Abstract
A wealth of data supports the notion that the hippocampus binds objects and events together in place and time. In support of this function, a cortical circuit that includes the retrosplenial cortex (RSC) and various structures in the parahippocampal region is thought to provide the hippocampus with essential information regarding the physical and temporal context in which the object/event occurs. However, it remains unclear if and how individual components of this so-called ‘where’ circuit make unique contributions to processing context-related information. Here we focus on the RSC and the postrhinal cortex (POR; homologous with parahippocampal cortex (PHC) in primates), two of the most strongly interconnected components of the where pathway and the foci of an increasing amount of recent research. Much of the behavioral evidence to date suggests that RSC and POR/PHC work closely together as a functional unit. We begin by briefly reviewing studies that have investigated the involvement of RSC and POR/PHC in contextual and spatial learning, both of which involve learning associations and relationships between the individual stimuli that compose an environment (i.e., where information). However, we propose that potential differences have been overlooked because most studies to date have relied on behavioral paradigms and experimental approaches that are not well suited for distinguishing between different aspects of information processing. We then consider the anatomical differences between RSC and POR/PHC and emerging behavioral evidence that gives rise to a working model of how these regions may differentially contribute to hippocampal-dependent learning and memory. We then discuss experimental designs and behavioral methods that may be useful in testing the model. Finally, approaches are described that may be valuable in probing the nature of information processing and neuroplasticity in the myriad of local circuits that are nested within the where pathway.
Keywords: hippocampus, retrosplenial, postrhinal, non-spatial, where pathway
1. Introduction
Contemporary theories maintain that the hippocampus is essential for binding objects or events together in place and time (Cohen & Eichenbaum, 1993; Cohen et al. 1999; Davachi, 2006; Eichenbaum, 2004, 2011), a function that relies on processed sensory information that is provided to the hippocampus by distinct cortical circuits (Figure 1). One circuit, the ‘what’ pathway that includes regions such as the perirhinal cortex and lateral entorhinal cortex, provides the hippocampus with information about a specific object or event (Murray et al.,. 2000). A second circuit, which is the focus of this review, is often referred to as the ‘where’ pathway and includes the retrosplenial cortex (RSC), postrhinal cortex (POR; homologous with parahippocampal cortex, PHC in primates), and medial entorhinal cortex and provides the hippocampus with information regarding the physical and temporal context in which an object/event occurs (Aggleton, 2012; Barense, et al., 2005; Eichenbaum et al., 2011; Furtak et al., 2007; Hunsaker et al., 2013; Ranganath & Ritchey, 2012; Staresina et al., 2011). For example, processing sensory information about a specific object, such as a coffee cup, would be carried out by structures in the what pathway, whereas information regarding layout of the kitchen in which the cup is located would be processed by the where pathway.
Figure 1.
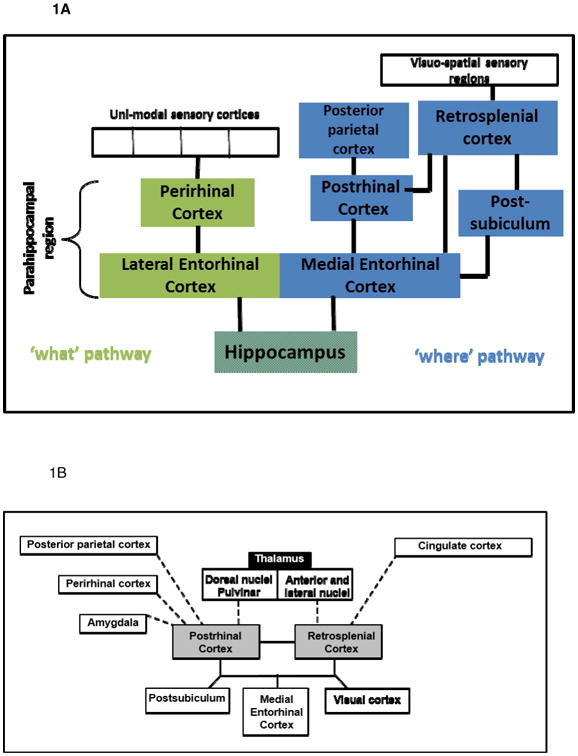
A. Schematic diagram of cortico-hippocampal circuitry. Only the densest connections (black lines) are illustrated for simplicity. B. Schematic diagram of differential connectivity of RSP and POR. The anatomical regions on the bottom half of the diagram (solid lines) represent similar connectivity to and from RSP and POR. The anatomical regions on the top half of the diagram (dotted lines) represent connections that differ between RSP and POR.
The functional distinctions between the what and where pathways are well established and have been valuable in defining the organization of the hippocampal memory system (Ranganath & Ritchey, 2012). However, beyond this dichotomy it continues to be unclear and actively debated if and how individual components of the where circuit make unique contributions to processing context-related information (Aggleton, 2010; 2012; Cowell et al., 2010, Eichenbaum et al., 2011; Kobayashi & Amaral, 2007; Ranganath & Ritchley, 2012; Vann et al., 2009; Wixted & Squire, 2011). Understanding the neural substrates involved in processing contextual information is important not only for episodic memory, but for guiding future behavior. Indeed, learning and recalling contextual information is critical for everyday life, such as remembering where you parked your car or how to get home. Not surprisingly, processing contextual information involves several different components, such as encoding and forming associations between individual stimuli that compose an environment, ascribing behavioral significance to those associations, updating stored associations to account for new information, and storing/retrieving associations made between stimuli. Among others, a present goal in the field is to resolve how individual regions within the where pathway contribute to these functions.
2. Outline and Goals
Here we focus on the RSC and POR/PHC, two of the most strongly interconnected components of the where pathway and the foci of an increasing amount of recent research. As described in an excellent review by Ranganath & Ritchey (2012), anatomical and behavioral evidence to date suggests that RSC and POR/PHC work closely together as a functional unit within the hippocampal memory system. We begin by briefly reviewing studies that have investigated the involvement of RSC and POR/PHC in contextual and spatial learning, both of which are processes that involve learning associations and relationships between individual stimuli that compose a particular environment (i.e., where information). Although the results of most of these studies suggest that RSC and POR/PHC have highly similar functions, it is likely that potential differences have been overlooked because most studies to date, including our own, have relied on behavioral paradigms and experimental approaches that have lacked the resolution to distinguish between specific aspects of information processing. We consider the anatomical differences between RSC and POR/PHC and emerging behavioral evidence that gives rise to a working model of how these regions may differentially contribute to hippocampal-dependent learning and memory. We then discuss experimental designs and behavioral methods that may be useful in testing the model. Finally, approaches are described that may be valuable in probing the nature of information processing and neuroplasticity in the myriad of local circuits that are nested within the where pathway.
3. Similarities between RSC and POR/PHC: Anatomical and Behavioral Evidence
The afferent and efferent connections of RSC and POR/PHC have been summarized recently in a diagrammatic and interactive connectome (a graphical connectivity map) that comprehensively describes the circuitry between the hippocampal formation (Ammons horn, dentate and subiculum), parahippocampal regions (perirhinal cortex, entorhinal cortex, and POR), and RSC (Sugar et al., 2011; van Strien et al., 2009). As these reports indicate, the RSC and POR/PHC are highly interconnected with each other and share similar patterns of cortical connectivity. RSC receives input from visuo-spatial cortical sensory areas and has strong reciprocal connections with visual cortex (areas 17, 18b), cingulate cortex, and multiple parahippocampal regions including POR, medial entorhinal cortex, and the postsubiculum (Agster & Burwell, 2009; Aggleton et al., 2012; Burwell & Amaral, 1998a; Kobayashi & Amaral, 2003, 2007; van Groen & Wyss, 1990; van Groen & Wyss, 1992a, 2003). The cortical connections of the POR/PHC are similar to those of RSC in that POR/PHC also receives input from visual cortex and is reciprocally connected the medial entorhinal cortex (Burwell & Amaral, 1998a, 1998b; Naber et al., 1997; Suzuki & Amaral, 1994) and the postsubiculum (Naber et al., 2001). The patterns of connectivity between RSC and POR/PHC and hippocampal/parahippocampal structures suggest that both RSC and POR/PHC are well-positioned to influence hippocampal-dependent functions.
Consistent with the connectivity of RSC and POR/PHC, substantial behavioral and functional data indicate that both regions are involved in processing where information. Here we highlight evidence from studies that use experimental lesions, neuroimaging methods or electrophysiological techniques to support a role for RSC and POR/PHC in two forms of learning and memory that depend critically on the hippocampus: contextual and spatial learning and memory.
3.1. Contextual Learning and Memory
Various behavioral methods have been used to demonstrate that RSC and POR/PHC contribute to both the acquisition and retrieval of contextual information. In a typical fear conditioning procedure for example, rats are placed in a novel environment (the testing chamber) and allowed to acclimate for several minutes, during which time stimulus-stimulus associations (those made between arbitrary and neutral visual, tactile and olfactory stimuli that comprise the context) are presumed to form (Anagnostaras et al., 2001; Holland & Bouton, 1999). Shortly thereafter, a neutral stimulus (a tone) is paired with an aversive stimulus (foot shock). Thus, during a single acquisition session, rats learn to associate novel arbitrary stimuli with each other as they form a representation of a new context, they learn to associate a single cue (the tone) with foot shock, and they also learn to associate the novel context with the shock. Accordingly, contextual learning not only involves the formation of associations between various sensory stimuli that compose the environment, but it also requires an updating of those associations when tone and shock are presented later in the acquisition session. Contextual fear memory is subsequently assessed by returning the subject to the training context in the absence of the tone or the aversive stimulus. Similarly, retrieval of cue-specific fear memory is assessed by placing the rat in a different environment and measuring freezing behavior in response to presentations of the tone (Fanselow, 1980).
Damage to either RSC or POR has consistently been shown to impair contextual fear conditioning while sparing cue-specific fear conditioning (Bucci et al., 2000, Burwell et al., 2004a, 2004b; Keene & Bucci, 2008a, 2008c). Importantly, these effects have been observed regardless of whether lesions were made prior to or after acquisition. In this regard, the effects of RSC or POR damage on contextual fear memory appear to be more pervasive than hippocampal lesions since hippocampal damage prior to training has been shown to be without effect in several studies (e.g., Maren et al., 1997). Moreover, consistent with the effects of permanent lesions, temporary blockade of NMDA receptors in RSC just prior to retrieval impairs contextual fear memory in mice (Corcoran et al., 2011). In addition, lesions of POR have also been shown to impair the ability to discriminate between different contexts (Bucci et al., 2002), and POR damage disrupts contextual memory in a spontaneous recognition paradigm while sparing object memory (Eacott & Gaffan, 2005; Norman & Eacott, 2005).
Further evidence is provided by complementary neurochemical, electrophysiological and neuroimaging studies that examine activation in RSC and POR during contextual learning and memory. Specifically, expression of the immediate-early genes Fos and Arc are elevated in RSC during contextual fear learning and during the retrieval of contextual fear memories (Beck & Fibiger, 1995; Robinson et al., 2012; Tayler et al., 2013). Similarly, electrophysiological data indicate that RSC is involved in distinguishing between different contexts (Freeman et al., 1996; Smith et al., 2004, 2012; Talk et al., 2004) and that cell firing in primate PHC is sensitive to changes in context (Vidyasagar et al., 1991). Neuroimaging studies in humans likewise demonstrate that both RSC and PHC are active during tasks that require the processing of contextual information or the formation of contextual associations (Aminoff et al., 2007; Bar & Aminoff, 2003; Bar et al., 2008a, 2008b; Brown et al., 2012; Fenske et al., 2006; Hayes et al., 2007; Kveraga et al., 2011).
3.2. Spatial Learning and Memory
In a typical spatial learning task, subjects use the associations between individual landmarks in the environment to navigate to a rewarded location or escape platform. For example, in the Morris water maze, the location of an invisible submerged platform is not indicated by an individual cue, but rather by the configuration of multiple visual cues present in the surrounding environment. Like contextual fear conditioning, there is substantial evidence that damage to RSC, in the form of permanent lesions or temporary inactivation, impairs performance on a variety of spatial navigation tasks (Cain et al., 2006; Cooper & Mizumori, 1999; Cooper et al, 2001; Harker & Whishaw, 2002, 2004; Lukoyanov et al., 2005; Pothuizen et al., 2010; van Groen et al., 2004; Vann & Aggleton, 2002, 2004; Vann et al., 2003), although the effects may vary based on lesion methods and extent of damage to surrounding areas such as the cingulum bundle. Damage to RSC also produces deficits in spatial working memory, in which a new location is learned during each set of trials (Keene & Bucci, 2009). Similarly, damage to POR/PHC has been shown to impair spatial learning (Liu & Bilkey, 2002; Mair et al., 2003; but see Burwell et al., 2004) and lesions of RSC or POR also impair long-term spatial memory (Haijima & Ichitani, 2008; Maviel et al., 2004; Ramos, 2013). Other studies have demonstrated that RSC damage disrupts the ability to perform spatial alternation tasks (Pothuizen et al., 2008, 2010), while recognition of the objects themselves is unaffected (Ennaceur et al., 1997; Parron & Save, 2004; Vann & Aggleton, 2002); a finding that is consistent with the notion that RSC participates in the where pathway, but has little intersection with the what pathway. Similarly, humans with RSC damage exhibit disorientation (Maeshima et al., 2001, Maguire, 2001; Takahashi et al., 1997) and it has been repeatedly shown that damage to PHC in monkeys or humans impairs the ability to use spatial cues to make object-place discriminations (Bachevalier & Nemanic, 2008; Malkova & Mishkin, 2003; Ploner et al., 2000; Epstein et al., 2001). Collectively, these data indicate that both RSC and POR/PHC are critically involved in processing spatial information.
Relatedly, previous reports documenting the activation of RSC and POR/PHC during spatial learning have revealed that RSC and POR are constituents of head-direction and/or place cell circuitry. Not only has the existence of head direction and place cells been observed in RSC (Chen et al., 1994; Cho & Sharp, 2001) and POR (Burwell & Hafeman, 2003), but additional reports have also demonstrated that permanent lesions of RSC influence head direction cell firing patterns in brain regions involved in spatial information processing (Clark et al., 2010). Likewise, electrophysiological evidence indicates that POR neurons encode object-location conjunctions (Furtak et al., 2012). Consistent with these findings neuroimaging studies in humans reveal that RSC is active during spatial navigation (Epstein, 2008; Epstein et al., 2007) and that PHC shows preferential activation during tasks that involve changes in scenes and during tasks that require spatial navigation (Epstein et al., 2003; Mullally & Maguire, 2011; Weniger et al., 2010).
3.3. Summary and Unanswered Questions
Collectively, the body of work described above provides converging evidence that both RSC and POR/PHC contribute to processing where information and that these regions are well positioned to influence hippocampal-dependent functions. Furthermore, the similarity in connectivity and in the findings from behavioral studies suggests that RSC and POR/PHC form an important functional unit. Hence, it may be that the functional contributions of RSC and POR/PHC are non-dissociable. Alternatively, there may be functional differences between RSC and POR/PHC, but they have yet to be revealed due to limitations in the experimental designs used to date. For example, the use of permanent lesions conducted prior to training makes it difficult to differentiate between effects on learning, consolidation, or memory retrieval. Similarly, prior studies have used behavioral designs that make it difficult to isolate different aspects of information processing related to contextual and spatial learning. Indeed, processing contextual and spatial information involves not only encoding and forming associations among individual stimuli that compose an environment, but also ascribing behavioral significance to those associations, updating them to account for new information, and storing/retrieving the associations. In the case of contextual fear conditioning, for instance, forming associations between the neutral contextual stimuli takes place in the same session as does the presentation of foot shock (i.e., behavioral significance), making it impossible to determine whether RSC or POR/PHC are involved in one process versus the other. Thus, there is a significant need for experimental designs that can be used to determine how RSC and PHC/POR contribute to processing where information (Ranganath & Ritchey, 2012). To address this, we present a theoretical model regarding the functions of RSC and POR/PHC, and then describe behavioral approaches and methods that can be used to test the hypotheses that emerge from this model.
4. Functional and Anatomical Distinctions between RSC and POR/PHC
Although RSC and POR/PHC are strongly interconnected and both contribute to processing where information, there are several notable neuroanatomical differences between these regions. Compared to RSC, the POR/PHC is more heavily interconnected with the posterior parietal cortex (PPC; Burwell & Amaral, 1998a), an area strongly implicated in attentional function (Bucci, 2009; Constantinidis, 2011; Hutchinson et al., 2009). Consistent with this, the thalamic input to POR/PHC arises primarily from dorsal thalamic nuclei, in particular the lateral posterior nucleus (Furtak et al., 2007), and from the pulvinar nucleus in primates (Baleydier & Mauguiere, 1985), both of which are also involved in regulating attention (Reep & Corwin, 2009; Robinson, 1993). By comparison, thalamic input to RSC is provided by anterior and lateral thalamic nuclei (van Groen & Wyss, 1992b; Vogt et al., 1987) which are closely linked to spatial memory (Jankowski et al., 2013). Additionally, POR/PHC is interconnected with regions that process information regarding the affective value of stimuli, such as the perirhinal cortex and the amygdala, whereas RSC exhibits little or no connectivity with these regions (Furtak et al, 2007; van Groen & Wyss, 1990, 1992a, 2003).
4.1. POR/PHC and Attentional Processes
One interpretation of these anatomical data is that POR/PHC contributes to hippocampal function by altering attention to changes in contextual and spatial stimuli (Bucci & Burwell, 2004). Several pieces of behavioral evidence are consistent with this idea. For example, PHC is preferentially activated when tasks involve changes in scenes (Epstein et al., 2003; Mullally & Maguire, 2011; Weniger et al., 2010). Likewise, analysis of place cell firing suggests that rodent POR neurons detect changes in the environment (Burwell & Hafeman, 2003; Furtak et al., 2012) and cell firing in the primate PHC was found to be sensitive to changes in the training context (Vidyasagar et al., 1991). Collectively, these findings may reflect a role for POR/PHC in monitoring the environment for physical changes in stimuli, or changes in the meaning of stimuli (e.g., whether or not they are followed by an expected outcome) and for redirecting attention to those cues accordingly (Burwell & Hafeman, 2003; Furtak et al., 2012). This notion may also provide an explanation for the finding that POR lesions do not always impair spatial learning. For example, deficits in the Morris water maze have been observed when POR-lesioned rats were tested in a large room containing numerous spatial cues (Liu & Bilkey, 2002), but not when the maze was surrounded by curtains containing just a few visual stimuli (Burwell et al., 2004). The former may require additional attentional resources, thereby requiring an intact POR for normal performance. Moreover, Liu & Bilkey (2002) found that the effects of POR damage on spatial working memory were delay independent, consistent with an attention deficit rather than a mnemonic impairment (Baxter et al., 1995).
4.2. Retrosplenial Cortex and Stimulus-Stimulus Associations
The RSC, on the other hand, may function as an interface and/or gateway between sensory and information-processing regions, respectively (Kobayashi & Amaral, 2007; Sugar et al., 2011; van Strien et al., 2009; Vann et al., 2009). As described previously, RSC is a large polymodal region that receives input from various visuo-spatial cortical sensory areas and projects extensively to the hippocampal-parahippocampal network. Thus, RSC may contribute to processing where information by forming the initial associations between the various sensory stimuli that compose a learning environment. Indeed, it has been proposed that one of the functions of the hippocampal memory system is to form arbitrary associations among the cues that compose the place, or context, where an object or event occurs (Bunsey & Eichenbaum, 1993). Interestingly, however, two recent studies indicate that the hippocampus itself is not necessary for this function (Iordanova et al., 2011; Wimmer & Shohamy, 2012), further suggesting that another region, such as RSC, may be responsible for forming the initial associations between contextual stimuli.
Building from prior work by Gabriel and others (Freeman et al., 1996; Harker & Wishaw, 2002; Smith et al,. 2004, 2012; Talk et al. 2005; Vann & Aggleton, 2004), we recently conducted a series of behavioral studies using rats with permanent RSC lesions to test the hypothesis that RSC is essential when arbitrary associations are formed between multiple sensory stimuli. At the outset of our studies, we reasoned that if RSC has a fundamental role in linking together sensory stimuli, then damage should impair learning regardless of factors such as the modality of the stimuli or how the stimuli are presented, as long as the task requires forming associations between them. We found that RSC lesions impaired spatial learning (Keene & Bucci, 2009) as well as contextual fear conditioning (Keene & Bucci, 2008a). Hence, RSC was necessary when tasks required forming arbitrary associations among the background stimuli in the environment regardless of whether those associations were used to navigate or not. We also found that the effects of RSC lesions were not limited to situations in which the stimuli were static, continually present background cues. For example, RSC damage also impaired the ability to form associations among phasic stimuli (e.g., a light and a tone presented for a discrete time period; Keene & Bucci, 2008b; Robinson et al., 2011). RSC was also shown to be critical for associative learning regardless of whether stimuli were presented in conjunction with appetitive or aversive outcomes (Keene & Bucci, 2008a, 2008b, Robinson et al., 2011).
Conversely, data from these studies consistently demonstrated that RSC is not necessary for the formation of an association between a single cue and an outcome. Together these findings support a specific role for RSC in establishing new associations among multiple sensory stimuli that compose a context or location. One intriguing idea that has been proposed is that the hippocampus uses such associations to form contextual and spatial representations which are subsequently incorporated into existing schemas (Eichenbaum et al., 2011; Kobayashi & Amaral, 2007; Kubik et al., 2012; Smith et al., 2011; Wolbers & Buchel, 2005).
5. Working Model
Based on these findings, we propose a working model that is illustrated in Figure 2A. One arm of the model advances the premise that RSC has a critical role in forming associations between the individual sensory stimuli that compose an environment. Moreover, we claim that the formation of those associations does not necessarily include, or require, the presence of reinforcers (e.g., food, shock, etc.). A second arm of our model maintains that information regarding the biological significance or change in the meaning of contextual stimuli is processed by POR/PHC. Using the example of contextual fear conditioning, the model maintains that RSC encodes the initial relationships and associations among the multiple sensory stimuli that compose the context. When shock is delivered later in the acquisition session, our working model implies that POR/PHC serves to detect behaviorally relevant changes to contextual stimuli and relay that new status to RSC. In turn, we propose that RSC incorporates the updated information into the previously acquired contextual associations and then furnishes this information to the hippocampal formation. An alternative model wherein information processed by RSC and POR/PHC is maintained in segregated paths that independently inform the hippocampus is also conceivable (Figure 2B).
Figure 2.
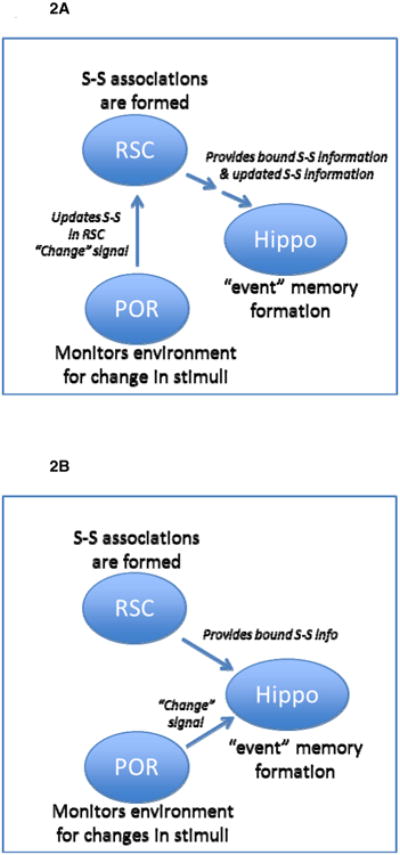
Working model of the functional contributions of RSC and POR/PHC to processing ‘where’ information.
6. Recent Evidence Supporting the Theoretical Model
The models presented above give rise to several testable hypotheses. First, if associations between sensory stimuli are formed in RSC, then RSC neurons should be active during contextual fear conditioning, and furthermore, RSC neurons should exhibit learning-related synaptic plasticity. We recently tested this by examining the expression of two immediate-early genes in RSC in rats that were sacrificed immediately after a contextual fear conditioning session (Figure 3a). Rats were placed in a conditioning chamber and three minutes later received three presentations of a mild foot shock. Control groups included home cage controls; rats that were placed in the conditioning chamber, but did not receive shock (context only); and rats that were placed in the chamber and immediately shocked (shock-only). The latter condition, which induces an immediate shock deficit, does not support fear learning (Fanselow, 1986; Frankland et al 2004; Kiernal & Cranney, 1992, Wiltgen et al, 2006). Sixty minutes later rats were sacrificed and brains were processed for FOS or ARC immunocytochemistry to assess general neural activity and learning-related plasticity, respectively (Beck and Fibiger, 1995; Campeau et al., 1991; Miyashita et al., 2009; Pezzone et al., 1992; Shepherd & Bear, 2011). Rats that were fear conditioned exhibited higher levels of FOS (suggestive of activation in general) and ARC (suggestive of learning-related plasticity) expression in RSC compared to each of the controls (Figure 3b, 3c). Moreover, a stair-step pattern of expression was noted (Figure 3b) in that the context-only group also exhibited greater expression compared to the shock-only and home cage groups. This further supports the notion that RSC is involved in forming the initial stimulus-stimulus associations between contextual stimuli, even when they are not associated with a significant event. In addition, these findings are consistent with other recent work demonstrating an elevation in ARC expression in RSC during hippocampal-dependent learning (Tse et al., 2011).
Figure 3.
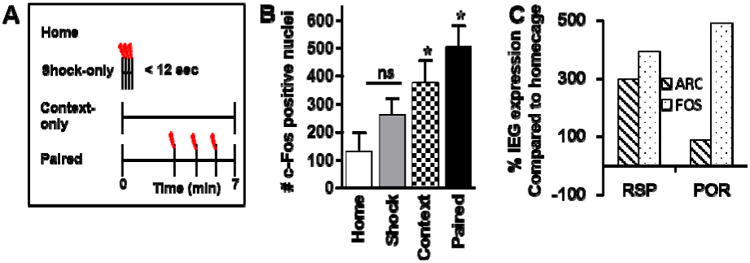
Behavioral design and immediate early gene protein expression in RSP and POR following contextual fear conditioning. A, A schematic depicting the four behavioral conditions included in the fear conditioning experiment. B, Average (mean ± SEM) c-Fos protein expression in RSP across behavior conditions. The profile of arc protein expression in RSP was comparable (data not shown). The asterisk (*) indicates significant (p < 0.05) differences from all other conditions; ns indicates no significant difference. C Percentage of c-fos (hashed bars) or Arc (dotted bars) positive cells in RSP or POR of contextually fear conditioned rats relative to the number of IEG positive neurons expressed in RSP and POR of homecage animals.
In contrast, our model predicts a subtle but important difference in the profile of immediate early gene expression in POR during fear conditioning. Specifically, if POR/PHC functions to provide RSC with information regarding the biological significance of contextual stimuli, POR should be active (i.e. elevated FOS expression) during acquisition of contextual fear conditioning, but should not necessarily exhibit learning-related plasticity (i.e., ARC expression should not differ across the experimental groups). Consistent with this hypothesis, we found that FOS but not ARC expression is increased in POR after contextual fear conditioning (Figure 3c, unpublished data).
Another prediction of the working model illustrated in Figure 2A is that communication between RSC and POR is necessary for contextual learning and memory. Consistent with this idea, we recently demonstrated that a functional disconnection of RSC and POR impairs contextual fear conditioning in rats (Robinson et al., 2012). In that study lesions were performed prior to fear conditioning and the behavior of rats in which the RSC was lesioned in one hemisphere and the POR was lesioned in the opposite hemisphere (i.e., bilateral disruption of communication between RSC and POR; contralateral lesion group) was compared to the behavior of rats in which unilateral lesions to RSC and POR were made in the same hemisphere (i.e., intact communication on one side; ipsilateral lesioned group). If each individual region, but not the interaction between RSC and POR, was important for learning we would have expected the two types of lesions to have similar effects since they involved damaging the same total amount of tissue (1 RSC and 1 POR). Instead, we found that bilateral disconnection (contralateral lesion group) produced a greater deficit than unilateral disconnection (ipsilateral lesion group), indicating that interaction between RSC and POR is required for contextual fear conditioning (Figure 4). Complementing these data, we also found that neurons in RSC that innervate POR were active during contextual fear conditioning (Robinson et al., 2012; Figure 5), further supporting the notion that RSC and POR communicate during contextual learning.
Figure 4.
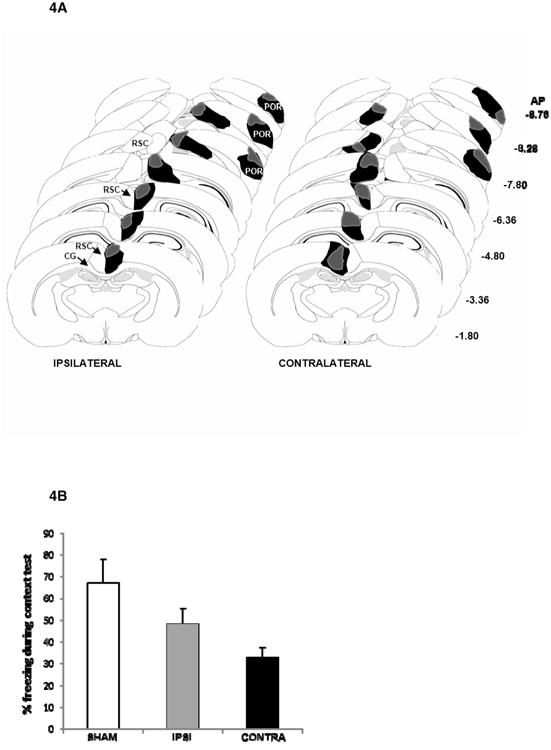
Unilateral lesion-induced disconnection of RSP and POR impairs contextual fear conditioning. A. Collages of brain diagrams (modified from Paxinos & Watson, 1988) along the rostrocaudal extent of the RSC and POR indicating the largest (black) and smallest (grey) ipsilateral and contralateral lesions of the RSC and POR. CG, Cingulum bundle; RSC, retrosplenial cortex; POR, postrhinal cortex. B. Freezing behavior of rats during a test of contextual fear learning. Rats sustained sham (open bars, SHAM), ipsilateral (grey bars, IPSI) or contralateral (black bars, CONTRA) lesions of RSC and POR prior to acquisition of fear conditioning and were tested 24 hr later for contextual fear memory.
Figure 5.
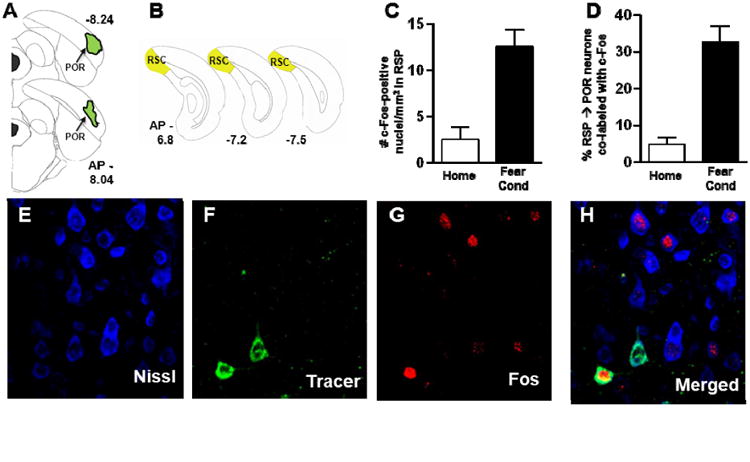
Histochemically verified neurons that project from RSC to POR are activated following fear conditioning. A. Brain diagrams depicting the infusion of a retrograde tracer (Cholera-toxin B coupled to Alexafluor-488) into POR. Half of the infused rats were underwent an acquisition session of a fear conditioning paradigm in which 3 foot shocks were delivered while the rats explored a novel context. The other half of the rats served as home-cage controls. B. Brain diagrams reflecting the rostrocaudal location from which confocal images of RSC neurons were collected. C. Average (mean and sem) c-Fos expression in RSC in homecage (HOME) compared to fear conditioned (FEAR COND) rats indicating that RSC is activated during contextual learning. D. Average (mean and sem) percentage of RSC neurons that project to POR that are co-labeled for tracer and c-Fos expression following fear learning indicating that the pathway between RSC and POR is activated. E-G. Photomicrographs of RSC neurons obtained with a confocal microscope equipped to detect Nissl (E), retrogradely labeled neurons (F) and c-Fos expression (G). H. Merged image of neurons co-labeled for Nissl, Tracer and c-Fos.
Lastly, some recent studies have compared the functions of RSC and POR/PHC in the same experimental preparation. For example, neuroimaging data indicate that RSC selectively processes the most permanent, or stable stimuli in an environment; conversely, the PHC is responsive to a broader range of attributes particularly when they guide behavior at a decision point (Auger et al., 2012). Relatedly, RSC was found to be more responsive to familiar scenes than PHC (Epstein et al., 2007). Other studies of spatial learning indicate that areas in PHC are involved in distinguishing between multiple views of a scene while RSC serves to integrate scenes within a specific context (Park & Chun, 2009). In addition, different response dynamics have been observed in PHC and RSC during scene or context processing (Henderson et al., 2008; Kveraga et al., 2011). These findings support the notion that RSC and POR/PHC may have dissociable functions that can be resolved using appropriate task designs and techniques.
7. Avenues for Future Study
7.1. Behavioral approaches that separate discrete phases of learning
Rigorous testing of ours or other models that seek to differentiate the contributions of RSC from those of POR/PHC will require the use of behavioral designs that explicitly separate functions that range from the formation of arbitrary associations among neutral sensory stimuli to the assignment of behavioral significance to those associations. This can be accomplished using paradigms such as sensory preconditioning (Blaisdell et al., 2009; Brogden, 1939; Holland & Ross, 1983; Ward-Robinson et al., 2001), which is illustrated in Figure 6. In the first phase of training, an auditory stimulus (a tone) is presented and followed immediately by a visual stimulus (a light) on half of the trials. During the other half of the trials in each session, another auditory stimulus (white noise) is presented alone. Importantly, no reinforcement is delivered during this phase of training. Thus, rats are given the opportunity to form a stimulus-stimulus association in the absence of reward. During the second phase of training, the same light is presented and followed by food reward. Therefore, unlike in a standard fear conditioning paradigm, the biological significance of arbitrary stimulus-stimulus associations are updated in a subsequent and distinct phase of learning. Lastly, during a final and single test session, the tone and white noise are presented alone on intermixed trials. Normal rats typically exhibit more conditioned responding to the preconditioned auditory cue (the tone) compared to the unpaired cue (white noise) during the test session (Blaisdell et al., 2009; Holland & Ross, 1983). This outcome is thought to reflect the formation of a stimulus-stimulus association between the two paired sensory stimuli during the initial training session, a phenomena referred to as sensory preconditioning.
Figure 6.
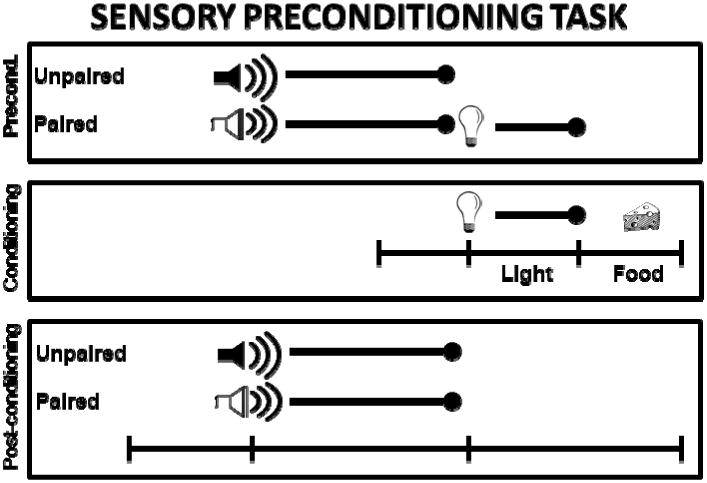
Schematic diagram of a sensory preconditioning procedure illustrating a behavioral paradigm that permits examination of distinct components of learning. During the Preconditioning phase (top panel) presentations of one type of auditory cue are paired with a visual stimulus whereas presentations of a second auditory cue occur unpaired. During this phase, stimulus-stimulus associations are presumed to occur. During the Conditioning phase (middle panel) the light is paired with food. During this phase, encoding of changes in the biological significance of stimuli is presumed to occur. In the third and final Post-Conditioning phase both auditory cues are presented in intermixed trials while conditioned responding is recorded.
As shown previously, permanent lesions of RSC impair sensory preconditioning, in that they respond similarly to both auditory stimuli during the test session. Notably, it has recently been shown that the hippocampus is not active (Wimmer & Shohamy, 2012) or necessary (Iordnova et al, 2011) for forming the initial stimulus-stimulus associations during the first phase of sensory preconditioning. Thus, the specific contribution of RSC to forming stimulus-stimulus associations could be tested by reversibly inactivating RSC during discrete phases of a sensory preconditioning task. Our model would predict that inactivating RSC during phase 1 would disrupt sensory preconditioning by eliminating the ability to form the stimulus-stimulus association between the light and the tone. Conversely, inactivating RSC during phase 2, when the light is paired with food, should be without effect. The opposite prediction could be tested by inactivating POR during phase 1 or phase 2. We would predict that silencing neurons in POR specifically during phase 2, when there is a change in the meaning of a stimulus, would disrupt performance during the final test session.
To date, these types of studies have been hampered by technical limitations. Specifically, the sheer size of RSC (almost 8mm along the rostro-caudal axis of the rat brain, Paxinos & Watson, 2008) has precluded the use of traditional inactivation techniques. Indeed, numerous surgically implanted cannulae are required on each side of the brain to inactive the RSC along its entire rosto-caudal axis. In our experience, this approach produces an unacceptable amount of mechanical damage and it is also very difficult to keep all cannulae patent over several daily training sessions. Yet, the importance of manipulating neurons along the entire rostro-caudal axis of RSC is underscored by recent anatomical data indicating that the intrinsic connectivity of the RSC is characterized by a significant amount of interconnectivity within the coronal plane (Shibata et al., 2012). This can now be surmounted using new chemogenetic techniques, whereby by systemic drug injection can be used to transiently activate inhibitory neurotransmitter channels introduced by prior injection of viral constructs (Armbruster et al, 2007, Rogan & Roth, 2011). For example, a viral construct containing the gene for a synthetic inhibitory G-protein coupled receptor is infused into one or more sites of a target region just like a traditional neurotoxin (e.g., NMDA, ibotenic acid). The G-protein is maximally expressed in neurons in the target region in ∼3 weeks and can be selectively activated by systemic administration of clozapine-N-oxide (CNO). Activation of the inhibitory G-protein coupled receptor by CNO silences neuronal activity for up to 2 hours. Using this method, the viral construct can be infused throughout the rostro-caudal extent of RSC, allowing for temporary silencing of neural activity of the entire RSC without introducing the mechanical damage and technical difficulties associated with traditional cannulation methods.
7.2. Other behavioral approaches
As described in the previous section, combining reversible inactivation methods with behavioral designs that separate discrete phases of learning will be valuable in testing for a double dissociation regarding the roles of RSC and POR in forming stimulus-stimulus associations versus altering attention when there are changes to those stimuli. However, there is also a need for behavioral studies that test the involvement of RSC and POR in more general aspects of these functions. For example, the model predicts that RSC has little involvement in altering attention when there are changes in contextual and spatial stimuli. Yet, very few published studies to date have examined the role of RSC in any aspect of attention. To our knowledge, the only exception is with regard to orienting behavior in humans or rats with unilateral damage to RSC (Heilman et al., 1990; Kwon et al., 1990). Thus, it remains to be fully tested whether RSC contributes to the changes in attentional processing that are proposed for POR. Similarly, there is a lack of studies that test whether POR is involved in forming stimulus-stimulus associations. Our model would predict that manipulations of POR would not impair the formation of associations between sensory stimuli. Consistent with this, a recent study by Gastelum et al. (2012) demonstrated that lesions involving POR did not impair (and in fact enhanced) the formation of associations between stimuli in a feature-positive feature-negative discrimination task. Additional studies such as these are essential in order to fully test the predicted dissociations predicated by a functional model.
7.3. Tract Tracing and Task-Induced Neural Activation
Our model further proposes that POR/PHC provides information directly to RSC regarding the behavioral significance of contextual stimuli (Figure 2A). However, an alternative to this hypothesis is that information processed by RSC and POR/PHC may be maintained in segregated paths that independently inform the hippocampus (Figure 2B). These competing hypotheses can be readily tested by combining tract-tracing and neural activation methods to assess communication between RSC, POR/PHC, and hippocampus during learning. For example, we recently used this approach to demonstrate that RSC neurons that project to POR are active during contextual fear conditioning (Robinson et al., 2012). Similarly, separate retrograde tracers could be infused into RSC or hippocampus prior to fear conditioning, and brains subsequently processed for Fos immunoreactivity, a marker of neuronal activation. With this approach, learning-related activation within the pathway from POR to RSC could be directly compared to activation within the pathway from POR to the hippocampus, which has the potential to provide information regarding task-related communication between structures included in our working model.
8. Conclusion
Although several different cortical structures compose the where pathway, there has been little insight to date regarding the specific contributions of each region to processing contextual or spatial information. This may indicate that the function of structures such as RSC and POR/PHC are non-dissociable. However, as we argue here, it is also possible that functional differences have been overlooked because of the reliance on behavioral paradigms and experimental approaches that lack the resolution to distinguish between specific aspects of information processing. In proposing a working model of how RSC and POR/PHC may differentially contribute to hippocampal-dependent learning and memory, we presented several experimental designs and behavioral methods that may be useful in testing emerging hypotheses. These same approaches may also guide future research in determining the functional significance of the host of other local circuits nested within the where pathway, thereby contributing to a more complete understanding of information processing within the hippocampal memory system.
Highlights.
The retrosplenial and postrhinal cortices are strongly interconnected components of the ‘where’ pathway.
Here we propose a theoretical model of their individual contributions to learning and memory.
Experimental designs and methods are described that may be useful in testing the functional contributions and interactions between these regions.
Acknowledgments
Research supported by NSF Grants IOS 0922075 and 0441934 (DJB) and NIH Grant MH092991 (SR).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aggleton JP. Multiple anatomical systems embedded within the primate medial temporal lobe: Implications for hippocampal function. Neurosci Biobehav Rev. 2012;36:1579–96. doi: 10.1016/j.neubiorev.2011.09.005. [DOI] [PubMed] [Google Scholar]
- Aggleton JP. Understanding retrosplenial amnesia: insights from animal studies. Neuropsychologia. 2010;48(8):2328–38. doi: 10.1016/j.neuropsychologia.2009.09.030. [DOI] [PubMed] [Google Scholar]
- Aggleton JP, Wright NF, Vann SD, Saunders RC. Medial temporal lobe projections to the retrosplenial cortex of the macaque monkey. Hippocampus. 2012;22(9):1883–900. doi: 10.1002/hipo.22024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agster KL, Burwell RD. Cortical efferents of the perirhinal, postrhinal, and entorhinal cortices of the rat. Hippocampus. 2009;19(12):1159–86. doi: 10.1002/hipo.20578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aminoff E, Gronau N, Bar M. The parahippocampal cortex mediates spatial and nonspatial associations. Cereb Cortex. 2007;17(7):1493–503. doi: 10.1093/cercor/bhl078. [DOI] [PubMed] [Google Scholar]
- Anagnostaras SG, Gale GD, Fanselow MS. Hippocampus and contextual fear conditioning: Recent controversies and advances. Hippocampus. 2001;11:8–17. doi: 10.1002/1098-1063(2001)11:1<8::AID-HIPO1015>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Armbruster BN, Li X, Pausch MH, Herlitze S, Roth BL. Evolving the lock to fit the key to create a family of G protein-coupled receptors potently activated by an inert ligand. Proc Natl Acad Sci U S A. 2007;104:5163–8. doi: 10.1073/pnas.0700293104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auger SD, Mullally SL, Maguire EA. Retrosplenial cortex codes for permanent landmarks. PLoS One. 2012;7(8):e43620. doi: 10.1371/journal.pone.0043620. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baleydier C, Mauguiere F. Anatomical evidence for medial pulvinar connections with the posterior cingulate cortex, the retrosplenial area, and the posterior parahippocampal gyrus in monkeys. J Comp Neurol. 1985;232(2):219–28. doi: 10.1002/cne.902320207. [DOI] [PubMed] [Google Scholar]
- Bar M, Aminoff E. Cortical analysis of visual context. Neuron. 2003;38:347–358. doi: 10.1016/s0896-6273(03)00167-3. [DOI] [PubMed] [Google Scholar]
- Bar M, Aminoff E, Schacter DL. Scenes unseen: the parahippocampal cortex intrinsically subserves contextual associations, not scenes or places per se. J Neurosci. 2008;28(34):8539–44. doi: 10.1523/JNEUROSCI.0987-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar M, Aminoff E, Ishai A. Famous faces activate contextual associations in the parahippocampal cortex. Cereb Cortex. 2008;18(6):1233–8. doi: 10.1093/cercor/bhm170. [DOI] [PubMed] [Google Scholar]
- Baxter MG, Bucci DJ, Gorman LK, Wiley RG, Gallagher M. Selective immunotoxic lesions of basal forebrain cholinergic cells: Effects on learning and memory in rats. Behavioral Neuroscience. 1995;109(4):714–722. doi: 10.1037//0735-7044.109.4.714. [DOI] [PubMed] [Google Scholar]
- Bachevalier J, Nemanic S. Memory for spatial location and object-place associations are differently processed by the hippocampal formation, parahippocampal areas TH/TF and perirhinal cortex. Hippocampus. 2008;18(1):64–80. doi: 10.1002/hipo.20369. [DOI] [PubMed] [Google Scholar]
- Barense MD, Bussey TJ, Lee AC, Rogers TT, Davies RR, Saksida LM, Murray EA, Graham KS. Functional specialization in the human medial temporal lobe. J Neurosci. 2005;25(44):10239–46. doi: 10.1523/JNEUROSCI.2704-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck CH, Fibiger HC. Conditioned fear-induced changes in behavior and in the expression of the immediate early gene c-fos: with and without diazepam pretreatment. J Neurosci. 1995;15(1 Pt 2):709–20. doi: 10.1523/JNEUROSCI.15-01-00709.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaisdell AP, Leising KJ, Stahlman WD, Waldmann MS. Rats distinguish between absence of events and lack of information. in sensory preconditioning. International Journal of Comparative Psychology. 2009;22:1–18. [Google Scholar]
- Brogden WJ. Sensory preconditioning. Journal of Experimental Psychology. 1939;25:323–332. [Google Scholar]
- Bucci DJ, Phillips RG, Burwell RB. Contributions of postrhinal and perirhinal cortices to contextual information processing. Behavioral Neuroscience. 2000;114(5):882–894. doi: 10.1037//0735-7044.114.5.882. [DOI] [PubMed] [Google Scholar]
- Bucci DJ, Saddoris MP, Burwell RD. Damage to postrhinal or perirhinal cortex impairs performance of a contextual fear discrimination. Behavioral Neuroscience. 2002;116(3):479–488. [PubMed] [Google Scholar]
- Bucci DJ. Posterior parietal cortex: An interface between attention and learning? Neurobiology of Learning and Memory. 2009;91:114–120. doi: 10.1016/j.nlm.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucci DJ, Burwell RD. Deficits in attentional orienting following damage to postrhinal or perirhinal cortex. Behavioral Neuroscience. 2004;118:1117–1122. doi: 10.1037/0735-7044.118.5.1117. [DOI] [PubMed] [Google Scholar]
- Bunsey M, Eichenbaum H. Critical role of the parahippocampal region for paired-associate learning in rats. Behav Neurosci. 1993;107(5):740–7. doi: 10.1037//0735-7044.107.5.740. [DOI] [PubMed] [Google Scholar]
- Burwell RD, Bucci DJ, Sanborn MR, Jutras Mj. Perirhinal and postrhinal contribution to remote memory for context. Journal of Neuroscience. 2004;24:11023–11028. doi: 10.1523/JNEUROSCI.3781-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burwell RD, Saddoris MP, Bucci DJ, Wiig KA. Corticohippocampal contributions to spatial and contextual learning. Journal of Neuroscience. 2004;24:3826–3836. doi: 10.1523/JNEUROSCI.0410-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burwell RD, Amaral DG. Cortical afferents of the perirhinal, postrhinal, and entorhinal cortices of the rat. Journal of Comparative Neurology. 1998a;398:1–27. doi: 10.1002/(sici)1096-9861(19980824)398:2<179::aid-cne3>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Burwell RD, Amaral DG. Perirhinal and postrhinal cortices of the rat: interconnectivity and connections with the entorhinal cortex. J Comp Neurol. 1998b;391(3):293–321. doi: 10.1002/(sici)1096-9861(19980216)391:3<293::aid-cne2>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Burwell RD, Hafeman DM. Positional firing properties of postrhinal cortex neurons. Neuroscience. 2003;119:577–88. doi: 10.1016/s0306-4522(03)00160-x. [DOI] [PubMed] [Google Scholar]
- Cain DP, Humpartzoomian R, Boon F. Retrosplenial cortex lesions impair water maze strategies learning or spatial place learning depending on prior experience of the rat. Behavioural Brain Research. 2006;170:316–325. doi: 10.1016/j.bbr.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Campeau S, Hayward MD, Hope BT, Rosen JB, Nestler EJ, Davis M. Induction of the cfos proto-oncogene in rat amygdala during unconditioned and conditioned fear. Brain Res. 1991;565(2):349–52. doi: 10.1016/0006-8993(91)91669-r. [DOI] [PubMed] [Google Scholar]
- Chen LL, Lin LH, Green EJ, Barnes CA, McNaughton BL. Head-direction cells in the rat posterior cortex. I. Anatomical distribution and behavioral modulation. Exp Brain Res. 1994;101(1):8–23. doi: 10.1007/BF00243212. [DOI] [PubMed] [Google Scholar]
- Clark BJ, Bassett JP, Wang SS, Taube JS. Impaired head direction cell representation in the anterodorsal thalamus after lesions of the retrosplenial cortex. J Neurosci. 2010;30(15):5289–302. doi: 10.1523/JNEUROSCI.3380-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen NJ, Eichenbaum H. Memory, amnesia, and the hippocampal system. MIT, Cambridge, MA: 1993. [Google Scholar]
- Cohen NJ, Ryan J, Hunt C, Romine L, Wszalek T, Nash C. Hippocampal system and declarative (relational) memory: summarizing the data from functional neuroimaging studies. Hippocampus. 1999;9:83–98. doi: 10.1002/(SICI)1098-1063(1999)9:1<83::AID-HIPO9>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Cho J, Sharp PE. Head direction, place, and movement correlates for cells in the rat retrosplenial cortex. Behav Neurosci. 2001;115(1):3–25. doi: 10.1037/0735-7044.115.1.3. [DOI] [PubMed] [Google Scholar]
- Constantinidis C. Posterior parietal mechanisms of visual attention. Rev Neurosci. 2006;17(4):415–27. doi: 10.1515/revneuro.2006.17.4.415. [DOI] [PubMed] [Google Scholar]
- Cooper BG, Mizumori SJ. Temporary inactivation of the retrosplenial cortex causes a transient reorganization of spatial coding in the hippocampus. J Neurosci. 2001;21(11):3986–4001. doi: 10.1523/JNEUROSCI.21-11-03986.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper BG, Mizumori SJ. Temporary inactivation of the retrosplenial cortex causes a transient reorganization of spatial coding in the hippocampus. J Neurosci. 2001;21(11):3986–4001. doi: 10.1523/JNEUROSCI.21-11-03986.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran KA, Donnan MD, Tronson NC, Guzman YF, Gao C, Jovasevic V, Guedea AL, Radulovic J. NMDA receptors in retrosplenial cortex are necessary for retrieval of recent and remote context fear memory. J Neurosci. 2011;31:11655–11659. doi: 10.1523/JNEUROSCI.2107-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowell RA, Bussey TJ, Saksida LM. Components of recognition memory: dissociable cognitive processes or just differences in representational complexity? Hippocampus. 2010;20(11):1245–62. doi: 10.1002/hipo.20865. [DOI] [PubMed] [Google Scholar]
- Davachi L. Item, context and relational episodic encoding in humans. Curr Opin Neurobiol. 2006;16:693–700. doi: 10.1016/j.conb.2006.10.012. [DOI] [PubMed] [Google Scholar]
- Eacott MJ, Gaffan EA. The roles of perirhinal cortex, postrhinal cortex, and the fornix in memory for objects, contexts, and events in the rat. Q J Exp Psychol B. 2005;58(3-4):202–17. doi: 10.1080/02724990444000203. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H. Hippocampus: cognitive processes and neural representations that underlie declarative memory. Neuron. 2004;44(1):109–20. doi: 10.1016/j.neuron.2004.08.028. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H. The Cognitive Neuroscience of Memory: An Introduction. 2nd. Oxford: Oxford University Press; 2011. [Google Scholar]
- Eichenbaum H, Sauvage M, Fortin N, Komorowski R, Lipton P. Towards a functional organization of episodic memory in the medial temporal lobe. Neurosci Biobehav Rev. 2011;36:1597–608. doi: 10.1016/j.neubiorev.2011.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ennaceur A, Neave N, Aggleton JP. Spontaneous object recognition and object location memory in rats: The effects of lesions in the cingulate cortices, the medial prefrontal cortex, the cingulum bundle and the fornix. Experimental Brain Research. 1997;113:509–519. doi: 10.1007/pl00005603. [DOI] [PubMed] [Google Scholar]
- Epstein R, Deyoe EA, Press DZ, Rosen AC, Kanwisher N. Neuropsychological evidence for a topographical learning mechanism in parahippocampal cortex. Cogn Neuropsychol. 2001;18(6):481–508. doi: 10.1080/02643290125929. [DOI] [PubMed] [Google Scholar]
- Epstein R, Graham KS, Downing PE. Viewpoint-specific scene representations in human parahippocampal cortex. Neuron. 2003;37(5):865–76. doi: 10.1016/s0896-6273(03)00117-x. [DOI] [PubMed] [Google Scholar]
- Epstein RA. Parahippocampal and retrosplenial contributions to human spatial navigation. Trends in Cognitive Sciences. 2008;12:388–396. doi: 10.1016/j.tics.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein RA, Higgins JS, Jablonski K, Feiler AM. Visual scene processing in familiar and unfamiliar environments. Journal of Neurophysiology. 2007;97:3670–3683. doi: 10.1152/jn.00003.2007. [DOI] [PubMed] [Google Scholar]
- Fanselow MS. Conditioned fear-induced opiate analgesia: a competing motivational state theory of stress analgesia. Ann N Y Acad Sci. 1986;467:40–54. doi: 10.1111/j.1749-6632.1986.tb14617.x. [DOI] [PubMed] [Google Scholar]
- Fanselow MS. Conditional and unconditional components of post-shock freezing. Pavlovian Journal of Biological Sciences. 1980;15:177–182. doi: 10.1007/BF03001163. [DOI] [PubMed] [Google Scholar]
- Fenske MJ, Aminoff E, Gronau N, Bar M. Top-down facilitation of visual object recognition: Object-based and context-based contributions. Progress in Brain Research. 2006;155:3–21. doi: 10.1016/S0079-6123(06)55001-0. [DOI] [PubMed] [Google Scholar]
- Frankland PW, Josselyn SA, Anagnostaras SG, Kogan JH, Takahashi E, Silva AJ. Consolidation of CS and US representations in associative fear conditioning. Hippocampus. 2004;14(5):557–69. doi: 10.1002/hipo.10208. [DOI] [PubMed] [Google Scholar]
- Freeman JH, Jr, Cuppernell C, Flannery K, Gabriel M. Limbic thalamic, cingulate cortical and hippocampal neuronal correlates of discriminative approach learning in rabbits. Behav Brain Res. 1996;80:123–136. doi: 10.1016/0166-4328(96)00027-7. [DOI] [PubMed] [Google Scholar]
- Furtak SC, Wei SM, Agster KL, Burwell RD. Functional neuroanatomy of the parahippocampal region in the rat: the perirhinal and postrhinal cortices. Hippocampus. 2007;17(9):709–22. doi: 10.1002/hipo.20314. [DOI] [PubMed] [Google Scholar]
- Furtak SC, Ahmed OJ, Burwell RD. Single Neuron Activity and Theta Modulation in Postrhinal Cortex during Visual Object Discrimination. Neuron. 2012;76:976–88. doi: 10.1016/j.neuron.2012.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gastelum ED, Guilhardi P, Burwell RD. The effects of combined perirhinal and postrhinal damage on complex discrimination tasks. Hippocampus. 2012;22(10):2059–67. doi: 10.1002/hipo.22063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haijima A, Ichitani Y. Anterograde and retrograde amnesia of place discrimination in retrosplenial cortex and hippocampal lesioned rats. Learn Mem. 2008;15(7):477–82. doi: 10.1101/lm.862308. [DOI] [PubMed] [Google Scholar]
- Harker KT, Whishaw IQ. Impaired spatial performance in rats with retrosplenial lesions: importance of the spatial problem and the rat strain in identifying lesion effects in a swimming pool. Journal of Neuroscience. 2002;22:1155–1164. doi: 10.1523/JNEUROSCI.22-03-01155.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harker KT, Whishaw IQ. Impaired place navigation in place and matching-to-place swimming pool tasks follows both retrosplenial cortex lesions and cingulum bundle lesions in rats. Hippocampus. 2004;14(2):224–31. doi: 10.1002/hipo.10159. [DOI] [PubMed] [Google Scholar]
- Hayes SM, Nadel L, Ryan L. The effect of scene context on episodic object recognition: parahippocampal cortex mediates memory encoding and retrieval success. Hippocampus. 2007;17(9):873–89. doi: 10.1002/hipo.20319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilman KM, Bowers D, Watson RT, Day A, Valenstein E, Hammond E, Duara R. Frontal hypermetabolism and thalamic hypometabolism in a patient with abnormal orienting and retrosplenial amnesia. Neuropsychologia. 1990;28(2):161–9. doi: 10.1016/0028-3932(90)90098-9. [DOI] [PubMed] [Google Scholar]
- Henderson JM, Larson CL, Zhu DC. Full scenes produce more activation than close-up scenes and scene-diagnostic objects in parahippocampal and retrosplenial cortex: an fMRI study. Brain Cogn. 2008;66(1):40–9. doi: 10.1016/j.bandc.2007.05.001. [DOI] [PubMed] [Google Scholar]
- Hutchinson JB, Uncapher MR, Wagner AD. Posterior parietal cortex and episodic retrieval: convergent and divergent effects of attention and memory. Learn Mem. 2009;16(6):343–56. doi: 10.1101/lm.919109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland PC, Bouton ME. Hippocampus and context in classical conditioning. Curr Opin Neurobiol. 1999;9(2):195–202. doi: 10.1016/s0959-4388(99)80027-0. [DOI] [PubMed] [Google Scholar]
- Holland PC, Ross RT. The savings test for associations between neutral stimuli. Animal Learning & Behavior. 1983;11:83–90. [Google Scholar]
- Hunsaker MR, 1, Chen V, Tran GT, Kesner RP. The medial and lateral entorhinal cortex both contribute to contextual and item recognition memory: a test of the binding of items and context model. Hippocampus. 2013;23(5):380–91. doi: 10.1002/hipo.22097. [DOI] [PubMed] [Google Scholar]
- Iordanova MD, Good M, Honey RC. Retrieval-mediated learning involving episodes requires synaptic plasticity in the hippocampus. J Neurosci. 2011;31:7156–62. doi: 10.1523/JNEUROSCI.0295-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowski MM, Ronnqvist KC, Tsanov M, Vann SD, Wright NF, Erichsen JT, Aggleton JP, O'Mara SM. The anterior thalamus provides a subcortical circuit supporting memory and spatial navigation. Front Syst Neurosci. 2013;7:45. doi: 10.3389/fnsys.2013.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keene CS, Bucci DJ. Contributions of the retrosplenial and posterior parietal cortices to cue-specific and contextual fear conditioning. Behavioral Neuroscience. 2008a;122:89–97. doi: 10.1037/0735-7044.122.1.89. [DOI] [PubMed] [Google Scholar]
- Keene CS, Bucci DJ. Involvement of the retrosplenial cortex in processing multiple conditioned stimuli. Behavioral Neuroscience. 2008b;122:651–658. doi: 10.1037/0735-7044.122.3.651. [DOI] [PubMed] [Google Scholar]
- Keene CS, Bucci DJ. Neurotoxic lesions of retrosplenial cortex disrupt signaled and unsignalled contextual fear conditioning. Behavioral Neuroscience. 2008c;122:1070–1077. doi: 10.1037/a0012895. [DOI] [PubMed] [Google Scholar]
- Keene CS, Bucci DJ. Damage to the retrosplenial cortex produces specific impairments in spatial working memory. Neurobiology of Learning and Memory. 2009;91:408–414. doi: 10.1016/j.nlm.2008.10.009. [DOI] [PubMed] [Google Scholar]
- Kiernan M, Cranney J. Immediate-startle stimulus presentation fails to condition freezing responses to contextual cues. Behav Neurosci. 1992;106(1):121–4. doi: 10.1037//0735-7044.106.1.121. [DOI] [PubMed] [Google Scholar]
- Kobayashi Y, Amaral DG. Macaque monkey retrosplenial cortex: II. Cortical afferents. J Comp Neurol. 2003;466(1):48–79. doi: 10.1002/cne.10883. [DOI] [PubMed] [Google Scholar]
- Kobayashi Y, Amaral DG. Macaque monkey retrosplenial cortex: III. Cortical efferents. Journal of Comparative Neurology. 2007;502:10–33. doi: 10.1002/cne.21346. [DOI] [PubMed] [Google Scholar]
- Kubik S, Miyashita T, Kubik-Zahorodna A, Guzowski JF. Loss of activity-dependent Arc gene expression in the retrosplenial cortex after hippocampal inactivation: Interaction in a higher-order memory circuit. Neurobiol Learn Mem. 2012;97(1):124–31. doi: 10.1016/j.nlm.2011.10.004. [DOI] [PubMed] [Google Scholar]
- Kveraga K, Ghuman AS, Kassam KS, Aminoff EA, Hämäläinen MS, Chaumon M, Bar M. Early onset of neural synchronization in the contextual associations network. Proc Natl Acad Sci U S A. 2011;108(8):3389–94. doi: 10.1073/pnas.1013760108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon SE, Nadeau SE, Heilman KM. Retrosplenial cortex: possible role in habituation of the orienting response. J Neurosci. 1990;10(11):3559–63. doi: 10.1523/JNEUROSCI.10-11-03559.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P, Bilkey DK. The effects of NMDA lesions centered on the postrhinal cortex on spatial memory tasks in the rat. Behav Neurosci. 2002;116(5):860–73. doi: 10.1037//0735-7044.116.5.860. [DOI] [PubMed] [Google Scholar]
- Lukoyanov NV, Lukoyanova EA, Andrade JP, Paula-Barbosa MM. Impaired water maze navigation of Wistar rats with retrosplenial cortex lesions: Effect of nonspatial pretraining. Behavioral Brain Research. 2005;158:175–182. doi: 10.1016/j.bbr.2004.08.023. [DOI] [PubMed] [Google Scholar]
- Maeshima S, Ozaki F, Masuo O, Yamaga H, Okita R, Moriwaki H. Memory impairment and spatial disorientation following a left retrosplenial lesion. J Clin Neurosci. 2001;8(5):450–1. doi: 10.1054/jocn.2000.0801. [DOI] [PubMed] [Google Scholar]
- Maguire EA. The retrosplenial contribution to human navigation: A review of lesion and neuroimaging findings. Scandinavian Journal of Psychology. 2001;42:225–238. doi: 10.1111/1467-9450.00233. [DOI] [PubMed] [Google Scholar]
- Mair RG, Burk JA, Porter MC. Impairment of radial maze delayed nonmatching after lesions of anterior thalamus and parahippocampal cortex. Behav Neurosci. 2003;117(3):596–605. doi: 10.1037/0735-7044.117.3.596. [DOI] [PubMed] [Google Scholar]
- Malkova L, Mishkin M. One-trial memory for object-place associations after separate lesions of hippocampus and posterior parahippocampal region in the monkey. J Neurosci. 2003;23(5):1956–65. doi: 10.1523/JNEUROSCI.23-05-01956.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S, Aharonov G, Fanselow MS. Neurotoxic lesions of the dorsal hippocampus and pavlovian fear conditioning in rats. Behavioural Brain Research. 1997;88:261–274. doi: 10.1016/s0166-4328(97)00088-0. [DOI] [PubMed] [Google Scholar]
- Maviel T, Durkin TP, Menzaghi F, Bontempi B. Sites of neocortical reorganization critical for remote spatial memory. Science. 2004;305(5680):96–9. doi: 10.1126/science.1098180. [DOI] [PubMed] [Google Scholar]
- Miyashita T, Kubik S, Haghighi N, Steward O, Guzowski JF. Rapid activation of plasticity-associated gene transcription in hippocampal neurons provides a mechanism for encoding of one-trial experience. J Neurosci. 2009;29:898–906. doi: 10.1523/JNEUROSCI.4588-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullally SL, Maguire EA. A new role for the parahippocampal cortex in representing space. J Neurosci. 2011;31(20):7441–9. doi: 10.1523/JNEUROSCI.0267-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray EA, Bussey TJ, Hampton RR, Saksida LM. The parahippocampal region and object identification. Ann N Y Acad Sci. 2000;911:166–74. doi: 10.1111/j.1749-6632.2000.tb06725.x. [DOI] [PubMed] [Google Scholar]
- Naber PA, Caballero-Bleda M, Jorritsma-Byham B, Witter MP. Parallel input to the hippocampal memory system through peri- and postrhinal cortices. NeuroReport. 1997;8:2617–2621. doi: 10.1097/00001756-199707280-00039. [DOI] [PubMed] [Google Scholar]
- Naber PA, Witter MP, Lopes da Silva FH. Evidence for a direct projection from the postrhinal cortex to the subiculum in the rat. Hippocampus. 2001;11(2):105–17. doi: 10.1002/hipo.1029. [DOI] [PubMed] [Google Scholar]
- Norman G, Eacott MJ. Dissociable effects of lesions to the perirhinal cortex and the postrhinal cortex on memory for context and objects in rats. Behav Neurosci. 2005;119(2):557–66. doi: 10.1037/0735-7044.119.2.557. [DOI] [PubMed] [Google Scholar]
- Park S, 1, Chun MM. Different roles of the parahippocampal place area (PPA) and retrosplenial cortex (RSC) in panoramic scene perception. Neuroimage. 2009;47(4):1747–56. doi: 10.1016/j.neuroimage.2009.04.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parron C, Save E. Comparison of the effects of entorhinal and retrosplenial cortical lesions on habituation, reaction to spatial and non-spatial changes during object exploration in the rat. Neurobiology of Learning and Memory. 2004;82:1–11. doi: 10.1016/j.nlm.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 4th. San Diego, CA: Academic Press; 1998. [Google Scholar]
- Pezzone MA, Lee WS, Hoffman GE, Rabin BS. Induction of c-Fos immunoreactivity in the rat forebrain by conditioned and unconditioned aversive stimuli. Brain Res. 1992;597:41–50. doi: 10.1016/0006-8993(92)91503-7. [DOI] [PubMed] [Google Scholar]
- Ploner CJ, Gaymard BM, Rivaud-Péchoux S, Baulac M, Clémenceau S, Samson S, PierrotDeseilligny C. Lesions affecting the parahippocampal cortex yield spatial memory deficits in humans. Cereb Cortex. 2000;10(12):1211–6. doi: 10.1093/cercor/10.12.1211. [DOI] [PubMed] [Google Scholar]
- Pothuizen HH, Davies M, Aggleton JP, Vann SD. Effects of selective granular retrosplenial cortex lesions on spatial working memory in rats. Behav Brain Res. 2010;208(2):566–7. doi: 10.1016/j.bbr.2010.01.001. [DOI] [PubMed] [Google Scholar]
- Pothuizen HH, Aggleton JP, Vann SD. Do rats with retrosplenial cortex lesions lack direction? Eur J Neurosci. 2008;28(12):2486–98. doi: 10.1111/j.1460-9568.2008.06550.x. [DOI] [PubMed] [Google Scholar]
- Ramos JM. Differential contribution of hippocampus, perirhinal cortex and postrhinal cortex to allocentric spatial memory in the radial maze. Behav Brain Res. 2013;247:59–64. doi: 10.1016/j.bbr.2013.03.017. [DOI] [PubMed] [Google Scholar]
- Ranganath C, Ritchey M. Two cortical systems for memory-guided behaviour. Nat Rev Neurosci. 2012;13:713–26. doi: 10.1038/nrn3338. [DOI] [PubMed] [Google Scholar]
- Reep RL, Corwin JV. Posterior parietal cortex as part of a neural network for directed attention in rats. Neurobiol Learn Mem. 2009;91(2):104–13. doi: 10.1016/j.nlm.2008.08.010. [DOI] [PubMed] [Google Scholar]
- Robinson DL. Functional contributions of the primate pulvinar. Prog Brain Res. 1993;95:371–80. doi: 10.1016/s0079-6123(08)60382-9. [DOI] [PubMed] [Google Scholar]
- Robinson S, Poorman CA, Marder TJ, Bucci DJ. Identification of functional circuitry between retrosplenial and postrhinal cortices during fear conditioning. Journal of Neuroscience. 2012;32:12076–12086. doi: 10.1523/JNEUROSCI.2814-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson S, Keene CS, Iaccarino HF, Duan D, Bucci DJ. Involvement of the retrosplenial cortex in forming associations between multiple sensory stimuli. Behavioral Neuroscience. 2011;125:578–587. doi: 10.1037/a0024262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogan SC, Roth BL. Remote control of neuronal signaling. Pharmacol Rev. 2011;63:291–315. doi: 10.1124/pr.110.003020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd JD, Bear MF. New views of Arc, a master regulator of synaptic plasticity. Nat Neurosci. 2011;14(3):279–84. doi: 10.1038/nn.2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata H, Honda Y, Sasaki H, Naito J. Organization of intrinsic connections of the retrosplenial cortex in the rat. Anat Sci Int. 2009;84(4):280–92. doi: 10.1007/s12565-009-0035-0. [DOI] [PubMed] [Google Scholar]
- Smith DM, Wakeman D, Patel J, Gabriel M. Fornix lesions impair context-related cingulothalamic neuronal patterns and concurrent discrimination learning in rabbits (Oryctolagus cuniculus) Behavioral Neuroscience. 2004;118:1225–1239. doi: 10.1037/0735-7044.118.6.1225. [DOI] [PubMed] [Google Scholar]
- Smith DM, Barredo J, Mizumori SJ. Complimentary roles of the hippocampus and retrosplenial cortex in behavioral context discrimination. Hippocampus. 2012;22(5):1121–33. doi: 10.1002/hipo.20958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staresina BP, Duncan KD, Davachi L. Perirhinal and parahippocampal cortices differentially contribute to later recollection of object- and scene-related event details. J Neurosci. 2011;31(24):8739–47. doi: 10.1523/JNEUROSCI.4978-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugar J, Witter MP, van Strien NM, Cappaert NL. The retrosplenial cortex: intrinsic connectivity and connections with the (para)hippocampal region in the rat. An interactive connectome. Front Neuroinform. 2011;5:7. doi: 10.3389/fninf.2011.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki WA, Amaral DG. The perirhinal and parahippocampal cortices of the Macaque monkey: Cortical afferents. Journal of Comparative Neurology. 1994;350:497–533. doi: 10.1002/cne.903500402. [DOI] [PubMed] [Google Scholar]
- Takahashi N, Kawamura M, Shiota J, Kasahata N, Hirayama K. Pure topographic disorientation due to right retrosplenial lesion. Neurology. 1997;49(2):464–9. doi: 10.1212/wnl.49.2.464. [DOI] [PubMed] [Google Scholar]
- Talk A, Stoll E, Gabriel M. Cingulate cortical coding of context dependent latent inhibition. Behav Neurosci. 2005;119:1524–1532. doi: 10.1037/0735-7044.119.6.1524. [DOI] [PubMed] [Google Scholar]
- Tayler KK, Tanaka KZ, Reijmers LG, Wiltgen BJ. Reactivation of neural ensembles during the retrieval of recent and remote memory. Curr Biol. 2013;23(2):99–106. doi: 10.1016/j.cub.2012.11.019. [DOI] [PubMed] [Google Scholar]
- Tse D, Takeuchi T, Kakeyama M, Kajii Y, Okuno H, Tohyama C, Bito H, Morris RG. Schema-dependent gene activation and memory encoding in neocortex. Science. 2011;333:891–895. doi: 10.1126/science.1205274. [DOI] [PubMed] [Google Scholar]
- Brown TI, Ross RS, Tobyne SM, Stern CE. Cooperative interactions between hippocampal and striatal systems support flexible navigation. Neuroimage. 2012;60(2):1316–30. doi: 10.1016/j.neuroimage.2012.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Groen T, Wyss JM. Connections of the retrosplenial granular b cortex in the rat. Journal of Comparative Neurology. 2003;463:249–263. doi: 10.1002/cne.10757. [DOI] [PubMed] [Google Scholar]
- van Groen T, Wyss JM. Connections of the retrosplenial dysgranular cortex in the rat. Journal of Comparative Neurolog. 1992;315:200–16. doi: 10.1002/cne.903150207. [DOI] [PubMed] [Google Scholar]
- van Groen T, Wyss JM. Connections of the retrosplenial granular a cortex in the rat. Journal of Comparative Neurology. 1990;300:593–606. doi: 10.1002/cne.903000412. [DOI] [PubMed] [Google Scholar]
- van Groen T, Kadish I, Wyss JM. Retrosplenial cortex lesions of area Rgb (but not of area Rga) impair spatial learning and memory in the rat. Behavioural Brain Research. 2004;154:483–491. doi: 10.1016/j.bbr.2004.03.016. [DOI] [PubMed] [Google Scholar]
- van Strien NM, Cappaert NL, Witter MP. The anatomy of memory: an interactive overview of the parahippocampal-hippocampal network. Nat Rev Neurosci. 2009;10(4):272–82. doi: 10.1038/nrn2614. [DOI] [PubMed] [Google Scholar]
- Vann SD, Aggleton JP. Extensive cytotoxic lesions of the rat retrosplenial cortex reveal consistent deficits on tasks that tax allocentric spatial memory. Behavioral Neuroscience. 2002;116:85–94. [PubMed] [Google Scholar]
- Vann SD, Aggleton JP. Testing the importance of the retrosplenial guidance system: effects of different sized retrosplenial cortex lesions on heading direction and spatial working memory. Behavioural Brain Research. 2004;155:97–108. doi: 10.1016/j.bbr.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Vann SD, Kristina Wilton LA, Muir JL, Aggleton JP. Testing the importance of the caudal retrosplenial cortex for spatial memory in rats. Behav Brain Res. 2003;140(1-2):107–18. doi: 10.1016/s0166-4328(02)00274-7. [DOI] [PubMed] [Google Scholar]
- Vann SD, Aggleton JP, Maguire EA. What does the retrosplenial cortex do? Nature Reviews. Neuroscience. 2009;10:792–802. doi: 10.1038/nrn2733. [DOI] [PubMed] [Google Scholar]
- Vidyasagar TR, Salzmann E, Creutzfeldt OD. Unit activity in the hippocampus and the parahippocampal temporobasal association cortex related to memory and complex behaviour in the awake monkey. Brain Res. 1991;544:269–78. doi: 10.1016/0006-8993(91)90064-3. [DOI] [PubMed] [Google Scholar]
- Vogt BA, Pandya DN, Rosene DL. Cingulate cortex of the rhesus monkey: I. Cytoarchitecture and thalamic afferents. J Comp Neurol. 1987;262(2):256–70. doi: 10.1002/cne.902620207. [DOI] [PubMed] [Google Scholar]
- Ward-Robinson J, Coutureau E, Good M, Honey RC, Killcross AS, et al. Oswald CJ. Excitotoxic lesions of the hippocampus leave sensory preconditioning intact: Implications for models of hippocampal function. Behavioral Neuroscience. 2001;115:1357–1362. doi: 10.1037//0735-7044.115.6.1357. [DOI] [PubMed] [Google Scholar]
- Weniger G, Siemerkus J, Schmidt-Samoa C, Mehlitz M, Baudewig J, Dechent P, Irle E. The human parahippocampal cortex subserves egocentric spatial learning during navigation in a virtual maze. Neurobiol Learn Mem. 2010;93(1):46–55. doi: 10.1016/j.nlm.2009.08.003. [DOI] [PubMed] [Google Scholar]
- Wiltgen BJ, Sanders MJ, Anagnostaras SG, Sage JR, Fanselow MS. Context fear learning in the absence of the hippocampus. Journal of Neuroscience. 2006;26:5484–9541. doi: 10.1523/JNEUROSCI.2685-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wimmer GE, Shohamy D. Preference by association: how memory mechanisms in the hippocampus bias decisions. Science. 2012;338:270–3. doi: 10.1126/science.1223252. [DOI] [PubMed] [Google Scholar]
- Wixted JT, Squire LR. The medial temporal lobe and the attributes of memory. Trends Cogn Sci. 2011 May;15(5):210–7. doi: 10.1016/j.tics.2011.03.005. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolbers T, Buchel C. Dissociable retrosplenial and hippocampal contributions to successful formation of survey representations. Journal of Neuroscience. 2005;25:3333–3340. doi: 10.1523/JNEUROSCI.4705-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]


