Abstract
Background
The plant respiratory chain contains several energy-dissipating enzymes, these being type II NAD(P)H dehydrogenases and the alternative oxidase, not present in mammals. The physiological functions of type II NAD(P)H dehydrogenases are largely unclear and little is known about their responses to stress. In this investigation, potato plants (Solanum tuberosum L., cv. Desiree) were sprayed with antimycin A, an inhibitor of the cytochrome pathway. Enzyme capacities of NAD(P)H dehydrogenases (EC 1.6.5.3) and the alternative oxidase were then analysed in isolated leaf mitochondria.
Results
We report a specific decrease in internal rotenone-insensitive NADH dehydrogenase capacity in mitochondria from antimycin A-treated leaves. External NADPH dehydrogenase and alternative oxidase capacities remained unaffected by the treatment. Western blotting revealed no change in protein abundance for two characterised NAD(P)H dehydrogenase homologues, NDA1 and NDB1, nor for two subunits of complex I. The alternative oxidase was at most only slightly increased. Transcript levels of nda1, as well as an expressed sequence tag derived from a previously uninvestigated closely related potato homologue, remained unchanged by the treatment. As compared to the daily rhythm-regulated nda1, the novel homologue displayed steady transcript levels over the time investigated.
Conclusions
The internal rotenone-insensitive NADH oxidation decreases after antimycin A treatment of potato leaves. However, the decrease is not due to changes in expression of known nda genes. One consequence of the lower NADH dehydrogenase capacity may be a stabilisation of the respiratory chain reduction level, should the overall capacity of the cytochrome and the alternative pathway be restricted.
Background
In contrast to mammals, the respiratory chain in plant mitochondria contains alternative, energy-dissipating pathways for the transfer of electrons from NAD(P)H to ubiquinone. In addition to the rotenone-sensitive and proton-pumping NADH dehydrogenase, complex I (EC 1.6.5.3), the plant respiratory chain contains at least four different rotenone-insensitive, non-proton-pumping NAD(P)H dehydrogenases [1,2]. In potato tuber mitochondria, NADH and NADPH can be oxidised by two separate Ca2+-dependent external dehydrogenases on the outer surface of the inner membrane, one specific for NADH, and one relatively specific for NADPH [3]. Similarly, at the internal side of the inner membrane, separate rotenone-insensitive dehydrogenases oxidise matrix NADH and NAD(P)H [4-6]. Plants can further make use of a non-proton-pumping alternative oxidase (AOX) (reviewed in [7,8]). This enzyme bypasses the proton-pumping cytochrome pathway, consisting of the antimycin A-sensitive bc1 complex (EC 1.10.2.2) and the cytochrome c oxidase (EC 1.9.3.1).
Two homologues of non-proton-pumping NADH dehydrogenases in yeast and E. coli, nda1 and ndb1, have been characterised in potato. The gene products, NDA1 and NDB1, are directed to the inner and the outer surface of the inner membrane, respectively [9]. Gene expression studies on respiratory chain proteins revealed that expression of nda1 is elevated during leaf development, is completely light-dependent and displays a diurnal rhythm in mature potato leaves, suggesting a role of NDA1 in photorespiration [10]. Recently, a specific down-regulation of NDA1 transcript and protein, as well as internal rotenone-insensitive NADH oxidation, was observed after cold stress [11].
Regarding cold effects on gene expression, the AOX has been studied more extensively than the rotenone-insensitive NAD(P)H dehydrogenases. However, diverse responses of the AOX have been reported for cold treatment. Tobacco cells and mung bean hypocotyls show an up-regulation of AOX protein and/or capacity during growth at low temperature [12,13], whereas AOX protein amounts are not affected in soybean cotyledons or potato leaves [11,13].
Several reports describe an induction of the alternative pathway by treatment with antimycin A, an inhibitor of the bc1 complex. AOX protein, as well as enzymatic capacity, increase in tobacco and petunia cells following antimycin A treatment [14,15]. Antimycin A treatment also causes an up-regulation of transcript levels of an aox gene in tobacco cell suspensions and in Arabidopsis thaliana leaves [16,17]. Conversely, Arabidopsis cells treated with a high concentration of antimycin A show only a slight increase in AOX protein. Instead, the degradation or decrease in proteins of the citric acid cycle, two subunits of complex I and one subunit of the ATP synthase complex is observed [18]. Other reported effects of antimycin A are a decrease in ATP in tobacco suspension cells and pea protoplasts, as well as decreased photosynthetic oxygen evolution in the latter case [14,19].
In vitro inhibition of the Hansenula anomala and rat heart bc1 complex by antimycin A leads to elevated amounts of reactive oxygen species (ROS) in a semiquinone-dependent process [20,21]. Consistent observations have been made in plants [2,22]. It has been suggested that an inhibitor-induced over-reduction of the electron transport chain increases the formation of ROS, and that this effect can be diminished by the AOX [23,24]. Over-expression of the AOX results in lower amounts of ROS in transgenic tobacco cell suspensions as compared to wild-type cells after addition of antimycin A [25]. Up-regulation of the AOX may therefore compensate for a decreased ubiquinol oxidation capacity of the inhibited cytochrome pathway. This could stabilise the reduction level of the ubiquinone pool and may prevent an increase in the formation of ROS [23,24,26].
As yet, no similar experiments have been carried out to investigate how the rotenone-insensitive NAD(P)H dehydrogenases respond to antimycin A treatment. Here we report a specific decrease in internal rotenone-insensitive NADH oxidation capacity in antimycin A-treated potato leaves and discuss possible consequences for the redox state of the respiratory chain.
Results
Internal rotenone-insensitive NADH oxidation capacity is lower in mitochondria from antimycin A-treated leaves
Potato plants were sprayed with different concentrations of antimycin A or with solvent as control. The treatments were not lethal to the plants, but necrotic lesions were visible on the antimycin A-sprayed leaves 10 days after spraying. Lesions were more abundant at the higher antimycin A concentrations (data not shown). Twenty-four hours after spraying, mitochondria were isolated from the leaves. NAD(P)H and succinate oxidation was measured on intact mitochondria, and internal NADH oxidation after osmotic rupture to permeabilise the inner membrane. Internal rotenone-insensitive NADH oxidation was found to be substantially lower in mitochondria isolated from antimycin A-treated leaves as compared to control leaves, this activity being affected more by the inhibitor treatment than the oxidation of succinate and external NAD(P)H (Fig. 1). A lower rate of internal rotenone-insensitive NADH oxidation is observed already after treatment with 3 μM antimycin A, and after 30 μM, the activity is about 38% of the control rate. The decrease in rotenone-insensitive NADH oxidation at 3 μM antimycin A, as compared to solvent control, is statistically significant at P < 0.05.
Figure 1.
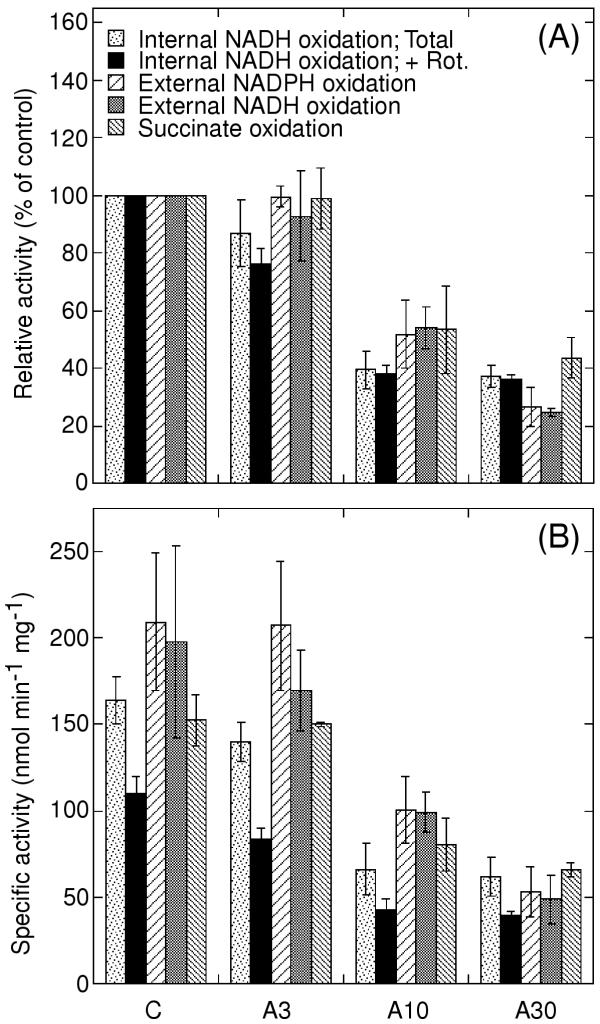
Oxidation of NAD(P)H and succinate in leaf mitochondria purified from control and antimycin A-sprayed potato plants. Data are presented as percentage of control in each experiment (A) and as absolute values in nmol NAD(P)H or succinate min-1 mg-1 (B). Internal NADH oxidation was measured on osmotically burst mitochondria in the absence (Total) and presence (+ Rot.) of the complex I inhibitor rotenone. External NAD(P)H oxidation was measured in the presence of Ca2+. A3, A10 and A30 denote mitochondria isolated from leaves sprayed with 3, 10 and 30 μM antimycin A, respectively. Control leaves (C) were sprayed with solvent only. Error bars indicate standard error for 3 independent experiments, except for succinate oxidation (standard deviation, n = 2).
Ubiquinol oxidation is restricted by leaf treatment with high antimycin A
The activity of external NADH and NADPH oxidation was substantially higher than the internal rotenone-insensitive NADH oxidation (Fig. 1). Treatment of leaves with 10 and 30 μM antimycin A led in both cases to a decreased rate in the isolated mitochondria. However, external NAD(P)H oxidation activity remained unchanged at 3 μM antimycin A as compared to the control. Similar results were observed with succinate as substrate (Fig. 1).
Since all other enzymes potentially capable of catalysing NAD(P)H oxidation to short chained quinone analogues, (e.g. complex I and dihydrolipoamide dehydrogenase), are located inside the inner membrane permeability barrier, external NADPH dehydrogenase can be measured directly using decyl-ubiquinone (DcQ) as acceptor. This activity is also independent of the capacity of enzymes further downstream in the electron transport chain. Fig. 2 shows that NADPH oxidation to DcQ in isolated mitochondria was mainly unaffected by leaf treatment with antimycin A. Activities varied somewhat between experiments but the normalised averages allow the conclusion that no decrease in external NADPH dehydrogenase capacity occurred as a result of antimycin A leaf treatment. The activity of cytochrome c oxidase, located downstream of the bc1 complex, was similar in mitochondria from control and antimycin A-treated leaves (data not shown).
Figure 2.
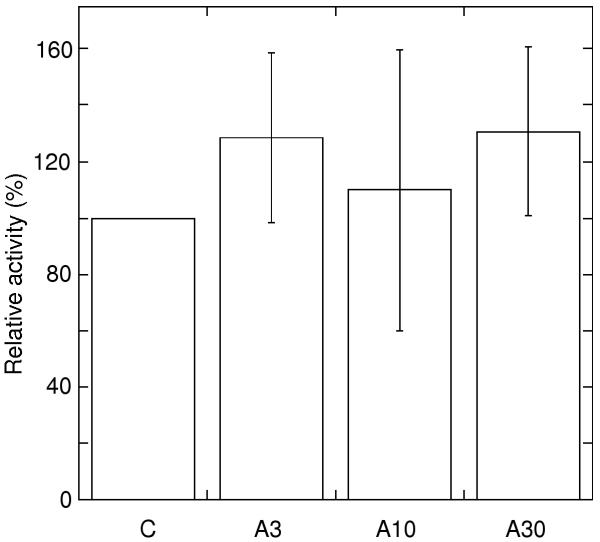
External NADPH oxidation with DcQ as electron acceptor. NADPH dehydrogenase activities in the presence of Ca2+ and antimycin A are presented as percentage of control in each experiment. No activity was seen without Ca2+ added to the reaction. Error bars indicate standard deviation (n = 2). Control activities were 315 and 479 nmol NADPH min-1 mg-1 in the two experiments. Samples are denoted as for Fig. 1.
The results show that in mitochondria from in vivo antimycin A treated leaves, the external NAD(P)H oxidation activities are limited by another component downstream of the dehydrogenases. However, internal rotenone-insensitive NADH oxidation, as measured in osmotically disrupted mitochondria, was apparently not limited in this way. The total internal NADH oxidation, measured before the addition of rotenone, was 1.5–1.7 times higher than the rotenone-insensitive component (Fig. 1B), showing that sufficient quinol oxidation capacity was present to make the rotenone-insensitive rate mainly dependent on the dehydrogenase capacity. Although the external NAD(P)H oxidation activity was higher and thus more likely to be affected by a downstream inhibition, these activities were affected to a lesser degree than the internal rotenone-insensitive NADH oxidation after leaf treatment with 3 and 10 μM antimycin A. It can thus be concluded that the lower rate of internal rotenone-insensitive NADH oxidation in mitochondria from leaves treated with 3 and 10 μM antimycin A is due to a lower dehydrogenase capacity, whereas the decrease in external NAD(P)H oxidation after treatment with 10 and 30 μM is a consequence of restricted ubiquinol oxidation.
AOX capacity is not induced by antimycin A treatment in potato leaves
To determine the capacity of the AOX, the cytochrome pathway activity was completely inhibited by adding antimycin A to the reaction medium. Fig. 3 summarises the rates after antimycin A addition with succinate and external NAD(P)H as substrates. No difference in AOX capacity is seen between mitochondria from antimycin A-treated leaves and control leaves. With the 30 μM antimycin A treatment, the rates were completely insensitive to in vitro addition of antimycin A (data not shown), indicating a high degree of in vivo bc1 complex inhibition at this concentration.
Figure 3.
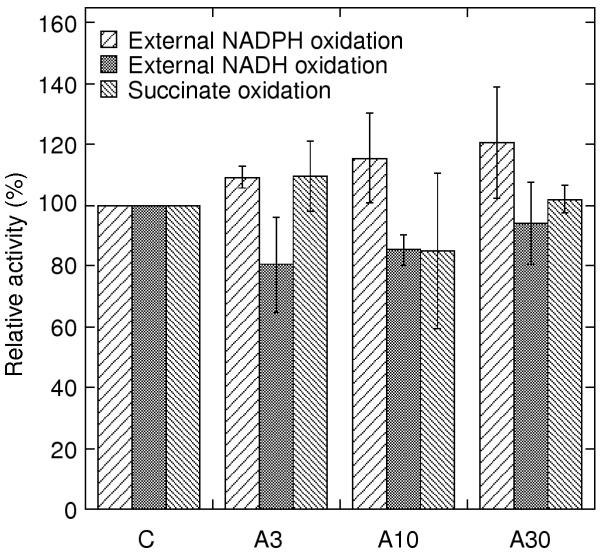
Alternative pathway activity. Antimycin A-insensitive oxidation of NADPH, NADH and succinate was measured to oxygen in the presence of DTT and pyruvate to assure maximum rates. Respiratory chain activities are displayed as percentage of control activity in each experiment. Control activities were: 12, 52 and 59 nmol NADPH min-1 mg-1; 25, 40 and 57 nmol NADH min-1 mg-1; or 60 and 52 nmol succinate min-1 mg-1 in the independent experiments. Rates of succinate oxidation were corrected for a small rate remaining after addition of antimycin A and salicylhydroxamic acid; 3–6 and 7–10 nmol O2 min-1 mg-1 for control/A3, and A10/A30, respectively. Samples and error bars are denoted as for Fig. 1, where the corresponding rates for total respiration (cytochrome path plus alternative path) are presented.
Gene expression of nda-type genes after antimycin A treatment
The isolated leaf mitochondria were analysed by western blotting to investigate if antimycin A leaf treatment had any effects on the protein abundance of respiratory chain enzymes. The antisera against NDA1 and NDB1 specifically recognised proteins of 48 kDa and 61 kDa, respectively, as previously seen for isolated potato leaf mitochondria [27]. However, the immunosignals for NDA, NDB, as well as the NAD9 and 76 kDa subunits of complex I showed no significant changes between the treatments and the control (Fig. 4). In two experiments, AOX was seen to increase slightly after leaf treatment with 3 and 10 μM antimycin A. The blot with the clearest increase is shown in Fig. 4. In a third experiment, this increase could not be seen. These results suggest that the AOX protein may be slightly increased by treatment of potato leaves with antimycin A.
Figure 4.
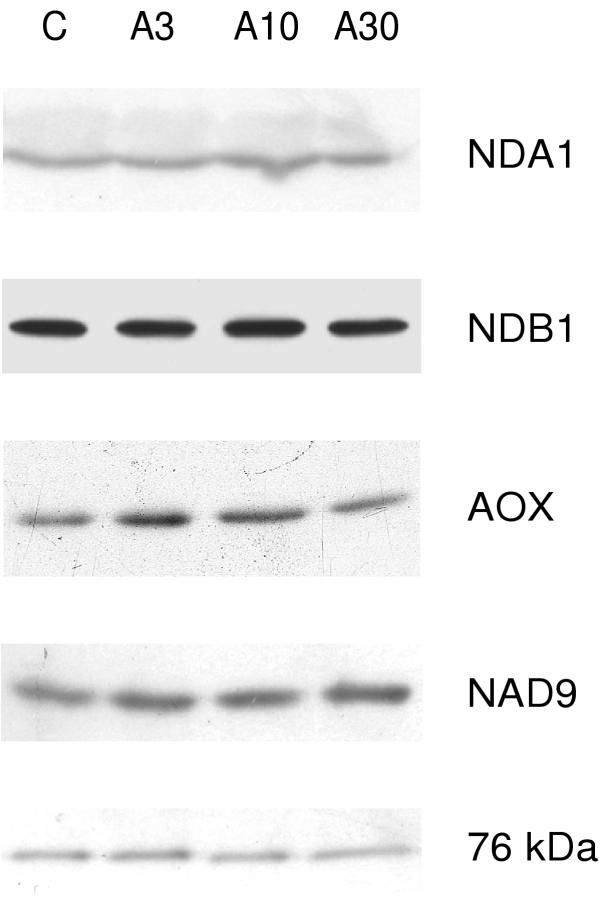
Western analyses of respiratory chain proteins. Potato leaf mitochondria were purified from control and antimycin A-sprayed plants. Twenty μg of mitochondrial protein was loaded in each lane. The proteins analysed for are indicated to the right. For NDA, NDB and complex I subunits, blots from one representative experiment out of 3 are shown. For AOX, the experiment with highest induction is depicted. Samples are denoted as for Fig. 1.
Since antibodies may vary in detecting different isoenzymes, gene expression of nda1 was additionally analysed at transcript level using a real-time PCR system. Plants were sprayed 1 h before light onset (03:00 h) and leaf transcript levels for nda1 were quantified at the time of treatment (03:00 h), after 4 h (07:00 h) and 8 h (11:00 h). Antimycin A treatment affected neither the level of nda1 transcripts nor its diurnal change in expression during this part of the day (Fig. 5A). The transcript for the 28.5 kDa subunit of complex I was similar in all isolates (Fig. 5C). In a separate experiment, total RNA was isolated from the same leaf material that was used for mitochondrial purification. No appreciable changes were seen in nda1 transcript amounts 24 h after spraying with 0, 3, 10 and 30 μM antimycin A (data not shown).
Figure 5.
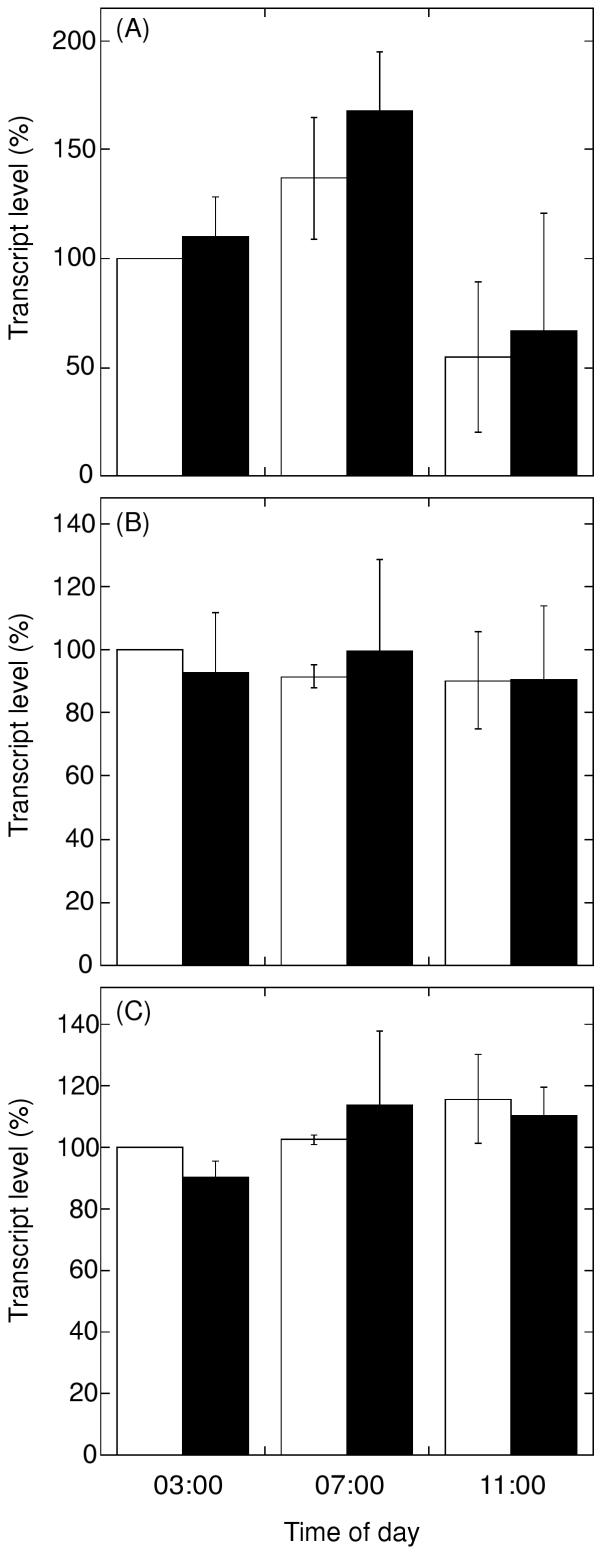
Transcript quantification by real-time PCR in control and antimycin A-treated potato leaves. Potato plants were sprayed at 03:00 h, one hour before light, and leaf samples were collected at start of the treatment (03:00 h), after 4 h (at 07:00 h) and after 8 h (at 11:00 h). White columns, leaves sprayed with solvent only. Black columns, leaves sprayed with 10 μM antimycin A. Transcript abundance is shown for nda1 (A), TC54504 (B) and the 28.5 kDa subunit of complex I (C). Transcript levels are given as percent of the level in the control plants at the start of the treatment (03:00 h). The error bars indicate standard deviation (n = 2).
To investigate whether other NDA-type proteins are present in potato, expressed sequence tag (EST) databases were searched. The potato NDA1 full-length protein sequence was used to query the TIGR and GenBank potato EST databases. The only highly significantly scoring entry was the EST contig TC54504 with a length of 1547 bp. This contig consists of 6 overlapping ESTs, three of which cover a region showing a high deduced amino acid sequence similarity to NDA1. The downstream part, made up by a single EST 156H09 (BI406197) appears to contain unspliced intron sequences as seen by comparison to the database intron annotations for the nda-type genes identified in Arabidopsis thaliana [28]. These were excluded from comparisons. In pair-wise sequence alignments, a 222 residues long translation of TC54504 shows 72% identity to the protein sequence of potato NDA1. On comparison to the potato NDB1, only 43% amino acid sequence identity is seen in a 216 residue overlap, indicating that TC54504 derives from an nda-type gene. The deduced protein sequence of TC54504 contains two highly conserved nucleotide-binding motifs, similar to other plant homologues [9].
In order to see if antimycin A leaf treatment has any effect on TC54504 mRNA, real-time PCR was carried out as for NDA1. Leaf transcript levels for TC54504 (Fig. 5B) revealed no change in mRNA abundance between treatment and control. In addition, no time-dependent change in expression of TC54504 was observed.
Discussion
Inhibition of the bc1 complex by antimycin A treatment of potato leaves resulted in a substantially decreased capacity for internal rotenone-insensitive NADH oxidation in isolated mitochondria as compared to mitochondria from control leaves (Fig. 1). A significant decrease specific to internal rotenone-insensitive NADH oxidation was seen already after leaf treatment with 3 μM antimycin A. The oxidation of external NAD(P)H and succinate was decreased only at higher concentrations of antimycin A (Fig. 1), most probably as a result of inhibitor-induced rate limitations of the cytochrome pathway further downstream in the electron transport chain. However, it is possible that other unspecific damage to the electron transport enzymes may occur at the highest antimycin A concentration used.
For antimycin A treatment to have affected the respiratory chain enzyme capacities in vivo, an inhibition of the bc1 complex should have occurred. The inhibition of the bc1-complex observed in isolated mitochondria after leaf treatment with 10 μM antimycin A was 45–50%, using external NAD(P)H as substrate (Fig. 1). Thus, some inhibition should be expected also after treatment with 3 μM antimycin A, but was not observed. However, substrate oxidation measurements may underestimate the quinol oxidation capacity in the control mitochondria, consequently underestimating the inhibitory effect of the leaf treatment. Alternatively, antimycin A may have been lost from the mitochondria during isolation, especially since the isolation media contains high concentrations of bovine serum albumin which efficiently binds hydrophobic molecules. It has also been suggested that antimycin A is degraded in cells [14]. Thus, a transient, partial bc1 complex inhibition may have taken place in vivo also after leaf treatment with 3 μM antimycin A, inducing the decrease in internal rotenone-insensitive NADH dehydrogenase capacity (Fig. 1). The lower in vitro internal NADH dehydrogenase capacity should reflect a lower in vivo enzyme capacity, which may restrict the uptake of electrons into the respiratory chain.
AOX capacity is not up-regulated following in vivo antimycin A leaf treatment in potato, and the AOX protein amount is unchanged or slightly increased (Figs. 3 and 4). This is in contrast to findings on other species, where AOX protein amount and capacity are induced, e.g. tobacco and petunia cell suspensions [14,15]. In Arabidopsis plants, leaves sprayed with 10 μM antimycin A specifically increased aox1a transcript abundance, but other aox genes were unaffected [17]. However, antimycin A treatment of Arabidopsis cells only slightly induced AOX protein, possibly because high amounts of the protein are already present in the controls [18]. Also in the present study, the AOX protein concentration could have been sufficiently high to compensate for an in vivo restriction of the cytochrome pathway. Alternatively, regulation of AOX expression could be different in potato than in other investigated species. There are variations between species regarding the effects of temperature decrease on AOX expression. Low growth temperature results in a higher AOX protein level and enzyme capacity in tobacco suspension cells [12] but no effect is seen in soybean [13] or in potato leaves [11], where AOX protein and capacity remain unaffected by cold treatment.
Taken together, a transient or persistent restriction in capacity of the bc1 complex and an unchanged AOX capacity caused by the antimycin A treatment, may have resulted in a higher in vivo reduction state of the ubiquinone pool. One secondary effect deriving from an over-reduction could be an elevated production of ROS from the electron transport chain [2,26]. Purvis and Shewfelt [23] hypothesised that the AOX prevents formation of ROS, should the electron flow from the NAD(P)H dehydrogenases exceed the capacity of the cytochrome pathway. The results from our study suggest that a specific decrease in internal rotenone-insensitive NADH oxidation capacity may prevent over-reduction, and possibly ROS generation, instead by decreasing ubiquinol formation in a situation where the ubiquinol oxidation is restricted. Consistent with this, the internal rotenone-insensitive NADH oxidation capacity was decreased under cold treatment [11], a stress that as one of its consequences leads to ROS formation [29]. In Arabidopsis cells, oxidative stress and antimycin A treatment resulted in a lower protein abundance of the 76 and 24 kDa subunits of complex I which may restrict ubiquinone reduction via rotenone-sensitive NADH oxidation [18]. However, in potato, the protein levels of the 76 kDa and NAD9 complex I subunits were unaffected by antimycin A leaf spray (Fig. 4), indicating that complex I capacity is not decreased by this treatment.
Apart from ROS production, other secondary effects of antimycin A have been reported. Lower levels of ATP are seen in tobacco suspension cells [14] and in pea mesophyll protoplasts, which additionally show a lower photosynthetic oxygen evolution due to impaired mitochondrial electron transport after antimycin A application [19]. These effects were observed after treatment of cells and protoplasts with lower concentrations of antimycin A than in the present study. Therefore, similar consequences for the ATP level, which would also cause metabolic changes, cannot be excluded in the present investigation. It is also known that antimycin A can inhibit cyclic electron transport around photosystem I in the chloroplast [30]. A direct effect on cyclic electron transport, resulting in a lower ATP generation in chloroplasts, is seen after application of 5 μM antimycin A on leaf discs with stripped off lower epidermis [31]. Another study shows that photosynthetic electron transport is not affected by 1 μM antimycin A applied to protoplasts [19]. In both studies, a much more efficient inhibitor uptake must be expected as compared to the treatment of intact leaves. In the present investigation, an effect on internal rotenone-insensitive NADH oxidation was seen already at 3 μM antimycin A applied to leaves with intact epidermis (Fig. 1). Thus, a direct inhibition of electron transport in the chloroplast is unlikely at this antimycin A concentration.
The lower capacity of internal rotenone-insensitive NADH oxidation (Fig. 1) correlated neither with nda1 transcript levels (Fig. 5A) nor with the amount of immunodetected NDA protein (Fig. 4), both of which were unaffected by the treatment. A possible explanation could be that the internal NADH oxidation is regulated directly at the enzyme level or that additional, as yet undiscovered, genes for rotenone-insensitive NADH dehydrogenases are present in potato. Previous suggestions that NDA1 is an internal NADH dehydrogenase are based on protein localisation, bioinformatics and correlation of gene expression and activity [9-11,32]. Additionally, two closely related NDA-type mitochondrial proteins are present in Arabidopsis [33], and import and mutant experiments have strongly suggested the NDA1/NDI1 to be an internal NADH dehydrogenase [34].
In the present study, a potato EST-contig (TC54504) potentially derived from a second nda-type gene was found by database searching. Previous investigations have indicated that there may be at least one additional NDA isoenzyme present in potato, based on correlation between protein and activity levels during inductions [10], and differences in molecular mass between immunorecognised NDA protein in different organs [27]. Recently, At-nda1 and At-nda2, two genes closely related to potato nda1, were found in Arabidopsis thaliana [28]. At-nda1 shows light-dependence, in contrast to At-nda2. In our study, transcript abundance of the potato gene corresponding to TC54504 is not affected by antimycin A treatment (Fig. 5B). Also, whereas the amount of nda1 transcript varies over the day, as previously reported [10], TC54504 transcript abundance is stable over the time investigated. Further experiments are needed to determine if TC54504 derives from a light-independent nda gene.
Conclusions
This investigation shows that in vivo antimycin A treatment of potato leaves specifically lowers the capacity for respiratory internal rotenone-insensitive NADH oxidation (Fig. 1). Recently, down-regulation of internal rotenone-insensitive NADH oxidation and nda1 expression was reported for cold-stressed potato leaves [11]. The absence of nda1 suppression in the present investigation suggests different ways of controlling rotenone-insensitive NADH oxidation in these two studies. Rotenone-insensitive internal NADH oxidation and/or nda1 gene expression has previously been shown to vary in response to leaf development, time of day, light and cold treatment [10,11]. Considering the wide variety of signals affecting AOX expression in plants [8], it is likely that several yet undiscovered external cues influence NAD(P)H dehydrogenases in plant mitochondria.
Neither cold stress [11] nor antimycin A treatment (Figs. 3 and 4) appears to substantially affect the capacity of the AOX in potato, indicating differences in respiratory chain regulation between potato and other investigated plants. The present study underlines that more attention should be given to the interplay between the AOX and the rotenone-insensitive NAD(P)H dehydrogenases in response to stress.
Methods
Plant material, plant treatment and isolation of mitochondria
Leaves of potato plants (Solanum tuberosum L., cv. Desiree) were used in all experiments. Plants were grown 4–5 weeks in the greenhouse at 20–25°C and 40–70% relative humidity. For plants grown in winter, the photoperiod was 16 h of extra light (04:00–20:00 h) to give 300 μmol m-2 s-1. In summer, natural light was supplemented with 12 h of extra light (04:00–10:00 h, 14:00–20:00 h) to give approximately the same light conditions of 300 μmol m-2 s-1. Leaves of 3 plants per treatment were sprayed with a solution of antimycin A in 0.01% (v/v) Tween-20, using a volume of approximately 50 ml per plant. Control plants were sprayed with 0.01% Tween-20. In order to prevent root uptake, the soil was covered with aluminium foil during spraying. For isolation of mitochondria, 40 g of leaf material was sampled 24 h after spraying and leaves were extensively washed for at least 5 min at 4°C in 5 L distilled water with slow agitation to remove any externally persisting detergent or inhibitor. Mitochondria were purified from leaves principally according to [35], frozen in liquid N2 in the presence of 5% dimethyl sulfoxide, and stored at -80°C. Protein concentration was determined with the bicinchoninic acid method (Sigma) using bovine serum albumin as a standard.
Enzymatic activities
Oxidation of NAD(P)H was measured at 340 nm using an Aminco DW 2 dual wavelength spectrophotometer, essentially as in [10]. The reaction medium (Medium A) contained 0.3 M sucrose, 10 mM MOPS, 0.5 mM EGTA, 2.5 mM MgCl2/KOH (pH 7.2) and 0.4 μM carbonyl cyanide p-(trifluoromethoxy)phenylhydrazone (FCCP). To certify maximum activity of the AOX during oxygen consumption, 20 μM of the reducing agent dithiothreitol (DTT) and 5 mM pyruvate were present during the reaction. The mitochondria (approximately 16 μg) were pre-incubated with the dithiothreitol (at 6–7 mM) for 10 min on ice, before the mitochondria were added to the assay. The assay was started by addition of 100 μM NAD(P)H and 1 mM CaCl2. To inhibit bc1 complex activity for the determination of AOX capacity, 0.375 μM antimycin A was added. Inhibition of AOX was done with 1 mM salicylhydroxamic acid.
For measurement of internal NADH oxidation, mitochondrial protein pre-incubated with DTT, was incubated for 6 min in a low osmolarity medium (1 mM MOPS, 0.1 mM EGTA, pH 7.2) at 2–3 mOsm, to disrupt the inner membrane [36,37]. The suspension was then supplemented to make up Medium A minus sucrose with, additionally 5 mM pyruvate, and the reaction was started by adding 100 μM NADH. Internal rotenone-insensitive NADH oxidation was measured in the presence of 15 μM rotenone. The rotenone-sensitive activity was determined as the difference plus and minus rotenone.
NADPH oxidation with DcQ as final electron acceptor was measured in Medium A including 0.375 μM antimycin A. Approximately 1.5 μg of mitochondrial protein was incubated with 20 μM DcQ and the reaction was started with the addition of 100 μM NADPH and 1 mM CaCl2.
Succinate oxidation was measured as oxygen consumption at 25°C with an oxygen electrode (Rank Brothers, Cambridge, UK). The medium contained 0.3 M sucrose, 5 mM MOPS, pH 7.2, 5 mM KH2PO4, 2.5 mM MgCl2, 10 mM pyruvate, 250 μM ATP, 10 mM succinate and 0.4 μM carbonyl cyanide p-(trifluoromethoxy)phenylhydrazone. Mitochondria were pre-incubated for 10 min with 6–7 mM dithiothreitol, giving a final concentration of 130 μM in the assay. AOX capacity was determined by additions of 0.375 μM antimycin A and 1 mM salicylhydroxamic acid. Activities were recalculated into succinate consumption using a factor of 2 succinate per O2 molecule.
Malate dehydrogenase (EC 1.1.1.37) activity and mitochondrial latency were determined as in [38]. Cytochrome c oxidase activity was measured as in [39].
Statistical analysis of the enzyme activity data was done by one-way analysis of variance at P > 0.05, using the SPSS Win 8.0.0 software package (SPSS Inc., Chicago, Ill.).
Western analysis
The same mitochondrial preparations used for activity measurements were resolved by SDS-PAGE according to [40], and western blotted as in [41]. Antisera raised against NDA1 and NDB1 are described in [10,27]. The 76 kDa subunit of complex I was detected by antibodies against the 78 kDa subunit of Neurospora crassa [42] as described in [1]. The NAD9 subunit of complex I was recognised by antibodies against the wheat homologue [43] and the AOX by monoclonal antibodies against the maize protein [44]. Antisera were used as 1:1000 dilutions. Antibody detection was visualised by the ECL system (Amersham Life Science Ltd, Little Chalfont, Bucks, UK).
Database screening and transcript analyses
The screening for additional NDA-type genes was done by the tblastn algorithm [45] using the potato NDA1 protein sequence as probe against the TIGR Unique Gene Indices database [46] and the EST-subset at National Centre of Biotechnology Information [47]. Pair-wise sequence alignments were done in SIM [48] at [49].
RNA preparation, cDNA synthesis and real-time PCR amplification was carried out as in [11]. For TC54504, real-time PCR was carried out for 45 cycles, each consisting of 20 s at 95°C, 30 s at 60°C, 45 s at 72°C and 20 s at 84°C (data acquisition). A specific primer pair was designed against the sequence of two overlapping ESTs, EST536929 and 156H09, of contig TC54504, avoiding regions of sequence variations: 5'-TGC TGA ACC AGT TAC CCA GA-3'; 5'-TCT TCC GAA ATG CCT GGA G-3'. The correct identity of the amplification product was verified by restriction cleavage. A control without reverse transcription was performed to certify that contamination by genomic DNA did not perturb the quantification of cDNA templates.
Authors' contributions
DAG had a major role in experiments, interpretation and drafting of the manuscript. FIJ participated in the Western analysis and interpretation. ÅSS participated in design, preliminary experiments and coordination. AGR participated in design, coordination, interpretation and writing of the manuscript. All authors read, modified and approved the manuscript.
Acknowledgments
Acknowledgements
Drs. T. E. Elthon, University of Nebraska-Lincoln, NE, USA, T. Friedrich, Albert-Ludwigs Universität, Freiburg, Germany and J. M. Grienenberger, Université Louis-Pasteur, Strasbourg, France, are acknowledged for generous donations of antibodies. We are also grateful to Dr. Jacqueline Postma, Wageningen University, The Netherlands, for help with statistics, and Dr. Ian M. Møller, Risø National Laboratory, Denmark, for constructive comments on the manuscript. This investigation was supported by Carl Tryggers Stiftelse, Carl Tesdorpfs Stiftelse and The Swedish Research Council for Environment, Agricultural Sciences and Spatial Planning.
Contributor Information
Daniela A Geisler, Email: daniela.geisler@cob.lu.se.
Fredrik I Johansson, Email: fredrik.johansson@cob.lu.se.
Å Staffan Svensson, Email: sts@kvl.dk.
Allan G Rasmusson, Email: allan.rasmusson@cob.lu.se.
References
- Rasmusson AG, Heiser V, Zabaleta E, Brennicke A, Grohmann L. Physiological, biochemical and molecular aspects of mitochondrial complex I in plants. Biochim Biophys Acta. 1998;1364:101–111. doi: 10.1016/S0005-2728(98)00021-8. [DOI] [PubMed] [Google Scholar]
- Møller IM. Plant mitochondria and oxidative stress: Electron transport, NADPH turnover, and metabolism of reactive oxygen species. Annu Rev Plant Physiol Plant Mol Biol. 2001;52:561–591. doi: 10.1146/annurev.arplant.52.1.561. [DOI] [PubMed] [Google Scholar]
- Roberts TH, Fredlund KM, Møller IM. Direct evidence for the presence of 2 external NAD(P)H dehydrogenases coupled to the electron transport chain in plant mitochondria. FEBS Lett. 1995;373:307–309. doi: 10.1016/0014-5793(95)01059-N. [DOI] [PubMed] [Google Scholar]
- Møller IM, Palmer JM. Direct evidence for the presence of a rotenone-resistant NADH dehydrogenase on the inner surface of the inner membrane of plant mitochondria. Physiol Plant. 1982;54:267–274. [Google Scholar]
- Rasmusson AG, Møller IM. NAD(P)H dehydrogenases on the inner surface of the inner mitochondrial membrane studied using inside-out submitochondrial particles. Physiol Plant. 1991;83:357–365. doi: 10.1034/j.1399-3054.1991.830305.x. [DOI] [Google Scholar]
- Melo AMP, Roberts TH, Møller IM. Evidence for the presence of two rotenone-insensitive NAD(P)H dehydrogenases on the inner surface of the inner membrane of potato tuber mitochondria. Biochim Biophys Acta. 1996;1276:133–139. doi: 10.1016/0005-2728(96)00068-0. [DOI] [Google Scholar]
- Vanlerberghe GC, McIntosh L. Alternative oxidase: From gene to function. Annu Rev Plant Physiol Plant Mol Biol. 1997;48:703–734. doi: 10.1146/annurev.arplant.48.1.703. [DOI] [PubMed] [Google Scholar]
- Millenaar FF, Lambers H. The alternative oxidase: in vivo regulation and function. Plant Biology. 2003;5:2–15. doi: 10.1055/s-2003-37974. [DOI] [Google Scholar]
- Rasmusson AG, Svensson AS, Knoop V, Grohmann L, Brennicke A. Homologues of yeast and bacterial rotenone-insensitive NADH dehydrogenases in higher eukaryotes: two enzymes are present in potato mitochondria. Plant J. 1999;20:79–87. doi: 10.1046/j.1365-313X.1999.00576.x. [DOI] [PubMed] [Google Scholar]
- Svensson ÅS, Rasmusson AG. Light-dependent gene expression for proteins in the respiratory chain of potato leaves. Plant J. 2001;28:73–82. doi: 10.1046/j.1365-313X.2001.01128.x. [DOI] [PubMed] [Google Scholar]
- Svensson ÅS, Johansson FI, Møller IM, Rasmusson AG. Cold stress decreases the capacity for respiratory NADH oxidation in potato leaves. FEBS Lett. 2002;517:79–82. doi: 10.1016/S0014-5793(02)02581-4. [DOI] [PubMed] [Google Scholar]
- Vanlerberghe GC, McIntosh L. Lower Growth Temperature Increases Alternative Pathway Capacity and Alternative Oxidase Protein in Tobacco. Plant Physiol. 1992;100:115–119. doi: 10.1104/pp.100.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzàlez-Meler MA, Ribas-Carbo M, Giles L, Siedow JN. The effect of growth and measurement temperature on the activity of the alternative respiratory pathway. Plant Physiol. 1999;120:765–772. doi: 10.1104/pp.120.3.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanlerberghe GC, McIntosh L. Coordinate Regulation of Cytochrome and Alternative Pathway Respiration in Tobacco. Plant Physiol. 1992;100:1846–1851. doi: 10.1104/pp.100.4.1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner AM, Wagner MJ. Changes in mitochondrial respiratory chain components of petunia cells during culture in the presence of antimycin A. Plant Physiol. 1997;115:617–622. doi: 10.1104/pp.115.2.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanlerberghe GC, McIntosh L. Mitochondrial Electron Transport Regulation of Nuclear Gene Expression. Studies with the Alternative Oxidase Gene of Tobacco. Plant Physiol. 1994;105:867–874. doi: 10.1104/pp.105.3.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saisho D, Nambara E, Naito S, Tsutsumi N, Hirai A, Nakazono M. Characterization of the gene family for alternative oxidase from Arabidopsis thaliana. Plant Mol Biol. 1997;35:585–596. doi: 10.1023/A:1005818507743. [DOI] [PubMed] [Google Scholar]
- Sweetlove LJ, Heazlewood JL, Herald V, Holtzapffel R, Day DA, Leaver CJ, Millar AH. The impact of oxidative stress on Arabidopsis mitochondria. Plant J. 2002;32:891–904. doi: 10.1046/j.1365-313X.2002.01474.x. [DOI] [PubMed] [Google Scholar]
- Padmasree K, Raghavendra AS. Importance of oxidative electron transport over oxidative phosphorylation in optimizing photosynthesis in mesophyll protoplasts of pea (Pisum sativum L.) Physiol Plant. 1999;105:546–553. doi: 10.1034/j.1399-3054.1999.105321.x. [DOI] [Google Scholar]
- Minagawa N, Koga S, Nakano M, Sakajo S, Yoshimoto A. Possible Involvement of Superoxide Anion in the Induction of Cyanide-Resistant Respiration in Hansenula anomala. FEBS Lett. 1992;302:217–219. doi: 10.1016/0014-5793(92)80444-L. [DOI] [PubMed] [Google Scholar]
- Raha S, McEachern GE, Myint AT, Robinson BH. Superoxides from mitochondrial complex III: The role of manganese superoxide dismutase. Free Radical Biol Medi. 2000;29:170–180. doi: 10.1016/S0891-5849(00)00338-5. [DOI] [PubMed] [Google Scholar]
- Rich PR, Bonner WD. The sites of superoxide anion generation in higher plant mitochondria. Arch Biochem Biophys. 1978;188:206–213. doi: 10.1016/0003-9861(78)90373-9. [DOI] [PubMed] [Google Scholar]
- Purvis AC, Shewfelt RL. Does the Alternative Pathway Ameliorate Chilling Injury in Sensitive Plant Tissues. Physiol Plant. 1993;88:712–718. doi: 10.1034/j.1399-3054.1993.880426.x. [DOI] [PubMed] [Google Scholar]
- Wagner AM, Wagner MJ. Measurements of in Vivo Ubiquinone Reduction Levels in Plant Cells. Plant Physiol. 1995;108:277–283. doi: 10.1104/pp.108.1.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell DP, Wang Y, McIntosh L. The alternative oxidase lowers mitochondrial reactive oxygen production in plant cells. Proc Natl Acad Sci USA. 1999;96:8271–8276. doi: 10.1073/pnas.96.14.8271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millenaar FF, Benschop JJ, Wagner AM, Lambers H. The role of the alternative oxidase in stabilizing the in vivo reduction state of the ubiquinone pool and the activation state of the alternative oxidase. Plant Physiol. 1998;118:599–607. doi: 10.1104/pp.118.2.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmusson AG, Agius SC. Rotenone-insensitive NAD(P)H dehydrogenases in plants: Immunodetection and distribution of native proteins in mitochondria. Plant Physiol Biochem. 2001;39:1057–1066. doi: 10.1016/S0981-9428(01)01334-1. [DOI] [Google Scholar]
- Svensson ÅS. PhD Thesis. Department of Cell and Organism Biology, Lund University; 2002. Respiratory NAD(P)H dehydrogenases of plants – Gene identity and expression in response to light and cold. [Google Scholar]
- Wise RR. Chilling-Enhanced Photooxidation - the Production, Action and Study of Reactive Oxygen Species Produced During Chilling in the Light. Photosynth Res. 1995;45:79–97. doi: 10.1007/BF00032579. [DOI] [PubMed] [Google Scholar]
- Slovacek RE, Crowther D, Hind G. Cytochrome function in the cyclic electron transport pathway of chloroplasts. Biochimica Biophysica Acta. 1979;547:138–148. doi: 10.1016/0005-2728(79)90102-6. [DOI] [PubMed] [Google Scholar]
- Joët T, Cournac L, Horvath EM, Medgyesy P, Peltier G. Increased sensitivity of photosynthesis to antimycin A induced by inactivation of the chloroplast ndhB gene. Evidence for a participation of the NADH dehydrogenase complex to cyclic electron flow around photosystem I. Plant Physiol. 2001;125:1919–1929. doi: 10.1104/pp.125.4.1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalecka Agnieszka M., Agius Stephanie C., Møller Ian M., Rasmusson Allan G. Identification of a mitochondrial external NADPH dehydrogenase by overexpression in transgenic Nicotiana sylvestris. Plant J. 2004;37:415–425. doi: 10.1046/j.1365-313X.2003.01970.x. [DOI] [PubMed] [Google Scholar]
- Michalecka Agnieszka M., Svensson Å. Staffan, Johansson Fredrik I., Agius Stephanie C., Johanson Urban, Brennicke Axel, Binder Stefan, Rasmusson Allan G. Arabidopsis Genes Encoding Mitochondrial Type II NAD(P)H Dehydrogenases Have Different Evolutionary Origin and Show Distinct Responses to Light. Plant Physiol. 2003;133:642–652. doi: 10.1104/pp.103.024208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore CS, Cook-Johnson RJ, Rudhe C, Whelan J, Day DA, Wiskich JT, Soole KL. Identification of AtNDI1, an Internal Non-Phosphorylating NAD(P)H Dehydrogenase in Arabidopsis Mitochondria. Plant Physiol. 2003;133:1968–1978. doi: 10.1104/pp.103.029363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutry M, Faber AM, Charbonnier M, Briquet M. Microanalysis of Plant Mitochondrial Protein Synthesis Products: Detection of Variant Polypeptides Associated with Cytoplasmic Male Sterility. Plant Mol Biol. 1984;3:445–452. doi: 10.1007/BF00033392. [DOI] [PubMed] [Google Scholar]
- Fredlund KM. PhD Thesis. Department of Plant Physiology, Lund University; 1996. NAD(P)H dehydrogenases in plant mitochondria. [Google Scholar]
- Douce R, Mannella CA, Bonner WD. External NADH dehydrogenases of intact plant mitochondria. Biochim Biophys Acta. 1973;292:105–116. doi: 10.1016/0005-2728(73)90255-7. [DOI] [PubMed] [Google Scholar]
- Møller IM, Lidén AC, Ericson I, Gardeström P. Isolation of submitochondrial particles with different polarities. Meth Enzymol. 1987;148:442–453. [Google Scholar]
- Rasmusson AG, Møller IM. NADP-utilizing enzymes in the matrix of plant mitochondria. Plant Physiol. 1990;94:1012–1018. doi: 10.1104/pp.94.3.1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during assembly of head of bacteriophage T4. Nature. 1970;227:680–&. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Moos M, Nguyen NY, Liu TY. Reproducible high-yield sequencing of proteins electrophoretically separated and transferred to an inert support. J Biol Chem. 1988;263:6005–6008. [PubMed] [Google Scholar]
- Friedrich T, Hofhaus G, Ise W, Nehls U, Schmitz B, Weiss H. A Small Isoform of NADH: Ubiquinone Oxidoreductase (Complex I) without Mitochondrially Encoded Subunits Is Made in Chloramphenicol-Treated Neurospora crassa. Eur J Biochem. 1989;180:173–180. doi: 10.1111/j.1432-1033.1989.tb14629.x. [DOI] [PubMed] [Google Scholar]
- Lamattina L, Gonzalez D, Gualberto J, Grienenberger JM. Higher plant mitochondria encode an homolog of the nuclear-encoded 30-kda subunit of bovine mitochondrial complex I. Eur J Biochem. 1993;217:831–838. doi: 10.1111/j.1432-1033.1993.tb18311.x. [DOI] [PubMed] [Google Scholar]
- Elthon TE, Nickels RL, Mcintosh L. Monoclonal Antibodies to the Alternative Oxidase of Higher Plant Mitochondria. Plant Physiol. 1989;89:1311–1317. doi: 10.1104/pp.89.4.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Research. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The TIGR Gene Indices. The Institute of Genomic Research; http://tigrblast.tigr.org/tgi/ [Google Scholar]
- BLAST http://ncbi.nlm.nih.gov/blast/
- Huang XQ, Miller W. A time-efficient, linear-space local similarity algorithm. Adv Appl Math. 1991;12:337–357. [Google Scholar]
- SIM - Alignment Tool for protein sequences http://www.expasy.org/tools/sim-prot.html


