Abstract
The direct conversion of human fibroblasts to neuronal cells, termed human induced neuronal (hiN) cells, has great potential for future clinical advances. However, previous studies have not provided an in-depth analysis of electrophysiological properties of adult fibroblast-derived hiN cultures. We have examined the electrophysiological profile of hiN cells by measuring passive and active membrane properties, as well as spontaneous and evoked neurotransmission. We found that hiN cells exhibited passive membrane properties equivalent to perinatal rodent neurons. In addition, 30% of hiN cells were incapable of action potential (AP) generation and did not exhibit rectifying membrane currents, and none of the cells displayed firing patterns of typical glutamatergic pyramidal neurons. Finally, hiN cells exhibited neither spontaneous nor evoked neurotransmission. Our results suggest that current methods used to produce hiN cells provide preparations in which cells do not achieve the cellular properties of fully mature neurons, rendering these cells inadequate to investigate pathophysiological mechanisms.
Introduction
Stem cell research is a promising and rapidly advancing field of modern science with the future prospect of developing patient-specific cells of any type to treat a variety of diseases. Recently, new methods have been developed to allow for the direct conversion of terminally differentiated human cells, such as fibroblasts, into neuronal cells, which have been termed human induced neuronal (hiN) cells (Ambasudhan et al., 2011; Pang et al., 2011; Pfisterer et al., 2011a; Pfisterer et al., 2011b; Qiang et al., 2011; Son et al., 2011; Yoo et al., 2011). A major benefit of this novel technology in comparison with neurons derived from induced pluripotent stem cells (iPSCs) is omitting an intermediate stem cell state. Thus, the risk of uncontrolled cell growth due to incomplete differentiation as reported in iPSCs (Pera, 2011) is not present in hiN cells. This suggests higher safety of the hiN technology for potential clinical application.
Physiological maturation of neuronal properties is important for proper neuronal functioning and functional network formation. For example, spontaneous synaptic activity after birth serves as a guidance signal for synaptogenesis in immature neurons (Kavalali et al., 2011; Spitzer, 2006). The early postnatal development of the brain is of critical importance to assure correct wiring and firing of neuronal circuits in later life. Several studies have described postnatal changes in electrophysiological properties in a variety of rodent brain structures, including hippocampal, cortical, thalamic, and cerebellar brain areas (Belleau and Warren, 2000; Cui et al., 2010; Etherington and Williams, 2011; Kinnischtzke et al., 2012; Koppensteiner et al., 2014; McCormick and Prince, 1987; McKay and Turner, 2005; Pirchio et al., 1997; Spigelman et al., 1992; Tyzio et al., 2003). Thus, a reliable method to investigate the extent of neuronal differentiation and functionality of transdifferentiated neurons is the measurement of their electrophysiological properties. Here, we provide a detailed examination of the electrophysiological profile of hiN cells to quantify the extent of neuronal conversion and functionality.
Materials and Methods
hiN cultures
Cells for electrophysiological recording were provided by the laboratory of Asa Abeliovich at Columbia University; for details on the method to prepare hiN cells from human fibroblasts, see Qiang et al. (2011). We received hiN cells plated on 3-cm glass-bottomed petri dishes that contained high densities of fibroblasts and approximately 5–10 cells with neuronal-like morphology per dish. These neuronal cells appeared either isolated or in small clusters of two to three cells and had one or two short processes protruding from small cell bodies. Our data derived from a total of six independent transfections, and we measured spontaneous neurotransmission in hiN cells derived from all six transfections. Passive and active membrane properties were measured in four of those six badges (10 cells from transfection 1, seven cells from transfection 2, nine cells from transfection 3, and one cell from transfection 4). Outwardly rectifying currents and paired patch clamp recordings of evoked neurotransmission were each measured in hiN cells derived from one transfection. hiN cells from all transfections appeared morphologically similar, and, in the analysis of our results, none of the hiN badges stood out as particularly different from the others.
Electrophysiology
Patch clamp recordings of hiN cells (21–28 days after transduction) were performed in whole-cell mode using a Multiclamp 700B amplifier (Molecular Devices, Sunnydale, CA, USA) with a Digidata 1440A Digitizer (Molecular Devices). Signals were filtered at 1 kHz, sampled at 10 kHz, and recorded with pClamp 10 software (Molecular Devices). Cells were examined with a TS100 ECLIPSE microscope (Nikon, Tokyo, Japan), and only hiN cells with neuronal-like morphology were used in this study.
The intracellular solution consisted of 130 mM K-gluconate, 10 mM KCl, 10 mM HEPES, 1 mM MgCl2, 0.06 mM CaCl2, 0.1 mM EGTA, 4 mM MgATP, 0.3 mM Na2GTP, 10 mM phosphocreatine; 290 mOsm, pH 7.4. The extracellular solution consisted of 119 mM NaCl, 5 mM KCl, 20 mM HEPES, 30 mM glucose, 2 mM CaCl2, 2 mM MgCl2, 0.001 mM glycine; 330 mOsm, pH ∼7.3. The calculated liquid junction potential with these solutions was −5.8 mV, and the data presented were not corrected.
Recording electrodes were crafted from thick-walled borosilicate glass tubes (World Precision Instruments, Sarasota, FL, USA) to give tip resistances of 4–7 MΩ using a PIP5 Pipette Puller (HEKA Instruments Inc., Bellmore, NY, USA). In some experiments, 1 μM tetrodotoxin (TTX; Ascent Scientific, Cambridge, MA, USA) was added to the extracellular solution to confirm the involvement of Nav channels in action potential (AP) generation. In measurements of outward-rectification experiments, voltage-steps ranged from −120 to +40 mV at 20-mV increments, starting from a holding potential of −70 mV. Synaptic activity was monitored at a holding potential of −70 mV. In current-clamp recordings, because of resting membrane potentials (RMPs) close to the threshold potential of APs, hyperpolarizing holding currents were applied to produce a physiological membrane potential close to −70 mV. Under these conditions, current steps of −50 to +110 pA were applied in 20-pA increments to elicit APs. In simultaneous whole-cell recordings of hiN cells, one cell was stimulated by applying depolarizing currents of 70 pA for 1 sec, and membrane currents were measured in a neighboring cell clamped at −70 mV. Recordings were discarded when a strong leak current was detected (more negative than or equal to −100 pA) at a holding potential of −70 mV or when the access resistance exceeded 25 Ω. This was the case in approximately <5% of the recorded cells.
Reagents
All reagents, with the exception of TTX, were obtained from Sigma (Sigma-Aldrich Corp).
Data analysis
Input resistance (Rin) was measured as the slope of the linear relationship between the intensity of applied currents and the resulting changes in the membrane potential. The membrane time constant tau was calculated by exponential fitting of the membrane potential change in response to current injection. For the analysis of AP properties, only the first AP in a current-step protocol was analyzed manually using pClamp 10 software (Molecular Devices). Cells were considered to have overshooting APs when the first AP surpassed 0 mV. Statistical analysis and figure preparation was done using Prism 5 (GraphPad Software, Inc., La Jolla, CA, USA). All data are presented as mean±standard error of the mean (SEM) and was considered statistically significant when p<0.05.
Results
hiN cells display passive membrane properties similar to neurons in perinatal rodents
The conversion of human fibroblasts to neuronal-like cells is a process that requires rearrangements of the cell membrane, such as the insertion of various ion channels. Similar to physiological neuronal development, this is reflected in changes in passive membrane properties of the converted cells. Therefore, we first analyzed passive membrane properties of 27 hiN cells to characterize their stage of neuronal development (summarized in Table 1). We measured high Rin and membrane time constant tau values (Rin, 1.06±0.08 GΩ, n=27; tau, 35.39±4.24 ms, n=27). Furthermore, membrane capacitance (Cm) was low (33.32±3.50 pF, n=27) and RMP values were slightly depolarized (−42.12±3.80 mV, n=27).
Table 1.
Passive Membrane Properties and Action Potential Properties of hiN Cells
| Passive membrane properties | ||||
|---|---|---|---|---|
| Rin (GΩ) | Cm (pF) | tau (ms) | RMP (mV) | |
| Mean | 1.06 | 33.32 | 35.39 | −42.12 |
| SEM | 0.08 | 3.50 | 4.24 | 3.80 |
| N | 27 | 27 | 27 | 27 |
| Action potential properties | |||
|---|---|---|---|
| Amplitude (mV) | FWHM (ms) | Threshold (mV) | |
| Mean | 39.23 | 24.12 | −30.54 |
| SEM | 4.97 | 6.48 | 1.71 |
| N | 19 | 19 | 19 |
Passive membrane properties, including input resistance (Rin), membrane capacitance (Cm), membrane time constant tau, and resting membrane potential (RMP) were measured in 27 hiN cells. Analysis of action potential (AP) properties in 19 human induced neuronal (hiN) cells that showed AP firing revealed small AP amplitudes, broad full-widths at half-maximum (FWHM), and depolarized AP threshold potentials.
SEM, standard error of the mean.
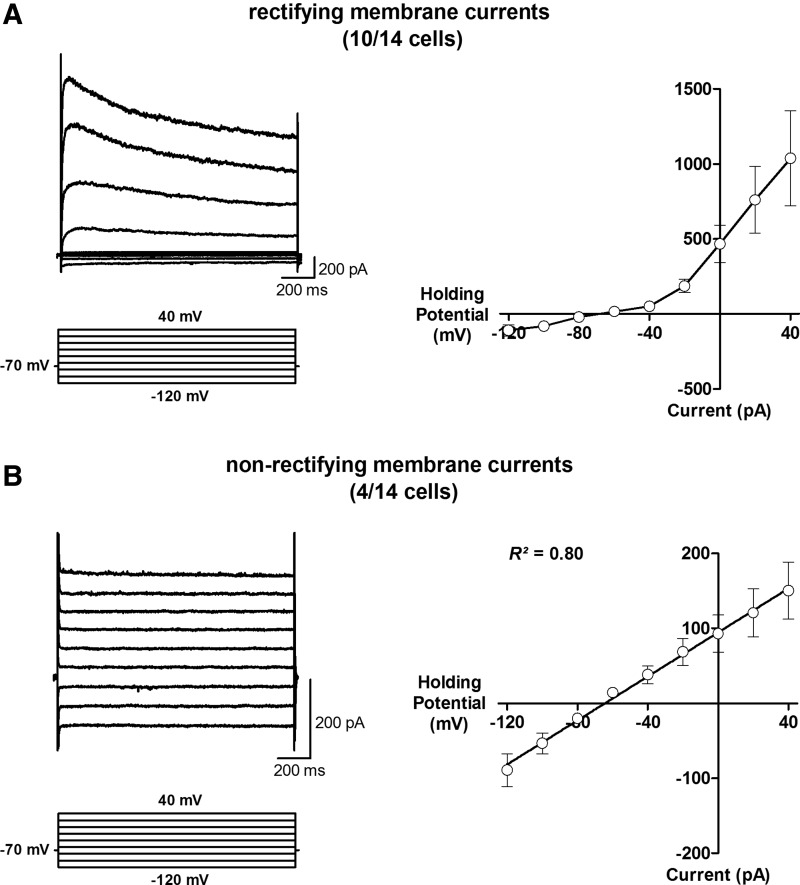
The development of electrophysiological membrane properties is correlated with progressively increasing numbers of ion channels in the neuronal membrane, including voltage-gated K+ channels (Spigelman et al., 1992). An important aspect of excitable neuronal membranes is the presence of rectifying K+ currents, meaning that the amplitude of K+ currents is disproportionally larger at positive membrane potentials as compared with currents at negative membrane potentials. Therefore, we measured membrane currents in 14 hiN cells at holding potentials ranging from −120 mV to +40 mV (Fig. 1). These 14 cells were a subset of the 27 cells analyzed for their passive membrane properties. We found that 71% of hiN cells showed rectifying currents (10 of 14 cells, Fig. 1A) with maximal currents of 1036±316 pA (n=10) at +40 mV. In contrast, 29% of hiN cells (four of 14 cells; Fig. 1B) showed a linear current/voltage relationship (R2=0.80, linear regression analysis) with significantly lower currents of 150.3±37.9 pA (n=4) at +40 mV (p<0.01, Mann–Whitney test). Thus, outwardly rectifying K+ currents were absent in 29% of hiN cells, indicating that neuronal conversion was not progressing equally in all hiN cells with neuronal morphology. Furthermore, these results indicate the presence of subpopulations of hiN cells with different electrophysiological profiles.
FIG. 1.
Analysis of rectifying membrane currents in response to voltage-step applications from −120 to +40 mV in 14 hiN cells. (A) (Left) Example traces of large, outwardly rectifying currents present in 71% of hiN cells. (Right) Rectifying membrane currents in response to voltage-step applications are indicated by larger currents in response to stronger depolarization. (B) (Left) Example traces of small, nonrectifying membrane currents found in 29% hiN cells. (Right) Linear relationship between holding potentials and membrane currents suggests an absence of rectifying K+-channels.
High variability in AP properties of hiN cells
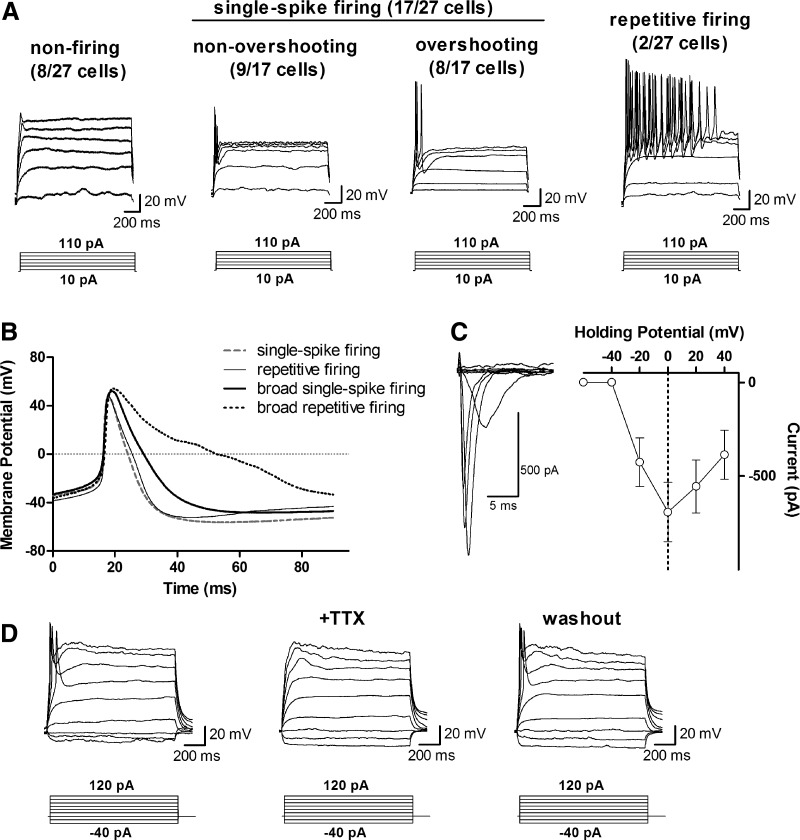
The ability to generate APs in response to suprathreshold current injection is a central characteristic of all neurons. Similar to passive membrane properties, AP properties are strongly influenced by and rapidly changing with progressive neuronal development (Spigelman et al., 1992). Therefore, we analyzed AP firing properties in the same 27 hiN cells that were used in passive membrane property analysis. Surprisingly, we found three subpopulations of hiN cells (Fig. 2A): Eight out of 27 cells failed to generate APs (30%), 17 out of 27 cells generated a single AP (63%), and two out of 27 cells showed repetitive AP firing (7%). In those hiN cells that generated APs, 10 out of 19 cells presented overshooting APs (53%) and nine out of 19 AP-generating hiN cells showed APs that did not surpass 0 mV (47%). Overall, only 10 out of 27 cells had overshooting APs (37%). In addition, rising phases of all overshooting APs were uniformly fast, but the repolarization times varied greatly between cells (Fig. 2B).
FIG. 2.
Action potential firing patterns and tetrodotoxin-sensitive Na+ currents. (A) We identified four subpopulations of hiN cells characterized by different AP firing patterns: Nonfiring (30%), non-overshooting (33%), and overshooting (30%) single-spike firing, and repetitive firing (7%) hiN cells. (B) Expanded view of AP traces of the two hiN subpopulations with overshooting APs reveals strong variability in AP broadness due to heterogeneous repolarization times. (C) (Left) Example traces of sodium currents in response to voltage-step applications from −20 to +40 mV. (Right) Plot of the relationship between holding potential and the amplitude of resulting Na+ currents in 4 hiN cells. (D) Application of the Nav-blocker TTX (1 μM) abolished AP firing, confirming the involvement of sodium currents in the observed APs.
We also confirmed the presence of Na+ currents with peak amplitudes of −692.5±158.1 pA (n=4) at a holding potential of 0 mV (Fig. 2C). In addition, we confirmed the involvement of voltage-gated Na+ channels (Nav) in AP generation by applying 1 μM TTX, which reversibly blocked AP generation (Fig. 2D). These results indicates that two-thirds of hiN cells were partially capable of generating APs, but showed considerable variability in firing patterns.
To gain further insight into the neurodevelopmental stage of hiN cells, we analyzed AP properties of the 19 cells that generated APs in response to depolarizing current injections (summarized in Table 1). Consistent with neurons in an early developmental stage, APs were characterized by small amplitudes (39.23±4.97 mV, n=19), broad full-widths at half-maximum (FWHM, 24.12±6.48 ms, n=19), and depolarized threshold potentials (−30.54±1.71 mV, n=19). It is noteworthy that FWHMs varied greatly between cells, with values ranging from 4.1 ms to 129.7 ms, due to high variability in the duration of AP repolarization (Fig. 2B). These results suggest that APs of hiN cells are characterized by properties similar to those of neurons in early development (McCormick and Prince, 1987; Spigelman et al., 1992) and demonstrate large heterogeneity in AP FWHM between individual hiN cells.
Absence of synaptic neurotransmission in hiN cell cultures
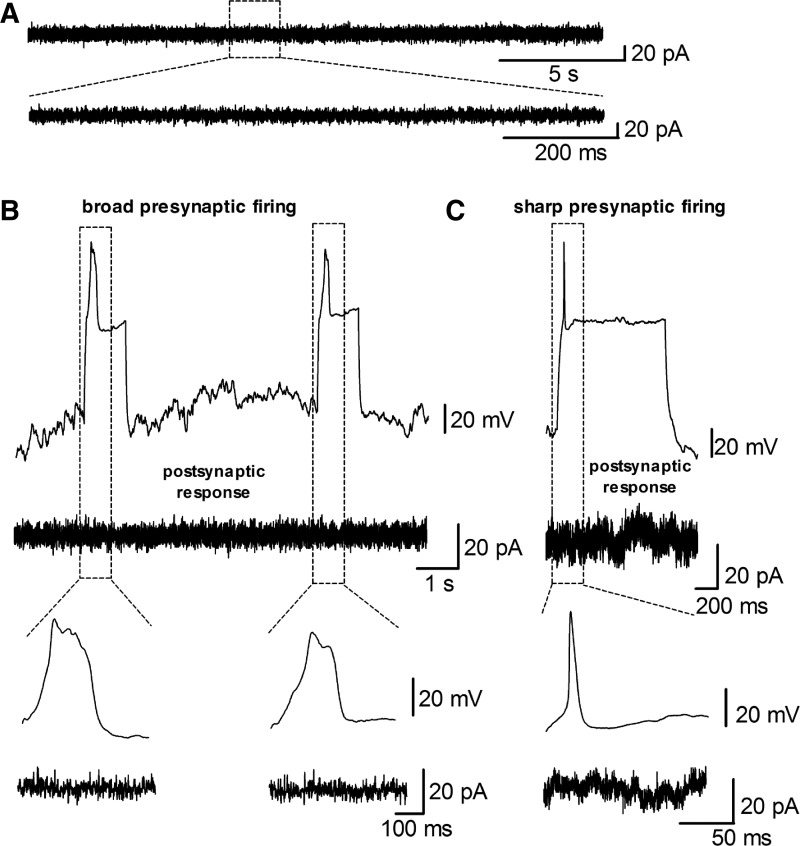
The formation of synaptic connections is a crucial aspect of neuronal development and serves to promote neuronal survival and network formation (Kavalali et al., 2011; Verhage et al., 2000). Therefore, we recorded membrane currents in 57 hiN cells at a holding potential of −70 mV to detect spontaneous neurotransmission but did not detect any events (see Fig. 3A for sample traces). To investigate whether synaptic neurotransmission was absent due to a lack of spontaneous presynaptic AP generation, we next performed simultaneous whole-cell patch-clamp recordings in nine pairs of hiN cells with visibly overlapping neuronal processes. In these experiments, we applied depolarizing currents in one cell and measured current responses in a neighboring cell. Of these nine pairs, six pairs showed presynaptic AP generation, and each pair was stimulated at least five times. However, we did not observe evoked neurotransmission events in any of the recorded cell pairs (see Fig. 3B, C for example traces). Thus, a lack of presynaptic AP generation is likely not the reason for the absence of spontaneous neurotransmission. These results indicate that, under the conditions of pure hiN cultures 3–4 weeks after transfection, hiN cells do not exhibit spontaneous or evoked neurotransmission.
FIG. 3.
Absence of spontaneous and evoked synaptic neurotransmission in hiN cells. (A) Example traces of gap-free current recordings at a holding potential of −70 mV. No membrane currents with typical characteristics of neurotransmission were observed in any of the 57 recorded hiN cells. (B, C) Example traces of simultaneous whole-cell recordings in pairs of neighboring hiN cells. Depolarization of a (potentially presynaptic) hiN cell failed to elicit current responses in a neighboring (potentially postsynaptic) cell in nine of nine paired recordings in hiN subpopulations with both broad (B) and sharp (C) APs.
Discussion
The direct conversion of human fibroblasts to neuronal-like cells presents unique opportunities in studying and potentially treating various diseases of the human central nervous system on a cellular level without the potential danger of tumorigenesis inherent in stem cell-derived neurons. Here, we provide an in-depth analysis of passive and active membrane properties, as well as synaptic activity of hiN cells. Our results suggest that the conversion of human fibroblasts to neurons was incomplete and the resulting cells were electrophysiologically heterogeneous: (1) Passive membrane properties resembled those of highly immature neurons, (2) 30% of hiN cells did not show outwardly rectifying membrane currents, (3) 30% of hiN cells did not fire APs, (4) only 37% of hiN cells had overshooting APs, (5) only 7% of hiN cells showed repetitive AP firing, and (6) hiN cells exhibited neither spontaneous nor evoked neurotransmission. Nevertheless, given the fact that only 7% of hiN cells showed repetitive AP firing, a much higher number of paired recordings might be able to evoke neurotransmission in some pairs of hiN cells. In addition, we cannot exclude the possibility that our hiN cells would be able to form functional synaptic connections under other conditions, such as in neuronal co-culture with other neurons or glial cells, or at a later time point (more than 4 weeks).
Passive membrane properties are an important index of neuronal maturation. The average values of Rin, Cm, and tau, three parameters of passive membrane properties investigated in the present study, were characteristic of immature neurons (McCormick and Prince, 1987; Spigelman et al., 1992; Tyzio et al., 2003). Specifically, Rin values in the GΩ range, Cm values below 50 pF, and tau values higher than 20 msec are typical for neurons in early developmental stages. These values indicate that the cell size of hiN cells (reflected in Rin and Cm), the number of open ion channels at rest (reflected in Rin), and the rate with which the membrane potential can change (reflected in tau) are lower compared with mature neurons (Spruston and Johnston, 1992). Similar passive membrane properties were reported in neurons differentiated from iPSCs (Israel et al., 2012) and in neurons obtained through direct conversion from human and rodent fibroblasts (Ambasudhan et al., 2011; Caiazzo et al., 2011; Pfisterer et al., 2011b; Qiang et al., 2011). The findings of considerable variability are not unusual for neurons in early development, and we cannot distinguish whether variability of these passive membrane properties reflects heterogeneity in the course of neuronal conversion or abnormal electrophysiological development. However, the presence of outwardly rectifying K+ channels has been reported in already highly immature neurons of perinatal rodents (Spigelman et al., 1992). Thus, the absence of outwardly rectifying currents is unusual, even for developing neurons, and indicates aberrant cellular electrophysiology in a subpopulation of hiN cells.
Importantly, mouse-derived iN cells present progressive maturation of passive membrane and AP properties over the first 3 weeks after conversion (Vierbuchen et al., 2010). Interestingly, electrophysiological profiles of our hiN cells 3 weeks after transduction were similar to those of mouse-derived iN cells 8 days after transduction (Vierbuchen et al., 2010). Thus, as described in a recent study (Zhang et al., 2013), the method of directly converting fibroblasts to neurons might be more suitable for mouse than for human fibroblasts, or longer time periods might be necessary for neuronal conversion to complete. Taken together, the present study indicates that passive membrane properties of our hiN cells were not yet fully matured.
APs reflect active membrane properties of neurons. Interestingly, 7% of hiN cells showed repetitive firing similar to neonatal interneurons (Zhong et al., 2006), as indicated by a lack of AP accommodation and large AP afterhyperpolarization, whereas AP firing of typical glutamatergic pyramidal neurons (Connors and Gutnick, 1990) was not observed in any of the recorded cells. The lack of repetitive firing in 93% of hiN cells indicates that neuronal conversion might be incomplete because even highly immature rodent neurons do fire multiple APs in response to suprathreshold current injections (Spigelman et al., 1992). Thus, it is likely that active membrane properties are not yet fully matured in our hiN cells.
Synaptic activity is another important neuronal feature, and iN cells obtained by direct neuronal conversion of mouse fibroblasts show synaptic neurotransmission in co-cultures with primary neurons or astrocytes (Chanda et al., 2013; Vierbuchen et al., 2010). In our study, we could detect neither spontaneous nor evoked synaptic neurotransmission 3 weeks after conversion. Furthermore, hiN cells directly converted from human fetal and early postnatal fibroblasts were shown to be inefficient and required the addition of another transcription factor, NeuroD1 (Pang et al., 2011). In the case of hiN cells obtained through the method used by Pang and colleagues, synaptic activity was only observed in hiN co-cultures with cortical neurons after 4–5 weeks and was mostly derived from gamma-aminobutyric acid release. In addition, neuronal conversion appeared to produce more mature neurons when derived from fetal compared to postnatal fibroblasts, as electrophysiological profiles of postnatal fibroblasts remained rather immature (Pang et al., 2011). Therefore, it is possible that the method of producing neurons capable of synaptic neurotransmission through direct neuronal conversion of fibroblasts might be inefficient when using adult human fibroblasts.
Importantly, a recently retracted study using hiN cells reported the presence of functional synapse formation. In their study (Qiang et al., 2011), hiN cells showed glutamatergic spontaneous neurotransmission when co-cultured with murine glial cells and after transplantation into embryonic mouse brains. However, close examination of these previous results reveals that these membrane currents appear not to possess fast rise and slow decay kinetics characteristic of typical glutamatergic neurotransmission. Although frequency and kinetics were not analyzed in the study by Qiang et al., the displayed frequency of spontaneous neurotransmission appeared unusually high for neurons of mice at postnatal day 7 (Gao et al., 2011; Qiang et al., 2011). Furthermore, an important feature of early neuronal networks is the occurrence network-wide bursts of neurotransmission that provide important signals for synaptic development (Ben-Ari et al., 1989; Kasyanov et al., 2004). These early network activities have been reported in mouse-derived induced neurons (Vierbuchen et al., 2010) but not in hiN cells (Ambasudhan et al., 2011; Qiang et al., 2011), further indicating impaired synaptic development of hiN cells. In contrast to the study by Qiang and colleagues, our findings indicate that hiN cells might be incapable of functional synaptic development and do not present firing patterns of pyramidal glutamatergic forebrain neurons.
Nonelectrophysiological cell differentiation markers help to understand the developmental stage of hiN cells. In a previous study, immunocytochemical analysis using markers such as Tuj1, MAP2, Tau1, NeuN, and vGLUT1 or GAD65 in hiN cells grown for 3 weeks after transfection in parallel with the cells used in our electrophysiological recordings showed that the number of our hiN cells expressing MAP2 and vGLUT1was highest 3 weeks after transfection and the amount of MAP2- and vGLUT1-positive cells was progressively decreasing thereafter due to apoptosis of the converted cells (Qiang et al., 2011). Furthermore, total neurite length was shown to have reached the maximum length of 100–200 μm 3–4 weeks after transduction (Qiang et al., 2011). Thus, our findings stress the relevance of performing an electrophysiological assessment to make sure that converted cells behave as mature neurons.
In many neurodegenerative disorders such as Alzheimer's disease, Parkinson's disease, Huntington's disease, or amyotrophic lateral sclerosis, patients show neurological symptoms in early to late adulthood. Given the incomplete, highly variable and perinatal electrophysiological profile, and the inability of functional synapse formation of hiN cells investigated by us, the use of these cells might not be suitable to study pathophysiological processes of early- or late-onset neurodegenerative disorders. Therefore, we conclude that the current method of directly converting adult human cells to neurons might require further refinement to produce fully mature neurons and assuring pathophysiological significance of any data obtained from them.
Acknowledgements
This work was supported by National Institutes of Health (NIH) grant no. NS049442 to OA. Cells were provided by Asa Abeliovich and Liang Qiang and were prepared according to the method published in Qiang et al. (2011), which was recently retracted by the authors for reasons that were not related to the electrophysiological characterization of the hiN cells (see retraction notice Cell 157, April 10, 2014).
Author Disclosure Statement
The authors declare that no conflicting financial interests exist.
References
- Ambasudhan R., Talantova M., Coleman R., Yuan X., Zhu S., Lipton S.A., and Ding S. (2011). Direct reprogramming of adult human fibroblasts to functional neurons under defined conditions. Cell Stem Cell 9, 113–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belleau M.L., and Warren R.A. (2000). Postnatal development of electrophysiological properties of nucleus accumbens neurons. J. Neurophysiol. 84, 2204–2216 [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y., Cherubini E., Corradetti R., and Gaiarsa J.L. (1989). Giant synaptic potentials in immature rat CA3 hippocampal neurones. J. Physiol. 416, 303–325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caiazzo M., Dell'Anno M., Dvoretskova E., Lazarevic D., Taverna S., Leo D., Sotnikova T., Menegon A., Roncaglia P., and Colciago G. (2011). Direct generation of functional dopaminergic neurons from mouse and human fibroblasts. Nature 476, 224–227 [DOI] [PubMed] [Google Scholar]
- Chanda S., Marro S., Wernig M., and Sudhof T.C. (2013). Neurons generated by direct conversion of fibroblasts reproduce synaptic phenotype caused by autism-associated neuroligin-3 mutation. Proc. Natl. Acad. Sci. USA 110, 16622–16627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connors B.W., and Gutnick M.J. (1990). Intrinsic firing patterns of diverse neocortical neurons. Trends Neurosci. 13, 99–104 [DOI] [PubMed] [Google Scholar]
- Cui J., Wang F., Wang K., and Xiang H. (2010). GABAergic signaling increases through the postnatal development to provide the potent inhibitory capability for the maturing demands of the prefrontal cortex. Cell. Mol. Neurobiol. 30, 543–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etherington S.J., and Williams S.R. (2011). Postnatal development of intrinsic and synaptic properties transforms signaling in the layer 5 excitatory neural network of the visual cortex. J. Neurosci. 31, 9526–9537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X.P., Liu Q.S., Liu Q., and Wong-Riley M.T. (2011). Excitatory-inhibitory imbalance in hypoglossal neurons during the critical period of postnatal development in the rat. J. Physiol. 589, 1991–2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israel M., Yuan S., Bardy C., Reyna S., Mu Y., Herrera C., Hefferan M., Van Gorp S., Nazor K., and Boscolo F. (2012). Probing sporadic and familial Alzheimer/'s disease using induced pluripotent stem cells. Nature 482:, 216–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasyanov A.M., Safiulina V.F., Voronin L.L., and Cherubini E. (2004). GABA-mediated giant depolarizing potentials as coincidence detectors for enhancing synaptic efficacy in the developing hippocampus. Proc. Natl. Acad. Sci. USA 101, 3967–3972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavalali E.T., Chung C., Khvotchev M., Leitz J., Nosyreva E., Raingo J., and Ramirez D.M. (2011). Spontaneous neurotransmission: An independent pathway for neuronal signaling? Physiology (Bethesda) 26, 45–53 [DOI] [PubMed] [Google Scholar]
- Kinnischtzke A.K., Sewall A.M., Berkepile J.M., and Fanselow E.E. (2012). Postnatal maturation of somatostatin-expressing inhibitory cells in the somatosensory cortex of GIN mice. Front. Neural Circuits 6, 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koppensteiner P., Aizawa S., Yamada D., Kabuta T., Boehm S., Wada K., and Sekiguchi M. (2014). Age-dependent sensitivity to glucocorticoids in the developing mouse basolateral nucleus of the amygdala. Psychoneuroendocrinology 46, 64–77 [DOI] [PubMed] [Google Scholar]
- McCormick D.A., and Prince D.A. (1987). Post-natal development of electrophysiological properties of rat cerebral cortical pyramidal neurones. J. Physiol. 393, 743–762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay B.E., and Turner R.W. (2005). Physiological and morphological development of the rat cerebellar Purkinje cell. J. Physiol. 567, 829–850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang Z.P., Yang N., Vierbuchen T., Ostermeier A., Fuentes D.R., Yang T.Q., Citri A., Sebastiano V., Marro S., Sudhof T.C., and Wernig M. (2011). Induction of human neuronal cells by defined transcription factors. Nature 476, 220–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pera M. (2011). Stem cells: The dark side of induced pluripotency. Nature 471, 46–47 [DOI] [PubMed] [Google Scholar]
- Pfisterer U., Kirkeby A., Torper O., Wood J., Nelander J., Dufour A., Bjorklund A., Lindvall O., Jakobsson J., and Parmar M. (2011b). Direct conversion of human fibroblasts to dopaminergic neurons. Proc. Natl. Acad. Sci. USA 108, 10343–10348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfisterer U., Wood J., Nihlberg K., Hallgren O., Bjermer L., Westergren-Thorsson G., Lindvall O., and Parmar M. (2011a). Efficient induction of functional neurons from adult human fibroblasts. Cell Cycle 10, 3311–3316 [DOI] [PubMed] [Google Scholar]
- Pirchio M., Turner J.P., Williams S.R., Asprodini E., and Crunelli V. (1997). Postnatal development of membrane properties and delta oscillations in thalamocortical neurons of the cat dorsal lateral geniculate nucleus. J. Neurosci. 17, 5428–5444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiang L., Fujita R., Yamashita T., Angulo S., Rhinn H., Rhee D., Doege C., Chau L., Aubry L., and Vanti W.B. (2011). Directed conversion of Alzheimer's disease patient skin fibroblasts into functional neurons. Cell 146, 359–371. (Retraction notice Cell 157, April 10, 2014, p. 514). [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Son E.Y., Ichida J.K., Wainger B.J., Toma J.S., Rafuse VF, Woolf CJ, Eggan K. (2011). Conversion of mouse and human fibroblasts into functional spinal motor neurons. Cell Stem Cell 9, 205–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spigelman I., Zhang L., and Carlen P.L. (1992). Patch-clamp study of postnatal development of CA1 neurons in rat hippocampal slices: Membrane excitability and K+ currents. J. Neurophysiol. 68, 55–69 [DOI] [PubMed] [Google Scholar]
- Spitzer N.C. (2006). Electrical activity in early neuronal development. Nature 444, 707–712 [DOI] [PubMed] [Google Scholar]
- Spruston N., and Johnston D. (1992). Perforated patch-clamp analysis of the passive membrane properties of three classes of hippocampal neurons. J. Neurophysiol. 67, 508–529 [DOI] [PubMed] [Google Scholar]
- Tyzio R., Ivanov A., Bernard C., Holmes G.L., Ben-Ari Y., and Khazipov R. (2003). Membrane potential of CA3 hippocampal pyramidal cells during postnatal development. J. Neurophysiol. 90, 2964–2972 [DOI] [PubMed] [Google Scholar]
- Verhage M., Maia A., Plomp J., Brussaard A., Heeroma J., Vermeer H., Toonen R., Hammer R., Missler M., and Geuze H. (2000). Synaptic assembly of the brain in the absence of neurotransmitter secretion. Science 287, 864–869 [DOI] [PubMed] [Google Scholar]
- Vierbuchen T., Ostermeier A., Pang Z.P., Kokubu Y., Sudhof T.C., and Wernig M. (2010). Direct conversion of fibroblasts to functional neurons by defined factors. Nature 463, 1035–1041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo A.S., Sun A.X., Li L., Shcheglovitov A., Portmann T., Li Y., Lee-Messer C., Dolmetsch R.E., Tsien R.W., and Crabtree G.R. (2011). MicroRNA-mediated conversion of human fibroblasts to neurons. Nature 476, 228–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Pak C., Han Y., Ahlenius H., Zhang Z., Chanda S., Marro S., Patzke C., Acuna C., Covy J., Xu W., Yang N., Danko T., Chen L., Wernig M., and Sudhof T.C. (2013). Rapid single-step induction of functional neurons from human pluripotent stem cells. Neuron 78, 785–798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong G., Diaz-Rios M., and Harris-Warrick R.M. (2006). Intrinsic and functional differences among commissural interneurons during fictive locomotion and serotonergic modulation in the neonatal mouse. J. Neurosci. 26, 6509–6517 [DOI] [PMC free article] [PubMed] [Google Scholar]