Abstract
S100A4, a biomarker of epithelial mesenchymal transition (EMT), plays an important role in invasion and metastasis by promoting cancer cell motility. In oral squamous cell carcinoma (OSCC), metastasis results in 90% of cancer associated mortality.
Objective
To investigate the role of S100A4 expression as an important component of the epithelial mesenchymal transition (EMT) program in oral squamous cell carcinoma (OSCC).
Material and Methods
S100A4 protein expression was assessed semi-quantitatively by immunohistochemistry in 47 histologically confirmed cases of oral squamous cell carcinoma (OSCC) and 10 normal oral mucosal biopsies. The association between the S100A4 overexpression and the aggressive features of OSCC were analyzed by X2 test.
Results
Moderate to strong cytoplasmic expression of S100A4 was observed in 30 out of 47 specimens of OSCC (64%). Overexpression of S100A4 was significantly associated with the clinical stage, lymph node involvement, metastases, pattern of invasion and recurrence (p<0.05).
Conclusion
S100A4 expression represents an important biomarker of prognostic significance that may be used to identify a subset of patients at high risk of invasion and metast
Keywords: Biomarker, Epithelial-mesenchymal transition, Oral cancer, Metastasis, Prognosis
INTRODUCTION
Initiation and early growth of primary epithelial cancers, and acquisition of invasiveness is thought to herald the advanced stages of this multistep process that eventually leads to metastatic dissemination, with life-threatening consequences11. The genetic control and biochemical mechanisms underlying the acquisition of the invasive phenotype are mediated by the activation of an epithelial mesenchymal transition (EMT) program13. EMT is a biologic process that allows an epithelial cell to acquire the properties of a mesenchymal cell, thereby promoting invasion into the surrounding stroma and intravasation of tumor cells27. A number of distinct molecular markers are engaged in order to initiate EMT and enable its completion. One such marker is the S100A4, a flament-associated calcium-binding protein, which is associated with the cells of mesenchymal origin, and deemed to play a significant role in EMT7.
S100A4 expression levels strongly correlate with motility, which is a central element of the metastatic cascade25. In oral squamous cell carcinoma (OSCC), metastasis results in 90% of cancer associated mortality. In this work, we studied the role of S100A4 in oral cancer as a biomarker of metastasis by assessing its expression in primary tumors from all the clinical stages of oral cancer and determined the association of S100A4 expression with aggressive clinico-pathological parameters including the pattern of invasion, distant metastasis and recurrence. Assessment of S100A4 in oral cancer may be related to the phenotypic alteration and thereby predict its metastatic potential.
MATERIAL AND METHODS
Patient details
The material for present study included a total of 57 formalin fixed paraffin embedded tissue sections retrieved from the departmental archives. Out of these, 47 were patients with carcinoma of the bucco-alveolar mucosal complex and of the foor of the mouth. fhe remaining 10 were healthy mucosal tissues, which were obtained at the time of extraction of impacted teeth. Only surgically treated cases of oral squamous cell carcinoma (OSCC) with a minimum follow-up period of 5 years were included in the study. Institutional Ethics Committee approval (IEC 36/2012) was obtained from Kasturba Hospital, Manipal, prior to carrying out the study. The patient demographics and other details of the cases included in the study are presented in Table 1. A chart analysis was carried out to study the expression of S100A4 in all these cases by immunohistochemistry (IHC). Tissue sections obtained from the lymph node constituted the positive control.
Table 1.
The demographic variable of the cases included in the study
| Particulars | Subset | Number | (%) | Total |
|---|---|---|---|---|
| Gender | Male | 37 | 78.72 | 47 |
| Female | 10 | 21.28 | ||
| Site | Buccal mucosa | 21 | 44.68 | 47 |
| Alveolus | 18 | 38.30 | ||
| Floor of the mouth | 8 | 17.02 | ||
| Presentation | Ulceroproliferative | 26 | 55.32 | 47 |
| Ulcer | 8 | 17.02 | ||
| Exophytic | 8 | 17.02 | ||
| Swelling | 5 | 10.64 | ||
| Habit | SLT | 26 | 55.32 | 47 |
| ST | 12 | 25.53 | ||
| SLT + ST | 3 | 6.38 | ||
| NT | 6 | 12.77 |
SLT - Smokeless tobacco. S - Smoking tobacco. NT- No tobacco
Immunohistochemistry
Immunohistochemical staining was carried out using indirect streptavidin biotin immunoperoxidase technique. The antibodies and reagents were obtained from Sigma – Aldrich Co. (St. Louis, MO, USA). Sections of 4 μm thickness taken on amino propyl triethoxysilane (APES) coated slides were incubated overnight at 48°C in a slide warmer to ensure complete adhesion of sections. Following tissue sections deparaffinization and hydration through descending grades of alcohol, sections were placed in phosphate buffered saline (PBS) for 30 minutes. The slides were immersed in a microwave resistant plastic staining jar containing antigen retrieval solution (10 mM sodium citrate buffer, pH 6.0) and maintained at 700 W power for 10 minutes. Subsequently, the solution was allowed to cool at room temperature for at least 20 minutes. The slides were incubated in 3% hydrogen peroxide for 5 minutes at room temperature, following which slides were washed with PBS. To decrease the background staining due to nonspecific binding of antibodies, the slides were incubated with 5% bovine serum albumin (BSA) for 10 minutes at room temperature and washed with PBS for 5 minutes. The researchers applied 100 μL of the primary antibody (Rabbit polyclonal anti-S100A4 antibody, 1:250) over the section completely, which was incubated for 60 minutes at 37°C in a humidified chamber. The slides were rinsed with PBS and placed in the PBS bath for 5 minutes. After draining and wiping of the slides, sections were incubated in 100 μl of biotinylated secondary antibody, Anti-Rabbit IgG (whole molecule - Peroxidase antibody produced in goat) for 30 minutes at 37°C. The slides were washed with PBS and placed in PBS bath for 5 minutes. For visualization, sections were incubated with freshly prepared substrate solution (SIGMAFAST™ 3, 3'-Diaminobenzidine tablets) for 5-10 minutes. This was followed by washing the slides in running tap water and counter staining with Mayer's haematoxylin (MHS1- Haematoxylin Solution, Mayer's 1 gil certified hematoxylin *pH 2.4) for 5 minutes, after which the slides were washed in running tap water for 1 minute. Briefy, the sections were dehydrated through ascending grades of alcohol (70%, 95% and 100%) for 2 minutes each, cleared in xylene for 1 minute, mounted in a resinous media and coverslipped.
Staining interpretation
The neoplastic cell with cytoplasmic staining was viewed at 200× magnification, using a light microscope. The distribution of immunolabelling in neoplastic epithelial cells was determined from a minimum of five representative high-power fields at the invasive front. A positive cell demonstrated a diffuse brown signal in the cytoplasm, independent of its intensity. To eliminate any inter-observer bias, the scoring was carried out independently by two observers. The degree of staining for S100A4 was evaluated using a semi-quantitative scale29. Tissues were graded as absent (-) when there was no staining, low (1+) when less than 25% of the tumor cells were positive, moderate (2+) when 25 to 75% of the tumor cells stained positively and diffuse (3+) when more than 75% of the tumor cells were positive for S100A4. S100A4 expression was then correlated with clinicopathological parameters including primary tumor site, stage of tumor, degree of differentiation, lymph node metastasis and mode (pattern) of invasion28.
Statistics
Statistical analysis was carried out using SPSS version 16.0 for Windows. Kendall's tau-b statistics was applied to assess the measure of agreement between two observers. Chi-square test was used to study the association between the S100A4 staining and clinicopathological parameters. Results were considered statistically significant if p<0.05.
RESULTS
Positive S100A4 expression in the cytoplasm of the tumor cells was observed in 30 out of 47 cases (63.82%) of OSCC. Kendall's tau-b test between the two observers showed a very high level of agreement for the expression of S100A4 (0.8, p<0.001). In the control group, none of oral mucosal tissues were positive for S100A4 in the cytoplasm of the epithelium.
Out of 47 cases studied, 1/6 of stage I, 1/2 of stage II, 13/17 of stage III and 15/22 of stage IV cases showed positive S100A4 expression (Table 2). A strong association was noted between the stage of the tumor and the expression score of S100A4 (p=0.007). S100A4 expression was positive in 12/14 (85.7%) node positive stage III cases, of which 7/12 were (3+) and 5/12 were (2+) for S100A4 expression. Among the node negative cases, 1/3 cases showed (3+) for S100A4 expression. Likewise, S100A4 expression was positive in 14/15 (93.3%) stage IV cases, of which 13/14 were (3+) and 1/14 was (2+). Among the 7 node negative cases, 1/7 was (3+) and the remaining 6/7 cases were negative for S100A4 expression. Although the cytoplasmic expression of S100A4 was strongly associated with lymph node involvement (p<0.001), there was no association between S100A4 expression in lymph node positive cases and primary size of the tumor (p=0.15).
Table 2.
Association of S100A4 expression with clinical stage, lymph node involvement, tumor size, metastasis, recurrence, grade of tumor and pattern of invasion
| Parameters | 0 | 2+ | 3+ | Total | Chi-square | p value |
|---|---|---|---|---|---|---|
| Stage | ||||||
| Stage I | 5 | 1 | 0 | 6 | ||
| Stage II | 1 | 1 | 0 | 2 | ||
| Stage III | 4 | 5 | 8 | 17 | 14.5 | 0.007 |
| Stage IV | 7 | 1 | 14 | 22 | ||
| Total | 17 | 8 | 22 | 47 | ||
| Lymphnode | ||||||
| No | 8 | 0 | 2 | 10 | ||
| Yes | 3 | 6 | 20 | 29 | 22.7 | <0.001 |
| Total | 11 | 6 | 22 | 39 | ||
| Tumor Size | ||||||
| T1 | 5 | 2 | 4 | 11 | ||
| T2 | 2 | 5 | 7 | 14 | ||
| T3 | 3 | 0 | 1 | 4 | 8.7 | 0.15 |
| T4 | 7 | 1 | 10 | 18 | ||
| Total | 17 | 8 | 22 | 47 | ||
| Metastasis | ||||||
| No | 7 | 0 | 4 | 11 | ||
| Yes | 0 | 1 | 10 | 11 | 10.71 | 0.004 |
| Total | 7 | 1 | 14 | 22 | ||
| Recurrence | ||||||
| No | 11 | 6 | 11 | 28 | ||
| Yes | 0 | 0 | 11 | 11 | 11.4 | 0.002 |
| Total | 11 | 6 | 22 | 39 | ||
| Grade | ||||||
| WDSCC | 6 | 4 | 5 | 15 | ||
| MDSCC | 8 | 4 | 9 | 21 | 5.07 | 0.27 |
| PDSCC | 3 | 0 | 8 | 11 | ||
| Total | 17 | 8 | 22 | 47 | ||
| Pattern of invasion | ||||||
| Type 1 | 2 | 1 | 1 | 4 | ||
| Type 2 | 6 | 2 | 0 | 8 | ||
| Type 3 | 6 | 3 | 2 | 11 | 27.4 | <0.001 |
| Type 4C | 3 | 2 | 7 | 12 | ||
| Type 4D | 0 | 0 | 12 | 12 | ||
| Total | 17 | 8 | 22 | 47 |
WDSCC - Well differentiated squamous cell carcinoma; MDSCC - Moderately differentiated squamous cell carcinoma; PDSCC - Poorly differentiated squamous cell carcinoma
With regard to distant metastasis (M), 11/22 (50%) cases in stage IV were positive for S100A4 expression, out of which 10/11 were (3+) and 1/11 was (2+) for S100A4 expression. A strong association could be established between the S100A4 expression in tumor cells and distant metastasis (p=0.004). Likewise, 11/47 (23.4%) cases that recurred after treatment were either in stage III (2/11) or stage IV (9/11). All these cases showed (3+) expression for S100A4. Among the stage III cases that recurred, one was node positive and the other was negative, whereas all the 9 stage IV cases that recurred were node positive. There was a strong positive association between the S100A4 expression and rate of recurrence (p=0.002).
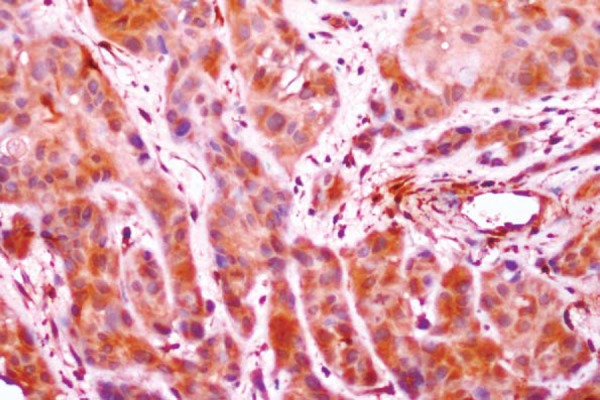
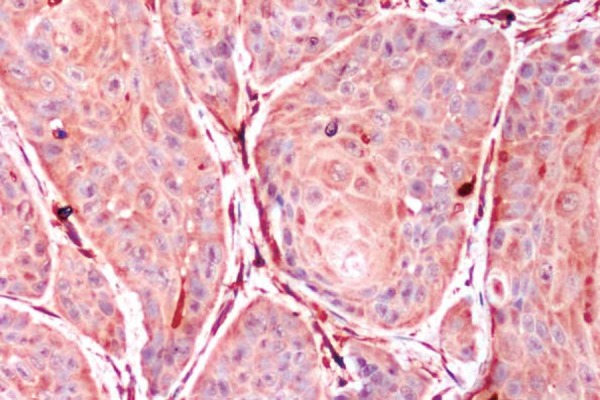
With regard to the pattern of invasion among the positive cases, 1/6 cases of stage I showed a type 1 (Figure 1) pattern and 1/2 cases in stage II showed type 2 (Figure 2) pattern. Out of the 13 stage III cases that showed positive S100A4 expression, 2 were type 1, 2 others were type 2, 3 were type 3 (Figure 3), 3 were type 4C (Figure 4) and 3 were type 4D (Figure 5). In the same manner, out of the 15 stage IV cases that showed positive S100A4 expression, 2 were type 3, 4 were type 4C and 9 were type 4D. There was a strong association (p<0.001) between S100A4 expression and pattern of invasion of OSCC. However, there was no association seen between the S100A4 expression and histological grade of the OSCC (p=0.27).
Figure 1.
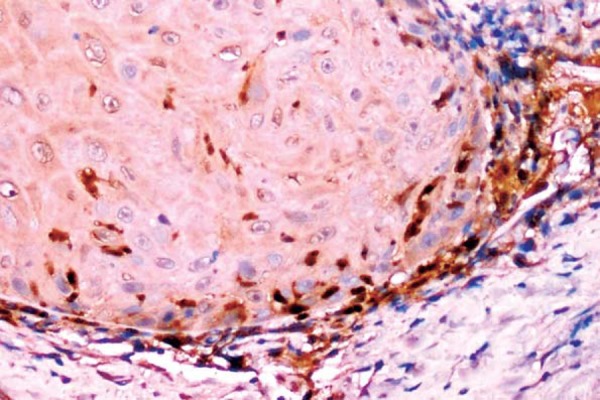
Oral squamous cell carcinoma (OSCC) showing type 1 well defined borderline pattern of invasion (S100A4, 20x)
Figure 2.
Oral squamous cell carcinoma (OSCC) showing type 2 cord like pattern of invasion (S100 A4, 20x)
Figure 3.
Oral squamous cell carcinoma (OSCC) showing type 3 pattern of invasion with groups of cells with no distinct borderline (S100A4, 20x)
Figure 4.
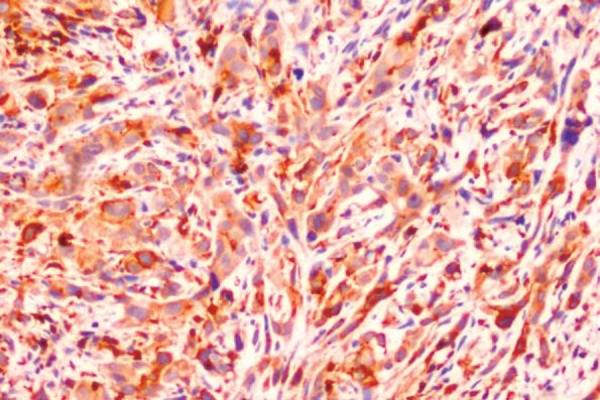
Oral squamous cell carcinoma (OSCC) showing type 4C diffuse cord like pattern of invasion (S100A4, 20x)
Figure 5.
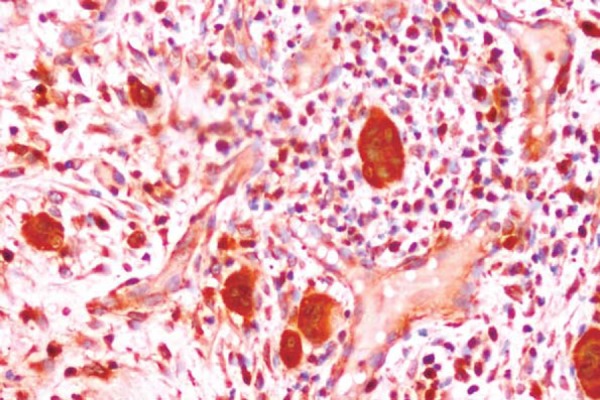
Oral squamous cell carcinoma (OSCC) showing type 4D diffuse widespread pattern of invasion (S100A4, 20x)
DISCUSSION
Oral cancer is one of the major cancers worldwide, with a low five-year survival rate that has not significantly changed over the past few decades12. Metastasis, which is responsible for 90% of the cancer associated mortality, is one of the poorly understood factors in cancer progression. An important step for the invasion and metastasis is the epithelial mesenchymal transition (EMT), a complex process induced by modifications of multiple pathways leading to a spectrum of epithelial cellular changes, including loss of polarity and cohesion, increased motility, and the acquisition of mesenchymal phenotype. EMT is critical for the invasion, progression, and metastasis in epithelial carcinogenesis8. Understanding the modulators of EMT may provide information of specific molecular targets.
Metastasis promoting S100A4 is a typical representative of S100 family of Ca2+-binding multifunctional proteins with dual extra- and intracellular function5. The increased expression of the S100A4 in invasive front of the tumor suggests the pivotal role played by S100A4 in invasion of tumor cells into the surrounding matrix. The acquisition of invasive phenotype with altered pattern of adhesion is a result of E-cadherin down regulation by S100A4, which the tumor cells acquire14. S100A4 further influences cell-matrix adhesions by interacting with liprin [1 and masking their protein kinase C-mediated phosphorylation sites17. Inhibition of the liprin [1-lAR complex by S100A4 loosens cell adhesion and allows cell invasion. Cell invasion is also facilitated by matrix metalloproteinases (MMPs), as S100A4 causes metastasis not only by affecting the motility of cells but also by affecting its invasive properties through influencing the expression of MMPs and their endogenous inhibitors2. A positive correlation of the S100A4 and MMP2 expression in oesophageal squamous cell carcinoma has been reported29.
S100A4 is located at the leading edge of migrating cells15, where it induces the formation of fexible protrusions. Moreover, in the presence of a chemoattractant, S100A4 enhances directed migration. Directed migration is dependent on the interaction of S100A4 with myosin II-A. Thus, the S100A4-myosin II-A interaction does not only increase cell motility, but also enhances cell polarization and directed migration19. S100A4 also binds to the septins 2, 6 and 7, which play a central role in cytokinesis, cell polarity determination, cytoskeletal reorganization, and membrane dynamics10,16. Therefore, the S100A4septin interaction also contributes to the process of migration.
S100A4 protein contributes to tumor angiogenesis by stimulating the motility of endothelial cells. S100A4 binds to annexin II on the endothelial surface, a complex that activates the tissue plasminogen activator, which in turn converts the plasminogen to plasmin, activating proMMPs. Active MMPs and plasmin together induce extracellular matrix remodelling and thereby facilitate angiogenesis26. Intracellular S100A4 also enhances angiogenesis by interacting with methionine aminopeptidase 2 (MetAP2)6. Ca2+-dependent binding of S100A4 to MetAP2 modulates the MetAP2 activity, which could promote endothelial growth and angiogenesis. This process of angiogenesis favors the metastasis of the tumor to the regional lymph nodes.
Elevated S100A4 expression has shown to be the marker of metastasis and poor prognosis in patients with pancreatic ductal adenocarcinoma23, prostate adenocarcinoma9, colorectal cancer4,18 and breast cancer24. A higher level of S100A4 expression has shown to correlate with auxillary lymph node metastasis and overall patient survival24.
Evidence suggesting the role for S100A4 in HNSCC, especially in OSCC, is scarce. Elevated S100A4 as a prognostic factor in metastatic cases of OSCC was demonstrated by Moriyama-Kita, et al.21 (2004).Our findings showing increased expression of S100A4 in cases with regional lymphnode involvement and metastasis further emphasizes the role of S100A4 in oral cancer patients with poor prognosis.
The final step in the metastatic cascade is the migration of the cells to a distant site and secondary colonization. In our study we found a significant correlation with the elevated S100A4 expression and distant metastasis. This finding has been elucidated earlier in papillary thyroid carcinoma30 and pancreatic cancer22, in which poor outcome of patients was related to increased S100A4 expression coupled with decreased E cadherin expression.
The increased expression of S100A4 in our study showed a significant correlation with the overall clinical stage of tumor, a finding that has been noted also in colorectal carcinoma4 and pancreatic cancer22. However, no association could be drawn between the elevated S100A4 level and the primary tumor size or histological grading in our analysis. fhese findings are in agreement with Moriyama-Kita, et al.21 (2004) in OSCC and Chen, et al.3 (2014) in lung cancer cells, in which S100A4 was frequently overexpressed, irrespective of histological subtype.
In our study a strong association between S100A4 expression and pattern of invasion could be elicited, with an increased expression noted in type 4C and type 4D pattern of invasion. Similar observations have been made in malignant melanomas where increased expression of S100A4 in the nodular variant was noted, linking this association to the pattern of invasion1.
Significantly, our observation of high S100A4 expression with tumor recurrence is a novel finding. S100A4 has been identified as a candidate marker for maintaining the stemness in head and neck cancer initiating cells. S100A4 controls the Notch and PTEN/PI3K/Akt signaling pathways, which regulate the self-renewal and tumorigenicity of stem cells or cancer stem cells20. S100A4 signaling pathways thus play a major role in the maintenance of cancer initiating cell population and contribute for the tumor recurrence.
CONCLUSION
In summary, the data from our study adds weight to the growing body of evidence that S100A4 can be used to identify the subgroup of patients at high risk of metastasis and recurrence in OSCC cases, and that targeting S100A4 signaling might be a potential therapeutic target for increasing the patient survival.
References
- 1.Andersen K, Nesland JM, Holm R, Flørenes VA, Fodstad Ø, Maelandsmo GM. Expression of S100A4 combined with reduced E-cadherin expression predicts patient outcome in malignant melanoma. Mod Pathol. 2004;17:990–997. doi: 10.1038/modpathol.3800151. [DOI] [PubMed] [Google Scholar]
- 2.Bjornland K, Winberg JO, Odegaard OT, Hovig E, Loennechen T, Aasen AO, et al. S100A4 involvement in metastasis: deregulation of matrix metalloproteinases and tissue inhibitors of matrix metalloproteinases in osteosarcoma cells transfected with an anti-S100A4 ribozyme. Cancer Res. 1999;59:4702–4708. [PubMed] [Google Scholar]
- 3.Chen N, Sato D, Saiki Y, Sunamura M, Fukushige S, Horii A. S100A4 is frequently overexpressed in lung cancer cells and promotes cell growth and cell motility. Biochem Biophys Res Commun. 2014;447:459–464. doi: 10.1016/j.bbrc.2014.04.025. [DOI] [PubMed] [Google Scholar]
- 4.Cho YG, Kim CJ, Nam SW, Yoon SH, Lee SH, Yoo NJ, et al. Overexpression of S100A4 is closely associated with progression of colorectal cancer. World J Gastroenterol. 2005;11:4852–4856. doi: 10.3748/wjg.v11.i31.4852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Donato R. Intracellular and extracellular roles of S100 proteins. Microsc Res Tech. 2003;60:540–551. doi: 10.1002/jemt.10296. [DOI] [PubMed] [Google Scholar]
- 6.Endo H, Takenaga K, Kanno T, Satoh H, Mori S. Methionine aminopeptidase 2 is a new target for the metastasis-associated protein, S100A4. J Biol Chem. 2002;277:26396–26402. doi: 10.1074/jbc.M202244200. [DOI] [PubMed] [Google Scholar]
- 7.Garrett SC, Varney KM, Weber DJ, Bresnick AR. S100A4, a mediator of metastasis. J Biol Chem. 2006;281:677–680. doi: 10.1074/jbc.R500017200. [DOI] [PubMed] [Google Scholar]
- 8.Gupta GP, Massagué J. Cancer metastasis: building a framework. Cell. 2006;127:679–695. doi: 10.1016/j.cell.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 9.Gupta S, Hussain T, MacLennan GT, Fu P, Patel J, Mukhtar H. Differential expression of S100A2 and S100A4 during progression of human prostate adenocarcinoma. J Clin Oncol. 2003;21:106–112. doi: 10.1200/JCO.2003.03.024. [DOI] [PubMed] [Google Scholar]
- 10.Hall PA, Russell SE. The pathobiology of the septin gene family. J Pathol. 2004;204:489–505. doi: 10.1002/path.1654. [DOI] [PubMed] [Google Scholar]
- 11.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 12.Jemal A, Thomas A, Murray T, Thun M. Cancer statistics, 2002. CA Cancer J Clin. 2002;52:23–47. doi: 10.3322/canjclin.52.1.23. [DOI] [PubMed] [Google Scholar]
- 13.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119:1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Keirsebilck A, Bonné S, Bruyneel E, Vermassen P, Lukanidin E, Mareel M, et al. E-cadherin and metastasin (mts-1/S100A4) expression levels are inversely regulated in two tumor cell families. Cancer Res. 1998;58:4587–4591. [PubMed] [Google Scholar]
- 15.Kim EJ, Helfman DM. Characterization of the metastasis- associated protein, S100A4. Roles of calcium binding and dimerization in cellular localization and interaction with myosin. J Biol Chem. 2003;278:30063–30073. doi: 10.1074/jbc.M304909200. [DOI] [PubMed] [Google Scholar]
- 16.Koshelev YA, Kiselev SL, Georgiev GP. Interaction of the S100A4 (Mts1) protein with septins Sept2, Sept6, and Sept7 in vitro. Dokl Biochem Biophys. 2003;391:195–197. doi: 10.1023/a:1025149005902. [DOI] [PubMed] [Google Scholar]
- 17.Kriajevska M, Fischer-Larsen M, Moertz E, Vorm O, Tulchinsky E, Grigorian M, et al. Liprin beta 1, a member of the family of LAR transmembrane tyrosine phosphatase-interacting proteins, is a new target for the metastasis-associated protein S100A4 (Mts1) J Biol Chem. 2002;277:5229–5235. doi: 10.1074/jbc.M110976200. [DOI] [PubMed] [Google Scholar]
- 18.Kwak JM, Lee HJ, Kim SH, Kim HK, Mok YJ, Park YT, et al. Expression of protein S100A4 is a predictor of recurrence in colorectal cancer. World J Gastroenterol. 2010;16:3897–3904. doi: 10.3748/wjg.v16.i31.3897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li ZH, Spektor A, Varlamova O, Bresnick AR. Mts1 regulates the assembly of nonmuscle myosin-IIA. Biochemistry. 2003;42:14258–14266. doi: 10.1021/bi0354379. [DOI] [PubMed] [Google Scholar]
- 20.Lo JF, Yu CC, Chiou SH, Huang CY, Jan CI, Lin SC, et al. The epithelial-mesenchymal transition mediator S100A4 maintains cancer-initiating cells in head and neck cancers. Cancer Res. 2011;71:1912–1923. doi: 10.1158/0008-5472.CAN-10-2350. [DOI] [PubMed] [Google Scholar]
- 21.Moriyama-Kita M, Endo Y, Yonemura Y, Heizmann CW, Schäfer BW, Sasaki T, et al. Correlation of S100A4 expression with invasion and metastasis in oral squamous cell carcinoma. Oral Oncol. 2004;40:496–500. doi: 10.1016/j.oraloncology.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 22.Oida Y, Yamazaki H, Tobita K, Mukai M, Ohtani Y, Miyazaki N, et al. Increased S100A4 expression combined with decreased E-cadherin expression predicts a poor outcome of patients with pancreatic cancer. Oncol Rep. 2006;16:457–463. [PubMed] [Google Scholar]
- 23.Rosty C, Ueki T, Argani P, Jansen M, Yeo CJ, Cameron JL, et al. Overexpression of S100A4 in pancreatic ductal adenocarcinomas is associated with poor differentiation and DNA hypomethylation. Am J Pathol. 2002;160:45–50. doi: 10.1016/S0002-9440(10)64347-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rudland PS, Platt-Higgins A, Renshaw C, West CR, Winstanley JH, Robertson L, et al. Prognostic significance of the metastasis-inducing protein S100A4 (p9Ka) in human breast cancer. Cancer Res. 2000;60:1595–1603. [PubMed] [Google Scholar]
- 25.Santamaria-Kisiel L, Rintala-Dempsey AC, Shaw GS. Calcium-dependent and -independent interactions of the S100 protein family. Biochem J. 2006;396:201–214. doi: 10.1042/BJ20060195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Semov A, Moreno MJ, Onichtchenko A, Abulrob A, Ball M, Ekiel I, et al. Metastasis-associated protein S100A4 induces angiogenesis through interaction with Annexin II and accelerated plasmin formation. J Biol Chem. 2005;280:20833–20841. doi: 10.1074/jbc.M412653200. [DOI] [PubMed] [Google Scholar]
- 27.Smit MA, Peeper DS. Epithelial-mesenchymal transition and senescence: two cancer-related processes are crossing paths. Aging (Albany NY) 2010;2:735–741. doi: 10.18632/aging.100209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yamamoto E, Miyakawa A, Kohama G. Mode of invasion and lymph node metastasis in squamous cell carcinoma of the oral cavity. Head Neck Surg. 1984;6:938–947. doi: 10.1002/hed.2890060508. [DOI] [PubMed] [Google Scholar]
- 29.Zhang HY, Zheng XZ, Wang XH, Xuan XY, Wang F, Li SS. S100A4 mediated cell invasion and metastasis of esophageal squamous cell carcinoma via the regulation of MMP-2 and E-cadherin activity. Mol Biol Rep. 2012;39:199–208. doi: 10.1007/s11033-011-0726-1. [DOI] [PubMed] [Google Scholar]
- 30.Zou M, Al-Baradie RS, Al-Hindi H, Farid NR, Shi Y. S100A4 (Mts1) gene overexpression is associated with invasion and metastasis of papillary thyroid carcinoma. Br J Cancer. 2005;93:1277–1284. doi: 10.1038/sj.bjc.6602856. [DOI] [PMC free article] [PubMed] [Google Scholar]


