Abstract
Background
The repair and restoration of function after chronic rotator cuff tears are often complicated by muscle atrophy, fibrosis, and fatty degeneration of the diseased muscle. The inflammatory response has been implicated in the development of fatty degeneration after cuff injuries. Licofelone is a novel anti-inflammatory drug that inhibits 5-lipoxygenase (5-LOX), as well as cyclooxygenase (COX)-1 and COX-2 enzymes, which play important roles in inducing inflammation after injuries. While previous studies have demonstrated that nonsteroidal anti-inflammatory drugs and selective inhibitors of COX-2 (coxibs) may prevent the proper healing of muscles and tendons, studies about bone and cartilage have demonstrated that drugs that inhibit 5-LOX concurrently with COX-1 and COX-2 may enhance tissue regeneration.
Hypothesis
After the repair of a chronic rotator cuff tear in rats, licofelone would increase the load to failure of repaired tendons and increase the force production of muscle fibers.
Study Design
Controlled laboratory study.
Methods
Rats underwent supraspinatus release followed by repair 28 days later. After repair, rats began a treatment regimen of either licofelone or a vehicle for 14 days, at which time animals were euthanized. Supraspinatus muscles and tendons were then subjected to contractile, mechanical, histological, and biochemical analyses.
Results
Compared with controls, licofelone-treated rats had a grossly apparent decrease in inflammation and increased fibro-cartilage formation at the enthesis, along with a 62% increase in the maximum load to failure and a 51 % increase in peak stress to failure. Licofelone resulted in a marked reduction in fibrosis and lipid content in supraspinatus muscles as well as reduced expression of several genes involved in fatty infiltration. Despite the decline in fibrosis and fat accumulation, muscle fiber specific force production was reduced by 23%.
Conclusion
The postoperative treatment of cuff repair with licofelone may reduce fatty degeneration and enhance the development of a stable bone-tendon interface, although decreases in muscle fiber specific force production were observed, and force production in fact declined.
Clinical Relevance
This study demonstrates that the inhibition of 5-LOX, COX-1, and COX-2 modulates the healing process of repaired rotator cuff tendons. Although further studies are necessary, the treatment of patients with licofelone after cuff repair may improve the development of a stable enthesis and enhance postoperative outcomes.
Keywords: rotator cuff, fatty degeneration, myosteatosis, licofelone
Rotator cuff tears are among the most frequent and devastating upper extremity injuries, with over a quarter of a million surgical repairs performed in the United States each year.6 Currently, the ability to repair the cuff and allow for normal strength and function is often complicated by atrophy, retraction, and fatty infiltration of the diseased muscle.1,14 The degree of these changes, termed “fatty degeneration,” increases with time and is a limiting factor for adequate repair as well as postoperative function and recovery.12,21 Fatty degeneration is a common pathological change that occurs in torn rotator cuff muscles; however, little is known behind the pathophysiological pathway of this phenomenon.18 It has been shown that even after surgical repair, fatty degeneration does not improve and in some cases can continue to worsen.13 The amount of fatty degeneration has been correlated to poor functional outcomes,15 and therapies that prevent or reverse fatty degeneration will likely lead to improved clinical outcomes for patients who suffer from chronic rotator cuff tears.
Inflammatory and fibroproliferative responses have been implicated in the development of fatty infiltration and muscle atrophy after rotator cuff injuries.16,23,30,45 Cyclooxygenase (COX)-1, COX-2, and 5-lipoxygenase (5-LOX) are fundamental enzymes in this inflammatory cascade.31 The COX enzymes convert arachidonic acid into prostaglandin H2, which can then be further converted to other prostaglandins that have potent proinflammatory effects.38 Arachidonic acid is also a substrate of 5-LOX, but it produces various classes of leukotrienes that also can promote inflammation and regulate immune function.38 COX inhibitors currently on the market are effective in reducing pain and inflammation but are associated with impaired muscle regeneration, weakened tendon-to-bone healing, gastric and renal damage, and cardiovascular side effects.5,22,27,35 Moreover, isolated COX enzyme inhibition can shunt arachidonic acid to the 5-LOX pathway and increase the production of leukotrienes.41 The increase in leukotriene production is thought to be one of the factors that causes side effects from the use of COX inhibitors.41 The selective blockade of both the COX and 5-LOX pathways may offer the ability to reduce inflammation with fewer side effects and improved tissue healing.
Licofelone is an anti-inflammatory drug, not currently approved by the Food and Drug Administration, that inhibits COX-1, COX-2, and 5-LOX and has a better cardiovascular profile and gastrointestinal tolerability than other nonsteroidal anti-inflammatory drugs (NSAIDs).10,36,42 In a large clinical trial of patients with knee osteoarthritis compared with patients given the COX-1/COX-2 inhibitor naproxen, patients who received licofelone had significantly reduced cartilage volume loss and reduced levels of circulating biomarkers of cartilage degradation and inflammation. Licofelone has also been shown to decrease subchondral bone resorption, prevent chondrocyte apoptosis, and decrease the production of collagenases that are implicated in osteoarthritis and are upregulated in rotator cuff tears.20,39
While blocking the biological activity of 5-LOX concurrently with the inhibition of COX-1 and COX-2 appears to offer improved efficacy in treating cartilage and bone injuries more than COX inhibition alone, it is unknown if a similar approach could improve the regeneration of other musculoskeletal tissues. The ability of licofelone to decrease bone resorption and MMP production and promote normal chondrocyte development suggests a potential use in enhancing the development of a stable bone-tendon interface. Further, the involvement of leukotrienes in lipid metabolism and muscle atrophy could result in licofelone having a positive effect on improving muscle strength and reducing fatty degeneration after rotator cuff repairs. We hypothesized that after the repair of a chronic rotator cuff tear in rats, licofelone would increase the load to failure of repaired tendons and increase the force production of supraspinatus muscle fibers.
MATERIALS AND METHODS
Animal Model and Surgical Procedure
This study was approved by the University of Michigan Committee on the Use and Care of Animals. Six-month-old male Sprague-Dawley rats (n = 7 rats in the vehicle control group, n = 11 rats in the drug group), maintained in specific pathogen-free conditions, were used in this study. The general surgical procedure has been previously described.16,19 To induce a supraspinatus tear, rats were anesthetized with 2% isoflurane and placed in a lateral decubitus position, and the skin above the shoulder was shaved and scrubbed with ChloraPrep (CareFusion). A deltoid-splitting transacromial approach was used to expose the supraspinatus tendon, which was then sharply detached from its footprint on the humeral head. The tendon was then enclosed in sterile nonpyrogenic surgical tubing (Pharmed BPT, Saint Gobain) that was secured to the tendon using a modified Mason-Allen stitch, which prevented the tendon from forming adhesions to the surrounding tissue and allowed the muscle to freely retract. The deltoid muscle and skin were closed, and the rats were allowed to recover for 28 days. To repair the torn tendon, rats were anesthetized and prepared as described above. The tendon was freed from surgical tubing, and a modified Mason-Allen stitch using 5-0 two-arm Ethibond sutures (Johnson & Johnson) was placed in the tendon stump. After complete debridement of the native enthesis on the greater tuberosity, crossed bone tunnels were drilled using a 0.7-mm K-wire (gSource) at the anterior and posterior margins of the cuff footprint and 2 mm lateral to the articular surface. Suture ends were passed through the bone tunnels and tied over the humeral metaphyseal cortex to anatomically repair the supraspinatus to the native footprint. In all surgical procedures, a splash block of 1% lidocaine was administered, the deltoid was closed using 4-0 chromic gut sutures (Johnson & Johnson), and the skin was closed using a subcutaneous running suture of 4-0 Vicryl (Johnson & Johnson) with GLUture (Abbott Laboratories) applied over the incision. Subcutaneous buprenorphine at a dose of 0.05 mg/kg was administered for analgesia during postoperative recovery. Ad libitum weightbearing and cage activity were allowed, and rats were monitored for signs of distress or infection. Rats were all grossly ambulatory to the same extent, and all demonstrated signs of adequate food and water intake.
After surgical repair of the torn supraspinatus tendon, the administration of the drug or vehicle began. Licofelone (ML3000, Cayman Chemical) was dissolved in a 1% hydroxypropyl methylcellulose (HPMC) solution and administered via oral gavage at a dose of 40 mg/kg twice daily for 2 weeks. This dose was selected based on a previous study that demonstrated the efficacy of licofelone in inhibiting 5-LOX, COX-1, and COX-2 activity and preventing joint damage in a rat model of adjuvant-induced arthritis.11 Control rats received the HPMC solution twice daily for 2 weeks. At the end of the 2-week period, rats were anesthetized with sodium pentobarbital, and supraspinatus muscles, tendons, and humeral heads were removed. After the removal of tissue, rats were euthanized by an overdose of sodium pentobarbital, followed by an induction of bilateral pneumothorax. The proximal third of the left supraspinatus was used for single fiber contractility measurements, while the distal two-thirds plus the tendon and humeral head were used for measurements of mechanical properties. For the right shoulder, the proximal two-thirds were finely minced and used for gene expression analyses and hydroxyproline assays, while the distal third of the muscle and humeral head with the enthesis were used for histology. All assays were performed in a blinded fashion.
Histology
Distal segments of the supraspinatus were frozen in Tissue-Tek (Sakura) using isopentane-cooled liquid nitrogen and stored at −80°C until use. Muscles were cryosectioned at a thickness of 10 µm and labeled with antibodies against myosin heavy chain type I (Developmental Studies Hybridoma Bank [DSHB]), myosin heavy chain type IIA (DSHB), and myosin heavy chain type IIB (DSHB). Type IIX fibers were detected by the absence of fluorescent signals. The extracellular matrix was identified with the lectin wheat germ agglutinin conjugated to AlexaFluor 488 (Invitrogen). Primary antibodies were labeled using highly cross-adsorbed secondary antibodies conjugated to AlexaFluor fluorescent probes (Invitrogen).
The entheses were fixed overnight in 10% neutral buffered formalin (Thermo Fisher), briefly rinsed in phosphate buffered saline, and then decalcified in Immunocal (Decal) for 5 days. Samples were then dehydrated in 70% ethanol, embedded in paraffin, sectioned, and stained with safranin O/fast green (Thermo Fisher).
Images were obtained using an Axioplan 2 microscope (Zeiss) equipped with AxioCam cameras (Zeiss). The assessment of muscle fiber type and size was performed using ImageJ software (National Institutes of Health).
Muscle Fiber Contractility
Single fiber contractility experiments were performed as previously described.16,17,25 Briefly, fiber bundles were dissected from the proximal portion of supraspinatus muscles, placed in a skinning solution for 30 minutes and then in a storage solution for 16 hours at 4°C, and stored at −80°C. For contractility testing, fibers were isolated from bundles freshly thawed on ice, placed in a chamber filled with a relaxing solution, and secured to a servomotor (model 322C, Aurora Scientific) and force transducer (model 403A, Aurora Scientific). Fiber length was adjusted to obtain a sarcomere length of 2.5 µm, and the fiber’s cross-sectional area (CSA) was measured. Maximum isometric force (Fo) was elicited by immersing the fiber in a high-[Ca2+] solution, and specific force (sFo) was derived by dividing Fo by the CSA. Ten to 20 type II fibers were tested from each muscle.
Gene Expression
Gene expression analysis was performed as previously described.16,17 Briefly, RNA was isolated from 100-mg portions of the supraspinatus muscles using a miRNeasy kit (Qiagen), and genomic DNA was eliminated with the use of DNase I (Qiagen). After reverse transcription of RNA with the RT2 First Strand kit (Qiagen), polymerase chain reaction (PCR) products were amplified using a CFX96 real-time thermal cycler (Bio-Rad) with RT2 SYBR Green quantitative PCR reagents (Qiagen). The 2−ΔCt technique33 was used to normalize the expression of mRNA transcripts to the stable housekeeping gene β-actin. A list of RNA transcripts and corresponding RefSeq information is provided in the Appendix (available in the online version of this article at http://ajsm.sagepub.com/supplemental).
Hydroxyproline Assay
Hydroxyproline measurements of muscle tissue were performed as previously described.26 Briefly, 25-mg portions of finely minced supraspinatus muscles were desiccated for 4 hours at 90°C, and the dry mass was then recorded. Samples were then digested into free amino acids in 6.0 N hydrochloric acid overnight at 110°C and neutralized in an equal volume of 6.0 N sodium hydroxide. The hydroxyproline content was then determined using a colorimetric assay44 that was measured in a SpectraMax microplate reader (Molecular Devices) and normalized to the dry mass of the muscle tissue.
Lipid Analysis
Fifty milligrams of muscle tissue was homogenized and suspended in 0.9% NaCl to a concentration of 20 µg/mL. Lipids were extracted according to Bligh and Dyer3 in a 2:2:1.8 chloroform:methanol:aqueous mixture. Samples were stored in 500 µL of chloroform and spotted on 10 × 10-cm silica high-performance thin-layer chromatography plates (EMD Millipore). Plates were developed in a 60:30:5 chloroform:methanol:water solution to separate phospholipids and then dried and developed in an 80:20:1.5 hexane: diethyl ether:acetic acid solution to separate apolar lipids. Plates were incubated in rhodamine 6G solution (Sigma) and imaged in a ChemiDoc XRS system (Bio-Rad). Densitometry of triglyceride and phospholipid bands was performed using ImageJ.
Biomechanical Testing
Biomechanical testing of the repaired enthesis interface was performed using a modified ElectroForce ELF3200 uniaxial testing system (Bose) equipped with a 100-N load cell (Omega). The humerus and attached supraspinatus were meticulously dissected under a stereo microscope. The humerus was potted in polymethyl methacrylate (DentsPly) and secured in a vise grip, and the muscle was secured in a screw grip using sandpaper and ethyl cyanoacrylate (Elmer’s Products Inc). The shoulder was preloaded to 1.0 N, and 2 cameras (CM-200MCL, JAI) mounted 90° apart captured the front and side views of the tendon, which were then used to calculate the tendon’s CSA by fitting the width and length measurements to an ellipse. The specimen was then stretched until failure at a rate of 10 mm/s, and the maximum displacement to yield, maximum load to yield, peak stress, and peak stiffness were determined from testing. One sample from the control group and 3 samples from the licofelone group experienced slippage between the tissue and grips during testing and were excluded from analyses.
Statistical Analysis
Data are presented as mean ± SE. A power analysis (β = .80) based on the muscle fiber sFo values from a previous work16 was used to determine the appropriate sample size to detect a 30% difference between the licofelone and control groups. Differences between vehicle- and licofelone-treated groups were tested using t tests (α = .05) in GraphPad Prism 6.0.
RESULTS
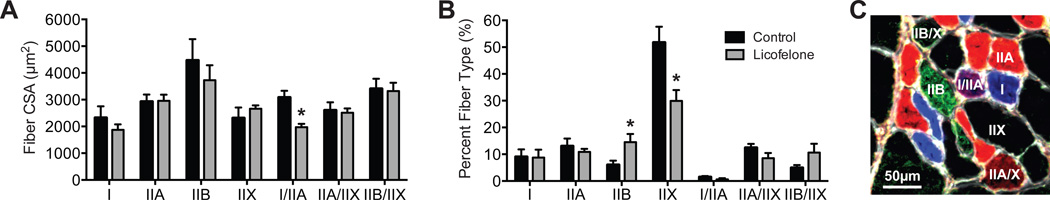
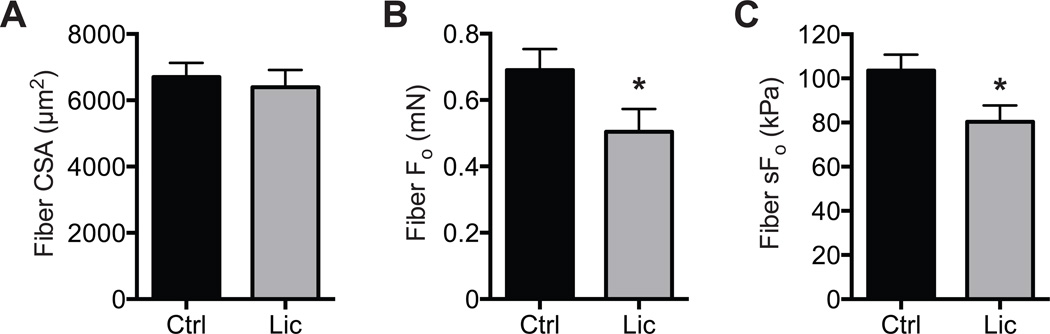
All rotator cuff repairs in both groups were intact postoperatively at the time of sacrifice, with no signs of humeral fractures, damage to transosseous tunnels, or failed repairs. No differences were observed in the muscle mass of the 2 groups (671 ± 56.3 mg for controls and 720.3 ± 35.4 mg for licofelone-treated groups; P = .223). The CSA of different types of muscle fibers was generally similar between control and licofelone-treated animals, with a 36% reduction in size (P = .004) observed only in type I/IIA muscles (Figure 1A). While fiber sizes were generally similar, there was a doubling (P = .015) in the number of type IIB muscle fibers present in licofelone-treated muscles and a decrease in the percentage of type IIX muscle fibers (P = .005) of a similar magnitude (Figure 1B). A representative image of different muscle fiber types is shown in Figure 1C. For muscle fiber contractility, no differences (P = .446) were observed in fiber CSAs (Figure 2A), but compared with controls, licofelone-treated animals had a 27% decrease (P = .041) in maximum isometric force production (Fo) (Figure 2B) and a 23% decrease (P = .024) in specific force (sFo) (Figure 2C).
Figure 1.
Muscle fiber type size and percentage of composition. (A) Cross-sectional area (CSA) and (B) percentage of distribution of fibers containing different myosin heavy chain (MHC) isoforms from control and licofelone-treated supraspinatus muscles. (C) Representative image indicating different fiber types. White, extracellular matrix (wheat germ agglutinin); blue, MHC I; red, MHC IIA; green, MHC IIB; black, MHC IIX; blue-red, hybrid MHC l/IIA; red-black, hybrid MHC IIA/IIX; green-black, hybrid MHC IIB/IIX. Values are reported as mean ± SE (n = 7 muscles from controls and n = 11 from licofelone-treated muscles). *Significantly different from the control group (P < .05).
Figure 2.
Permeabilized fiber contractility. (A) Permeabilized fiber cross-sectional area (CSA), (B) maximum isometric force (Fo), and (C) specific force (sFo) of control and licofelone-treated supraspinatus muscles. Values are reported as mean ± SE (n = 7 muscles from controls and n = 11 from licofelone-treated muscles). *Significantly different from the control group (P < .05).
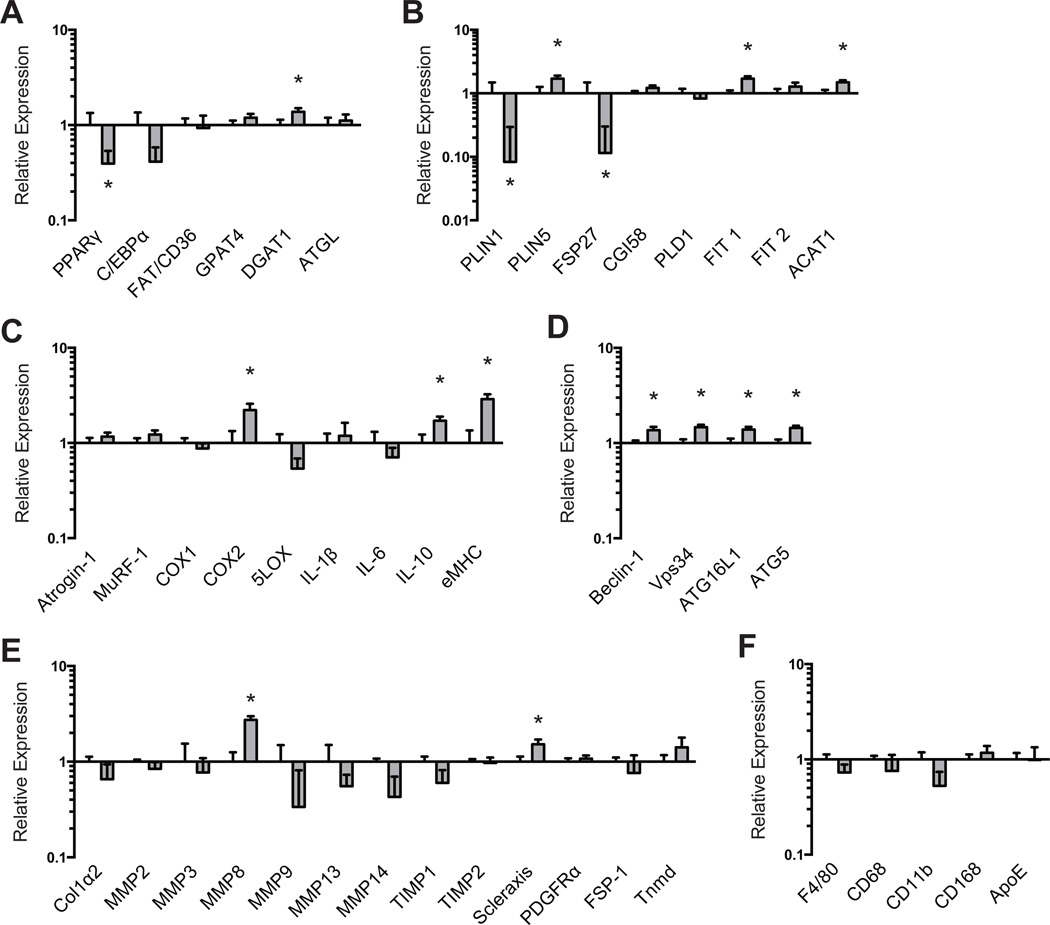
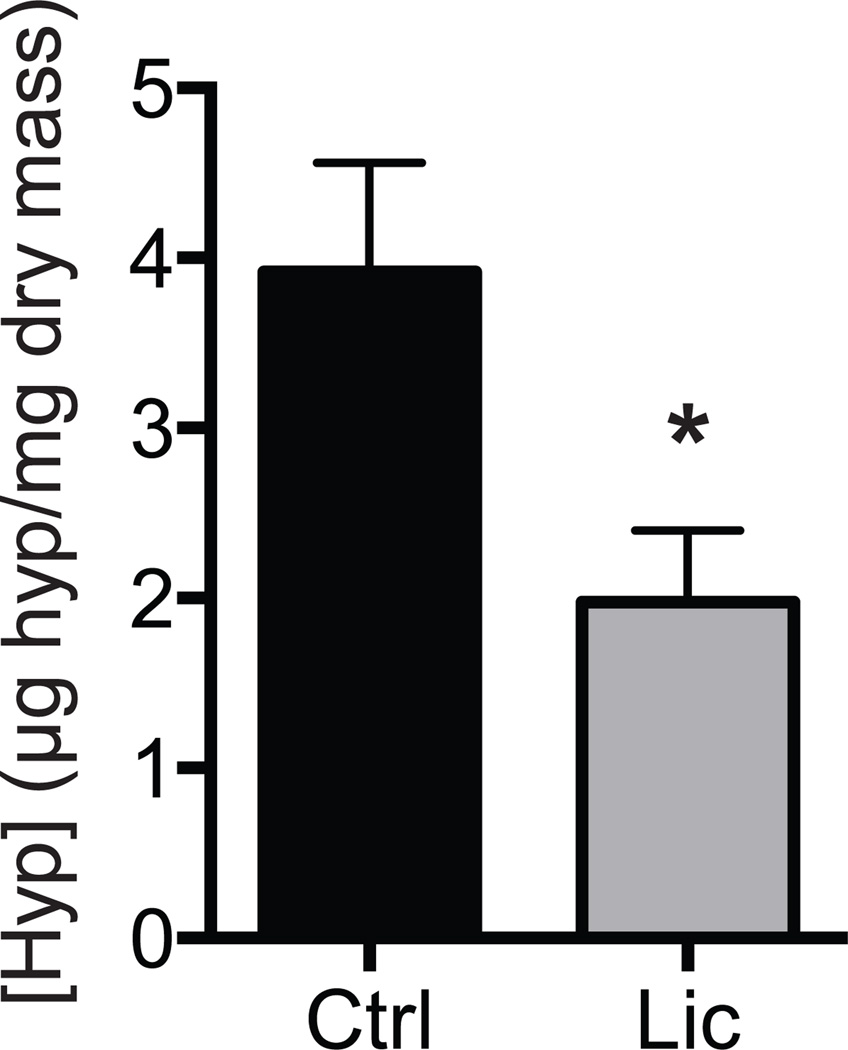
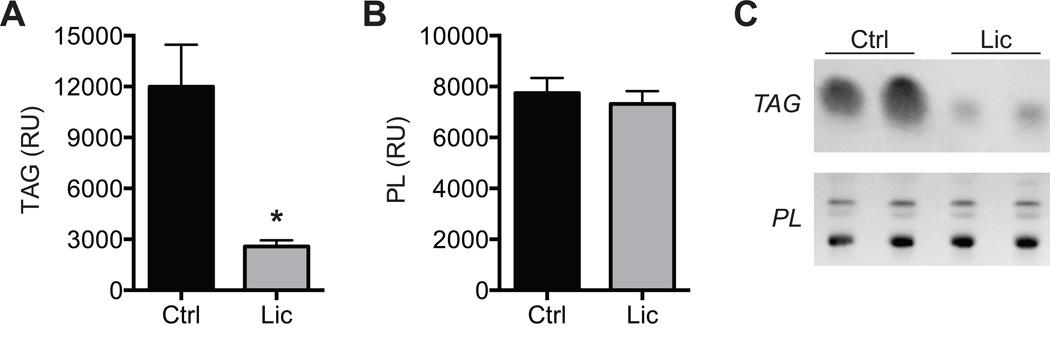
We next measured differences in molecular and biochemical markers of fibrosis, atrophy, and lipid accumulation in supraspinatus muscles. For genes related to adipogenesis (Figure 3A) and lipid storage (Figure 3B), licofelone treatment resulted in a modest decrease in PPAR-γ (P = .039) expression, with marked decreases in perilipin 1 (P = .033) and FSP27 (P = .032) expression and slight increases in DGAT1 (P = .037), perilipin 5 (P = .021), FIT1 (P = .003), and ACAT1 expression (P = .009). Licofelone treatment also resulted in an increase in the expression of the inflammation- and atrophy-related genes (Figure 3C) COX-2 (P = .020), IL-10 (P = .012), and embryonic myosin heavy chain and the autophagy-related genes (Figure 3D) beclin-1 (P = .015), Vps34 (P = .002), ATG16L1 (P = .008), and ATG5 (P = .001). For most genes related to fibrosis (Figure 3E) and macrophage (Figure 3F), no differences between groups were observed, with the exception of increases in MMP-8 (P < .001) and scleraxis (P = .027) in the licofelone group. Hydroxyproline is an amino acid that makes up approximately 14% of the dry mass of fibrillar collagen and is commonly used as a marker of collagen content.28 Although no differences in type I collagen, MMP, or TIMP expression were observed, the hydroxyproline content of licofelone-treated muscles was approximately half (P = .009) of the values in control muscles (Figure 4). The triglyceride and phospholipid content of supraspinatus muscle samples was measured using thin-layer chromatography (Figure 5), and we observed a 78% reduction in triglyceride levels (P = .002). Phospholipids, which are mainly found in the plasma membranes of cells and act as a loading control, were present in similar levels between groups (P = .296).
Figure 3.
Expression of mRNA transcripts related to (A) adipogenesis, (B) lipid storage, (C) extracellular matrix synthesis and fibrosis, (D) autophagy, (E) inflammation and atrophy, and (F) macrophage and fatty macrophage accumulation. Target gene expression was normalized to β-actin. Values are reported as mean ± SE (n = 7 muscles from controls and n = 11 from licofelone-treated muscles). *Significantly different from the control group (P < .05).
Figure 4.
The content of hydroxyproline in the control and licofelone-treated muscles. Values are reported as mean ± SE (n = 7 muscles from controls and n = 11 from licofelone-treated muscles). *Significantly different from the control group (P < .05).
Figure 5.
Lipid content: The content of (A) triglycerides (TAG) and (B) phospholipids (PL) in control and licofelone-treated muscles. Values are expressed as relative units (RU) of pixel density. (C) Representative rhodamine 6G–stained thin-layer chro-matography plates. Values for A and B are reported as mean ± SE (n = 6 muscles per group). *Significantly different from the control group (P < .05).
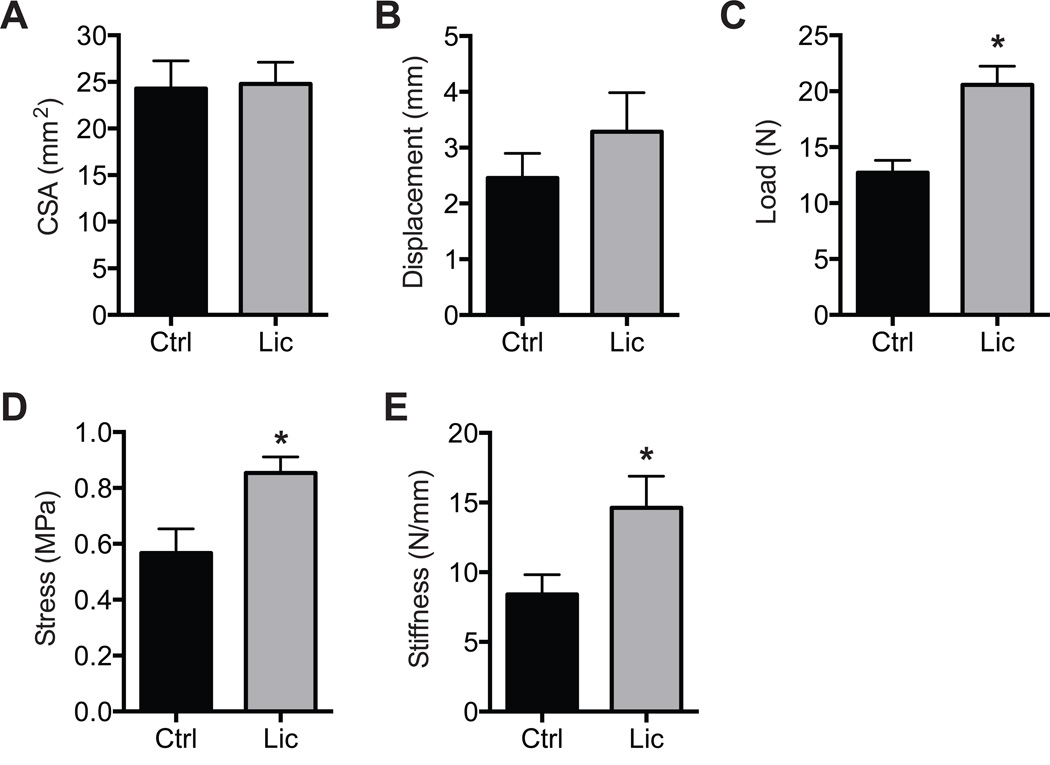
Finally, we evaluated the morphological characteristics and mechanics of the repaired enthesis. Gross views of the supraspinatus tendon and humerus (Figure 6A) demonstrate larger and more inflamed connective tissue in control shoulders compared with the licofelone-treated group. At the level of the enthesis, more fibrocartilage was also noted in the licofelone-treated group (Figure 6B and 6C). No differences in tendon CSAs (P = .446) (Figure 7A) or displacement to yield (P = .186) (Figure 7B) were observed, but compared with controls, licofelone-treated animals had a 62% increase in maximum load (P = .002) (Figure 7C), a 51% increase in peak stress (P = .007) (Figure 7D), and a 73% increase in stiffness (P = .026) (Figure 7E).
Figure 6.
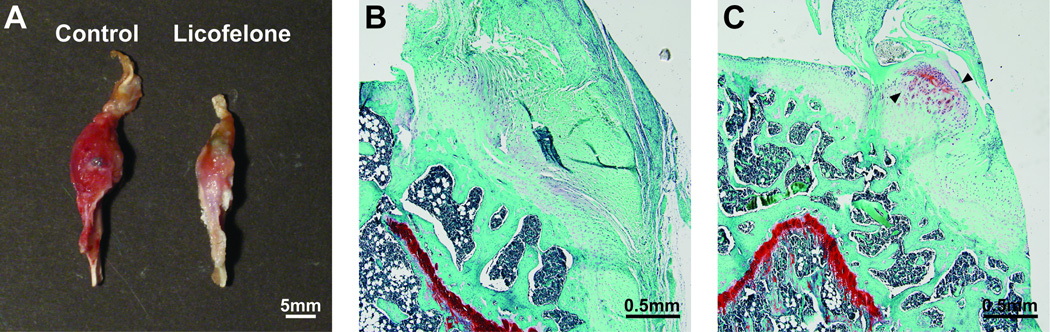
Gross pathological and histological results of the enthesis. (A) Gross view of representative shoulders in the sagittal plane from control and licofelone-treated animals. Representative fast green/safranin O histology of (B) control and (C) licofelone-treated muscles. Arrowheads in C demonstrate the presence of cartilage formation at the tendon-bone interface.
Figure 7.
Mechanical properties of entheses subjected to failure testing. (A) Cross-sectional area (CSA), (B) maximum displacement to yield, (C) maximum load to yield, (D) peak stress, and (E) peak stiffness of tendon-bone interfaces from control and licofelone-treated animals. Values are reported as mean ± SE (n = 6 muscles from controls and n = 8 from licofelone-treated muscles). *Significantly different from the control group (P < .05).
DISCUSSION
Anti-inflammatory medications are commonly used in the treatment of rotator cuff injuries and include both NSAIDs, which target COX-1 and COX-2, and coxib drugs, which specifically target COX-2.24 While these drugs can offer pain relief and limit inflammation, several studies in animal models have suggested a detrimental effect of NSAIDs and coxibs on tendon and enthesis regeneration5,9 and on muscle regeneration.4,27,34 The diversion of arachidonic acid to the 5-LOX pathway and increased production of leukotrienes are thought to be the primary factors that cause side effects from the use of NSAIDs and coxibs.41 Our objective was to determine whether the use of the 5-LOX, COX-1, and COX-2 inhibitor licofelone could prevent inflammation while enhancing the regeneration of torn rotator cuff muscles. The combined results from this study suggest that, similar to NSAIDs and coxibs, licofelone reduces functional muscle regeneration but is effective at enhancing healing at the tendon-bone interface after repair.
Structural failure after rotator cuff repair is a common finding, with reports in the literature ranging from 11% to 95% at 2-year follow-up.8 In a rat model of rotator cuff tear and repair, Cohen and colleagues5 reported that the use of either indomethacin or Celebrex (celecoxib), which belong to the NSAID and coxib classes, respectively, resulted in up to a 50% reduction in the load to failure compared with controls. Similar results were observed in a rat model of patellar tendon tears.9 In the current study, consistent with our hypothesis, licofelone administration resulted in a nearly 2-fold increase in both the load and stress to failure compared with vehicle-treated rats. In support of the observed changes in tendon mechanical properties, we observed a grossly apparent reduction in redness and inflammation at the enthesis in licofelone-treated animals as well as an increase in fibrocartilage formation at the newly repaired enthesis. In a rat model of fracture repair,7 the inhibition of 5-LOX resulted in increased chondrocyte proliferation and hypertrophy at the fibrocartilage callus as well as an increase in type II collagen expression. The inhibition of 5-LOX also protects against the loss of articular cartilage in preclinical animal studies11,29 and in clinical trials of patients with osteoarthritis.32 Bedi and colleagues2 reported that 14 days after repairing an acutely torn supraspinatus tendon, the load to failure was slightly less than 10 N, and this improved to over 20 N by 28 days. In comparison with our results, the load to failure for controls is roughly consistent with the 14-day repair values of Bedi et al,2 but for the licofelone-treated group, the load to failure is closer to what is observed 28 days after repair, suggesting a substantial positive effect of licofelone on tendon healing. Overall, the results from the current study suggest that the combined inhibition of 5-LOX, COX-1, and COX-2 can enhance the formation of a stable enthesis after chronic rotator cuff tears.
Muscle atrophy and fibrosis are commonly observed after chronic rotator cuff tears. COX-2 expression is elevated after muscle injuries,43 which was also observed in the current study, and although anti-inflammatory medications are commonly used to treat pain and swelling that can occur after acute muscle injuries, several studies have indicated that both NSAIDs and coxibs are detrimental to muscle regeneration and strength after injuries.4,27,34 In the current study, for the most common muscle fiber types, we observed no differences in fiber CSAs. This is supported at the gene expression level, as no differences in the expression of the muscle-specific E3 ubiquitin ligases atrogin-1 and MuRF-1 were observed between groups. We did surprisingly observe an increase in the number of pathological type IIB fibers and a subsequent decrease in the abundance of type IIX fibers in licofelone-treated animals. An increase in embryonic myosin heavy chain and slight increases in autophagy-related genes were observed in the licofelone group, which suggests that the muscle fibers have delayed regeneration and are undergoing an active remodeling phase. In studies of human exercise training, Trappe and colleagues40 demonstrated that nonspecific COX inhibitors could suppress muscle fiber protein synthesis. Consistent with these observations, there was a reduction in muscle fiber maximum isometric force and specific force production.
Licofelone also had an effect on fibrosis and fatty infiltration. Although we did not detect a difference in type I collagen expression at the transcript level, licofelone treatment resulted in an approximately 50% reduction in hydroxyproline content of muscles. This is likely because of an increase in the expression of the collagenase MMP-8. Licofelone also markedly decreased the expression of the adipogenic markers PPAR-γ and perilipin 1, which is consistent with the observation of a decrease in whole muscle triglyceride levels observed on thin-layer chromatography plates. While licofelone reduced the expression of adipocyte-related genes and overall muscle triglyceride content, there were slight increases in the intramyocellular lipid droplet–related genes perilipin 5, FIT1, and DGAT1. Combined, these findings indicate that licofelone had a favorable effect on markers of fibrosis and fatty accumulation that are often seen after rotator cuff tears. However, contrary to our hypothesis, but similar to NSAIDs and coxibs, licofelone also reduced muscle force production. While an improvement in muscle force production was not observed, it is possible that including postoperative rehabilitation exercise concurrently with licofelone treatment could provide a mechanical stimulus to muscle that could enhance protein synthesis and promote the functional recovery of muscle fibers.
There are several limitations to this study. While the rat is frequently utilized as a model of rotator cuff tears,37 it does not develop fatty infiltration and atrophy to the same extent as humans and demonstrates a much better rate of structural healing after repair of chronic tears. Because of their relatively short half-life, we did not directly measure leukotriene and prostaglandin levels in tissues, but we did select a dose of licofelone that has previously been demonstrated to effectively reduce the levels of these proinflammatory signaling molecules. While we measured the function of both muscles and tendons in this study, we only measured changes in gene expression in muscles, as we used the whole tendons of both animals for histology and mechanics. We performed failure testing with tendons in this study and did not measure changes in mechanical properties or hysteresis with repetitive loading. Based on our previous work and the work of others, we selected a single time point for the tear duration and repair duration that we think are predictive of long-term outcomes after the repair of chronically torn rotator cuff muscles. Despite these limitations, this study provided important insight into the biology of rotator cuff regeneration and identified a pharmacological compound that could potentially enhance the formation of a stable bone-tendon interface after rotator cuff repair. Future studies that utilize multiple doses of licofelone, include several time points, and measure changes in tendon and enthesis gene expression will provide further insight into the biology of prostaglandins and leukotrienes in the context of rotator cuff repair and potentially help to design a clinical trial in patients with chronic rotator cuff tears or other avulsion injuries.
CONCLUSION
The functional outcomes after the surgical repair of chronic rotator cuff tears can be poor because of tendon retraction, muscle atrophy, and fatty degeneration of the muscle. This study demonstrates that the inhibition of 5-LOX, COX-1, and COX-2 enzymes modulates the healing process of repaired rotator cuff tendons. It appears that the postoperative treatment of rotator cuff repair with licofelone reduces the markers of fatty degeneration and enhances the development of a stable bone-tendon interface in a rat model. These results suggest that therapeutic interventions, such as the use of licofelone, to inhibit 5-LOX and COX enzymes after rotator cuff repair surgery could improve the development of a stable enthesis and enhance postoperative outcomes.
Supplementary Material
Acknowledgments
One or more of the authors has declared the following potential conflict of interest or source of funding: This work was supported by National Institutes of Health grants R01-AR063649 and T32-GM008322.
Footnotes
Investigation performed at the University of Michigan Medical School, Ann Arbor, Michigan, USA
Presented as a poster at the 39th annual meeting of the AOSSM, Chicago, Illinois, July 2013.
REFERENCES
- 1.Bedi A, Dines J, Warren RF, Dines DM. Massive tears of the rotator cuff. J Bone Joint Surg Am. 2010;92(9):1894–1908. doi: 10.2106/JBJS.I.01531. [DOI] [PubMed] [Google Scholar]
- 2.Bedi A, Fox AJ, Kovacevic D, Deng XH, Warren RF, Rodeo SA. Doxy-cycline-mediated inhibition of matrix metalloproteinases improves healing after rotator cuff repair. Am J Sports Med. 2010;38(2):308–317. doi: 10.1177/0363546509347366. [DOI] [PubMed] [Google Scholar]
- 3.Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 4.Bondesen BA, Mills ST, Kegley KM, Pavlath GK. The COX-2 pathway is essential during early stages of skeletal muscle regeneration. Am J Physiol Cell Physiol. 2004;287(2):C475–C483. doi: 10.1152/ajpcell.00088.2004. [DOI] [PubMed] [Google Scholar]
- 5.Cohen DB, Kawamura S, Ehteshami JR, Rodeo SA. Indomethacin and celecoxib impair rotator cuff tendon-to-bone healing. Am J Sports Med. 2006;34(3):362–369. doi: 10.1177/0363546505280428. [DOI] [PubMed] [Google Scholar]
- 6.Colvin AC, Egorova N, Harrison AK, Moskowitz A, Flatow EL. National trends in rotator cuff repair. J Bone Joint Surg Am. 2012;94(3):227–233. doi: 10.2106/JBJS.J.00739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cottrell JA, O’Connor JP. Pharmacological inhibition of 5-lipoxygenase accelerates and enhances fracture-healing. J Bone Joint Surg Am. 2009;91(11):2653–2665. doi: 10.2106/JBJS.H.01844. [DOI] [PubMed] [Google Scholar]
- 8.Edwards SL, Lynch TS, Saltzman MD, Terry MA, Nuber GW. Biologic and pharmacologic augmentation of rotator cuff repairs. J Am Acad Orthop Surg. 2011;19(10):583–589. doi: 10.5435/00124635-201110000-00002. [DOI] [PubMed] [Google Scholar]
- 9.Ferry ST, Dahners LE, Afshari HM, Weinhold PS. The effects of common anti-inflammatory drugs on the healing rat patellar tendon. Am J Sports Med. 2007;35(8):1326–1333. doi: 10.1177/0363546507301584. [DOI] [PubMed] [Google Scholar]
- 10.Fischer L, Hornig M, Pergola C, et al. The molecular mechanism of the inhibition by licofelone of the biosynthesis of 5-lipoxygenase products. Br J Pharmacol. 2007;152(4):471–480. doi: 10.1038/sj.bjp.0707416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gay RE, Neidhart M, Pataky F, Tries S, Laufer S, Gay S. Dual inhibition of 5-lipoxygenase and cyclooxygenases 1 and 2 by ML3000 reduces joint destruction in adjuvant arthritis. J Rheumatol. 2001;28(9):2060–2065. [PubMed] [Google Scholar]
- 12.Gerber C, Schneeberger AG, Hoppeler H, Meyer DC. Correlation of atrophy and fatty infiltration on strength and integrity of rotator cuff repairs: a study in thirteen patients. J Shoulder Elbow Surg. 2007;16(6):691–696. doi: 10.1016/j.jse.2007.02.122. [DOI] [PubMed] [Google Scholar]
- 13.Gladstone JN, Bishop JY, Lo IK, Flatow EL. Fatty infiltration and atrophy of the rotator cuff do not improve after rotator cuff repair and correlate with poor functional outcome. Am J Sports Med. 2007;35(5):719–728. doi: 10.1177/0363546506297539. [DOI] [PubMed] [Google Scholar]
- 14.Goutallier D, Postel JM, Bernageau J, Lavau L, Voisin MO. Fatty muscle degeneration in cuff ruptures: pre- and postoperative evaluation by CT scan. Clin Orthop Relat Res. 1994;304:78–83. [PubMed] [Google Scholar]
- 15.Goutallier D, Postel JM, Gleyze P, Leguilloux P, Van Driessche S. Influence of cuff muscle fatty degeneration on anatomic and functional outcomes after simple suture of full-thickness tears. J Shoulder Elbow Surg. 2003;12(6):550–554. doi: 10.1016/s1058-2746(03)00211-8. [DOI] [PubMed] [Google Scholar]
- 16.Gumucio JP, Davis ME, Bradley JR, et al. Rotator cuff tear reduces muscle fiber specific force production and induces macrophage accumulation and autophagy. J Orthop Res. 2012;30(12):1963–1970. doi: 10.1002/jor.22168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gumucio JP, Korn MA, Saripalli AL, et al. Aging-associated exacerbation in fatty degeneration and infiltration after rotator cuff tear. J Shoulder Elbow Surg. 2014;23(1):99–108. doi: 10.1016/j.jse.2013.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kang JR, Gupta R. Mechanisms of fatty degeneration in massive rotator cuff tears. J Shoulder Elbow Surg. 2012;21(2):175–180. doi: 10.1016/j.jse.2011.11.017. [DOI] [PubMed] [Google Scholar]
- 19.Kovacevic D, Fox AJ, Bedi A, et al. Calcium-phosphate matrix with or without TGF-β3 improves tendon-bone healing after rotator cuff repair. Am J Sports Med. 2011;39(4):811–819. doi: 10.1177/0363546511399378. [DOI] [PubMed] [Google Scholar]
- 20.Kulkarni SK, Singh VP. Licofelone: the answer to unmet needs in osteoarthritis therapy? Curr Rheumatol Rep. 2008;10(1):43–48. doi: 10.1007/s11926-008-0008-7. [DOI] [PubMed] [Google Scholar]
- 21.Laron D, Samagh SP, Liu X, Kim HT, Feeley BT. Muscle degeneration in rotator cuff tears. J Shoulder Elbow Surg. 2012;21(2):164–174. doi: 10.1016/j.jse.2011.09.027. [DOI] [PubMed] [Google Scholar]
- 22.Leval X, Julemont F, Delarge J, Pirotte B, Dogne JM. New trends in dual 5-LOX/COX inhibition. Curr Med Chem. 2002;9(9):941–962. doi: 10.2174/0929867024606713. [DOI] [PubMed] [Google Scholar]
- 23.Li Z, Yang G, Khan M, Stone D, Woo SL, Wang JH. Inflammatory response of human tendon fibroblasts to cyclic mechanical stretching. Am J Sports Med. 2004;32(2):435–440. doi: 10.1177/0095399703258680. [DOI] [PubMed] [Google Scholar]
- 24.Longo UG, Franceschi F, Berton A, Maffulli N, Droena V. Conservative treatment and rotator cuff tear progression. Med Sport Sci. 2012;57:90–99. doi: 10.1159/000328910. [DOI] [PubMed] [Google Scholar]
- 25.Mendias CL, Kayupov E, Bradley JR, Brooks SV, Claflin DR. Decreased specific force and power production of muscle fibers from myostatin-deficient mice are associated with a suppression of protein degradation. J Appl Physiol (1985) 2011;111(1):185–191. doi: 10.1152/japplphysiol.00126.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mendias CL, Marcin JE, Calerdon DR, Faulkner JA. Contractile properties of EDL and soleus muscles of myostatin-deficient mice. J Appl Physiol. 2006;101(3):898–905. doi: 10.1152/japplphysiol.00126.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mishra DK, Fridén J, Schmitz MC, Lieber RL. Anti-inflammatory medication after muscle injury: a treatment resulting in short-term improvement but subsequent loss of muscle function. J Bone Joint Surg Am. 1995;77(10):1510–1519. doi: 10.2106/00004623-199510000-00005. [DOI] [PubMed] [Google Scholar]
- 28.Neuman RE, Logan MA. The determination of hydroxyproline. J Biol Chem. 1950;184(1):299–306. [PubMed] [Google Scholar]
- 29.Pelletier J-P, Boileau C, Boily M, et al. The protective effect of licofelone on experimental osteoarthritis is correlated with the downregulation of gene expression and protein synthesis of several major cartilage catabolic factors: MMP-13, cathepsin K and aggrecanases. Arthritis Res Ther. 2005;7(5):R1091–R1102. doi: 10.1186/ar1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perry SM, Mcllhenny SE, Hoffman MC, Soslowsky LJ. Inflammatory and angiogenic mRNA levels are altered in a supraspinatus tendon overuse animal model. J Shoulder Elbow Surg. 2005;14(1 Suppl S):79S–83S. doi: 10.1016/j.jse.2004.09.020. [DOI] [PubMed] [Google Scholar]
- 31.Peters-Golden M, Henderson WR., Jr Leukotrienes. N Engl J Med. 2007;357(18):1841–1854. doi: 10.1056/NEJMra071371. [DOI] [PubMed] [Google Scholar]
- 32.Raynauld J-P, Martel-Pelletier J, Bias P, et al. Protective effects of licofelone, a 5-lipoxygenase and cyclo-oxygenase inhibitor, versus naproxen on cartilage loss in knee osteoarthritis: a first multicentre clinical trial using quantitative MRI. Ann Rheum Dis. 2009;68(6):938–947. doi: 10.1136/ard.2008.088732. [DOI] [PubMed] [Google Scholar]
- 33.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3(6):1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 34.Shen W, Li Y, Tang Y, Cummins J, Huard J. NS-398, a cyclooxygenase-2-specific inhibitor, delays skeletal muscle healing by decreasing regeneration and promoting fibrosis. Am J Pathol. 2005;167(4):1105–1117. doi: 10.1016/S0002-9440(10)61199-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shen W, Prisk V, Li Y, Foster W, Huard J. Inhibited skeletal muscle healing in cyclooxygenase-2 gene-deficient mice: the role of PGE2 and PGF2alpha. J Appl Physiol (1985) 2006;101(4):1215–1221. doi: 10.1152/japplphysiol.01331.2005. [DOI] [PubMed] [Google Scholar]
- 36.Singh VP, Patil CS, Kulkarni SK. Anti-inflammatory effect of licofelone against various inflammatory challenges. Fundam Clin Pharmacol. 2006;20(1):65–71. doi: 10.1111/j.1472-8206.2005.00387.x. [DOI] [PubMed] [Google Scholar]
- 37.Soslowsky LJ, Carpenter JE, DeBano CM, Banerji I, Moalli MR. Development and use of an animal model for investigations on rotator cuff disease. J Shoulder Elbow Surg. 1996;5(5):383–392. doi: 10.1016/s1058-2746(96)80070-x. [DOI] [PubMed] [Google Scholar]
- 38.Stables MJ, Gilroy DW. Old and new generation lipid mediators in acute inflammation and resolution. Prog Lipid Res. 2011;50(1):35–51. doi: 10.1016/j.plipres.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 39.Sun HB, Li Y, Fung DT, Majeska RJ, Schaffler MB, Flatow EL. Coordinate regulation of IL-1beta and MMP-13 in rat tendons following subrupture fatigue damage. Clin Orthop Relat Res. 2008;466(7):1555–1561. doi: 10.1007/s11999-008-0278-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Trappe TA, White F, Lambert CP, Cesar D, Hellerstein M, Evans WJ. Effect of ibuprofen and acetaminophen on postexercise muscle protein synthesis. Am J Physiol Endocrinol Metab. 2002;282(3):E551–E556. doi: 10.1152/ajpendo.00352.2001. [DOI] [PubMed] [Google Scholar]
- 41.Tries S, Neupert W, Laufer S. The mechanism of action of the new antiinflammatory compound ML3000: inhibition of 5-LOX and COX-1/2. Inflamm Res. 2002;51(3):135–143. doi: 10.1007/pl00000285. [DOI] [PubMed] [Google Scholar]
- 42.Vidal C, Gomez-Hernandez A, Sanchez-Galan E, et al. Licofelone, a balanced inhibitor of cyclooxygenase and 5-lipoxygenase, reduces inflammation in a rabbit model of atherosclerosis. J Pharmacol Exp Ther. 2007;320(1):108–116. doi: 10.1124/jpet.106.110361. [DOI] [PubMed] [Google Scholar]
- 43.Weinheimer EM, Jemiolo B, Carroll CC, et al. Resistance exercise and cyclooxygenase (COX) expression in human skeletal muscle: implications for COX-inhibiting drugs and protein synthesis. Am J Physiol Regul Integr Comp Physiol. 2007;292(6):R2241–R2248. doi: 10.1152/ajpregu.00718.2006. [DOI] [PubMed] [Google Scholar]
- 44.Woessner JF. The determination of hydroxyproline in tissue and protein samples containing small proportions of this imino acid. Arch Biochem Biophys. 1961;93:440–447. doi: 10.1016/0003-9861(61)90291-0. [DOI] [PubMed] [Google Scholar]
- 45.Yadav H, Nho S, Romeo A, MacGillivray JD. Rotator cuff tears: pathology and repair. Knee Surg Sports Traumatol Arthrosc. 2009;17(4):409–421. doi: 10.1007/s00167-008-0686-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.