Abstract
Retinoblastoma has the unique capacity to accelerate its own intra-ocular propagation by adopting semi-solid or even liquid growth properties through seeding. Until recently, the presence of any degree of seeding was mostly incompatible with successful conservative management, due to the multiresistant nature of the seeds. Surprisingly, this well-recognized retinoblastoma behavior has not undergone any detailed description of seeding patterns and anatomic sites. In this paper, we describe the phenotypic variability of seeds across the four possible intraocular seeding compartments and classify them into three fundamental types: namely dust, spheres, and clouds. We also provide an overview of the different therapeutic strategies developed for seeding, with special attention to intravitreal chemotherapy as the treatment of choice for vitreous and retro-hyaloid seeding. Finally, we propose criteria to enable assessment of the response to treatment by reporting seed regression patterns, as well as a clinical grading system for the retinal toxicity observed following intravitreal melphalan.
Keywords: Classification, intravitreal chemotherapy, retinoblastoma, seeding
DEFINITION
Retinoblastoma seeding refers to any type of tumor dispersion into an adjacent liquid or semi-liquid compartment. Seeding is typically seen in advanced intra-ocular retinoblastoma and represents a major determinant for eye grouping at presentation (see International Classification of Retinoblastoma:1 groups C, D, and E). It can also be a feature observed in extra-ocular retinoblastoma (see Retinoblastoma Staging System:2 stages IIN3 and IVb3).
THE SEEDING ANATOMIC SITES
Intra-ocular retinoblastoma (Figure 1A) may seed into four distinct anatomic sites (Figure 1B–E): (1) tumor dispersion into the vitreous gel following endophytic disruption of the internal limiting membrane (ILM) and hyaloid at tumor apex; (2) tumor suspension spreading into the retro-hyaloidal space secondary to endophytic disruption of the ILM at tumor base alone, and partial or complete posterior vitreous detachment; (3) tumor suspension into the subretinal space created by exophytic growth; and (4) tumor suspension into the aqueous fluid of the posterior and anterior chambers secondary to disruption of the anterior hyaloid. In addition, retinoblastoma growth extending anteriorly to the ciliary body can cause a contaminated supraciliary effusion.3 Extra-ocular retinoblastoma may seed in two different anatomic sites: (1) anteriorly into the amniotic fluid in the case of fetal fungating retinoblastoma,4, 5 and (2) posteriorly in the case of retro-laminar invasion with seeding into the circulating subarachnoidal fluid and progress from localized micrometastasis (stage IIN3) with negative lumbar punction cytology6 to diffuse CNS disease with leptomeningeal carcinomatosis (stage IVb3). Alternatively, direct seeding into the CSF can occur from a pinealoblastoma or a parasellar tumor in the case of trilateral retinoblastoma.7
FIGURE 1.
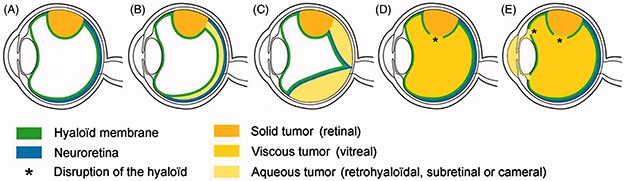
Schematic view of the natural history of intra-ocular retinoblastoma growth with respect to the invasion of five clinically recognizable anatomic sites: (A) retina; (B) retro-hyaloid space; (C) subretinal space; (D) vitreous cavity; (E) posterior and anterior chambers.
SEED CLASSIFICATION
There are three distinct seeding patterns in retinoblastoma (Figure 2), namely (1) dust formation resulting from cellular infiltration (Figure 2A), (2) cloud formation resulting from translocation of the primary tumor content (Figure 2C), and (3) sphere formation resulting indirectly from clonal expansion of the dust (Figure 2B) or the cloud, or directly by sprouting of the primary retinal tumor (Figure 2D and E). The spheres may be either translucent (Figure 2H and I) or may have a whitish center (necrosis) surrounded by translucent mono- or multilayered tumor cells (Figure 2F–K).
FIGURE 2.
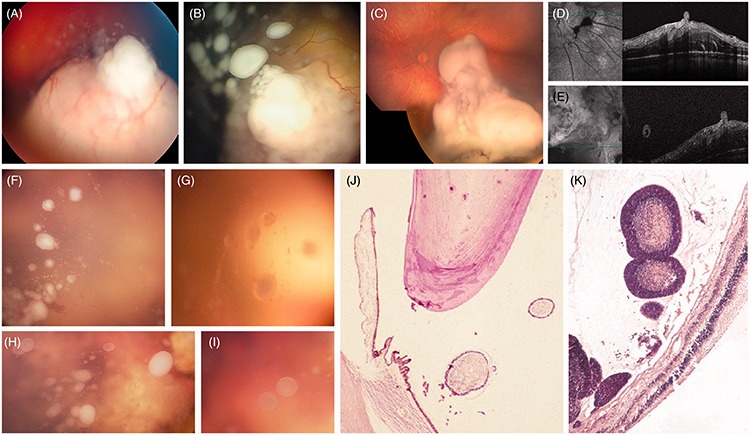
Seeding patterns of retinoblastoma: (A) dust following the apical disruption of the ILM/hyaloid complex; (B) spheres resulting either from clonal expansion of dust (or cloud) or (D and E) from sprouting; (C) cloud(s) following massive disruption of the ILM/hyaloid complex. Ophthalmoscopic aspect of vitreous seeds (F–I) white or translucent with a whitish (necrotic upon histopathology and hyper-reflective upon OCT) or translucent center (cystic on OCT, cf. Figure 7E and F). Histopathologic aspect of vitreous seeds (J and K) mono- or multilayered tumor cells surrounding necrotic material.
The three seeding types can occur in any of the four retro-hyaloidal, vitreous, subretinal, or intra-cameral seeding compartments. The tumor appearance and growth patterns will differ according to the physical nature of the invaded compartments. Tumor spread into the viscous vitreous gel initially resembles dusty (loose cellular spread) or cloudy (dense cumulus-like spread) infiltrates, resulting from localized (Figures 3 and 4) versus massive ILM/hyaloid disruption, respectively (Figure 5). The vast majority of the cells composing dust or clouds will not survive the hypoxic environment of the newly invaded compartment unless they develop the necessary metabolic reprogramming8 and/or resistance to anoikis.9 Based on our observations in the vitreous, the capacity for clonal expansion of the surviving cells can be divided into two populations with distinct phenotypic behaviors: (1) cells capable of adherence-independent growth forming free floating spheres (Figure 3D–H) and (2) cells under the control of adherence-dependent growth leading to the formation of seaweed-like or oval-shaped tumors anchored to the internal face of the posterior hyaloid (Figure 4A–F). Under the effect of gravity, the free-floating vitreous tumors slowly migrate and tend to accumulate inferiorly and to project anteriorly to the ora serrata. Finally, tumor spread into the retro-hyaloid (Figure 6) or subretinal (Figure 7) compartments is also associated with the formation of spheres, which differ from those in the vitreous by their ability to rapidly migrate according to gravitational stimuli, tending to accumulate inferiorly posterior to the vitreous base (Figure 6H and I) or the ora serrata (Figure 7C), respectively. In contrast to the free-floating vitreous seeds, the pre-hyaloid and retro-hyaloid seeds are all located at the same level, attach to the internal face of the hyaloid and ILM, respectively and tend to coalesce (Figures 4 and 7C and D). The retro-hyaloid seeds with adherence-independent properties behave either as free spheres (Figure 8E–H) or pavementous-like seeds in the meniscus of liquid created by a partial posterior hyaloid detachment (Figure 8A and B), in contrast with the hemispheric or oval-shaped aspect of seeds with adherence-dependent growth. The subretinal seeds may be free running (Figure 9A and B) or fixed to the external retinal surface (Figure 9C and D) or to the retinal pigment epithelium (Figure 9F and G). Finally, a large ILM disruption at tumor base can be followed by a massive transfer of tumor material into the retro-hyloidal space, creating a position-dependent circular level (Figure 10) masking the optic nerve head. The same phenomenon of cloud formation can occur under a detached retina, leaving the optic nerve head visible (Figure 7G–L).
FIGURE 3.
Characteristics of endophytic seeding into the vitreous cavity: (A and B) fine dust visible around the retinal tumor; (C) dust originating from the upper tumor; (D and E) multiple free-floating spheres growing within the initial dust, (F) sphere formation in the same patient 4 months after focal treatment of the retinal tumors. Note the concentration of localized spheres in the upper quadrant and their migration inferiorly; (G and H) diffuse migration of spheres projecting anterior to the ora serrata, (I) spheres anterior to the ora serrata, masking the corpus ciliaris except the pars plicata and corresponding UBM imaging (J).
FIGURE 4.
Characteristics of prehyaloid seeding, (A–H) partially coalescent seeds attached to the internal face of the posterior hyaloid; (I and J) OCT of prehyaloid seeds (same patient as G and H) showing signs of fragmentation and hyper-reflectivity and progressive hyaloid detachment following intravitreal injections of melphalan.
FIGURE 5.
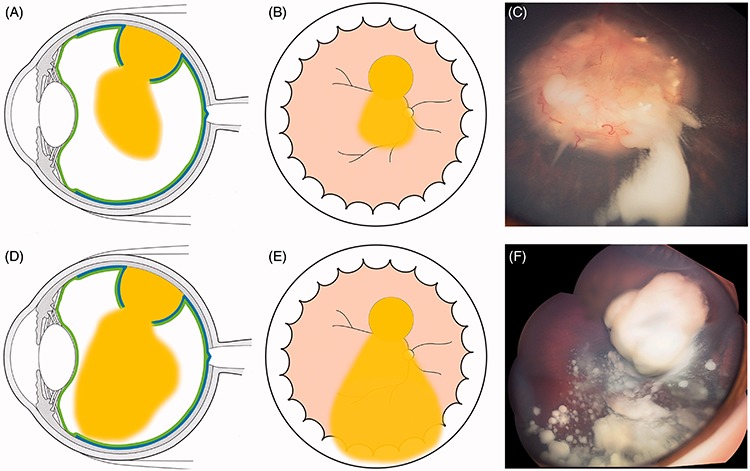
Localized (A–C) and diffuse (D–F) cloud formation with concomitant clonal growth (F). The cloud can be multiple as a result of iterative translocations of tumor material into the vitreous.
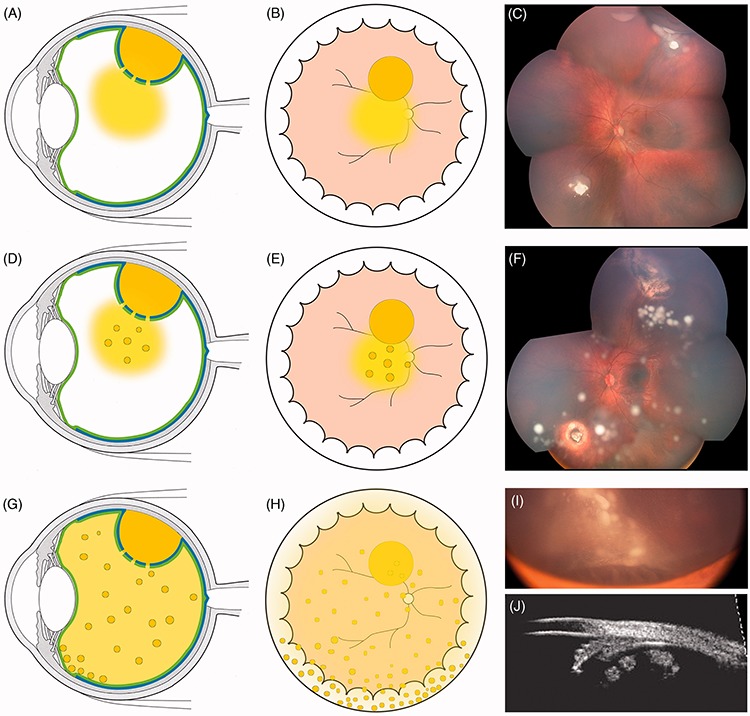
FIGURE 6.
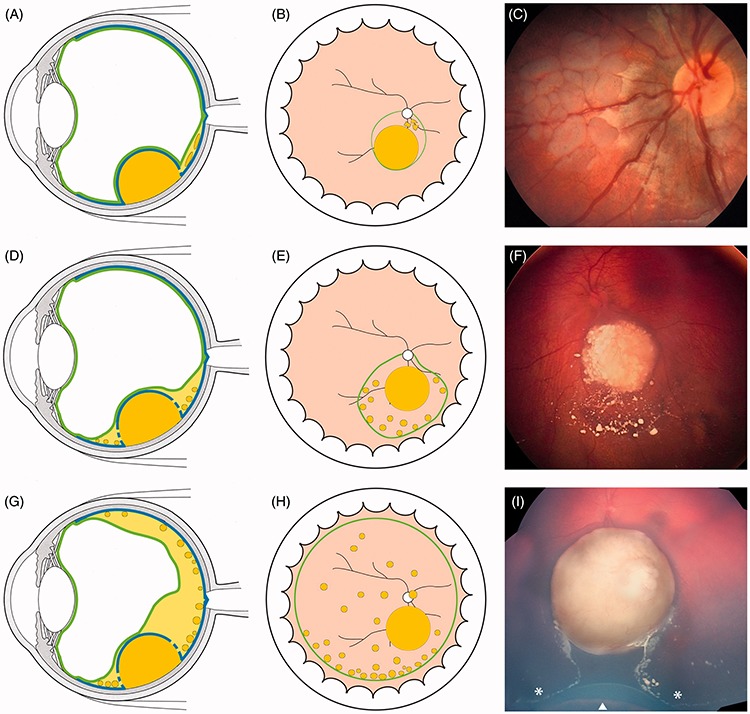
Retro-hyaloid seeding following partial (A–F) or complete (G–H) hyaloid detachment; (I) tumor at presentation with subtotal hyaloid detachment sparing the 6 o’clock meridian. The white arrow indicates the ora serrata and the white asterisks the anterior extension of the hyaloid detachment corresponding to the posterior border of the vitreous base.
FIGURE 7.
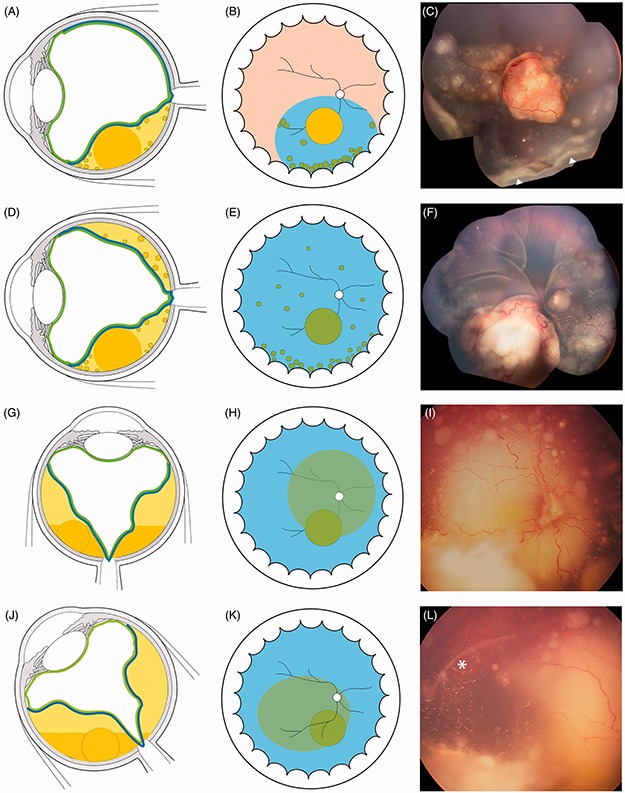
Characteristics of subretinal seeding (A–D) partial and total retinal detachment with subretinal seeding showing typical accumulation inferiorly at the ora serrata (Figure 9C white triangles) and tending to coalesce. (G–K) Subretinal cloud with position-dependent ophthalmoscopic contours and spirit level. The white asterisk highlights the limit of cloud extension in lateral decubitus.
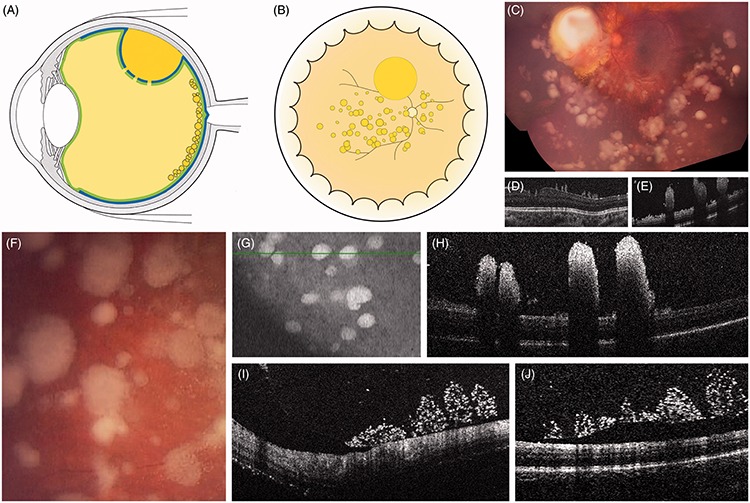
FIGURE 8.
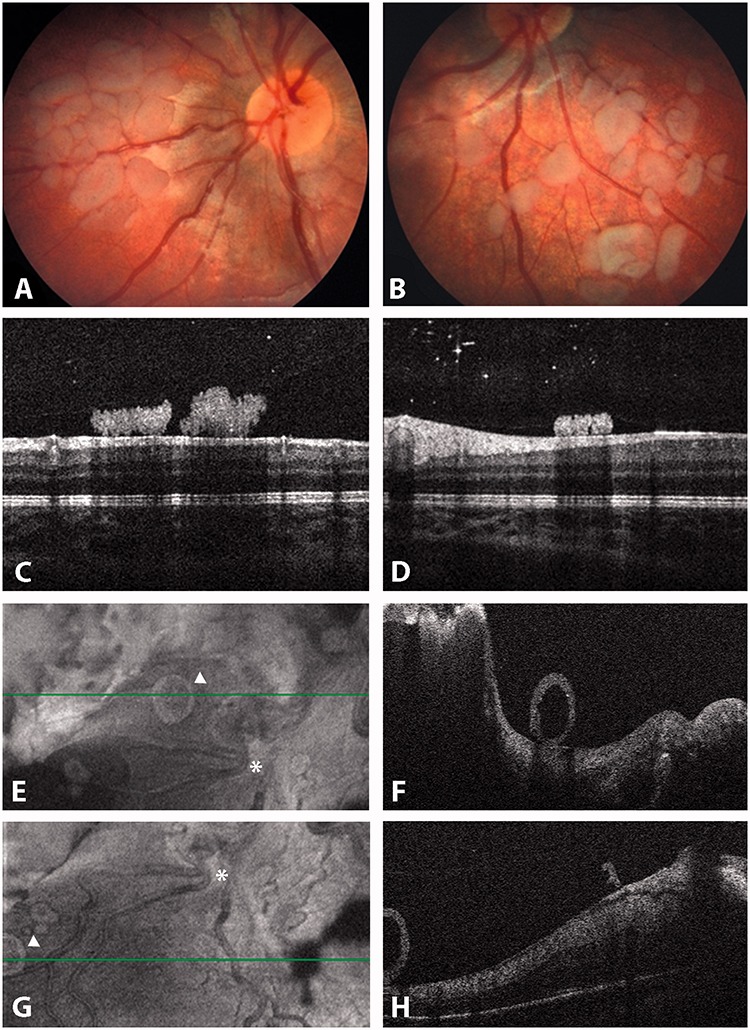
Retro-hyaloid seeding: (A–D) free pavementous-like seeds changing position with eye movements within a very shallow retro-hyaloid aqueous space; (F–H) cystic sphere rolling at the posterior pole. See the position of the seed highlighted by the white triangle (E and G) with respect to the vascular bifurcation (white asterisk).
FIGURE 9.
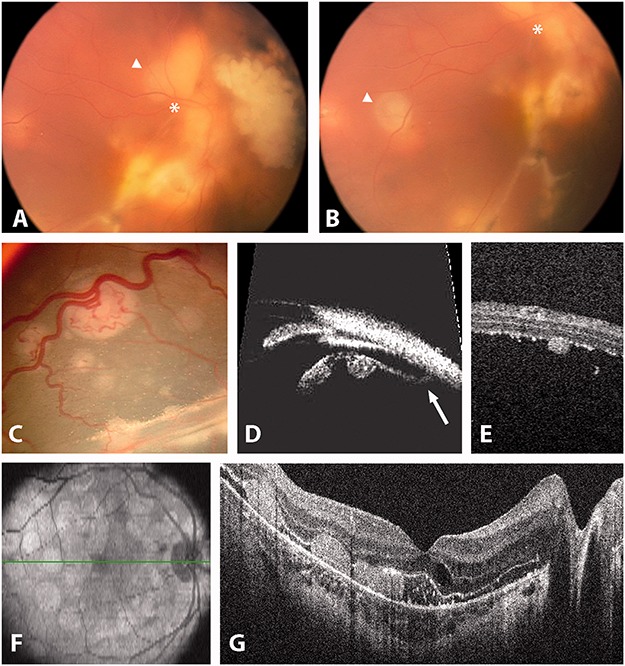
Subretinal seeding. (A and B) free subretinal sphere (white asterisks and triangles show the mobility of the seed). (C and D) Fixed subretinal seeds attached to the external retina as seen by ophthalmoscopy. (D) UBM. The white arrow highlights the oral insertion of the detached retina. (E) OCT showing a seed attached to the external retina. (F and G) OCT showing retinal pigment epithelium attachment of subretinal seeding following retinal reattachment.
FIGURE 10.
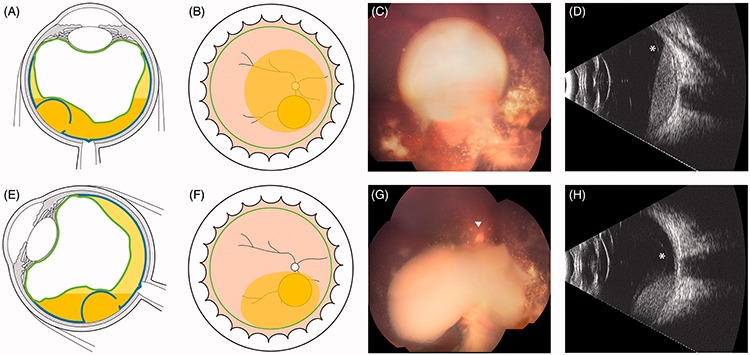
(A–H) Retro-hyaloid cloud with typical position-dependent ophthalmoscopic contours and spirit level on ultrasonography (white asterisks: detachment of the posterior hyaloid).
With tumor invasion of the aqueous fluid of the anterior segment following disruption of the anterior hyaloid, the clinical features can be divided into free-floating spheres or tumor cells, possibly forming a pseudo-hypopion, or tumors attached to the iris (spheres) and corneal endothelium (pavementous-like growth) (Figure 11).
FIGURE 11.
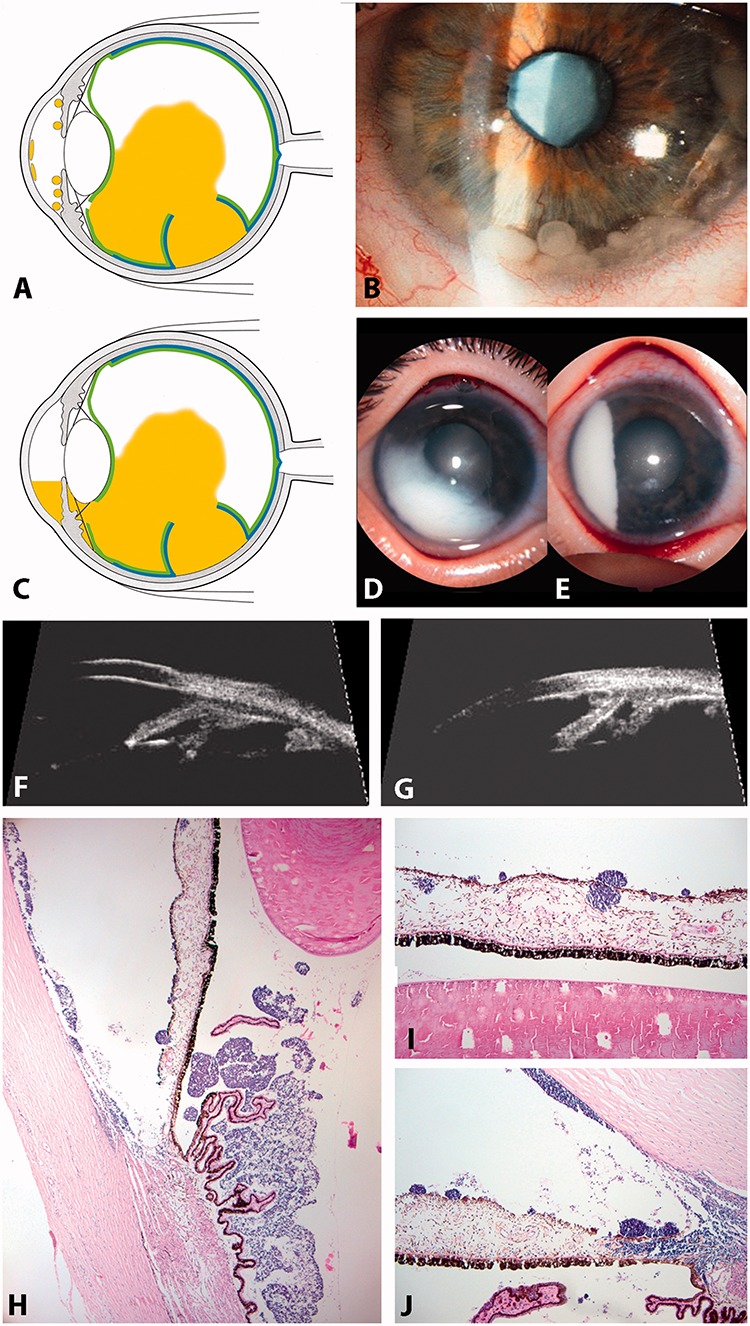
Anterior and posterior chamber seedings. (A and B) spheres in the anterior chamber; (C and D) pseudo-hypopyon (anterior chamber equivalent of a cloud); (E and F) UBM showing spheres invasion of the posterior chamber (E) and spheres and plaque formation in the anterior chamber (F); (G–I) HE staining of seeds in the anterior segment showing spheres and tumor plaques growing at the corneal endothelial surface (G and I).
THE THERAPEUTIC CHALLENGE OF SEEDING
While solid vascularized retinal tumors are easily accessible to various treatment modalities, the tumoral avascular counterpart involving the other ocular sites are either poorly controlled by conventional therapies or beyond any conservative treatment as in the case of anterior segment invasion (absolute criteria for enucleation). Lack of response of these avascular tumors may be explained by the inability of the present routes of antimitotic administration to achieve tumoricidal concentrations in the corresponding eye compartments. In addition, these tumors are virtually inaccessible to focal treatments and are highly radio resistant due to their hypoxic nature.
Primary Vitreous Seeding
The presence of diffuse vitreous seeding is a hallmark of advanced retinoblastoma characterizing group D eyes in the International Classification of Retinoblastoma,1 a feature typically necessitating enucleation or external beam radiotherapy. Reese10 already noted that the prognosis of eyes with vitreous seeding at presentation was “very unfavorable” and classified them in the most severe group (Vb).
Historically, the best salvage rates reported with first-line external beam radiotherapy barely exceeded 50% for group Vb eyes.11 The shift to first-line systemic (IVC) chemotherapy (with or without pre-chemo cryorupture of the external hemato-retinal barrier to increase the vitreous drug concentration) failed to improve eye survival of advanced retinoblastoma with only 47% avoiding enucleation and EBR at 5 years follow-up.12 In a recent study on 55 group D eyes, IVC and focal therapy resulted in Kaplan–Meyer estimates of eye survival to be 68% at 5 years, with additional low dose (36 Gy) intensity-modulated radiation therapy being necessary in 44% of the eyes.13 The probability of ocular salvage without EBR in eyes with vitreous seeding significantly increased to 64% at 2 years follow-up after the introduction of first-line intra-arterial chemotherapy.14 However, this figure may be optimistic since Suzuki et al.15 reported an eye preservation rate of 45% in group D eyes using the same approach with a longer follow-up (79 months).
Secondary Vitreous Seeding
Vitreous seeding may also appear during the treatment course in eyes devoid of vitreous seeds at diagnosis.16 A possible iatrogenic component is plausible since the occurrence of secondary vitreous seeding is observed in only 10% of eyes treated with chemotherapy alone versus 21% in eyes treated with thermochemotherapy.16 Another cause of secondary vitreous involvement is the sudden vitreous dispersion of large tumors shortly after the initiation of chemotherapy due to a necrotic disruption of the internal limiting membrane.17 However, given such a complication, it is mandatory to distinguish active vitreous seeding from inactive mostly calcified seeding.18
Vitreous recurrence in eyes with vitreous seeds at presentation is a frequent finding after chemoreduction. The mean interval to first and last recurrent seedings is 14 months (3–37 months) and 21 months (6–50 months), respectively.19 Not surprisingly, the probability for ocular survival in the case of recurrent and/or refractory vitreous seeding is only 20–24%.16, 19 We published a slightly better prognosis of 76% tumor control for localized vitreous seeding confined to the apex of recurrent retinal tumors accessible to ruthenium brachytherapy.20 Vitreous seeding recurring after EBR has a worse prognosis with only 2% salvage following a second course of EBR.21 Intra-arterial chemotherapy as salvage treatment for recurrent vitreous seeding was recently granted a 50–76% eye survival rate at 2 years.14
Despite tremendous advances in the conservative management of advanced retinoblastoma, the major cause of failure remains the persistence or recurrence of resistant vitreous seeding. Pharmacokinetic studies in the preclinical model have recently shown that if the novel routes of administration such as intra-arterial chemotherapy have greatly improved the ocular penetration of the drugs compared with systemic chemotherapy, the achieved vitreous concentration is barely tumoricidal and does not last long enough for tumor control. The C max obtained in the vitreous following intra-arterial infusion of melphalan remains lower than the 50% inhibiting concentration (IC50).22
To circumvent this concentration problem, intravitreal delivery of chemotherapy would offer the highest drug bioavailability in the vitreous. Despite the obvious risk of tumor spread, this invasive approach was first explored by Ericson and Rosengren23 who performed intravitreal injections of thiotepa as a heroic treatment in six eyes with recurrent vitreous disease, achieving success in three eyes with a mean follow-up of only 8 months. This initial experience was pursued more than 30 years later by Seregard et al.,24 who treated three eyes using the same approach followed by vitrectomy with a mean follow-up of 54 months, avoiding enucleation in two eyes. More recently, Kivela et al. reported the use of intravitreal methotrexate in five eyes with relapse following chemoreduction, only two of them having vitreous seeding.25 Each eye received 20–27 injections of methothrexate over a period ranging between 10 and 12 months. Two eyes were enucleated, including one eye with vitreous seeds and one eye required external beam irradiation.
The literature owes to Kaneko and Suzuki26 the pioneering role in IViC, for publishing the largest series of IViC treatments in eyes with retinoblastoma. These authors performed intravitreal injections of 8 μg of melphalan combined with ocular hyperthermia for vitreous tumor and claimed an eye-preservation rate of 51% at 50 months follow-up. The choice of melphalan was based on in vitro studies by Inomata and Kaneko,27 who found this drug to be the most efficient among the 12 tested, achieving complete suppression of colony formation at a concentration of 4 μg/ml. Preclinical studies in albino rabbits28 have established that melphalan at a vitreous concentration of 5.9 μg/ml is functionally and structurally non-toxic to the retina. When extrapolated to the human vitreous volume, the injected rabbit dose corresponds to 20–30 μg which is to be injected depending on the patient’s age.
Since their initial pioneering report, Kaneko and Suzuki29 have performed 896 IViCs in 237 eyes of 227 patients. They reported the occurrence of extraocular subconjunctival extension in one eye (0.4%), which had anterior chamber involvement and dense vitreous seeds. The patient received adjuvant chemotherapy after enucleation and is reported to be in complete remission. Among the 10 patients (4.4%) who developed metastases, one was potentially due to IViC (0.4%). However, it should be emphasized that the Japanese injection procedure significantly differs from our protocol (see below). Specifically, the absence of well-defined contraindications, as well as the lack of antireflux measures and needle tract sterilization, despite injected volumes of 0.1–0.2 ml, might have contributed to the incidence of the reported adverse events.
Conditional Rehabilitation of Intravitreal Chemotherapy
Until recently, the intravitreal approach remained virtually banished from the therapeutic armamentarium against retinoblastoma, due to the risk of loco-regional and systemic tumor spread. This risk results from two distinct underlying mechanisms, the one active post-operative and the other passive per-operative. Active post-operative exteriorization may occur via tumor growth along a contaminated surgical wound, or in consequence to co-localization of the entry site with a parietal tumor. Passive per-operative tumor spread may occur due to vitreous incarceration or spilling of tumor cells adherent to surgical instruments when removed from the eye, or to the reflux of contaminated humors secondary to variations of intraocular pressure.
In the light of these data, we decided to revisit the feasibility of injecting the chemotherapeutic agent directly into the vitreous cavity through the pars plana as the best way to achieve the appropriate drug concentration. As intravitreal injection constitutes a violation of the “metastatic grace period” typically operated during conservative management of intraocular retinoblastoma, reappraisal of this route of administration was subjected to three pre-requisites susceptible to prevent tumor spread (1) to validate a method reliably assessing the anterior extension of retinoblastoma, hidden in the dead angle of ophthalmoscopy (anterior hyaloid and posterior chamber), in order to secure the needle entry site; (2) to delineate eligibility criteria for the procedure; and (3) to describe a safety-enhanced injection technique.
Prevention of Tumor Spread
As a first step, we tested the value of ultrasonic bio-microscopic (UBM) imaging of the anterior segment, using a 35 m Hz transducer, to predict the safety of a pars plana route of administration. This allowed us to show that tumoral contamination of the posterior chamber can be assessed by UBM with high sensitivity and specificity even in the absence of anterior chamber involvement.30
Our next step was to profile eligibility criteria for intravitreal injection by parametring all risk factors for tumor spread and then by designing an injection technique minimizing the addressed risks.31 To be effective in preventing extra-ocular tumor spread, the injection procedure should minimize both active and passive mechanisms of exteriorization.
Concerning the prevention of active mechanisms of exteriorization, UBM-based contra-indications to IViC were identified. IViC was considered a threat for survival, and thus an absolute contra-indication, in the case of parietal tumor or seeding co-localizing with the entry site, especially if the posterior chamber is invaded. IViC was considered as a threat for eye survival, and thus a relative contra-indication, in the case of anterior hyaloid or retinal detachment at the meridian of the entry site. The risk here is to convert (a) vitreous seeding to anterior segment seeding through the perforated hyaloid, or (b) exudative retinal detachment to the rhegmatogenous form. This is also the reason why we excluded other injection routes of administration other than the pars plana, such as the trans-corneal approach. The technique of injection through the peripheral cornea and iris root specifically creates not only perforation of the cornea but also perforation of the iris and anterior hyaloid. If the first can be secured by cryotherapy, the risk of contamination remains for the latter two, the worst being the creation of a communication between the vitreous cavity and the posterior chamber. The danger is real since anyone who has performed intravitreal injections knows that vitreous incarceration through the sclera can occur while retracting the needle.
The second level of tumor spread prevention is aimed at addressing all risk factors linked to the per-operative passive mechanisms of exteriorization, i.e. the gradient of pressure across the sclera, the size and the number of surgical entries, and the duration of surgery. This led us to develop a safety-enhanced injection technique,31 consisting of an antireflux technique and sterilization of the needle tract. Specifically, we first create a transient hypotony by anterior chamber paracentesis, aspirating the same volume as that to be injected into the vitreous. A 32 G needle mounted on a tuberculin syringe is then introduced perpendicularly 2.5–3.5 mm from the limbus at the desired meridian opposite the seeds, through the conjunctiva and sclera viewed under the surgical microscope, until the needle tip reaches the center of the vitreous cavity immediately behind the lens (with care not to inject in a retro-hyaloid space). The injected dose is 20 μg in most cases but can be cumulatively increased by 2–4 μg up to 30 μ g for each of the following situations: (1) age over 2 years; (2) diffuse nature and/or high density of the seeding; (3) previous intra-arterial exposure to melphalan; and (4) relapse after previous IViC. Upon removal of the needle three cycles of freeze and thaw cryo applications are given at the injection site. The eye is then carefully shaken with forceps in all directions to enable even distribution of the drug.
PRESENT MANAGEMENT OF VITREOUS AND RETRO-HYALOID SEEDING
Using the above-mentioned technique, we reported the first case series showing the efficacy and the safety of intravitreal chemotherapy (IViC) in retinoblastoma patients presenting with vitreous disease.32 Twenty-three consecutive heavily pretreated patients presenting vitreous seeding and eligible for IViC were included in this retrospective non-comparative study. The study population consisted of 18 bilaterally affected patients, 10 of whom had only one eye, and five patients with unilateral retinoblastoma. IViC was proposed as an alternative to external beam irradiation or enucleation for recurrent (74%) or refractory (26%) seeds. Almost two-thirds of this population received intra-arterial melphalan chemotherapy before IViC. Overall success with control of vitreous seeds was achieved in 21 of 23 eyes (91%) after a mean number of four injections. Globe retention was achieved in 87% of cases with only two eyes enucleated for progressive disease and one for phthisis bulbi unrelated to IViC. All retained eyes were in complete remission, and there were no cases of orbital or systemic retinoblastoma recurrence over a mean 22 months’ follow-up. The Kaplan–Meier estimate of ocular survival rates at 2 years was 84.14% (95% CI 62.48–95.28%). All patients were alive without evidence of extraocular spread (95% CI 82.19–100%). We have now extended the follow-up of this initial cohort of 20 conserved eyes with a mean tumor-free eye survival (unpublished data) of 32 months (17–42 months).
Retinal toxicity appeared to be limited to the site of injection in the form of a peripheral well-demarcated salt-and-pepper retinopathy in 10 eyes (43%). In fact, this local toxicity confined to the site of a higher concentration of melphalan along the needle passage serves to increase the security level of the procedure. There was no ophthalmoscopic or fluoroangiographic evidence of retinal toxicity at other locations. Similarly, we detected no optic coherence tomography (OCT) changes within the macula after IViC (unpublished data). A transient localized vitreous hemorrhage in two eyes (8.5%) was the only ocular complication observed. Specifically, IViC was not found to cause corneal endothelium insufficiency, cataract (one case was radiation induced), uveitis, endophthalmitis, or retinal detachment.
For the first time, the eye retention rate of the worst retinoblastoma eye group (group D and all cases with recurrent or refractory vitreous seeding) appeared to parallel that of groups A–C without external beam radiotherapy.
Clinical guidelines and rules conditioning the prescription of IViC for vitreous and/or retro-hyaloid seeding
(a) the tumoral nature of the seeding is unequivocal and differentiated from other mimicking conditions, such as old vitreous hemorrhage or vitritis;
(b) the tumoral viability of the seeding is obvious, which can sometimes require an observation period to document the vitreous growth;
(c) all UBM-based contra-indications have been ruled out;
(d) finally, the retinal source of the seeding must be identified and, if still active and accessible to focal treatments, must be concomitantly eradicated. If the retinal source is not amenable to focal treatment, combined intra-arterial and intravitreal chemotherapy may be considered.
RESPONSE MONITORING AND REGRESSION PATTERNS
At each visit, the residual vitreous tumor burden is reassessed and IViC is carried out every 7–10 d, up to eight injections if a response can be documented, until complete seed fragmentation and dispersion are observed or complete response is achieved (Figure 12). Complete response is established if the seeds (1) completely disappear (vitreous seeding regression type 0) or convert into (2) refringent and/or calcified residues (vitreous seeding regression type I), (3) amorphous often non-spherical inactive residues with or without pigment (vitreous seeding regression type II), or (4) a combination of the latter two (vitreous seeding regression type III). An injection of consolidation is usually given once complete response is observed. IViC can be repeated if vitreous recurrence occurs from another source.
FIGURE 12.
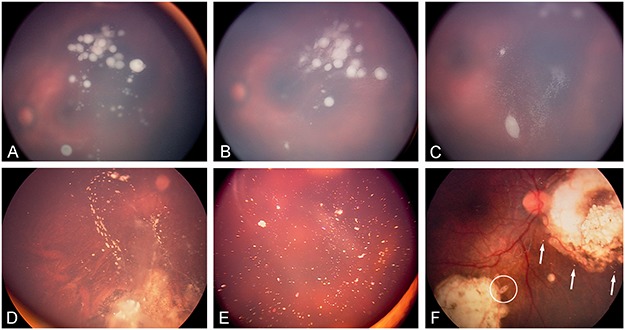
Regression patterns of vitreous seeding: (A) vitreous seeds prior to IViC, (B) partial response following first IViC characterized by fragmentation of the spheres sometimes accompanied by a pseudo-growth, (C) further fragmentation after additional injections before complete extinction. Complete response can be classified into complete disappearance (type 0), conversion into either (D) calcified seeds (type Ia), (E) crystalline refringent dust (type Ib), (F) amorphous non-spherical (see asterisk) seeds (type II), or (F) a combination of regression types I and II (type III).
Interestingly (unpublished data), the timing to complete regression, regression type, number of intravitreal injections, and total dose of melphalan differ significantly among dust, spheres, and clouds. The median number of injections is 3 (a median total dose of 60 μg) in case of dust compared with 4 (107 μg) and 6 (203 μg) for spheres and clouds, respectively. Similarly, the median time to complete regression is 0.5, 1.4, and 6.6 months for dust, spheres, and clouds, respectively. Finally, the regression pattern was type 0 in 100% of dust, 90% of spheres (10% types I and II), and 55% of clouds (45% types I, II, and III).
Clinical Grading of Retinal Toxicity
In our initial report,32 a localized peripheral salt-and-pepper retinopathy was noted in 43% of the cases at the site of injection. We propose a clinical grading system to enable assessment of retinal toxicity. Retinal toxicity grade I is defined as salt-and-pepper retinopathy no greater than 2 clock hours of peripheral retina anterior to or at the equator. Grade II refers to any retinopathy extending greater than 2 clock hours anteriorly or at the equator. In grade III, the retinopathy extends posterior to the equator but sparing the macula. In grade IV, the retinopathy is complicated by a maculopthy, while grade V is characterized by a pan-retinopathy with optic atrophy (Figure 13).
FIGURE 13.
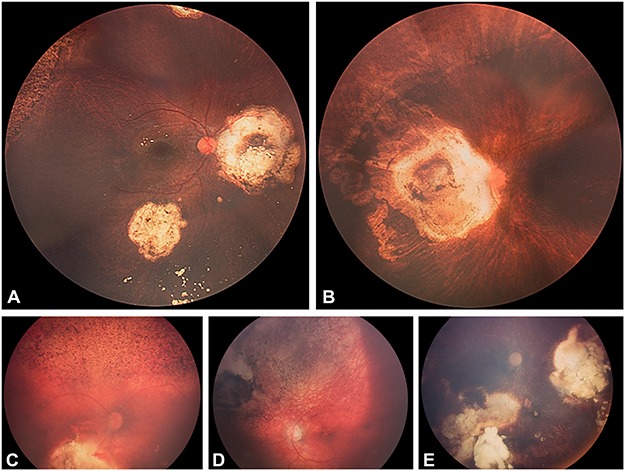
Clinical grading system of retinal toxicity: (A) grade I, (B) grade II, (C) grade III, (D) grade IV, (E) grade V.
We believe that this complication is technique related and can be minimized by (1) choosing a pars plana entry site 3–3.5 mm from the limbus, (2) using a 12-mm 32G needle placed centrally under the operating microscope in the back of the lens to avoid injecting close to the retina or in the retro-hyaloid space, (3) performing a paracentesis of the anterior chamber to promote the use of higher injection volumes of diluted drug, (4) injecting systematically along the same meridian to avoid the circular extension of the retinopathy, and (5) shaking the eye in all directions to enable even distribution of the drug. At least in our hands, the strict application of these rules completely prevented the occurrence of grades IV and V toxicity after more than 400 procedures.
CONCLUSIONS AND PERSPECTIVES
In our experience, the introduction of IViC (combined with intra-arterial chemotherapy if the retinal source of the seeding is not accessible to focal therapy) is associated with an eye survival of 95% (paper in preparation). In addition, external beam irradiation is now virtually eradicated with no indication in the last 6 years of our practice.
Although IViC appears to offer a safe and efficient salvage option, its validation awaits the results of a prospective phase II clinical trial (EudraCT number 2013-002006-31). Special attention will be paid to long-term safety and retinal toxicity assessed by electroretinogram, fluorescein angiography, optic coherence tomography (OCT), and adaptive optics. In a preliminary report, we have shown that photopic ERG amplitudes were unchanged compared with those recorded prior to the intravitreal injection treatments.33
If validated, IViC will not only be useful as salvage treatment for recurrent or resistant vitreous seeds but also as a prophylactic measure in cases of iatrogenic seeding after photocoagulation and plaque surgery, or for group B eyes with ruptured internal limiting membrane (as assessed by fluorescein angiography or OCT, i.e. presumptive submicroscopic infraclinical vitreous disease at presentation). In addition, confirmation of IViC safety will pave the way for the development of trials with novel, possibly customized, molecules.
Finally, we want to emphasize that although IViC does not replace standard treatment care for group C and D eyes, we expect that the addition of front-line IViC to state-of-the-art treatment in eligible group C and D eyes may significantly reduce the exposure to systemic or intra-arterial chemotherapy, as well as the indications for EBR and/or enucleation.
ACKNOWLEDGMENTS
I would like to thank Dr Marie-Claire Gaillard and Dr Aubin Balmer for their continuous support, to Dr Alexandre Moulin for providing the histopathologic iconography and to Marc Curchod and Yann Leuba for the ophthalmic photographies and fundus montages. My gratitude extends to Dr David Abramson for his input in my efforts to classify seeds, and to Dr Yacoub Yousef and Dr Donata Montvilaité for their contributions to the iconography of this paper. Finally, I am thankful to Susan Houghton for correcting and editing this manuscript.
Footnotes
Declaration of interest The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
REFERENCES
- 1.Murphree AL. Intraocular retinoblastoma: the case for a new group classification. Ophthalmol Clin North Am. 2005;18:41–53. doi: 10.1016/j.ohc.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 2.Chantada G, Doz F, Antoneli CB, et al. A proposal for an international retinoblastoma staging system. Pediatr Blood Cancer. 2006;47:801–805. doi: 10.1002/pbc.20606. [DOI] [PubMed] [Google Scholar]
- 3.Chhablani J, Romanzo A, Balmer A, et al. 106)Ruthenium brachytherapy for ciliary recurrence with supraciliary effusion in retinoblastoma. Ophthal Genet. 2010;31:190–192. doi: 10.3109/13816810.2010.499888. [DOI] [PubMed] [Google Scholar]
- 4.Maat-Kievit JA, Oepkes D, Hartwig NG, et al. A large retinoblastoma detected in a fetus at 21 weeks of gestation. Prenat Diagn. 1993;13:377–384. doi: 10.1002/pd.1970130510. [DOI] [PubMed] [Google Scholar]
- 5.Salim A, Wiknjosastro GH, Danukusumo D, et al. Fetal retinoblastoma. J Ultrasound Med. 1998;17:717–720. doi: 10.7863/jum.1998.17.11.717. [DOI] [PubMed] [Google Scholar]
- 6.Gimblett ML, Wellings PC, Lewis M, et al. Retinoblastoma with micrometastasis to CSF. Pathology. 1995;27:27–29. doi: 10.1080/00313029500169412. [DOI] [PubMed] [Google Scholar]
- 7.Popovic MB, Diezi M, Kuchler H, et al. Trilateral retinoblastoma with suprasellar tumor and associated pineal cyst. J Pediatr Hematol Oncol. 2007;29:53–56. doi: 10.1097/MPH.0b013e3180308782. [DOI] [PubMed] [Google Scholar]
- 8.Phan LM, Yeung SC, Lee MH. Cancer metabolic reprogramming: importance, main features, and potentials for precise targeted anti-cancer therapies. Cancer Biol Med. 2014;11:1–19. doi: 10.7497/j.issn.2095-3941.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Horbinski C, Mojesky C, Kyprianou N. Live free or die: tales of homeless (cells) in cancer. Am J Pathol. 2010;177:1044–1052. doi: 10.2353/ajpath.2010.091270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reese AB. Tumors of the eye. New York: Harper & Row; 1963. pp. 368–377. [Google Scholar]
- 11.Abramson DH, Beaverson KL, Chang ST, et al. Outcome following initial external beam radiotherapy in patients with Reese-Ellsworth group Vb retinoblastoma. Arch Ophthalmol. 2004;122:1316–1323. doi: 10.1001/archopht.122.9.1316. [DOI] [PubMed] [Google Scholar]
- 12.Shields CL, Honavar SG, Meadows AT, et al. Chemoreduction plus focal therapy for retinoblastoma: factors predictive of need for treatment with external beam radiotherapy or enucleation. Am J Ophthalmol. 2002;133:657–664. doi: 10.1016/s0002-9394(02)01348-x. [DOI] [PubMed] [Google Scholar]
- 13.Berry JL, Jubran R, Kim JW, et al. Long-term outcomes of Group D eyes in bilateral retinoblastoma patients treated with chemoreduction and low-dose IMRT salvage. Pediatr Blood Cancer. 2013;60:688–693. doi: 10.1002/pbc.24303. [DOI] [PubMed] [Google Scholar]
- 14.Abramson DH, Marr BP, Dunkel IJ, et al. Intra-arterial chemotherapy for retinoblastoma in eyes with vitreous and/or subretinal seeding: 2-year results. Br J Ophthalmol. 2012;96:499–502. doi: 10.1136/bjophthalmol-2011-300498. [DOI] [PubMed] [Google Scholar]
- 15.Suzuki S, Yamane T, Mohri M, et al. Selective ophthalmic arterial injection therapy for intraocular retinoblastoma: the long-term prognosis. Ophthalmology. 2011;118:2081–2087. doi: 10.1016/j.ophtha.2011.03.013. [DOI] [PubMed] [Google Scholar]
- 16.Gombos DS, Cauchi PA, Hungerford JL, et al. Vitreous relapse following primary chemotherapy for retinoblastoma: is adjuvant diode laser a risk factor? Br J Ophthalmol. 2006;90:1168–1172. doi: 10.1136/bjo.2006.091223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parness-Yossifon R, Bryar PJ, Weinstein JL, et al. Sudden dispersion of retinoblastoma shortly after initial chemotherapy treatment. Am J Ophthalmol. 2009;147:903–906. doi: 10.1016/j.ajo.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 18.Tawansy KA, Samuel MA, Shammas M, et al. Vitreoretinal complications of retinoblastoma treatment. Retina. 2006;26:S47–S52. doi: 10.1097/01.iae.0000225350.83931.f6. [DOI] [PubMed] [Google Scholar]
- 19.Shields CL, Honavar SG, Shields JA, et al. Factors predictive of recurrence of retinal tumors, vitreous seeds, and subretinal seeds following chemoreduction for retinoblastoma. Arch Ophthalmol. 2002;120:460–464. [PubMed] [Google Scholar]
- 20.Abouzeid H, Moeckli R, Gaillard MC, et al. 106)Ruthenium brachytherapy for retinoblastoma. Int J Radiat Oncol Biol Phys. 2008;71:821–828. doi: 10.1016/j.ijrobp.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 21.Abramson DH, Ellsworth RM, Rosenblatt M, et al. Retreatment of retinoblastoma with external beam irradiation. Arch Ophthalmol. 1982;100:1257–1260. doi: 10.1001/archopht.1982.01030040235004. [DOI] [PubMed] [Google Scholar]
- 22.Schaiquevich P, Ceciliano A, Millan N, et al. Intra-arterial chemotherapy is more effective than sequential periocular and intravenous chemotherapy as salvage treatment for relapsed retinoblastoma. Pediatr Blood Cancer. 2013;60:766–770. doi: 10.1002/pbc.24356. [DOI] [PubMed] [Google Scholar]
- 23.Ericson LA, Rosengren BH. Present therapeutic resources in retinoblastoma. Acta Ophthalmol (Copenh) 1961;39:569–576. doi: 10.1111/j.1755-3768.1961.tb00269.x. [DOI] [PubMed] [Google Scholar]
- 24.Seregard S, Kock E, af Trampe E. Intravitreal chemotherapy for recurrent retinoblastoma in an only eye. Br J Ophthalmol. 1995;79:194–195. doi: 10.1136/bjo.79.2.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kivela T, Eskelin S, Paloheimo M. Intravitreal methotrexate for retinoblastoma. Ophthalmology. 2011;118:1689, 1689.e1681–1686. doi: 10.1016/j.ophtha.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 26.Kaneko A, Suzuki S. Eye-preservation treatment of retinoblastoma with vitreous seeding. Jpn J Clin Oncol. 2003;33:601–607. [PubMed] [Google Scholar]
- 27.Inomata M, Kaneko A. Chemosensitivity profiles of primary and cultured human retinoblastoma cells in a human tumor clonogenic assay. Jpn J Cancer Res. 1987;78:858–868. [PubMed] [Google Scholar]
- 28.Ueda M, Tanabe J, Inomata M, et al. Study on conservative treatment of retinoblastoma – effect of intravitreal injection of melphalan on the rabbit retina. Nihon Ganka Gakkai Zasshi. 1995;99:1230–1235. [PubMed] [Google Scholar]
- 29. Suzuki S, Kaneko A. Vitreous injection therapy of melphalan for retinoblastoma. XV Biannual Meeting ISOO 2011 International Society of Ocular Oncology Committee. Buenos Aires 2011.
- 30.Moulin AP, Gaillard MC, Balmer A, et al. Ultrasound biomicroscopy evaluation of anterior extension in retinoblastoma: a clinicopathological study. Br J Ophthalmol. 2012;96:337–340. doi: 10.1136/bjophthalmol-2011-300051. [DOI] [PubMed] [Google Scholar]
- 31.Munier FL, Soliman S, Moulin AP, et al. Profiling safety of intravitreal injections for retinoblastoma using an anti-reflux procedure and sterilisation of the needle track. Br J Ophthalmol. 2012;96:1084–1087. doi: 10.1136/bjophthalmol-2011-301016. [DOI] [PubMed] [Google Scholar]
- 32.Munier FL, Gaillard MC, Balmer A, et al. Intravitreal chemotherapy for vitreous disease in retinoblastoma revisited: from prohibition to conditional indications. Br J Ophthalmol. 2012;96:1078–1083. doi: 10.1136/bjophthalmol-2011-301450. [DOI] [PubMed] [Google Scholar]
- 33.Brodie SE, Munier FL, Francis JH, et al. Persistence of retinal function after intravitreal melphalan injection for retinoblastoma. Doc Ophthalmol. 2013;126:79–84. doi: 10.1007/s10633-012-9358-6. [DOI] [PubMed] [Google Scholar]


