Abstract
The adhesion of biofilm on dental prostheses is a prerequisite for the occurrence of oral diseases.
Objective
To assess the antimicrobial activity and the mechanical properties of an acrylic resin embedded with nanostructured silver vanadate (β-AgVO3).
Material and Methods
The minimum inhibitory concentration (MIC) of β-AgVO3 was studied in relation to the species Staphylococcus aureus ATCC 25923, Streptococcus mutans ATCC 25175, Pseudomonas aeruginosa ATCC 27853, and Candida albicans ATCC 10231. The halo zone of inhibition method was performed in triplicate to determine the inhibitory effect of the modified self-curing acrylic resin Dencor Lay - Clássico®. The surface hardness and compressive strength were examined. The specimens were prepared according to the percentage of β-AgVO3 (0%-control, 0.5%, 1%, 2.5%, 5%, and 10%), with a sample size of 9x2 mm for surface hardness and antimicrobial activity tests, and 8x4 mm for the compression test. The values of the microbiologic analysis were compared and evaluated using the Kruskal-Wallis test (α=0.05); the mechanical analysis used the Shapiro-Wilk's tests, Levene's test, ANOVA (one-way), and Tukey's test (α=0.05).
Results
The addition of 10% β-AgVO3 promoted antimicrobial activity against all strains. The antimicrobial effect was observed at a minimum concentration of 1% for P. aeruginosa, 2.5% for S. aureus, 5% for C. albicans, and 10% for S. mutans. Surface hardness and compressive strength increased significantly with the addition of 0.5% β-AgVO3 (p<0.05). Higher rates of the nanomaterial did not alter the mechanical properties of the resin in comparison with the control group (p>0.05).
Conclusions
The incorporation of β-AgVO3 has the potential to promote antimicrobial activity in the acrylic resin. At reduced rates, it improves the mechanical properties, and, at higher rates, it does not promote changes in the control.
Keywords: Acrylic resins, Nanotechnology, Products with antimicrobial action
INTRODUCTION
The installation of prosthetic devices within the oral cavity changes the oral conditions and promotes the deposit of biofilms on dental surfaces and on the prosthetic device25. The porous surface and irregularities of acrylic resins favor the accumulation of microorganisms, which are determining agents in the vast majority of oral problems, such as candidiasis, caries, gingivitis, and periodontitis28,29.
The solution to these problems includes the use of drugs and repairs or replacement of the prosthesis4,5,7,8. Prosthetic devices with antimicrobial properties could be ideal to improve the quality of life of patients and decrease discomfort and additional costs. With the addition of antimicrobial agents, several studies have focused on reducing or eliminating bacterial and fungal contamination8,9 without impairing the mechanical properties of dental materials.
Nanotechnology is currently the most promising field to generate new applications in healthcare, including dentistry6,13-20, with a special emphasis on silver nanoparticles (Ago). It is widely known for its antimicrobial properties and long application track record17. Therefore, compounds containing silver nanoparticles are effective because their broad-spectrum antimicrobial properties include the bacterium Streptococcus mutans, which is the main and most virulent cariogenic microorganism found in oral biofilm2,6,18. A major problem with the use of AgNPs is the difficulty of homogeneously dispersing and incorporating the resin8; nanostructured silver vanadate is an alternative material for use in health care as it avoids the agglomeration of nanoparticles, providing high surface contact with the microorganisms and greater antibacterial activity11.
This study is an unprecedented exploration of the effect of the addition of nanostructured silver vanadate on antimicrobial activity, surface hardness, and compressive strength of dental acrylic resin. The null hypothesis tested was that incorporating nanostructured silver vanadate would not affect the antimicrobial and mechanical properties of the acrylic resin. The antimicrobial behavior of the material was also determined and compared with the antimicrobial properties after the additive was incorporated (0.5%, 1%, 2.5%, 5%, and 10% by mass).
MATERIAL AND METHODS
Synthesis of nanostructured silver vanadate decorated with AgNPs
The synthesis of the material was performed by a precipitation reaction between silver nitrate (AgNO3, Merck 99.8%) and ammonium metavanadate (NH4VO3, Merck 99%)12.
First, 0.9736 grams of NH4VO3 were dissolved in 200 mL of distilled water at 65°C, under magnetic stirring for 10 min. Next, 1.3569 grams of AgNO3 were dissolved in 200 mL of distilled water under the same conditions. The AgNO3 solution was added dropwise to the NH4VO3 and stirred for 30 min until it formed an ammonium vanadate solution. The precipitate obtained was fltered under vacuum, washed with distilled water and absolute ethanol, and dried in a vacuum line for 4 hours.
Minimum Inhibitory Concentration
The minimum inhibitory concentration (MIC) values of the nanostructured silver vanadate compared with the microorganisms Staphylococcus aureus ATCC 25923, Streptococcus mutans ATCC 25175, Pseudomonas aeruginosa ATCC27853, and Candida albicans ATCC 10231(American Type Culture Collection, Manassas, VA, EUA) were determined based on successive dilutions outlined by the Clinical and Laboratory Standards Institute (CLSI)7 in cell culture plates (TPP, Trasadingen, Unterklettgau, Switzerland) containing 96 wells. The plates contained the culture medium supplemented with increasing concentrations of the antimicrobial agent. Each well was inoculated with the microorganism under testing, and after 24 hours of incubation, the microbial growth was assessed by the turbidity of the culture.
Experimental design
The MIC of the nanostructured silver vanadate against microorganisms of interest has been determined, and it corresponds to the addition of nanomaterial to the resin structure. According to the literature11, the addition of 1% (w/v) of silver vanadate to a commercial sample of water-based paint is sufficient to create antibacterial activity against Staphylococcus aureus. Thus, due to the unpublished nature of this study, to determine the effective concentration of vanadate silver incorporated in acrylic resin against proposed microorganisms, a greater number of concentrations were tested: (a) no antimicrobial agent, (b) 0.5% of β-AgVO3 (mass/mass), (c) 1% of β-AgVO3 (mass/ mass), (d) 2.5% of β-AgVO3 (mass/mass), (e) 5% of β-AgVO3 (mass/mass), and (f) 10% of β-AgVO3 (mass/mass).
To test compressive strength, assuming a standard deviation of 7.95, an error of 5%, and a power to 100% N=9, it was necessary to perform a surface hardness test, assuming a standard deviation of 2.63, an error of 5%, and power to 100% N=2. Both analyses used N=10 for each concentration of vanadate and control group.
For the antimicrobial analysis, 12 specimens were prepared for each concentration (vanadate and control group), which were distributed in groups of 3 specimens for each microorganism. The results of the analysis were replicated 3 times.
Sample preparation
The samples were prepared according to the specifications of the IS0 (IS0 10477:1992/ Amd 1:1998) and followed the manufacturer's recommended proportions. The nanostructured silver vanadate samples were prepared by mixing 0.5%, 1%, 2.5%, 5%, and 10% by mass of the antimicrobial agent with the polymer acrylic resin. After mixing the two powders, the monomer was added in the amount specified by the manufacturer, considering the sum of the two powders (polymer and vanadate).
Once it reached the plastic phase, the resin was adjusted to the muffle mold and pressed together using a hydraulic press (Midas Dental Products VH, Araraquara, SP, Brazil) gradually until reaching a pressure of 1000 Kgf, which was maintained during the curing time of the resin. The excess material was removed using a milling cutter (Maxi-cut, Maillefer AS, Ballaigues, Switzerland), followed by polishing with water sandpaper (Norton; Saint-Gobain Abrasivos Ltda, Vinhedo, SP, Brazil).
Microbiologic analysis
The agar diffusion method was used to determine the inhibitory effect of the specimens on the proposed species.
The standardized microbial inocula were prepared in a saline solution (~106 108 CFU/mL) from recent cultures at 37°C for 24 h using a spectrophotometer (λ=625 nm and absorbance=0.08 to 0.100). Next, 1 mL of these inocula was seeded onto the surface of Petri dishes (20x100 mm) containing 20 ml of the culture medium, and the excess was removed with a pipette. The culture medium was Tryptic Soy Agar for S. mutans, Sabouraud Dextrose Agar for C. albicans, and Mueller Hinton Agar for S. aureus and P. aeruginosa.
The specimens were placed on the surface of the culture medium with the bacterial inoculum. Aliquots of 50 μL of a chlorhexidine gluconate solution of 0.05%, a positive control for antimicrobial activity, were applied to wells prepared in the center of the plates.
After the 2 h pre-incubation to disperse the products, the Petri dishes were incubated at 37°C for 24 h under aerobic conditions.
The zone of inhibition was determined by its diameter (mm). The size of the zone of inhibition included the diameter of the specimen (9 mm).
Compressive strength
The specimens were submitted to a mechanical compressive strength test using the universal testing machine EMIC DL-2000®, with a load cell of 2000 kgf and speed of 1 mm/min, according to ASTM D 695 and ISO 20795-1:2008. The Tesc® program, version 2.0, was used (EMIC, São José dos Pinhais, PR, Brazil), which stores the results in a graph database with numerical values in MPa.
Surface hardness
The surface hardness test was carried with a Shimadzu microdurometer model HMV-2000 (Kyoto, Japan). Three random measurements were performed for each specimen with a Knoop diamond indenter under a load of 25 gf for 5 seconds. The penetrations were measured by two markings on the vertices of the lozenge of a 40x magnified image, which determined the length of the longest diagonal, and the Knoop hardness was automatically calculated by the software.
Statistical analysis
For the mechanical tests, once confirmed the normal distribution (Shapiro-Wilk's test) and homogeneous data (Levene's test), one-way ANOVA test and Tukey's test for comparison of means were performed.
The statistical analysis of microbiological assay was performed using the Kruskal-Wallis test.
For both analyses, a p value of <0.05 was considered significant.
RESULTS
Minimum inhibitory concentration
Figure 1 shows the MIC results.
Figure 1.
Standard microorganisms used in the microbiological experiments and minimum inhibitory concentration (MIC) values for Ag vanadate
| Code | Microorganisms | Source | Morphotintorial Characteristics | MIC (μg/mL) |
|---|---|---|---|---|
| Sa | Staphylococcus aureus | ATCC 25923 | Gram-positive cocci | 31.25 |
| Sm | Streptococcus mutans | ATCC 25175 | Gram-positive cocci | 250 |
| Pa | Pseudomonas aeruginosa | ATCC 27853 | Gram-negative rods | 31.25 |
| Ca | Candida albicans | ATCC 10231 | Gram-positive yeast | 62.5 |
ATCC=American Type Culture Collection
The MIC value compared with the strains of Staphylococcus aureus and Pseudomonas aeruginosa was 31.25 μg/mL; for Candida albicans, it was 62.5 μg/mL; and compared with Streptococcus mutans, it was 250 μg/mL. These are important values as they demonstrate the antimicrobial effectiveness of silver vanadate against yeasts, gram-negative, and gram-positive bacteria responsible for oral problems commonly associated with dental prostheses.
Antimicrobial activity
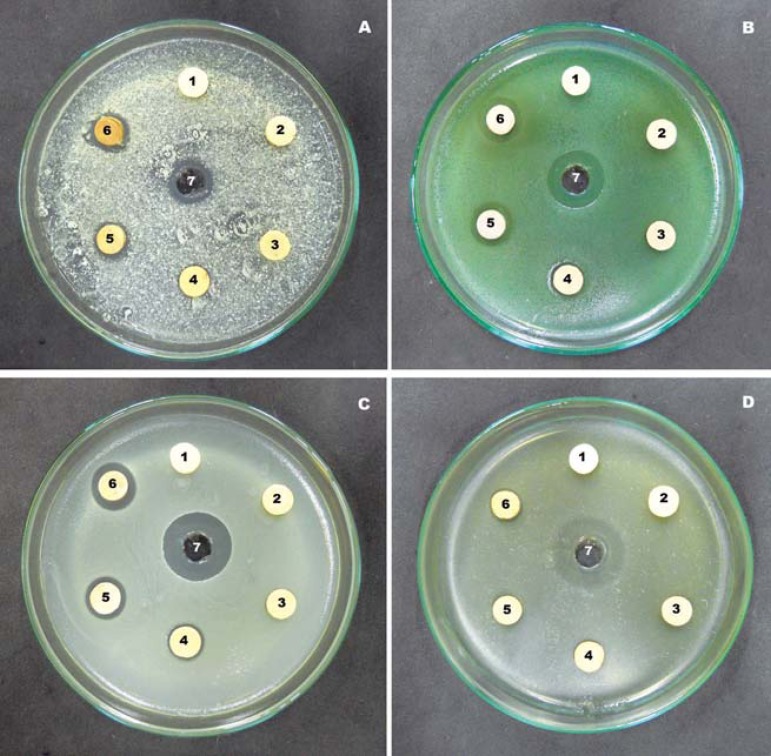
Figure 2 shows the inhibition zone results. None of the negative control groups, with no addition of the antimicrobial agent, showed inhibitory activity.
Figure 2.
Zone of inhibition formed: A- Candida albicans (ATCC 10231); B- Pseudomonas aeruginosa (ATCC 27853); C- Staphylococcus aureus (ATCC 25923); D- Streptococcus mutans (ATCC 25175). 1 – 0%; 2 – 0.5%; 3 – 1.0%; 4 – 2.5%; 5 – 5.0%; 6 – 10%. 7 – solution of chlorhexidine gluconate at 0.05%
Table 1 shows the average zones of inhibition diameter (in mm) of the acrylic resin with different percentages of nanostructured silver vanadate, as well as the standard deviation and the differences between groups. For the acrylic resin specimens, there was an overall increase in the levels of inhibition with the increasing percentage of nanostructured silver vanadate.
Table 1.
Mean standard deviation (SD) of inhibition halos of self-cured acrylic resin incorporated with different percentages of nanostructured silver vanadate
| Microorganisms | 0% | 0.5% | 1% | 2.5% | 5% | 10% | |
|---|---|---|---|---|---|---|---|
| Candida albicans | 0.0 | (0.0)a | 0.0 (0.0)a | 0.0 (0.0)a | 0.0 (0.0)a | 11.0 (0.0)b | 12.3 (0.6)b |
| Streptococcus mutans | 0.0 | (0.0)a | 0.0 (0.0)a | 0.0 (0.0)a | 0.0 (0.0)a | 0.0 (0.0)a | 12.0 (0.0)b |
| Staphylococcus aureus | 0.0 | (0.0)a | 0.0 (0.0)a | 0.0 (0.0)a | 12.3 (0.6)b | 14.6 (0.6)c | 15.6 (0.6)c |
| Pseudomonas aeruginosa | 0.0 | (0.0)a | 0.0 (0.0)a | 10.6 (9.1)b | 13.0 (0.0)c | 14.6 (0.6)c | 15.0 (0.0)c |
Different letters indicate significant differences between the groups for the same microorganism
The largest inhibition zone was observed with the addition of 10% in comparison with S. aureus (15.6±0.6).
There was no statistically significant difference between the antimicrobial activity promoted by the addition of 5 to 10% compared with S. aureus, P. aeruginosa, and C. albicans. Regarding P. aeruginosa, the percentage of 2.5% also showed microbial activity similar to 5 and 10% (Table 2).
Table 2.
Descriptive analysis
| % Nanomaterial | Minimum | Maximum | Mean | Median | SD | p value | |
|---|---|---|---|---|---|---|---|
| Candida albicans | 10% | 12.0 | 13.0 | 12.3 | 12.0 | 0.57 | 0.000 |
| Staphylococcus aureus | 2.5% | 12.0 | 13.0 | 12.3 | 12.0 | 0.57 | 0.000 |
| 5% | 14.0 | 15.0 | 14.6 | 15.0 | 0.57 | ||
| 10% | 15.0 | 16.0 | 15.6 | 16.0 | 0.57 | ||
| Pseudomonas aeruginosa | 1% | 1.0 | 19.0 | 10.66 | 12.0 | 9.07 | 0.000 |
| 5% | 14.0 | 15.0 | 14.6 | 15.0 | 0.57 |
SD=standard deviation
To assist in understanding the differences between the groups, the descriptive statistical analysis of groups with greater than a 0.0 standard deviation is described.
Compressive strength
Table 3 shows the compressive strength values. Compared with the control group, a statistically significant difference (p<0.05) was observed only for the group with 0.5% of the nanostructured silver vanadate, which showed the highest value (119.34±5.72 MPa), and which was statistically similar to the group with 2.5%. Compared to the other groups, there was no statistically significant difference between 0%, 1%, and 5% and 10%, 0%, 2.5%, and 10%.
Table 3.
Mean standard deviation (SD) of compressive strength of self-cured acrylic resin incorporated with different percentages of nanostructured silver vanadate
| 0% | 0.5% | 1% | 2.5% | 5% | 10% | p value |
|---|---|---|---|---|---|---|
| 103.6 | 119.34 | 99.7 | 111.6 | 100.11 | 103.65 | 0.000 |
| (8.9)ac | (5.72)b | (6.9)a | (6.08)cb | (8.44)a | (9.44)ac |
Different letters indicate significant differences between the groups
Surface hardness
Table 4 shows the surface hardness values. Compared with the control group, a statistically significant difference (p<0.05) was observed for the group with 0.5% of the nanostructured silver vanadate, which showed the highest value (26.14±5.13 HK). There was no statistically significant difference (p> 0.05) between the groups with 0%, 1%, 2.5%, 5%, and 10%.
Table 4.
Mean standard deviation (SD) of surface hardness of self-cured acrylic resin incorporated with different percentages of nanostructured silver vanadate
| 0% | 0.5% | 1% | 2.5% | 5% | 10% | p value |
|---|---|---|---|---|---|---|
| 16.88 | 26.14 | 19.23 | 17.85 | 17.45 | 15.65 | 0.000 |
| (2.0)a | (5.13)b | (1.41)a | (2.11)a | (1.71)a | (1.61)a |
Different letters indicate significant differences between the groups
DISCUSSION
Biofilm formation on the surface of dental prostheses is a prerequisite for diverse microbial colonization and the occurrence of dental diseases28. It is related to factors such as the structure and composition of the surface material and the properties of microbial cells27. In addition, cleaning the prosthesis may be difficult to perform for geriatric patients who have cognitive impairment, reduced motor dexterity, and memory loss22. Thus, the need to develop a simple and effective system of care for the prosthesis exists, and an acrylic resin with antimicrobial properties could provide a significant advancement in the prevention of diseases and improvement of oral hygiene.
For this purpose, various substances have been incorporated into the acrylic resins for dentures and other dental materials27. These include antifungal agents6,8-23, antiseptics3, antimicrobial monomers24, and silver-based materials1. However, although some compounds showed good results, there is no reliable evidence in the literature to provide a true recommendation on the addition of these materials. An assessment of the actual effectiveness of the material, including the optimal therapeutic concentration, which does not deteriorate the mechanical properties of the substrate, produces no toxic effects on the oral cavity is required21-27.
Silver has a long history of application and is known to be superior to other metals and nonmetals in terms of antimicrobial properties. These properties are increased when prepared as nanoparticles. The combination of derivatives of ions and other silver compounds is highly toxic to microorganisms, but produces low toxicity for humans when in appropriate doses5,7-16.
Although studies show that dental materials incorporated with antimicrobial agents, when in contact with the oral cavity, lose their effectiveness quickly21-27, when silver-based materials are used, it is biologically safe and efficacious in the long term due to the slow release of ions30.
Even though the nanoparticles are important for diverse technical applications and provide several advantages when used alone, they have some common problems, such as the agglomeration of nanoparticles26. Thus, to expand the use of silver nanoparticles and improve some problems inherent to them, silver vanadate (AgVO3) was developed, nanostructured, and decorated with AgNPs.
This paper proposed the incorporation of this material into acrylic resin, promoting antimicrobial activity and increasing its mechanical properties. 0nce combined with β-AgVO3, the resin can potentially provide satisfactory results since this material presents high antibacterial activity in a broad spectrum of gram-positive and gram-negative bacteria11, and it functions as a carrier for silver nanoparticles (AgNPs). Contact with the microorganism changes its morphology and metabolism, leading to cell death20. It also reduces the loss of silver nanoparticles through leaching and has the form of a skein of yarn with nanometer dimensions. It can also adhere to the surface of the acrylic resin more easily than only AgNP12.
β-AgVO3 also promotes a high dispersion of silver nanoparticles on nanowires of silver vanadates, which is important to maintain a large surface area with pathogenic microorganisms. This also has a greater effect and duration of action in terms of anti-microbial material characteristics11.
In this study, the control group samples (without the addition of nanostructured silver vanadate) showed no antimicrobial activity. As already demonstrated with other antimicrobial compounds, the effect was dose-dependent19-22. The addition of 10% of the nanomaterial was sufficient to produce antimicrobial activity against all strains tested, although at smaller percentages, the antimicrobial activity was observed in smaller proportions.
Some authors have suggested that the antimicrobial efficiency of polymers incorporated with silver-based materials is related to the release of silver ions or AgNPs; others report that the inhibitory effect is probably due to direct contact between the microorganisms and the material29. This may explain why the group with 10% of vanadate showed more sensitivity when compared to others, as a structure with more nanomaterial.
Marra, et al.19 (2012) incorporated an antimicrobial polymer acrylic resin and obtained good results using 10% and 25% against S. aureus and S. mutans, but there was no significant effect on C. albicans. In the present study, the antimicrobial effect was observed at a minimum concentration of 1% for P. aeruginosa, 2.5% for S. aureus, 5% for C. albicans, and 10% for S. mutans. The largest inhibition zone was observed with the addition of 10% against S. aureus (15.6±0.6). Thus, the higher the vanadate percentage, the greater the antimicrobial activity. This shows that even relatively inert material, such as acrylic resin, can be modified to have useful antimicrobial properties.
In a study by Holtz, et al.11 (2012), adding 1% of the material to a water-based pigment was sufficient to promote a 4 mm zone of inhibition against S. aureus. With this same concentration in the acrylic resin, a small non-measurable inhibition against S. aureus was observed around the specimen, and against P. aeruginosa, there was a greater halo of inhibition (10.6±9.1). These results demonstrate that although the acrylic resin matrix encapsulates the nanomaterial, it does not prevent it from acting on the microorganisms.
While promoting desirable effects in microbiological terms, the incorporation of antimicrobial agents in acrylic resin can promote undesirable changes in their mechanical and/or physical properties4-21. By incorporating methacrylate 2-tert-butylaminoethyl (TBAEMA) in acrylic resin, Paleari, et al.21 (2012) noted that the addition of 1% is sufficient to cause significant changes in fexural strength of the resin and justifes this change by incomplete polymerization of the same and permanence of residual monomer.
However, this study used a nanomaterial. The presence of unsaturated bonds on the nanomaterials' surface causes them to have high surface free energy, which also promotes strong bonding to other materials, hence promoting better physical and mechanical properties13. Thus, it was expected that in addition to promoting good antimicrobial activity, changing the composition of the resin would not reduce or improve the mechanical properties.
To confirm this hypothesis, the mechanical performance was evaluated in order to predict the durability of the material when exposed to forces in the oral cavity by compressive strength and surface hardness tests. These analyses are important because compressive forces are transmitted to the supporting tissues of the oral cavity. In the presence of parafunctional habits, this factor multiplies, with the frequent occurrence of fractures of prosthetic devices14, therefore laboratory compressive strength tests are helpful to observe in vitro the fractures that can occur in clinical practice15. While surface hardness represents the material's resistance to scratching, abrasion, or cutting, it is directly related to its wearing, which can result in more roughness and the adhesion of microorganisms6,10-18.
The addition of 0.5% silver vanadate to the acrylic resin resulted in higher surface hardness and compressive strength values, indicating that a small percentage of nanometric particles can reinforce the material's matrix, which confirms the effectiveness of this material as a reinforcement structure, a widely required condition in dental materials. This percentage may be ideal to obtain this goal, though not to promote antimicrobial activity. Moreover, the addition of higher percentages promoted antimicrobial activity against microorganisms studied and has maintained the same values of surface hardness and compressive strength compared to the control group.
Significant results were found in the study. β-AgVO3 may be associated with acrylic resin as a potential antimicrobial agent, including S. mutans, the bacteria involved in the early stages of biofilm formation, and the production of extracellular polysaccharide, which facilitates connections with other microorganisms such as C. albicans (associated with denture stomatitis, or lesions commonly found in users of removable dentures) 19. This association did not influence negatively the mechanical properties and is a low-cost method that does not depend on patient cooperation.
However, this study has some limitations. First, the literature is scarce on the subject, and no studies were found describing the incorporation of β-AgVO3 in polymers or other materials for dental applications or even its cytotoxicity in biological systems. Furthermore, only two mechanical properties were evaluated. One should evaluate the effect of incorporating other important mechanical properties, such as surface roughness and fexural strength, as well as the physical properties of the resin. Studies should include more complex biofilm models as well as cytotoxicity studies and long-term stability before these results can be applied in clinical research.
CONCLUSIONS
The incorporation of nanostructured silver vanadate in acrylic resin indicates possible antimicrobial activity directly related to the percentage of the nanomaterial applied to the microorganisms evaluated; P. aeruginosa and S. aureus are the most sensitive. Furthermore, the addition of 0.5% of the nanomaterial can promote improvement in the mechanical properties of hardness and resistance to compression.
ACKNOWLEDGMENTS
This study was conducted in partnership with: FAPESP (2012/09124-1 and 2012/09347-0); Solid State Chemistry Laboratory – UNICAMP; Oral Rehabilitation Research Laboratory – FORP/USP; Integrated Laboratory of Materials Biocompatibility Research – FORP/USP; Laboratory of Laser Welding and Corrosion Analysis – FORP/USP.
References
- 1.Acosta-Torres LS, Mendieta I, Nuñez-Anita RE, Cajero-Juárez M, Castaño VM. Cytocompatible antifungal acrylic resin containing silver nanoparticles for dentures. Int J Nanomedicine. 2012;7:4777–4786. doi: 10.2147/IJN.S32391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allaker RP. The use of nanoparticles to control oral biofilm formation. J Dent Res. 2010;89:1175–1186. doi: 10.1177/0022034510377794. [DOI] [PubMed] [Google Scholar]
- 3.Amin WM, Al-Ali MH, Salim NA, Al-Tarawneh SK. A new form of intraoral delivery of antifungal drugs for the treatment of denture-induced oral candidosis. Eur J Dent. 2009;3(4):257–266. [PMC free article] [PubMed] [Google Scholar]
- 4.Casemiro LA, Gomes Martins CH, Pires-de-Souza FC, Panzeri H. Antimicrobial and mechanical properties of acrylic resins with incorporated silver-zinc - part I. Gerodontology. 2008;25:187–194. doi: 10.1111/j.1741-2358.2007.00198.x. [DOI] [PubMed] [Google Scholar]
- 5.Cheng H, Li Y, Huo K, Gao B, Xiong W. Long-lasting in vivo and in vitro antibacterial ability of nanostructured titania coating incorporated with silver nanoparticles. J Biomed Mater Res A. 2014;102(10):3488–3499. doi: 10.1002/jbm.a.35019. [DOI] [PubMed] [Google Scholar]
- 6.Cheng L, Weir MD, Zhang K, Wu EJ, Xu SM, Zhou X, et al. Dental plaque microcosm biofilm behavior on calcium phosphate nanocomposite with quaternary ammonium. Dent Mater. 2012;28:853–862. doi: 10.1016/j.dental.2012.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clinical and Laboratory Standards Institute . Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. 6th ed. Wayne: CLSI; 2003. [Google Scholar]
- 8.Fan C, Chu L, Rawls HR, Norling BK, Cardenas HL, Whang K. Development of an antimicrobial resin - a pilot study. Dent Mater. 2011;27:322–328. doi: 10.1016/j.dental.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 9.Gjorgievska E, Apostolska S, Dimkov A, Nicholson JW, Kaftandzieva A. Incorporation of antimicrobial agents can be used to enhance the antibacterial effect of endodontic sealers. Dent Mater. 2013;29:e29–e34. doi: 10.1016/j.dental.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 10.Goiato MC, Santos DM, Baptista GT, Moreno A, Andreaotti AM, Dekon SF. Effect of thermal cycling and disinfection on microhardness of acrylic resin denture base. J Med Eng Technol. 2013;37:203–207. doi: 10.3109/03091902.2013.774444. [DOI] [PubMed] [Google Scholar]
- 11.Holtz RD, Lima BA, Souza AG, Filho, Brocchi M, Alves OL. Nanostructured silver vanadate as a promising antibacterial additive to water-based paints. Nanomedicine. 2012;8:935–940. doi: 10.1016/j.nano.2011.11.012. [DOI] [PubMed] [Google Scholar]
- 12.Holtz RD, Souza AG, Filho, Brocchi M, Martins D, Durán N, Alves OL. Development of nanostructured silver vanadates decorated with silver nanoparticles as a novel antibacterial agent. Nanotechnology. 2010;21:185102. doi: 10.1088/0957-4484/21/18/185102. [DOI] [PubMed] [Google Scholar]
- 13.Jandt KD, Sigusch BW. Future perspectives of resin-based dental materials. Dent Mater. 2009;25:1001–1006. doi: 10.1016/j.dental.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 14.Johansson A, Omar R, Carlsson GE. Bruxism and prosthetic treatment: a critical review. J Prosthodont Res. 2011;55:127–136. doi: 10.1016/j.jpor.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 15.Kamble VD, Parkhedkar RD. In vitro comparative evaluation of the effect of two different fiber reinforcements on the fracture toughness of provisional restorative resins. Indian J Dent Res. 2012;23:140–144. doi: 10.4103/0970-9290.100415. [DOI] [PubMed] [Google Scholar]
- 16.Khurana C, Vala AK, Andhariya N, Pandey OP, Chudasama B. Antibacterial activity of silver: the role of hydrodynamic particle size at nanoscale. J Biomed Mater Res A. 2014;102(10):3361–3368. doi: 10.1002/jbm.a.35005. [DOI] [PubMed] [Google Scholar]
- 17.Kumar V, Jolivalt C, Pulpytel J, Jafari R, Aref-Khonsari F. Development of silver nanoparticle loaded antibacterial polymer mesh using plasma polymerization process. J Biomed Mater Res A. 2013;101:1121–1132. doi: 10.1002/jbm.a.34419. [DOI] [PubMed] [Google Scholar]
- 18.Li F, Chen J, Chai Z, Zhang L, Xiao Y, Fang M, et al. Effects of a dental adhesive incorporating antibacterial monomer on the growth, adherence and membrane integrity of Streptococcus mutans. J Dent. 2009;37:289–296. doi: 10.1016/j.jdent.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 19.Marra J, Paleari AG, Rodriguez LS, Leite AR, Pero AC, Compagnoni MA. Effect of an acrylic resin combined with antimicrobial polymer on biofilm formation. J Appl Oral Sci. 2012;20:643–648. doi: 10.1590/S1678-77572012000600009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ozak ST, Ozkan P. Nanotechnology and dentistry. Eur J Dent. 2013;7:145–151. [PMC free article] [PubMed] [Google Scholar]
- 21.Paleari AG, Marra J, Pero AC, Rodriguez LS, Ruvolo-Filho A, Compagnoni MA. Effect of incorporation of 2-tert-butylaminoethyl methacrylate on fexural strength of a denture base acrylic resin. J Appl Oral Sci. 2011;19:195–199. doi: 10.1590/S1678-77572011000300003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pesci-Bardon C, Fosse T, Serre D, Madinier I. In vitro antiseptic properties of an ammonium compound combined with denture base acrylic resin. Gerodontology. 2006;23:111–116. doi: 10.1111/j.1741-2358.2006.00088.x. [DOI] [PubMed] [Google Scholar]
- 23.Redding S, Bhatt B, Rawls HR, Siegel G, Scott K, Lopez-Ribot J. Inhibition of Candida albicans biofilm formation on denture material. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;107:669–672. doi: 10.1016/j.tripleo.2009.01.021. [DOI] [PubMed] [Google Scholar]
- 24.Regis RR, Della Vecchia MP, Pizzolitto AC, Compagnoni MA, Souza PP, Souza RF. Antimicrobial properties and cytotoxicity of an antimicrobial monomer for application in prosthodontics. J Prosthodont. 2012;21:283–290. doi: 10.1111/j.1532-849X.2011.00815.x. [DOI] [PubMed] [Google Scholar]
- 25.Rodriguez LS, Paleari AG, Giro G, Oliveira NM, Junior, Pero AC, Compagnoni MA. Chemical characterization and fexural strength of a denture base acrylic resin with monomer 2-tert-butylaminoethyl methacrylate. J Prosthodont. 2013;22:292–297. doi: 10.1111/j.1532-849X.2012.00942.x. [DOI] [PubMed] [Google Scholar]
- 26.Shameli K, Ahmad MB, Zargar M, Yunus WM, Rustaiyan A, Ibrahim NA. Synthesis of silver nanoparticles in montmorillonite and their antibacterial behavior. Int J Nanomedicine. 2011;6:581–590. doi: 10.2147/IJN.S17112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Skupien JA, Valentini F, Boscato N, Pereira-Cenci T. Prevention and treatment of Candida colonization on denture liners: a systematic review. J Prosthet Dent. 2013;110:356–362. doi: 10.1016/j.prosdent.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 28.Valentini F, Luz MS, Boscato N, Pereira-Cenci T. Biofilm formation on denture liners in a randomised controlled in situ trial. J Dent. 2013;41:420–427. doi: 10.1016/j.jdent.2013.02.012. [DOI] [PubMed] [Google Scholar]
- 29.Wady AF, Machado AL, Zucolotto V, Zamperini CA, Berni E, Vergani CE. Evaluation of Candida albicans adhesion and biofilm formation on a denture base acrylic resin containing silver nanoparticles. J Appl Microbiol. 2012;112:1163–1172. doi: 10.1111/j.1365-2672.2012.05293.x. [DOI] [PubMed] [Google Scholar]
- 30.Yu RY, Zhou YS, Feng HL, Liu XY. Silver-ion release and particle distribution of denture base resin containing nanometer-sized silver-supported antimicrobical agent. Zhonghua Kou Qiang Yi Xue Za Zhi. 2008;43(1):54–56. [PubMed] [Google Scholar]