Abstract
Aims: Liver injury and regeneration involve complicated processes and are affected by various physio-pathological factors. We investigated the mechanisms of steatosis-associated liver injury and delayed regeneration in a mouse model of partial hepatectomy. Results: Initial regeneration of the steatotic liver was significantly delayed after hepatectomy. Although hepatocyte proliferation was not significantly suppressed, severe liver injury with oxidative stress (OS) occurred immediately after hepatectomy in the steatotic liver. Fas-ligand (FasL)/Fas expression was upregulated in the steatotic liver, whereas the expression of antioxidant and anti-apoptotic molecules (catalase/MnSOD/Ref-1 and Bcl-2/Bcl-xL/FLIP, respectively) and p62/SQSTM1, a steatosis-associated protein, was downregulated. Interestingly, pro-survival Akt was not activated in response to hepatectomy, although it was sufficiently expressed even before hepatectomy. Suppression of p62/SQSTM1 increased FasL/Fas expression and reduced nuclear factor erythroid 2-related factor-2 (Nrf-2)-dependent antioxidant response elements activity and antioxidant responses in steatotic and nonsteatotic hepatocytes. Exogenously added FasL induced severe cellular OS and necrosis/apoptosis in steatotic hepatocytes, with only the necrosis being inhibited by pretreatment with antioxidants, suggesting that FasL/Fas-induced OS mainly leads to necrosis. Furthermore, p62/SQSTM1 re-expression in the steatotic liver markedly reduced liver injury and improved liver regeneration. Innovation: This study is the first which demonstrates that reduced expression of p62/SQSTM1 plays a crucial role in posthepatectomy acute injury and delayed regeneration of steatotic liver, mainly via redox-dependent mechanisms. Conclusion: In the steatotic liver, reduced expression of p62/SQSTM1 induced FasL/Fas overexpression and suppressed antioxidant genes, mainly through Nrf-2 inactivation, which, along with the hypo-responsiveness of Akt, caused posthepatectomy necrotic/apoptotic liver injury and delayed regeneration, both mainly via a redox-dependent mechanism. Antioxid. Redox Signal. 21, 2515–2530.
Introduction
Liver regeneration occurs as a series of physio-pathological events that result in quantitative recovery from loss of liver mass, compensating for decreased hepatic volume and impaired function. The liver has the ability to restore lost volume, a phenomenon that is rarely seen in other organs (10, 22). It is well established that normal adult hepatocytes are usually quiescent but have the potential to replicate. After surgical procedures that reduce liver mass, such as partial hepatectomy (PH) or live-donor liver transplantation, a rapid enlargement of the residual or grafted liver commonly occurs to restore liver mass and function (9). Poor or insufficient regeneration of diseased liver, for example in nonalcoholic steatohepatitis (NASH) and liver cirrhosis, or of aged liver, is potentially fatal for these patients (1, 25, 32, 33). Therefore, a better understanding of the molecular mechanisms of liver injury and delayed regeneration in various pathological conditions may lead to clinical benefits.
Innovation.
Clinically, steatotic liver has been considered the likely cause both of increased mortality after hepatectomy, mainly as a result of reduced tolerance to ischemic injury and oxidative stress, and of impaired mitotic response. This study is the first which demonstrates that the reduced expression of p62/SQSTM1 in steatotic liver directly causes post-partial hepatectomy liver injury, mainly in a redox-dependent manner, and that restoration of p62/SQSTM1 reduces injury and restores the regeneration of steatotic liver. Furthermore, the study importantly suggests the potential role of p62/SQSTM1 in protecting the liver from all kinds of injury.
Hepatic steatosis (fatty liver) is a commonly encountered hepatic disorder that may be caused by various factors, such as obesity, diabetes mellitus, and alcohol consumption (33). It is often considered a benign condition, because it does not usually cause severe clinical symptoms. However, living-donor liver transplantation from a donor with a steatotic liver is problematic, because steatosis often causes immediate graft failure (primary nonfunction) (33).
In the normal liver, various mitotic factors and cytokines promptly activate various cellular signals and events, eventually leading to sufficient liver regeneration after PH (9, 10, 22, 36). In the steatotic liver, impairment of signaling mechanisms due to adaptation to chronic metabolic abnormalities and decreased adenosine triphosphate (ATP) production has been reported to be the likely cause of increased mortality and impaired regeneration after PH (24, 33, 35). In addition, hepatic steatosis is considered to reduce tolerance to ischemic injury and oxidative stress (OS) (2, 33).
This study was designed to explore the root causes of post-PH liver injury and delayed regeneration of the steatotic liver, and to identify the molecules responsible for these phenomena. Here, we report that the reduction of steatosis-associated p62/sequestosome1 (p62/SQSTM1) accelerates post-PH liver injury with OS and is involved in impairment of the initial regeneration of steatotic liver after PH.
Results
Initial liver regeneration was disturbed in the steatotic liver after PH, despite sufficient hepatocyte proliferation
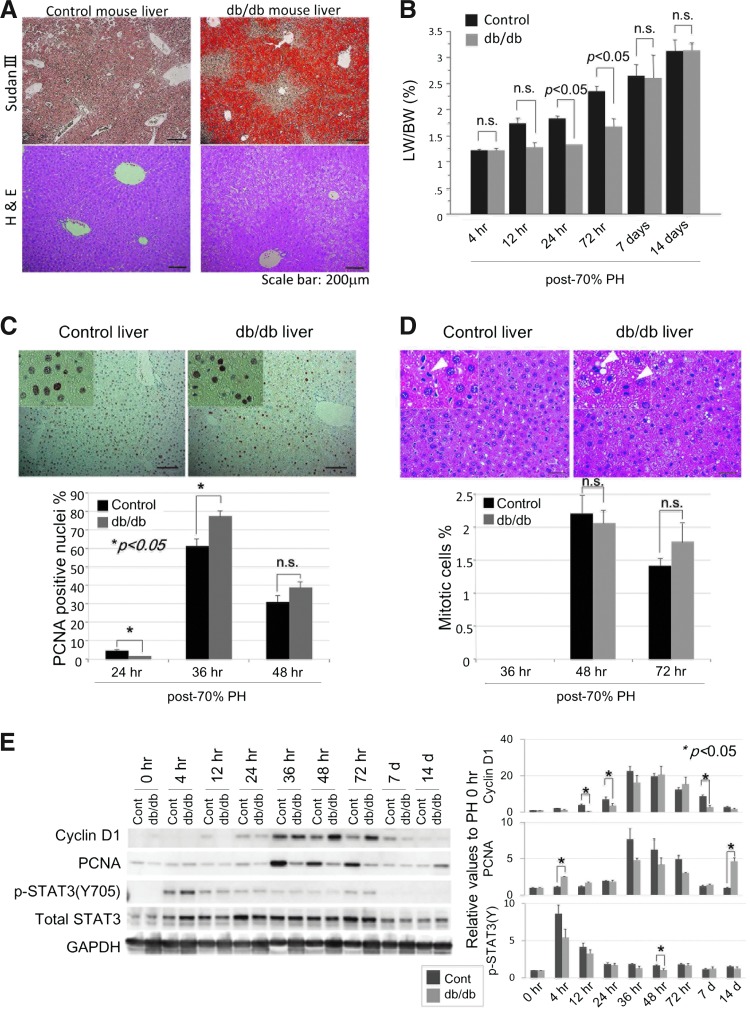
First, we monitored liver recovery in db/db steatotic mice and lean littermate controls after two-thirds PH (Fig. 1). The liver lipid content was determined by Sudan III stain in the lean controls and the db/db steatotic mice (Fig. 1A), which showed evident lipid accumulation only in the db/db mouse liver. Liver recovery occurred immediately after PH in the nonsteatotic liver of the control mice, whereas it was markedly disturbed in the steatotic liver of the db/db mice, at least until 72 h post-PH (Fig. 1B). The recovery of the steatotic liver, however, was equivalent to that of the control liver at 7 days post-PH. This observation suggests that the regeneration of the steatotic liver may be initially disturbed by a reduced mitotic response after PH.
FIG. 1.
Although post-PH initial liver regeneration is disturbed in db/db mice, mitotic responses occur equally in lean control and db/db steatotic mice. (A) Lipid content was confirmed by Sudan III stain in the liver of control and db/db mice before PH. Lipid accumulation was more evident in the central area than in the portal area of the db/db mouse liver. (B) Initial liver regeneration was disturbed in the db/db mice after PH. (C) Counts of PCNA-positive hepatocytes in the regenerating liver showed that the mitotic response peaked at 36 h post-PH in lean control and db/db mouse liver. (D) Histological examination (H&E staining) revealed similar mitotic responses at 36, 48, and 72 h post-PH in the liver of lean control and db/db steatotic mice. Scale bar: 50 μm. At least five mice were used for each experiment (B–D). Data are expressed as mean±SEM. (E) Expression of cell cycle-associated proteins was examined in the liver. Each blot represents at least three independent experiments. The intensity of each band was quantified by densitometry, showing the chronological relative changes for each protein, and is plotted on the right. The results are expressed as mean±SEM of at least three independent experiments; p<0.05 was considered statistically significant (B–E). Groups without an asterisk (*) were not statistically different (E). H&E, hematoxylin and eosin; PCNA, proliferating cell nuclear antigen; PH, partial hepatectomy; SEM, standard error of the mean. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
To investigate the mitotic response in the steatotic liver, we examined and counted proliferating cell nuclear antigen (PCNA)-positive hepatocytes (Fig. 1C) and mitotic hepatocytes (Fig. 1D) in the regenerating liver after PH. These histological analyses showed that PCNA positivity and mitosis in the regenerating liver peaked at 36 and 48 h post-PH, respectively, and thereafter decreased. Western blot analysis showed that cyclinD1 and PCNA similarly peaked at 36 h post-PH and thereafter decreased. Signal transducer and activator of transcription 3 (STAT3), which is mainly associated with cell proliferation, was found to be expressed at equivalent levels in both livers and to be phosphorylated at 4 h post-PH in both livers (Fig. 1E). Although post-PH expression of cyclinD1 and PCNA was more evident in the db/db mouse liver and the control liver, respectively, the counts of mitotic cells were almost equal in both groups (Fig. 1D).
In light of what has been said earlier, we can say that hepatocyte proliferation in the db/db mouse liver was equivalent to that in the control liver, suggesting that a mechanism other than delayed or disturbed mitotic response was the main cause of the delayed regeneration of the steatotic liver.
OS and injury occurred in the steatotic liver of db/db mice immediately after PH
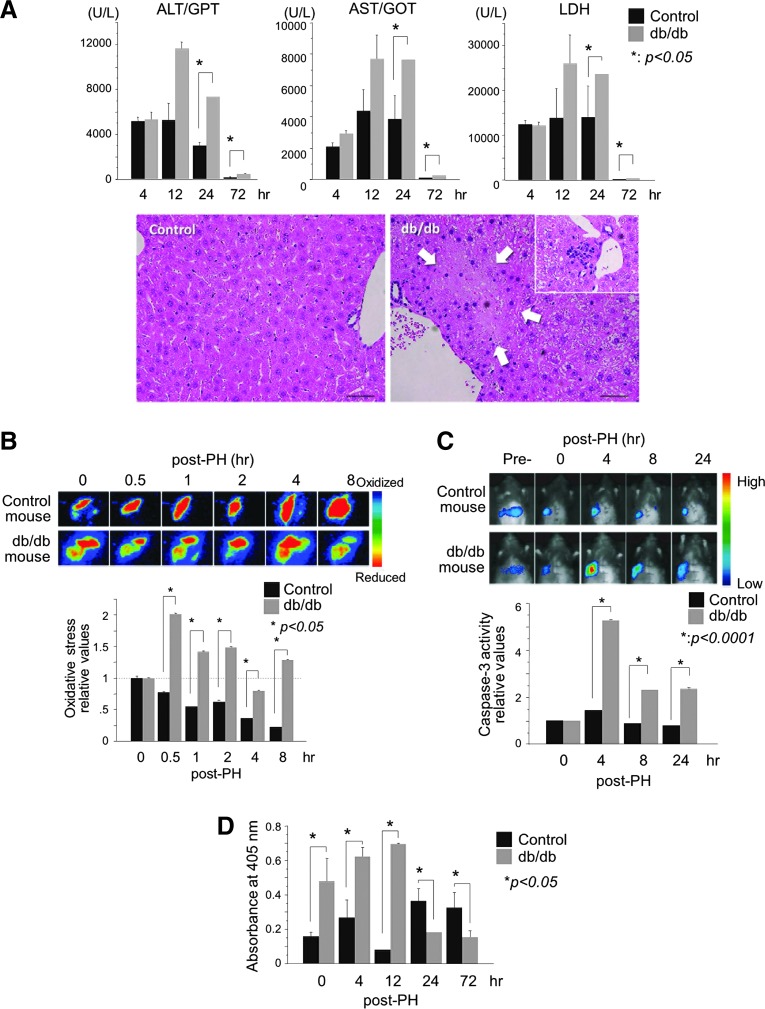
Next, we monitored post-PH liver injury in the db/db mice to elucidate the mechanism underlying delayed liver regeneration. Marked liver injury occurred within 12 h of PH in the db/db mice (Fig. 2A). The serum levels of aspartate aminotransferase (AST), alanine aminotransferase (ALT), and lactate dehydrogenase (LDH) were markedly elevated at 12–24 h post-PH in the db/db mice but had recovered to baseline levels at 3 days post-PH, suggesting that the harmful cellular events associated with PH occurred immediately. Histological examination also confirmed liver injury with sporadic necrosis and neutrophil infiltration observed especially in the peri-portal areas of the steatotic liver at 24 h after PH (Fig. 2A).
FIG. 2.
Steatotic liver shows acute OS and injury immediately after PH. (A) Blood biochemistry shows immediate liver injury after PH in the db/db steatotic mouse liver. Histologically, sporadic necrosis in the peri-portal areas (area surrounded by arrows) and neutrophil infiltration (inset) are observed at 24 h after PH. (B) Bio-imaging of liver OS after PH. Emission from the roGFP fluorescent probe was measured directly at the exposed liver surface, imaged, and quantified. Photographs are representative images of the dynamic changes in hepatic redox states (oxidation: green to blue; reduction: orange to red). Early post-PH, OS is observed in the db/db steatotic liver. For each experiment, the intensities of the hepatic roGFP signals are plotted relative to the pre-PH values. (C) Bio-imaging of liver caspase-3 activity after PH. The pcFluc-DEVD probe emitted signals at 4–24 h post-PH in the remnant liver of db/db mice, with no evident signal in the control liver. Data in the graph are expressed as mean±SEM and are expressed relative to the pre-PH control. (D) Apoptotic cell death is markedly increased at 4 h post-PH in the steatotic liver. Results are expressed as mean±SEM of five independent experiments; p<0.05 was considered statistically significant (A–D). Each experiment was performed five times, and representative photographs are shown (B, C). OS, oxidative stress; roGFP, reduction-oxidation–sensitive green fluorescent protein. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
To determine whether OS is induced in the steatotic liver in the early post-PH period, we studied hepatic OS using a reduction-oxidation–sensitive green fluorescent protein (roGFP) probe (Fig. 2B) (7, 15). This redox-sensitive roGFP probe showed the immediate generation of robust OS only in the steatotic liver, which peaked within 30 min of PH and continued for at least 8 h.
We next examined whether and when apoptotic cell death occurred in the post-PH steatotic liver. Bio-imaging of the post-PH remnant liver showed immediate activation of caspase-3, peaking at 4 h post-PH and continuing for at least 24 h (Fig. 2C). Hepatic apoptosis in the steatotic liver also began increasing immediately after PH and peaked at 12 h post-PH (Fig. 2D). These analyses reveal that apoptotic cell death occurred at 4 h post-PH in the steatotic liver, but did not occur in the control liver.
Given that hepatic OS and apoptosis occurred sequentially and that some caspases are redox sensitive (17), we considered that the severe post-PH OS might have activated redox-sensitive caspases, thereby inducing apoptosis-mediated injury in the db/db mice.
Analysis of hepatic signals associated with cell proliferation, cell survival/death, and OS
To elucidate the mechanisms of hepatic OS, injury and delayed regeneration in the db/db mice, we investigated the hepatic signal molecules involved.
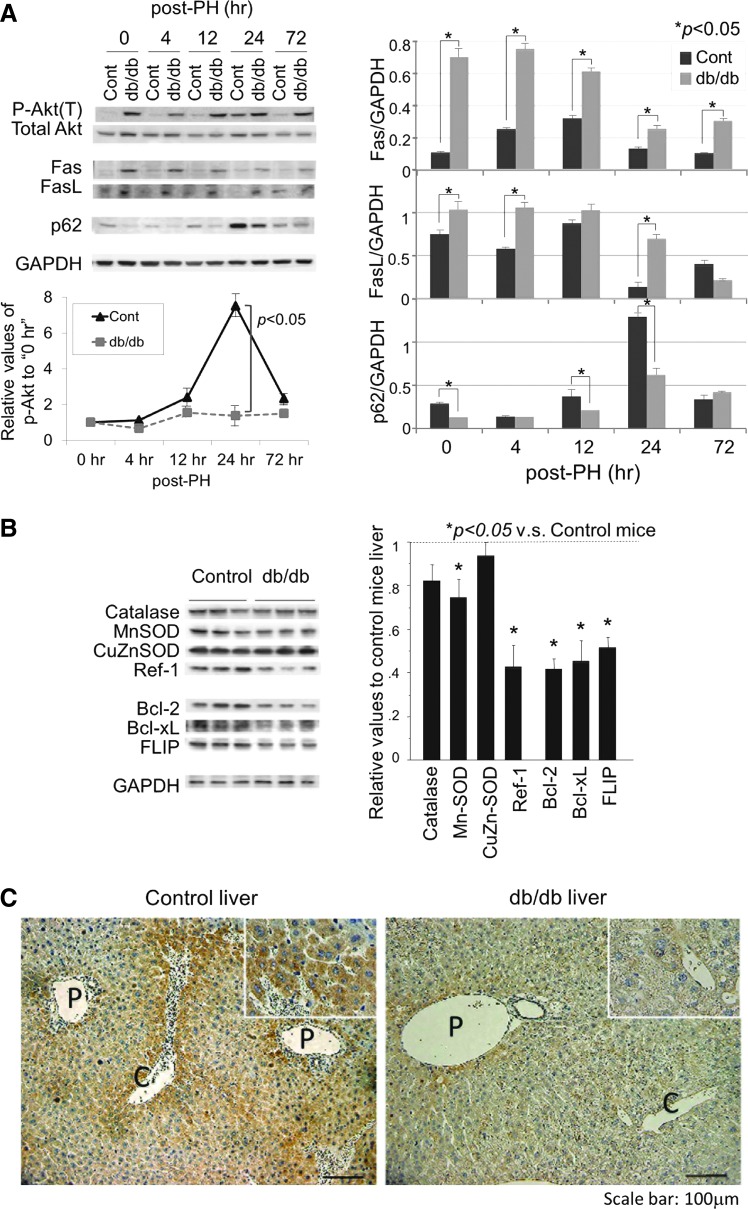
Both Fas and Fas-ligand (FasL) were expressed at higher levels in the steatotic liver before and after PH as compared with the control liver (Fig. 3A). Akt, mainly associated with cell survival, was expressed at equivalent levels in both livers, but was transiently phosphorylated after PH with a peak at 24 h in the control liver. Interestingly, in the steatotic liver, it was activated even before PH and there was no increase in activation after PH.
FIG. 3.
Analysis of signals associated with cell proliferation and survival, apoptosis, and OS in hepatocytes. (A) Protein expression of phospho-Akt, Akt, Fas, FasL, and p62/SQSTM1 was analyzed by Western blotting. (B) Anti-oxidant/anti-apoptotic proteins were analyzed in the liver of the control and db/db mice. Each blot represents at least three independent experiments. The intensity of each band was quantified by densitometry and is plotted on the right. The data are expressed as mean±SEM relative to the lean control values; p<0.05 was considered statistically significant (A, B). (C) Immunohistochemical study of the p62/SQSTM1 expression in mouse liver, demonstrating the reduced expression of p62/SQSTM1 in the db/db steatotic liver. (P: portal vein; C: central vein). FasL, Fas-ligand. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Antioxidant proteins, catalase, manganese superoxide dismutase (MnSOD), and redox factor-1 (Ref-1) were expressed at lower levels in the steatotic liver than in the control liver, and only copper zinc superoxide dismutase (CuZnSOD) was expressed at equivalent levels (Fig. 3B). Anti-apoptotic proteins such as Bcl-2/-xL and FILCE-like inhibitory protein (FLIP) were expressed at lower levels in the steatotic liver. Along with the hypo-responsiveness of Akt and the decreased levels of anti-apoptotic and antioxidant molecules, these harmful signals mediated by highly expressed FasL/Fas may play a central role in post-PH liver injury and delayed liver regeneration.
FasL/Fas signals induce cellular reactive oxygen species and death in steatotic hepatocytes
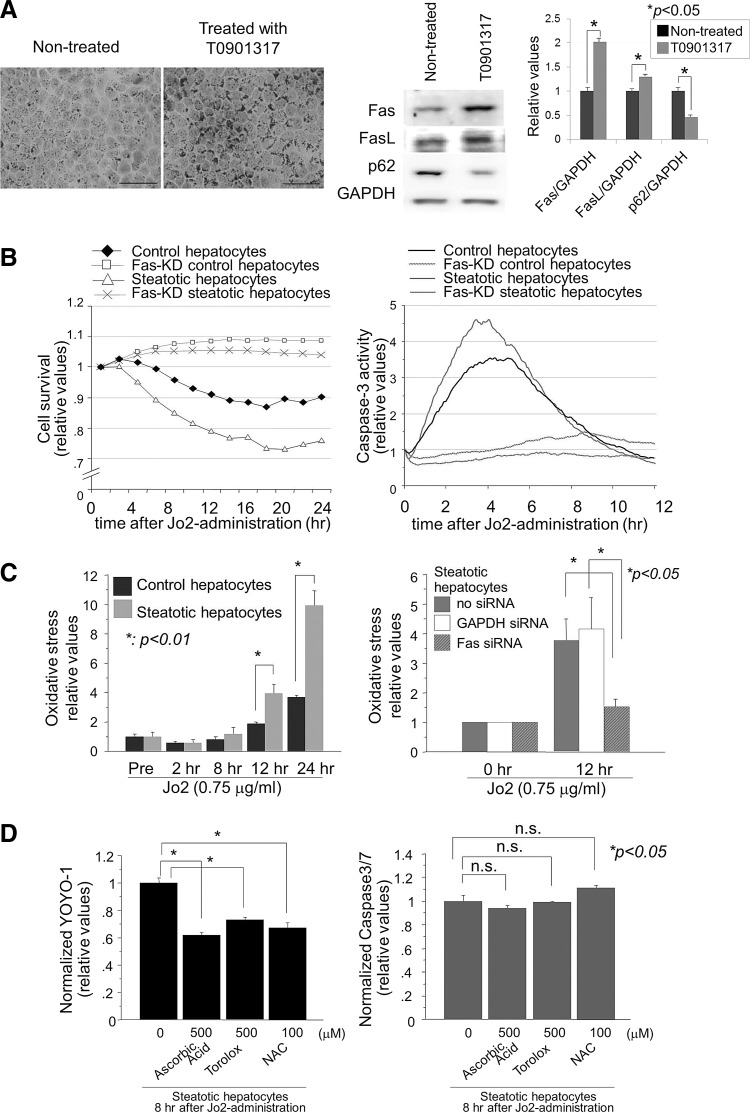
Next, we determined whether the induction of steatosis in hepatocytes leads to FasL/Fas overexpression and whether FasL stimuli induce cellular reactive oxygen species (ROS) and more cell death (apoptosis/necrosis) in steatotic hepatocytes. Treatment of AML12 liver cells with T0901317, an agonist for Liver X Receptor-α (4), induced a marked steatotic change in hepatocytes within 5 days (Fig. 4A). Similar to the in vivo observations, the steatotic hepatocytes showed overexpression of both FasL and Fas, but less expression of p62/SQSTM1.
FIG. 4.
Fas/FasL expression, ROS generation, and cell death (necrosis/apoptosis) in steatotic hepatocytes. (A) Steatosis was induced in AML12 liver cells by treatment with 1 μM T0901317 for 5 days. Oil Red O stain showed a marked and homogenous accumulation of lipid in the treated hepatocytes. Fas and FasL expression was upregulated in the T0901317-treated steatotic hepatocytes. Scale bar: 50 μm. Photographs are representative of three independent experiments. The intensities of each band are expressed relative to the intensities of the “Nontreated” band; p<0.05 was considered significant. (B) Cell survival and caspase-3 activity were monitored in the control and steatotic hepatocytes with or without Fas after anti-Fas antibody challenge (0.75 μg/ml of Jo2). (C) Anti-Fas-induced OS in the steatotic hepatocytes was evaluated using a redox-sensitive roGFP probe. For each group, the experiment was performed thrice and the data are expressed relative to values before anti-Fas treatment. (D) The effect of antioxidants on necrosis and apoptosis was examined in the steatotic hepatocytes. Antioxidants (ascorbic acid, Trolox, and NAC) suppressed anti-Fas-induced necrotic death but not apoptotic death in steatotic hepatocytes. Cell apoptosis and necrosis were determined using the fluorogenic probes Caspase-Glo 3/7 and YOYO-1, respectively. For each group, the experiment was performed thrice and the data are expressed relative to the values of the untreated cells. NAC, N-acetyl cysteine; ROS, reactive oxygen species.
Steatotic hepatocytes were more prone to die than nonsteatotic hepatocytes when challenged with exogenous FasL (Jo2 antibody) (Fig. 4B). Steatotic hepatocytes stimulated by FasL showed more cell death and caspase-3 activity as compared with nonsteatotic hepatocytes. Although cell death continued for 20–24 h after Jo2 administration, caspase-3 was transiently activated with a peak at 4 h. These facts indicate that FasL/Fas-mediated death signals induce hepatocyte death through caspase-3-mediated apoptosis and/or nonapoptotic cell death, and that steatotic hepatocytes are more susceptible than control hepatocytes to FasL/Fas-induced cell death.
To examine the involvement of ROS in Fas-induced cell death and to exclude neutrophil-derived ROS in post-PH liver injury, we next examined ROS generation in hepatocytes with and without steatosis (Fig. 4C). Steatotic hepatocytes expressing greater amounts of FasL and Fas molecules, as compared with nonsteatotic hepatocytes, clearly generated cellular ROS after stimulation of FasL. Hepatocytes in which Fas was depleted by small-interfering RNAs (siRNA), however, generated little cellular ROS, even when stimulated with Jo2, which confirmed that FasL/Fas signals induce more cellular ROS in steatotic hepatocytes.
To investigate the involvement of FasL/Fas-mediated cellular ROS in the death of steatotic cells, we studied the effects of antioxidants on cell death (apoptosis and necrosis) (Fig. 4D). Unexpectedly, FasL/Fas-induced necrotic cell death was significantly inhibited by pretreatment with the antioxidants ascorbic acid, Torolox and N-acetyl cysteine (NAC); whereas apoptotic cell death was not inhibited at all. These data indicate that FasL/Fas-induced cell death occurred through apoptosis and necrosis in a caspase-dependent manner and an ROS-dependent manner, respectively.
Reduced expression of p62/SQSTM1 in the steatotic liver and hepatocytes is responsible for increased FasL/Fas expression and OS
To examine the molecular mechanism underlying the increased FasL/Fas signaling and OS in the steatotic liver, we focused on p62/SQSTM1, a molecule associated with steatosis (23), whose expression was reduced only in the steatotic liver and hepatocytes (Figs. 3A, 3C, and 4A). It has been reported that p62/SQSTM1 is negatively associated with hepatic steatosis (23, 28) and that its reduced expression may impair the nuclear factor erythroid 2-related factor-2 (Nrf-2)-dependent cellular antioxidant system (16, 19).
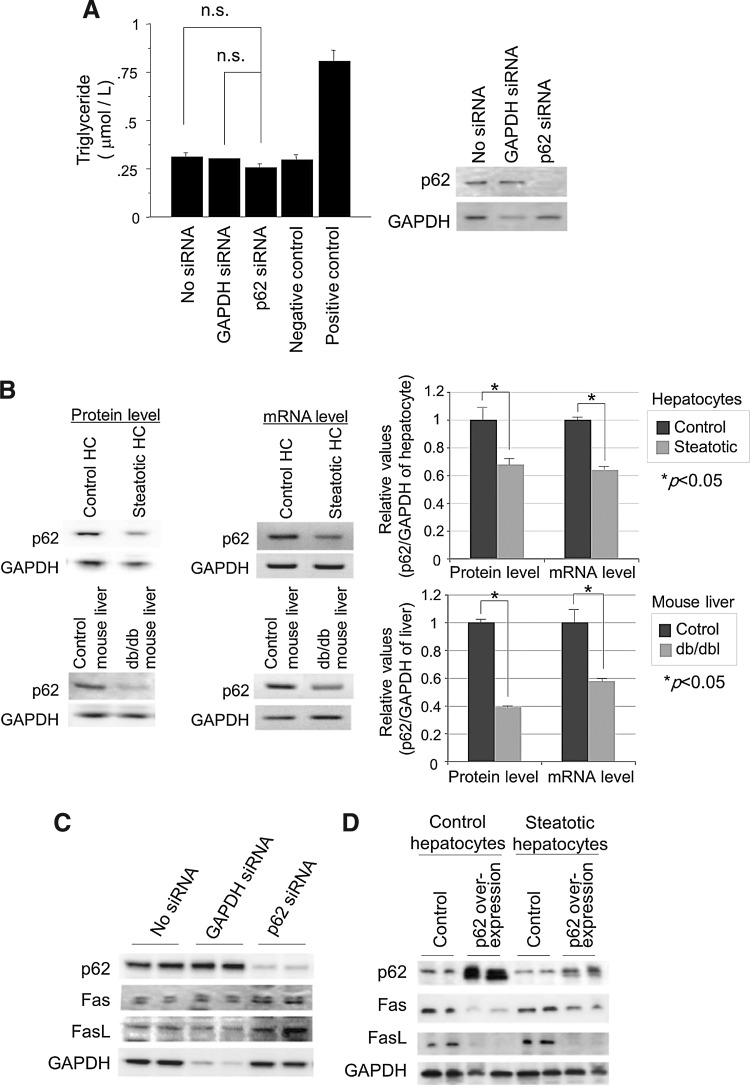
Acute reduction of p62/SQSTM1 by siRNA did not affect the cellular triglyceride content of hepatocytes (Fig. 5A), suggesting that p62/SQSTM1 does not primarily induce steatotic changes in hepatocytes and that its reduced expression occurs secondary to the steatotic changes in hepatocytes. In the steatotic liver, p62/SQSTM1 was expressed at much lower levels before PH, although it was induced transiently at 24 h post-PH in both the normal and the steatotic liver (Fig. 3A). The expression of p62/SQSTM1 mRNA and protein (Figs. 3A, 4A, and 5B) was downregulated in the steatotic liver and hepatocytes. Conversely, the expression of FasL and Fas was upregulated in the steatotic liver and hepatocytes (Figs. 3A and 4A). Interestingly, even nonsteatotic hepatocytes strongly expressed Fas and FasL proteins when depleted of p62/SQSTM1 (Fig. 5C). In contrast, induction of p62/SQSTM1 in normal and steatotic hepatocytes reduced FasL and Fas expression (Fig. 5D). It is interesting that the induction of p62/SQSTM1 was less efficient in steatotic hepatocytes than in nonsteatotic hepatocytes, resulting in an insufficient reduction of FasL/Fas molecules in steatotic hepatocytes (Fig. 5D). This suggests that p62/SQSTM1 negatively regulates these harmful molecules.
FIG. 5.
Cellular steatosis reduces the expression of p62/SQSTM1, and the expression of FasL and Fas is negatively regulated by p62/SQSTM1, regardless of steatosis. (A) Adipogenesis assay of p62/SQSTM1-ablated AML12 cells. Ablation of p62/SQSTM1 alone did not induce steatosis in AML12 liver cells. Untreated AML12 cells were used as a negative control, and T0901317-treated AML12 cells were used as a positive control. (B) mRNA and protein expression levels of p62/SQSTM1 are reduced in steatotic hepatocytes and steatotic liver. The intensity of each band was quantified and normalized to control hepatocytes by densitometry and is plotted on the right; p<0.05 was considered statistically significant. (C) Ablation of p62/SQSTM1 alone induced FasL and Fas expression in AML12 hepatocytes, even without steatosis. (D) Overexpression of p62/SQSTM1 in both nonsteatotic and steatotic hepatocytes led to a reduction of FasL and Fas. Each experiment was performed at least thrice independently, and representative data are shown.
p62/SQSTM1 is responsible for the antioxidant property of hepatocytes
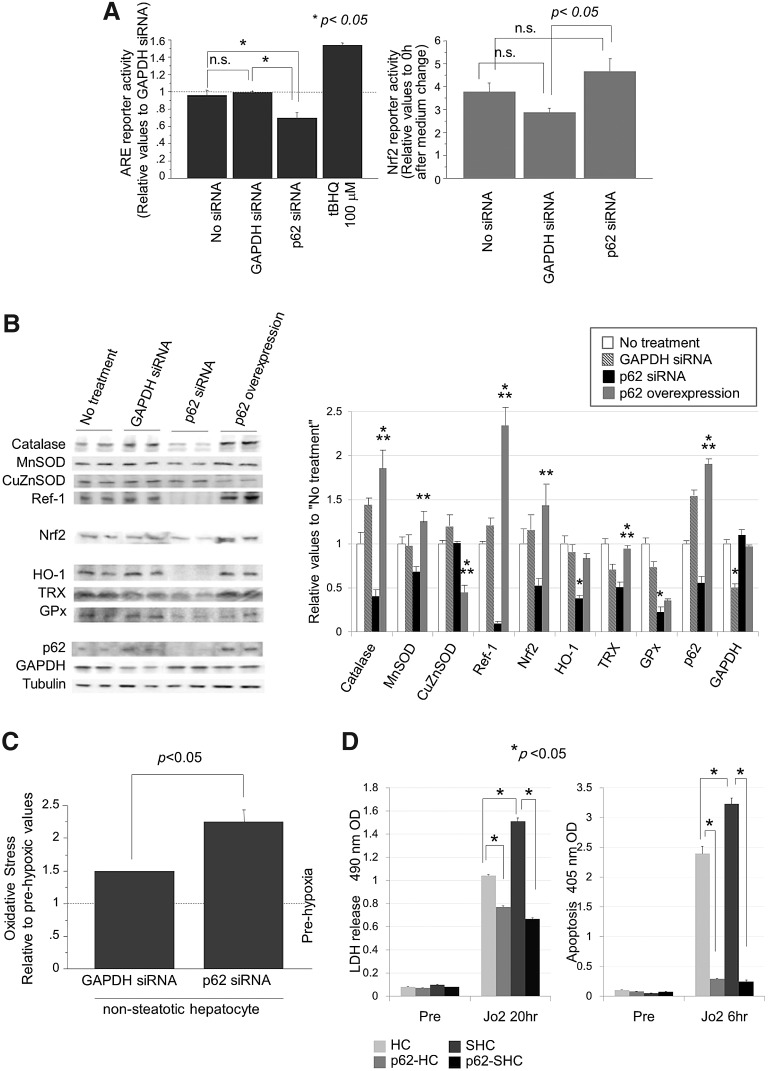
p62/SQSTM1 is known to positively regulate the DNA-binding activity of Nrf-2 by physical association with Kelch-like ECH-associated protein-1 (Keap-1), which binds directly to Nrf-2 and inactivates its transcriptional activity on anti-oxidant response elements (ARE) (16, 19). Therefore, we examined the regulatory role of p62/SQSTM1 in Nrf-2-mediated ARE-transcriptional activity in hepatocytes. As expected, reduction of p62/SQSTM1 expression by siRNA decreased ARE-transcription significantly, but did not decrease Nrf-2 reporter activity (Fig. 6A). Although the transcription activity of Nrf-2 was increased by p62/SQSTM1 depletion, possibly by a negative feedback mechanism, its protein expression was slightly decreased (Fig. 6B). These data suggest that free Keap-1, increased by the reduced expression of p62/SQSTM1, alternatively bound to Nrf-2, eventually leading to the suppression of ARE transcription.
FIG. 6.
Reduced p62/SQSTM1 leads to a decrease in cellular antioxidant properties by suppressing ARE activity. (A) Ablation of p62/SQSTM1-suppressed ARE reporter activity in AML12 cells (left panel), but not Nrf-2 reporter activity (right panel). ARE reporter activity is expressed relative to the ARE activity against GAPDH siRNA. tBHQ was used as a positive control of the ARE reporter probe. (B) Ablation of p62/SQSTM1 reduced the expression of antioxidant molecules, whereas its overexpression increased the levels of these molecules. Photographs are representative of three independent experiments. The intensity of each band was quantified, normalized against tubulin, and expressed relative to the “no treatment” band (*p<0.05 vs. GAPDH-siRNA; **p<0.05 vs. GAPDH-siRNA and vs. p62-siRNA). (C) A roGFP redox-sensitive probe showed that ablation of p62/SQSTM1 enhanced hypoxia/reoxygenation-induced ROS generation in AML12 liver cells (4-h hypoxia, 10-min reoxygenation). (D) Induction of p62/SQSTM1 suppressed Jo2-induced cell death (LDH release and apoptosis) in both control and steatotic hepatocytes (0.75 μg/ml of Jo2, anti-Fas antibody). In each group, the data are expressed as mean±SEM values relative to the corresponding prehypoxic cells; p<0.05 was considered significant. ARE, antioxidant response elements; LDH, lactate dehydrogenase; Nrf-2, nuclear factor erythroid 2-related factor-2; siRNA, small-interfering RNA; tBHQ, tert-butylhydroquinone.
The expression of a series of antioxidant proteins (catalase, MnSOD, Ref-1, heme oxygenase 1 [HO-1], thioredoxin [TRX], and glutathione peroxidase [GPx]) in hepatocytes was reduced by depletion of p62/SQSTM1 (Fig. 6B), probably through the suppression of ARE activity (Fig. 6A). Among these proteins, catalase, MnSOD, Ref-1, and Nrf-2 were significantly increased by overexpression of p62/SQSTM1. These data clearly indicate that p62/SQSTM1 is responsible for the expression of antioxidants both in cultured hepatocytes and in the liver. Overall, p62/SQSTM1 is critically involved in the increased FasL/Fas expression and the decreased antioxidant properties of steatotic hepatocytes, with its expression being suppressed by cellular steatosis.
Since hypoxia/reoxygenation (H/R) of hepatocytes induces cellular ROS and injury, we examined whether H/R-induced ROS is exacerbated in hepatocytes by previous ablation of p62/SQSTM1 (Fig. 6C). Four hours of hypoxia followed by reoxygenation induced cellular ROS in control hepatocytes, and this was significantly exacerbated in hepatocytes without p62/SQSTM1. These data confirm the general role of p62/SQSTM1 in protection from various kinds of OS in hepatocytes. Furthermore, the induction of p62/SQSTM1 in nonsteatotic and steatotic hepatocytes equally and sufficiently suppressed Jo2-induced necrotic and apoptotic cell death, although Jo2 induced cell death more in steatotic hepatocytes than in nonsteatotic hepatocytes (Fig. 6D). These data demonstrate the antioxidant and cytoprotective capacities (against both necrosis and apoptosis) of p62/SQSTM1 in hepatocytes with or without steatosis.
Induction of p62/SQSTM1 in the db/db steatotic liver reduced liver injury after PH and improved liver regeneration
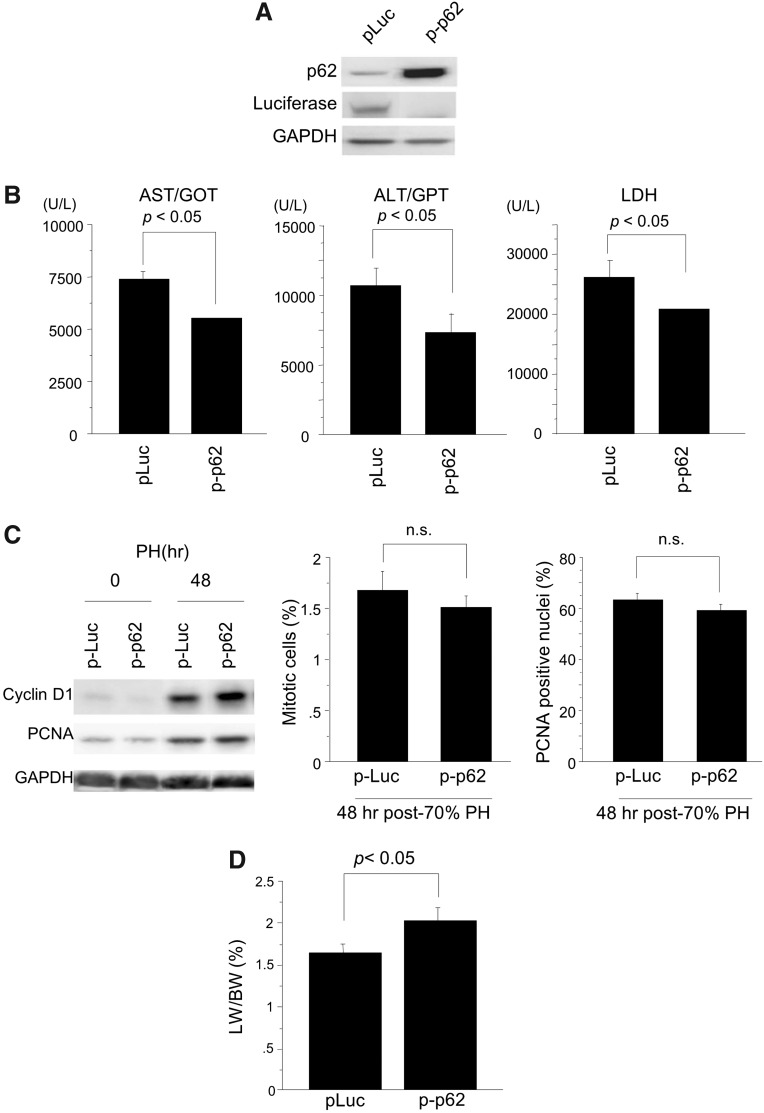
In order to examine the in vivo effect of p62/SQSTM1, we determined whether or not re-expression of p62/SQSTM1 in the steatotic liver of db/db mice improves liver regeneration after PH by reducing injury. PH was performed at 36 h after a hydrodynamic injection of the p62/SQSTM1 gene. p62/SQSTM1 protein was robustly expressed in the liver of db/db mice at the time of liver resection (Fig. 7A). Induction of p62/SQSTM1 in the steatotic liver clearly protected it from PH-induced injury (Fig. 7B), although some liver injury remained. The expression levels of PCNA and cyclinD1 proteins peaked at 48 h post-PH similarly in both the control and p62/SQSTM1-expressing liver (Fig. 7C). In addition, there was no significant difference in the mitotic index and the counts of PCNA-positive nuclei in hepatocytes between the two livers. Induction of p62/SQSTM1 gene did not affect post-PH cell proliferation in the db/db mouse liver.
FIG. 7.
Hepatic transduction of the p62/SQSTM1 gene markedly reduces post-PH liver injury and improves regeneration of the steatotic liver of db/db mice. Post-PH liver injury and regeneration were evaluated in db/db mouse liver transduced with the p62/SQSTM1 gene. (A) Western blot analysis shows the robust induction of p62/SQSTM1 protein in the liver of db/db mice by hydrodynamic injection of the p62/SQSTM1 gene (p-p62). Plasmid DNA of luciferase gene was injected as a control (p-Luc). (B) Serum levels of AST, ALT, and LDH were reduced at 12 h post-PH in the p62/SQSTM1-transduced steatotic liver, as compared with the luciferase-transduced control liver. (C) Expression of PCNA and cyclinD1 protein peaked at 48 h post-PH similarly in both the control and p62/SQSTM1-expressing liver; in addition, there was no significant difference in the mitotic index and the counts of PCNA-positive hepatocytes between the two types of liver. (D) Liver regeneration recovered partially, but not completely, after transduction of p62/SQSTM1 into the steatotic liver. At least five mice were used for each experiment. Data are expressed as mean±SEM; p<0.05 was considered statistically significant. ALT, alanine aminotransferase; AST, aspartate aminotransferase.
By re-introducing p62/SQSTM1 into the db/db mouse liver and clearly reducing liver injury, post-PH liver regeneration was partially but consistently improved at 72 h post-PH (Fig. 7D).
A different model of liver steatosis (high-fat-diet mice) also shows reduced p62/SQSTM1 in liver, leading to increased FasL/Fas and decreased antioxidant/anti-apoptotic molecules
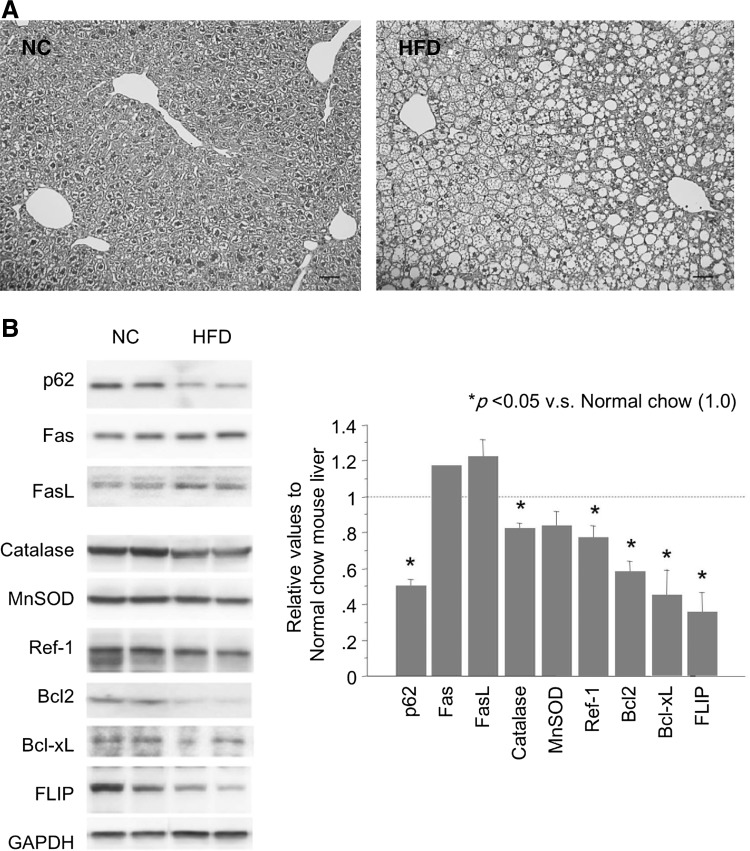
In order to determine whether or not the reduction of p62/SQSTM1 and its effects are limited to the liver in db/db mice, we studied a different model of liver steatosis (high-fat-diet [HFD] model). In this model, mice were fed on chow containing 35% fat by weight from 4 to 16 weeks of age, which made hepatocytes sufficiently steatotic (Fig. 8A). As shown in Figure 8B, the protein level of p62/SQSTM1 was reduced in the steatotic liver tissue. FasL and Fas were increased, and antioxidant molecules such as catalase, MnSOD, and Ref-1 and anti-apoptotic molecules such as Bcl-2, Bcl-xL, and FLIP were reduced. These changes were similar to those observed in the db/db mouse liver. Thus, reduction of p62/SQSTM1 and p62/SQSTM1-dependent regulation of these pathways/signals are not confined to the db/db mouse model, but occur in the steatotic liver of various origins.
FIG. 8.
HFD induces liver steatosis and p62/SQSTM1 reduction in mouse liver, leading to an increase in FasL/Fas and a decrease in antioxidant/-apoptotic molecules. (A) HFD feeding made hepatocytes sufficiently steatotic in the liver tissue (H&E staining). Photographs are representative of three independent experiments. NC: Normal chow. (B) Western blot analysis showed reduced expression of p62/SQSTM1, increased FasL/Fas, and decreased antioxidant (catalase, MnSOD, and Ref-1) and anti-apoptotic (Bcl-2, Bcl-xL, and FLIP) molecules. Each blot represents at least three independent experiments. The intensity of each band was quantified, normalized against GAPDH, and expressed relative to the “NC mouse liver” band. FLIP, FILCE-like inhibitory protein; HFD, high fat diet; MnSOD, manganese superoxide dismutase; Ref-1, redox factor-1.
Discussion
Fas expression and apoptosis are well-recognized features of human NASH (11) and other steatotic liver diseases (6, 38), suggesting the potential role of Fas in injury of the steatotic liver. However, the precise mechanism of injury of the steatotic liver has not been thoroughly elucidated. In this study, using a mouse model of hepatectomy, we first demonstrated that lack of p62/SQSTM1 plays a pivotal role in post-PH acute injury of the steatotic liver (Fig. 9). Initial regeneration of the steatotic liver after PH was delayed not by a suppressed mitotic response but by an immediate post-PH liver injury consisting of Fas/caspase-mediated apoptosis and Fas/OS-dependent necrosis.
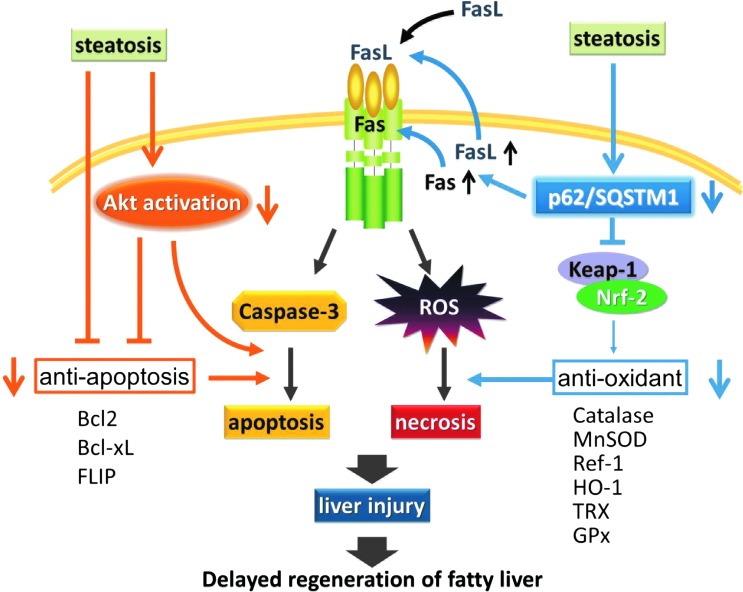
FIG. 9.
Schematic showing the pivotal role of p62/SQSTM1 in injury of the steatotic liver. Steatosis-associated reduction of p62/SQSTM1 induces FasL/Fas in the steatotic liver. Along with exogenous FasL, this directly causes post-PH necrotic and apoptotic acute liver injury in, respectively, a redox-dependent and redox-independent manner. Reduction of p62/SQSTM1 reduces Keap-1/Nrf-2 binding, which suppresses the expression of antioxidant molecules (catalase, MnSOD, Ref-1, HO-1, TRX, and GPx) and, therefore, makes the liver susceptible to OS. Furthermore, the hypo-responsiveness of Akt enhances necrotic and apoptotic injury, along with the reduced expression of antioxidant molecules and anti-apoptotic molecules (Bcl-2, Bcl-xL, and FLIP), respectively. These mechanisms may collectively underlie steatosis-associated liver injury in the mouse. GPx, glutathione peroxidase; HO-1, heme oxygenase 1; Keap-1, Kelch-like ECH-associated protein 1; TRX, thioredoxin. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
In terms of the mechanism underlying acute injury of the steatotic liver, we demonstrated that steatosis-induced reduction of p62/SQSTM1, by enhancing the cellular FasL/Fas pathway, causes post-PH necrotic and apoptotic acute liver injury in a redox-dependent and redox-independent manner, respectively. Of particular note, reduction of p62/SQSTM1 lowered the expression of antioxidant molecules (catalase, MnSOD, Ref-1, HO-1, TRX, and GPx) by decreasing Keap-1/Nrf-2 binding (i.e., ARE activity), making the liver susceptible to OS. In addition, we demonstrated that OS induced by FasL/Fas in the steatotic liver mainly caused the necrotic cell death of hepatocytes. Just as important was the finding that the hypo-responsiveness of Akt, a pro-survival and antioxidant molecule, enhanced/promoted OS-induced necrotic injury and caspase-mediated apoptotic liver injury (14, 30), along with reduced expression of antioxidant molecules and anti-apoptotic molecules, respectively.
In this study, steatotic changes in the liver or hepatocytes reduced the expression of p62/SQSTM1, which may have induced mild but continuous liver injury by causing overexpression of FasL/Fas even under basal conditions. Serum levels of AST, ALT, and LDH without PH showed a slight but significant elevation of these enzymes [AST (IU/L): 13.0±0.0 and 70.0±24.0; ALT (IU/L): 35.5±5.0 and 54.5±5.0; LDH (IU/L): 349.5±12.0 and 635.5±132.2, in control and db/db mice, respectively; p<0.05] (Fig. 2A), indicating continuous mild liver injury even without PH. Increased expression and activation of Akt was also observed without PH, which again may have been induced by continuous liver injury under basal conditions. Surgical stress such as PH might exacerbate the liver injury or trigger marked liver injury, because surgery induces a strong inflammatory reaction and OS. In particular, the steatotic liver may be more vulnerable to such insults, because Fas-receptor expression is elevated and antioxidative capacity is reduced by p62-associated Nrf-2/ARE inactivation. These mechanisms may underlie steatosis-associated acute liver injury in the mouse. The compensatory regeneration (mitotic response) was not clearly observed under basal conditions (Fig. 1A); however, phosphorylation of STAT3 (Supplementary Fig. S1; Supplementary Data are available online at www.liebertpub.com/ars) and Akt (Fig. 3A) was observed even without PH, as previously reported (24, 37). This may explain the cellular response for proliferation (p-STAT3) and survival (p-Akt) against mild and continuous hepatocyte injury. After PH, acute liver injury may be enhanced by the PH-associated rapid increase in exogenous FasL, and the ensuing apoptosis and OS-mediated necrosis in the steatotic liver. Hepatic OS might also be induced/enhanced by ROS generated from mitochondria and the NOX system after PH more in the steatotic liver than in the normal liver (3, 13).
Although expression of p62/SQSTM1 was markedly decreased in the steatotic liver and hepatocytes (Figs. 3A, 3C, 4A, and 5B), depletion of p62/SQSTM1 did not induce steatotic changes in hepatocytes (Fig. 5A), suggesting that hepatic steatosis is the primary cause of the reduced expression of p62/SQSTM1. In addition, the db/db mice showed moderate hyperglycemia in addition to steatotic liver, raising the question of whether hyperglycemia might cause the reduction of p62/SQSTM1. As shown by the in vitro assay in Figure 4, however, chemically induced steatosis in hepatocytes with a normal glucose level consistently decreased p62/SQSTM1 and increased FasL/Fas. In addition, in an alternative in vivo steatotic liver model, in which steatotic liver was induced by high-fat feeding, the p62/SQSTM1 level in the liver was similarly reduced (Fig. 8). Thus, we conclude that steatosis, and not hyperglycemia, mainly leads to the reduction of p62/SQSTM1 in hepatocytes.
The exact mechanism underlying the reduced expression of p62/SQSTM1 and overexpression of FasL/Fas was not elucidated in this study; however, a study on p62/SQSTM1 and its anti-apoptotic capacity showed that p62/SQSTM1 exerts anti-apoptotic capacity by inhibiting BID and caspase-3 activities (20). Generally, it seems that p62/SQSTM1, when it is present, serves as a cell-protection molecule by providing an anti-apoptotic function. In this study, when p62/SQSTM1 was not present, the expression of both assaultive FasL and Fas genes/proteins was upregulated. p62/SQSTM1, when present, may also “positively” suppress FasL/Fas expression at the transcriptional level as a “gatekeeper molecule.” Furthermore, the negative regulation of FasL/Fas expression by p62/SQSTM1 was observed in hepatocytes without steatosis (Fig. 5C, D), suggesting that p62/SQSTM1 is a key molecule in the protection of hepatocytes and other types of cells which potentially express the Fas molecule.
Deterioration of the antioxidant defense mechanism in the steatotic liver may have enhanced OS-induced acute liver injury after PH. If this is the case, then the steatotic liver with reduced levels of p62/SQSTM1 may be very vulnerable to OS, even in a static condition. p62/SQSTM1 itself is involved in the regulation of cellular OS through a Keap-1/Nrf-2-dependent antioxidant mechanism involving glutathione S-transferase, cytochrome p450, HO-1, SOD, catalase, or p62/STSQM1 (8, 16, 19, 23, 28, 31, 40). Indeed, in this study, these antioxidant molecules were found to be downregulated in the steatotic liver (Fig. 3B) and the ablation of p62/SQSTM1 was found to reduce both ARE reporter activity and their expression, even in nonsteatotic hepatocytes (Fig. 6A, B). These facts strongly suggest that p62/SQSTM1 confers antioxidant properties on hepatocytes through the Keap-1/Nrf-2 system. Therefore, a decrease in the p62/SQSTM1 level of hepatocytes, whatever the cause, might make the liver and hepatocytes vulnerable to OS.
Our previous paper (24) analyzed delayed regeneration of steatotic liver from the aspect of cell cycle progression and proliferation. It revealed that hepatocyte proliferation was mildly reduced by the suppressed expression of Wee1/Myt1 at 1–3 days post-PH, but that it continued even after 3 days post-PH, until the lost liver mass was made up. In the control liver, however, hepatocyte proliferation ceased after 3 days post-PH. These observations indicate that post-PH hepatocytes in the db/db mouse liver can proliferate until 7 days post-PH, despite the severe injury early after PH. We consider that transient but severe liver injury early after PH in the steatotic liver, along with somewhat slow cell proliferation, causes the delayed liver regeneration but that hepatocyte proliferation continues until the lost liver mass is made up (3–7 days post-PH). In this study, re-expression of p62/SQSTM1 in the steatotic liver did not affect cell proliferation after PH (Fig. 7C).
In an acute stress model such as PH, the increase in FasL/Fas caused by reduction of p62/SQSTM1 seems to be highly relevant in inducing acute liver injury, because the increased FasL/Fas signal induced post-PH liver injury in a positive manner (caspase-dependent apoptosis and OS-dependent necrosis). By contrast, the reduced Nrf-2/Keap-1 signal accelerated the injurious signals described earlier by reducing the expression of antioxidant molecules. Previous reports have described the pathological relevance of p62/SQSTM1 in acute and chronic situations in humans, such as neuronal, musculo-skeletal, and infectious diseases (5, 12, 27, 34). Many studies focused on analyses of p62/SQSTM1 with regard to autophagy, because p62/SQSTM1 has been known to be a good in vivo marker of autophagy. However, one report indicated the relevance of p62/SQSTM1 and the FasL/Fas pathway in the acute lung injury that occurs in acute respiratory distress syndrome (20). Thus, p62/SQSTM1 might also play an important role in human diseases other than the steatotic liver.
This study is the first which shows the critical role of p62/SQSTM1 in redox-dependent steatotic liver injury and disturbed early regeneration after PH. The lack of p62/SQSTM1 in some pathological conditions such as steatosis induces acute liver injury. Further studies are required to thoroughly elucidate the mechanism of liver protection and injury, but these data clearly demonstrate the critical role of p62/SQSTM1 in the liver antioxidant system and provide important clues toward the development of new therapies for various pathological liver conditions.
Materials and Methods
Animal experiments
Male homozygous db/db mice (38–42 g body weight, 9–10 weeks old) and lean littermate controls (20–25 g body weight, 9–10 weeks old) were obtained from CLEA Japan and used for the 2/3 PH experiment. In the HFD mouse model, mice (C57BL6/J, male) were fed on chow containing 35% fat by weight from 4 to 16 weeks of age (#D12492; Research Diets, Inc.). The mice were fasted overnight before the experiments and were anesthetized with 1.5%–2.0% isoflurane (Forane©; Abbott). The percentage of the whole liver constituted by each lobe was similar, and surgical resection of the middle and left lobes resulted in 2/3 PH in both mice. Sudan III stained lipid droplets in more than 90% of the hepatocytes in the liver of db/db mice but very few droplets in the hepatocytes of the control mice. After laparotomy, the left and median liver lobes were surgically resected. The mice were sacrificed for the collection of liver specimens at the time points indicated before or after hepatectomy, and the liver/body weight ratios were calculated to estimate the recovery of liver mass. The animals were maintained under standard conditions and treated according to the Guidelines for the Care and Use of Laboratory Animals of Hokkaido University.
Histological analysis
To visualize lipid accumulation in the liver and hepatocytes, frozen sections of formalin-fixed liver tissue and hepatocytes were stained with Sudan III (Fig. 1A) and Oil Red O dye (Fig. 4A), respectively. To evaluate the proliferation of hepatocytes after PH, PCNA-positive and mitotic hepatocytes were histologically examined in liver tissues at 36, 48, and 72 h post-PH. To assess the expression of p62/SQSTM1 in mouse liver, immunohistochemical analysis of p62/SQSTM1 was performed in the untreated liver tissues. These tissues were fixed in 10% buffered formalin, paraffin embedded, and subjected to hematoxylin and eosin staining and immunohistochemical staining with anti-PCNA and anti-p62/SQSTM1. At least 500 hepatocytes were counted for mitotic index or PCNA positivity at least in triplicate in different sections in each group.
Western blot analysis
Whole-liver protein extracts (30 μg) were separated by 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred to a polyvinylidene difluoride membrane. The following primary antibodies were used: Akt/phospho-Akt, signal STAT3/phospho-STAT3, cyclinD1, p62/SQSTM1 (Cell Signaling Technology), Fas, Ref-1, CuZnSOD, Bcl-2/xL, Nrf-2, HO-1 (Santa Cruz Biotechnology), FasL, GPx (Abcam), Catalase, FLIP (EMD Biosciences), MnSOD, and TRX (BD Transduction Laboratories).
In vivo imaging of mouse liver redox states (OS) and caspase-3 activity (apoptosis)
We developed a recombinant adenovirus encoding roGFP, which enables real-time visualization of the oxidation/reduction potentials of various cells in vitro (7). Three days before each experiment, AdroGFP was administered intravenously via the tail vein in a volume of 100 μl (5×107 pfu/mouse). For in vivo liver imaging, the liver was exposed under anesthesia to enable a CCD camera to record images directly using a Photon Imager (Biospace Co.). Fluorescence intensities at 530 nm from excitation at 480 nm were measured and plotted reciprocally [1/(em. 530 at ex. 480 nm)]. This enabled the signal to increase with oxidation and to decrease with reduction and was used as an index of in vivo redox states.
An optical probe termed pcFirefly luciferase (pcFluc)-DEVD was used to detect caspase-3 activity in real time (18, 29). We also developed a recombinant adenovirus encoding pcFluc-DEVD and used it to infect mice (5×107 pfu/mouse). D-Luciferin, a luciferase substrate, was injected intraperitoneally at 1 mg in 100 μl of PBS. In vivo imaging of the mouse liver was performed using an in vivo imager for 5 min at 5–10 min after the injection.
Biochemical measurement of liver injury (apoptosis/necrosis)
For evaluation of apoptotic cell death, an ELISA kit (Cell Death Detection ELISAPLUS; Roche) was used according to the manufacturer's instructions. Biochemical analyses, such as serum AST, ALT, and LDH levels, as indices of liver injury, were performed at the time points indicated before and after PH.
In vitro and in vivo gene silencing with RNA interference and gene transfer with cDNA
For in vitro and in vivo experiments, siRNAs for mouse p62/SQSTM1 (sense 5′-GGAACUCGCUAUAAGUGCATT-3′, antisense 5′-UGCACUUAUAGCGAGUUCCCA-3′) and GAPDH used as the control were purchased from Ambion, Inc., and cDNA for mouse p62/SQSTM1 was purchased from Origene, Inc. Luciferase cDNA was used as a control. For the in vitro experiments, transfection of siRNAs and cDNA into AML12 liver cells was accomplished using Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions. p62/SQSTM1 and GAPDH expression was evaluated by polymerase chain reaction (PCR) and Western blot analyses. In the in vivo experiments, hydrodynamic injections were used to introduce the DNA into mouse liver. Plasmid DNA of luciferase (pLuc as a control) or p62/SQSTM1 gene (p-p62) was injected rapidly into the tail veins of the db/db mice (10 μg in 2 ml of saline). The livers were harvested at 48 h after injection, and the luciferase activity and mRNA and protein levels of p62/SQSTM1 were measured to detect expression. The supernatants of liver tissue homogenates were examined for luciferase activity by a Luciferase Assay Systems kit (Promega).
Reverse transcription-PCR assay
First-strand cDNA synthesis used 5 μg of total RNA, Superscript III reverse transcriptase and oligo (dT) 20 primers (Invitrogen), according to the manufacturer's instructions. The cDNA was amplified by PCR with specific primers for mouse p62/SQSTM1 (225 bp): sense 5′-GATGTGGAACATGGAGGGAAGAG-3′, antisense 5′-AGTCATCGTCTCCTCCTGAGCA-3′. PCR was performed by 30–25 cycles of denaturation at 94°C for 30 s, annealing at 55°C for 30 s, and extension at 72°C for 30 s.
Reporter probes for ARE/Nrf-2 transcriptional activation
The ARE-luciferase reporter probe was constructed from the ARE sequences of human NQO1 in the pGL4.17 firefly luciferase reporter vector (Promega) (26). The secreting type of metridia luciferase reporter probe was used for Nrf-2 transcriptional activity assay. The promoter region of nrf2 (21, 39) was isolated by PCR amplification from genomic DNA of human gastric cells and ligated into pMetLuc2-Reporter (Clontech). Next, the CMV promoter-driven Renilla luciferase gene from pGL4.75 was introduced into this reporter plasmid for normalization of the reporter gene assay. After 48 h of incubation, the cell lysate or the culture medium was subjected to ARE-luciferase reporter assay (Dual-luciferase reporter assay system; Promega) or Nrf-2-metridia luciferase reporter assay (Ready-To-Glow secreted luciferase reporter assay; Clontech), respectively.
Monitoring of viable, apoptotic, and necrotic cells
Cells at 70%–80% confluence were plated in a 24-well plate. Growth curves were determined by plating the cells in the IncuCyte system (Essen Instruments), which enables the automated noninvasive monitoring of live cells in culture. Cell apoptosis and necrosis were determined using Caspase-Glo 3/7 (Essen BioScience) and YOYO-1 (Molecular Probe), respectively.
Evaluation of cellular redox states in vitro
The redox-sensitive roGFP was adenovirally transduced into AML12 cells (5 moi) at 48 h before the experiment. The cellular redox potentials were imaged using fluorescence microscopy with an emission wavelength of 530 nm and an excitation wavelength of 400/480 nm. Fluorescence intensities resulting from excitation at 400/480 nm were quantified using BZ-analyzer software (Keyence Corp.). At least 20 cells were measured in each experiment, and the ratio ex 400/480 nm was used as a cellular redox index.
Statistical analysis
All results were expressed as means±standard error of the mean (SEM). Data were compared by Fisher's test, and p-values of less than 0.05 were considered statistically significant.
Supplementary Material
Abbreviations Used
- ALT
alanine aminotransferase
- ARE
antioxidant response elements
- AST
aspartate aminotransferase
- ATP
adenosine triphosphate
- CuZnSOD
copper zinc superoxide dismutase
- FasL
Fas-ligand
- FLIP
FILCE-like inhibitory protein
- GPx
glutathione peroxidase
- H&E
hematoxylin and eosin
- HFD
high fat diet
- HO-1
heme oxygenase 1
- Keap-1
Kelch-like ECH-associated protein 1
- LDH
lactate dehydrogenase
- MnSOD
manganese superoxide dismutase
- NAC
N-acetyl cysteine
- NASH
nonalcoholic steatohepatitis
- Nrf-2
nuclear factor erythroid 2-related factor-2
- OS
oxidative stress
- p62/SQSTM1
p62/sequestosome1
- pcFluc
pc firefly luciferase
- PCNA
proliferating cell nuclear antigen
- PH
partial hepatectomy
- PCR
polymerase chain reaction
- Ref-1
redox factor-1
- roGFP
reduction-oxidation–sensitive green fluorescent protein
- ROS
reactive oxygen species
- SEM
standard error of the mean
- siRNA(s)
small-interfering RNA(s)
- STAT3
signal transducer and activator of transcription 3
- TRX
thioredoxin
Acknowledgments
This work was supported by Grants in Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (No. 23659631, No. 23249066, and No. 20249060 to M.O.), a JSPS Grant for Young Scientists (No. 10J06373 to S.H.), and the heartfelt donation from Mr. & Mrs. Fujikawa (to M.O.). The authors are grateful to Y. Yasuzaki for her excellent technical support.
Author Disclosure Statement
The authors declare that no conflicts of interest exist.
References
- 1.Aoyama T, Ikejima K, Kon K, Okumura K, Arai K, and Watanabe S. Pioglitazone promotes survival and prevents hepatic regeneration failure after partial hepatectomy in obese and diabetic KK-A(y) mice. Hepatology 49: 1636–1644, 2009 [DOI] [PubMed] [Google Scholar]
- 2.Caraceni P, Domenicali M, Vendemiale G, Grattagliano I, Pertosa A, Nardo B, Morselli-Labate AM, Trevisani F, Palasciano G, Altomare E, and Bernardi M. The reduced tolerance of rat steatotic liver to ischemia reperfusion is associated with mitochondrial oxidative injury. J Surg Res 124: 160–168, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Cardoso AR, Kakimoto PA, and Kowaltowski AJ. Diet-sensitive sources of reactive oxygen species in liver mitochondria: role of very long chain acyl-CoA dehydrogenases. PLoS One 8: e77088, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cha JY. and Repa JJ. The liver X receptor (LXR) and hepatic lipogenesis. The carbohydrate-response element-binding protein is a target gene of LXR. J Biol Chem 282: 743–751, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Deretic V. Autophagy in infection. Curr Opin Cell Biol 22: 252–262, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Donohue TM, Jr., Clemens DL, Galli A, Crabb D, Nieto N, Kato J, and Barve SS. Use of cultured cells in assessing ethanol toxicity and ethanol-related metabolism. Alcohol Clin Exp Res 25: 87S–93S, 2001 [DOI] [PubMed] [Google Scholar]
- 7.Dooley CT, Dore TM, Hanson GT, Jackson WC, Remington SJ, and Tsien RY. Imaging dynamic redox changes in mammalian cells with green fluorescent protein indicators. J Biol Chem 279: 22284–22293, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Faraonio R, Vergara P, Di Marzo D, Pierantoni MG, Napolitano M, Russo T, and Cimino F. p53 suppresses the Nrf2-dependent transcription of antioxidant response genes. J Biol Chem 281: 39776–39784, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Fausto N. Liver regeneration. J Hepatol 32: 19–31, 2000 [DOI] [PubMed] [Google Scholar]
- 10.Fausto N. Liver regeneration: from laboratory to clinic. Liver Transpl 7: 835–844, 2001 [DOI] [PubMed] [Google Scholar]
- 11.Feldstein AE, Canbay A, Angulo P, Taniai M, Burgart LJ, Lindor KD, and Gores GJ. Hepatocyte apoptosis and fas expression are prominent features of human nonalcoholic steatohepatitis. Gastroenterology 125: 437–443, 2003 [DOI] [PubMed] [Google Scholar]
- 12.Garner TP, Long J, Layfield R, and Searle MS. Impact of p62/SQSTM1 UBA domain mutations linked to Paget's disease of bone on ubiquitin recognition. Biochemistry 50: 4665–4674, 2011 [DOI] [PubMed] [Google Scholar]
- 13.Guichard C, Moreau R, Pessayre D, Epperson TK, and Krause KH. NOX family NADPH oxidases in liver and in pancreatic islets: a role in the metabolic syndrome and diabetes? Biochem Soc Trans 36: 920–929, 2008 [DOI] [PubMed] [Google Scholar]
- 14.Haga S, Morita N, Irani K, Fujiyoshi M, Ogino T, Ozawa T, and Ozaki M. p66(Shc) has a pivotal function in impaired liver regeneration in aged mice by a redox-dependent mechanism. Lab Invest 90: 1718–1726, 2010 [DOI] [PubMed] [Google Scholar]
- 15.Haga S, Remington SJ, Morita N, Terui K, and Ozaki M. Hepatic ischemia induced immediate oxidative stress after reperfusion and determined the severity of the reperfusion-induced damage. Antioxid Redox Signal 11: 2563–2572, 2009 [DOI] [PubMed] [Google Scholar]
- 16.Jain A, Lamark T, Sjøttem E, Larsen KB, Awuh JA, Øvervatn A, McMahon M, Hayes JD, and Johansen T. p62/SQSTM1 is a target gene for transcription factor NRF2 and creates a positive feedback loop by inducing antioxidant response element-driven gene transcription. J Biol Chem 285: 22576–22591, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kannan K. and Jain SK. Oxidative stress and apoptosis. Pathophysiology 7: 153–163, 2000 [DOI] [PubMed] [Google Scholar]
- 18.Kanno A, Yamanaka Y, Hirano H, Umezawa Y, and Ozawa T. Cyclic luciferase for real-time sensing of caspase-3 activities in living mammals. Angew Chem Int Ed Engl 46: 7595–7599, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Komatsu M, Kurokawa H, Waguri S, Taguchi K, Kobayashi A, Ichimura Y, Sou YS, Ueno I, Sakamoto A, Tong KI, Kim M, Nishito Y, Iemura S, Natsume T, Ueno T, Kominami E, Motohashi H, Tanaka K, and Yamamoto M. The selective autophagy substrate p62 activates the stress responsive transcription factor Nrf2 through inactivation of Keap1. Nat Cell Biol 12: 213–223, 2010 [DOI] [PubMed] [Google Scholar]
- 20.Liang X, Wei SQ, Lee SJ, Fung JK, Zhang M, Tanaka A, Choi AM, and Jin Y. p62 sequestosome 1/light chain 3b complex confers cytoprotection on lung epithelial cells after hyperoxia. Am J Respir Cell Mol Biol 48: 489–496, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marzec JM, Christie JD, Reddy SP, Jedlicka AE, Vuong H, Lanken PN, Aplenc R, Yamamoto T, Yamamoto M, Cho HY, and Kleeberger SR. Functional polymorphisms in the transcription factor NRF2 in humans increase the risk of acute lung injury. FASEB J 21: 2237–2246, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Michalopoulos GK. and DeFrances MC. Liver regeneration. Science 276: 60–66, 1997 [DOI] [PubMed] [Google Scholar]
- 23.Moscat J. and Diaz-Meco MT. Feedback on fat: p62-mTORC1-autophagy connections. Cell 147: 724–727, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murata H, Yagi T, Iwagaki H, Ogino T, Sadamori H, Matsukawa H, Umeda Y, Haga S, Takaka N, and Ozaki M. Mechanism of impaired regeneration of steatotic liver in mouse partial hepatectomy model. J Gastroenterol Hepatol 22: 2173–2180, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Murata S, Hashimoto I, Nakano Y, Myronovych A, Watanabe M, and Ohkohchi N. Single administration of thrombopoietin prevents progression of liver fibrosis and promotes liver regeneration after partial hepatectomy in cirrhotic rats. Ann Surg 248: 821–828, 2008 [DOI] [PubMed] [Google Scholar]
- 26.Niitsu Y, Hakamata M, Goto Y, Higashi T, Shoji M, Sugai T, and Umezawa K. Chemoenzymatic synthesis of (2R,3R,4R)-dehydroxymethylepoxyquinomicin (DHMEQ), a new activator of antioxidant transcription factor Nrf2. Org Biomol Chem 9: 4635–4641, 2011 [DOI] [PubMed] [Google Scholar]
- 27.Nogalska A, Terracciano C, D'Agostino C, King Engel W, and Askanas V. p62/SQSTM1 is overexpressed and prominently accumulated in inclusions of sporadic inclusion-body myositis muscle fibers, and can help differentiating it from polymyositis and dermatomyositis. Acta Neuropathol 118: 407–413, 2009 [DOI] [PubMed] [Google Scholar]
- 28.Okada K, Yanagawa T, Warabi E, Yamastu K, Uwayama J, Takeda K, Utsunomiya H, Yoshida H, Shoda J, and Ishii T. The alpha-glucosidase inhibitor acarbose prevents obesity and simple steatosis in sequestosome 1/A170/p62 deficient mice. Hepatol Res 39: 490–500, 2009 [DOI] [PubMed] [Google Scholar]
- 29.Ozaki M, Haga S, and Ozawa T. In vivo monitoring of liver damage using caspase-3 probe. Theranostics 2: 207–214, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ozaki M, Haga S, Zhang HQ, Irani K, and Suzuki S. Inhibition of hypoxia/reoxygenation-induced oxidative stress in HGF-stimulated antiapoptotic signaling: role of PI3-K and Akt kinase upon rac1. Cell Death Differ 10: 508–515, 2003 [DOI] [PubMed] [Google Scholar]
- 31.Ramos-Gomez M, Dolan PM, Itoh K, Yamamoto M, and Kensler TW. Interactive effects of nrf2 genotype and oltipraz on benzo[a]pyrene-DNA adducts and tumor yield in mice. Carcinogenesis 24: 461–467, 2003 [DOI] [PubMed] [Google Scholar]
- 32.Schmucker DL. Age-related changes in liver structure and function: implications for disease? Exp Gerontol 40: 650–659, 2005 [DOI] [PubMed] [Google Scholar]
- 33.Selzner M. and Clavien PA. Steatotic liver in liver transplantation and surgery. Semin Liver Dis 21: 105–113, 2001 [DOI] [PubMed] [Google Scholar]
- 34.Shen YF, Tang Y, Zhang XJ, Huang KX, and Le WD. Adaptive changes in autophagy after UPS impairment in Parkinson's disease. Acta Pharmacol Sin 34: 667–673, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tanoue S, Uto H, Kumamoto R, Arima S, Hashimoto S, Nasu Y, Takami Y, Moriuchi A, Sakiyama T, Oketani M, Ido A, and Tsubouchi H. Liver regeneration after partial hepatectomy in rat is more impaired in a steatotic liver induced by dietary fructose compared to dietary fat. Biochem Biophys Res Commun 407: 163–168, 2011 [DOI] [PubMed] [Google Scholar]
- 36.Taub R. Liver regeneration: from myth to mechanism. Nat Rev Mol Cell Biol 5: 836–847, 2004 [DOI] [PubMed] [Google Scholar]
- 37.Torbenson M, Yang SQ, Liu HZ, Huang J, Gage W, and Diehl AM. STAT-3 overexpression and p21 up-regulation accompany impaired regeneration of fatty livers. Am J Pathol 161: 155–161, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wedemeyer I, Bechmann LP, Odenthal M, Jochum C, Marquitan G, Drebber U, Gerken G, Gieseler RK, Dienes HP, and Canbay A. Adiponectin inhibits steatotic CD95/Fas up-regulation by hepatocytes: therapeutic implications for hepatitis C. J Hepatol 50: 140–149, 2009 [DOI] [PubMed] [Google Scholar]
- 39.Yamamoto T, Yoh K, Kobayashi A, Ishii Y, Kure S, Koyama A, Sakamoto T, Sekizawa K, Motohashi H, and Yamamoto M. Identification of polymorphisms in the promoter region of the human NRF2 gene. Biochem Biophys Res Commun 321: 72–79, 2004 [DOI] [PubMed] [Google Scholar]
- 40.Zou X, Feng Z, Li Y, Wang Y, Wertz K, Weber P, Fu Y, and Liu J. Stimulation of GSH synthesis to prevent oxidative stress-induced apoptosis by hydroxytyrosol in human retinal pigment epithelial cells: activation of Nrf2 and JNK-p62/SQSTM1 pathways. J Nutr Biochem 23: 994–1006, 2012 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.