Abstract
This article explores the applicability of fluorescence and absorbance spectroscopy for estimating organic pollution in polluted rivers. The relationship between absorbance, fluorescence intensity, dissolved organic carbon, biochemical oxygen demand (BOD), chemical oxygen demand (COD), and other water quality parameters were used to characterize and identify the origin and the spatial variability of the organic pollution in a highly polluted watershed. Analyses were performed for the Iguassu River, located in southern Brazil, with area about 2,700 km2 and ∼3 million inhabitants. Samples were collect at six monitoring sites covering 107 km of the main river. BOD, COD, nitrogen, and phosphorus concentration indicates a high input of sewage to the river. Specific absorbance at 254 and 285 nm (SUVA254 and A285/COD) did not show significant variation between sites monitored, indicating the presence of both dissolved compounds found in domestic effluents and humic and fulvic compounds derived from allochthonous organic matter. Correlations between BOD and tryptophan-like fluorescence peak (peak T2, r=0.7560, and peak T1, r=0.6949) and tyrosine-like fluorescence peak (peak B, r=0.7321) indicated the presence of labile organic matter and thus confirmed the presence of sewage in the river. Results showed that fluorescence and absorbance spectroscopy provide useful information on pollution in rivers from critical watersheds and together are a robust method that is simpler and more rapid than traditional methods employed by regulatory agencies.
Key words: : organic pollution, spectrophotometry, surface water quality, urban rivers, water quality planning and management
Introduction
It is well known that water quality deterioration is a multivariate problem. Changes in land use and occupation, urban development, and anthropogenic activities may affect the water resources with multiple consequences with significant impact on the physical, chemical, and biological water properties. To track such changes, it is necessary to consider a distinct monitoring strategy that uses both quantitative and qualitative approaches flexible enough to take into account the main characteristics that can be relevant for planning and management purposes. Thus, the identification of the pollution sources and the respective action to minimize its impacts on water quality depend on in-depth studies and better strategies for monitoring activities. Up until now, however, management and restoration efforts often focus only on a set of quantitative variables that typically includes biological and chemical oxygen demand (COD), nutrients, and sediments (Stanley et al., 2012).
Organic matter content in the aquatic ecosystem consists of a complex mixture of distinct chemical species. Therefore, its characterization based on the analysis of each individual compound and their properties is still not possible (Frimmel, 1998; Leenheer and Croué, 2003; Sharma et al., 2011). Instead, the common approach focus to identify and characterize the occurrence of different classes of compounds (Sharma et al., 2011). Consequently, to perform these analyses, both indirect and direct quantitative measurements, and qualitative evaluations are commonly used.
Two indirect measurements are commonly used for a primary evaluation of the organic content in water samples: biochemical oxygen demand (BOD) and COD. The main principle of both analyses is the measurement of the oxygen or a chemical oxidant, respectively, required for the oxidation of the organic matter. While BOD requires 5 days for its incubation and evaluation, COD uses a chemical oxidant that allows the quantification in several hours. Despite this, COD does not measure the same oxydisable compounds, since BOD can mostly measure the biodegradable fraction (Thomas et al., 2007). However, both measurements presents a subjectivity interpretation, since the amount of oxygen consumed will depend on factors that are difficult to be rigorously controlled and compared (Comber et al., 1996; Nataraja et al., 2006; Thomas et al., 2007).
In addition to BOD and COD, the direct measurement of the organic carbon can be used to evaluate the amount of organic matter in surface waters. Total organic carbon (TOC) is considered as the most comprehensive measurement to quantify the presence of organic matter in aquatic systems (Leenheer and Croué, 2003). Operationally, the organic carbon can be defined as dissolved organic carbon (DOC) and particulate organic carbon (POC). The determination involves the filtration to separate DOC from POC, the elimination of inorganic carbon by acidification, oxidation of the organic carbon (combustion or wet oxidation), and detection of the resulting CO2 (Leenheer and Croué, 2003; Matilainen et al. 2011).
In terms of water quality planning and management, it is necessary to evaluate the presence of organic pollution, as the respective degradation mechanisms in the aquatic systems. The degradation mechanism depends on the presence of labile or refractory compounds. In addition, the composition of organic matter is a function of its sources in the environment (Leenheer and Croué, 2003). Consequently, qualitative information about the organic matter sources and degradability characteristics are important for an effective monitoring strategy in polluted rivers.
In recent years, spectrophotometric techniques such as UV-visible and fluorescence intensity have been proposed as an alternative to qualitatively evaluate the organic pollution in surface waters. These spectrophotometric analyses are based on properties such as the absorption on both visible and ultraviolet light and the presence of fluorescence compounds (Carstea, 2012). Due to its easy and fast analyses, these techniques have been widely applied for compounds identification and evaluation (Westerhoff and Anning, 2000; Peuravuori et al., 2002; Pons et al., 2004; Henderson et al., 2009; Quaranta et al., 2012), statistical modeling (Murphy et al., 2010; Hur and Cho, 2012; Carter et al., 2012; Cohen et al., 2014), in situ monitoring tools (Kowalczuk et al., 2010; Carstea, 2012; Shutova et al., 2014), and tracking of pollution sources (Goldman et al., 2012; Meng et al., 2013).
Fluorescence and absorbance spectroscopy has already been applied as a surrogate method for surface waters characterization and organic matter sources identification (Thomas et al., 2005; Hudson et al., 2008; Hur and Cho, 2012; Kwak et al., 2013), wastewater characterization (Reynolds and Ahmad, 1997; Escalas et al., 2003; Nataraja et al., 2006; Hur et al., 2010; Melendez-Pastor et al., 2013; Yang et al., 2014), industrial effluents (Chevakidagarn, 2007), and for organic matter identification in reservoirs (Westphal et al., 2007; Nguyen et al., 2011). Table 1 presents a summary of previous work relating different samples and the respective correlation between conventional parameters (BOD, COD, TOC, or DOC) and spectrophotometric measurements (absorbance and fluorescence intensity).
Table 1.
Correlations Relating Absorbance and Fluorescence Peak Intensities to Traditional Water Quality Parameters for River, Sewage, and Sewage-Impacted Waters
| Sample | Number of samples | Parameter | Optical parameter | Correlation coefficient (Pearson's r) | Reference |
|---|---|---|---|---|---|
| River | 124 (river) and 141 (sewage) | BOD | Tryptophan-like fluorescence—T1 | 0.612 | Hudson et al. (2007) |
| Effluenta | 0.714 | ||||
| River | TOC | 0.457 | |||
| Effluent | 0.714 | ||||
| River | 64 | TOCb | Fluorescence-like peak and humic-like peak | 0.409/0.463 | Cumberland and Baker (2007) |
| BOG | 49 | 0.756/0.504 | |||
| Groundwater (SPRG) | 16 | 0.631/0.63 | |||
| Pond | 14 | 0.657/0.609 | |||
| Treated final effluent (STW) | 16 | 0.167/0.277 | |||
| Raw wastewaterc | 24 | BOD | UV280 | 0.73 | Nataraja et al. (2006) |
| Filtered raw wastewater | 12 | 0.63 | |||
| Primary wastewaterc | 34 | 0.49 | |||
| Filtered primary wastewater | 17 | 0.24 | |||
| NSB | 19 | 0.11 | |||
| Raw+primary | 29 | 0.95 | |||
| Filtered raw+primary | 29 | 0.79 | |||
| River (non-affected by sewage)d | 55 | BOD | Peak I and Ae | 0.892/0.901f | Hur et al. (2008) |
| UV254 | 0.778f | ||||
| Conductivity | 0.684f | ||||
| EEM peak | 0.91f | ||||
| River (non-affected by sewage)d | 31 | Peak I and Ae | 0.615/0.591f | ||
| UV254 | 0.249f | ||||
| Conductivity | 0.102f | ||||
| EEM peak | 0.654f | ||||
| River (affected by sewage)d | 24 | Peak I and Ae | 0.627/0.755f | ||
| UV254 | 0.545f | ||||
| Conductivity | 0.706f | ||||
| EEM peak | 0.737f | ||||
| River | 91 | BOD | T peak | 0.2f | Baker (2002) |
| F peak | 0.68f | ||||
| River (affected by sewage)g | 35 | TN | UV200 and UV254 | 0.911/0.914 | Hur and Cho (2012) |
| C1, C2, and C3h | 0.951/0.927/0.950 | ||||
| BOD | UV200 and UV254 | 0.706–0.892 | |||
| C1, C2, and C3h | 0.948/0.938/0.948 | ||||
| DOC | UV200 and UV254 | 0.770–0.973 | |||
| C1, C2, and C3h | 0.977/0.967/0.977 |
Effluent derived from domestic and industrial effluent.
Filtered samples were considered as TOC.
Raw: inlet wastewater; Primary: outlet of the primary settling basin.
Sampling in three different times.
Peak I corresponding to Δλ=30 nm at 285 nm, and peak A corresponding to Δλ=60 nm at 285 nm.
Sperman's rho coefficient.
Sampling in 18 sites along the main river in 2 days.
PARAFAC components: C1 and C2 being humic-like substances and C3 being related to tryptophan-like fluorescence (Acidified samples used for fluorescence analysis).
BOD, biochemical oxygen demand; DOC, dissolved organic carbon; EEM, excitation-emission matrix; NSB, nitrification settling basin; TN, total nitrogen; TOC, total organic carbon.
For example, the specific absorbance at 200 nm wavelength (UV200), 254 nm (UV254), and 280 nm (UV280) have been analyzed with BOD, DOC, and total nitrogen (TN) concentration considering wastewater samples (Nataraja et al., 2006) and surface waters affected by sewage (Hur et al., 2008; Hur and Cho, 2012). The results of Nataraja et al. (2006) indicated that UV280 could be useful to estimate the BOD concentration, with good correlations for raw nonfiltrate effluent. However, the results did not present a unique pattern of correlation (Table 1), indicating that the relationship between absorbance and BOD may be wastewater and treatment plant-specific and variable with time and treatment (Nataraja et al., 2006). Hudson et al. (2008) also emphasized that there is a strong influence of the site-specific properties and the relationship between tryptophan-like fluorescence and BOD5. Complementarily, Hur and Cho (2012) found strong correlation between UV200 and UV254 and TN (r=0.911 and r=0.914, respectively) and BOD (r=0.706 and r=0.892, respectively). However, these results considered only two samplings with a short time interval in a river affected by sewage. Consequently, the strong correlations may not be representative to extrapolate as a surrogate method for other flow conditions.
Based upon the same principles, tryptophan-like fluorescence peak and humic-like fluorescence peak are often correlated with BOD, TOC, and DOC concentration (Baker, 2002; Cumberland and Baker, 2007; Hudson et al., 2007; Hur et al., 2008; Hur and Cho, 2012). In a study to evaluate the possibility of using fluorescence spectrometry as a substitute for BOD testing, Hudson et al. (2007) found strong correlations for a wide variety of samples. However, the authors emphasize that the potential use of the technique is not as a surrogate, but as an independent indicator test for the presence of bio-available organic matter, associated biological activity, and oxidizing potential with probable associated impacts on water quality. In addition, sewage samples often presents stronger correlations between spectrophotometric measurements and BOD than river water samples (Nataraja et al., 2006; Cumberland and Baker, 2007; Hudson et al., 2007).
Most of studies found in the literature do not present an overall analysis toward more efficient water resources planning and management research. The challenge is to establish a strategy that combines and compares the water quality database considering its interpretation and application by regulatory agencies. Thus, this article aims to evaluate the applicability of fluorescence and absorbance spectroscopy to evaluate the variability of organic matter in polluted rivers. Moreover, according to some differences about the analytical methods, equipment, and monitoring techniques reported by some authors (Bisutti et al., 2004; Spencer et al., 2007; Henderson et al., 2009; Bayram et al., 2011), this article also explores the interactions associated with the dynamics of organic matter in polluted rivers. As a consequence, a final objective is to consolidate an integrated approach to evaluate the organic pollution using different water quality parameters.
Materials and Methods
Study area
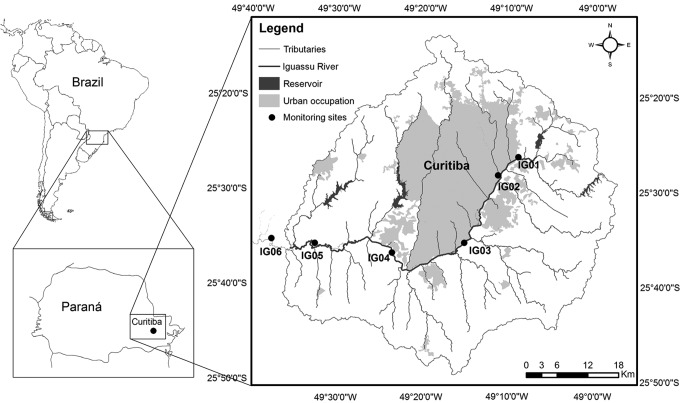
Figure 1 presents the Iguassu River, located in an important watershed in Southern Brazil. The selected watershed occupies an area about 2,700 km2 with 26 major tributaries contributing to the main river. About 3 million people reside within the basin in 14 municipalities. A total of 32 municipal sewage treatment plants are located within the watershed, with 60% of collection, and 89% of sewage treatment with an average of 70% of efficiency (168 ton BOD/day). About 26.3% of the basin can be considered as a highly urbanized region, 62.4% of the catchment area is composed of agriculture and 9.6% by forest. As a consequence, problems have been encountered in the water supply system, the level of domestic effluents treatment, urban drainage system, and irregular occupation of headwater areas.
FIG. 1.
Location of the Iguassu River and the monitoring sites IG01 (headwater) to IG06.
Samples were collected at six sites along a 107 km reach of the Iguassu River (Fig. 1) from Aug/2012 to Dec/2013 (10 sampling: 4 during Spring, 2 during Summer,1 during Fall, and 3 during Winter). One site (IG01) was located upstream of the urbanization area, three sites were located in the most impacted area and downstream from wastewater treatment plants (IG02, IG03 and IG04), and two sites were selected downstream of the urbanization area (IG05 and IG06). Samples from site IG02 were collected in two margins (IG02A and IG02B). Table 2 presents a summary of main information about the number of inhabitants, land use and occupation, and the respective drainage area for each monitoring site.
Table 2.
Population, Land Occupation, and Density for Each Monitoring Site
| % Land occupation | |||||||
|---|---|---|---|---|---|---|---|
| Monitoring site | Inhabitantsa | Incremental area (km2)a | Accumulated area (km2) | Urban | Agricultural | Forest | Density (hab/km2) |
| IG01 | 109,479 | 321.4 | 321.4 | 16.0 | 65.4 | 18.5 | 364.9 |
| IG02 | 751,341 | 220.2 | 541.6 | 57.2 | 42.8 | 0.0 | 3,001.8 |
| IG03 | 1,130,605 | 666.2 | 1,207.8 | 28.6 | 63.4 | 8.0 | 1,811.5 |
| IG04 | 854,031 | 778.3 | 1,986.1 | 30.7 | 63.1 | 6.2 | 1,039.9 |
| IG05 | 109,195 | 410.5 | 2,396.6 | 19.6 | 68.0 | 12.4 | 249.4 |
| IG06 | 185,392 | 268.7 | 2,665.3 | 9.7 | 72.5 | 17.8 | 665.4 |
Inhabitants and incremental drainage area of each monitoring site.
Chemical and spectrophotometric analyses
The chemical parameters measured were as follows: dissolved oxygen (DO; Hach), BOD5 (respirometric method), COD (closed reflux, titrimetric method), ammonia nitrogen (NH3, phenate method), orthophosphate (PO4−, ascorbic acid method), DOC (TOC-VCPH Shimadzu, 680°C combustion catalytic oxidation and nondispersive infrared method, prewashed 0.45 μm acetate cellulose membrane), and specific absorbance and fluorescence peak intensities (filtered samples with prewashed 0.45 μm acetate cellulose membrane), according to the APHA (1998). All sampling bottles were acid washed and baked during 5 h at 550°C. Samples were collected in thoroughly rinsed 2 L glass bottles and stored in the dark at 4°C.
Fluorescence was measured in 4 cm3 quartz cuvettes using a Varian Cary Eclipse fluorescence spectrophotometer. Excitation wavelengths were scanned from 200 to 600 nm in 10 nm steps, and the emitted fluorescence detected between 200 and 600 nm in 5 nm steps. The emission and excitation slits widths were fixed in 5 nm. Scan speed was 3,000 nm/min and the photomultiplier tube (PMT) voltage was set on 950 V. The results of fluorescence excitation-emission matrices (EEMs) were corrected for inner filter effects (McKnight et al., 2001; Carstea, 2012) and normalized in Raman Units (at excitation of 350 nm and emitted fluorescence detected between 395 at 400 nm). The peaks intensities identification was determined by an algorithm implemented in Visual Basic for applications. The range of emission and excitation wavelengths used for fluorescence peak identification was based on a classification proposed by Coble (1996) as follows: humic substances in peak A (λex=230 nm/λem=400–500 nm) and peak C (λex=300–500 nm/λem=400–500 nm), tryptophan as peak T1 (λex=290 nm/λem=350 nm) and T2 (λex=230 nm/λem=350 nm) and tyrosine as peak B (λex=230–275 nm/λem=310 nm). The fluorescence ratio (FR), calculated by the ratio of the emission intensity at λ=450 nm and the emission intensity at λ=500 nm (FR=λ450/λ500) was used to evaluate the occurrence of autochthonous sources (FR>1.8) and allochthonous source of humic substances (FR≤1.5) (Westerhoff and Anning, 2000). Table 3 presents additional information about different wavelengths commonly applied to evaluate the organic matter sources and differentiate the organic compounds.
Table 3.
Spectroscopic Analyses Used in Monitoring Surface Water Quality and Organic Matter Characterization
| Wavelength (nm) | Application | Reference |
|---|---|---|
| Absorbance | ||
| 254 nm | Organic matter characterization and source identification, DOC, and conductivity correlation | Westerhoff and Anning (2000); Pons et al. (2004) |
| 280 nm | Domestic effluent characterization, BOD, and DOC correlation | Nataraja et al. (2006) |
| 285 nm | DOC composition, fulvic acids, and labil organic matter | Rostan and Cellot (1995) |
| Synchronous fluorescence (Δλ=λem Δ λex) | ||
| Δλ=18 nm | Aquatic humic matter | Peuravuori et al. (2002) |
| Δλ=20 nm | Wastewater | Ahmad and Reynolds (1995) |
| Δλ=60 nm | Detection of domestic wastewater | Galapate et al. (1998); Reynolds (2003) |
| Fluorescence (excitation/emission wavelength) | ||
| 230–275/310 nm | Tyrosine-like fluorescence (peak B) | Coble (1996); Hudson et al. (2007) |
| 230–248/340–350 nm | Tryptophan peak (peak T2), NOM, and domestic effluents characterization, BOD correlation | Coble (1996); Ahmad and Reynolds (1999); Henderson et al. (2009) |
| 280–290/350 nm | Tryptophan-like fluorescence (peak T1) and sewage evaluation, BOD correlation, biodegradable organic matter differentiation | Coble (1996); Pons et al. (2004); Reynolds (2002); Hudson et al. (2007) |
| 280/440 nm | Nonbiodegradable organic matter differentiation | Reynolds (2002) |
| 324 nm | Natural organic matter and DOC characterization | Frimmel (1998); Westerhoff and Anning (2000) |
| 370 nm | DOC and humic content characterization in water, soil, and sediments | Westerhoff and Anning (2000) |
UV-Vis absorbance was measured using a UV-1601 PC spectrometer (Shimadzu), with a 1 cm quartz cuvette and ultrapure water as a blank, in the range of 200 to 600 nm. The specific absorbance in 254 wavelength, SUVA254 (Westerhoff and Anning, 2000), was calculated by the ratio between UV254 (a.u.) absorbance, the respective DOC concentration (mg/L), and corrected by the optical path (m). According to Westerhoff and Anning (2000), values of SUVA254 close to 1.2 L/[mg·m] indicate the presence of autochthone compounds or organic matter derived from biological degradation (e.g., sewage), while values close to 4.4 L/[mg·m] indicate the presence of humic and fulvic compounds. The ratio between absorbance at 285 nm and the DOC concentration, A285/DOC (Rostan and Cellot, 1995), was used to differentiate fulvic acid (≈20 L/g) and labile organic matter (≈10 L/g). Table 3 presents a summary of absorptivity in different wavelength ranges commonly used to evaluate the organic matter sources.
Statistical analyses
A descriptive statistical analysis was applied to evaluate the water quality parameters monitored through time and space variability. The identification of patterns and correlations between all the observed data and especially with the parameters that represent the organic content was evaluated using Software R (Ihaka and Gentleman, 1996). The significance of the difference between median was tested by the Wilcox rank sum test (p<0.05).
Results and Discussion
Water quality assessment
Table 4 presents the median results of the chemical stream water measurements. According to the results, it was generally possible to identify a different pattern for most of the parameters at the monitoring sites affected by urban development (IG02–IG06) and the monitoring site located in a less impacted area (IG01). The median of BOD, NH3, and PO4− from sites downstream of the urban area (IG02 to IG06) were significantly higher than the median from samples collected at site IG01, (Wilcox rank sum test, p<0.05). For DO, the median downstream of the site IG02 were significantly lower than site IG01 (Wilcox rank sum test, p<0.05). For COD, sites IG02 to IG05 were significant higher than site IG01, but not with site IG06 (Wilcox rank sum test, p<0.05). SUVA254, and A285/COD did not showed significant variation between the sites monitored. For DOC, only sites IG03 and IG04 presented higher concentration than the median concentration from other sites monitored (Wilcox rank sum test, p<0.05).
Table 4.
Stream Water Characteristics at Sites Monitored (Median±Standard Deviation)
| Monitoring sites | ||||||
|---|---|---|---|---|---|---|
| Parameters | IG01 (n=10) | IG02 (n=10) | IG03 (n=10) | IG04 (n=9) | IG05 (n=10) | IG06 (n=10) |
| BOD (mg/L) | 4.1±3.1 | 19.4±5.3 | 31.3±14.1 | 26.3±15.4 | 19.6±7.4 | 10.6±3.2 |
| COD (mg/L) | 20.0±16.0 | 41.4±13.9 | 72.5±46.8 | 49.9±27.3 | 42.2±24.3 | 28.0±16.8 |
| DOC (mg/L) | 4.7±1.5 | 5.8±1.5 | 8.2±3.4 | 6.0±1.4 | 5.7±1.6 | 4.7±1.2 |
| DO (mg/L) | 5.5±1.2 | 3.3±1.4 | 1.6±1.3 | 1.2±0.6 | 1.3±0.9 | 1.6±0.8 |
| NH3 (mg/L) | 0.2±0.1 | 5.9±3.8 | 8.1±7.3 | 6.0±5.0 | 6.6±6.0 | 3.9±3.0 |
| PO4− (mg/L) | 0.03±0.06 | 0.21±0.16 | 0.41±0.34 | 0.35±0.21 | 0.37±0.20 | 0.23±0.13 |
| FR | 1.4±0.0 | 1.7±0.1 | 1.7±0.1 | 1.7±0.1 | 1.6±0.1 | 1.6±0.1 |
| SUVA254 (L/[mg·m]) | 2.9±0.4 | 2.7±0.7 | 2.4±0.9 | 2.8±0.7 | 2.9±1.3 | 3.0±1.2 |
| A285/DOC (L/[g·cm]) | 21.1±3.3 | 19.1±4.8 | 16.9±6.3 | 19.8±5.1 | 20.5±8.8 | 21.5±7.6 |
| Peak A | 0.88±0.73 | 1.99±0.52 | 2.14±0.58 | 2.03±0.29 | 1.80±0.22 | 1.54±0.21 |
| Peak C | 0.57±0.21 | 1.03±0.24 | 1.39±0.27 | 1.23±0.16 | 1.08±0.14 | 0.90±0.15 |
| Peak B | 0.41±0.26 | 2.77±1.03 | 3.04±1.15 | 2.98±1.00 | 2.07±0.91 | 1.58±0.60 |
| Peak T1 | 0.46±0.13 | 1.18±0.80 | 1.03±0.45 | 1.36±0.50 | 0.86±0.23 | 1.00±0.34 |
| Peak T2 | 0.53±0.21 | 2.01±0.79 | 2.46±0.91 | 2.51±0.58 | 1.96±0.38 | 1.71±0.35 |
A285/DOC (L/[g·cm]), specific ultraviolet absorbance in the wavelength 285 nm normalized by DOC (g/L) and the optical path (cm); A, C, B, T1, and T2 are the fluorescence intensity peaks according do Coble (1996); COD, chemical oxygen demand; DO, dissolved oxygen; FR, ratio between the intensities of fluorescence emitted at 450 and 500 nm wavelengths, with an excitation of 370 nm; n, number of samples; SUVA254 (L/[mg·m]), specific ultraviolet absorbance in the wavelength 254 nm normalized by DOC (mg/L) and the optical path (m).
Average values of SUVA254 and A285/DOC (Table 4) indicate a probable mixture of autochthonous sources of biological activity and allochthonous sources of dissolved organic matter. SUVA254 values lower than 4.4 L/[mg·m] indicates that the dissolved organic matter may arise from anthropogenic allochthonous sources, such as domestic effluents, since DOC present in effluents has low absorption in the UV region, thus diminishing both the SUVA254, and the A285/DOC ratio (Rostan and Cellot, 1995; Westerhoff and Anning, 2000). Musikavong and Wattanachira (2007) also indicate that SUVA254 values tend to increase in advanced levels of biological treatment in effluents, since biological activity tends to remove the fraction of the organic matter that is not sensitive to UV emission, that is, the easily biodegradable organic compounds such as carbohydrates (Imai et al., 2002) and other aliphatic functional groups (Ma et al., 2001), diminishing DOC and maintaining absorbance. Complementarily, the FR values (Table 4) indicate the presence of either autochthonous DOC, and DOC formed by compounds that do not present fluorescence emission in 450/500 nm with excitation of 370 nm wavelength, such as dissolved organic substances in domestic effluents (Westerhoff and Anning, 2000).
Fluorescence EEM analyses: evidence of anthropogenic sources of pollution
The EEMs peak intensities showed to be related to the level of organic pollution between the monitoring sites. Figure 2 presents a sequence of EEMs for sites IG01 (headwater) through IG06, considering sampling performed on Nov/2012. Figure 3 presents the variation of fluorescence intensity peaks B, T2, and T1, representing the labile organic matter among the six sites monitored in the Iguassu River. Figure 4 presents the variation of fluorescence intensity peaks C and A, representing the refractory organic matter. The results from EEMs indicated an increase of fluorescence peaks intensity at site IG02, which can be related to domestic effluents loads. Site IG02 is located after a sewage treatment plant in the most urbanized area of the watershed. The presence of labile organic matter (peaks B, T2, and T1), were evident at sites IG02, IG03, and IG04. The higher intensity of the fluorescence peaks indicated in Fig. 2 confirms the relative concentration of sewage in the intermediate sites of the watershed. The low rates of anthropogenic sources at site IG01 can be identified by the EEM analysis, with little contribution of labile organic matter (lower intensity of peaks B, T2, and T1) and presence of humic compounds (peak A and C).
FIG. 2.
Example of three-dimensional excitation-emission matrices (EEMs) for all sites monitored along the main river (IG01 is located at headwater and IG06 is the most downstream site). Samples collected in Nov/2012. Color scale represents the fluorescence intensities (Raman units [r.u.]).
FIG. 3.

Variation of fluorescence intensity peaks B, T2, and T1, relating to the labile organic matter for all sites monitored along the main river (IG01 is located at headwater and IG06 is the most downstream site).
FIG. 4.
Variation of fluorescence intensity peaks A and C, relating to the refractory organic matter for all sites monitored along the main river (IG01 is located at headwater and IG06 is the most downstream site).
These combinations of commonly used analytical analyses with spectroscopic techniques (Table 4 and Fig. 2) have potential for the evaluation of organic matter dynamics in rivers. The results of fluorescence spectra and the identification of different regions with higher fluorescence intensity peaks (Hudson et al., 2007; Henderson et al., 2009; Goldman et al., 2012), allows the identification of the presence of labile organic matter (Westerhoff and Anning, 2000) and the identification of the location of the critical points due to the inputs of wastewater along the main river (Fig. 2). The analyses of these results show, for example, a nonaffected part of the basin (site IG01), the evolution of the presence of sewage (peaks B, T2, and T1) along the most urbanized points within the watershed (IG02 to IG04), and the recovery of the water quality as a result of the degradation of the labile compounds (sites IG05 and IG06). The refractory organic matter shows small variation when comparing fluorescence peaks A and C. These results indicate that even though the labile organic matter predominates and are a direct consequence of the urbanization and organic pollution derived from domestic wastewater, there is still a percentage of refractory organic matter in the water column. Due to its rapid determination, these methods can be useful for regulatory agencies for a primary identification of the most impacted areas in an urban river.
Correlations between water quality parameters
Correlations between parameters were evaluated to explore interactions associated with the organic matter in the Iguassu River. Figure 5 shows COD and DOC correlations with BOD, and the respective correlation between DOC and COD. The linear correlations with BOD considering the data from all monitoring sites (n=66) were r=0.6217 (p<0.0001, COD), and r=0.5439 (p<0.0001, DOC). DOC and COD also presented a linear correlation, r=0.6779 (p<0.0001). While DOC ranged from 2.5 to 14.4 mg/L, values of COD above 100 mg/L were observed at sites with an elevated number of inhabitants and population density (Table 2), IG02 and IG03. In essence, COD, TOC, DOC, and BOD represent a different approach to measure the same organic matter in a water sample. Different organic compounds are suitable for determination by a set of analytical methods. However, not all the analytical methods can identify all the same compounds. One example is the BOD. While BOD allows the evaluation of the biodegradable organic matter, carbohydrates and oxydisable minerals, this test cannot measure complex compounds such as humic substances and aliphatic and aromatic hydrocarbons (Thomas et al., 2007). Since BOD is an indirect measure of the organic matter by the equivalent of oxygen consumed, the results will depend on the amount of biodegradable organic compounds and the adequate presence of microorganisms and other experimental conditions. Complementarily, COD and TOC data account for complex compounds, but while TOC measures only organic carbon compounds, COD can oxidize other substances (APHA, 1998). Thus, several factors can impact the interpretation and the strategy to establish a well-defined rank to compare data in an environment with heterogeneous (natural and anthropogenic) sources of organic matter.
FIG. 5.
X-Y plots for COD and DOC×BOD (left) and DOC×COD (right). Solid lines represent the corresponding regression. r is the Pearson's linear coefficient and p is the significant level (n=66 data; site IG02 considered for left margin, IG02A, and right margin, IG02B). BOD, biochemical oxygen demand; COD, chemical oxygen demand; DOC, dissolved organic carbon.
The relationship between spectroscopic fluorescence peaks intensities and BOD also evidenced the presence of effluent-derived organic matter in the Iguassu River. Figure 6 presents the relationship along the intensity of fluorescence peaks B, T2, and T1 and the concentration of BOD, corresponding to the labile organic matter. The linear correlations (Table 5) considering the data from all monitoring sites were r=0.7321 (p<0.0001, peak B, n=56), r=0.7560 (p<0.0001, peak T2, n=57), and r=0.6949 (p<0.0001, peak T1, n=57). The fluorescence peaks A and C, related to the presence of humic compounds, also correlated with BOD (Fig. 7). The linear correlations considering the data from all monitoring sites were r=0.5523 (p<0.0001, peak A, n=57), and r=0.6708 (p<0.0001, peak C, n=57).
FIG. 6.

X-Y plots for (a) peak B×BOD, (b) peak T2×BOD, and (c) peak T1×BOD. Solid lines represent the corresponding regression. r is the Pearson's linear coefficient and p is the significant level (n=66 data, all sampling sites. Site IG02 considered for left margin, IG02A, and right margin, IG02B).
Table 5.
Pearson's r Linear Coefficient and p-Value for Each Regression Considering BOD, COD, DOC, and Fluorescence Intensity Peaks A, C, B, T1, and T2 (All p<0.0001)
| Dependent variables | |||||
|---|---|---|---|---|---|
| Independent variable | Peak A | Peak C | Peak B | Peak T1 | Peak T2 |
| BOD | 0.5523 (n=57) | 0.6708 (n=57) | 0.7321 (n=56) | 0.6949 (n=57) | 0.7560 (n=57) |
| COD | 0.4573 (n=60) | 0.5025 (n=60) | 0.5512 (n=59) | 0.3333 (n=60) | 0.5579 (n=60) |
| DOC | 0.4402 (n=64) | 0.4778 (n=64) | 0.5403 (n=63) | 0.3810 (n=64) | 0.5308 (n=64) |
FIG. 7.
X-Y plots for (a) peak A×BOD, (b) peak C×BOD. Solid lines represent the corresponding regression. r is the Pearson's linear coefficient and p is the significant level (n=66 data, all sampling sites. Site IG02 considered for left margin, IG02A, and right margin, IG02B).
Complementarily, it can be observed that the fluorescence intensity peaks showed a stronger correlation with BOD rather than for COD or DOC (Table 5). While the tryptophan-like fluorescence peak (T1) is commonly associated with the presence of labile organic matter (Carstea, 2012), the direct correlation with BOD may be not necessarily significant for all kinds of samples (Baker, 2002; Cumberland and Baker, 2007). Effluent samples presents, generally, a strong correlation between T1 and BOD than unpolluted river samples (Table 1). Hudson et al. (2007) found a good linear correlation between T1 and BOD for rivers (r=0.612, n=124 samples) and effluents (r=0.714, n=141 samples), while Baker (2002) did not find strong correlations for unpolluted river samples (r=0.2, n=91 samples). Since the organic matter dynamics depends on factors such as composition, origin of compounds, and physical and biological conditions for degradation, the estimated correlation between the parameters analyzed will also depend on site-specific characteristics (Nataraja et al., 2006). In addition, for unpolluted waters, the weak correlations between BOD and tryptophan-like fluorescence (Table 1) may be caused by the inaccuracies of the BOD analysis on samples with low organic content (Comber et al., 1996; Henderson et al., 2009).
The different sources of organic pollution in rivers (sewage, industrial effluents, and urban and agricultural runoff) result in a complex mixture of compounds with distinct fluorescence and absorbance intensities. Consequently, both fluorescence and nonfluorescence compounds are quantified through organic carbon analyses, but not all compounds are qualitatively identified (Henderson et al., 2009). In such a context, it is important to highlight that both sampling spatial and temporal scale could properly affect the data interpretation. One example is the results presented by Hur et al. (2012), which relies on two samplings in a short time difference. The authors found correlations along BOD, TN, COD, absorbance, and PARAFAC components (Table 1). While the strong correlations found by Hur et al. (2012) may indicate that fluorescence intensity peaks can be used as surrogates for BOD analysis, it also may indicate that under the conditions of samplings performed, no variation was observed on flow regime, changes on sewage inputs, or seasonality factors. Additionally, Kokorite et al. (2012) also discusses some factors that may affect concentrations of dissolved organic matter in surface waters, especially in large rivers basins. In their studies, discharge and correlated complex factors (such as long flow trajectories, different land-use types, soil, and climate) can make the interpretation of a river's dissolved organic matter content more complex, confirming the results herein included.
A common conclusion from reported studies is that fluorescence and absorbance spectroscopy can be used to characterize natural waters or effluents. For a highly polluted river such as the case study presented in this article, such methods enable the water resources manager to better characterize and evaluate the organic matter content in a river. But the use of these techniques as surrogates for standard water quality parameters requires caution. First, the site-specific nature of the correlations considering spectroscopic measurements, interpretation could be limited because relationships with traditional parameters may not be applicable to other sites. Second, since the organic pollution of an urban river is a result from a complex mixture, spectroscopic analyses may indicate more than one type of organic matter source. Results reported from earlier research suggests that if a surrogate parameter is used to estimate the organic content in terms of BOD or other water quality parameter, it is important that the results retain a degree of comparability with historical data (Comber et al., 1996).
Conclusions
This article explores the applicability of the complementary use of fluorescence and absorbance spectroscopy along with commonly used water quality parameters to investigate the organic matter content in a polluted river. Parameters such as BOD, COD, NH3, PO4−, and DO directly indicated the most polluted areas, while DOC did not show significant variation according to the levels of urbanization and organic pollution in the river. The median values of specific absorbance SUVA254 and A285/DOC also did not significantly vary among the sites monitored. The respective results of absorbance indicated a mixture of anthropogenic allochthonous sources, that is, domestic effluents, and compounds associated with humic and fulvic acids.
Considering the characterization of organic pollution in a river with different spatial characteristics, the analysis of fluorescence spectroscopy showed to be more representative than specific index of the absorbance spectrum. Stronger correlations were found between fluorescence peaks B, T2, and T1 and BOD, indicating the presence of labile organic matter. In addition, the results of fluorescence EEMs also indicate the spatial change on the anthropogenic-derived organic matter. Monitoring sites IG02, IG03, and IG04, located in the most urbanized region of the study, were more affected by the occurrence of tryptophan-like and tyrosine-like fluorescence.
Finally, the results presented in this study confirm the existence of qualitative relationships between spectroscopy and parameters commonly used in water quality monitoring strategies. Considering the necessity of a rapid and direct analysis to the identification of organic pollution by regulatory agencies focusing on better management strategies, fluorescence spectroscopy can be applied to identify the presence of labile organic matter. Due to its qualitative characteristic, fluorescence data can be used as a complementarily parameter in water quality planning and management strategies for basins with high inputs of organic pollution.
Acknowledgments
The authors are grateful for financial support from MCT/CNPq no. 14/2010 (471456/2010-1); doctorate scholarship of the first author (MCT/CNPq/CT-Hidro no. 22/2009 (grant 142130/2010-9); and Capes-Fulbright (DRI/CGCI no. 040/2010). This research was also partially funded by MCT/FINEP/CT-Hidro-GRH 01/2004 no. 01 41000 00 (Project Bacias Críticas) and MCT/FINEP/CT-Hidro-IGRH 01/2007 (Project Integra).
Author Disclosure Statement
No competing financial interests exist.
References
- Ahmad S.R., and Reynolds D.M. (1995). Synchronous fluorescence spectroscopy of wastewater and some potential constituents. Water Res. 29, 1599 [Google Scholar]
- Ahmad S.R., and Reynolds D.M. (1999). Monitoring of water quality using fluorescence technique: prospect of on-line process control. Water Res. 33, 2069 [Google Scholar]
- APHA. (1998). Standard Methods for the Examination of Water and Wastewater. 20th edition, American Public Health Association/American Water Works Association/Water Environment Federation, Washington, DC [Google Scholar]
- Baker A. (2002). Fluorescence properties of some farm waster: implications for water quality monitoring. Water Res. 36, 189. [DOI] [PubMed] [Google Scholar]
- Bayram A., Önsoy H., Akinci G., and Bulut V.N. (2011). Variation of total organic carbon content along the stream Harsit, Eastern Black Sea Basin, Turkey. Environ. Monit. Assess. 182, 85. [DOI] [PubMed] [Google Scholar]
- Bisutti I., Hilke I., and Raessler M. (2004). Determination of total organic carbon—an overview of current methods. Trends Anal. Chem. 23, 716 [Google Scholar]
- Carstea E.M. (2012). Fluorescence spectroscopy as a potential tool for in-situ monitoring of dissolved organic matter in surface water systems. In Balkis N., Ed., Water Pollution. Croatia: Intech, pp. 47–68 [Google Scholar]
- Carter H.T., Tipping E., Koprivnjak J., Miller M.P., Cookson B., and Hamilton-Taylor J. (2012). Freshwater DOM quantity and quality from a two-component model of UV absorbance. Water Res. 46, 4532. [DOI] [PubMed] [Google Scholar]
- Chevakidagarn P. (2007). BOD5 Estimation by using UV absorption and COD for rapid industrial effluent monitoring. Environ. Monit. Assess. 131, 445. [DOI] [PubMed] [Google Scholar]
- Coble P.G. (1996). Characterization of marine and terrestrial DOM in seawater using excitation-emission matrix spectroscopy. Mar. Chem. 51, 325 [Google Scholar]
- Cohen E., Levy G.J., and Borisover M. (2014). Fluorescent components of organic matter in wastewater: efficacy and selectivity of the water treatment. Water Res. 55, 323. [DOI] [PubMed] [Google Scholar]
- Comber S.D.W., Gardner M.J., and Gunn A.M. (1996). Measurement of absorbance and fluorescence as potential alternatives to BOD. Environ. Technol. 17, 771 [Google Scholar]
- Cumberland S.A., and Baker A. (2007). The freshwater dissolved organic matter fluorescence-total organic carbon relationship. Hydrol. Process. 21, 2093 [Google Scholar]
- Escalas A., Droguet M., Guadayol J.M., and Caixach J. (2003). Estimating DOC regime in a wastewater treatment plant by UV deconvolution. Water Res. 37, 2627. [DOI] [PubMed] [Google Scholar]
- Frimmel F.H. (1998). Characterization of natural organic matter as major constituents in aquatic systems. J. Contam. Hydrol. 35, 201 [Google Scholar]
- Galapate R.P., Baes A.U., Ito K., Mukai T., Shoto E., and Okada M. (1998). Detection of domestic wastes in Kurose River using synchronous fluorescence spectroscopy. Water Res. 32, 2232 [Google Scholar]
- Goldman J.H., Rounds S.A., and Needoba J.A. (2012). Applications of fluorescence spectroscopy for predicting percent wastewater in an urban stream. Environ. Sci. Technol. 46, 4374. [DOI] [PubMed] [Google Scholar]
- Henderson R.K., Baker A., Murphy K.R., Hambly A., Stuetz R.M., and Khan S.J. (2009). Fluorescence as a potential monitoring tool for recycled water systems: a review. Water Res. 43, 863. [DOI] [PubMed] [Google Scholar]
- Hudson N., Baker A., and Reynolds D. (2007). Fluorescence analysis of dissolved organic matter in natural, waste and polluted waters—a review. River Res. Appl. 23, 631 [Google Scholar]
- Hudson N., Baker A., Ward D., Reynolds D.M., Brunsdon C., Carliell-Marquet C., and Browing S. (2008). Can fluorescence spectrometry be used as a surrogate for the biochemical oxygen demand (BOD) test in water quality assessment? An example from South West England. Sci. Total Environ. 391, 149. [DOI] [PubMed] [Google Scholar]
- Hur J., and Cho J. (2012). Prediction of BOD, COD, and total nitrogen concentrations in a typical urban river using a fluorescence excitation-emission matrix with PARAFAC and UV absorption indices. Sensors 12, 972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hur J., Hwan S.J., and Shin J.K. (2008). Using synchronous fluorescence technique as a water quality monitoring tool for an urban river. Water Air Soil Pollut. 191, 231 [Google Scholar]
- Hur J., Lee B., Lee T., and Park D. (2010). Estimation of biological oxygen demand and chemical oxygen demand for combined sewer systems using synchronous fluorescence spectra. Sensors 10, 2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihaka R., and Gentleman R. (1996). R: a language for data analysis and graphics. J. Comput. Graph. Stat. 5, 299 [Google Scholar]
- Imai A., Fukushima T., Matsushige K., Kim Y., and Choi K. (2002). Characterization of dissolved organic matter in effluents from wastewater treatment plants. Water Res. 36, 859. [DOI] [PubMed] [Google Scholar]
- Kokorite I., Klavins M., Rodinov V., and Springe G. (2012). Trends of natural organic matter concentrations in river waters of Latvia. Environ. Monit. Assess. 184, 499. [DOI] [PubMed] [Google Scholar]
- Kowalczuk P., Zablocka M., Sagan S., and Kulinski K. (2010). Fluorescence measured in situ as a proxy of CDOM absorption and DOC concentration in the Baltic Sea. Oceanologia 52, 431 [Google Scholar]
- Kwak J., Khang B., Kim E., and Kim H. (2013). Estimation of biochemical oxygen demand based on dissolved organic carbon, UV absorption, and fluorescence measurements. J. Chem. 2013, Article ID 243769 [Google Scholar]
- Leenheer J.A., and Croué J.P. (2003). Characterizing dissolved aquatic organic matter. Environ. Sci. Technol. 37, p. 18–26 [PubMed] [Google Scholar]
- Ma H., Allen H.E., and Yin Y. (2001). Characterization of isolated fractions of dissolved organic matter from natural waters and a wastewater effluent. Water Res. 35, 985. [DOI] [PubMed] [Google Scholar]
- Matilainen A., Gjessing E.T., Lahtinen T., Hed L., Bhatnagar A., and Sillanpaa M. (2011). An overview of the methods used in the characterization of natural organic matter in relation to drinking water treatment. Chemosphere 83, 1431. [DOI] [PubMed] [Google Scholar]
- Mcknight D.M., Boyer E.W., Westerhoff P.K., Doran P.T., Kulbe T., and Andersen D.T. (2001). Spectrofluorometric characterization of dissolved organic matter for indication of precursor organic material and aromaticity. Limnol. Oceanogr. 46, 38 [Google Scholar]
- Melendez-Pastor I., Almendro-Candel M.B., Navarro-Pedreño J., Gómez I., Lilllo M.G., Hernández E.I. (2013). Monitoring urban wastewaters characteristics by visible and short wave near-infrared spectroscopy. Water 5, 2026 [Google Scholar]
- Meng F., Huang G., Yang X., Li Z., Li J., Cao J., Wang Z., and Sun L. (2013). Identifying the sources and fate of anthropogenically impacted dissolved organic matter (DOM) in urbanized rivers. Water Res. 47, 5027. [DOI] [PubMed] [Google Scholar]
- Murphy K.R., Butler K.D., Spencer R.G.M., Stedmon C.A., Boehme J.R., and Aiken G.R. (2010). Measurement of dissolved organic matter fluorescence in aquatic environments: an interlaboratory comparison. Environ. Sci. Technol. 44, 9405. [DOI] [PubMed] [Google Scholar]
- Musikavong C., and Wattanachira S. (2007). Reduction of dissolved organic matter in terms of DOC, UV-254, SUVA and THMFP in industrial estate wastewater treated by stabilization ponds. Environ. Monit. Assess. 134, 489. [DOI] [PubMed] [Google Scholar]
- Nataraja M., Qin Y., and Seagren E.A. (2006). Ultraviolet spectrophotometry as an index parameter for estimating the biochemical oxygen demand of domestic wastewater. Environ. Technol. 27, 789. [DOI] [PubMed] [Google Scholar]
- Nguyen H.V.M., Shin J.K., and Hur J. (2011). Multivariate analysis for spatial distribution of dissolved organic matters in a large rive-type dam reservoir. Environ. Monit. Assess. 183, 425. [DOI] [PubMed] [Google Scholar]
- Peuravuori J., Koivikko R., and Pihlaja K. (2002). Characterization, differentiation and classification of aquatic humic matter separated with different sorbents: synchronous scanning fluorescence spectroscopy. Water Res. 36, 4552. [DOI] [PubMed] [Google Scholar]
- Pons M., Bonté S.L., and Potier O. (2004). Spectral analysis and fingerprinting for biomedia characterization. J. Biotechnol. 113, 211. [DOI] [PubMed] [Google Scholar]
- Quaranta M.L., Mendes M.D., and MacKay A.A. (2012). Similarities in effluent organic matter characteristics from Connecticut wastewater treatment plants. Water Res. 46, 284. [DOI] [PubMed] [Google Scholar]
- Reynolds D.M. (2002). The differentiation of biodegradable and non-biodegradable dissolved organic matter in wastewaters using fluorescence spectroscopy. J. Chem. Technol. Biotechnol. 77, 965 [Google Scholar]
- Reynolds D.M. (2003). Rapid and direct determination of tryptophan in water using synchronous fluorescence spectroscopy. Water Res. 37, 3055. [DOI] [PubMed] [Google Scholar]
- Reynolds D.M., and Ahmad S.R. (1997). Rapid and direct determination of wastewater BOD values using a fluorescence technique. Water Res. 31, 2012 [Google Scholar]
- Rostan J.C., and Cellot B. (1995). On the use of UV spectroscopy to assess dissolved organic carbon origin variations in the upper Rhône River. Aquat. Sci. 57, 70 [Google Scholar]
- Sharma S.K., Maeng S.K., and Nam S.-N. (2011). Characterization tools for differentiating natural organic matter from effluent organic matter. In Wilderer P., Rogers P., Uhlenbrook S., Frimmel F., Hanaki K., Vereijken T., Eds. Treatise on Water Science. Vol. 3, Chapter 3.15. Amsterdam: Elsevier Science, pp. 417–427 [Google Scholar]
- Shutova Y., Baker A., Bridgeman J., and Henderson R.K. (2014). Spectroscopic characterization of dissolved organic matter changes in drinking water treatment: from PARAFAC analysis to online monitoring wavelengths. Water Res. 54, 159. [DOI] [PubMed] [Google Scholar]
- Spencer R.G.M., Baker A., Ahad J.M.E., Cowie G.L., Ganeshram R., Upstill-Goddard R.C., and Uher G. (2007). Discriminatory classification of natural and anthropogenic waters in two U. K. estuaries. Sci. Total Environ. 373, 305. [DOI] [PubMed] [Google Scholar]
- Stanley E.H., Powers S.M., Lottig N.R., Buffam I., and Crawford J.T. (2012). Contemporary changes in dissolved organic carbon (DOC) in human-dominated rivers: is there a role for DOC management? Freshw. Biol. 57, 26 [Google Scholar]
- Thomas O., Baures E., and Pouet M.F. (2005). UV spectrophotometry as a non-parametric measurement of water and wastewater quality variability. Water Qual. Res. J. Can. 40, 51 [Google Scholar]
- Thomas O., Theraulaz F., Vaillant S., and Pouet M.F. (2007). Urban wastewater. In Thomas O., and Burgess C., Eds., UV-Visible Spectrophotometry of Water and Wastewater. Techniques and Instrumentation in Analytical Chemistry. Amsterdam: Elsevier Science, Vol. 27, pp. 189–216 [Google Scholar]
- Westerhoff P., and Anning D. (2000). Concentrations and characteristics of organic carbon in surface water in Arizona: influence of urbanization. J. Hydrol. 236, 202 [Google Scholar]
- Westphal K.S., Chapra S.C., and Sung W. (2007). Modeling TOC and UV254 absorbance for reservoir planning and operation. JAWRA J. Am. Water Resour. Assoc. 40, 795 [Google Scholar]
- Yang L., Shin H., and Hur J. (2014). Estimating the concentration and biodegradability of organic matter in 22 wastewater treatment plants using fluorescence excitation emission matrices and parallel factor analysis. Sensors 14, 1771. [DOI] [PMC free article] [PubMed] [Google Scholar]