Abstract
The present study compared the potential of porcine bone marrow mesenchymal stem cells (pBMSCs) at different passages as nuclear transfer (NT) donors and the developmental efficiency of NT embryos from donor cells transfected with/without Oct4 and Sox2. Early-passage pBMSCs showed higher proliferation and expression of Oct4 and Sox2 and differentiation potential into mesenchymal lineages than middle- and late-passage pBMSCs. Cleavage rate did not differ among pBMSCs at different passages, but NT embryos with early-passage pBMSCs and middle-passage pBMSCs transfected with Oct4 (Oct4-pBMSCs) had significantly (p<0.05) higher blastocyst development than those with middle-passage pBMSCs. The incidence of apoptotic bodies in NT blastocysts from late-passage pBMSCs and Sox2-transfected middle-passage pBMSCs (Sox2-pBMSCs) was significantly (p<0.05) higher than others. The transcriptional levels of Oct4, Sox2, Nanog, Cdx2, Dnmt3a, and Igf2r genes were significantly (p<0.05) higher in Oct4- and Sox2-pBMSCs NT embryos. Middle-passage pBMSCs NT embryos revealed lower transcriptional levels of Bcl2 than others, except Sox2-pBMSCs NT embryos. The transcriptional level of Bax increased gradually in NT embryos derived from pBMSCs following extended passages and was significantly (p<0.05) higher in Sox2-pBMSCs NT embryos. Our results demonstrated that early-passage pBMSCs are more potent in expressing transcription factors and displayed higher differentiation ability, and middle-passage pBMSCs transfected with Oct4 improved the developmental efficiency of NT embryos, suggesting that high Oct4 expression cells are more efficient as NT donors.
Introduction
Embryo cloning by nuclear transfer (NT) is the most efficient and commonly used technique to generate animal models to understand fundamental findings of genetic and epigenetic diversity (Ibrahim et al., 2006; Schook et al., 2005; Wilmut et al., 2002). Despite recent improvements in embryo cloning by NT technology, its efficiency in pig remains low, with few live offspring being born. This is thought to be due to improper reprogramming of donor nuclei by enucleated ooplasm recipients, which subsequently causes the dysregulation of gene expression, resulting in retarded early embryonic development, increased fetal death, and stillbirth. Reprogramming is influenced by various factors, such as donor cell type (Jin et al., 2007; Kumar et al., 2007; Wakayama et al., 2005), oocyte activation method (Kim et al., 2005; Ogura et al., 2000), and embryo culture system (Kishigami et al., 2006; Ock et al., 2007). Among these, the donor cell remains an important factor for improving developmental efficiency of NT embryos.
Earlier NT was centered around using embryos as a source of donor nuclei for producing cloned embryos and offspring (Prather et al., 1989); later fetal-derived fibroblasts cells and mesenchymal stem cells (MSCs) cultured in vitro were used to clone animals that also resulted in live offspring (Chen et al., 2013; Polejaeva et al., 2000; Reggio et al., 2001). Porcine embryos cloned with MSCs have shown similar gene expression patterns, including embryonic growth, apoptosis, DNA methylation, histone deacetylation, and transcription, to their in vivo counterparts (Kumar et al., 2007). These results clearly suggest that undifferentiated cells like MSCs have high potential as NT donors. In accordance with the previous observations, we have demonstrated the enhanced potential of MSCs derived from bone marrow (BM) as nuclear donors in achieving increased production of porcine NT embryos and offspring compared to fetal fibroblasts (Jin et al., 2007; Kumar et al., 2007; Lee et al., 2010). DNA methylation analysis showed that MSCs derived from human BM and skin fibroblasts have different DNA methylation patterns, and, when compared to skin fibroblasts, BM MSCs displayed lower methylation levels in development-related genes (Koch et al., 2011).
Since the first identification of MSCs derived from BM (Gnecchi and Melo, 2009), BM extracts are considered an important source of MSCs with easy access for isolation and expansion. Bone marrow–derived MSCs (BMSCs) from different sources and passages revealed different characteristics regarding differentiation ability, fibroblast-like morphology, proliferation ability, and expression of transcription factors. Oct4, Sox2, and Nanog, which are known as important early transcription factors for regulation of stem cells pluripotency, are expressed in MSCs (Do and Schöler, 2009; Ock et al., 2011). Deletion of Oct4, Sox2, and Nanog in embryonic stem cells (ESCs) resulted in loss of pluripotency and failure to maintain the epiblast (Welstead et al., 2008). Recently, Oct4 and Sox2 are known as major reprogramming factors for generating induced pluripotent stem cells (iPSCs) (Sommer et al., 2009). MSCs derived from young rat BM expressed Oct4, Sox2, and Nanog; however, those from old rats BM did not express Sox2 and Nanog (Asumda and Chase, 2011). Expression of Oct4 and Nanog in BMSCs decreased at late passages (Yew et al., 2011).
It has been shown that embryos cloned with highly expressed Oct4 cells enhanced blastocyst development, indicating that those cells underwent initial reprogramming events quite similar to normal fertilization (Pfeiffer et al., 2010). In embryos cloned with Nanog overexpression cells, Nanog overexpression did not have an effect on blastocyst rate or total cell number (Zhang et al., 2011).
We designed the present study to compare the expression level of Oct4, Sox2, and Nanog in porcine (p) BMSCs at different passages and to determine the developmental efficiency of cloned embryos derived from pBMSCs at different passages. Finally, the effect of Oct4 and Sox2 transfection to middle-passage pBMSCs was investigated at the cellular and molecular levels, as well as the development efficiency of resulting NT embryos.
Materials and Methods
Reagents and media
Unless otherwise specified, all chemicals were purchased from Sigma Chemical Company (St. Louis, MO, USA) and media from Gibco (Invitrogen, Burlington, ON, Canada).
Isolation and culture
All experiments were authorized by the Animal Center for Biomedical Experimentation at Gyeongsang National University. BM extracts were recovered from 1-year-old pigs under standard surgical procedures, and MSCs were isolated as previously described (Kumar et al., 2007). A total of 1×105 cells were cultured in a 35-mm culture dish in Advanced Dulbecco's Modified Eagle Medium (ADMEM) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin (10,000 IU and 10,000 μg/mL, Pen-Strep) at 38.5°C in a humidified atmosphere of 5% CO2 in air. Culture medium was changed every 3 days until confluence was reached at ∼10 days, when cells were dissociated with 0.25% trypsin–ethylenediaminetetraacetic acid (EDTA) solution and pelleted at 300×g for 5 min. Cells were then regrown for further analysis.
Transfection of MSCs with Oct4 and Sox2
A total of 1×105 pBMSCs at passage 8 were transfected with 20 multiplicity of infection (MOI) hOct3/4-RFP (OR) (ABP-SC-LVIOCT3R, Allele) (Oct4-pBMSCs) and Polybrene (final concentration, 4 μg/mL) in 2 mL of ADMEM supplemented with 10% FCS and 1% Pen-Strep. The cells were incubated at 38.5°C in a humidified atmosphere of 5% CO2 in air, with medium change 10 h after transfection. For Sox2 gene transfection into pBMSCs (Sox2-pBMSCs), piPSC-hSox2 (SR10062PA-1, SBI, WI, USA) and FuGENE 6 (Promega, CA, USA) were transfected according to manufacturer's protocol. Briefly, a total of 1×106 293T cells at 80–90% confluence were cultured in 2 mL of ADMEM supplemented with the mixture of piPSC-hSox2 and FuGENE 6, as previously described by Takahashi et al. (2007). After filtration by a 0.45-μm cellulose acetate filter, 600 μL of virus-containing medium was added to the MSC culture dish containing 1.4 mL of ADMEM supplemented with Polybrene (final concentration, 4 μg/mL). Confirmation of transfected cells was made under a fluorescence microscope by evaluating red fluorescent protein (RFP)-positive expression.
Expression of cell-surface marker
Early- (passages 3–5), middle- (passages 8–10), and late- (passages 14–16) passage pBMSCs at confluence were harvested by dissociation with 0.25% trypsin-EDTA solution and labeled with fluorescein isothiocyanate (FITC) conjugated at 4°C or unconjugated primary antibodies at room temperature for 1 h, and followed by fixation with 3.7% formaldehyde solution for 30 min. After being washed with Dulbecco's phosphate-buffered saline (DPBS), cells were labeled with FITC-conjugated secondary antibody for 45 min. MSC-positive cell-surface markers, FITC-conjugated mouse anti-CD44 (1:100, BD Pharmingen™), CD90 (1:100, BD Pharmingen™), vimentin (1:100, Sigma) and unconjugated CD29 (1:100, BD Pharmingen™), and negative marker, FITC-conjugated mouse anti-CD45 (1:100, Santa Cruz biotechnology) were used for this experiment. The standard was established using isotype controls. FITC-labeled cells were analyzed by a BD FACSCalibur (Becton Dickinson, USA) in the 488-nm wavelength.
In vitro differentiation potential
pBMSCs at 70–80% confluence were induced into adipogenic, osteogenic, and chondrogenic lineages for 3 weeks following previously reported protocols (Pittenger et al., 1999; Vacanti et al., 2005) with minor modifications, and media were changed twice a week. Adipogenic differentiation was performed in ADMEM supplemented with 1 μM dexamethasone, 10 μM insulin, and 200 μM indomethacin. Intracellular accumulation of neutral lipids was evaluated by incubating the cells with 0.5% Oil Red O solution for 30 min.
Osteogenic differentiation was performed in ADMEM supplemented with 0.1 μM dexamethasone, 50 mμ ascorbate-2-phosphate, and 10 mM β-glycerol phosphate. Osteogenic differentiation was evaluated with von Kossa, Alizarin Red, and alkaline phosphatase staining [Western Blue® Stabilized Substrate for Alkaline Phosphatase (BCIP/NBT) of calcium accumulation].
Chondrogenic differentiation was performed using commercial medium (HyClone Advanced STEM™, Thermo Fisher Scientific Inc., US). Alcian Blue staining was used to detect extracellular proteoglycan production.
Oocyte preparation
Oocytes were collected from ovaries harvested at a local slaughterhouse and subjected to in vitro maturation (IVM) by a previously described protocol (Fan and Sun, 2004). Briefly, cumulus–oocyte complexes (COCs) from follicles 3–6 mm in diameter were aspirated using a 19-gauge needle attached to a 10-mL syringe. Sets of 100 COCs with uniform cytoplasm and multilayered cumulus cells were matured in 500 μL of tissue culture medium-199 (TCM-199) supplemented with 5% (wt/vol) FBS, 1% Pen-Strep, 10 ng/mL (wt/vol) epidermal growth factor (EGF), 0.57 mM cysteine, 2.5 mM sodium pyruvate, 1 mM l-glutamine, 0.5 μg/mL (wt/vol) follicle-stimulating hormone (FSH), and 0.5 μg/mL (wt/vol) luteinizing hormone (LH) for 22 h at 38.5°C in a humidified atmosphere of 5% CO2 in air, and further cultured for additional 19 h in the same medium without hormone supplementations.
Embryo production
To generate embryo clones, NT was carried out with minor modifications according to the previously described protocol (Kumar et al., 2012). After 41 h of IVM, cumulus cells were removed from oocytes by pipetting for 1 min in Ca2+- and Mg2+-free DPBS supplemented with 0.1% (wt/vol) hyaluronidase. The cumulus-free oocytes with even ooplasm, intact cytoplasmic membrane and visible first polar body (PB1) were selected for further experiments. Selected metaphase II–stage oocytes were enucleated. Their nuclei and the PB1 with a small volume of bordering cytoplasm were placed in HEPES-buffered TCM-199 supplemented with 7.5 μg/mL cytochalasin B (CCB), 0.3% bovine serum albumin (BSA), and 12 mM sorbitol, and coupled with qualified nuclear donor cells (pBMSCs at early, middle, and later passage, Sox2- and Oct4-pBMSCs). The couplets were fused in 0.28 M mannitol solution containing 0.5 mM HEPES, 0.1 mM MgSO4, 0.05 mM CaCl2, and 0.01% BSA, by applying two pulses with 2.0 KV/cm direct current for 30 μsec using a BTX Electro Square Porator (ECM 830, BTX, Inc., San Diego, CA, USA), and followed by culturing in 1.9 mM 6-dimethylaminopurine (DMAP) for 3 h. Parthenotes produced using the combination of electric stimulation and 6-DMAP treatment were controls for NT embryos.
All eggs were then cultured in sets of 30 embryos per 30-μL droplet in porcine zygote medium 5 (PZM5) supplemented with 3 mg/mL BSA, 20 μg/mL Eagle's amino acids (EAA), and 10 μg/mL nonessential amino acids (NEAA) under paraffin oil for 2 days at 38.5°C in humidified atmosphere of 5% O2, 5% CO2, and 90% N2. They were further cultured in the same medium supplemented with 10% FBS for additional 5 days, as described in a previously described protocol (Ock et al., 2007).
Embryo assessments
The rates of cleavage and blastocyst development were evaluated on days 2 and 7, respectively. Day-7 blastocysts were analyzed for total cell number, apoptotic bodies, and expression of genes. The incidence of apoptotic body formation was evaluated by the TUNEL[terminal deoxynucleotidyl transferase deoxyuridine triphosphate (dUTP) nick end labeling] reaction using an In Situ Cell Death Detection Kit (Roche, Germany) following the manufacturer's protocol. Briefly, blastocysts were fixed overnight in 4% formaldehyde solution at 4°C, permeabilized in 0.5% Triton X-100 containing 0.1% (wt/vol) sodium citrate for 1 h, incubated in fluorescein-conjugated dUTP and terminal deoxynucleotidyl transferase (TdT) for 1 h at 37°C under dark, further incubated with RNase A (50 μg/mL) for 1 h, and counterstained with propidium iodine (40 μg/mL) for 2 h. After being mounted with VECTASHIELD™ (Vector Laboratories Inc., CA) medium, the samples were observed under a fluorescence microscope (Leica CTR600, Switzerland). Apoptotic cells appeared with red nuclei and yellow-green cytoplasm.
Quantitative real-time polymerase chain reaction
The expression of transcription factors (Oct4, Sox2, and Nanog) in pBMSCs and lineage differentiation genes (Oct4 and Cdx2), transcription and pluripotency genes (Nanog and Sox2), DNA methylation genes (Dnmt3a), imprinting genes (Igf2r), and apoptosis genes (Bcl2 and Bax) in day-7 blastocysts were analyzed by quantitative real-time polymerase chain reaction (qRT-PCR). Total RNA isolated from pools of 10 embryos in five replicates was purified using an RNeasy Micro Kit (Qiagen Valencia, Canada) and quantified using an OPTIZEN 3220 UV Bio Spectrophotometer (Mecasys Co., Ltd., Korea). Reverse transcription of purified total RNA was performed using a Sensiscript Reverse Transcription kit (Qiagen Valencia, Canada) with oligo(dT) primer (Invitrogen, USA). The cDNA was synthesized from a total of 500 ng of RNA in a 20-μL reaction mixture at 37°C for 1 h.
The cDNA products were amplified on a LightCycler™ with FastStart DNA Master SYBR Green I (Roche Diagnostics, Germany) by the real-time PCR method. The PCR mixture consisted of 2 mM MgCl2, 2 μL of SYBR Green mix and 0.25 μM each of specific forward and reverse primers (Table 1) in a PCR capillary.
Table 1.
Sequences of qRT-PCR Primers Used for the Expression of Genes
| Gene | Sequence (5′–3′) | Annealing temp. (°C) | Reference |
|---|---|---|---|
| Oct4 | F-ACAAGGAGAAGCTGGAGCCGA | 59 | NM_001113060.1 |
| R-GAGCTTGGCAAATTGTTCGAGAT | |||
| Cdx2 | F-TCTTCCCTGCAAGGCTCGGT | 57 | GU017420.1 |
| R-GACGGTGGGGTTTAGCACGC | |||
| Nanog | F-TGGAAACCTGCCCGTGTGGG | 59 | NM_001129971.1 |
| R-TGGCTGTTCCAGGTCTGGTTGC | |||
| Sox2 | F-CATGCACCGCTACGACGTGA | 56 | NM_001123197.1 |
| R-GGCGAGCCGTTCATGTAGGT | |||
| Dnmt3a | F-CTGAGAAGCCCAAGGTCAAG | 56 | NM_001097437 |
| R-CAGCAGATGGTGCAGTAGGA | |||
| Igf2r | F-CGCTCTCTGCCTCTAGCAGT | 58 | AF342812 |
| R-CCTACACCCCAAGTCTGGAA | |||
| Bcl2 | F-GAAACCCCTAGTGCCATCAA | 58 | NM_214285.1 |
| R-GGGACGTCAGGTCACTGAAT | |||
| Bax | F-AAGCGCATTGGAGATGAACT | 52 | AJ606301.1 |
| R-AAAGTAGAAAAGCGCGACCA | |||
| HMBS2 | F- AGGATGGGCCAACTCTACCTG | 58 | DQ845174 |
| R- GATGGTGGCCTGCATAGTCT |
F, forward; R, reverse.
The program consisted of denaturation at 95°C for 1 min; 45 cycles of PCR (at 95°C for 10 sec, 52–59°C for 6 sec, and 72°C for 4 sec); a melting curve from 65°C to 95°C by 0.1°C/sec; and cooling at 40°C for 30 sec. The relative quantification of gene transcription level was calculated against a reference gene, HMBS2. The amplification of PCR products was quantified by melting curve analysis.
Statistical analysis
One-way analysis of variance (ANOVA) and the Games–Howell post hoc test were used to analyze data using SPSS (v. 18.0). Statistical significance was set at p<0.05. Data are expressed as mean±standard error of the mean (SEM).
Results
Morphology of MSCs
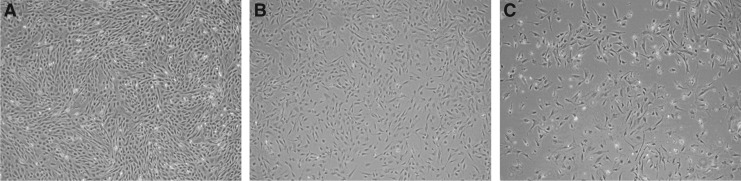
As shown in Figure 1, all pBMSCs from early, middle, and later passages acquired the characteristic properties of attachment to plastic culture dishes and expansion in culture medium. pBMSCs at early and middle passages exhibited a population of fibroblast-like cells; however, those at late passage lacked fibroblast-like morphology.
FIG. 1.
Morphology of pBMSCs at different passages: (A) Early-passage pBMSCs (passages 3–5); (B) middle-passage pBMSCs (passages 8–10); (C) late-passage pBMSCs (passages 14–16). All cells were cultured for 5 days, after seeding the same number of cells. Magnification, 40×.
Expression of transcription factors
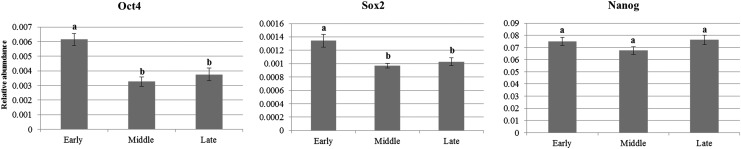
The expression of transcription factors, including Oct4, Sox2, and Nanog, were quantified using qRT-PCR and measured the relative abundance (RA) to the expression level of pBMSCs at early passage, as shown in Figure 2. pBMSCs at early passage showed significantly (p<0.05) higher expression of Oct4 and Sox2 compared to those at middle and late passages. The RA of Nanog showed no significant (p<0.05) difference among different passages.
FIG. 2.
Relative gene expression levels of transcriptional factors by qRT-PCR. Early-passage pBMSCs (passages 3–5); middle-passage pBMSCs (passages 8–10); late-passage pBMSCs (passages 14–16). Data are the mean±SEM, five replicates; p>0.05.
Cell-surface markers
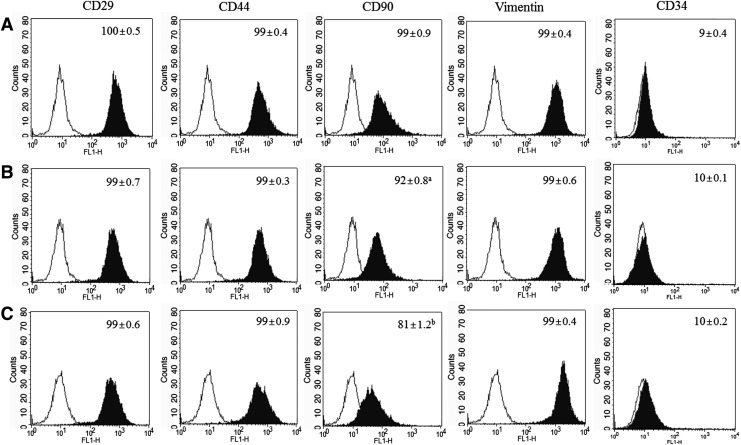
All pBMSCs at early, middle, and late passages strongly expressed CD29, CD44, vimentin, and CD90, with more than 92% of cells showing expression (Fig. 3). However, CD90 in late-passage pBMSCs was significantly (p<0.05) lower than those in early- and middle-passage pBMSCs (81±1.2 vs. 99±0.9 and 92±0.8, respectively). CD34 as a negative marker was rarely expressed in all pBMSCs, and it did not differ among different passages.
FIG. 3.
Analysis of cell-surface markers in pBMSCs at different passages by flow cytometry. (A) Early-passage pBMSCs (passages 3–5); (B) middle-passage pBMSCs (passages 8–10); (C) late-passage pBMSCs (passages 14–16). Gates were drawn on the basis of isotype controls (black line), and positive expression is marked as black fill. Data are the mean±SEM, three replicates; p>0.05.
In vitro differentiation
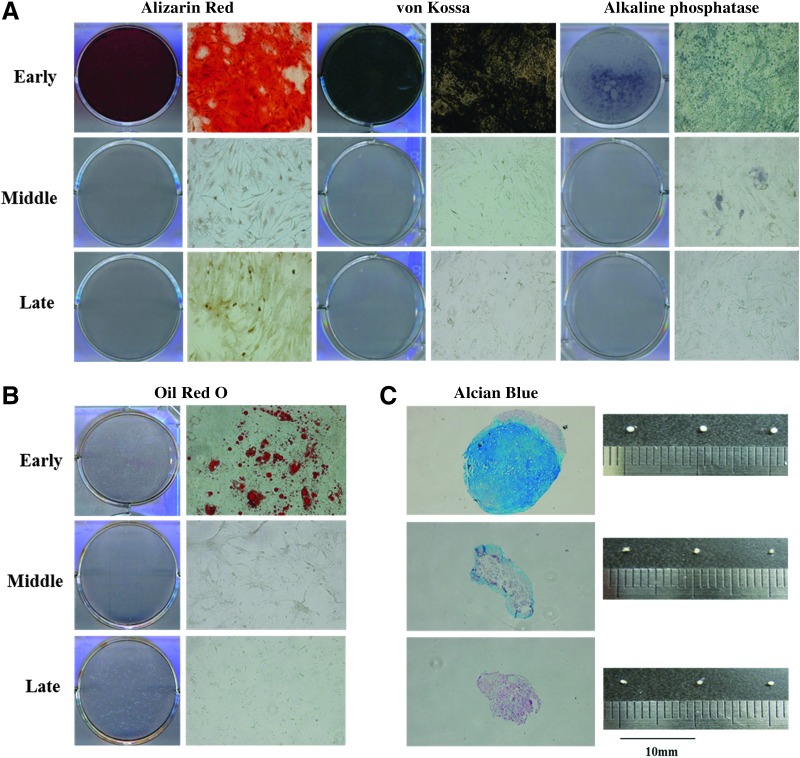
The differentiation potential of pBMSCs at early, middle, and late passages was evaluated for in vitro differentiation into mesenchymal lineages, adipocytes, osteocytes and chondrocytes, under conditioned media. As shown in Figure 4, pBMSCs at early and middle passage were confirmed to be differentiated into the three lineages; however, pBMSCs at late passages did not exhibit intracellular accumulation of lipid droplets and calcium accumulation with mineralized matrix.
FIG. 4.
In vitro differentiation of pBMSCs at different passages: (A) After induction for 21 days, osteogenesis was demonstrated by Alizarin Red S, von Kossa, and alkaline phosphatase; (B) after induction for 21 days, adipogenesis was demonstrated by Oil Red O; (C) after induction for 21 days, chondrogenesis were demonstrated by Alcian Blue staining. Early-passage pBMSCs (passages 3–5); middle-passage pBMSCs (passages 8–10); late-passage pBMSCs (passages 14–16). Magnification, 100×, three replicates.
During in vitro chondrogenesis, extracellular cartilage matrix was observed in a whole section of cells in early pBMSCs and in the periphery of middle-passage pBMSCs. However, no extracellular cartilage matrix was found in late-passage pBMSCs (Fig. 4).
Developmental rates, number of cells, and apoptotic body of parthenotes and NT embryos
The developmental efficiency of NT embryos derived from pBMSCs at early, middle, and late passages, and with Oct4- and Sox2-transfected pBMSCs at middle passage was compared to that of parthenote controls. The results of cleavage and blastocyst rates, total cell number, and apoptotic body incidence are presented in Table 2. Significantly (p<0.05) higher blastocyst rates were observed in parthenote controls than in cloned embryos. Among the NT embryos, no differences were observed in cleavage rates; however, blastocyst rates of NT embryos derived from early-passage pBMSCs were significantly (p<0.05) higher than those from middle- and late-passage pBMSCs donor cells (17.2±0.8 vs. 10.2±0.6 and 5.6±0.4, respectively). In addition, the blastocyst rate in NT embryos from Oct4-pBMSCs cells (13.6±0.5) was similar to those from early-passage pBMSCs NT, and significantly (p<0.05) higher than those derived from middle-passage and Sox2-pBMSCs donor cells (8.8±0.5).
Table 2.
Developmental Rates, Total Cell and Apoptotic Body Number in Porcine NT and Parthenotic Embryos
| No. of embryos (mean%±SEM) | |||||
|---|---|---|---|---|---|
| Groups | Oocytes used | Cleavage | Blastocyst | Total cell number (Mean±SEM) | Apoptotic body (Mean±SEM) |
| Control | 600 | 460 (76.7±1.4)a | 132 (22.0±1.3)a | 55.0±4.0a | 1.5±0.4a |
| Early-passage pBMSCs NT | 402 | 274 (71.8±3.3)a | 69 (17.2±0.8)b | 52.0±2.8a | 1.6±0.6a |
| Middle-passage pBMSCs NT | 388 | 297 (74.2±2.1)a | 41 (10.2±0.6)c | 48.2±2.7a | 3.1±0.5ab |
| Late-passage pBMSCs NT | 381 | 267 (70.4±4.1)a | 21 (5.6±0.4)d | 46.6±2.8a | 6.9±0.6c |
| Oct4-pBMSCs NT | 498 | 331 (67.9±2.5)a | 76 (13.6±0.5)b | 48.8±3.8a | 1.4±0.3a |
| Sox2-pBMSCs NT | 479 | 316 (66.3±2.4)a | 42 (8.8±0.5)c | 46.8±2.7a | 4.6±0.7bc |
Different superscripts in the same column indicate a significant difference (p<0.05), eight replicates.
SEM, standard error of the mean; NT, nuclear transfer; pBMSCs, porcine bone marrow–derived mesenchymal stem cells; control, parthenote; early-passage pBMSCs NT, pBMSCs at passages 3–5; middle-passage pBMSCs NT, pBMSCs at passages 8–10; late-passage pBMSCs NT, pBMSCs at passages 14–16; Oct4-pBMSCs NT, Oct4-transfected pBMSCs at passages 8–10; Sox2-pBMSCs NT, Sox2-transfected pBMSCs at passages 8–10.
The total cell numbers of day-7 blastocysts were not significantly (p<0.05) different between embryos cloned with pBMSCs and parthenotes. The incidence of apoptotic bodies in NT embryos from late-passage pBMSCs and Sox2-pBMSCs donor cells was significantly (p<0.05) higher than that in other NT embryos and parthenotes.
Gene expressions of parthenotes and NT embryos
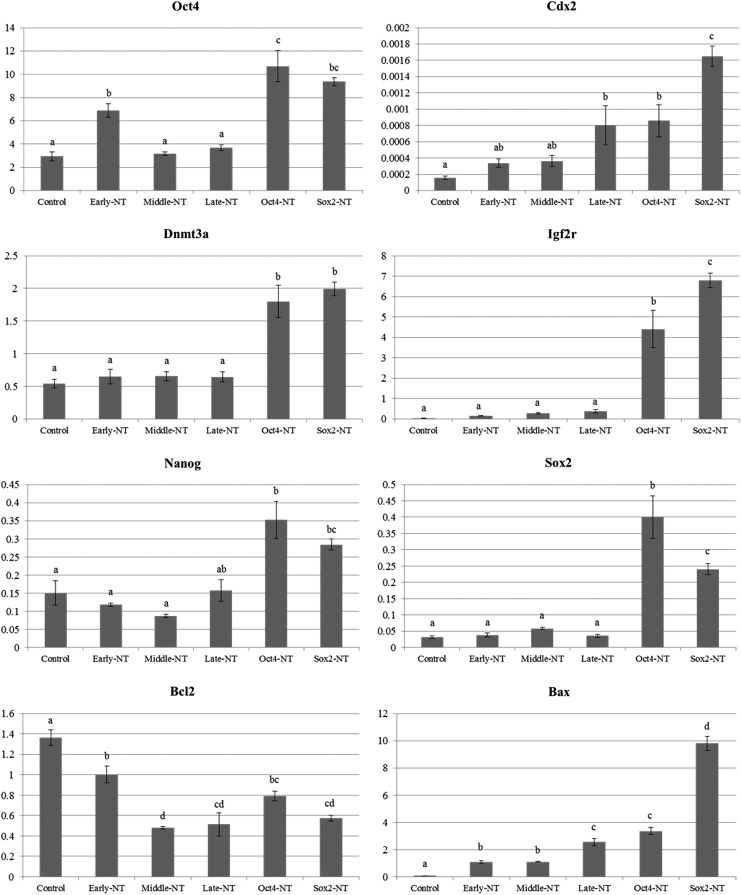
The expression of lineage differentiation (Oct4 and Cdx2), transcription and pluripotency (Nanog and Sox2), DNA methylation (Dnmt3a), imprinting (Igf2r), and apoptosis (Bcl2 and Bax) genes in day-7 NT and parthenote blastocysts was quantified for transcriptional level by qRT-PCR (Fig. 5). The transcriptional level of Oct4 was significantly (p<0.05) higher in early-passage pBMSCs NT embryos compared to middle- and late-passage pBMSC-derived NT and parthenogenetic embryos, but the level was significantly (p<0.05) lower than those in Oct4- and Sox2-pBMSCs–derived NT embryos. In contrast, the level of Cdx2 transcription was significantly (p<0.05) higher in late-passage pBMSCs, Oct4- and Sox2-pBMSCs–derived NT embryos than early- and middle-passage derived ones. For Nanog transcripts, the level amongst NT and parthenote embryos was higher in early-passage pBMSCs, Oct4- and Sox2-pBMSC–derived NT embryos and lower in middle-passage pBMSCs-derived NT embryos. Middle-passage pBMSCs-derived NT embryos revealed significantly (p<0.05) higher transcriptional levels of Sox2 compared to controls and early- and late-passage pBMSCs-derived NT embryos, but the level was significantly (p<0.05) lower compared to Oct4- and Sox2-pBMSCs NT embryos.
FIG. 5.
Relative gene expression levels of lineage differentiation genes (Oct4 and Cdx2), transcription and pluripotency genes (Nanog and Sox2), DNA methylation (Dnmt3a), imprinting genes (Igf2r), and apoptosis genes (Bcl2 and Bax) in day-7 blastocysts was analyzed by qRT-PCR. Early-NT, pBMSCs at passages 3–5 donor; middle-NT, pBMSCs at passages 8–10 donor; late-NT, pBMSCs at passages 14–16 donor; Oct4-NT, Oct4-transfected pBMSCs at passages 8–10 donor; Sox2-NT, Sox2-transfected pBMSCs at passages 8–10 donor; control, parthenote. Data are the mean±SEM, five replicates; p>0.05.
The transcription levels of Dnmt3a and Igf2r were significantly (p<0.05) higher in Oct4- and Sox2-pBMSCs–derived NT embryos compared to those in control, early-, middle-, and late-passage pBMSCs-derived NT embryos. The level in NT embryos derived from middle-passage pBMSCs was similar to ones from Oct4-pBMSCs, but significantly (p<0.05) lower than those from Sox2-pBMSCs. Middle-passage pBMSCs-derived NT embryos revealed a lower transcriptional level of Bcl2 than all other groups, except for Sox2-pBMSCs–derived NT embryos. The transcriptional level of Bax increased gradually following extended passages and attained the minimum level in parthenotes and controls and significantly (p<0.05) higher in Sox2-pBMSCs NT embryos.
Discussion
In the present study, the basic biological characteristics of pBMSCs at early, middle, and late passages were compared. Furthermore, these cells along with Oct4- and Sox2-transfected middle-passage pBMSCs were evaluated for their ability to generate NT cloned embryos by examining the developmental efficiency and embryo-specific gene expression patterns.
Earlier studies have shown that MSCs derived from old donor and at late passages failed to maintain fibroblast-like morphology and increased cell size compared to their counterparts at early passages and those derived from young donors (Dimri et al., 1995; Mauney et al., 2004; Stenderup et al., 2003), suggesting inherent morphological changes during in vitro and in vivo aging. Also, the adhesion capacity of MSCs to plastic culture dishes is reduced by passaging, resulting in reduced cell density with the individual colonies and enlarged cells at late passages (Vacanti et al., 2005). Similarly, in our study, pBMSCs exhibited the characteristic property of attaching to plastic culture dishes and expanding in culture medium. Early- and middle-passage pBMSCs grew into a population of fibroblast-like cells; however, late-passage pBMSCs exhibited a lack of fibroblast-like morphology in culture and instead appeared bigger and had a more spread out morphology.
MSCs are known to express specific cluster of differentiation (CD) markers, but the levels of expression differ considerably according to species and tissue of origin (Chen et al., 1999). Furthermore, expression levels of CD44, CD90, and CD105 depend upon donor age and culture duration (Stolzing et al., 2008). In stem cells, CD90 plays a role in cell adhesion, growth, and differentiation (Clark and Springer, 1999; Rege and Hagood, 2006) and is known to be a differentiation marker for the development of osteoblasts (Chen et al., 1999). Here, we also report that pig bone marrow MSCs with similar phenotypical aspects at different passages expressed CD29, CD44, and vimentin; however, the expression level of CD90 gradually decreased with later passages, supporting the previous observations.
The results of our qRT-PCR analysis showed that pBMSCs at early passage had significantly higher expression of Oct4 and Sox2 than middle- and late-passage pBMSCs. However, expression of Nanog did not differ in pBMSCs at different passages, contradicting the previous report that expression of Oct4 and Nanog decreased with late passage (Yew et al., 2011). Rat BMSCs derived from old donors did not express Sox2 and Nanog (Asumda and Chase, 2011); in human BMSCs, the mRNA levels of Sox2 and Nanog declined with passage, but Oct4 expression levels remain unchanged (Yoon et al., 2011).
MSCs possess broad differentiation ability under specific in vitro culture conditions not only into mesenchymal lineages but also into nonmesenchymal lineages (Jaiswal et al., 1997; Johnstone et al., 1998; Kumar et al., 2012; Ock et al., 2010; Purpura et al., 2004). The in vitro differentiation into adipocytes, osteocytes, and chondrocytes described above obviously showed that pMSCs at early passage have a higher potential capacity than those at middle, and late passages on the basis of morphological observation; however, late-passage pBMSCs failed to differentiate into adipocytes, osteocytes, or chondrocytes. Still, in this regard there are many contrasting reports in the literature. Porcine MSCs at early passage were reported to be capable of maintaining adipogenic, osteogenic, and chondrogenic differentiation, whereas those at late passage maintained only adipogenic differentiation ability (Vacanti et al., 2005). In another report, the osteogenic differentiation ability of MSCs at late passage (passage 7) was higher than that at early passage (passage 1), adipogenic differentiation ability of late passage was lower than that in early-passage MSCs, and chondrogenic differentiation ability was not present in late-passage MSCs (Yoon et al., 2011). Therefore, it is likely that these differences in observation could be related to species, different induction media, and preferentially amplifying different cell populations upon prolonged passaging.
The present study showed that Oct4 and Sox2 gene expression decreased in pBMSCs as culture duration increased, indicating that expression of these genes is directly related to proliferation and differentiation ability. Therefore, to examine the importance of Oct4 and Sox2 in embryo development, overexpression of Oct4 and Sox2 genes in middle-passage pBMSCs, which showed a drop in Oct4 and Sox2 levels, was induced and those cells were used as NT donor nuclei to determine if restoring their levels would restore embryo development rates. We then compared the developmental efficiency of embryos derived from middle-passage pBMSCs transfected with Oct4 and Sox2 with embryos derived from different passage pBMSCs and control parthenotes. Significantly (p<0.05) higher cleavage and blastocyst rates were observed in controls than in cloned embryos. Among the NT embryos, no differences were observed in cleavage rate; however, blastocyst rates in early-passage pBMSCs NT embryos and Oct4-pBMSCs NT embryos were significantly (p<0.05) higher than those in Sox2-pBMSCs NT and middle- and late-passage pBMSCs NT embryos. This finding emphasizes the importance of Oct4 in embryo development, suggesting that pBMSCs at early passage and overexpression of Oct4 in pBMSCs at middle passage as NT donor-produced embryos with similar capability of reprogramming and epigenetic modification of their genome as fertilization-derived counterparts.
Previously, it was reported that overexpression of Oct4 in human MSCs activated the expression of other pluripotent genes, including Sox2, Nanog, c-Myc, and Klf4 (Palma et al., 2013). Another study reported that blastocysts rate and total cell number were increased using Oct4-positive bovine fibroblast donors, but overall cloning efficiency remained unchanged (Rodríguez-Alvarez et al., 2013). The dysregulation of imprinted genes, which leads to prevention of early embryonic and fetal development, may occur due to repeated passage along with long-term culture of donor cells (Wakayama et al., 1999). But in constrast, there were no differences on blastocyst development between different passages (Heidari et al., 2010) ranging from five to 15 (Kubota et al., 2000).
In early embryos, assessment of apoptosis and total cell number were considered as major parameters for embryo quality and subsequent postimplantation development (Hao et al., 2003; Machaty et al., 1998). In pigs, fetal stem cells and MSC-NT embryos had a higher total cell number than fibroblast NT embryos (Jin et al., 2007; Zhu et al., 2004). Our study showed that total cell numbers in day-7 blastocysts were not significantly (p<0.05) different among embryos cloned with pBMSCs and parthenotes, and the presence of the apoptotic body in late-passage pBMSCs NT and Sox2-pBMSCs NT embryos was significantly (p<0.05) higher than those in other NT embryos and parthenotes. This observation supports a relationship between apoptosis incidence and embryo developmental potential, as indicated by decreased blastocyst development in late-passage pBMSCs NT.
DNA methylation is a major regulator of mammalian development. Dnmt3a encodes a DNA methyltransferase that is thought to be responsible for de novo methylation, rather than the maintenance of existing methylated sites (Okano et al., 1998; Xie et al., 1999). Among all NT and parthenote embryos in the present study, the transcription level of Dnmt3a was significantly (p<0.05) higher in Oct4- and Sox2-pBMSCs NT embryos as compared to those in control, early-, middle-, and late-passage pBMSCs NT embryos. Igf2r, which is most well-studied imprinted gene, is essential for fetal growth and normal development (Latham et al., 1994). Igf2r null mice displayed fetal lethality at the late gestation period due to insufficient insulin-like growth factor II (IGFII) signaling activation (Filson et al., 1993; Lau et al., 1994; Wang et al., 1994). Epigenetic silencing of Igf2r is associated with the large offspring syndrome in sheep (Young et al., 2001), causing increased lethality. In the present study, transcriptional levels of Igf2r in Oct4- and Sox2-pBMSCs NT embryos were significantly (p<0.05) higher than in control, early-, middle-, and late-passage pBMSCs NT embryos.
Bcl2 (cell survival) and Bax (cell death) are apoptosis-related genes and are important during embryo development (Metcalfe et al., 2004). Expression of Bax is elevated and that of Bcl2 is reduced in embryos undergoing fragmentation resulting from unsuitable culture condition and remodeling (Kumar et al., 2007). The present study showed that middle-passage pBMSCs NT embryos revealed a lower transcriptional level of Bcl2 than any other groups, except for Sox2-pBMSCs NT embryos. The transcriptional level of Bax increased gradually following extended passages; it attained the minimum in parthenote controls and was significantly (p<0.05) higher in Sox2-pBMSCs NT embryos. It is unlikely that the presence or absence of a single transcript will determine the survival or death of preimplanted embryos because most cell death–causing gene products work through a complex network of homo- and heterodimerization (Jurisicova et al., 2003).
Our experimental results clearly indicate that early-passage pBMSCs are more potent as NT donor cells because of their clear expression of transcription factors and in vitro differentiation ability; hence, these cells could be suitable as NT donor cells. We also confirm that Oct4 transfection of middle-passage pBMSCs improved the developmental efficiency of NT cloned embryos, evidenced by the fact that cells with high expression of Oct4 are most efficient as NT donor cells.
Acknowledgments
This work was supported by a grant from the Next-Generation BioGreen 21 Program (no. PJ009021) of Rural Development Administration, and Bio-industry Technology Development Program (IPET, no. 312060-5) of the Ministry for Food, Agriculture, Forestry, and Fisheries, Republic of Korea. W.A.K. was supported by the MSIP (Ministry of Science, Ict & future Planning), Republic of Korea.
Author Disclosure Statement
The authors declare that there are no conflicts of interest.
J.H.L. was involved in study conception and design, collection and assembly of data, data analysis and interpretation, and manuscript writing; W.J.L., R.H.J., Y.M.L., and S.J.J. were involved in the provision of study material and patients and the collection and assembly of data; B.M.K. and W.A.K. were involved in data analysis and interpretation and manuscript writing; S.L.L. and B.G.J. were involved in administrative support; and G.J.R. was involved in financial support, data analysis and interpretation, administrative support, and final approval of the manuscript.
References
- Asumda F.Z., and Chase P.B. (2011). Age-related changes in rat bone-marrow mesenchymal stem cell plasticity. BMC Cell Biol. 12, 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C.H., Jiang B.H., Huang S.Y., Yang T.S., Lee K.H., Tu C.F., and Wu S.C. (2013). Genetic polymorphisms, growth performance, hematological parameters, serum enzymes, and reproductive characteristics in phenotypically normal Landrace boars produced by somatic cell nuclear transfer. Theriogenology 80, 1088–1096 [DOI] [PubMed] [Google Scholar]
- Chen X.D., Qian H.Y., Neff L., Satomura K., and Horowitz M.C. (1999). Thy-1 antigen expression by cells in the osteoblast lineage. J. Bone Miner. Res. 14, 362–375 [DOI] [PubMed] [Google Scholar]
- Clark R.A., and Springer T.A. (1999). Protein reviews on the Web, CD90
- Dimri G.P., Lee X., Basile G., Acosta M., Scott G., Roskelley C., Medrano E.E., Linskens M., Rubelj I., and Pereira-Smith O. (1995). A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc. Natl. Acad. Sci. USA 92, 9363–9367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do J.T., and Schöler H.R. (2009). Regulatory circuits underlying pluripotency and reprogramming. Trends Pharmacol. Sci. 30, 296–302 [DOI] [PubMed] [Google Scholar]
- Fan H.Y., and Sun Q.Y. (2004). In vitro maturation and fertilization of pig oocytes. Methods Mol. Biol. 253, 227–234 [DOI] [PubMed] [Google Scholar]
- Filson A.J., Louvi A., Efstratiadis A., and Robertson E.J. (1993). Rescue of the T associated maternal effect in mice carrying null mutations in Igf-2 and Igf2r, two reciprocally imprinted genes. Development 118, 731–736 [DOI] [PubMed] [Google Scholar]
- Gnecchi M., and Melo L.G. (2009). Bone marrow-derived mesenchymal stem cells: Isolation, expansion, characterization, viral transduction, and production of conditioned medium. Methods Mol. Biol. 482, 281–294 [DOI] [PubMed] [Google Scholar]
- Hao Y., Lai L., Mao J., Im G.S., Bonk A., Randall S., and Prather R.S. (2003). Apoptosis and in vitro development of preimplantation porcine embryos derived in vitro or by nuclear transfer. Biol. Reprod. 69, 501–507 [DOI] [PubMed] [Google Scholar]
- Heidari B., Shirazi A., Tajic P., Ahmadi E., Nazari H., Shams-Esfandabadi N., and Ghasemzadeh-Nava H. (2010). Effect of donor cell age on development of ovine nuclear transfer embryos in vitro. Zygote 18, 331–338 [DOI] [PubMed] [Google Scholar]
- Ibrahim Z., Busch J., Awwad M., Wagner R., Wells K., and Cooper D.K. (2006). Selected physiologic compatibilities and incompatibilities between human and porcine organ systems. Xenotransplantation 13, 488–499 [DOI] [PubMed] [Google Scholar]
- Jaiswal N., Haynesworth S.E., Caplan A.I., and Bruder S.P. (1997). Osteogenic differentiation of purified, culture-expanded human mesenchymal stem cells in vitro. J. Cell. Biochem. 64, 295–312 [PubMed] [Google Scholar]
- Jin H.F., Kumar B.M., Kim J.G., Song H.J., Jeong Y.J., Cho S.K., Balasubramanian S., Choe S.Y., and Rho G.J. (2007). Enhanced development of porcine embryos cloned from bone marrow mesenchymal stem cells. Int. J. Dev. Biol. 51, 85–90 [DOI] [PubMed] [Google Scholar]
- Johnstone B., Hering T.M., Caplan A.I., Goldberg V.M., and Yoo J.U. (1998). In vitro chondrogenesis of bone marrow-derived mesenchymal progenitor cells. Exp. Cell Res. 238, 265–272 [DOI] [PubMed] [Google Scholar]
- Jurisicova A., Antenos M., Varmuza S., Tilly J.L., and Casper R.F. (2003). Expression of apoptosis-related genes during human preimplantation embryo development: Potential roles for the Harakiri gene product and Caspase-3 in blastomere fragmentation. Mol. Hum. Reprod. 9, 133–141 [DOI] [PubMed] [Google Scholar]
- Kim Y.S., Lee S.L., Ock S.A., Balasubramanian S., Choe S.Y., and Rho G.J. (2005). Development of cloned pig embryos by nuclear transfer following different activation treatments. Mol. Reprod. Dev. 70, 308–313 [DOI] [PubMed] [Google Scholar]
- Kishigami S., Mizutani E., Ohta H., Hikichi T., Thuan N.V., Wakayama S., Bui H.T., and Wakayama T. (2006). Significant improvement of mouse cloning technique by treatment with trichostatin A after somatic nuclear transfer. Biochem. Biophys. Res. Commun. 340, 183–189 [DOI] [PubMed] [Google Scholar]
- Koch C.M., Suschek C.V., Lin Q., Bork S., Goergens M., Joussen S., Pallua N., Ho A.D., Zenke M., and Wagner W. (2011). Specific age-associated DNA methylation changes in human dermal fibroblasts. PLoS One 6, e16679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota C., Yamakuchi H., Todoroki J., Mizoshita K., Tabara N., Barber M., and Yang X. (2000). Six cloned calves produced from adult fibroblast cells after long-term culture. Proc. Natl. Acad. Sci. USA 97, 990–995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar B.M., Jin H.F., Kim J.G., Ock S.A., Hong Y., Balasubramanian S., Choe S.Y., and Rho G.J. (2007). Differential gene expression patterns in porcine nuclear transfer embryos reconstructed with fetal fibroblasts and mesenchymal stem cells. Dev. Dyn. 236, 435–446 [DOI] [PubMed] [Google Scholar]
- Kumar B.M., Maeng G.H., Lee Y.M., Kim T.H., Lee J.H., Jeon B.G., Ock S.A., Yoo J.G., and Rho G.J. (2012). Neurogenic and cardiomyogenic differentiation of mesenchymal stem cells isolated from minipig bone marrow. Res. Vet. Sci. 93, 749–757 [DOI] [PubMed] [Google Scholar]
- Latham K., Doherty A.S., Scott C.D., and Schultz R.M. (1994). Igf2r and Igf2 gene expression in androgenetic, gynogenetic and parthenogenetic preimplantation mouse embryos: Absence of regulation by genomic imprinting. Genes Dev. 8, 290–299 [DOI] [PubMed] [Google Scholar]
- Lau M.M.H., Stewart C.E.H., Liu Z., Bhatt H., Rotwein P., and Stewart C.L. (1994). Loss of the imprinted IGF2/cation-independent mannose 6-phosphate receptor results in fetal overgrowth and perinatal lethality. Genes Dev. 8, 2953–2963 [DOI] [PubMed] [Google Scholar]
- Lee S.L., Kang E.J., Maeng G.H., Kim M.J., Park J.K., Kim T.S., Hyun S.H., Lee E.S., and Rho G.J. (2010). Developmental ability of miniature pig embryos cloned with mesenchymal stem cells. J. Reprod. Dev. 56, 256–262 [DOI] [PubMed] [Google Scholar]
- Machaty Z., Day B.N., and Prather R.S. (1998). Development of early porcine embryos in vitro and in vivo. Biol. Reprod. 59, 451–455 [DOI] [PubMed] [Google Scholar]
- Mauney J.R., Kaplan D.L., and Volloch V. (2004). Matrix-mediated retention of osteogenic differentiation potential by human adult bone marrow stromal cells during ex vivo expansion. Biomaterials 25, 3233–3243 [DOI] [PubMed] [Google Scholar]
- Metcalfe A.D., Hunter H.R., Bloor D.J., Lieberman B.A., Picton H.M., Leese H.J., Kimber S.J., and Brison D.R. (2004). Expression of 11 members of the BCL-2 family of apoptosis regulatory molecules during human preimplantation embryo development and fragmentation. Mol. Reprod. Dev. 68, 35–50 [DOI] [PubMed] [Google Scholar]
- Ock S.A., Lee S.L., Kim J.G., Kumar B.M., Balasubramanian S., Choe S.Y., and Rho G.J. (2007). Development and quality of porcine embryos in different culture system and embryo-producing methods. Zygote 15, 1–8 [DOI] [PubMed] [Google Scholar]
- Ock S.A., Jeon B.G., and Rho G.J. (2010). Comparative characterization of porcine mesenchymal stem cells derived from bone marrow extract and skin tissues. Tissue Eng. Part C Methods 16, 1481–1491 [DOI] [PubMed] [Google Scholar]
- Ock S.A., Kwack D.O., Mohana , Kumar B., Han J., Kim S.W., and Rho G.J. (2011). Effects of activation methods on DNA synthesis and development of parthenogenetic porcine embryos. Reprod. Dom. Anim. 46, 1082–1089 [DOI] [PubMed] [Google Scholar]
- Ogura A., Inoue K., Takano K., Wakayama T., and Yanagimachi R. (2000). Birth of mice after nuclear transfer by electrofusion using tail tip cells. Mol. Reprod. 57, 55–59 [DOI] [PubMed] [Google Scholar]
- Okano M., Xie S., and Li E. (1998). Cloning and characterization of a family of novel mammalian DNA (cytosine-5) methyltransferases. Nat. Genet. 19, 219–220 [DOI] [PubMed] [Google Scholar]
- Palma C.S., Tannous M.A., Malta T.M., Russo E.M., Covas D.T., and Picanço-Castro V. (2013). Forced expression of OCT4 influences the expression of pluripotent genes in human mesenchymal stem cells and fibroblasts. Genet. Mol. Res. 12, 1054–1060 [DOI] [PubMed] [Google Scholar]
- Pfeiffer M.J., Balbach S.T., Esteves T.C., Crosetto N., and Boiani M. (2010). Enhancing somatic nuclear reprogramming by Oct4 gain-of-function in cloned mouse embryos. Int. J. Dev. Biol. 54, 1649–1657 [DOI] [PubMed] [Google Scholar]
- Pittenger M.F., Mackay A.M., Beck S.C., Jaiswal R.K., Douglas R., Mosca J.D., Moorman M.A., Simonetti D.W., Craig S., and Marshak D.R. (1999). Multilineage potential of adult human mesenchymal stem cells. Science 284, 143–147 [DOI] [PubMed] [Google Scholar]
- Polejaeva I.A., Chen S.H., Vaught T.D., Page R.L., Mullins J., Ball S., Dai Y., Boone J., Walker S., Ayares D.L., Colman A., and Campbell K.H. (2000). Cloned pigs produced by nuclear transfer from adult somatic cells. Nature 7, 86–90 [DOI] [PubMed] [Google Scholar]
- Prather R.S., Sims M.M., and First N.L. (1989). Nuclear transplantation in early pig embryos. Biol. Reprod. 41, 414–418 [DOI] [PubMed] [Google Scholar]
- Purpura K.A., Aubin J.E., and Zandstra P.W. (2004). Sustained in vitro expansion of bone progenitors is cell density dependent. Stem Cells 22, 39–50 [DOI] [PubMed] [Google Scholar]
- Rege T.A., and Hagood J.S. (2006). Thy-1 as a regulator of cell–cell and cell–matrix interactions in axon regeneration, apoptosis, adhesion, migration, cancer, and fibrosis. FASEB J. 20, 1045–1054 [DOI] [PubMed] [Google Scholar]
- Reggio B.C., James A.N., Green H.L., Gavin W.G., Behboodi E., Echelard Y., and Godke R.A. (2001). Cloned transgenic offspring resulting from somatic cell nuclear transfer in the goat: Oocytes derived from both follicle-stimulating hormone-stimulated and nonstimulated abattoir-derived ovaries. Biol. Reprod. 65, 1528–1533 [DOI] [PubMed] [Google Scholar]
- Rodríguez-Alvarez L., Manriquez J., Velasquez A., and Castro F.O. (2013). Constitutive expression of the embryonic stem cell marker OCT4 in bovine somatic donor cells influences blastocysts rate and quality after nucleus transfer. In Vitro Cell Dev. Biol. Anim. 49, 657–667 [DOI] [PubMed] [Google Scholar]
- Schook L., Beattie C., Beever J., Donovan S., Jamison R., Zuckermann F., Niemi S., Rothschild M., Rutherford M., and Smith D. (2005). Swine in biomedical research: creating the building blocks of animal models. Anim. Biotechnol. 16, 183–190 [DOI] [PubMed] [Google Scholar]
- Sommer C.A., Stadtfeld M., Murphy G.J., Hochedlinger K., Kotton D.N., and Mostoslavsky G. (2009). Induced pluripotent stem cell generation using a single lentiviral stem cell cassette. Stem Cells 27, 543–549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenderup K., Justesen J., Clausen C., and Kassem M. (2003). Aging is associated with decreased maximal life span and accelerated senescence of bone marrow stromal cells. Bone 33, 919–926 [DOI] [PubMed] [Google Scholar]
- Stolzing A., Jones E., McGonagle D., and Scutt A. (2008). Age-related changes in human bone marrow-derived mesenchymal stem cells: Consequences for cell therapies. Mech. Ageing Dev. 129, 163–173 [DOI] [PubMed] [Google Scholar]
- Takahashi K., Okita K., Nakagawa M., and Yamanaka S. (2007). Induction of pluripotent stem cells from fibroblast cultures. Nat. Protoc. 2, 3081–3089 [DOI] [PubMed] [Google Scholar]
- Vacanti V., Kong E., Suzuki G., Sato K., Canty J.M., and Lee T. (2005). Phenotypic changes of adult porcine mesenchymal stem cells induced by prolonged passaging in culture. J. Cell Physiol. 205, 194–201 [DOI] [PubMed] [Google Scholar]
- Wakayama T., Rodriguez I., Perry A.C.F., Yanagimachi R., and Mombaerts P. (1999). Mice cloned from embryonic stem cells. Proc. Natl. Acad. Sci. USA 96, 14984–14989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakayama S., Mizutani E., Kishigami S., Thuan N.V., Ohta H., Hikichi T., Bui H.T., Miyake M., and Wakayama T. (2005). Mice cloned by nuclear transfer from somatic and ntES cells derived from the same individuals. J. Reprod. Dev. 51, 765–772 [DOI] [PubMed] [Google Scholar]
- Wang Z.Q., Fung M.R., Barlow D.P., and Wagner E.F. (1994). Regulation of embryonic growth and lysosomal targeting by the imprinted Igf2/Mpr gene. Nature 372, 464–467 [DOI] [PubMed] [Google Scholar]
- Welstead G.G., Schorderet P., and Boyer L.A. (2008). The reprogramming language of pluripotency. Curr. Opin. Genet. Dev. 18, 123–129 [DOI] [PubMed] [Google Scholar]
- Wilmut I., Beaujean N., de Sousa P.A., Dinnyes A., King T.J., Paterson L.A., Wells D.N., and Young L.E. (2002). Somatic cell nuclear transfer. Nature. 419, 583–586 [DOI] [PubMed] [Google Scholar]
- Xie S., Wang Z., Okano M., Nogami M., Li Y., He W.W., Okumura K., and Li E. (1999). Cloning, expression and chromosome locations of the human DNMT3 gene family. Gene 236, 87–95 [DOI] [PubMed] [Google Scholar]
- Yew T.L., Chiu F.Y., Tsai C.C., Chen H.L., Lee W.P., Chen Y.J., Chang M.C., and Hung S.C. (2011). Knockdown of P3-51Cip1/Waf1 enhances proliferation, the expression of stemness markers, and osteogenic potential in human mesenchymal stem cells. Aging Cell 10, 349–361 [DOI] [PubMed] [Google Scholar]
- Yoon D.S., Kim Y.H., Jung H.S., Paik S., and Lee J.W. (2011). Importance of Sox2 in maintenance of cell proliferation and multipotency of mesenchymal stem cells in low-density culture. Cell Prolif. 44, 428–440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young L.E., Fernandes K., McEvoy T.G., Butterwith S.C., Gutierrez C.G., Carolan C., Broadbent P.J., Robinson J.J., Wilmut I., and Sinclair K.D. (2001). Epigenetic change in IGF2R is associated with fetal overgrowth after sheep embryo culture. Nature Genet. 27, 153–154 [DOI] [PubMed] [Google Scholar]
- Zhang L., Luo Y.B., Bou G., Kong Q.R., Huan Y.J., Zhu J., Wang J.Y., Li H., Wang F., Shi Y.Q., Wei Y.C., and Liu Z.H. (2011). Overexpression Nanog activates pluripotent genes in porcine fetal fibroblasts and nuclear transfer embryos. Anat. Rec. 294, 1809–1817 [DOI] [PubMed] [Google Scholar]
- Zhu H., Craig J.A., Dyce P.W., Sunnen N., and Li J. (2004). Embryos derived from porcine skin-derived stem cells exhibit enhanced preimplantation development. Biol. Reprod. 71, 1890–1897 [DOI] [PubMed] [Google Scholar]