chlorhexidine digluconate mixture as disinfectant during retreatment of failed endodontic cases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;100:756–761. doi: 10.1016/j.tripleo.2005.05.072. [DOI] [PubMed] [Google Scholar]