Abstract

Professor Helmut Sies
Dr. Helmut Sies (MD, 1967) is recognized as a Redox Pioneer, because he authored five articles on oxidative stress, lycopene, and glutathione, each of which has been cited more than 1000 times, and coauthored an article on hydroperoxide metabolism in mammalian systems cited more than 5000 times (Google Scholar). He obtained preclinical education at the University of Tübingen and the University of Munich, clinical training at Munich (MD, 1967) and Paris, and completed Habilitation at Munich (Physiological Chemistry and Physical Biochemistry, 1972). In early research, he first identified hydrogen peroxide (H2O2) as a normal aerobic metabolite and devised a method to quantify H2O2 concentration and turnover in cells. He quantified central redox systems for energy metabolism (NAD, NADP systems) and antioxidant GSH in subcellular compartments. He first described ebselen, a selenoorganic compound, as a glutathione peroxidase mimic. He contributed a fundamental discovery to the physiology of GSH, selenium nutrition, singlet oxygen biochemistry, and health benefits of dietary lycopene and cocoa flavonoids. He has published more than 600 articles, 134 of which are cited at least 100 times, and edited 28 books. His h-index is 115. During the last quarter of the 20th century and well into the 21st, he has served as a scout, trailblazer, and pioneer in redox biology. His formulation of the concept of oxidative stress stimulated and guided research in oxidants and antioxidants; his pioneering research on carotenoids and flavonoids informed nutritional strategies against cancer, cardiovascular disease, and aging; and his quantitative approach to redox biochemistry provides a foundation for modern redox systems biology. Helmut Sies is a true Redox Pioneer. Antioxid. Redox Signal. 21, 2459–2468.
The joy of exploring the unknown and finding something novel and noteworthy: what a privilege!
—Prof. Helmut Sies
Background Development and Training
The 20th century culminated with the triumph of sequencing the human genome, but achievements in physiological chemistry and redox biology dominated life science research for most of the century. Warburg's description of Atmungsferment (cytochrome c oxidase) as the central catalyst of aerobic life and Keilin's description of cytochromes provide highlights. Krebs connected these to the citric acid cycle; Lipmann described ATP as the central energy currency; Lehninger and Chance demonstrated associations of these processes with mitochondria; and Mitchell provided the mechanism for electrochemical coupling of oxidative phosphorylation. Seminal discoveries linked oxidative mechanisms to disease, and included discovery of peroxisomes, the respiratory burst of phagocytes, radical mechanisms of CCl4 toxicity, radical scavenging of vitamin E, selenium requirement for antioxidant proteins, discovery of thioredoxin and superoxide dismutase, and elucidation of nitric oxide (NO) and hydrogen peroxide (H2O2) signaling. Helmut Sies stands among the preeminent scientists with his contributions to this pioneering redox research.
Sies was born in 1942 and grew up in northern Germany “at the border of meadows and hillside forests,” a place enchanted by colorful kingfishers, springtime lush with wildflowers, and metamorphosis of tadpoles into frogs (64). He cites his first elementary school teacher, Georg Henkel, for fostering curiosity that led to a lifelong pursuit of discovery. His formative education was rich with culture, achievement, and opportunity. He took demanding experimental physics, mathematics, and chemistry and credits study of Latin for providing logic and long-term perspective. At 17 years of age, he boarded a Greek liner along with 200 European exchange students traveling to the United States for studying abroad. He lived with a medical doctor in a small town near Cincinnati; the impressive life and dedication of the country doctor had a lifelong impact (64). Sies graduated high school in Kankakee, near Chicago, in 1960, and returned to Germany, where he graduated from Jakobson-Schule in Seesen in 1961.
Subsequent education at Tübingen (studium generale and Medicine), extending to Marburg and Munich for thesis research, and at the Sorbonne in Paris for clinical medicine, was extraordinary. Already in 1962, he sought out Theodor Bücher, Professor of Biochemistry, for dissertation research on steady-state enzyme kinetics. In 1963, he met and conversed with eminent scientists at what he described as an “Olympic event” (64), the 13th Nobel Prize winners meeting in Lindau, on Lake Constance. Imagine a 21-year-old aspiring scientist listening to Theorell discuss the biochemistry of alcohol, Ochoa present the chemical basis of heredity, Warburg talk about the chemistry of photosynthesis, Burnet explain the role of thymus in immunity, and Krebs talk about regulation of metabolism.
Sies finished MD certification in Munich in 1967 and began postdoctoral study with Bücher, surrounded by a technology genius. Bolko Brauser had enhanced a rapid-scanning spectrophotometer to measure spectral changes in biologic systems, and Sies, Brauser, and Bücher used this with a light guide and a photomultiplier to measure cytochrome changes in intact, perfused rat liver (68, 69). In a certain regard, this represented a steady march of science: the best minds, the best tools, and the best environment. More critically for Sies, this provided a launching pad for discovery. Michaelis, Briggs, and Haldane had enlightened science with their understanding of enzyme catalysis. However, key questions of respiratory enzymology were unanswered: two-electron reactions to generate H2O2 were known, but efforts to detect H2O2 in mammalian systems had failed. Chance had used spectrophotometry to detect a steady-state intermediate of H2O2 with isolated peroxidases and catalase. In Munich, Sies identified the spectrophotometer wavelengths in perfused liver specific for catalase Compound I. On infusing an electron donor (ethanol) to test for reduction of the Compound I, Sies showed for the first time the presence of H2O2 in an intact functioning mammalian tissue. This seminal finding, published along with Chance in 1970 (71), remains unsurpassed in laying the groundwork for quantification of H2O2 production and concentration in mammalian cells (12, 37, 56, 66, 79).
In his early career, Sies was a trailblazer in physiological chemistry, applying quantitative principles to redox biochemistry and physiology throughout the spectrum of structural complexity (56). As his career matured, he integrated disparate observations of chemistry and medicine into a central definition of oxidative stress (58, 59). In this, he emerged as a scout for new principles in redox biology, discovering redox cycling as a central process in oxidative toxicities (23), elucidating roles of GSH (63) and selenium as antioxidants (4, 31), and demonstrating the role of carotenoids in protection against singlet oxygen (60, 77). In fulfillment of his medical training, he translated his studies of dietary lycopene and cocoa flavanols to improve human health (14, 44).
Physiological Chemistry and Enzymology in Complex Systems: Quantitative Foundation for Redox Systems Biology
The key finding of H2O2 in living tissues (71) highlighted an integrated quantitative framework that Sies developed for redox systems biology of intact, functioning organs (8, 71, 72). Contemporary redox scholars are well served to revisit his pioneering contributions of the 1970s as he completed his habilitation [formal process for being accepted as faculty (52)], established his career in redox biochemistry, and took leadership of the Biochemistry Department at the University of Düsseldorf. The isolated, perfused organ and noninvasive monitoring techniques that Sies adopted (55) allowed him to quantify rates while monitoring redox steady-state changes by spectrophotometry and fluorometry (Fig. 1). With noninvasive readout methods to distinguish free thermodynamically active redox partners from bound ones, he obtained accurate quantitative descriptions of specific redox processes within intact functional organs (8, 57).
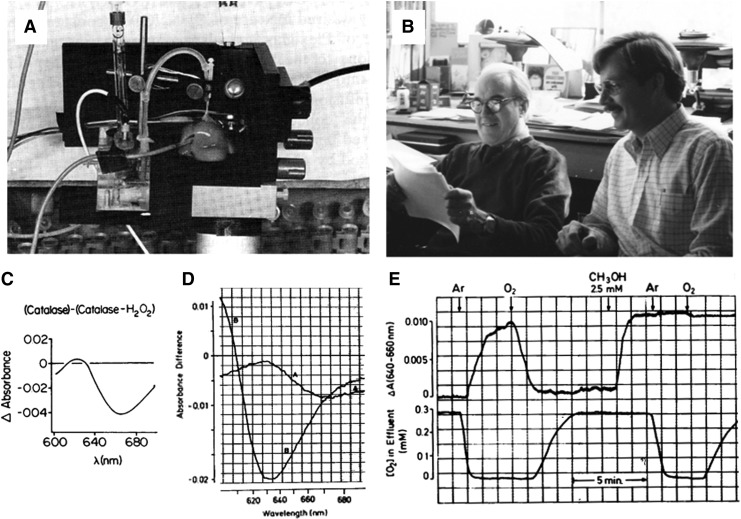
FIG. 1.
First detection of hydrogen peroxide (H2O2) in living tissue. (A) Study of biochemical functions in the isolated perfused liver was enabled by rapid scanning organ spectrophotometry using light guides and a sensitive photomultiplier tube. This configuration allowed quantitative measurement of removal and production of biochemicals in the perfusate while measuring steady states of chromophores (e.g., cytochromes, NADH, NADPH, and catalase). Sies' discovery of H2O2 in tissue was based on earlier enzymology studies of Britton Chance. (B) After initial discovery of H2O2 in perfused liver in Munich, Sies (right) met with Chance, and the two published the initial finding (71). (C) Measurement in the liver relied on characteristics of the purified enzyme. In the catalase reaction cycle, the first molecule of H2O2 reacts to form a stable intermediate, Compound I, with different light-absorbing properties. This is visualized as the difference spectrum of catalase and catalase with H2O2. (D) A second molecule of H2O2 serves as an electron donor to reduce Compound I in the normal catalase turnover cycle (2H2O2→O2+2H2O). Methanol can also serve as an electron donor for the reduction of Compound I, and addition of methanol to the perfused liver preparation generated the characteristic difference spectrum of catalase and catalase+H2O2. (E) Spectral changes at the wavelengths specific for detection of catalase Compound I after transition between aerobic and anaerobic conditions and with and without methanol provided experimental proof that H2O2 is generated in the functioning liver. See recent presentation in greater detail (66).
In a 10-year span, Sies extended these approaches to obtain the first measures of peroxisome function in an intact organ (79) and to understand tissue O2 gradients (54), mitochondrial function during hypoxia (53), integrated pathways of ethanol metabolism (34), contributions of mitochondria and endoplasmic reticulum to NADPH and NADH dependent metabolism (51, 57, 68), drug metabolism (55, 98, 99), hepatic release of GSSG and GSH (1, 2, 73), and selenium dependence of peroxide elimination. Parallel tissue fractionation showed the relationships of redox systems in mitochondria, cytosolic and nuclear compartments (56, 57, 80). This pioneering research remains a foundation for quantitative redox systems biology.
Integration of Chemistry, Toxicology, and Nutrition into Central Concept of Oxidative Stress
During a visit by Britton Chance to Benno Hess at the Max-Planck-Institut für Ernährungsphysiologie, Dortmund, Enrique Cadenas recalls Chance's reference to Sies as an “outstanding biologist who could see how concepts developed in the future.” In redox biology, Sies led others to discovery through his vision of oxidative stress, defined in 6.5 pages in Oxidative Stress: Introductory Remarks (58) and developed in a review in Angewandte Chemie (59). He brought together findings from biochemistry, chemistry, physics, radiology, medicine, nutrition, cell biology, physiology, and cancer biology, to provide a roadmap for upcoming decades as he conceptualized the consequences of an imbalance of pro-oxidants and antioxidants in terms of macromolecule damage to DNA, lipids, and protein (Fig. 2).
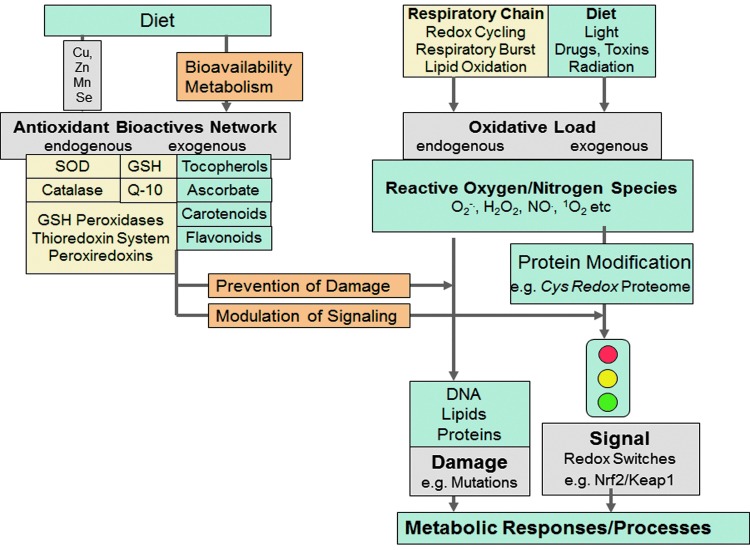
FIG. 2.
First formulation of the concept of oxidative stress. Shown here is a contemporary depiction of oxidative stress (Sies H and Stahl W, 2014, unpublished) that encompasses the original definition (58, 59) with inclusion of redox signaling (75), which was unknown at the time of the original formulation of the concept. The original concept presented an overview of reactive oxygen species, antioxidant defenses, the nature of oxidative damage, processes elicited by oxidative stress, and cellular and medical aspects (59). This clear delineation has served to guide research in a broad range of disciplines, including diet and nutrition, cardiology, diabetes, neurodegeneration, dermatology, ophthalmology, toxicology, environmental health, drug development, immune function, and carcinogenesis.
During this seminal period, he discovered that ebselen, an organoselenium compound, is a GSH peroxidase mimetic (33, 38, 39, 61, 62). His description of redox cycling linked a broad range of redox-active enzymes to mechanisms of macromolecular damage and cell toxicity (23) in a process driven by enzymatic activity and not requiring radical propagation for toxicity. Sies contributed to the understanding of •NO (24, 25, 27), the nitroxyl anion (35), a method to capture •NO (26), detect S-nitrosylated and S-glutathionylated proteins (22) and nitrite as a precursor to •NO (93). He characterized reactions of organoselenocompounds with peroxynitrite (3, 6, 7, 29) and showed that GSH peroxidase is a peroxynitrite reductase (76). He showed that plasma selenoprotein P protected low-density lipoprotein (LDL) against oxidation (94) and astrocytes (90) and endothelial cells from damage (91). He also examined a relationship of high selenium intake to type 2 diabetes risk (40, 92).
Translation of Basic Science to Health Practices in Studies of Carotenoids and Flavonoids
In a third key finding, Sies (14) showed lycopene to be the most efficient carotenoid quencher of singlet oxygen (Fig. 3). Lycopene, present in different foods (83–87), showed preferential availability from processed tomato products (86). With Stahl, he extended lycopene research to humans, notably regarding skin damage from sunlight (Fig. 3). Applying noninvasive measurement and identifying dermal carotenoid products (82), his pioneering research demonstrated protection against ultraviolet light in humans (5, 17, 81, 82), and it is now extended to lycopene research worldwide, particularly in prostate cancer and cardiovascular disease. It should be noted that Sies studied carotenoids in a nutritional biochemical context, not as dietary supplements which, at high doses, proved counterproductive in human studies.
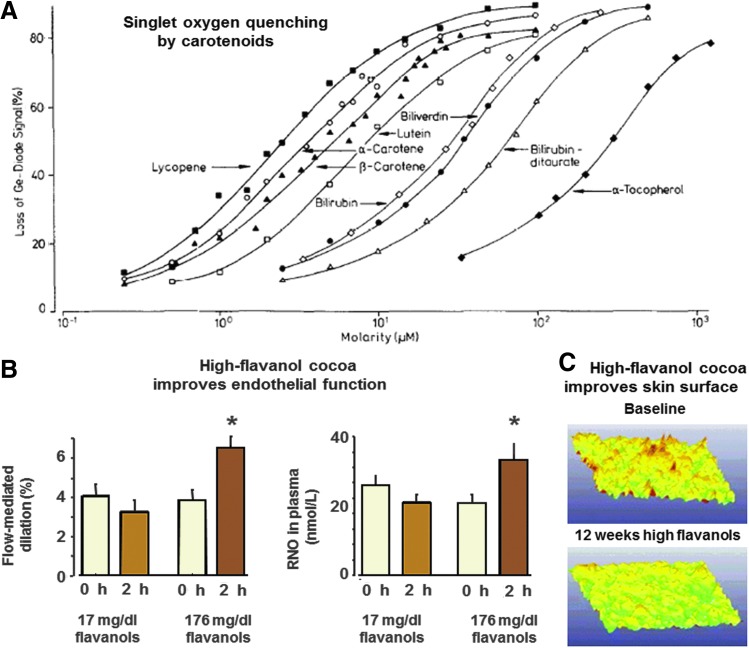
FIG. 3.
Translation of basic science to human health. (A) Singlet oxygen quenching by carotenoids. The original discovery of lycopene as a potent scavenger of singlet oxygen (14), shown here, was followed by translational studies demonstrating greater uptake kinetics from processed tomato products (86) and protection against skin damage from sunlight (78). (B) Individuals were given either a low-flavanol or a high-flavanol drink and studied for flow-mediated dilation (FMD) as a measure of endothelial function (left) and nitrosylated protein as a measure of bioavailability of nitric oxide (NO) (right). The results on the left show that FMD is not improved after consumption of a beverage with low flavanol content, while significant improvement occurs after a beverage with high flavanol content. On the right, results show that increased bioavailability of NO is associated with improved endothelial function (19). (C) Translation of basic research to humans is illustrated by results from a 12-week study of a high-flavanol cocoa drink. The images show how the rough skin surface profile at baseline is converted to a smooth skin surface profile by the cocoa drink. The cocoa drink was also found to decrease UV-induced erythema, increase blood flow of cutaneous and subcutaneous tissues, increase skin density and skin hydration, and decrease skin scaling (18).
In diet and health research, Sies advanced a mechanistic understanding of flavanols on pro-oxidative enzymes (46–49, 88, 89) and showed that flavanol-rich cocoa protects against inflammatory events (30). In human volunteers, high-flavanol cocoa increased the level of bioactive protein-bound NO in the blood and improved endothelial function (Fig. 3) (19, 20). The effect was mimicked by (-)epicatechin isolated from cocoa (50). He found flavanol-rich cocoa to diminish plasma F2-isoprostane in humans (100) and showed beneficial vascular effects in smokers (21), photoprotective and cosmetic effects in the skin (18, 36), and longer-term health benefits (20) distinct from acute vascular responses (65). In other research, Sies studied vitamin E in viral hepatitis (95–97) and inactivation of virus in human plasma (32). He contributed to human research on aging (43), diabetics, congestive heart failure, ischemic stroke, and dementia (28, 41–43, 45). Stretching forward from his key findings on singlet oxygen, reactive carbonyls, carotenoids, and other antioxidants, Sies provided pioneering leadership in the roles of vitamins, essential minerals, and phytochemicals in promoting optimum health and preventing disease.
Other Achievements and Current Position
As founder of the concept of oxidative stress, Professor Sies set the foundation for the integration in a rational way of the biochemical observations toward the field of biomedicine. With the advance in knowledge on the role of redox processes in biology, the concept of oxidative stress was updated to include the role in redox signaling (75). He is a pioneer in quantitative redox biology and a trailblazer in exploring mechanisms and impacts of dietary constituents on a large variety of disease conditions and even in the aging process. His concepts opened new avenues for the possibility of modulating redox processes in vivo through dietary or pharmacological means, an area that continues to be highly active and now extends to roles of oxidative processes in cell signaling. He continues his leadership role in basic and translational science as Professor Emeritus, Department of Biochemistry and Molecular Biology I, Faculty of Medicine, Heinrich Heine University Düsseldorf, Düsseldorf, Germany.
Professor Sies has frequently lectured at international scientific meetings and has also been active in dissemination of research to the public, through lectures to lay audiences, at nutrition meetings, at academies, and through television interviews. He frequently credits his close associates, Dieter Häussinger (13, 15, 16, 74), Enrique Cadenas (9–11, 67, 70), Paolo diMascio (14), Theo Akerboom (1, 2), Wilhelm Stahl (78, 83–87), and many others cited in the references, as well as support from the National Foundation for Cancer Research (Bethesda), Alexander von Humboldt Foundation, Deutsche Forschungsgemeinschaft, and others. In 1996, he received an Honorary Ph.D. degree from the University of Buenos Aires from Prof. Boveris' institution, and in 2010, he received an Honorary M.D. from Dr. Radi's institution, Facultad de Medicina, Universidad de la República, Montevideo, for his outstanding contributions to unravel molecular mechanisms in physiology and pathology and to celebrate the 25th anniversary of the emergence of the Oxidative Stress concept.
He has received FEBS Anniversary Prize (1978), the Ernst-Jung-Prize for Medicine (1988), the Claudius-Galenus-Prize (1990), the Werner-Heisenberg-Medal of the Alexander von Humboldt Foundation (1999), the Linus Pauling Institute Prize for Health Research (2013), and the Trevor Slater Award of the Society for Free Radical Research International (2014).
He has been an instrumental force in scientific leadership for decades, editing scientific journals and books and providing leadership, such as President of the Society for Free Radical Research International, an umbrella organization that encompasses the regional societies in America, Europe, Asia, and Australasia, and of the Oxygen Club of California. He has served as President of the Northrhine-Westphalian Academy of Sciences and is a Member of the German National Academy of Sciences Leopoldina. He has helped lead scientific education, fostering the highest standards in research conduct and dissemination. He stands staunchly on the shoulders of his distinguished scientific pedigree: a student of Theodor Bücher, who descends from Nobel Prize awardees Otto Warburg and Emil Fischer, who traces back to Justus von Liebig, the founder of nutritional biochemistry. Professor Sies extends this tradition with research transcending science to address the health of our society.
Participants at the 25. Konferenz der Gesellschaft für Biologische Chemie at Reisensburg Castle, Germany, July 8–11, 1978; organized by Helmut Sies and Albrecht Wendel. Next to GSH sign: Sir Hans Krebs, right, Alton Meister, left. Front row, Leopold Flohé and Albrecht Wendel, 3rd and 4th from right. Helmut Sies (with sunglasses) is two rows at the back of A. Wendel. Coauthor, Dean Jones (with glasses), is two rows at the back of A. Meister.
Alberto Boveris, Nozomu Oshino, and Helmut Sies (left to right) at the Society for Free Radical Research International meeting in Kyoto, 2000.
Participants at the National Foundation for Cancer Research Conference in Montecito, CA, February 11–12, 1983. Front row, from left: Harold Swartz; Lester Packer, Franklin Salisbury, Albert Szent-György, Trevor Slater, Patrick Riley, Hermann Esterbauer. Second row, from left: Keith Ingold, Bill Pryor, John Ward, Rolf Mehlhorn, Helmut Sies, Alexandre Quintanilha, Norman Krinsky, Peter Gascoyne, Bruce Demple, Martyn Smith, and Robin Willson.
Supplementary Material
Abbreviations Used
- H2O2
hydrogen peroxide
- NO
nitric oxide
Acknowledgments
Helmut Sies writes in gratitude: “I was fortunate to start with Theodor Bücher, an ingenious and generous mentor, and have students and fellows who enthusiastically propelled the research, notably Dieter Häussinger, Theo Akerboom, Sibylle Soboll, Herbert de Groot, Karlis Briviba, Heribert Wefers, Regina Brigelius, Wilhelm Stahl, Wolfgang Schulz, Lars-Oliver Klotz, Peter Brenneisen, Cristina Polidori, Tankred Schewe, Christian Heiss, Holger Steinbrenner.
Associates from other countries and Alexander-von-Humboldt Foundation support were crucial: Enrique Cadenas, Gianna Bartoli, Lionel Gil, Denis Crane, José Estrela, Francisco Romero, Toshihisa Ishikawa, Marisa Medeiros, Paolo di Mascio, Mike Murphy, Vasanthy Narayanaswami, Paul Devasagayam, Greg Bartosz, Motoko Oarada, Wilhelm Hansberg, Lubica Horakova, Takeshi Nikawa, Kazuhiko Rokutan, Hiroshi Masumoto, Victor Sharov, Ivan Roussyn, Oleg Panasenko, Elena Ostrakhovitch, Alcir Dafré, Gavin Arteel, Jens Thiele, Claus Jacob, Govind Mugesh. Visiting scientists: Ray Burk, Toru Komai, Paul Talalay, Raj Sohal, Jim Kehrer, Gail Gurtner, Frank Meyskens, Jim Thomas, Dan Ziegler, Keith Ingold, Brian Ketterer, Etelvino Bechara. Of the many colleagues on the Düsseldorf campus, I would like to especially mention Karin Scharffetter-Kochanek, Jean Krutmann and Thomas Ruzicka from Dermatology, Malte Kelm and Christian Heiss from Cardiology, Hans-Jürgen Bidmon from Anatomy, and Hans-Dieter Martin from Organic Chemistry.
I had good fortune of early contact with Leopold Flohé and Albrecht Wendel on glutathione, longstanding relationship with Britton Chance, working with Nozomu Oshino on H2O2 and compiling the hydroperoxide metabolism review with Alberto Boveris. Deep thanks to sabbatical hosts: Alberto Boveris (Buenos Aires, 1979); Bruce Ames (Berkeley, 1984); and Roland Stocker (Sydney, 1992). The Berkeley sabbatical in 1984 led to a lasting friendship with Lester Packer, the founder of the ‘Oxygen Club of California (OCC),’ with whom I coedited a number of books in the redox field, for example, in Methods in Enzymology.
I am grateful to the National Foundation for Cancer Research, founded by Franklin Salisbury to support cancer research by Albert Szent-György, for research funding since 1984, and to Deutsche Forschungsgemeinschaft for funding since the 1970s.
Finally, I would like to express warm personal thanks to Dean Jones and Rafael Radi for this article, and last-not-least to my wife, Nancy, for her understanding and support throughout the years.”
References
- 1.Akerboom TP, Bilzer M, and Sies H. The relationship of biliary glutathione disulfide efflux and intracellular glutathione disulfide content in perfused rat liver. J Biol Chem 257: 4248–4252, 1982 [PubMed] [Google Scholar]
- 2.Akerboom TP. and Sies H. Transport of glutathione, glutathione disulfide, and glutathione conjugates across the hepatocyte plasma membrane. Methods Enzymol 173: 523–534, 1989 [DOI] [PubMed] [Google Scholar]
- 3.Arteel GE, Mostert V, Oubrahim H, Briviba K, Abel J, and Sies H. Protection by selenoprotein P in human plasma against peroxynitrite-mediated oxidation and nitration. Biol Chem 379: 1201–1205, 1998 [PubMed] [Google Scholar]
- 4.Arteel GE. and Sies H. The biochemistry of selenium and the glutathione system. Environ Toxicol Pharmacol 10: 153–158, 2001 [DOI] [PubMed] [Google Scholar]
- 5.Aust O, Stahl W, Sies H, Tronnier H, and Heinrich U. Supplementation with tomato-based products increases lycopene, phytofluene, and phytoene levels in human serum and protects against UV-light-induced erythema. Int J Vitam Nutr Res 75: 54–60, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Briviba K, Klotz LO, and Sies H. Defenses against peroxynitrite. Methods Enzymol 301: 301–311, 1999 [DOI] [PubMed] [Google Scholar]
- 7.Briviba K, Roussyn I, Sharov VS, and Sies H. Attenuation of oxidation and nitration reactions of peroxynitrite by selenomethionine, selenocystine and ebselen. Biochem J 319 (Pt 1): 13–15, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bucher T, Brauser B, Conze A, Klein F, Langguth O, and Sies H. State of oxidation-reduction and state of binding in the cytosolic NADH-system as disclosed by equilibration with extracellular lactate-pyruvate in hemoglobin-free perfused rat liver. Eur J Biochem 27: 301–317, 1972 [DOI] [PubMed] [Google Scholar]
- 9.Cadenas E. and Sies H. Oxidative stress: excited oxygen species and enzyme activity. Adv Enzyme Regul 23: 217–237, 1985 [DOI] [PubMed] [Google Scholar]
- 10.Cadenas E. and Sies H. Formation of electronically excited states during the oxidation of arachidonic acid by prostaglandin endoperoxide synthase. Methods Enzymol 319: 67–77, 2000 [DOI] [PubMed] [Google Scholar]
- 11.Cadenas E, Wefers H, and Sies H. Low-level chemiluminescence of isolated hepatocytes. Eur J Biochem 119: 531–536, 1981 [DOI] [PubMed] [Google Scholar]
- 12.Chance B, Sies H, and Boveris A. Hydroperoxide metabolism in mammalian organs. Physiol Rev 59: 527–605, 1979 [DOI] [PubMed] [Google Scholar]
- 13.Crane D, Haussinger D, and Sies H. Rise of coenzyme A-glutathione mixed disulfide during hydroperoxide metabolism in perfused rat liver. Eur J Biochem 127: 575–578, 1982 [DOI] [PubMed] [Google Scholar]
- 14.Di Mascio P, Kaiser S, and Sies H. Lycopene as the most efficient biological carotenoid singlet oxygen quencher. Arch Biochem Biophys 274: 532–538, 1989 [DOI] [PubMed] [Google Scholar]
- 15.Haussinger D. and Sies H. Hepatic glutamine metabolism under the influence of the portal ammonia concentration in the perfused rat liver. Eur J Biochem 101: 179–184, 1979 [DOI] [PubMed] [Google Scholar]
- 16.Haussinger D. and Sies H. Hepatic encephalopathy: clinical aspects and pathogenetic concept. Arch Biochem Biophys 536: 97–100, 2013 [DOI] [PubMed] [Google Scholar]
- 17.Heinrich U, Gartner C, Wiebusch M, Eichler O, Sies H, Tronnier H, and Stahl W. Supplementation with beta-carotene or a similar amount of mixed carotenoids protects humans from UV-induced erythema. J Nutr 133: 98–101, 2003 [DOI] [PubMed] [Google Scholar]
- 18.Heinrich U, Neukam K, Tronnier H, Sies H, and Stahl W. Long-term ingestion of high flavanol cocoa provides photoprotection against UV-induced erythema and improves skin condition in women. J Nutr 136: 1565–1569, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Heiss C, Dejam A, Kleinbongard P, Schewe T, Sies H, and Kelm M. Vascular effects of cocoa rich in flavan-3-ols. JAMA 290: 1030–1031, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Heiss C, Finis D, Kleinbongard P, Hoffmann A, Rassaf T, Kelm M, and Sies H. Sustained increase in flow-mediated dilation after daily intake of high-flavanol cocoa drink over 1 week. J Cardiovasc Pharmacol 49: 74–80, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Heiss C, Kleinbongard P, Dejam A, Perre S, Schroeter H, Sies H, and Kelm M. Acute consumption of flavanol-rich cocoa and the reversal of endothelial dysfunction in smokers. J Am Coll Cardiol 46: 1276–1283, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Ji Y, Akerboom TP, Sies H, and Thomas JA. S-nitrosylation and S-glutathiolation of protein sulfhydryls by S-nitroso glutathione. Arch Biochem Biophys 362: 67–78, 1999 [DOI] [PubMed] [Google Scholar]
- 23.Kappus H. and Sies H. Toxic drug effects associated with oxygen metabolism: redox cycling and lipid peroxidation. Experientia 37: 1233–1241, 1981 [DOI] [PubMed] [Google Scholar]
- 24.Klotz LO, Schroeder P, and Sies H. Peroxynitrite signaling: receptor tyrosine kinases and activation of stress-responsive pathways. Free Radic Biol Med 33: 737–743, 2002 [DOI] [PubMed] [Google Scholar]
- 25.Klotz LO. and Sies H. Reversible conversion of nitroxyl anion to nitric oxide. Methods Enzymol 349: 101–106, 2002 [DOI] [PubMed] [Google Scholar]
- 26.Korth H-G, Ingold KU, Sustmann R, de Groot H, and Sies H. Tetramethyl‐Ortho‐quinodimethane, first member of a family of custom‐tailored cheletropic spin traps for nitric oxide. Angew. Chem. Int. Ed. Engl. 31: 891–893, 1992 [Google Scholar]
- 27.Kroncke KD, Klotz LO, Suschek CV, and Sies H. Comparing nitrosative versus oxidative stress toward zinc finger-dependent transcription. Unique role for NO. J Biol Chem 277: 13294–13301, 2002 [DOI] [PubMed] [Google Scholar]
- 28.Li L, Willets RS, Polidori MC, Stahl W, Nelles G, Sies H, and Griffiths HR. Oxidative LDL modification is increased in vascular dementia and is inversely associated with cognitive performance. Free Radic Res 44: 241–248, 2010 [DOI] [PubMed] [Google Scholar]
- 29.Masumoto H, Kissner R, Koppenol WH, and Sies H. Kinetic study of the reaction of ebselen with peroxynitrite. FEBS Lett 398: 179–182, 1996 [DOI] [PubMed] [Google Scholar]
- 30.McKim SE, Konno A, Gabele E, Uesugi T, Froh M, Sies H, Thurman RG, and Arteel GE. Cocoa extract protects against early alcohol-induced liver injury in the rat. Arch Biochem Biophys 406: 40–46, 2002 [DOI] [PubMed] [Google Scholar]
- 31.Mugesh G, du Mont WW, and Sies H. Chemistry of biologically important synthetic organoselenium compounds. Chem Rev 101: 2125–2179, 2001 [DOI] [PubMed] [Google Scholar]
- 32.Muller-Breitkreutz K, Mohr H, Briviba K, and Sies H. Inactivation of viruses by chemically and photochemically generated singlet molecular oxygen. J Photochem Photobiol B 30: 63–70, 1995 [DOI] [PubMed] [Google Scholar]
- 33.Muller A, Cadenas E, Graf P, and Sies H. A novel biologically active seleno-organic compound—I. Glutathione peroxidase-like activity in vitro and antioxidant capacity of PZ 51 (Ebselen). Biochem Pharmacol 33: 3235–3239, 1984 [DOI] [PubMed] [Google Scholar]
- 34.Muller A. and Sies H. Role of alcohol dehydrogenase activity and the acetaldehyde in ethanol-induced ethane and pentane production by isolated perfused rat liver. Biochem J 206: 153–156, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Murphy ME. and Sies H. Reversible conversion of nitroxyl anion to nitric oxide by superoxide dismutase. Proc Natl Acad Sci U S A 88: 10860–10864, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Neukam K, Stahl W, Tronnier H, Sies H, and Heinrich U. Consumption of flavanol-rich cocoa acutely increases microcirculation in human skin. Eur J Nutr 46: 53–56, 2007 [DOI] [PubMed] [Google Scholar]
- 37.Oshino N, Chance B, Sies H, and Bucher T. The role of H2O2 generation in perfused rat liver and the reaction of catalase compound I and hydrogen donors. Arch Biochem Biophys 154: 117–131, 1973 [DOI] [PubMed] [Google Scholar]
- 38.Parnham M. and Sies H. Ebselen: prospective therapy for cerebral ischaemia. Expert Opin Investig Drugs 9: 607–619, 2000 [DOI] [PubMed] [Google Scholar]
- 39.Parnham MJ. and Sies H. The early research and development of ebselen. Biochem Pharmacol 86: 1248–1253, 2013 [DOI] [PubMed] [Google Scholar]
- 40.Pinto A, Speckmann B, Heisler M, Sies H, and Steinbrenner H. Delaying of insulin signal transduction in skeletal muscle cells by selenium compounds. J Inorg Biochem 105: 812–820, 2011 [DOI] [PubMed] [Google Scholar]
- 41.Polidori MC, Cherubini A, Stahl W, Senin U, Sies H, and Mecocci P. Plasma carotenoid and malondialdehyde levels in ischemic stroke patients: relationship to early outcome. Free Radic Res 36: 265–268, 2002 [DOI] [PubMed] [Google Scholar]
- 42.Polidori MC, Mattioli P, Aldred S, Cecchetti R, Stahl W, Griffiths H, Senin U, Sies H, and Mecocci P. Plasma antioxidant status, immunoglobulin g oxidation and lipid peroxidation in demented patients: relevance to Alzheimer disease and vascular dementia. Dement Geriatr Cogn Disord 18: 265–270, 2004 [DOI] [PubMed] [Google Scholar]
- 43.Polidori MC, Mecocci P, Reimann A, Cherubini A, Cecchetti R, Briviba K, Stahl W, Sies H, and Senin U. Plasma lipid peroxidation and vitamin C status in healthy centenarians. J Am Geriatr Soc 47: 1038–1039, 1999 [DOI] [PubMed] [Google Scholar]
- 44.Polidori MC, Pratico D, Mangialasche F, Mariani E, Aust O, Anlasik T, Mang N, Pientka L, Stahl W, Sies H, Mecocci P, and Nelles G. High fruit and vegetable intake is positively correlated with antioxidant status and cognitive performance in healthy subjects. J Alzheimers Dis 17: 921–927, 2009 [DOI] [PubMed] [Google Scholar]
- 45.Polidori MC, Savino K, Alunni G, Freddio M, Senin U, Sies H, Stahl W, and Mecocci P. Plasma lipophilic antioxidants and malondialdehyde in congestive heart failure patients: relationship to disease severity. Free Radic Biol Med 32: 148–152, 2002 [DOI] [PubMed] [Google Scholar]
- 46.Sadik CD, Sies H, and Schewe T. Inhibition of 15-lipoxygenases by flavonoids: structure-activity relations and mode of action. Biochem Pharmacol 65: 773–781, 2003 [DOI] [PubMed] [Google Scholar]
- 47.Schewe T, Kuhn H, and Sies H. Flavonoids of cocoa inhibit recombinant human 5-lipoxygenase. J Nutr 132: 1825–1829, 2002 [DOI] [PubMed] [Google Scholar]
- 48.Schewe T, Sadik C, Klotz LO, Yoshimoto T, Kuhn H, and Sies H. Polyphenols of cocoa: inhibition of mammalian 15-lipoxygenase. Biol Chem 382: 1687–1696, 2001 [DOI] [PubMed] [Google Scholar]
- 49.Schewe T. and Sies H. Myeloperoxidase-induced lipid peroxidation of LDL in the presence of nitrite. Protection by cocoa flavanols. Biofactors 24: 49–58, 2005 [DOI] [PubMed] [Google Scholar]
- 50.Schroeter H, Heiss C, Balzer J, Kleinbongard P, Keen CL, Hollenberg NK, Sies H, Kwik-Uribe C, Schmitz HH, and Kelm M. (-)-Epicatechin mediates beneficial effects of flavanol-rich cocoa on vascular function in humans. Proc Natl Acad Sci U S A 103: 1024–1029, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sies H. The state of NADPH-NADP+during mixed-function oxidation of hexobarbital in hemoglobin-free perfused rat liver. Chem Biol Interact 3: 308–309, 1971 [DOI] [PubMed] [Google Scholar]
- 52.Sies H. Biochemistry of the peroxisome in the liver cell. Angew Chem Int Ed Engl 13: 706–718, 1974 [DOI] [PubMed] [Google Scholar]
- 53.Sies H. Cytochrome oxidase and urate oxidase as intracellular O2 indicators in studies of O2 gradients during hypoxia in liver. Adv Exp Med Biol 94: 561–566, 1977 [DOI] [PubMed] [Google Scholar]
- 54.Sies H. Oxygen gradients during hypoxic steady states in liver. Urate oxidase and cytochrome oxidase as intracellular O2 indicators. Hoppe Seylers Z Physiol Chem 358: 1021–1032, 1977 [DOI] [PubMed] [Google Scholar]
- 55.Sies H. The use of perfusion of liver and other organs for the study of microsomal electron-transport and cytochrome P-450 systems. Methods Enzymol 52: 48–59, 1978 [DOI] [PubMed] [Google Scholar]
- 56.Sies H. Measurement of hydrogen peroxide formation in situ. Methods Enzymol 77: 15–20, 1981 [DOI] [PubMed] [Google Scholar]
- 57.Sies H. Nicotinamide nucleotide compartmentation. In: Metabolic Compartmentation, edited by Sies H. London: Academic Press, 1982, pp. 205–231. [Google Scholar]
- 58.Sies H. Oxidative stress: introductory remarks. In: Oxidative Stress, edited by Sies H. London: Academic Press, 1985, pp. 1–8. [Google Scholar]
- 59.Sies H. Biochemistry of oxidative stress. Angew. Chem. Int. Ed. Engl. 25: 1058–1071, 1986 [Google Scholar]
- 60.Sies H. Carotenoids and tocopherols as antioxidants and singlet oxygen quenchers. J Nutr Sci Vitaminol (Tokyo) Spec No: 27–33, 1992 [DOI] [PubMed] [Google Scholar]
- 61.Sies H. Ebselen, a selenoorganic compound as glutathione peroxidase mimic. Free Radic Biol Med 14: 313–323, 1993 [DOI] [PubMed] [Google Scholar]
- 62.Sies H. Ebselen. Methods Enzymol 252: 341–342, 1995 [DOI] [PubMed] [Google Scholar]
- 63.Sies H. Glutathione and its role in cellular functions. Free Radic Biol Med 27: 916–921, 1999 [DOI] [PubMed] [Google Scholar]
- 64.Sies H. How I became a biochemist. IUBMB Life 59: 469–473, 2007 [DOI] [PubMed] [Google Scholar]
- 65.Sies H. Polyphenols and health: update and perspectives. Arch Biochem Biophys 501: 2–5, 2010 [DOI] [PubMed] [Google Scholar]
- 66.Sies H. Role of metabolic H2O2 generation: redox signaling and oxidative stress. J Biol Chem 289: 8735–8741, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sies H, Akerboom TP, and Cadenas E. The role of glutathione in hepatic hydroperoxide metabolism. Biochem Soc Trans 10: 79–80, 1982 [DOI] [PubMed] [Google Scholar]
- 68.Sies H. and Brauser B. Interaction of mixed function oxidase with its substrates and associated redox transitions of cytochrome P-450 and pyridine nucleotides in perfused rat liver. Eur J Biochem 15: 531–540, 1970 [DOI] [PubMed] [Google Scholar]
- 69.Sies H, Brauser B, and Bucher T. On the state of mitochondria in perfused liver: action of sodium azide on respiratory carriers and respiration. FEBS Lett 5: 319–323, 1969 [DOI] [PubMed] [Google Scholar]
- 70.Sies H. and Cadenas E. Oxidative stress: damage to intact cells and organs. Philos Trans R Soc Lond B Biol Sci 311: 617–631, 1985 [DOI] [PubMed] [Google Scholar]
- 71.Sies H. and Chance B. The steady state level of catalase compound I in isolated hemoglobin-free perfused rat liver. FEBS Lett 11: 172–176, 1970 [DOI] [PubMed] [Google Scholar]
- 72.Sies H, Gerstenecker C, Menzel H, and Flohe L. Oxidation in the NADP system and release of GSSG from hemoglobin-free perfused rat liver during peroxidatic oxidation of glutathione by hydroperoxides. FEBS Lett 27: 171–175, 1972 [DOI] [PubMed] [Google Scholar]
- 73.Sies H. and Graf P. Hepatic thiol and glutathione efflux under the influence of vasopressin, phenylephrine and adrenaline. Biochem J 226: 545–549, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sies H, Haussinger D, and Grosskopf M. Mitochondrial nicotinamide nucleotide systems: ammonium chloride responses and associated metabolic transitions in hemoglobin-free perfused rat liver. Hoppe Seylers Z Physiol Chem 355: 305–320, 1974 [DOI] [PubMed] [Google Scholar]
- 75.Sies H. and Jones DP. Oxidative stress. In: Encyclopedia of Stress, edited by Fink G. San Diego: Academic Press, 2007, pp. 45–47. [Google Scholar]
- 76.Sies H, Sharov VS, Klotz LO, and Briviba K. Glutathione peroxidase protects against peroxynitrite-mediated oxidations. A new function for selenoproteins as peroxynitrite reductase. J Biol Chem 272: 27812–27817, 1997 [DOI] [PubMed] [Google Scholar]
- 77.Sies H. and Stahl W. Vitamins E and C, beta-carotene, and other carotenoids as antioxidants. Am J Clin Nutr 62: 1315S–1321S, 1995 [DOI] [PubMed] [Google Scholar]
- 78.Sies H. and Stahl W. Nutritional protection against skin damage from sunlight. Annu Rev Nutr 24: 173–200, 2004 [DOI] [PubMed] [Google Scholar]
- 79.Sies H. and Summer KH. Hydroperoxide-metabolizing systems in rat liver. Eur J Biochem 57: 503–512, 1975 [DOI] [PubMed] [Google Scholar]
- 80.Soboll S, Gründel S, Harris J, Kolb-Bachofen V, Sies H, and Ketterer B. The content of glutathione and glutathione S-transferases and the glutathione peroxidase activity in rat liver nuclei determined by a non-aqueous technique of liver cell fractionation. Biochem J 311: 889–894, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Stahl W, Heinrich U, Jungmann H, Sies H, and Tronnier H. Carotenoids and carotenoids plus vitamin E protect against ultraviolet light-induced erythema in humans. Am J Clin Nutr 71: 795–798, 2000 [DOI] [PubMed] [Google Scholar]
- 82.Stahl W, Heinrich U, Jungmann H, Tronnier H, and Sies H. Carotenoids in human skin: noninvasive measurement and identification of dermal carotenoids and carotenol esters. Methods Enzymol 319: 494–502, 2000 [DOI] [PubMed] [Google Scholar]
- 83.Stahl W, Schwarz W, and Sies H. Human serum concentrations of all-trans beta- and alpha-carotene but not 9-cis beta-carotene increase upon ingestion of a natural isomer mixture obtained from Dunaliella salina (Betatene). J Nutr 123: 847–851, 1993 [DOI] [PubMed] [Google Scholar]
- 84.Stahl W, Schwarz W, Sundquist AR, and Sies H. cis-trans isomers of lycopene and beta-carotene in human serum and tissues. Arch Biochem Biophys 294: 173–177, 1992 [DOI] [PubMed] [Google Scholar]
- 85.Stahl W, Schwarz W, von Laar J, and Sies H. All-trans beta-carotene preferentially accumulates in human chylomicrons and very low density lipoproteins compared with the 9-cis geometrical isomer. J Nutr 125: 2128–2133, 1995 [DOI] [PubMed] [Google Scholar]
- 86.Stahl W. and Sies H. Uptake of lycopene and its geometrical isomers is greater from heat-processed than from unprocessed tomato juice in humans. J Nutr 122: 2161–2166, 1992 [DOI] [PubMed] [Google Scholar]
- 87.Stahl W. and Sies H. Lycopene: a biologically important carotenoid for humans? Arch Biochem Biophys 336: 1–9, 1996 [DOI] [PubMed] [Google Scholar]
- 88.Steffen Y, Gruber C, Schewe T, and Sies H. Mono-O-methylated flavanols and other flavonoids as inhibitors of endothelial NADPH oxidase. Arch Biochem Biophys 469: 209–219, 2008 [DOI] [PubMed] [Google Scholar]
- 89.Steffen Y, Schewe T, and Sies H. (-)-Epicatechin elevates nitric oxide in endothelial cells via inhibition of NADPH oxidase. Biochem Biophys Res Commun 359: 828–833, 2007 [DOI] [PubMed] [Google Scholar]
- 90.Steinbrenner H, Alili L, Bilgic E, Sies H, and Brenneisen P. Involvement of selenoprotein P in protection of human astrocytes from oxidative damage. Free Radic Biol Med 40: 1513–1523, 2006 [DOI] [PubMed] [Google Scholar]
- 91.Steinbrenner H, Bilgic E, Alili L, Sies H, and Brenneisen P. Selenoprotein P protects endothelial cells from oxidative damage by stimulation of glutathione peroxidase expression and activity. Free Radic Res 40: 936–943, 2006 [DOI] [PubMed] [Google Scholar]
- 92.Steinbrenner H, Speckmann B, Pinto A, and Sies H. High selenium intake and increased diabetes risk: experimental evidence for interplay between selenium and carbohydrate metabolism. J Clin Biochem Nutr 48: 40–45, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Suschek CV, Schewe T, Sies H, and Kroncke KD. Nitrite, a naturally occurring precursor of nitric oxide that acts like a ‘prodrug’. Biol Chem 387: 499–506, 2006 [DOI] [PubMed] [Google Scholar]
- 94.Traulsen H, Steinbrenner H, Buchczyk DP, Klotz LO, and Sies H. Selenoprotein P protects low-density lipoprotein against oxidation. Free Radic Res 38: 123–128, 2004 [DOI] [PubMed] [Google Scholar]
- 95.von Herbay A, de Groot H, Hegi U, Stremmel W, Strohmeyer G, and Sies H. Low vitamin E content in plasma of patients with alcoholic liver disease, hemochromatosis and Wilson's disease. J Hepatol 20: 41–46, 1994 [PubMed] [Google Scholar]
- 96.von Herbay A, Stahl W, Niederau C, and Sies H. Vitamin E improves the aminotransferase status of patients suffering from viral hepatitis C: a randomized, double-blind, placebo-controlled study. Free Radic Res 27: 599–605, 1997 [DOI] [PubMed] [Google Scholar]
- 97.von Herbay A, Stahl W, Niederau C, von Laar J, Strohmeyer G, and Sies H. Diminished plasma levels of vitamin E in patients with severe viral hepatitis. Free Radic Res 25: 461–466, 1996 [DOI] [PubMed] [Google Scholar]
- 98.Waydhas C, Weigl K, and Sies H. The disposition of formaldehyde and formate arising from drug N-demethylations dependent on cytochrome P-450 in hepatocytes and in perfused rat liver. Eur J Biochem 89: 143–150, 1978 [DOI] [PubMed] [Google Scholar]
- 99.Weigl K. and Sies H. Drug oxidations dependent on cytochrome P-450 in isolated hepatocytes. The role of the tricarboxylates and the aminotransferases in NADPH supply. Eur J Biochem 77: 401–408, 1977 [DOI] [PubMed] [Google Scholar]
- 100.Wiswedel I, Hirsch D, Kropf S, Gruening M, Pfister E, Schewe T, and Sies H. Flavanol-rich cocoa drink lowers plasma F(2)-isoprostane concentrations in humans. Free Radic Biol Med 37: 411–421, 2004 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.