Abstract
The avian bacterial pathogen Mycoplasma gallisepticum is a good model for systems studies due to small genome and simplicity of regulatory pathways. In this study, we used RNA-Seq and MS-based proteomics to accurately map coding sequences, transcription start sites (TSSs) and transcript 3′-ends (T3Es). We used obtained data to investigate roles of TSSs and T3Es in stress-induced transcriptional responses. We identified 1061 TSSs at a false discovery rate of 10% and showed that almost all transcription in M. gallisepticum is initiated from classic TATAAT promoters surrounded by A/T-rich sequences. Our analysis revealed the pronounced operon structure complexity: on average, each coding operon has one internal TSS and T3Es in addition to the primary ones. Our transcriptomic approach based on the intervals between the two nearest transcript ends allowed us to identify two classes of T3Es: strong, unregulated, hairpin-containing T3Es and weak, heat shock-regulated, hairpinless T3Es. Comparing gene expression levels under different conditions revealed widespread and divergent transcription regulation in M. gallisepticum. Modeling suggested that the core promoter structure plays an important role in gene expression regulation. We have shown that the heat stress activation of cryptic promoters combined with the hairpinless T3Es suppression leads to widespread, seemingly non-functional transcription.
INTRODUCTION
Mycoplasma gallisepticum belongs to the Mollicutes class—a specialized branch of microorganisms related to Gram-positive bacteria (1). M. gallisepticum is an important pathogen in poultry and wild birds, in which it causes chronic respiratory disease (2). Mollicutes feature reduced genomes with an average size of 1 Mb, and they lack a cell wall (3). Consequently, their cell physiology is considerably simplified compared to that of most bacteria, making Mollicutes a good model for systemic studies and, in particular, for studying the complex response to stress.M. gallisepticum, along with most Mollicutes, shows a reduced repertoire of transcription factors (TFs) compared to that of related bacteria, such as Bacillus subtilis (4). The only TF whose mechanism is known in M. gallisepticum is a heat-shock repressor (HrcA) that binds a palindromic sequence known as controlling inverted repeat of chaperone expression (CIRCE) in the promoters of several chaperone genes (5), whereas other common bacterial TFs, such as the LexA repressor of the SOS response (6), are lacking. Recent studies that have demonstrated widespread differential expression in response to a variety of stresses in the Mycoplasma species (7–9) raise questions about the underlying regulation of these responses. Experimental identification of transcription start sites (TSSs) and transcription terminators (TTs) could help to resolve this ‘regulation without regulators’ puzzle.
Classic views on bacterial transcription assume that coding sequences (CDSs) are organized into operons–genomic regions that are transcribed as a single RNA (10). On the contrary, recent genome-wide transcriptomics studies on several bacterial species (Escherichia coli (11), B. subtilis (12), Listeria monocytogenes (13), Helicobacter pylori (14) and Mycoplasma pneumoniae (7)) demonstrated that operons frequently include internal promoters and terminators that lead to the so-called ‘staircase’ transcription (7). These results cast doubts on the operon paradigm and call for a new, more realistic approach.
Common RNA-Seq experiments do not allow for the precise identification of TSSs; however, several techniques that address this problem have recently been developed. These techniques include either tagging of the 5′-ends by the ligation of a specific adapter (11) or 5′-end enrichment procedures using a 5′-phosphate-dependent nuclease (14).
In the current work, we systematically investigated transcription and translation in M. gallisepticum under several conditions using common RNA-Seq, primary 5′-end enriched RNA-Seq and mass spectrometry-based proteomics. We for the first time performed genome-wide identification of TSSs and measured their activity under two different conditions (control and heat shock) that allowed us to identify promoter features correlated with heat shock-induced activity changes. We introduced a new approach to study bacterial transcription that is based on intervals (genomic regions between the two nearest transcript ends) instead of operons. By combining both experimental evidence and in silico prediction, we identified hundreds of promoters, ribosome-binding sites (RBS), transcript 3′-ends (T3Es), operons and non-coding RNAs (Figure 1A).
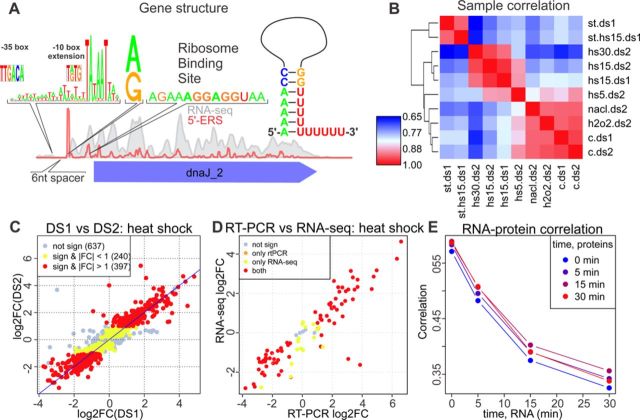
Figure 1.
(A) Typical RNA-Seq and 5′-ERS coverage profiles near the dnaJ2 gene. The diagram of the promoter sequence, the first nucleotide of the transcript, RBS and a schematic representation of the TT are shown above the plot. (B) Correlation heatmap (correlation increases from 0.65 (blue) to 1 (red); one minus the Spearman's correlation coefficient was used as the distance metric for clustering for different conditions (c, h2o2, nacl, hs5, hs15 and hs30 denote the control, oxidative stress, osmotic stress and heat stress for 5, 15 and 30 min, respectively) and data sets (ds1 and ds2). The expression values represent the average values of the replicates. (C) Agreement of the heat shock (15 min)-related expression changes (log2-fold change) between DS1 (x-axis) and DS2 (y-axis). One dot denotes one CDS. Not significant, significant but with a fold change below 2 and significant with a fold change above 2 are shown in gray, yellow and red, respectively. (D) Agreement of the heat shock (15 min)-related expression changes between RT-PCR (x-axis, differences in cycle number) and DS2 (y-axis, log2-fold change). The same color scheme as in panel C was used. (E) Dependence of the Spearman's correlation coefficient between the protein abundance (PAI) measured for different heat shock durations (shown with different colors) and the mRNA abundance (RPKM) on the heat shock duration used for the RNA-Seq experiments.
MATERIALS AND METHODS
Cell culturing
M. gallisepticum S6 was cultivated on a liquid medium containing tryptose (20 g/l), Tris (3 g/l), NaCl (5 g/l), KCl (5 g/l), yeast dialysate (5%), horse serum (10%) and glucose (1%) at pH = 7.4 and 37°C in aerobic conditions and exposed to stress conditions as described previously (9).
RNA extraction
Aliquots of the cell culture were directly lysed in TRIzol LS reagent (Life Technologies) at a 1:3 ratio of culture medium:TRIzol LS (v/v). The lysates were extracted with chloroform, and the aqueous phase was purified with a PureLink RNA Mini Kit (Ambion) to remove tRNA or was used directly to precipitate RNA by the addition of an equal volume of isopropanol.
Real-time polymerase chain reaction (PCR)
RNA was treated by DNAse I (Thermo Scientific), and cDNA was synthesized from random hexamer primers by H-minus Mu-MLV reverse transcriptase (Thermo Scientific). Real-time PCR was performed using iQ SYBR Green Supermix (Bio-Rad) and a CFX96 Real-Time PCR Detection System (Bio-Rad) PCR machine. Quantitative data were normalized to the 23S rRNA transcript as described previously (9).
Preparation of libraries for RNA-Seq
RNA (either total or tRNA-depleted) was fragmented into 200 bp by chemical fragmentation (100 mM ZnSO4, 100 mM Tris, pH = 7.0 at 70°C for 15 min). The fragmentation reaction was stopped with 20 mM ethylenediaminetetraacetic acid (EDTA) (pH = 8.0). The fragmented RNA was end-repaired with T4 polynucleotide kinase according to the manufacturer's protocol (Thermo). Strand-specific double-stranded cDNA (ds-cDNA) libraries for standard RNA-Seq on a SOLiD platform were prepared according to the manufacturer's protocol using a Total RNA-Seq Kit and a SOLiD RNA Barcoding Kit (Ambion). The quality of the RNA, fragmented RNA and cDNA libraries was assayed with an Agilent 2100 Bioanalyzer system (Agilent).
Amplified ds-cDNA was subjected to a normalization procedure with DSN (double-strand specific nuclease, Evrogen). First, 400–1000 ng of ds-cDNA (12 μl) was mixed with 4 μl of hybridization buffer (200 mM HEPES, 2 M NaCl, pH = 7.5). The procedure was performed in a PCR thermocycler. The samples of ds-cDNA were denatured at 98°C for 2 min and then re-annealed at 68°C for 5 h. Then, 32 μl of DSN 2× master-buffer (Evrogen) that was pre-warmed to 68°C was added. The mixture was incubated at 68°C for 10 min. Subsequently, 0.5 μl of DSN enzyme was added, and the samples were incubated at 68°C for 15 min. The reaction was stopped by the addition of 64 μl of 200 mM EDTA (pH = 8.0). Then, an equal volume of isopropanol was added, the samples were incubated at −20°C overnight and the cDNA was recovered by centrifugation (20 min at 4°C, 16 000 Relative Centrifugal Force (rcf)). Subsequently, the cDNA was amplified and purified using a PureLink PCR Micro Kit (Invitrogen). Then, the procedure was repeated, resulting in two rounds of normalization in total. The normalized cDNA was selected for size by agarose gel electrophoresis (2% agarose, 1×TBE, 4 V/cm). Sample cDNA in the 200–300 bp range was extracted from the agarose blocks using a SOLiD Library Quick Gel Extraction Kit (Life Technologies; E1 buffer from the SOLiD Library Column Purification Kit was used for cDNA elution) and used for downstream preparations according to the standard protocol.
All samples were prepared in two biological replicates with one technical replicate per biological replicate.
Preparation of libraries for 5′-ERS
To prepare 5′-enriched libraries, at least 20 μg of total RNA was fragmented, end-repaired (as described above) and treated with Terminator exonuclease (Epicentre). This process resulted in the degradation of the non-primary 5′-end RNA fragments, whereas the primary 5′-fragments were protected by the tri-phosphate groups on their 5′-ends. As chemical fragmentation leaves phosphates randomly on fragments ends, end-repair procedure was used to enhance degradation of non-primary 5′-end fragments (e.g. with 5′-OH), which otherwise undergo adapter ligation and cDNA synthesis (if they have 3′-OH) and to rescue primary 5′-end fragments with 3′-phosphate (by 3′-phosphatase activity of T4 PNK). Addition of end-repair procedure increases signal (e.g. coverage of primary 5′-ends) and decreases background. Then, the RNA was precipitated by isopropanol and treated with tobacco acid phosphatase (Epicentre) to remove the pyrophosphate groups. Next, the RNA was precipitated by isopropanol and used for strand-specific ds-cDNA preparation according to the standard protocol for SOLiD libraries. The sample cDNA was normalized in one round as described above and used to prepare SOLiD libraries according to the standard protocol.
Sequencing
Sequencing was performed on a SOLiD 4 (Life Technologies) platform using SOLiD EZ Bead E80 System Consumables and SOLiD ToP Sequencing Kit, MM50 (Applied Biosystems).
Protein extraction and 1D electrophoresis
Cells harvested by centrifugation at 10 000 × g at 4°С for 10 min were washed twice in a wash buffer (150 mM NaCl, 50 mM Tris-HCl, 2 mM MgCl2, pH = 7.4). The cells were lysed in 20 μl of 1% sodium dodecyl sulphate in 100 mM NH4HCO3 and incubated in an ultrasonic bath for 15 min followed by centrifugation at 10 000 × g at 4°С for 5 min. The supernatant was extracted, and the protein concentration was determined using a Bicinchoninic Acid Protein Assay Kit (Sigma). Next, 20 μl of 2× Laemmli reagents was added, and the samples were incubated at 95°С for 5 min. Then, 50 μg of protein was loaded onto a polyacrylamide gel (10 × 0.1 cm, 12% polyacrylamide), and electrophoresis was performed as described by Laemmli (15) (10 mA current). The electrophoresis was stopped when the front dye reached 1.5 cm in the separating gel.
Trypsinolysis in polyacrylamide gel
The polyacrylamide gel was fixed in a fixation buffer (20% CH3OH and 10% CH3COOH) for 30 min and washed twice in H2O. The gel was cut into 1 × 1 mm pieces, transferred into tubes and treated with 10 mM DTT and 100 mM NH4HCO3 for 30 min at 56°С. Then, the proteins were alkylated with 55 mM iodoacetamide in 100 mM NH4HCO3 for 20 min in the dark. Next, water was removed from the gel pieces by the addition of 100% acetonitrile.
The dehydrated samples were treated with a 150 μl of trypsin solution (40 mM NH4HCO3, 10% acetonitrile, 20 ng/μl Trypsin Gold, mass spectrometry grade; Promega). The samples were incubated for 60 min at 40°С and for 16–18 h at 37°С. Peptides were extracted once by 5% formic acid and twice by 50% acetonitrile with 5% formic acid. The extracts were joined and dried in a vacuum centrifuge at 45°С. The precipitate was diluted in 50 μl of 5% acetonitrile with 0.1% formic acid.
Chromato mass spectrometry
The peptides were analyzed using a TripleTOF 5600+ (ABSciex) mass spectrometer with a NanoSpray III ion source and a NanoLC Ultra 2D+ chromatograph (Eksigent). Chromatographic separation was performed in a gradient of acetonitrile in water (5–40% of acetonitrile in 120 min) with 0.1% formic acid on 75 × 150 μm columns with a Phenomenex Luna C18 3 μm sorbent and a flow rate of 300 nl/min.
The IDA mode of the mass spectrometer was used to analyze the peptides. Based on the first MS1 spectrum (the mass range for the analysis and subsequent ion selection for MS2 analysis was 300–1250 m/z; the signal accumulation was 250 ms), 50 parent ions with maximum intensity in the current spectrum were chosen for the subsequent MS/MS analysis (the resolution of the quadrupole unit was 0.7 Da, the mass measurement range was 200–1800 m/z, the ion beam focus was optimized to obtain maximal sensitivity, and the signal accumulation was 50 ms for each parent ion). Nitrogen was used for collision dissociation with a fixed average energy of 40 V. The collision energy was linearly increased from 25 to 55 V during the signal accumulation time (50 ms). The parental ions that had already been analyzed were excluded from the analysis for 15 s.
The mass spectrometry proteomics data have been deposited in the ProteomeXchange Consortium (http://proteomecentral.proteomexchange.org) via the PRIDE partner repository (16) with the data set identifier PXD000922 and DOI 10.6019/PXD000922.
Analysis of mass spectrometry data
Raw data files (.wiff file format) were converted to the Mascot generic format (.mgf file format) using AB SCIEX MS Data Converter version 1.3 and were searched using Mascot version 2.2.07 against a database of all proteins (836 amino acids sequences) of M. gallisepticum S6 (GI:604957178). The Mascot searches were performed with the following parameters: tryptic and semi-tryptic peptides; maximum of one missed cleavage; a peptide charge state limited to 1+, 2+ and 3+; a peptide mass tolerance of 10 ppm; a fragment mass tolerance of 0.5 Da and variable modifications caused by oxidation (M) and carbamidomethylation (C). Protein scores greater than 6 for protein-trypsin, 17 for protein-semitrypsin, 32 for genome-trypsin and 24 for genome-semitrypsin were assumed to be significant.
The proteogenomic profiling of M. gallisepticum was performed using the database of the chromosomal DNA sequence (GenBank) split by 3000 nucleotides with a shift of 1000 nucleotides (493 nucleotides sequences). We used the Protein Abundance Index (PAI) to evaluate the protein concentrations (as described elsewhere (17)).
Read mapping
All reads from DS1 with average quality values below 15 were discarded. Because many reads contain an adapter sequence in the 5′-end, we truncated all reads from both data sets to the first 25 read bases. Then, the reads were mapped to the M. gallisepticum strain S6 genome (GI:604957178) using Bowtie software (18) with the following parameters: bowtie–trim3 23 -f -C -v 3 -y -a–best–strata -S. Each match for the reads that was mapped to multiple positions was treated as an independent read. The results were nearly the same when only the uniquely mapped reads were used.
Transcription interval identification
To divide the M. gallisepticum genome into transcription intervals (intervals with a constant expression level), we combined all RNA-Seq samples from DS2 together and calculated the number of reads that mapped to each position in the genome (read coverage). A read was considered to be mapped to the given position only if its alignment started from that position. Then, we looked for local changes in the read coverage using a sliding window. For each genome position, we calculated the following function:
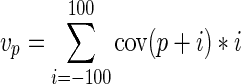 |
where cov(j) is the read coverage at a specific position and p is genome position. We considered all local maxima and minima of vp as possible borders of transcription intervals. Then, we sorted all borders by vp, and, for each border (starting from borders with highest vp), we removed all other borders within 25 nt. We used the intervals between the borders to estimate the dependence of the overdispersion parameter on the logarithm of the average coverage using generalized linear models (GLMs) and a quasi-Poisson distribution, od(cov). Then, we applied the following iterative procedure to merge the intervals with similar coverage together:
Use GLM with a quasi-Poisson distribution to model the read coverage in each pair of consecutive intervals.
Calculate the overdispersion parameter as a maximum of one, the overdispersion from the GLM model calculated in step 1 and the approximation by od(cov) calculated for each of two intervals.
Find the pair of intervals with the highest P-value (quasi-log likelihood test). Join these intervals if the P-value is lower than 0.05 divided by the genome length.
Repeat steps 1–3 until step 3 results in interval merging.
Each remaining border was called a coverage step, either up or down (up-CS and down-CS). For each coverage step and each sample (all union of samples), we calculated its size:
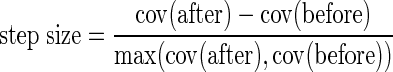 |
where cov(after) and cov(before) are the average coverage in interval after and before the step, respectively.
To detect the expressed intervals, we defined the coverage cutoff as the 5% quantile of the average coverage of the CDS-containing intervals. We defined an set of overlapping transcripts (SOT) as a set of consecutive expressed intervals.
TSS identification
To identify TSSs, we used the 5′-ERS data from DS2. Considering each sample separately, we searched for a local maximum in the read coverage (defined as described in the section on Transcription Interval Identification) that was supported by at least five reads. Then, we modeled the coverage at each local maximum while considering 5 nt in each direction as background using a GLM with a quasi-binomial distribution and controlling the overdispersion parameter to be not lower than 1. We used a quasi-log likelihood test to identify significant coverage peaks (BH-corrected P-value < 0.05). As a result, 32 148 peaks were detected in at least one (out of four) 5′-ERS sample from data set 2.
To investigate promoter structure, we focused on the peaks that lie within 300 nt from the nearest start codon and are supported by at least 200 reads. We observed a TATAAT-like sequence surrounded by an AT-rich region in the upstream peak. We built a position weight matrix (PWM) for the (−32,−3) peak region and then optimized it using the following procedure:
For each peak, find the best PWM match in the (−34,−1) region.
Order the peaks by match weight and take the top 60%.
Rebuild the PWM using the matches selected in step 2.
Repeat steps 1–3 until convergence is achieved.
The optimized PWM matrix is shown in Supplementary Table S9.
Then, we searched for the best match of the resulting PWM in all peaks. To this end, we separated the PWM into two parts: PWM1 corresponded to the TATAAT-like region, and PWM2 corresponded to the surrounding region. We first looked for positions with the best PWM1 match; if there was more than one position that had a maximum score, we selected the one with the highest PWM2 score. The results (Supplementary Figure S5) show that the positions that correspond to a 5–7 nt spacer between the TATAAT box and the TSS are preferred. Thus, we used these positions to predict the best promoter sequence for each peak. To evaluate the background distribution of the weight of both PWM parts, we scanned the whole genome. For every three consecutive positions, we chose the best PWM match. We divided the whole range of PWM1 and PWM2 scores into 20 bins that resulted in a 2D (20 × 20) PWM score distribution. In each bin of PWM1, we set a specific cutoff for the PWM2 score to achieve an false discovery rate (FDR) below 10% (Supplementary Figure S8), resulting in the identification of 1061 TSSs.
To identify TSSs that have a TRTGN extension or −35 box, we searched for the corresponding sequences (TRTG and TTGACA with no more than two mismatches) in the regions upstream of the TATAAT box. We compared the numbers of matches in the expected regions (−5 and −23 from TATAAT) and in the (−86,−36) region.CIRCE elements were annotated by a genome-wide search for the TTAGCACTC-N9-GAGTGCTAA sequence that allowed for up to two mismatches (Supplementary Table S8).
Hairpin prediction
We used the RNIE (19) program in sensitive mode to predict hairpins in the M. gallisepticum genome.
Differential expression analysis
The read counts and reads per kilobase of transcript per million mapped reads (RPKM) for the CDSs and intervals were calculated using SAJR software (20). Each location of the reads that mapped to multiple locations was treated independently and in the same manner as the locations of uniquely mapped reads. For most of the genes, the results did not change when only the uniquely mapped reads were considered. Only the expressed intervals that did not overlap with any known non-coding (tmRNA, tRNA and rRNA) genes were used. Library sizes were calculated as the sums of the coverage of all CDSs (or intervals). All library sizes (except for the two stationary samples) were adjusted by Relative Log Expression (RLE) normalization using the package edgeR (21). RNA-seq-based differential gene expression analysis is based on assumption that gene read counts are Poisson-like distributed with mean proportional to transcript abundance, transcript length and to library size (sum of all gene read counts). But in some cases few highly expressed genes could affect library sizes too much leading to biases in gene expression level estimates. RLE normalization scales library sizes in the way to remove such biases. For more details please see original paper (22). Then, we applied SAJR to detect differentially expressed genes while considering the number of reads mapped to a given CDS (or interval) as the result of the binomial trials with the number of trials equal to the library size. The CDSs (and intervals) with a Benjamini–Hochberg (BH)-corrected (23) P-value below 0.05 and with a fold change above two were considered to be significantly differentially expressed.A similar procedure, with two differences, was used to analyze TSS activity under heat shock: first, for each TSS, only the reads that mapped exactly to a transcription initiation site were used; second, we used the exact test from the edgeR package to assess the significance of the expression change.
Heat-shock gene clustering
Genes whose expression changed significantly after at least one heat-shock period were clustered using hierarchical clustering with complete linkage and using one minus the Pearson's correlation between the expression levels (control and heat-shock samples) as a distance measure. The expression levels (RPKMs) were averaged between the replicates and were logarithmized. The obtained dendrogram was divided to form nine clusters. The resulting clusters were reordered by size. For visualization, RPKMs were z-score transformed. The boxplots in Figure 4B represent the distribution of z-scores in the given condition for all genes for a particular cluster.
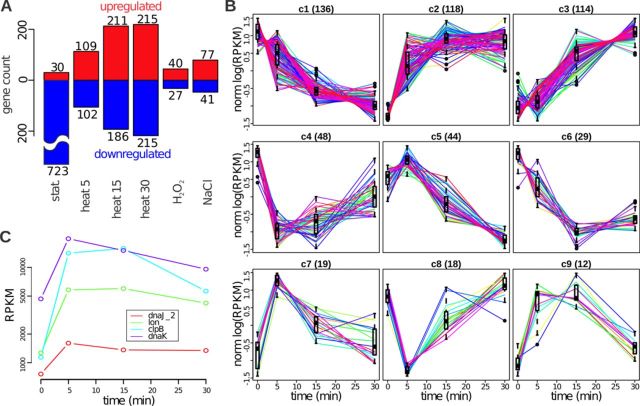
Figure 4.
Transcription regulation. (A) Number of CDSs whose expression changes significantly under different conditions. The up- and down-regulated genes are shown in red and blue, respectively. (B) Nine patterns of gene expression changes under heat shock. The patterns are ordered by their size (shown in brackets in the panel titles). The normalized log expression of individual genes is shown with lines, and the distribution of the normalized log expression at each time point is shown with a box. (C) Gene expression profiles (RPKM) of four genes that have the CIRCE motif in the upstream region.
Gene ontology (GO) enrichment analysis
GO annotation was conducted using the blast2GO program (24). Enrichment analysis was performed using the goseq package (25), and all annotated CDSs were used as the background. Only the terms with more than two genes were considered. All terms with a BH-corrected P-value above 0.2 were considered significant.
Modeling of TSS heat-shock response
We modeled the log fold change of the heat-shock-related TSS activity change using the randomForest package (26). The sequence of the −10 box (the sequences that met less than 10 times were considered a single class), the first nucleotide of the transcript and the spacer between the −10 box and the TSS were used as categorical predictors and the AT contents within 20 nt upstream and 3 nt downstream of the Pribnow box, nucleotide content in first 100 nt of transcript were used as continuous variables. We fitted the model with 10 000 trees 500 times and used it to perform self- (using all trees) and cross- (based on out-of-bag data) verification.
Modeling of the interval expression
We modeled the average coverage in each expressed interval under each condition (replicates were merged together, and the coverage was scaled by the sum of adjusted using RLE normalization (by intervals; see above) library sizes using the following model:
The coverage (cov) before the start of each SOT, was set to 0.
At the ith up-CS, the coverage is updated by cov = cov + effect(up-CSi), where effect(up-CSi) cannot be negative.
At the ith down-CS, the coverage is updated by cov = cov*effect(down-CSi), where effect(down-CSi) is between 0 and 1.
Such models perfectly explain (with a few negligible exceptions) the interval coverage under each condition. To decompose the effects of TSSs (effect(up-CS)) and TTs (effect(down-CS)) on the expression changes after 30 min of shock, we built two models—one for the control conditions and one for the heat shock. Then, we constructed an intermediate model that utilizes the TSS effects from the model built for the heat shock sample and the TT effects from the control model and used it to model the interval coverage. We considered the difference between the control condition and the results of the coverage prediction by the intermediate model to be TSS-related effects, and we considered the difference between the coverage prediction by the intermediate model and the interval coverage under heat shock to be TT-related effects. The feature with the greatest effect was considered the major driver of the expression change for a given interval. The results changed only moderately when the intermediate model was constructed in the opposite way (using the TSS effects from the control model and the TT effects from the model built for heat-shock samples).
Validation of weak down-CSs
To independently test weak down-CSs we selected four down-CSs with significant step size (more than 50%) and absence of antisense transcription. The second condition made a diversity of suitable down-CSs very narrow. For each we designed two pair of primers for regions up- and downstream of the down-CS. We performed real-time PCR under normal conditions and 30 min heat stress. Then we measured ratio between signals from upstream and downstream regions.
Construction of a vector for transformation of M. gallisepticum
To test identified promoter and RBS structure we constructed a transposon-based vector for M. gallisepticum transformation. Genetic construct, containing Tn4001 transposase and tetM (tetracycline resistance gene) with respective promoters, RBSs and terminators were chemically synthesized (see Supplementary Figure S9) and cloned into pBluescript SK+ plasmid between SacI and KpnI sites. Codon usage (and GC-content) for transposase and tetM was optimized to conform to average codon usage in M. gallisepticum. Transformation was performed by electroporation as described by Markham et al. (27).
Data access
M. gallisepticum S6 genome sequence and annotation were deposited in NCBI GenBank under GenBank id CP006916.2. Transcriptomics data was uploaded to NCBI SRA database under project id PRJNA243934. Proteomics data was uploaded to ProteomeXchange Consortium database via the PRIDE partner repository under dataset id PXD000922 and DOI 10.6019/PXD000922, reviewer account: Username: reviewer67732@ebi.ac.uk, Password: nXnmBoCV.
RESULTS
In this study, we strand-specifically sequenced two sets of samples (16 samples in each data set) of the total RNA extracted from M. gallisepticum that was cultured under different conditions (exponential and stationary growth phases; heat shock, osmotic and oxidative stresses) in at least two biological replicates using a SOLiD 4 sequencer (Life Technologies). The sequencing produced 989 million reads, 44% of which could be mapped to the genome. Although most of these reads correspond to rRNA and tRNA, 17% are mapped to CDSs and 2.6% are mapped to intergenic regions (Supplementary Table S1). In addition to common RNA-Seq experiments, we employed a 5′-end enrichment procedure (see Materials and Methods; Figure 1A) to precisely identify the primary 5′ ends of the transcripts (5′-enriched RNA sequencing, 5′-ERS). Correlation analysis shows good sample clustering determined by the biological conditions rather than by technical variations (Figure 1B; Supplementary Figure S1). For example, we observed a close agreement between the gene expression changes under heat shock measured in the two data sets (the Spearman's correlation coefficient (rho) between the fold changes is 0.92; Figure 1C). Additionally, we performed reverse transcriptase-PCR (RT-PCR) for 98 selected genes under the same conditions (Supplementary Table S2). The gene expression levels as well as the fold changes measured by these two techniques show high correlation (Pearson's rho > 0.7, Figure 1D, Supplementary Figures S2 and S3). We used mass spectrometry (see below) to assess the PAI under the heat shock and control conditions. The correlation between the PAI and mRNA abundance (RPKM) is ∼0.5 for the control conditions (Figure 1E), which is comparable to the estimates obtained in other works (28). The results confirm the high quality of our data and support their applicability in characterizing the transcription organization in M. gallisepticum strain S6.
Prediction of transcription units structure by read coverage
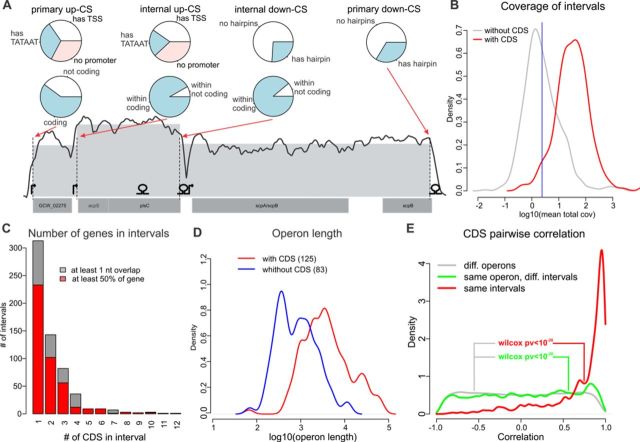
Traditionally, bacterial transcription is considered to be organized into operons, which are genomic regions with a single promoter and several CDSs that are transcribed as a single mRNA (10). Recent evidence indicates that bacterial transcripts seem to overlap with each other (11,13), resulting in a complex structure with internal TSSs, TTs and processed transcript ends. To denote it we introduce a new term ‘SOT’. SOTs can be divided into the intervals between two nearest transcript ends. Theoretically, the read coverage in such intervals should follow a Poisson distribution, but practically, this distribution is much more variable due to the differences in nucleotide composition, RNA secondary structure and sequencing biases (29). We used GLMs with a quasi-Poisson distribution to model the read coverage. We applied a quasi-log likelihood test to divide the M. gallisepticum transcriptome into 1059 equally covered intervals (see Materials and Methods, Figure 2A and Supplementary Table S3). The borders between the intervals are formed by either up- or down-coverage steps (up-CSs or down-CSs), which should theoretically correspond to transcript 5′ and 3′ ends, respectively. In total, we observed 499 up-CSs and 558 down-CSs. For each step we calculated relative step size as (ca-ab)/max(ca,cb), where cb and ca are the mean coverages in intervals before and after the step, respectively. We assigned annotated features (CDS, tRNA and rRNA) to the intervals if the overlap was greater than 50% of the length of the gene. Of 877 annotated genes, 868 could be assigned to intervals and 586 reside completely within a single interval. The read coverage strikingly differed between the intervals that contained genes and those that did not (Figure 2B). Assuming that 95% of the gene-containing intervals are expressed, we set the coverage threshold for expressed intervals to 2.38 read per position. The 372 intervals with a coverage value below the threshold were considered to be unexpressed. Most of the intervals overlap with just a few genes. The longest (in terms of the number of overlapped genes) expressed interval (12 638 nt) contains 24 genes that encode ribosomal proteins (Figure 2C). The definition of an SOT as a continuous sequence of expressed intervals results in 208 SOTs, 125 of which overlap 839 genes (in total) by at least 50% of the gene length. Furthermore, 772 genes reside completely within an SOT. In addition to the 208 primary up-CSs and down-CSs, the SOTs contain 218 and 261 internal up-CSs and down-CSs, respectively. Most of these CSs are observed within gene-containing SOTs, most likely because the latter are usually much longer than the SOTs that do not contain genes (Figure 2D). We compared our prediction for each consecutive pair of genes that resided within the same or in different intervals with the operon prediction obtained from proOpDB (30) and observed a high and significant overlap (Fisher's exact test P < 1e-14). The high correlation between expressions of the CDSs from the same interval (and, to some degree, from the same SOT) (Figure 2E) confirms the robustness of our procedure.
Figure 2.
SOT prediction. (A) Example of a predicted SOT. Annotated CDSs are shown at the bottom; the TSSs identified by 5′-ERS and the hairpins predicted by RNIE are shown above. The smoothed coverage (running mean in a 100 nt window; log scale) is shown with a solid line; the dashed vertical lines represent up- and down-CSs; and the mean interval coverage is represented by the gray area. Classifications of primary and internal CSs by TSS/hairpin existence and the coding potential of the SOT to which they belong are shown with pie charts. (B) Distribution of intervals with (red) and without (gray) CDSs by log coverage. The 5% quantile of the former is shown as a blue vertical line. (C) Distribution of intervals by the number of genes. (D) Distribution of SOTs with (red) and without (blue) CDSs by log length. (E) Distribution of Pearson's correlation coefficients for the pairs of genes that belong to the same interval (red), same SOT (but not interval, green) and genes from different SOTs (gray).
5′-ERS-driven identification of promoters
To further understand the transcription organization in M. gallisepticum, we used our 5′-ERS samples to identify the exact locations of TSSs. Briefly, we looked for the local maxima (peaks) in the 5′-ERS coverage, iteratively optimized the PWM for the upstream region and then used the PWM to identify the peaks with a significantly better PWM match than expected by chance (see Materials and Methods; Figure 1A). The procedure resulted in 1061 TSSs at an FDR < 10% (Supplementary Table S4). All of the identified promoters are sigma 70 promoters with a TA[T/A]AAT −10 element surrounded by an A/T-rich region. We searched for the TRTGN 5′-extension of the Pribnow box that was previously reported for B. subtilis and other Gram-positive bacteria (31). We found 25 TSSs (out of 1061) with such an extension; this result is 5-fold greater than expected by chance (see Materials and Methods). The primary sigma factor of M. gallisepticum (RpoD) along with the Pribnow box-binding domain contains the −35 element-binding domain (Supplementary Figure S4). To identify −35 box we used MEME suite (32) to search for any enriched motifs in upstream of −10 box (see Materials and Methods). Unfortunately, no one motif with e-value above 0.1 was found. To test whether M. gallisepticum promoters contain the −35 element, we searched up to 100 nt upstream of TSSs for a consensus TTGACA sequence (33) with no more than two mismatches. The results indicated that TTGACA sequence occurs twice as frequently at the −35 position than in other locations in average. We identified 122 TSSs with −35 box in total (Supplementary Figure S11). Promoters containing −35 elements with no more than two mismatches show higher than average transcription level (two-sided Wilcoxon test P-value < 0.004 under control condition and <0.002 under 15 min heat stress, Supplementary Figure S12). An analysis of the M. gallisepticum genome revealed one putative sigma factor-like TF (GCW_00440) that may function as an alternative sigma factor. However, the regions around the 5′-ERS peaks did not exhibit the enrichment of motifs that were dissimilar to TATAAT, leading us to the conclusion that M. gallisepticum has only one functional sigma factor and that the observed TATAAT-lacking 5′-ERS peaks represent experimental noise. Analysis of the promoter structure revealed that the −10 element is separated by a 6 nt (or, rarely, a 7 or 5 nt) spacer from the TSS (see Materials and Methods, Figure 3A and Supplementary Figure S5). Similarly to transcription in Mycobacterium tuberculosis (34), transcription in M. gallisepticum is initiated almost solely from purine (95% of TSS and 99% of overall transcription under both control condition and heat shock is initiated from purine). If the seventh position from the −10 element is occupied by a C or T, transcription initiates one nucleotide downstream, resulting in a 7 nt spacer. Unfortunately, there is no such simple explanation for the most rare spacer, the 5 nt spacer (Figure 3A). The promoters predicted based on 5′-ERS are located significantly closer to the up-CSs than expected by chance (Wilcoxon test, P < 1e-8, Figure 3B). Although only 35% of the up-CSs have promoters that are predicted from 5′-ERS within a ±20 nt interval, most of them (62%) have a good PWM match in the same region. Up-CSs that are not associated with any promoter-like sequences could represent processed 5′-ends of transcripts. Interestingly, most of the TSSs identified by 5′-ERS (857 TSSs, 81%) are not associated with up-CSs. Although some of these 857 TSSs might be false positives, 69% of them are associated with the increase in read coverage and are likely to contribute to transcription. The 5′-ERS-identified TSSs that are not associated with up-CSs have a low relative step size and coverage by 5′-ERS reads (6-fold lower on the average than the coverage of the up-CSs associated TSSs) and constitute only 43% of the overall transcription initiation activity. Similarly, the up-CSs that lack 5′-URS-identified TSSs have a lower step size than the up-CSs that have a TSS within 20 nt (Figure 3C).
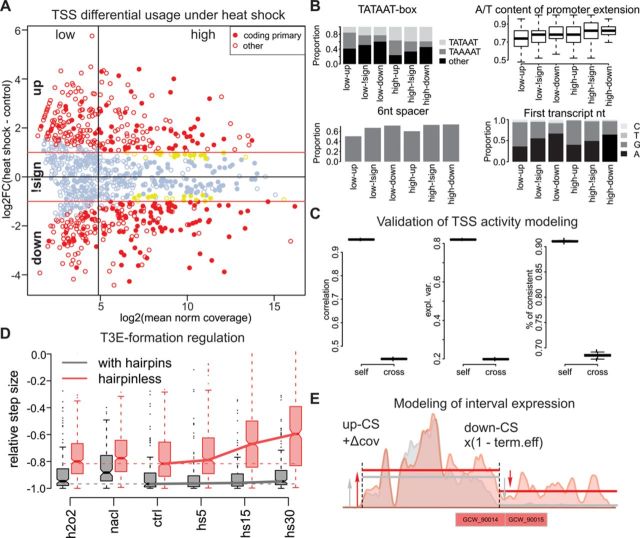
Figure 3.
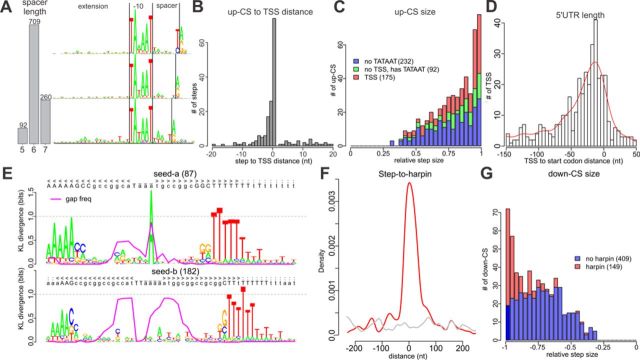
Structure of M. gallisepticum TSSs and T3Es. (A) Distribution of TSSs by spacer (between the −10 element and the TSS) length (left) and logo-images of the promoter region for each spacer length (7 to 5 nt from top to bottom). The −10 element and TSS are shown with vertical black lines. (B) Distribution of distances between the TSSs and the nearest up-CS. (C) Distribution of up-CSs by step size. The up-CSs that have 5′-ERS-detected TSSs, have only a good −10 element (detected by PWM) and have no signs of a TATAAT-like promoter are shown in red, green and blue, respectively. (D) Distribution of distances from the nearest start codon to a TSS (negative values correspond to TSSs placed before the start codon). (E) The logo-images of alignments of RNIE-predicted hairpins to two seeds. The proportion of gaps in a given position is shown with a magenta line. (F) Distribution of distances between down-CSs and RNIE-predicted hairpins. (G) Distribution of down-CSs by relative step size. The down-CSs with and without hairpins are shown in red and blue, respectively.
We observed that 39% of the predicted TSSs reside in close proximity (<200 nt) to a start codon. The distribution of the 5′ UTR length has a mode near 15 nt, and nine transcripts appear to be leaderless (Figure 3D). For eight of these transcripts, we detected peptides from the corresponding proteins in the proteomic data; three of these proteins are the first Open Reading Frame (ORFs) in polycistronic transcripts.
Two classes of T3Es in M. gallisepticum
T3Es could originate either from transcription termination either from post-transcriptional modification, such as endonuclease cleavage, and exonuclease activity.
Because no ORF-encoding Rho factor was found in the M. gallisepticum genome, the transcription termination in M. gallisepticum should occur in a Rho-independent manner and is likely to be associated with RNA secondary structures, such as hairpins (intrinsic terminators) (35). We predicted 256 hairpins genome-wide using the RNIE program (19). Our results suggest that the stems of the terminator hairpins in M. gallisepticum are reduced compared to those of the default RNIE models and usually consist of 5 A-T pairs and just two G-C pairs near the loop (Figure 3E).
The predicted hairpins cluster near down-CSs (Figure 3F) as well as near the stop codons, which indicates that they represent intrinsic terminators. We observed a weak but significant (Pearson's rho = −0.18, P < 0.002) correlation between the hairpin score and the relative coverage step size. The hairpins that cannot be associated with a down-CS have a significantly lower score (one-sided Wilcoxon test, P < 0.03) than that of the hairpins that reside within a ±200 nt interval from a down-CS. Interestingly, whereas most of the down-CSs with a relative step size close to −1 have a hairpin (102 of 166 down-CSs with a step size below −0.9), a large class of down-CSs with a step size distributed around −0.7 mostly lacks hairpins (only 47 of 392 down-CSs with a step size above −0.9 have a hairpin; Figure 3G). While this class of down-CSs could originate from RNA processing, the similarity of GC content profiles around both classes of down-CSs (GC content drops from 32% in the upstream region to 27% in the downstream region; Supplementary Figure S6), suggests similarity of mechanisms that are responsible for formation of these two classes of T3Es.
More than 95% of genes change expression under at least one stress
We used annotated CDSs to assess the differential expression in M. gallisepticum. All normalizations used in RNA-Seq-based transcriptomics assume that only a tiny fraction of the genes change their expression or, at least, that the numbers of up- and down-regulated genes are approximately equal (36). Here, we used RLE normalization from the edgeR package (21) to scale the library sizes. When applied to all samples but the stationary phase, the calculated normalization factors are within the 0.82–1.2 interval, and their effect is below our fold change threshold (2). However, if the stationary phase samples are included, the results change dramatically: the normalization factors for the stationary phase samples (0.13–0.32) are ∼10 times lower than the normalization factors for other samples (1.9–2.7). Such huge differences may arise if most of the genes are down-regulated in the stationary phase. Our RT-PCR experiments confirm this idea: when compared to 23S rRNA abundances, the abundances of most mRNAs decrease in the stationary phase (Supplementary Figure S3). Because no normalization could be applied in such circumstances, we did not scale the library sizes for differential expression analysis under the stationary phase (see Materials and Methods). We used the SAJR package (20) to perform a pairwise comparison for the control samples and each of the stressed samples. Genes with a q-value (BH-corrected P-value) below 0.05 and fold change above two were considered differentially expressed (for both up- and down-regulation).
The most significant change in the transcriptional landscape of M. gallisepticum was observed upon the transition to the stationary phase (Figure 4A, Supplementary Table S5). For the stationary phase, we identified 723 down- and 30 up-regulated genes. The up-regulated genes were significantly enriched in genes associated with the ‘response to stress,’ ‘oxidation-reduction process’ and ‘glycolysis’ (Supplementary Table S6). Only 67 and 118 genes change their expression under oxidative and osmotic stresses, respectively, and most of these genes are up-regulated (40 and 77, respectively).
Annotation of genes that are up-regulated under oxidative stress are significantly enriched in the ‘iron-sulfur cluster assembly’ and ‘oxidation-reduction process’ GO terms. These genes encode the Fe-S cluster assembly protein (SufB), the scaffold protein for Fe-S clusters assembly (NifU), methionine-sulfoxide reductase (MsrB), azoreductase (AsoR), cysteine desulfurase (CsdB), flavodoxin and several other proteins involved in Reactive Oxygen Species (ROS) protection (37–39).
Previously, we demonstrated that M. gallisepticum exhibited significant halotolerance and can survive in 1.2 M NaCl solution and grow in 0.5 M NaCl (9). The adaptation to hyperosmotic conditions usually involves the transport of low-molecular-weight osmoprotectors, including proline and/or glycine-betaine, via specialized transporters: ProP in E. coli (40), BetP in Corynebacterium glutamicum (41) and OpuA in B. subtilis (42). These transporters were not detected in the M. gallisepticum genome, suggesting an alternative adaptation mechanism. The genes that were up-regulated under hyperosmotic conditions are enriched in the ‘aldehyde dehydrogenase [NAD(P)+] activity,’ ‘cellular aldehyde metabolic process,’ ‘acetyl-CoA metabolic process,’ ‘endonuclease activity’ and ‘response to stress’ terms (Supplementary Table S5).
Compared with the oxidative and osmotic stresses, the heat stress induced stronger transcription response. Thus, we studied the kinetics of the heat stress response for 5, 15 and 30 min. The number of heat shock-affected genes increases with the stress duration (Figure 4A); in total, 538 genes show altered expression for at least one time point. To further classify the heat shock-related expression changes, we clustered the genes into nine clusters based on the similarity of their expression changes under heat shock (see Materials and Methods and Figure 4B). Most of the genes (for a total of 69%) correspond to continuous expression changes. Among them 232 genes increased expression (clusters 2 and 3) and 136 genes decreased expression (cluster 1). GO enrichment analysis revealed that the translation-associated genes as well as the genes involved in glycolysis were down-regulated (cluster 1), whereas the genes linked with ‘serine-type endopeptidase activity’ and ‘transposase activity’ were quickly up-regulated under heat shock (cluster 2). The genes that were activated later during the heat stress (cluster 3) are involved in ‘carbohydrate transport’ and some other functions. The genes involved in Guanosine Tri-Phosphate (GTP) catabolism are quickly inactivated under heat shock (cluster 4; Supplementary Table S7). At the same time, we observed no significant transcriptional changes during the heat stress applied to the cells at the stationary growth phase.
Antisense transcription in M. gallisepticum is induced under stress
The SOT-prediction analysis showed that an appreciable proportion of transcription in M. gallisepticum corresponds to non-coding regions (79 SOTs, 1.3% of mapped reads, excluding tRNA or rRNA reads). Fifty-six (71%) of the non-coding SOTs overlap with 31 coding SOTs in the antisense orientation. Most of the antisense SOTs (47) reside completely within the coding SOTs. We used the intervals identified above to examine the differential expression of non-coding transcripts. We repeated the analysis described above at the level of intervals, focusing on the 657 expressed intervals that do not overlap with known non-coding genes (rRNA, tRNA and tmRNA). Strikingly, under all stress conditions, the non-coding intervals were significantly more frequently activated than the coding intervals (Fisher exact test, P <3e-5, 5e-40, 3e-37, 0.02 and 2e-11 for 5, 15 and 30 min of heat shock and for the oxidative and osmotic stresses, respectively).
To rather investigate a cis-antisense expression and its possible regulatory role we counted sense and antisense Fragments (of transcripts) Per Kilobase (of a gene which fpkm is calculated for) Per Million (of fragments) (FPKMs) under each condition for each CDS and used it to calculate log fold changes (sense-logFC and antisense-logFC). Antisense-logFC exhibits low anticorrelation with sense-logFC (Pearson's correlation coefficient is −0.205, −0.23 and −0.269 for 30 min heat shock, osmotic and oxidative stresses, respectively) pointing to a possible contribution of antisense transcription to suppression of sense transcripts. However, antisense transcription activation under the heat stress seems to be pervasive (50% and 94% of total CDS length have antisense coverage in at least one sample under control conditions and after 30 min of heat shock, respectively, 96% of CDS exhibit increase in antisense transcription with median antisense-log2FC equal to 2.8) while numbers of up- and down-regulated CDS (sense expression) is the same. Under the control conditions, the sense and antisense expression exhibit a significant negative Pearson's correlation (95% confidence interval is [−0.39, −0.46]), which indicates its regulatory role, but after 30 min of heat stress, the correlation drops dramatically to [−0.14, −0.27]. It may indicate that under the heat stress antisense transcription loses its regulatory role.
MS-proteomics does not identify unannotated proteins
We used LC-MS/MS mass spectrometry to profile the M. gallisepticum proteins under the control conditions and after 5, 15 and 30 min of heat shock, and we obtained 278 615 MS2 spectra. Using these spectra, we performed an annotation-guided identification of peptides (see Materials and Methods), resulting in 6619 peptides from 622 genes. We used these data to calculate the PAI (17). Our analysis revealed a relatively high protein-to-RNA correlation (pro > 0.55): the PAI values measured under both the control and heat shock conditions correlate with the control levels of mRNA. The protein-to-RNA correlation drops during heat shock because the mRNA abundances change dramatically, whereas the protein levels seem to remain unaffected by such short periods (Figure 1E).
In attempt to identify the novel peptides, we performed a genome-wide peptide search (see Materials and Methods). The procedure resulted in 5634 peptides, most of which (5475) are tryptic peptides that originate from the known proteins. Thirty-three (of the 159 remaining) peptides originated from the ORFs of known genes and correspond to an ORF extension (by usage of the upstream start codon) of seven genes. In two of these seven genes, the extensions are supported by more than one peptide. Most (87) of the remaining 126 peptides are confirmed by only a single spectrum. Grouping these peptides by ORF revealed only four short ORFs supported by more than one peptide. All these ORFs appear to be part of known proteins disrupted by a frame shift that can be explained by sequencing errors.
The results agree with the lack of unannotated CDSs. Consequently, 79 SOTs with a total length of 115 590 nt (1.3% of mapped reads, excluding tRNA or rRNA reads) with no protein-coding ability are either transcription junk or serve other functions.
Prediction of RBS
To determine the strength of RBS, we used the RNAduplex program to search for the best (in terms of free energy) RNA duplex that was formed by the 3′-terminal region of 16S rRNA (UUACCUCCUUUCU, homologous to slightly extended Shine–Dalgarno sequence-binding region of E. coli (43)) and 100 nt regions upstream of annotated CDSs. Duplexes with a free energy below −8 kcal/mol show pronounced enrichment in the (−25,−1) region (Supplementary Figure S7). We used the (−100,−76) region as a negative control to estimate the FDR, and we found RBSs for 160 ORFs at an FDR of 40%. When the number of RBSs was restricted to 100, the FDR dropped to 30%. These 100 RBSs have consensus aAAAAGGAGGtaa (nucleotides shown in upped case form secondary structure with the 3′-terminal region of 16S rRNA in more than 50% cases according to RNAduplex). The duplex-free energy exhibits a weak but significant correlation with the average abundance of both protein and mRNA (rho = −0.22 and −0.27; P < 2e-7 and 1e-10, respectively).
Core promoter and hairpinless down-CS regulates gene expression under heat stress
Molecular mechanisms must be responsible for the observed dramatic changes in mRNA abundance under heat and other stresses. In the Mycoplasma species, the only TF with a known binding site is a heat-shock repressor, HrcA (GCW_02005), that binds a conserved inverted repeat TTAGCACTC-N9-GAGTGCTAA known as CIRCE (5,44). A genome-wide scan for CIRCE elements that allowed up to two mismatches revealed five CIRCE elements in M. gallisepticum S6 (Supplementary Table S8). Four of these elements are located upstream of the chaperone gene promoters (clpB, dnaK, lon and dnaJ_2). The remaining element is located in a pseudo-gene (dnaJ homolog split into three ORFs) promoter region. Logically, the respective genes are up-regulated under heat stress (Figure 4C). However, their expression fold changes are not significantly higher than those of the other heat-shock up-regulated genes.
We applied the edgeR (21) package to the 5′-ERS read counts to identify TSSs with significant activity changes (BH-corrected P < 0.05 and fold change above two) under heat shock (see Materials and Methods). We divided all TSSs into six non-overlapping classes by the mean activity (high or low) and by the change direction: up, not significant and down (Figure 5A). We applied the MEME suite (32) to the sequences that were within 100 nt up- or downstream of CDS-associated TSSs from all six classes but, unfortunately, found no enriched motifs. Even the CIRCE elements discussed above could not be identified, most likely due to their low abundance.
Figure 5.
TSS and T3S formation contributions to gene expression regulation. (A) Fold change versus mean activity plot (log scale) for TSSs under heat shock. The TSSs associated with the coding genes are shown with filled circles, the TSSs with significant changes and with |FC| > 1 are shown in red, and the TSSs with significant changes but with |FC| < 1 are shown in yellow. The TSSs are divided into six groups by the change direction (up, down and not significant (denoted by ‘!sign’)) and by the average activity (low or high; the vertical line represents the median). (B) Distributions of TSS properties among the six TSS groups defined in A: type of −10 box, A/T content of the −10 element extension, proportion of promoters with a 6-nt-long spacer between the −10 element and the TSS and frequencies of first transcript nucleotide. (C) Pearson's correlation, proportion of explained variance and proportion of TSSs with the correct direction of change prediction for the self- and cross-validation of the random forest modeling of the heat-shock fold change. In total, 500 permutations were performed, and in each case, the learning and test sets consisted of 90% and 10% of 495 significant TSSs, respectively. (D) Distributions of down-CS efficiency under different conditions and dependence on the presence of hairpins. (E) Modeling of the interval expression by additive TSS and multiplicative T3S formation activity. The colored areas represent a smoothed read coverage, the interval borders are shown with vertical lines, and the mean interval coverage is shown with solid lines. The read coverage profiles under the control conditions and heat shock are shown in gray and red, respectively.
Comparing the six TSS classes revealed that the TSSs activated under heat shock differ strikingly from the down-regulated TSSs independently of the mean activity. The heat shock-activated TSSs usually are not associated with CDSs, have a non-canonical spacer (5 or 7 nt) between the −10 element and the TSS, have a lower AT content in the −10 element extension and frequently have a canonical (TAWAAT) −10 element with a preference for TAAAAT (unlike the down-regulated promoters that prefer TATAAT and have a high fraction of non-canonical −10 elements). Additionally, heat shock-activated transcription is usually initiated from guanosine, whereas the preferred first nucleotide of most down-regulated transcripts is adenosine (Figure 5B). These observations suggest that an appreciable part of the heat shock-related expression variation can be explained by these few factors. We used the random forests algorithm (26) to predict the log fold change based on the −10 element sequence, the first nucleotide of the transcript, the spacer length, the A/T richness of −10 element extensions and nucleotide content of first 100 nt of transcript (see Materials and Methods). The model explained 82% and 20% of the total variance and achieved a Pearson's correlation equal to 0.95 and 0.45 in self- and cross-validation, respectively. The correlation reaches 0.56 for cross-validation when only heat shock-related TSSs are considered (Figure 5C).
In addition to the transcription initiation that is usually considered a major contributor to transcription regulation, other steps of mRNA synthesis, such as elongation, termination or processing (such as cleavage or degradation), may also play a role. For example, a change in the efficiency of T3E formation (through transcription termination or RNA cleavage) might change the expression levels of downstream intervals. In our data, we observed a sufficient drop (by 15%, from −0.72 to −0.56) in efficiency of the hairpinless, but not the hairpin-containing, down-CSs (Figure 5D). To further investigate the contribution of T3E formation to transcription regulation, we modeled the read coverage in each interval as result from the additive contributions of up-CSs and the multiplicative contribution of down-CSs (see Materials and Methods and Figure 5E). This modeling allowed us to decompose the effects of TSSs and T3E formation on the expression change of each interval. The results show that for most of the intervals (71% of the 657 expressed intervals that do not overlap with tRNA or rRNA), a single factor (either a TSS or T3E formation) explains more than 80% of the expression variability under heat shock. In general, 87–92% of the transcription variability under heat and other stresses could be explained solely by changes in TSS activity, but there are still many intervals with low expression that are mainly regulated by T3E formation. In most of the intervals (490), more than 50% of the observed expression variability under heat shock could be attributed to TSSs, whereas in the remaining 167 intervals, the effect of T3E formation dominates. Of these 167 intervals, 100 change expression significantly under heat shock, and most of them (84%) are activated. The 86 intervals regulated by T3E formation contain 158 CDSs. Of these, 114 change their expression significantly under heat shock. Most of these CDSs (77%) belong to clusters 2 and 3 (Figure 4B). The genes that are up-regulated under heat shock due to the T3E formation change are involved in the process of ‘carbohydrate transport,’ whereas the TSS-regulated genes play a role in transposition, ‘response to stress’ and proteolysis (Supplementary Table S9).
Interestingly, although under most conditions, the hairpin containing down-CS have nearly perfect efficiency (∼ −0.95), their efficiency drops by 8% under osmotic stress (Figure 5D).
To provide additional experimental confirmation of heat shock-related regulation of hairpinless down-CS, we performed RT-PCR experiments for four selected cases. For each case we measured transcript concentration by two pairs of primers: designed for upstream and downstream of down-CS (up- and down-abundance). For all but one cases up-abundance exceeds down-abundance as expected (Supplementary Table S14). One case (step id 1008) with down-abundance exceeding up-abundance could be explained by sequence dependent RT-PCR efficiency. Importantly, heat shock-induced increase in down-/up-abundance ratio in all cases, thus providing independent confirmation of heat shock-dependent regulation of hairpinless T3E formation.
Transformation of M. gallisepticum
To provide experimental confirmation of sufficiency of identified transcript structure for efficient transcription and translation we constructed a transformation vector with Tn4001 transposase and tetracycline resistance gene (see Materials and Methods) and successfully used it for establishing stable transformation of M. gallisepticum. Coding regions were constructed de novo. For 5′UTRs (including RBS) and promoter regions we used rpsP upstream region (identified as a highly active TSS with very short UTR) slightly modified (to improve transcription and translation efficiency). We used 3′UTRs (including terminator hairpins) from rRNA precursor and dps (Supplementary Figures S9 and S10).
Transformation efficiency was 10−6 (Supplementary Table S15) as reported by Pour-El et al. (45) for Tn4001-based vector. However, our vector allows use of 1 ng of plasmid DNA (resulting in about 30–50 CFU per 1 ml of late-logarithm cell culture) instead of 10 mkg as reported by Pour-El et al. for transposon vector (45) and Markham et al. for OriC-plasmid (27).
Integration of vector into genome was confirmed for three clones by chromosome walking (Supplementary Table S16). Transcription of tetM gene was measured by real-time PCR (Supplementary Table S17) and was in average (for four transformants) 4-fold higher than of glycerol-3-phosphate dehydrogenase (gapd) and 10-fold higher than of enolase. Number of vector integrations was identified as 1 per genome by real-time PCR and verified by chromosome walking. Translation of tetM was verified by mass spectrometry (10–12 TetM peptides were identified in 4 clones; see Data Access section in Materials and Methods). TetM abundance according to PAI is average: about 50% of detected M. gallisepticum proteins are more abundant than TetM.
DISCUSSION
Here, for the first time, we present a genome-wide analysis of the regulation of transcription initiation and formation of transcripts 3′ends in M. gallisepticum strain S6. Key difference of our study from previous research on M. pneumoniae (7) is that we applied experimental genome-wide identification of TSSs and measured their activity. An analysis of 5′-ERS libraries allowed us to identify 1061 TSSs genome-wide and to decipher promoter structure. Our interval-based analysis allowed us to split the genome into 1059 intervals with equal expression levels, to group them into 208 sets of overlapping transcripts and to decompose the effects of transcription initiation and T3E formation on the mRNA concentrations. We have shown a dramatic reorganization of transcription under different biological conditions, such as heat shock or the stationary growth phase. We found no regulatory sequence motifs that could be responsible for the observed regulation other than CIRCE, which was found using a priori knowledge. We speculate that other motifs, even if they do exist, could not be found due to their low abundance and thus cannot be responsible for the observed dramatic transcription changes under heat stress.TAWAAT sequences are avoided in M. gallisepticum (they are 2-fold less abundant than expected by chance), but their number (3088) is still greater than both: the number of annotated genes and the number of detected TSSs. Only a subset of these sequences is likely to constitute functional promoters. How does the M. gallisepticum transcription machinery distinguish the functional promoters from the cryptic ones? Additional features, such as the A/T-richness of the surrounding region, the correct spacer length and the first nucleotide of the transcript could play a role. Increased temperatures may facilitate transcription initiation on cryptic promoters via the enhanced melting of the DNA, leading to ‘promiscuous transcription’. Indeed, heat shock-activated promoters have a lower AT content, a spacer whose length is not 6 nt and an alteration in the first nucleotide of the transcript (G instead of A). Modeling revealed that these features explain a substantial proportion of the transcription variation under heat shock.
Most of the heat shock-activated promoters are not associated with CDSs. Because the major fraction of the M. gallisepticum genome encodes proteins, the activation of cryptic promoters leads to widespread antisense expression. Loss of negative coordination between sense and antisense transcription confirms the idea of ‘promiscuous transcription’. Observed changes in the antisense transcription could have an adaptive function. Widespread activation of the antisense transcription taken together with the loss of sense to antisense coordination rather indicates that in the heat stress these changes are not functional. Though, part of these RNAs seems to carry regulatory role under normal growth. We speculate that the down-regulation of promoters during heat stress may be the result of promoter competition for RNA polymerase as a result of widespread activation of transcription.
This finding suggests that a significant part of the heat-induced transcription changes are non-adaptive. In contrast, CDSs, whose expression follows specific patterns during heat shock or changes under oxidative stress are enriched in the relevant biological functions; this finding indicates a tight balance between the transcription noise and regulation.
The HrcA-CIRCE regulatory system is conserved among Mycoplasmas, and this conservation implies its functional importance. However, under heat-stress conditions, CIRCE-dependent promoters behave similarly to numerous CIRCE-less up-regulated promoters, raising questions about the true function of CIRCE. Non-adaptive stress-induced expression changes were previously identified in different bacterial species (46). We speculate that under heat shock in M. gallisepticum, a few adaptive changes, most likely guided by specific TFs, occur on the background of a noise-like response.
Our analysis of T3Es formation reveals two classes of T3Es: strong, hairpin-containing and weak, hairpinless. While first are likely to be primary T3Es, second could originate from RNA cleavage. Whereas hairpin-containing T3Es are mostly not regulated during 30 min of heat shock, the efficiency of the hairpinless T3Es drops dramatically, apparently contributing to the ‘promiscuous transcription’ described above. A significant portion of the up-regulated CDSs during heat stress can be attributed to regulated formation of T3E. As we demonstrated above, a set of the genes coding for carbohydrate uptake proteins is regulated by T3E formation rather than by TSSs. Carbohydrate transport under heat stress may be adaptive, as carbohydrates are a source of ATP for chaperones. We speculate that the gene expression regulation by T3E formation may represent a novel mechanism of adaptive regulation in genome-reduced bacteria.
Our proteomic study shows that the mRNA and protein concentrations correlate well under the control conditions. However, during heat shock, the mRNA, but not protein, levels change dramatically, resulting in a strong decrease in the mRNA-protein correlation. Such a discrepancy might be explained by the different time scales of mRNA and protein turnover (28).
Sequencing efficiency greatly depends on the sequence and, especially, on the secondary structure of RNA, resulting in a highly variable read coverage depth (29). In the current work, we attempted to address this issue by pooling all samples together to minimize the noise during interval prediction and allowing for overdispersion by using a quasi-Poisson distribution. As a side effect of this approach, we have likely lost certain condition-dependent information. The relatively low agreement between the TSSs predicted using up-CSs and those predicted using 5′-ERS might also be explained by a high coverage variability that may lead to both the identification of false up-CSs and the loss of correct TSSs. The design of our sequencing pipeline resulted in loss of RNAs shorter than 150–200 bp, which could have resulted in a loss of some regulatory RNAs.
In summary, we have taken a step toward understanding the stress-response mechanisms in M. gallisepticum and in genome-reduced bacteria in general. Our interval-based approach allowed us to look beyond the operon concept, identify two classes of T3Es and decipher their roles in transcription regulation. We found that properties of promoters and T3Es taken together can explain significant amount of transcriptional changes under stress conditions in M. gallisepticum without need of specific transcriptional factors. We believe that the constructed transcription map together with a comprehensive list of TSSs and T3Es in M. gallisepticum will enhance future research.
SUPPLEMENTARY DATA
Supplementary Data are available at NAR Online.
Footnotes
The authors wish it to be known that, in their opinion, the first two authors should be regarded as Joint First Authors.
Present address: Gleb Fisunov, Department of Molecular Biology and Genetics, Research Institute of Physical Chemical Medicine, Malaya Pirogovskaya 1a, Moscow 119435, Russia.
FUNDING
Russian Science Foundation [14–24–00159] «Systems research of minimal cell on a Mycoplasma gallisepticum model». Funding for open access charge: Russian Science Foundation [14–24–00159] «Systems research of minimal cell on a Mycoplasma gallisepticum model».
Conflict of interest statement. None declared.
REFERENCES
- 1.Davis J.J., Xia F., Overbeek R.A., Olsen G.J. Genomes of the class Erysipelotrichia clarify the firmicute origin of the class Mollicutes. International journal of systematic and evolutionary microbiology. 2013;63:2727–2741. doi: 10.1099/ijs.0.048983-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Levisohn S., Kleven S.H. Avian mycoplasmosis (Mycoplasma gallisepticum) Rev. sci. tech. Off. int. Epiz. 2000;19:425–442. [PubMed] [Google Scholar]
- 3.Razin S., Yogev D. Molecular biology and pathogenicity of mycoplasmas. Microbiology and Molecular Biology. 1998;62:1094–1156. doi: 10.1128/mmbr.62.4.1094-1156.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moreno-Campuzano S., Janga S.C., Pérez-Rueda E. Identification and analysis of DNA-binding transcription factors in Bacillus subtilis and other Firmicutes-a genomic approach. BMC genomics. 2006;7:147. doi: 10.1186/1471-2164-7-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang L.-J., Chen W.-H., Minion F.C., Shiuan D. Mycoplasmas regulate the expression of heat-shock protein genes through CIRCE-HrcA interactions. Biochemical and biophysical research communications. 2008;367:213–218. doi: 10.1016/j.bbrc.2007.12.124. [DOI] [PubMed] [Google Scholar]
- 6.Carvalho F.M., Fonseca M.M., Batistuzzo De Medeiros S., Scortecci K.C., Blaha C.A.G., Agnez-Lima L.F. DNA repair in reduced genome: the Mycoplasma model. Gene. 2005;360:111–119. doi: 10.1016/j.gene.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 7.Güell M., van Noort V., Yus E., Chen W.-H., Leigh-Bell J., Michalodimitrakis K., Yamada T., Arumugam M., Doerks T., Kühner S., et al. Transcriptome complexity in a genome-reduced bacterium. Science. 2009;326:1268–1271. doi: 10.1126/science.1176951. [DOI] [PubMed] [Google Scholar]
- 8.Weiner J., III, Zimmerman C.-U., GoÈhlmann H.W.H., Herrmann R. Transcription profiles of the bacterium Mycoplasma pneumoniae grown at different temperatures. Nucleic Acids Research. 2003;31:6306–6320. doi: 10.1093/nar/gkg841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gorbachev A.Y., Fisunov G.Y., Izraelson M., Evsyutina D.V., Mazin P.V., Alexeev D.G., Pobeguts O.V., Gorshkova T.N., Kovalchuk S.I., Kamashev D.E., et al. DNA repair in Mycoplasma gallisepticum. BMC Genomics. 2013;14:726. doi: 10.1186/1471-2164-14-726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jacob F., Monod J. Genetic regulatory mechanisms in the synthesis of proteins. Journal of molecular biology. 1961;3:318–356. doi: 10.1016/s0022-2836(61)80072-7. [DOI] [PubMed] [Google Scholar]
- 11.Cho B., Zengler K., Qiu Y., Park Y.S., Knight E.M., Barrett C., Gao Y., Palsson B.Ø. Elucidation of the Transcription Unit Architecture of the Escherichia coli K-12 MG1655 Genome. Nat Biotechnol. 2013;27 doi: 10.1038/nbt.1582. doi:10.1038/nbt.1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kobayashi H., Akitomi J., Fujii N., Kobayashi K., Altaf-Ul-Amin M., Kurokawa K., Ogasawara N., Kanaya S. The entire organization of transcription units on the Bacillus subtilis genome. BMC genomics. 2007;8:197. doi: 10.1186/1471-2164-8-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Toledo-Arana A., Dussurget O., Nikitas G., Sesto N., Guet-Revillet H., Balestrino D., Loh E., Gripenland J., Tiensuu T., Vaitkevicius K., et al. The Listeria transcriptional landscape from saprophytism to virulence. Nature. 2009;459:950–956. doi: 10.1038/nature08080. [DOI] [PubMed] [Google Scholar]
- 14.Sharma C.M., Hoffmann S., Darfeuille F., Reignier J., Findeiss S., Sittka A., Chabas S., Reiche K., Hackermüller J., Reinhardt R., et al. The primary transcriptome of the major human pathogen Helicobacter pylori. Nature. 2010;464:250–255. doi: 10.1038/nature08756. [DOI] [PubMed] [Google Scholar]
- 15.Laemmli U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 16.Vizcaíno J.A., Côté R., Reisinger F., Foster J.M., Mueller M., Rameseder J., Hermjakob H., Martens L. A guide to the Proteomics Identifications Database proteomics data repository. Proteomics. 2009;9:4276–4283. doi: 10.1002/pmic.200900402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ishihama Y., Schmidt T., Rappsilber J., Mann M., Hartl F.U., Kerner M.J., Frishman D. Protein abundance profiling of the Escherichia coli cytosol. BMC genomics. 2008;9:102. doi: 10.1186/1471-2164-9-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Langmead B., Trapnell C., Pop M., Salzberg S.L. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome biology. 2009;10:R25. doi: 10.1186/gb-2009-10-3-r25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gardner P.P., Barquist L., Bateman A., Nawrocki E.P., Weinberg Z. RNIE: genome-wide prediction of bacterial intrinsic terminators. Nucleic acids research. 2011;39:5845–5852. doi: 10.1093/nar/gkr168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mazin P., Xiong J., Liu X., Yan Z., Zhang X., Li M., He L., Somel M., Yuan Y., Phoebe Chen Y.-P., et al. Widespread splicing changes in human brain development and aging. Molecular systems biology. 2013;9:633. doi: 10.1038/msb.2012.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Robinson M.D., McCarthy D.J., Smyth G.K. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26:139–140. doi: 10.1093/bioinformatics/btp616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anders S., Huber W. Differential expression analysis for sequence count data. Genome biology. 2010;11:R106. doi: 10.1186/gb-2010-11-10-r106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Benjamini Y., Hochberg Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. Journal of the Royal Statistical Society. Series B. 2009;57:289–300. [Google Scholar]
- 24.Conesa A., Götz S., García-Gómez J.M., Terol J., Talón M., Robles M. Blast2GO: a universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics. 2005;21:3674–3676. doi: 10.1093/bioinformatics/bti610. [DOI] [PubMed] [Google Scholar]
- 25.Young M.D., Wakefield M.J., Smyth G.K., Oshlack A. Gene ontology analysis for RNA-seq: accounting for selection bias. Genome biology. 2010;11:R14. doi: 10.1186/gb-2010-11-2-r14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liaw A., Wiener M. Classification and Regression by randomForest. R news. 2002;2:18–22. [Google Scholar]
- 27.Lee S.-W., Browning G.F., Markham P.F. Development of a replicable oriC plasmid for Mycoplasma gallisepticum and Mycoplasma imitans, and gene disruption through homologous recombination in M. gallisepticum. Microbiology. 2008;154:2571–2580. doi: 10.1099/mic.0.2008/019208-0. [DOI] [PubMed] [Google Scholar]
- 28.Maier T., Schmidt A., Güell M. Quantification of mRNA and protein and integration with protein turnover in a bacterium. Molecular systems. 2011;7:511. doi: 10.1038/msb.2011.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khrameeva E.E., Gelfand M.S. Biases in read coverage demonstrated by interlaboratory and interplatform comparison of 117 mRNA and genome sequencing experiments. BMC bioinformatics. 2012;13(Suppl. 6):S4. doi: 10.1186/1471-2105-13-S6-S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taboada B., Ciria R., Martinez-Guerrero C.E., Merino E. ProOpDB: Prokaryotic Operon DataBase. Nucleic acids research. 2012;40:D627–D631. doi: 10.1093/nar/gkr1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Voskuil M.I., Chambliss G.H. The TRTGn Motif Stabilizes the Transcription Initiation Open Complex. Journal of Molecular Biology. 2002;322:521–532. doi: 10.1016/s0022-2836(02)00802-1. [DOI] [PubMed] [Google Scholar]
- 32.Bailey T.L., Elkan C. Fitting a mixture model by expectation maximization to discover motifs in biopolymers. Proceedings of the Second International Conference on Intelligent Systems for Molecular Biology. 1994;2:28–36. [PubMed] [Google Scholar]
- 33.Hinton D.M. Transcription Initiation by Mix and Match Elements: Flexibility for Polymerase Binding to Bacterial Promoters. Gene Regul Syst Bio. 2007;1:275–293. [PMC free article] [PubMed] [Google Scholar]
- 34.Cortes T., Schubert O.T., Rose G., Arnvig K.B., Comas I., Aebersold R., Young D.B. Genome-wide mapping of transcriptional start sites defines an extensive leaderless transcriptome in Mycobacterium tuberculosis. Cell reports. 2013;5:1121–1131. doi: 10.1016/j.celrep.2013.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Farnham P.J., Platt T. Rho-independent termination: dyad symmetry in DNA causes RNA polymerase to pause during transcription in vitro. Nucleic Acids Research. 1981;9:563–577. doi: 10.1093/nar/9.3.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dillies M.-A., Rau A., Aubert J., Hennequet-Antier C., Jeanmougin M., Servant N., Keime C., Marot G., Castel D., Estelle J., et al. A comprehensive evaluation of normalization methods for Illumina high-throughput RNA sequencing data analysis. Briefings in bioinformatics. 2013;14:671–683. doi: 10.1093/bib/bbs046. [DOI] [PubMed] [Google Scholar]
- 37.Kiley P.J., Beinert H. The role of Fe–S proteins in sensing and regulation in bacteria. Current Opinion in Microbiology. 2003;6:181–185. doi: 10.1016/s1369-5274(03)00039-0. [DOI] [PubMed] [Google Scholar]
- 38.Coba de la Peña T., Redondo F.J., Fillat M.F., Lucas M.M., Pueyo J.J. Flavodoxin overexpression confers tolerance to oxidative stress in beneficial soil bacteria and improves survival in the presence of the herbicides paraquat and atrazine. Journal of applied microbiology. 2013;115:236–246. doi: 10.1111/jam.12224. [DOI] [PubMed] [Google Scholar]
- 39.Liu G., Zhou J., Fu Q.S., Wang J. The Escherichia coli azoreductase AzoR Is involved in resistance to thiol-specific stress caused by electrophilic quinones. Journal of bacteriology. 2009;191:6394–6400. doi: 10.1128/JB.00552-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.MacMillan S., Alexander D. The ion coupling and organic substrate specificities of osmoregulatory transporter ProP in Escherichia coli. Biochimica et biophysica acta. 1999;1420:30–44. doi: 10.1016/s0005-2736(99)00085-1. [DOI] [PubMed] [Google Scholar]
- 41.Krämer R. Osmosensing and osmosignaling in Corynebacterium glutamicum. Amino acids. 2009;37:487–497. doi: 10.1007/s00726-009-0271-6. [DOI] [PubMed] [Google Scholar]
- 42.Patzlaff J.S., van der Heide T., Poolman B. The ATP/substrate stoichiometry of the ATP-binding cassette (ABC) transporter OpuA. The Journal of biological chemistry. 2003;278:29546–29551. doi: 10.1074/jbc.M304796200. [DOI] [PubMed] [Google Scholar]
- 43.Shine J., Dalgarno L. The 3′-terminal sequence of Escherichia coli 16S ribosomal RNA: complementarity to nonsense triplets and ribosome binding sites. Proc. Natl. Acad. Sci. U.S.A. 1974;71:1342–1346. doi: 10.1073/pnas.71.4.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zuber U., Schumann W. CIRCE, a novel heat shock element involved in regulation of heat shock operon dnaK of Bacillus subtilis. Journal of bacteriology. 1994;176:1359–1363. doi: 10.1128/jb.176.5.1359-1363.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pour-El I., Adams C., Minion F.C. Construction of mini-Tn4001tet and its use in Mycoplasma gallisepticum. Plasmid. 2002;47:129–37. doi: 10.1006/plas.2001.1558. [DOI] [PubMed] [Google Scholar]
- 46.Price M.N., Deutschbauer A.M., Skerker J.M., Wetmore K.M., Ruths T., Mar J.S., Kuehl J.V., Shao W., Arkin A.P. Indirect and suboptimal control of gene expression is widespread in bacteria. Molecular systems biology. 2013;9:660. doi: 10.1038/msb.2013.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.