Abstract
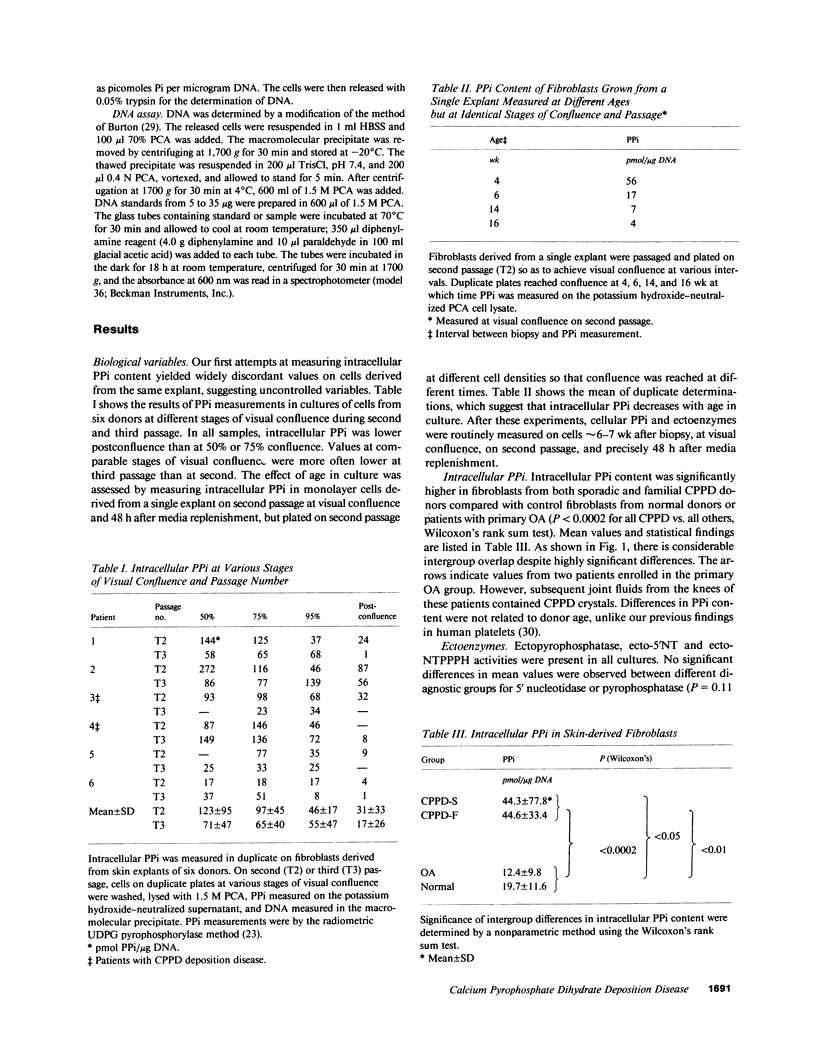
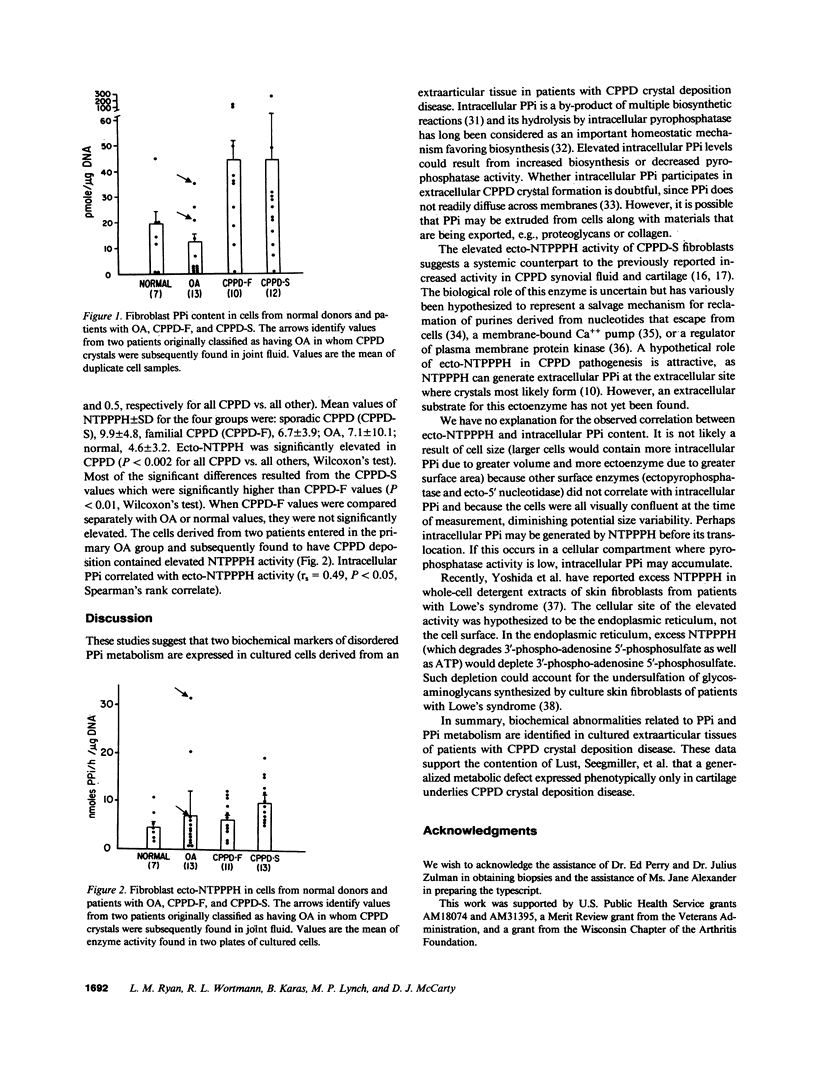
In calcium pyrophosphate dihydrate (CPPD) crystal deposition disease, metabolic abnormalities favoring extracellular inorganic pyrophosphate (PPi) accumulation have been suspected. Elevations of intracellular PPi in cultured skin fibroblasts from a single French kindred with familial CPPD deposition (19) and elevated nucleoside triphosphate pyrophosphohydrolase activity (NTPPPH), which generates PPi in extracts of CPPD crystal-containing cartilages (14) favor this suspicion. To determine whether NTPPPH activity or PPi content of cells might be a disease marker expressed in extraarticular cells, human skin-derived fibroblasts were obtained from control donors and patients affected with the sporadic and familial varieties of CPPD (CPPD-S and CPPD-F) deposition. Intracellular PPi was elevated in both CPPD-S (P less than 0.05) and CPPD-F (P less than 0.01) fibroblasts compared with control fibroblasts. Ecto-NTPPPH activity was elevated in CPPD-S (P less than 0.01) but not CPPD-F. Intracellular PPi correlated with ecto-NTPPPH (P less than 0.01). Elevated PPi levels in skin fibroblasts may serve as a biochemical marker for patients with familial or sporadic CPPD crystal deposition disease; ecto-NTPPPH activity further separates the sporadic and familial disease types. Expression of these biochemical abnormalities in nonarticular cells implies a generalized metabolic abnormality.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altman R. D., Muniz O. E., Pita J. C., Howell D. S. Articular chondrocalcinosis. Microanalysis of pyrophosphate (PPi) in synovial fluid and plasma. Arthritis Rheum. 1973 Mar-Apr;16(2):171–178. doi: 10.1002/art.1780160206. [DOI] [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camerlain M., McCarty D. J., Silcox D. C., Jung A. Inorganic pyrophosphate pool size and turnover rate in arthritic joints. J Clin Invest. 1975 Jun;55(6):1373–1381. doi: 10.1172/JCI108056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnemolla B., Cutolo M., Castellani P., Balza E., Raffanti S., Zardi L. Characterization of synovial fluid fibronectin from patients with rheumatic inflammatory diseases and healthy subjects. Arthritis Rheum. 1984 Aug;27(8):913–921. doi: 10.1002/art.1780270811. [DOI] [PubMed] [Google Scholar]
- Cheung C. P., Suhadolnik R. J. Analysis of inorganic pyrophosphate at the picomole level. Anal Biochem. 1977 Nov;83(1):61–63. doi: 10.1016/0003-2697(77)90510-3. [DOI] [PubMed] [Google Scholar]
- Eade A. W., Swannell A. J., Williamson N. Pyrophosphate arthropathy in hypophosphatasia. Ann Rheum Dis. 1981 Apr;40(2):164–170. doi: 10.1136/ard.40.2.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards N. L., Cassidy J. T., Fox I. H. Lymphocyte 5'-nucleotidase deficiency in hypogammaglobulinemia: clinical characteristics. Clin Immunol Immunopathol. 1980 Sep;17(1):76–88. doi: 10.1016/0090-1229(80)90075-6. [DOI] [PubMed] [Google Scholar]
- Felix R., Fleisch H. The effect of pyrophosphate and diphosphonates on calcium transport in red cells. Experientia. 1977 Aug 15;33(8):1003–1005. doi: 10.1007/BF01945933. [DOI] [PubMed] [Google Scholar]
- Flodgaard H., Torp-Pedersen C. A calcium ion-dependent adenosine triphosphate pyrophosphohydrolase in plasma membrane from rat liver. Demonstration that the adenosine triphosphate analogues adenosine 5'-[betagamma-imido]triphosphate and adenosine 5'-[betagamma-methylene]-triphosphate are substrates for the enzyme. Biochem J. 1978 Jun 1;171(3):817–820. doi: 10.1042/bj1710817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukui S., Yoshida H., Tanaka T., Sakano T., Usui T., Yamashina I. Glycosaminoglycan synthesis by cultured skin fibroblasts from a patient with Lowe's syndrome. J Biol Chem. 1981 Oct 25;256(20):10313–10318. [PubMed] [Google Scholar]
- Howell D. S., Muniz O., Pita J. C., Enis J. E. Extrusion of pyrophosphate into extracellular media by osteoarthritic cartilage incubates. J Clin Invest. 1975 Dec;56(6):1473–1480. doi: 10.1172/JCI108228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutson J. M., Fallat M. E., Kamagata S., Donahoe P. K., Budzik G. P. Phosphorylation events during Müllerian duct regression. Science. 1984 Feb 10;223(4636):586–589. doi: 10.1126/science.6607531. [DOI] [PubMed] [Google Scholar]
- Lust G., Faure G., Netter P., Gaucher A., Seegmiller J. E. Evidence of a generalized metabolic defect in patients with hereditary chondrocalcinosis. Increased inorganic pyrophosphate in cultured fibroblasts and lymphoblasts. Arthritis Rheum. 1981 Dec;24(12):1517–1521. doi: 10.1002/art.1780241210. [DOI] [PubMed] [Google Scholar]
- Lust G., Faure G., Netter P., Seegmiller J. E. Increased pyrophosphate in fibroblasts and lymphoblasts from patients with hereditary diffuse articular chondrocalcinosis. Science. 1981 Nov 13;214(4522):809–810. doi: 10.1126/science.6270793. [DOI] [PubMed] [Google Scholar]
- Lust G., Nuki G., Seegmiller J. E. Inorganic pyrophosphate and proteoglycan metabolism in cultured human articular chondrocytes and fibroblasts. Arthritis Rheum. 1976 May-Jun;19 (Suppl 3):479–487. doi: 10.1002/1529-0131(197605/06)19:3+<479::aid-art1780190723>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- McCarty D. J., Solomon S. D., Warnock M. L., Paloyan E. Inorganic pyrophosphate concentrations in the synovial fluid of arthritic patients. J Lab Clin Med. 1971 Aug;78(2):216–229. [PubMed] [Google Scholar]
- Muniz O., Pelletier J. P., Martel-Pelletier J., Morales S., Howell D. S. NTP pyrophosphohydrolase in human chondrocalcinotic and osteoarthritic cartilage. I. Some biochemical characteristics. Arthritis Rheum. 1984 Feb;27(2):186–192. doi: 10.1002/art.1780270210. [DOI] [PubMed] [Google Scholar]
- O'Duffy J. D. Hypophosphatasia associated with calcium pyrophosphate dihydrate deposits in cartilage. Report of a case. Arthritis Rheum. 1970 Jul-Aug;13(4):381–388. doi: 10.1002/art.1780130404. [DOI] [PubMed] [Google Scholar]
- Perry E. L., Overholt E. L., Newcomer K. L. Familial occurrence of chondrocalcinosis (pseudogout syndrome). Wis Med J. 1969 Nov;68(11):321–324. [PubMed] [Google Scholar]
- RUSSELL R. G. EXCRETION OF INORGANIC PYROPHOSPHATE IN HYPOPHOSPHATASIA. Lancet. 1965 Sep 4;2(7410):461–464. doi: 10.1016/s0140-6736(65)91422-4. [DOI] [PubMed] [Google Scholar]
- Richardson B. C., Chafetz N. I., Ferrell L. D., Zulman J. I., Genant H. K. Hereditary chondrocalcinosis in a Mexican-American family. Arthritis Rheum. 1983 Nov;26(11):1387–1396. doi: 10.1002/art.1780261112. [DOI] [PubMed] [Google Scholar]
- Russell R. G., Bisaz S., Donath A., Morgan D. B., Fleisch H. Inorganic pyrophosphate in plasma in normal persons and in patients with hypophosphatasia, osteogenesis imperfecta, and other disorders of bone. J Clin Invest. 1971 May;50(5):961–969. doi: 10.1172/JCI106589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell R. G., Bisaz S., Fleisch H., Currey H. L., Rubinstein H. M., Dietz A. A., Boussina I., Micheli A., Fallet G. Inorganic pyrophosphate in plasma, urine, and synovial fluid of patients with pyrophosphate arthropathy (chondrocalcinosis or pseudogout). Lancet. 1970 Oct 31;2(7679):899–902. doi: 10.1016/s0140-6736(70)92070-2. [DOI] [PubMed] [Google Scholar]
- Russell R. G. Metabolism of inorganic pyrophosphate (PPi). Arthritis Rheum. 1976 May-Jun;19 (Suppl 3):465–478. doi: 10.1002/1529-0131(197605/06)19:3+<465::aid-art1780190722>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Ryan L. M., Cheung H. S., McCarty D. J. Release of pyrophosphate by normal mammalian articular hyaline and fibrocartilage in organ culture. Arthritis Rheum. 1981 Dec;24(12):1522–1527. doi: 10.1002/art.1780241211. [DOI] [PubMed] [Google Scholar]
- Ryan L. M., Kozin F., McCarty D. J. Quantification of human plasma inorganic pyrophosphate. I. Normal values in osteoarthritis and calcium pyrophosphate dihydrate crystal deposition disease. Arthritis Rheum. 1979 Aug;22(8):886–891. doi: 10.1002/art.1780220812. [DOI] [PubMed] [Google Scholar]
- Ryan L. M., Lynch M. P., McCarty D. J. Inorganic pyrophosphate levels in blood platelets from normal donors and patients with calcium pyrophosphate dihydrate crystal deposition disease. Arthritis Rheum. 1983 Apr;26(4):564–566. doi: 10.1002/art.1780260419. [DOI] [PubMed] [Google Scholar]
- SUGINO Y., MIYOSHI Y. THE SPECIFIC PRECIPITATION OF ORTHOPHOSPHATE AND SOME BIOCHEMICAL APPLICATIONS. J Biol Chem. 1964 Jul;239:2360–2364. [PubMed] [Google Scholar]
- Silcox D. C., McCarty D. J., Jr Elevated inorganic pyrophosphate concentrations in synovial fluids in osteoarthritis and pseudogout. J Lab Clin Med. 1974 Apr;83(4):518–531. [PubMed] [Google Scholar]
- Tenenbaum J., Muniz O., Schumacher H. R., Good A. E., Howell D. S. Comparison of phosphohydrolase activities from articular cartilage in calcium pyrophosphate deposition disease and primary osteoarthritis. Arthritis Rheum. 1981 Mar;24(3):492–500. doi: 10.1002/art.1780240307. [DOI] [PubMed] [Google Scholar]
- Tran T. T., Phillips J. W., Schulze-Specking A., Rasenack J., Decker K. Properties and biosynthetic connection of the nucleotide pyrophosphatases of rat liver plasma membrane and endoplasmic reticulum. Hoppe Seylers Z Physiol Chem. 1981 Mar;362(3):305–316. doi: 10.1515/bchm2.1981.362.1.305. [DOI] [PubMed] [Google Scholar]
- Whyte M. P., Murphy W. A., Fallon M. D. Adult hypophosphatasia with chondrocalcinosis and arthropathy. Variable penetrance of hypophosphatasemia in a large Oklahoma kindred. Am J Med. 1982 Apr;72(4):631–641. doi: 10.1016/0002-9343(82)90474-0. [DOI] [PubMed] [Google Scholar]
- Yoshida H., Fukui S., Yamashina I., Tanaka T., Sakano T., Usui T., Shimotsuji T., Yabuuchi H., Owada M., Kitagawa T. Elevation of nucleotide pyrophosphatase activity in skin fibroblasts from patients with Lowe's syndrome. Biochem Biophys Res Commun. 1982 Aug;107(3):1144–1150. doi: 10.1016/0006-291x(82)90641-6. [DOI] [PubMed] [Google Scholar]



