Abstract
For peptide receptor targeting usually internalizing agonists are selected. There is increasing evidence that non-internalizing receptor antagonists can be used for this purpose. We investigated whether the glucagon-like peptide-1 receptor (GLP-1R) antagonist exendin(9-39) can be used for in vivo targeting of GLP-1R expressing tumours and compared the in vitro and in vivo characteristics to the GLP-1R agonists exendin-3 and exendin-4.
The binding and internalization kinetics of labelled [Lys40(DTPA)]exendin-3, [Lys40(DTPA)]exendin-4 and [Lys40(DTPA)]exendin(9-39) were determined in vitro using INS-1 cells. The in vivo targeting properties of [Lys40(111In-DTPA)]exendin-3, [Lys40(111In-DTPA)]exendin-4 and [Lys40(111In-DTPA)]exendin(9-39) were examined in BALB/c nude mice with subcutaneous INS-1 tumours. natIn-labelled [Lys40(DTPA)]exendin-3, [Lys40(DTPA)]exendin-4 and [Lys40(DTPA)]exendin(9-39) exhibited similar IC50 values (13.5, 14.4 and 13.4 nM, respectively) and bound to 26 × 103, 41 × 103 and 37 × 103 receptors/cell, respectively. [Lys40(111In-DTPA)]exendin-3 and [Lys40(111In-DTPA)]exendin-4 showed rapid in vitro binding and internalization kinetics, whereas [Lys40(111In-DTPA)]exendin(9-39) showed lower binding and minimal internalization in vitro. In mice, high specific uptake of [Lys40(111In-DTPA)]exendin-3 (25.0 ± 6.0 %ID/g) in the tumour was observed at 0.5 h p.i. with similar uptake up to 4 h p.i.. [Lys40(111In-DTPA)]exendin-4 showed higher tumour uptake at 1 and 4 h p.i. (40.8 ± 7.0 and 41.9 ± 7.2 %ID/g, respectively). Remarkably, [Lys40(111In-DTPA)]exendin(9-39) showed only low specific uptake in the tumour at 0.5 h p.i. (3.2 ± 0.7 %ID/g), rapidly decreasing over time.
In conclusion, the GLP-1R agonists [Lys40(DTPA)]exendin-3 and [Lys40(DTPA)]exendin-4 labelled with 111In could be useful for in vivo GLP-1R targeting, whereas [Lys40(DTPA)]exendin(9-39) is not suited for in vivo targeting of the GLP-1R.
Keywords: GLP-1, exendin, antagonist, agonist, insulinoma
Introduction
Peptide receptor targeting is a successful method for the diagnosis and therapy of neuroendocrine tumours (NET) (1) with somatostatin receptor targeting as the most successful example. In general, agonists are selected for receptor targeting, because agonists usually trigger the internalization of the ligand-receptor complex (2). Internalization of the ligand-receptor complex is the basis for increasing target-to-background ratios, due to retention of the radiolabelled tracer in the target tissue through metabolic trapping (1,3–6). Therefore, internalization is considered to be a crucial step for in vivo peptide receptor targeting (5,6). A correlation between internalization rate and in vivo accumulation of radiolabelled somatostatin analogues in somatostatin receptor subtype 2 (sstr2) has been found in rats with AR42J tumours (7).
Most peptide receptor antagonist do not internalize, while they do bind to the same receptor with high affinity. Antagonists were considered not be suitable for in vivo imaging of tumours due to the lack of internalization. This has been supported by several studies, for example a study showing that a bombesin agonist displayed superior in vivo targeting characteristics in comparison to the antagonist (8). However, recent studies showed favourable in vivo targeting of radiolabelled bombesin antagonists with higher tumour uptake and longer washout compared to bombesin agonists (9–11). Moreover, a recent study with somatostatin receptor type 2 (sstr 2) and type 3 (sstr 3) ligands showed a remarkably higher uptake in the tumour of the antagonists than the agonists (12). Therefore, there is increasing evidence that peptide receptor antagonists may be useful for the in vivo targeting of receptor expressing tissues.
Insulinomas are NET derived from pancreatic beta cells. Although somatostatin receptor scintigraphy is highly efficient in detecting NET and their metastases (13), the sensitivity for the detection of insulinomas is only 40–60% caused by a relatively low incidence of sstr2 and sstr5 receptor subtype expression in insulinomas (13–15). In comparison, the glucacon-like peptide-1 receptor (GLP-1R) is expressed on more than 90% of insulinomas and at a mean density that is two times higher than that of the sst2r (16). Exendin is a GLP-1 (glucagon-like peptide-1) analogue that targets the GLP-1R. For the detection of insulinomas, radiolabelled exendin has been developed recently, with promising preclinical as well as clinical results (17–20). Therefore, exendin analogues should be considered for insulinoma targeting (17–21).
For the targeting of the GLP-1R, only agonists (exendin-3 and exendin-4) labelled with 111In via DTPA conjugated to a C-terminal lysine residue have been examined (17,18,20–22). The GLP-1R antagonist exendin(9-39) is an amino-terminally truncated variant prepared by enzymatic degradation of exendin (Table 1). We aimed to investigate whether exendin(9-39) may also be suitable or – in analogy to the sstr2, sstr3 and bombesin antagonists (9–12) – may even be superior for insulinoma imaging as compared to exendin 3 and 4. Therefore, we investigated the in vitro and in vivo GLP-1R targeting characteristics of the GLP-1R antagonist exendin(9-39) conjugated with DTPA at a C-terminal Lysine: [Lys40(DTPA)]exendin(9-39) and compared these with these of agonists [Lys40(DTPA)]exendin-3 and [Lys40(DTPA)]exendin-4.
Table 1.
Amino acid sequence of GLP-1, exendin-3, exendin-4 and exendin(9-39). The homologous amino acids compared to GLP-1 are underlined.
| Peptide | Amino acid sequence |
|---|---|
| GLP-1 | HAEGTFTSDVSSYLEGQAAKEFIAWLVKGRG |
| exendin-3 | HSDGTFTSDLSKQMEEEAVRLFIEWLKNGGPSSGAPPPS-NH2 |
| exendin-4 | HGEGTFTSDLSKQMEEEAVRLFIEWLKNGGPSSGAPPPS-NH2 |
| exendin(9-39) | DLSKQMEEEAVRLFIEWLKNGGPSSGAPPPS-NH2 |
Results
Radiolabelling
All peptides could be labelled with 111In with a specific activity of up to 700 GBq/μmol. Radiochemical purity was >99% as determined by HPLC and ITLC. 111In-EDTA eluted from the column after 3 min whereas 111In-labelled [Lys40(DTPA)]exendin-3, [Lys40(DTPA)]exendin-4 and [Lys40(DTPA)]exendin(9-39) had a retention time of ~13 min.
Receptor binding assays
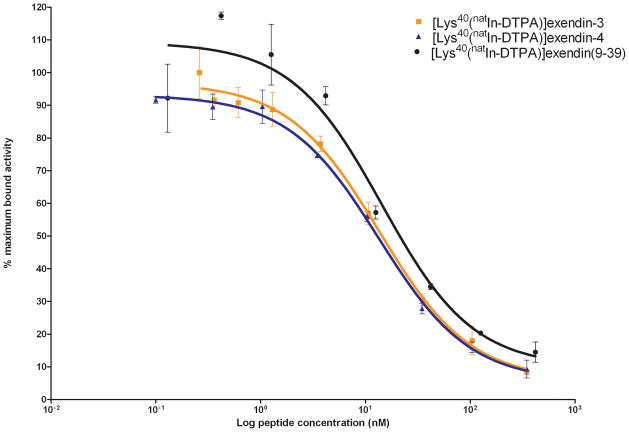
The results of the IC50 determination are shown in Figure 1 and Table 2. The IC50 of natIn-labelled [Lys40(DTPA)]exendin-3, [Lys40(DTPA)]exendin-4 and [Lys40(DTPA)]exendin(9-39) were 13.5 nM, 13.4 nM and 14.4 nM, respectively.
Figure 1.
Competition binding assay (IC50) of [Lys40(natIn-DTPA)]exendin-3, [Lys40(natIn-DTPA)]exendin-4 and [Lys40(natIn-DTPA)]exendin(9-39). [Lys40(111In-DTPA)]exendin-3 was used as tracer.
Table 2.
IC50 values (with the 95% confidence interval in parenthesis) of [Lys40(natIn-DTPA)]exendin-3, [Lys40(natIn-DTPA)]exendin-4 and [Lys40(natIn-DTPA)]exendin(9-39) determined in a competition binding assay in INS-1 cells. The dissociation constant (Kd) and the Bmax (with the 95% confidence interval in parenthesis) of [Lys40(111In-DTPA)]exendin-3, [Lys40(111In-DTPA)]exendin-4 and [Lys40(111In-DTPA)]exendin(9-39) determined by a binding saturation experiment in INS-1 cells.
| Compound | IC50 (nM) | Kd (nM) | Bmax (receptors/cell) |
|---|---|---|---|
| [Lys40(DTPA)]exendin-3 | 13.5 (9.9 – 18.5) | 8.7 (7.6 – 10.1) | 26,000 (24 × 103 – 29 × 103) |
| [Lys40(DTPA)]exendin-4 | 13.4 (10.5 – 17.0) | 17.6 (14.2 – 23.3) | 41,000 (35 × 103 – 50 × 103) |
| [Lys40(DTPA)]exendin(9-39) | 14.4 (4.8 – 43.2) | 15.1 (6.9 – 80.7) | 37,000 (13 × 103 – 60 × 103) |
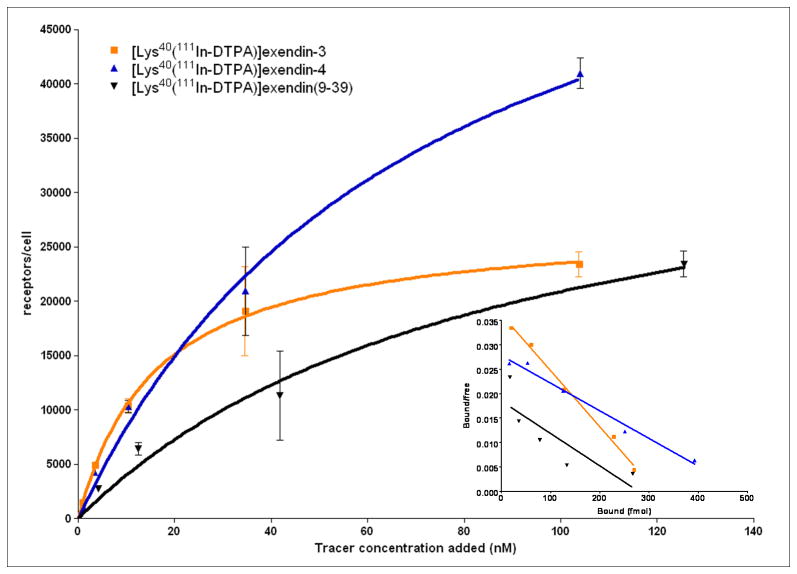
The results of the saturation binding assay are summarized in Figure 2 and Table 2. [Lys40(111In-DTPA)]exendin-3 bound 26,000 receptors/cell, [Lys40(111In-DTPA)]exendin-4 bound 41,000 receptors/cell and [Lys40(111In-DTPA)]exendin(9-39) bound 37,000 receptors/cell. The Kd for [Lys40(111In-DTPA)]exendin-3, [Lys40(111In-DTPA)]exendin-4 and [Lys40(111In-DTPA)]exendin(9-39) was 8.7 nM, 17.6 nM and 15.1 nM, respectively.
Figure 2.
Saturation binding assay and Scatchard plot (insert) of [Lys40(111In-DTPA)]exendin-3, [Lys40(111In-DTPA)]exendin-4 and [Lys40(111In-DTPA)]exendin(9-39).
The large standard deviation for the Kd and Bmax for [Lys40(111In-DTPA)]exendin(9-39) is due to the lower in vitro binding of this tracer, resulting in a low signal in this assay. This finding is in line with the results reported by Waser et al., which also showed lower in vitro binding of exendin(9-39) (23).
Internalization assay
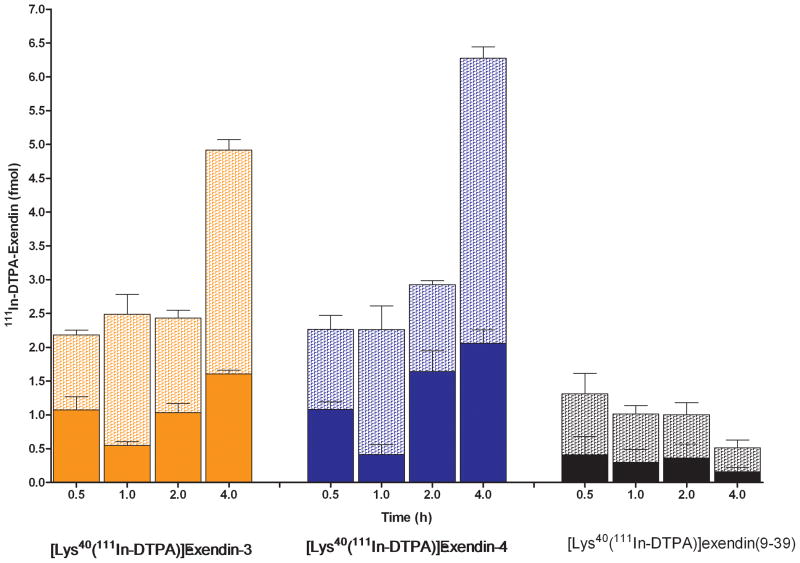
The internalization kinetics of [Lys40(111In-DTPA)]exendin-3, [Lys40(111In-DTPA)]exendin-4 and [Lys40(111In-DTPA)]exendin(9-39) are shown in Figure 3. After 0.5 hour 1.1 ± 0.1 fmol [Lys40(111In-DTPA)]exendin-3 was bound to the receptor and 1.1 ± 0.2 fmol was internalized, increasing to 3.3 ± 0.2 fmol cell bound and 1.6 ± 0.1 fmol internalized after 4 hours. The binding kinetics of [Lys40(111In-DTPA)]exendin-4 were similar to that of [Lys40(111In-DTPA)]exendin-3, with maximum binding and internalization after 4 hours: 4.2 ± 0.2 fmol and 2.1 ± 0.2 fmol, respectively. Membrane bound [Lys40(111In-DTPA)]exendin(9-39) after 0.5 h was 0.9 ± 0.3 fmol and decreased to 0.4 ±0.1 fmol after 4 h. Minimal internalization of [Lys40(111In-DTPA)]exendin(9-39) was observed
Figure 3.
Internalization kinetics of [Lys40(111In-DTPA)]-exendin-3, [Lys40(111In-DTPA)]-exendin-4 and [Lys40(111In-DTPA)]exendin(9-39). Specifically internalized (solid bars) and specifically cell surface bound (hatched bars) tracer are expressed in fmol.
Biodistribution studies
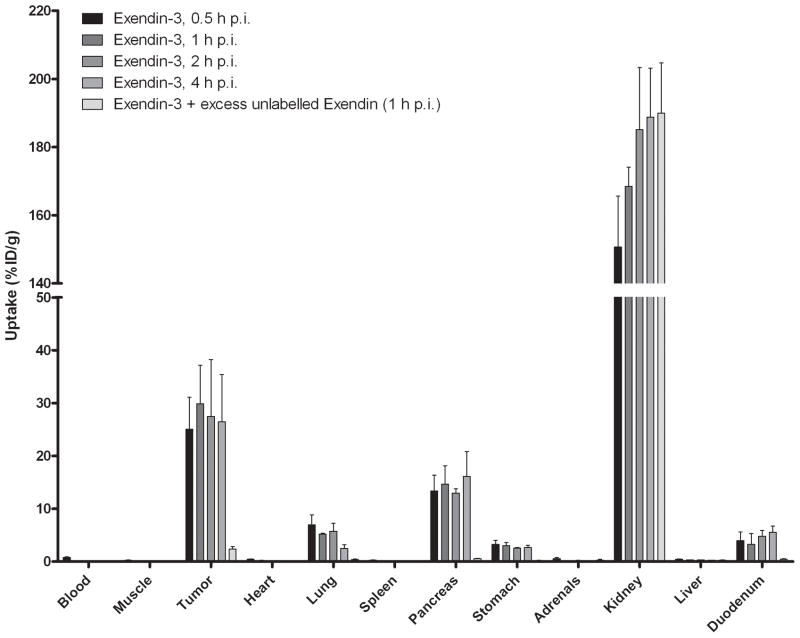
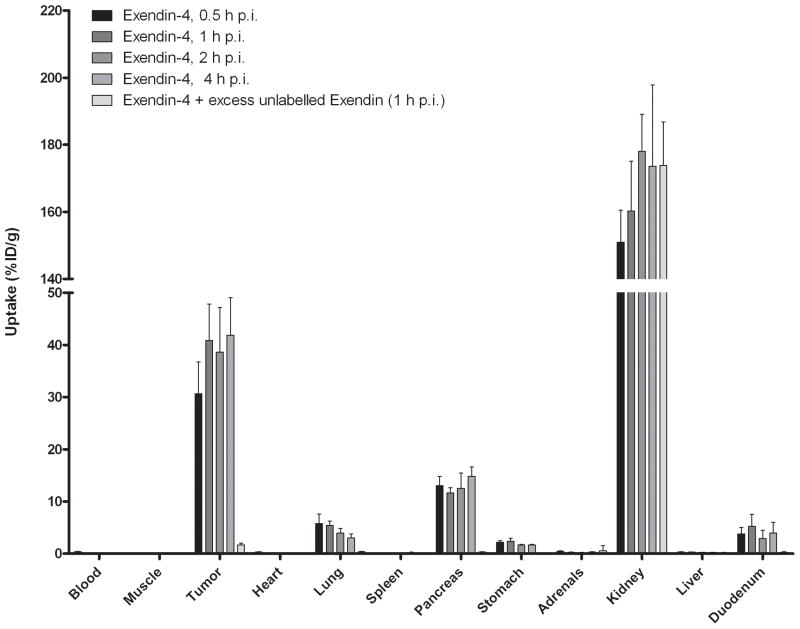
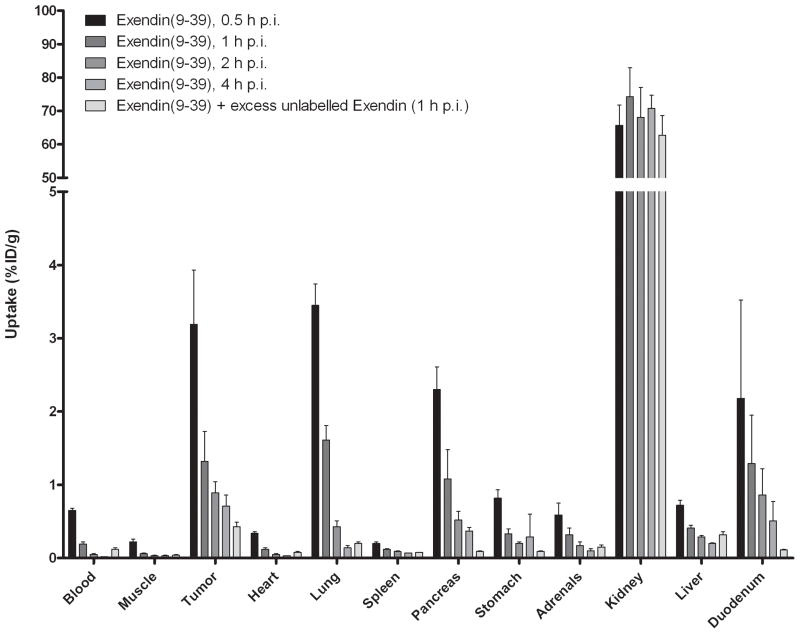
The results of the biodistribution studies are summarized in Figure 4. [Lys40(111In-DTPA)]exendin-3 showed high uptake in the tumour as soon as 30 min after injection (25.0 ± 6.0 %ID/g) and a similar uptake was observed up to 4 h p.i. (26.5 ± 8.9 %ID/g). There was no significant difference in tumour uptake between any time points. Co-administration of an excess unlabelled exendin resulted in much lower uptake: 2.4 ± 0.5 %ID/g, indicating that the uptake was GLP-1R-mediated. Specific uptake of [Lys40(111In-DTPA)]exendin-3 was also seen in pancreas, lung and stomach. [Lys40(111In-DTPA)]exendin-4 showed a higher tumour uptake: 30.7 ± 6.1 %ID/g at 0.5 h p.i. increasing to 41.9 ± 7.2 %ID/g at 4 h p.i. and was statistically higher than tumour uptake of [Lys40(111In-DTPA)]exendin-3 at 1 and 4 h p.i. (p = 0.04 and 0.02, respectively). There was no significant difference in pancreas, stomach and lung uptake between [Lys40(111In-DTPA)]exendin-3 and [Lys40(111In-DTPA)]exendin-4. In contrast, [Lys40(111In-DTPA)]exendin(9-39) showed much lower uptake in the tumour at 0.5 h p.i.: 3.2 ± 0.7 %ID/g and there was a rapid wash-out of [Lys40(111In-DTPA)]exendin(9-39) from the tumour. The same phenomenon was found in all GLP-1R positive organs (pancreas, lung, stomach and duodenum).
Figure 4.
Biodistribution study of [Lys40(111In-DTPA)]exendin-3 (A), [Lys40(111In-DTPA)]exendin-4 (B) and [Lys40(111In-DTPA)]exendin(9-39) (C) in BALB/c nude mice bearing a subcutaneous INS-1 tumour. Values are expressed as percentage injected dose per gram tissue (%ID/g). Blocking was performed by co-injection of a 100-fold molar excess of unlabelled [Lys40]exendin-3. Mice were dissected 4 hours after injection.
Renal uptake was significantly higher with [Lys40(111In-DTPA)]exendin-3 and [Lys40(111In-DTPA)]exendin-4 compared to renal uptake of [Lys40(111In-DTPA)]exendin(9-39) (p=0.0003 and p=0.0002 respectively).
Discussion
In this study, the tumour targeting characteristics of the GLP-1 receptor (GLP-1R) antagonist exendin(9-39) and the agonists exendin-3 and exendin-4 were evaluated in vitro and in vivo. We found that [Lys40(natIn-DTPA)]exendin(9-39) had a similar affinity for the GLP-1R as the agonists [Lys40(natIn-DTPA)]exendin-3 and [Lys40(natIn-DTPA)]exendin-4, as determined in the IC50 assay. [Lys40(111In-DTPA)]exendin(9-39) bound to a similar number of receptors per cell in vitro as compared to the 111In-labelled agonists. The results of the IC50 determination and the Scatchard analysis of exendin-3 were paradoxal: the IC50 of exendin-3 (13.5 nM) was significantly higher than the Kd as derived from the Scatchard analysis (8.7 nM). This could be due to the fact that in the Scatchard analysis 111In-labelled exendin-3 was used, while in the IC50 nonradioactive exendin-3 was used.
Despite the high affinity, [Lys40(111In-DTPA)]exendin(9-39) showed lower in vitro binding compared to the agonists and minimal internalization as compared to the agonists. The biodistribution study showed high uptake of the agonists in the tumour and in GLP-1R positive organs (pancreas, lung and stomach). In contrast, [Lys40(111In-DTPA)]exendin(9-39) showed much lower uptake in GLP-1R expressing tissues at 0.5 h p.i. and the uptake rapidly decreased at later time points. These results suggest that internalization is a prerequisite for specific accumulation of the ligand in GLP-1R expressing tissues in vitro and in vivo.
Previous studies showed favourable tumour targeting of antagonists compared to agonists, that could be explained by the fact that the antagonists bound to more receptors than the agonists (9,12,24,25). In our study there was no significant difference between the number of receptors bound by [Lys40(111In-DTPA)]exendin(9-39) and agonists [Lys40(111In-DTPA)]exendin-3 and [Lys40(111In-DTPA)]exendin-4. Our study showed that [Lys40(111In-DTPA)]exendin(9-39) is not suitable for the targeting of the GLP-1R in vivo. A recent study evaluated the potency of 125I-exendin(9-39) for pancreatic beta cell imaging and showed high uptake in the pancreas (approximately 20% ID/g) of ddY mice (26). A difference between the two studies is the peptide dose injected. We have previously shown that the optimal peptide dose for the GLP-1R agonist [Lys40(111In-DTPA)]exendin-3 in BALB/c nude mice with a subcutaneous tumour model is < 0.1μg (27). The peptide dose of 125I-exendin(9-39) was 60-fold lower than the peptide dose in our study (0.0017 μg vs. 0.1 μg), which might explain the enhanced pancreatic uptake of 125I-exendin(9-39). A straight comparison between the two tracers is needed to clarify the cause of the paradoxal results in the two studies. Although high pancreatic uptake was observed of 125I-exendin(9-39), the feasibility for beta-cell imaging remains to be determined, because for imaging purposes higher activity doses and thus higher peptide doses will be required.
By in vitro autoradiography Waser et al. found that 125I-exendin(9-39) had a high affinity for the GLP-1R in murine pancreatic tissue (2.6 ± 0.4 nM), whereas the affinity for the human GLP-1R is much lower (63 ± 47 nM) (28). It should be noted that this study does not provide data to show that GLP-1R antagonists are superior or inferior to GLP-1R agonists.
Our biodistribution study showed high uptake for all peptides in the kidney, which was not GLP-1R-mediated. The renal uptake of the radiolabelled tracer is caused by tubular reabsorbtion after glomerular filtration. The renal uptake of [Lys40(111In-DTPA)]exendin(9-39) was significantly lower than that of [Lys40(111In-DTPA)]exendin-3 and [Lys40(111In-DTPA)]exendin-4, indicating that the 8 N-terminal amino acid sequence caused more efficient tubular reabsorption. Another explanation might be a different uptake mechanism of [Lys40(111In-DTPA)]exendin(9-39) and [Lys40(111In-DTPA)]exendin-3 and [Lys40(111In-DTPA)]exendin-4 in the proximal tubules of the kidney.
Conclusion
The GLP-1R agonists exendin-3 and exendin-4 show high receptor affinity and efficient in vitro binding and internalization kinetics. [Lys40(111In-DTPA)]exendin-3 and [Lys40(111In-DTPA)]exendin-4 accumulated efficiently in INS-1 tumours and GLP-1R positive organs and could therefore be useful for detection and therapy of insulinomas. [Lys40(DTPA)]exendin(9-39) exhibited high affinity for the GLP-1R, but did not show internalization and low uptake in INS-1 tumours and GLP-1R positive organs and therefore is not suited for targeting the GLP-1R in vivo. This study indicates that internalization is crucial for efficient accumulation and retention in GLP-1R positive tissues.
Experimental
Radiolabelling of exendin
[Lys40(DTPA)]exendin-3, [Lys40(DTPA)]exendin-4 and [Lys40(DTPA)]exendin(9-39) were purchased from Peptide Specialty Laboratories (Heidelberg, Germany). Labelling of DTPA-conjugated [Lys40]exendin-3, [Lys40]exendin-4 or [Lys40]exendin(9-39) was performed under identical conditions. 150 MBq 111InCl3 was added to 1 μg of exendin in 5 volumes of 0.1 M MES buffer, pH 5.5. After 30 min of incubation at room temperature, EDTA was added to a final concentration of 5 mM and incubated at room temperature for 5 min. Tween80 was added to a final concentration of 0.1% and quality control was performed using reversed-phase high performance liquid chromatography (RP-HPLC) on a C18 reversed-phase column (Zorbax Rx-C18; 4.6 mm × 25 cm; Agilent echnologies). The column was eluted with 10 mM NH4Ac, pH 5.5 with a linear gradient to 100% acetonitrile over 10 min with a flow rate of 1 ml/min.
ITLC was performed on silica gel ITLC strips (Pall Corporation Life Sciences, New York, NY, USA). Two mobile phases were used: 0.1 M EDTA in 0.1 M NH4Ac, pH 5.5 (Rf 111In-labelled exendin = 0, Rf 111In-EDTA = 1) and 0.25 M NH4Ac, pH 5.5:methanol (1:1) (Rf colloidal residues = 0, Rf 111In-labelled [Lys40(DTPA)]exendin = 0.2–0.8: and Rf 111In-EDTA = 1).
Cell culture
The rat insulinoma cell line INS-1 (29) was maintained in RPMI-1640 medium supplemented with 10% fetal bovine serum, 2 mM glutamine, 10 mM HEPES, 50 μM β-mercaptoethanol, 1 mM sodium pyruvate, 100 units/ml penicillin and 100 μg/ml streptomycin, in a humidified 5% CO2 atmosphere at 37 °C. The cells were harvested by trypsinization with trypsin/EDTA.
Receptor binding assays
The 50% inhibitory concentration (IC50) was determined for [Lys40(natIn-DTPA)]exendin-3, [Lys40(natIn-DTPA)]exendin-4 and [Lys40(natIn-DTPA)]exendin(9-39) using suspensions of INS-1 cells. [Lys40(DTPA)]exendin-3 and [Lys40(DTPA)]exendin-4 were labelled with natIn by adding 21 μl natInCl3 (0.5 mM in 0.02 M HCl) to 105 μl 0.1 M MES buffer, pH 5.5 containing 10 μg peptide, representing equimolar conditions. [Lys40(DTPA)]exendin(9-39) was labelled by adding 25 μl natInCl3 (0.5 mM in 0.02 M HCl) to 125 μl 0.1 M MES buffer, pH 5.5 containing 10 μg peptide. The reaction mixtures were incubated at room temperature for 20 min and 64 μl 1% Tween80 in PBS was added. Serial dilutions of [Lys40(natIn-DTPA)]exendin-3, [Lys40(natIn-DTPA)]exendin-4 and [Lys40(natIn-DTPA)]exendin(9-39) were prepared in binding buffer (RPMI 1640 medium containing 1% BSA). INS-1 cells were resuspended in binding buffer to a concentration of 3 million cells/0.5 ml. After 10 min incubation on ice, [Lys40(natIn-DTPA)]exendin-3, [Lys40(natIn-DTPA)]exendin-4 and [Lys40(natIn-DTPA)]exendin(9-39) were added at final concentrations ranging from 0.10–350 nM along with radiolabelled [Lys40(111In-DTPA)]exendin-3 (1,000 Bq). To prevent internalization, cells were incubated on ice for 4 h. The cell suspension was centrifuged at 3,000 g for 5 min and the supernatant was discarded. The cell pellet was washed with 500 μl ice-cold PBS, centrifuged at 3,000 g for 5 min and the supernatant was discarded. The radioactivity in the cell pellet was determined in a gamma counter (Wizard, Wallac-Oy, Uppsala, Sweden) and the bound fraction was determined. Saturation binding assays were performed for [Lys40(111In-DTPA)]exendin-3, [Lys40(111In-DTPA)]exendin-4 and [Lys40(111In-DTPA)]exendin(9-39) in suspensions of INS-1 cells. Serial dilutions of the radiolabelled peptides, ranging from 0.1–125 nM, were added to 7 × 106 INS-1 cells in 600 μl binding buffer. The cells were incubated on ice, to prevent internalization, for 4 h. After incubation the cells were centrifuged at 3,000 g for 5 min, the supernatant was discarded and the cells were washed with 500 μl ice-cold PBS. The radioactivity in the cell pellet was determined in a counter. The bound fraction (in receptors/cell) was plotted against the tracer concentration added. In the scatchard plot the bound/free was plotted against the bound fraction and the dissociation constant (Kd) and the maximum number of binding sites was calculated by nonlinear regression using GraphPad Prism (version 4).
Internalization assay
The kinetics of internalization of [Lys40(111In-DTPA)]exendin-3, [Lys40(111In-DTPA)]exendin-4 and [Lys40(111In-DTPA)]exendin(9-39) were determined using suspensions of INS-1 cells as described above. Approximately 1,000 Bq of 111In-labelled peptide (55 fmol) was added and incubated for 0.5, 1, 2 or 4 h at 37 °C. After incubation, the cells were centrifuged and the supernatant was discarded. The cells were washed twice with 0.5 ml ice-cold PBS and subsequently 0.5 ml acid buffer (0.1 M acetic acid, 154 mM NaCl, pH 2.5) was added. After 10 min incubation at 4 °C, cells were spun down and the pellet was washed twice with ice-cold PBS. The radioactivity associated with the cell pellet (internalized fraction) and the supernatants (receptor-bound fraction) was determined.
Biodistribution studies
Animal experiments were performed after approval of the local ethical committee (RUDEC) for animal experiments (RUDEC). Six to eight weeks old female BALB/c nude mice were injected subcutaneously with 0.2 ml of a 1 × 107 cells/ml suspension of INS-1 cells. When the tumours were 2–5 mm in diameter, mice were randomly divided in groups of 5 mice. Mice were intravenously injected with 370 kBq (peptide dose: 20 pmol) of 111In-labelled [Lys40(DTPA)]exendin-3, [Lys40(DTPA)]exendin-4 or [Lys40(DTPA)]exendin(9-39) via a tail vein. At various time points (0.5, 1, 2 and 4 hours) after injection, mice were euthanized by CO2 suffocation and blood, tumour and relevant tissues (muscle, heart, lung, spleen, pancreas, kidney, liver, stomach and duodenum) were dissected, weighed and counted in a gamma counter. The percentage injected dose per gram tissue (%ID/g) was determined for each tissue. To determine whether the exendin uptake is GLP-1 receptor-mediated, a 100-fold excess (2 nmol) of unlabelled [Lys40]exendin-3 was co-injected in a separate group of mice (n=5).
Acknowledgments
Our work was supported by NIH grant 1R01 AG 030328-01 and the European Community’s Seventh Framework Programme (FP7/2007-2013), project BetaImage, under grant agreement n° 222980.
References
- 1.Reubi JC. Peptide receptors as molecular targets for cancer diagnosis and therapy. Endocrine reviews. 2003;24(4):389–427. doi: 10.1210/er.2002-0007. [DOI] [PubMed] [Google Scholar]
- 2.Koenig JA, Edwardson JM. Endocytosis and recycling of G protein-coupled receptors. Trends in pharmacological sciences. 1997;18(8):276–287. doi: 10.1016/s0165-6147(97)01091-2. [DOI] [PubMed] [Google Scholar]
- 3.Bodei L, Paganelli G, Mariani G. Receptor radionuclide therapy of tumors: a road from basic research to clinical applications. J Nucl Med. 2006;47(3):375–377. [PubMed] [Google Scholar]
- 4.Cescato R, Schulz S, Waser B, Eltschinger V, Rivier JE, Wester HJ, Culler M, Ginj M, Liu Q, Schonbrunn A, Reubi JC. Internalization of sst2, sst3, and sst5 receptors: effects of somatostatin agonists and antagonists. J Nucl Med. 2006;47(3):502–511. [PubMed] [Google Scholar]
- 5.Hofland LJ, Lamberts SW. The pathophysiological consequences of somatostatin receptor internalization and resistance. Endocrine reviews. 2003;24(1):28–47. doi: 10.1210/er.2000-0001. [DOI] [PubMed] [Google Scholar]
- 6.Weckbecker G, Lewis I, Albert R, Schmid HA, Hoyer D, Bruns C. Opportunities in somatostatin research: biological, chemical and therapeutic aspects. Nature reviews. 2003;2(12):999–1017. doi: 10.1038/nrd1255. [DOI] [PubMed] [Google Scholar]
- 7.Storch D, Behe M, Walter MA, Chen J, Powell P, Mikolajczak R, Macke HR. Evaluation of [99mTc/EDDA/HYNIC0]octreotide derivatives compared with [111In-DOTA0, Tyr3, Thr8]octreotide and [111In-DTPA0]octreotide: does tumor or pancreas uptake correlate with the rate of internalization? J Nucl Med. 2005;46(9):1561–1569. [PubMed] [Google Scholar]
- 8.Breeman WA, Hofland LJ, de Jong M, Bernard BF, Srinivasan A, Kwekkeboom DJ, Visser TJ, Krenning EP. Evaluation of radiolabelled bombesin analogues for receptor-targeted scintigraphy and radiotherapy. International journal of cancer. 1999;81(4):658–665. doi: 10.1002/(sici)1097-0215(19990517)81:4<658::aid-ijc24>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 9.Mansi R, Wang X, Forrer F, Kneifel S, Tamma ML, Waser B, Cescato R, Reubi JC, Maecke HR. Evaluation of a 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid-conjugated bombesin-based radioantagonist for the labeling with single-photon emission computed tomography, positron emission tomography, and therapeutic radionuclides. Clin Cancer Res. 2009;15(16):5240–5249. doi: 10.1158/1078-0432.CCR-08-3145. [DOI] [PubMed] [Google Scholar]
- 10.Cescato R, Maina T, Nock B, Nikolopoulou A, Charalambidis D, Piccand V, Reubi JC. Bombesin receptor antagonists may be preferable to agonists for tumor targeting. J Nucl Med. 2008;49(2):318–326. doi: 10.2967/jnumed.107.045054. [DOI] [PubMed] [Google Scholar]
- 11.Abd-Elgaliel WR, Gallazzi F, Garrison JC, Rold TL, Sieckman GL, Figueroa SD, Hoffman TJ, Lever SZ. Design, synthesis, and biological evaluation of an antagonist-bombesin analogue as targeting vector. Bioconjugate chemistry. 2008;19(10):2040–2048. doi: 10.1021/bc800290c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ginj M, Zhang H, Waser B, Cescato R, Wild D, Wang X, Erchegyi J, Rivier J, Macke HR, Reubi JC. Radiolabeled somatostatin receptor antagonists are preferable to agonists for in vivo peptide receptor targeting of tumors. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(44):16436–16441. doi: 10.1073/pnas.0607761103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Modlin IM, Tang LH. Approaches to the diagnosis of gut neuroendocrine tumors: the last word (today) Gastroenterology. 1997;112(2):583–590. doi: 10.1053/gast.1997.v112.pm9024313. [DOI] [PubMed] [Google Scholar]
- 14.Joseph K, Stapp J, Reinecke J, Skamel HJ, Hoffken H, Benning R, Neuhaus C, Lenze H, Trautmann ME, Arnold R. [Receptor scintigraphy using 111In-pentetreotide in endocrine gastroenteropancreatic tumors] Nuklearmedizin. 1993;32(6):299–305. [PubMed] [Google Scholar]
- 15.Krenning EP, Kwekkeboom DJ, Bakker WH, Breeman WA, Kooij PP, Oei HY, van Hagen M, Postema PT, de Jong M, Reubi JC, et al. Somatostatin receptor scintigraphy with [111In-DTPA-D-Phe1]- and [123I-Tyr3]-octreotide: the Rotterdam experience with more than 1000 patients. European journal of nuclear medicine. 1993;20(8):716–731. doi: 10.1007/BF00181765. [DOI] [PubMed] [Google Scholar]
- 16.Reubi JC, Waser B. Concomitant expression of several peptide receptors in neuroendocrine tumours: molecular basis for in vivo multireceptor tumour targeting. European journal of nuclear medicine and molecular imaging. 2003;30(5):781–793. doi: 10.1007/s00259-003-1184-3. [DOI] [PubMed] [Google Scholar]
- 17.Gotthardt M, Fischer M, Naeher I, Holz JB, Jungclas H, Fritsch HW, Behe M, Goke B, Joseph K, Behr TM. Use of the incretin hormone glucagon-like peptide-1 (GLP-1) for the detection of insulinomas: initial experimental results. European journal of nuclear medicine and molecular imaging. 2002;29(5):597–606. doi: 10.1007/s00259-002-0761-1. [DOI] [PubMed] [Google Scholar]
- 18.Wicki A, Wild D, Storch D, Seemayer C, Gotthardt M, Behe M, Kneifel S, Mihatsch MJ, Reubi JC, Macke HR, Christofori G. [Lys40(Ahx-DTPA-111In)NH2]-Exendin-4 is a highly efficient radiotherapeutic for glucagon-like peptide-1 receptor-targeted therapy for insulinoma. Clin Cancer Res. 2007;13(12):3696–3705. doi: 10.1158/1078-0432.CCR-06-2965. [DOI] [PubMed] [Google Scholar]
- 19.Wild D, Behe M, Wicki A, Storch D, Waser B, Gotthardt M, Keil B, Christofori G, Reubi JC, Macke HR. [Lys40(Ahx-DTPA-111In)NH2]exendin-4, a very promising ligand for glucagon-like peptide-1 (GLP-1) receptor targeting. J Nucl Med. 2006;47(12):2025–2033. [PubMed] [Google Scholar]
- 20.Wild D, Macke H, Christ E, Gloor B, Reubi JC. Glucagon-like peptide 1-receptor scans to localize occult insulinomas. The New England journal of medicine. 2008;359(7):766–768. doi: 10.1056/NEJMc0802045. [DOI] [PubMed] [Google Scholar]
- 21.Gotthardt M, Lalyko G, van Eerd-Vismale J, Keil B, Schurrat T, Hower M, Laverman P, Behr TM, Boerman OC, Goke B, Behe M. A new technique for in vivo imaging of specific GLP-1 binding sites: first results in small rodents. Regulatory peptides. 2006;137(3):162–167. doi: 10.1016/j.regpep.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 22.Raufman JP, Singh L, Eng J. Exendin-3, a novel peptide from Heloderma horridum venom, interacts with vasoactive intestinal peptide receptors and a newly described receptor on dispersed acini from guinea pig pancreas. Description of exendin-3(9–39) amide, a specific exendin receptor antagonist. The Journal of biological chemistry. 1991;266(5):2897–2902. [PubMed] [Google Scholar]
- 23.Waser B, Reubi JC. Value of the radiolabelled GLP-1 receptor antagonist exendin(9–39) for targeting of GLP-1 receptor-expressing pancreatic tissues in mice and humans. European journal of nuclear medicine and molecular imaging. 38(6):1054–1058. doi: 10.1007/s00259-010-1701-0. [DOI] [PubMed] [Google Scholar]
- 24.Perrin MH, Sutton SW, Cervini LA, Rivier JE, Vale WW. Comparison of an agonist, urocortin, and an antagonist, astressin, as radioligands for characterization of corticotropin-releasing factor receptors. The Journal of pharmacology and experimental therapeutics. 1999;288(2):729–734. [PubMed] [Google Scholar]
- 25.Sleight AJ, Stam NJ, Mutel V, Vanderheyden PM. Radiolabelling of the human 5-HT2A receptor with an agonist, a partial agonist and an antagonist: effects on apparent agonist affinities. Biochemical pharmacology. 1996;51(1):71–76. doi: 10.1016/0006-2952(95)02122-1. [DOI] [PubMed] [Google Scholar]
- 26.Mukai E, Toyoda K, Kimura H, Kawashima H, Fujimoto H, Ueda M, Temma T, Hirao K, Nagakawa K, Saji H, Inagaki N. GLP-1 receptor antagonist as a potential probe for pancreatic beta-cell imaging. Biochemical and biophysical research communications. 2009;389(3):523–526. doi: 10.1016/j.bbrc.2009.09.014. [DOI] [PubMed] [Google Scholar]
- 27.Brom M, Oyen WJ, Joosten L, Gotthardt M, Boerman OC. 68Ga-labelled exendin-3, a new agent for the detection of insulinomas with PET. European journal of nuclear medicine and molecular imaging. 37(7):1345–1355. doi: 10.1007/s00259-009-1363-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Waser B, Reubi JC. Value of the radiolabelled GLP-1 receptor antagonist exendin(9–39) for targeting of GLP-1 receptor-expressing pancreatic tissues in mice and humans. European journal of nuclear medicine and molecular imaging. 2011 Jan 6; doi: 10.1007/s00259-010-1701-0. (Epub ehead of print) [DOI] [PubMed] [Google Scholar]
- 29.Asfari M, Janjic D, Meda P, Li G, Halban PA, Wollheim CB. Establishment of 2-mercaptoethanol-dependent differentiated insulin-secreting cell lines. Endocrinology. 1992;130(1):167–178. doi: 10.1210/endo.130.1.1370150. [DOI] [PubMed] [Google Scholar]