Abstract
Nuclear receptors have generated substantial interest in the past decade as potential therapeutic targets for the treatment of neurodegenerative disorders. Despite years of effort, effective treatments for progressive neurodegenerative diseases such as Alzheimer’s disease, Parkinson’s disease, Huntington’s disease and ALS remain elusive, making non-classical drug targets such as nuclear receptors an attractive alternative. A substantial literature in mouse models of disease and several clinical trials have investigated the role of nuclear receptors in various neurodegenerative disorders, most prominently AD. These studies have met with mixed results, yet the majority of studies in mouse models report positive outcomes. The mechanisms by which nuclear receptor agonists affect disease pathology remain unclear. Deciphering the complex signaling underlying nuclear receptor action in neurodegenerative diseases is essential for understanding this variability in preclinical studies, and for the successful translation of nuclear receptor agonists into clinical therapies.
Keywords: nuclear receptors, peroxisome proliferator-activated receptors, liver X receptors, retinoid X receptors, microglia, inflammation, neurodegeneration, Alzheimer’s disease, Parkinson’s disease, amyotrophic lateral sclerosis, Huntington’s disease
Nuclear receptors are ligand activated transcription factors that act globally to regulate a diverse array of homeostatic processes (Chawla et al., 2001; Castrillo and Tontonoz, 2004). The best characterized of these are the type I receptors, which include estrogen and progesterone receptors. This review will focus on the more recently discovered type II nuclear receptors, which act as regulators of lipid and energy metabolism, and specifically on their actions in the brain. The predominant type II nuclear receptors in the brain are the peroxisome proliferator-activated receptors (PPAR) α, β/δ and γ, and liver X receptors (LXR) α and β. PPARs function as lipid sensors which bind dietary lipids or their metabolites, most prominently fatty acids and eicosanoids. LXRs act as cholesterol sensors, binding hydroxylated forms of cholesterol. Through association of the receptors with sequence specific promoter elements of genes of lipid and energy metabolism, PPARs and LXRs couple the size of the metabolic machinery to metabolic demand. These receptors play critical roles in CNS biology because the brain has a very high lipid content and is the most metabolically active organ in the body.
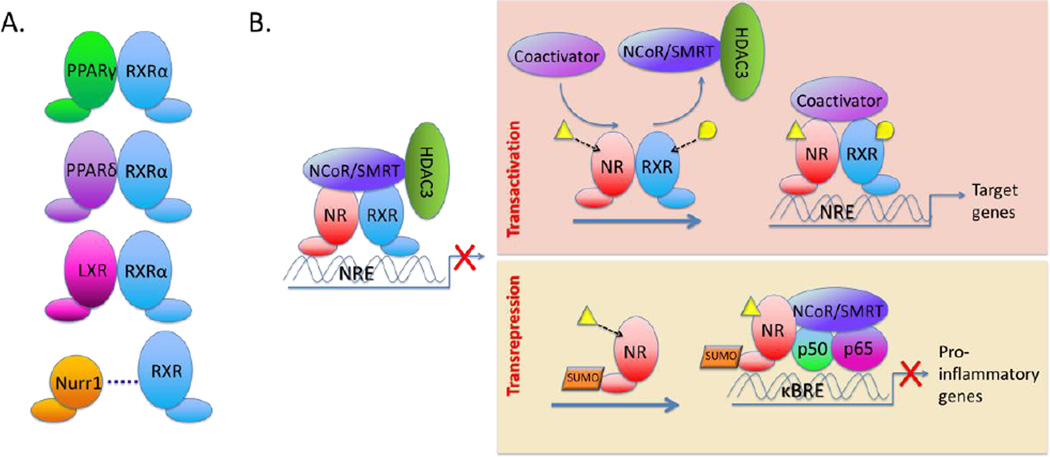
Nuclear receptors (type II) form obligate heterodimers with retinoid X receptors (RXR) α,β and γ to create a functional transcription factor (Figure 1A). In the nucleus, ligand bound or unbound receptor heterodimers associate with DNA response elements comprised of two direct repeat motifs. Unliganded dimeric receptors are transcriptionally silenced by their association with the corepressors NCoR or SMRT and HDAC3 (Figure 1B). Upon ligand binding, the corepressor complexes are dismissed and coactivator complexes then associate with the heterodimeric receptor, resulting in changes in local chromatin structure and the subsequent transcription of the target gene (Saijo et al., 2012). Unique exceptions to this mechanism are the NR4A receptors, including Nurr1, which are ligand independent receptors that can also signal as heterodimers with RXR. This review will focus on the PPARs and LXRs, as they are highly expressed in the brain, and discuss new data on the NR4A receptor Nurr1 that link it to CNS metabolism and disease.
Figure 1. Nuclear receptors are ligand-activated transcription factors.
Nuclear receptors (type II) form obligate heterodimers with RXR and comprise the functional transcription factor. The nuclear receptor complex transactivates its target genes by binding to sequence specific elements in their promoters. Ligand binding results in dismissal of a corepressor complex and association with coactivators, resulting in transcription of the target gene. Nuclear receptors can also act as transrepressors. Sumoylation induces their direct association with NFkB positioned on the promoters of proinflammatory genes, preventing the dismissal of corepressor complexes and the initiation of transcription.
Regulation of microglial phenotype and anti-inflammatory actions
Neurodegenerative diseases all exhibit a robust inflammatory component, reflective of the response of the innate immune system to disease-related perturbations in the brain (Mosher and Wyss-Coray, 2014). The brain is densely and uniformly populated by resident innate immune cells, the microglia (Nayak et al., 2014). The diseased brain is characterized by an increase in microglial number and their transformation from a surveillant, tissue maintenance mode to a protective host-defense mode and induction of proinflammatory genes. Typically, these ‘activated’ microglia are found associated with disease-related lesions or focal accumulations of abnormally folded proteins which stimulate host-defense responses normally directed to pathogens (Czirr and Wyss-Coray, 2012). This phenotypic conversion of microglia to a proinflammatory or ‘M1’ state is linked to the elaboration of a diverse array of immune mediators, including proinflammatory cytokines (Colton, 2009; Gordon and Martinez, 2010).
In the context of neurodegenerative diseases, this proinflammatory milieu within the brain acts to impair normal neuronal functions and synaptic activity and has coincident effects on CNS glia, including autocrine regulation of microglial and astrocyte phenotypes. Proinflammatory activation of microglia impedes their normal tissue surveillance and maintenance functions, prominently inhibiting their active monitoring of neuronal homeostasis and synapses (Morris et al., 2013). Inflammatory cytokines also act to impair neuronal integrity and have been postulated to mediate the loss of neurons at late stages of disease. The disease-related stimulation of the microglial inflammatory response is responsible for ‘bystander damage’ in the brain and contributes to disease pathogenesis and progression.
Examination of the brain in many neurodegenerative diseases reveals that activated microglia are generally unable to efficiently clear the initiating stimulus. An evolutionary adaption to this type of situation (e.g. parasitic infections) in other organ systems has been the ability of macrophages to acquire an ‘alternative activation’ phenotype, termed ‘M2’ (Gordon and Martinez, 2010). The M2 phenotype is associated with the inhibition of inflammatory gene expression and resolution of inflammation as well as the induction of a genetic program associated with tissue repair and enhanced phagocytosis. Importantly, it has only recently been appreciated that nuclear receptors act as master regulators of macrophage/microglia phenotype, governing the acquisition of ‘alternative activation’ states (Odegaard and Chawla, 2011). Macrophages in which PPARγ (Odegaard et al., 2007), PPARδ (Mukundan et al., 2009), LXRs (A-Gonzalez et al., 2009) and RXRα (Nunez et al., 2010) have been genetically inactivated exhibit reduced phagocytosis and are unable to acquire an M2 phenotype (Odegaard and Chawla, 2011). Each of these receptors has been shown to transactivate genes associated with the resolution of inflammation including anti-inflammatory cytokines such as IL-10 and TGFβ. This mechanism explains why phagocytosis of apoptotic cells by macrophages or microglia does not elicit an inflammatory response. There is a coincident stimulation of genes promoting phagocytosis.
Importantly, nuclear receptor activation also acts to suppress proinflammatory gene expression. Elegant work by Glass and colleagues have demonstrated that PPARs and LXRs undergo sumoylation upon ligand binding, allowing their targeted interaction with NFκB and AP1 positioned on the promoters of proinflammatory genes (Figure 1B) (Glass and Saijo, 2010). This interaction stabilizes the binding of NCoR/HDAC3 complexes with NFkB, repressing target proinflammatory gene expression. The phagocytic ingestion of the apoptotic corpse is associated with the catabolism of its membranes, yielding high levels of nuclear receptor ligands, most prominently fatty acids and cholesterol. Upon ligand binding, the receptors act to suppress NFκB-dependent inflammatory gene expression. This adaptive response is central to normal development and ongoing tissue maintenance.
It is now evident that nuclear receptors can be enlisted to intervene in disease pathogenesis, owing to the salutary effects of agonists of these receptors in a number of CNS disorders, including neurodegenerative diseases. The mechanisms subserving these effects in the brain are poorly understood. However, studies in other organ systems have revealed a complex interplay between the innate immune response and tissue metabolism (Odegaard and Chawla, 2013). The diseased brain has a well-documented alteration in metabolic state, with reduced glucose utilization and production associated with a broad range of metabolic changes. The innate immune system is an exquisitely responsive sensor of the metabolic state of the tissue in which these cells reside (Odegaard et al., 2007; Odegaard and Chawla, 2013). In the liver, muscle and fat, metabolic perturbations associated with obesity and type II diabetes result in an increased abundance of macrophages in the tissues and elevated inflammatory cytokines reflective of a low grade inflammatory state. The cytokines in turn elicit insulin resistance and impaired glucose uptake by the tissue. These reciprocal interactions between the tissue and its endogenous macrophages contribute to disease pathogenesis. It is of particular importance that nuclear receptor agonists act to normalize both metabolism and inflammation. This may account for their ability to attenuate disease-related pathologies and reverse behavioral impairment in animal models of neurodegenerative disease. These types of interactions have yet to be explored in the brain and this is clearly an area in need of investigation.
PPARγ
PPARγ plays critical roles in lipid homeostasis through its ability to interact with fatty acids and other lipid metabolites (Beaven and Tontonoz, 2006). Its activation leads to the induction of genes associated with lipid uptake and storage. In the periphery PPARγ activation is associated with enhanced insulin sensitivity and thus two PPARγ agonists have been FDA approved (pioglitazone, ActosTM; rosiglitazone, Avandia™) for the treatment of type II diabetes. In addition, PPARγ activation is reported to improve mitochondrial metabolism and biogenesis (Alaynick, 2008).
The first report of the neurodegenerative disease-relevant actions of PPARγ agonists was published in 2000 (Combs et al., 2000) and was quickly followed by a flurry of studies in animal models of AD (Mandrekar-Colucci and Landreth, 2011), PD (Carta and Pisanu, 2013), amyotrophic lateral sclerosis (ALS), Huntington’s disease (HD) (Kiaei, 2008), stroke (Ouk et al., 2013), and traumatic injuries (Semple and Noble-Haeusslein, 2011; Mandrekar-Colucci et al., 2013). The therapeutic relevance of targeting PPARγ has been documented in animal models for a number of neurodegenerative diseases and has resulted in several clinical trials for these disorders.
Alzheimer’s disease
The actions of PPARγ and its agonists in in vitro and murine models of AD have been well documented over the past decade and this work has been extensively reviewed (Heneka et al., 2007; Jiang et al., 2008a; Landreth et al., 2008; Nicolakakis and Hamel, 2010; Mandrekar-Colucci and Landreth, 2011; Sodhi et al., 2011; Mandrekar-Colucci et al., 2013). In 17 independent studies, oral administration of PPARγ agonists have been shown to be effective in many mouse models of AD, as measured by improved memory and cognition, suppression of inflammation and reduction of amyloid levels (Table I). There has been significant new work that has focused on the underlying mechanisms.
Table 1.
Effects of nuclear receptor agonists in neurodegenerative disease models.
| Neurodegenerative disease | Nuclear Receptor target |
Ligand | Dosage | Length of treatment |
Route of Administration |
Animal model | Pathology effects | Inflammatory Effects |
Behavioral Outcomes |
|---|---|---|---|---|---|---|---|---|---|
| Alzheimer’s disease | |||||||||
| Yan et al., 2003 | PPARγ | Pioglitazone | 20 mg/kg/day | 16 wks | oral: chow | Tg2576 | ↓ sol Aβ | ||
| Lacombe et al. 2004 | Pioglitazone | 18 mg/kg/day | 2 months | oral: chow | TGFbeta OE | ↓ sol Aβ42 | ↓ | ||
| Heneka et al., 2005 | Pioglitazone | 40 mg/kg/day | 7 days | oral: chow | APPV717I | ↓ plaques, sol Aβ | ↓ | ||
| Sastre et al., 2006 | Pioglitazone | 40 mg/kg/day | 7 days | oral: chow | APPV717I | ↓ intracellular Aβ | |||
| Nicolakakis et al., 2008 | Pioglitazone | 20 mg/kg/day | 6–8 wks | oral: chow | hAPP swe-ind | no effect | ↓ | n.c. MWM | |
| Mandrekar-Collucci et al., 2012 | Pioglitazone | 80 mg/kg/day | 9 days | oral: gavage | APPswe/PSEN1dE9 | ↓ plaques, sol Aβ | ↓ | ↑ CFC | |
| Searcy et al. 2012 | Pioglitazone | 18 mg/kg/day | 14 wks | oral: chow | 3xTg-AD | ↓ intracellular Aβ, ↓ p-tau | ↑ active avoidance learning | ||
| Masciopinto et al., 2012 | Pioglitazone | 20 mg/kg/day | 9 months | oral: chow | PS1-KIm146v | ↑ MWM, NOR in females | |||
| Pioglitazone | 20 mg/kg/day | 9 months | oral: chow | 3xTg-AD | n.c. | ||||
| Papadopoulos et al., 2013 | Pioglitazone | 20 mg/kg/day | 6 months | oral: chow | hAPP swe-ind/TGF-b1 | n.c. | ↓ | n.c. | |
| Pioglitazone | 20 mg/kg/day | 3 months | oral: chow | hAPP swe-ind/TGF-b1 | n.c. | ↓ | n.c. | ||
| Pedersen et al., 2006 | Rosiglitazone | 4 mg/kg/day | 15 wks | oral: chow | Tg2576 | ↓ sol Aβ42 | ↑ radial arm maze | ||
| Escribano et al. 2009 | Rosiglitazone | 5 mg/kg/day | 10 wks | oral: chow | hAPP swe-ind | ↑ NOR | |||
| Rosiglitazone | 5 mg/kg/day | 4 wks | oral: chow | hAPP swe-ind | ↑ NOR | ||||
| Toledo and Inestrosa, 2010 | Rosiglitazone | 3 mg/kg/day | 12 wks | oral: gavage | APPswe/PSEN1dE9 | ↓ plaques | ↓ | ↑ MWM | |
| Escribano et al., 2010 | Rosiglitazone | 5 mg/kg/day | 4–16 wks | oral: gavage | hAPP swe-ind | ↓ plaques, sol Aβ, p-tau | ↓ | ↑ NOR, MWM | |
| Rodriguez-Rivera et al., 2011 | Rosiglitazone | 0.18 mg/day | 1 month | oral: chow | Tg2576 | ↑ CFC, age dependent (9m only) | |||
| O'Reilly and Lynch, 2012 | Rosiglitazone | 6 mg/kg/day | 4 wks | oral | APPswe/PSEN1dE9 | ↓ plaques, insol Aβ42 | ↓ | ↑ MWM | |
| Denner et al., 2012 | Rosiglitazone | 0.18 mg/day | 1 month | oral: chow | Tg2576 | n.c. | ↑ CFC | ||
| Yamanaka et al., 2012 | DSP-8658 | 150 mg/kg/day | 3 months | oral: chow | APPswe/PSEN1dE9 | ↓ plaques, sol Aβ | ↑ phagocytosis | ↑ MWM | |
| Inestrosa et al., 2013 | PPARα | WY-14643 | 0.2 g/l | 60 days | oral: water | APPswe/PSEN1dE9 | ↓ plaques, p-tau | ↓ | ↑ MWM |
| 4-PB | 10 mg/l | 60 days | oral: water | APPswe/PSEN1dE9 | ↓ plaques, p-tau | ↓ | ↑ MWM | ||
| Kalinin et al., 2009 | PPARδ | GW742 | 5XFAD | ↓ plaques | ↓ | ||||
| Dumont et al. 2012 | pan-PPAR | bezafibrate | 0.5% chow | 9 months | oral: chow | P301S | ↓ tau pathology, p-tau | ↓ | ↓ locomotor deficits and anxiety |
| Jiang et al., 2008 | LXR | GW3965 | 33 mg/kg/day | 4 months | oral: chow | Tg2576 | ↓ plaques, sol Aβ | ↑ CFC | |
| Donkin et al., 2010 | GW3965 | 2.5 mg/kg/day | 8 or 24 wks | oral: chow | APPswe/PSEN1dE9 | ↑ sol Aβ | ↑ NOR/MWM | ||
| GW3965 | 33 mg/kg/day | 8 wks | oral: chow | APPswe/PSEN1dE9 | ↓ plaques, ↑ sol Aβ | ↑ NOR/MWM | |||
| Wesson et al., 2011 | GW3965 | 33 mg/kg/day | 2 wks | oral: gavage | Tg2576 | ↓ plaques, sol Aβ | ↑ olfactory behavior | ||
| Koldamova et al., 2005 | TO901317 | 50 mg/kg/day | 6 days | oral: gavage | APP23 | ↓ sol Aβ | |||
| Riddell et al., 2007 | TO901317 | 10 mg/kg/day | 7 days | oral: gavage | Tg2576 | n.c. | |||
| TO901317 | 30 mg/kg/day | 7 days | oral: gavage | Tg2576 | ↓ sol Aβ42 | ||||
| TO901317 | 50 mg/kg/day | 7 days | oral: gavage | Tg2576 | ↓ sol Aβ42 | ↑ CFC | |||
| Lefterov et al., 2007 | TO901317 | 50 mg/kg/day | 1 day | oral: gavage | APP23 | n.c. | |||
| TO901317 | 20 mg/kg/day | 4 wks | oral: gavage | APP23 | ↓ insol Aβ | ↓ | |||
| Fitz et al., 2010 | TO901317 | 25 mg/kg/day | 4 months | oral: chow | APP23 | ↓ plaques, sol Aβ | ↑ MWM | ||
| Vanmierlo et al., 2011 | TO901317 | 30 mg/kg/day | 6–9 wks | oral: chow | APPSLxPS1mut | n.c. in plaques | ↑ NOR and object location | ||
| Terwel et al., 2011 | TO901317 | 50 mg/kg/day | 7 wks | oral: gavage | APP23 | ↓ plaques, sol Aβ | (↑) MWM | ||
| TO901317 | 50 mg/kg/day | 6 days | oral: gavage | APP23 | ↑ glia/plaque association |
||||
| Cui et al., 2012 | TO901317 | 30 mg/kg/day | 30 days | oral: gavage | APPswe/PSEN1dE9 | ↓ plaques | ↓ | ↑ MWM | |
| Fitz et al., 2014 | TO901317 | 25 mg/kg/day | 15 days | oral: chow | APP23 | ↓ ISF Aβ42 | |||
| TO901317 | 25 mg/kg/day | 50 days | oral: chow | APP23 | n.c. in plaques, sol Aβ | ↑ CFC and RWM | |||
| Hu et al., 2013 | 19 | 10 mg/kg; 3x/wk | 6 wks | IP | APPswe/PSEN1dE9 | ↓ plaques, sol Aβ | |||
| 3, 7 or 14 | |||||||||
| Cramer et al., 2012 | RXR | bexarotene | 100 mg/kg/day | days | oral: gavage | APPswe/PSEN1dE9 | ↓ plaques, ↓sol Aβ | ↑ CFC/MWM | |
| bexarotene | 100 mg/kg/day | 90 days | oral: gavage | APPswe/PSEN1dE9 | ↓ sol Aβ, n.c. plaques | ↑ CFC/MWM | |||
| bexarotene | 100 mg/kg/day | 20 days | oral: gavage | APPPS1-21 | ↓ plaques, sol Aβ | ↑ CFC/MWM | |||
| bexarotene | 100 mg/kg/day | 3 or 9 days | oral: gavage | Tg2576 | ↑ olfactory behavior/nesting | ||||
| Price et al., 2013 | bexarotene | 100 mg/kg/day | 3 or 7 days | oral: gavage | APPswe/PSEN1dE9 | n.c. in plaques or sol Aβ | |||
| Fitz et al., 2013 | bexarotene | 100 mg/kg/day | 15 days | oral: gavage | APPswe/PSEN1dE9 | ↓ ISF Aβ, n.c. in plaques | ↑ RWM | ||
| Veeraraghavalu et al., 2013 | bexarotene | 100 mg/kg/day | 7 days | oral: gavage | APPswe/PSEN1dE9 | (↓) sol Aβ, n.c. in plaques | |||
| ↓ sol Aβ40, (↓) sol Ab4β, | |||||||||
| bexarotene | 100 mg/kg/day | 7 days | oral: gavage | 5XFAD | n.c. in plaques | ||||
| bexarotene | 100 mg/kg/day | 7 days | oral: gavage | APPPS1-21 | (↓) sol Aβ, n.c. in plaques | ||||
| Tesseur et al., 2013 | bexarotene | 100 mg/kg/day | 19 days | oral: gavage | APPPS1-21 | n.c. plaques/sol Aβ40 | unclear, possibly due to drug toxicity |
||
| Ulrich et al., 2013 | bexarotene | 100 mg/kg/day | 1 day 3, 7 or 14 |
oral: gavage | APPswe/PSEN1dE9 | ↓ ISF Aβ40 | |||
| LaClair et al., 2013 | bexarotene | 100 mg/kg/day | days | oral: gavage | APPswe/PSEN1dE9 | n.c. plaques | n.c. | n.c. in CFC | |
| Boehm-Cagan and Michaelson, 2014 | bexarotene | 2.5mg/day | 10 days | oral: gavage | ApoE4-TR | ↓ neuronal Aβ42, tau | ↑ MWM, NOR | ||
| Parkinson’s disease | |||||||||
| Breidert et al., 2002 | PPARγ | Pioglitazone | 20 mg/kg/day | 6–14 days | oral: chow | MPTP | ↓ TH+ neuron loss | ↓ | |
| Dehmer et al., 2004 | Pioglitazone | 20 mg/kg/day | 6–16 days | oral: chow | MPTP | ↓ TH+ neuron loss | ↓ | ||
| 20 mg/kg; | |||||||||
| Quinn et al., 2008 | Pioglitazone | 2x/day | 7 days | oral: gavage | MPTP | ↓ TH+ neuron loss | ↑ motor performance | ||
| 10 or 30 | |||||||||
| Kumar et al., 2009 | Pioglitazone | mg/kg/day | 35 days | oral: gavage | MPTP (rat) | ↓ oxidative stress | ↑ MWM/passive avoidance | ||
| Swanson et al., 2011 | Pioglitazone | 2.5 mg/kg/day | 3 months | oral | MPTP (monkey) | n.c. | n.c. | n.c. | |
| Pioglitazone | 5 mg/kg/day | 3 months | oral | MPTP (monkey) | ↓ TH+ neuron loss | ↓ | ↑ motor performance | ||
| Ulusoy et al., 2011 | Pioglitazone | 10 mg/kg/day | 15 days | IP | rotenone | ↑ striatal DA | ↑ motor performance | ||
| Laloux et al, 2012 | Pioglitazone | 50 mg/kg/day | 14 days | oral: gavage | MPTP | ↓ TH+ neuron loss | ↑ motor performance | ||
| Pioglitazone | 50 mg/kg/day | 14 days | oral: gavage | 6-OHDA (rat) | n.c. | n.c. | |||
| 20 mg/kg; | |||||||||
| Sadeghian et al., 2012 | Pioglitazone | 2x/day | 7 days | oral: gavage | 6-OHDA (rat) | ↓ TH+ neuron loss | ↓ | ||
| 15 mg/kg; | |||||||||
| GW855266X | 2x/day | 7 days | oral: gavage | 6-OHDA (rat) | ↓ TH+ neuron loss | ↓ | |||
| Schintu et al., 2009 | Rosiglitazone | 10 mg/kg/day | 5 wks | IP | MPTPp | ↓ TH+ neuron loss | ↓ | ↑ motor/olfactory performance | |
| Carta et al., 2011 | Rosiglitazone | 10 mg/kg/day | 1.5 wk | IP | MPTPp | ↓ TH+ neuron loss | ↓ | ||
| Martin et al., 2012 | Rosiglitazone | 10 mg/kg/day | 29 days | IP | MPTP | ||||
| Lee et al., 2012 | Rosiglitazone | 3 mg/kg; 2x/day | 1 day | IP | 6-OHDA (rat) | ↓ TH+ neuron loss | ↓/↑ | ||
| Swanson et al., 2013 | LSN862 | 30 mg/kg/day | 29 days | oral: gavage | MPTP | ↓ TH+ neuron loss | ↓ | ||
| Barbiero et al., 2014 | PPARα | fenofibrate | 100 mg/kg/day | 1 day | oral: gavage | MPTP (rat) | ↓ TH+ neuron loss | ↑ motor performance | |
| Uppalapati et al., 2014 | fenofibrate | 10 mg/kg/day | 30 days | oral: gavage | MPTP (rat) | ↓ TH+ cell loss | ↓ | ||
| fenofibrate | 30 mg/kg/day | 30 days | oral: gavage | MPTP (rat) | ↓ TH+ cell loss | ↓ | ↑ MWM | ||
| fenofibrate | 100 mg/kg/day | 30 days | oral: gavage | MPTP (rat) | ↓ TH+ cell loss | ↓ | ↑ passive avoidance, MWM | ||
| Sadeghian et al., 2012 | PPARδ | GW610742X | 10 mg/kg/day | 7 days | oral: gavage | 6-OHDA (rat) | n.c. | ↓ | |
| Martin et al., 2013 | GW0742 | 84 ug/day | 14 days | intra-striatal | MPTP | ↓ TH+ neuron loss | |||
| Iwashita et al., 2007 | L-165041 | 24 or 240 ug/day | 2 days | i.c.v. infusion | MPTP | ↑ striatal DA | |||
| GW501516 | 24 or 240 ug/day | 2 days | i.c.v. infusion | MPTP | ↑ striatal DA | ||||
| Dai et al., 2012 | LXR | GW3965 | 20 mg/kg/day | 7 days | s.c. | MPTP | ↓ TH+ neuron loss | ↓ | |
| McFarland et al., 2013 | RXR/Nurr1 | bexarotene | 6 ug/kg/day | 28 days | i.c.v. infusion | 6-OHDA (rat) | ↓ TH+ neuron loss | ↑ motor performance | |
| bexarotene | 0.3 mg/kg/day | 28 days | oral: gavage | 6-OHDA (rat) | n.c. | ||||
| bexarotene | 1 or 3 mg/kg/day | 28 days | oral: gavage | 6-OHDA (rat) | ↓ TH+ neuron loss | ↑ motor performance | |||
| ALS | |||||||||
| Kiaei et al., 2005 | PPARγ | Pioglitazone | 1200 ppm | 6 wks - death |
oral: chow | G93A SOD1 | ↓ motor neuron loss | ↓ | ↑ motor performance |
| Schutz et al., 2005 | Pioglitazone | 40 mg/kg/day | day 57 - death |
oral: chow | G93A SOD1 | ↓ motor neuron loss | ↓ | ↑ motor performance | |
| Shibata et al., 2008 | Pioglitazone | 1200 ppm | 6 wks - death |
oral: chow | G93A SOD1 | ↓ motor neuron loss | ↓ | ||
| Huntington’s disease | |||||||||
| Chiang et al., 2010 | PPARγ | Rosiglitazone | 0.01% in chow | 4 wks - death |
oral: chow | R6/2 | n.c. in neuronal death | ↑ motor performance/survival | |
| Jin et al., 2013 | Rosiglitazone | 10 mg/kg/day | 24 wks | oral: gavage | N171-82Q | ↓ neurodegeneration | ↑ motor performance | ||
| Johri et al., 2012 | pan-PPAR | bezafibrate | 0.5% in chow | 9 wks | oral: chow | R6/2 | ↓ neurodegeneration | ↑ motor performance/survival |
Key: IP: intraperitoneal; i.c.v.: intracerebroventricular; s.c: subcutaneous; sol Aβ: soluble amyloid β; insol Aβ: insoluble amyloid β; n.c: no change; ISF: interstitial fluid; TH: tyrosine hydroxylase; DA: dopamine; MWM: Morris water maze; CFC: contextual fear conditioning; NOR: novel object recognition; RWM: radial arm water maze
PPARγ agonist treatment of murine models of AD has been associated with the reversal of transgene-induced behavioral impairments, as evaluated in a number of different assays of cognition, memory and neural network function. It remains enigmatic exactly how the PPARγ-mediated improvement of behavior is effected. One proposed mechanism is that the robust anti-inflammatory effects of PPARγ suppress the levels of proinflammatory cytokines that have been linked to cognitive impairment. Whether anti-inflammatory effects are entirely responsible for reversal of the behavioral deficits remains to be convincingly demonstrated. Recent studies of PPARγ signaling in neurons argue that other mechanisms likely participate in the PPARγ-mediated enhancement of cognition and memory. The actions of PPARγ agonists on neurons have received comparatively little attention. PPARγ agonists are reported to stimulate Wnt signaling (Toledo and Inestrosa, 2010). Recent work by Dinely and colleagues have dissected the neuronal effects of PPARγ agonists and their underlying mechanisms which are summarized in this volume (Rodriguez-Rivera et al., 2011; Denner et al., 2012; Nenov et al., 2014). It has also been reported that PPAR agonists act to normalize synaptic function in AD mouse models (Searcy et al., 2012). The salutary effects of PPARγ agonists in AD mice have been postulated to arise from their ability to improve peripheral insulin sensitivity in type II diabetes and by extension work in analogous ways in the brain (Craft et al., 2013). However, there is no direct evidence to support the view that neurons are insensitive to insulin action, but they have been reported to exhibit changes in signal transduction pathways reflective of impaired insulin receptor signaling in AD models of rodents monkeys and in humans. Ferreira and colleagues have argued the insulin resistance results from the actions of microglia-derived TNFα. TNFa is elevated in the AD brain and causes the inactivation of elements necessary for insulin signaling (Ferreira et al., 2014).
One of the most compelling effects of chronic PPARγ agonist treatment documented in earlier work is the reduction of amyloid plaque burden owing to induction of microglial phagocytosis of Aβ deposits (Pedersen et al., 2006; Escribano et al., 2009; Toledo and Inestrosa, 2009; Escribano et al., 2010; Rodriguez-Rivera et al., 2011; Denner et al., 2012; Masciopinto et al., 2012; O'Reilly and Lynch, 2012; Searcy et al., 2012; Yamanaka et al., 2012). Recently, Mandrekar–Collucci reported that pioglitazone treatment as brief as 9 days was sufficient to clear up to 50% of plaques in 6 or 12 month old APP/PS1 mice and was associated with the appearance of amyloid-laden microglia in the cortex and hippocampus of the drug-treated mice (Mandrekar-Colucci et al., 2012). Similarly, Yamanaka reported that pioglitazone and a new PPARγ agonist, DSP-8658, stimulated the recruitment of microglia to plaques and promoted their clearance (Yamanaka et al., 2012). In vitro studies demonstrated that PPARγ agonists stimulated Aβ phagocytosis through induction of CD36 expression. The effect of the PPARγ agonists was dependent upon RXRa expression, and was additively enhanced by simultaneous treatment with an RXR agonist. The same study observed that DSP-8658 was also able to stimulate microglial recruitment to plaques and increase their phagocytosis of Aβ in an AD mouse model. In each of these latter studies behavioral improvement was observed.
A frequent comorbidity and contributor to AD pathogenesis is cerebral amyloidosis, or the accumulation of Aβ peptides within the vasculature, which is associated with impaired vascular function (Park et al., 2011). PPARγ agonist treatment of mouse models also restored vascular reactivity and improved blood flow to the brain (Nicolakakis and Hamel, 2010; Papadopoulos et al., 2013). Thus, PPARγ-mediated improvements in vascular function could provide another mechanism of therapeutic action.
There have been several phase I/II trials of pioglitazone and rosiglitazone in AD patients. A large phase III trial of rosiglitazone in mild/moderate AD failed to show clinical benefit (Gold et al., 2010). Currently, a phase III trial of pioglitazone is underway.
Parkinson’s disease
The initial report of the effects of PPARγ agonists in an MPTP model of PD by Breidert and colleagues found that pioglitazone prevented dopaminergic cell loss and suppressed the inflammatory response seen in this model (Breidert et al., 2002). Subsequently, there have been several studies implicating PPARγ in disease etiology. Many studies in murine models of PD (Schintu et al., 2009; Swanson et al., 2013) have found drug-induced prevention of dopaminergic terminal and cell loss as well as prevention of functional deficits. In an MPTP model in monkeys, Swanson et al. reported that pioglitazone treatment ameliorated behavioral deficits and prevented the loss of several markers of dopaminergic function. Importantly, pioglitazone prevented dopaminergic cell loss, with a reduction in inflammation, similar to the effects observed in rodents (Swanson et al., 2011). The underlying mechanisms that subserve these effects remain controversial and have been postulated to be due to anti-oxidant effects (Martin et al., 2012), MAO-B inhibition (Quinn et al., 2008), or the antiinflammatory effects of PPARγ activation (Breidert et al., 2002; Dehmer et al., 2004; Carta and Pisanu, 2012).
A principal cofactor and regulator of PPAR action is PPARγ-coactivator 1α (PCG-1a) (Katsouri et al., 2012). PCG-1α participates in transcriptional complexes mediating the activation of PPARγ and other nuclear receptor-responsive genes. PCG-1α has been shown to play critical roles in insulin sensitivity, mitochondrial biogenesis, energy production, and neuronal viability. In MPTP treated mice, transgenic expression of PCG-1α prevented the loss of dopaminergic neurons (Mudo et al., 2012). PGC-1α levels are reduced in genetic models of PD (Katsouri et al., 2012) and in parkin mutant mice (Shin et al., 2011). PPARγ agonists have been shown to stimulate the expression of PGC-1α (Hondares 2006), providing another possible mechanism for PPARγ action in PD. There is currently a phase II trial of pioglitazone in early stage Parkinson’s underway.
Amyotrophic lateral sclerosis
Amyotrophic lateral sclerosis (ALS) is characterized by extensive loss of motor neurons. Treatment of mouse SOD1 models of ALS with pioglitazone resulted in prevention of neuronal loss, preservation of neurological function and longer survival (Kiaei et al., 2005; Schutz, 2005; Shibata et al., 2008). Pioglitazone treatment was also shown to reduce inflammation. However, a clinical trial of pioglitazone in ALS patients showed no clinical benefit (Dupuis et al., 2012).
Huntington’s disease
Chiang et al. reported that PPARγ levels were reduced both in Huntington’s disease (HD) patient lymphocytes and the R6/2 HD mouse model. Treatment of HD mice with a PPARγ agonist prevented functional impairments in these mice, extended their survival, and also normalized the reduced expression of PCG-1α (Chiang et al., 2010). Administration of a pan-PPAR agonist resulted in elevation of PCG-1α levels, amelioration of behavioral deficits, and increased survival (Johri et al., 2012). A recent study using a different HD model found that rosiglitazone prevented neuronal loss, improved mitochondrial function and restored PGC-1α levels in the brain (Jin et al., 2013). These studies have led to the conclusion that PGC-1α deficiency underlies the mitochondrial dysfunction observed in HD (Johri et al., 2013).
LXR
LXRs are cholesterol sensors that play an essential modulatory role in cholesterol metabolism, lipogenesis, and the regulation of inflammation. Two isoforms of LXR exist, LXRα, which is prominently expressed in liver, adipose tissue, adrenal glands, intestine, kidney, and macrophages, and LXRβ , which is expressed 2–5 times higher than LXRα in the brain (Whitney et al., 2002) and ubiquitously expressed at low levels throughout the body. The two isoforms are activated by the same endogenous ligands, namely 24(S)-hydroxycholesterol, 22(R)-hydroxycholesterol 24(S),25-epoxycholesterol, and 27-hydroxycholesterol, and transcriptionally regulate genes involved in reverse cholesterol transport. The principal LXR ligand in the brain is 24(S)-hydroxycholesterol. Activated LXRs also exhibit antiinflammatory action, as they are sumoylated and repress transcription at NFκB target genes by a mechanism similar to that of PPARγ (Lee et al., 2009).
LXRs have been shown to play an essential role in the normal CNS. Neuronal development, synaptogenesis, and learning and memory are dependent on cholesterol, and dysregulation of cholesterol metabolism has been implicated in several neurodegenerative disorders (Brown 3rd et al., 2004). LXR double knockout mice have CNS abnormalities, including increased lipid deposition and neurodegeneration (Wang et al., 2002), and LXRβ knockout mice exhibit adult-onset motor neuron degeneration (Andersson et al., 2005). Two widely used synthetic ligands, T0901317 and GW3965, have been developed as tools for the study of LXRs. LXRs have been studied in a number of neurodegenerative diseases and CNS injury models and the development of new LXR agonists with acceptable side effect profiles remains an active area of interest.
Alzheimer’s disease
The elevated risk associated with possession of the apolipoprotein e4 allele for sporadic AD indicates the importance of cholesterol metabolism in AD. LXRs act to regulate the expression of the APOE gene and its lipid transporters, ABCA1 and ABCG1. Apolipoprotein E (apoE) is the most prominent apolipoprotein in the brain, where it functions as an acceptor of cholesterol and phospholipids effluxed from cells and facilitates their transport as HDL-like particles throughout the brain. Nascent apoE is lipidated by the lipid transporter ABCA1 and subsequently by ABCG1, which acts to transfer additional lipids to already lipidated apoE particles. Although the mechanism by which apoE isoforms confer AD risk remains unclear, it is generally thought that the comparatively poor lipid-carrying ability of apoE4 impairs its ability to facilitate soluble Aβ clearance, leading to accumulation of soluble amyloid species in the brain. Other mechanisms have also been postulated, reflective of a toxic gain of function associated with the E4 allele (Kim et al., 2009). ApoE has also been argued to play a role in clearance of amyloid from the brain into the peripheral vasculature and apoE4 is implicated in compromise of vascular integrity (Zlokovic, 2013). LXR, as a direct transcriptional regulator of apoE, ABCA1, and ABCG1, is an attractive therapeutic target and acts to increase Aβ clearance in mouse models of AD.
In the past 10 years, LXR agonists have been investigated in twelve separate studies (Table 1) which demonstrate their efficacy in improving behavioral impairments and amyloid clearance in AD models, with some mixed results in the stimulation of plaque clearance (Koldamova et al., 2005; Lefterov et al., 2007; Riddell et al., 2007; Jiang et al., 2008b; Donkin et al., 2010; Fitz et al., 2010; Terwel et al., 2011; Wesson et al., 2011; Vanmierlo et al., 2011; Cui et al., 2012; Hu et al., 2013; Fitz et al., 2014). Vanmierlo et al. observed improvements in hippocampal dependent memory but no change in plaque load with T0901317 treatment in APPSLxPS1mut mice (Vanmierlo et al., 2011). However, Riddell et al. only observed decreases in hippocampal Aβ42 with no change in cortical Aβ levels, although T0901317 treatment of Tg2576 mice mediated behavioral improvement (Riddell et al., 2007). The ability of LXR agonists to stimulate Aβ clearance and behavioral improvements is dependent on ABCA1 (Donkin et al., 2010; Fitz et al., 2010), indicating that LXR-mediated regulation of apoE lipidation state plays an important role in amyloid pathology.
Several groups have also shown that LXRs are able to modulate neuroinflammation in AD models primarily by modifying the responses of microglia and astrocytes (Zelcer et al., 2007; Cui et al., 2012). LXR-mediated transrepression of the expression of neurotoxic pro-inflammatory cytokines could be an additional mechanism for the behavioral improvements LXR agonists effect in AD models (Zhang-Gandhi and Drew, 2007; Ghisletti et al., 2009). The long-studied role of LXRs in peripheral macrophage inflammatory phenotype (Zelcer and Tontonoz, 2006; Hong and Tontonoz, 2008) and phagocytosis (A-Gonzalez et al., 2009) has recently sparked interest in the role of LXRs in microglia (Saijo et al., 2012) and the therapeutic value of LXR’s anti-inflammatory effect in AD models (Lefterov et al., 2007; Zhang-Gandhi et al., 2007; Cui et al., 2012).
Their anti-inflammatory properties and stimulation of reverse cholesterol transport makes LXRs an attractive therapeutic candidate for AD treatment, and one that is supported by an extensive literature in AD models. However, the poor side effect profile of LXR ligands precludes their clinical use. Better targeted or tissue-specific agonists of LXRs are currently in development (Hu et al., 2013) as potential AD therapeutics.
Parkinson’s disease
A novel role for LXRs in the developmental differentiation of dopaminergic neurons has recently been proposed by the Gustafsson group. Developmental midbrain neurogenesis is decreased in LXR double knockouts (Sacchetti et al., 2009). Moreover, activation of LXRs is important for in vivo development as well as sufficient for inducing ESCs to differentiate into dopaminergic neurons (Sacchetti et al., 2009; Theofilopoulos et al., 2012). This agrees with the observation that LXR double knockout mice have decreased neuronal numbers in the substantia nigra (Wang et al., 2002). Additionally, LXRβ knockout mice have increased death of dopaminergic neurons in the substantia nigra upon challenge with MPTP (Dai et al., 2012) and β-sitosterol (Kim et al., 2008), which is attributed to increased activation of microglia. Dai et al. also report that treatment with LXR agonist GW3965 can protect against loss of dopaminergic neurons induced by MPTP (Dai et al., 2012).
PPARδ
The role of PPAR β/δ (hereafter referred to as PPARδ) in neurodegeneration is far less studied than that of its related family member, PPARγ. PPARδ is ubiquitously expressed in all cell types in the central nervous system (CNS) (Moreno et al., 2004) and has the highest CNS expression of the three PPARs (Braissant et al., 1996). Several synthetic PPARδ activators have been produced and used in the research of CNS diseases although none are currently approved for clinical use.
PPARδ has important roles in neuronal function. Studies using mice deficient in PPARδ have revealed its vital role in many physiological processes, especially in the control of central and peripheral inflammatory reactions. PPARδ deficient mice are viable but show several defects in normal CNS biology and exhibit augmented inflammatory reactions. Specifically, PPARδ deficient mice show altered myelination (Peters et al., 2000) and impaired performance in memory tests with associated increases in inflammatory markers, astrogliosis and tau hyperphosphorylation (Barroso et al., 2013). PPARδ deficient mice show increased vulnerability to ischemic insults (Arsenijevic et al., 2006; Pialat et al., 2007) due to defects in antioxidant responses (Arsenijevic et al., 2006). PPARδ is also abundantly expressed in brain endothelia and controls vascular functions. Specific deletion of PPARδ in vascular smooth muscle cells leads to increased ischemic infarct size by increasing matrix metalloproteinase (MMP)-9 activity and by increasing the expression of several proinflammatory mediators (Yin et al., 2011).
The data generated from the use of PPARδ agonists show that PPARδ activation provides protection in many pathological CNS conditions largely due to its potent anti-inflammatory and antioxidant properties. PPARδ agonists have been shown to provide protection against neuronal degeneration in the MPTP model of Parkinson’s disease (Iwashita et al., 2007; Martin et al., 2013), stroke (Iwashita et al., 2007; Yin et al., 2010), EAE (Polak et al., 2005), spinal cord injury (Paterniti et al., 2010) and in a streptozotocin-induced experimental type 3 diabetes (La Monte et al., 2006), all mainly via reducing inflammation and oxidative stress. However, it should be noted that one study reported that a PPARδ agonist was not able to reduce 6-OHDA induced neuron loss in vivo even though it reduced microgliosis (Sadeghian et al., 2012). The protection elicited by PPARδ activation has several possible underlying mechanisms. PPARδ interferes with NFκB signaling, thus leading to a dampened inflammatory milieu and decreased oxidative stress (Paterniti et al., 2010; Barroso et al., 2013). PPARδ activation has been shown to decrease intracellular calcium concentration and reduce ROS production in vitro (Jin et al., 2012). In ischemic conditions PPARδ reduces MMP-9 activity, possibly by binding directly to the PPAR response element site in the MMP-9 promoter region (Yin et al., 2011). In addition, PPARδ activation has also been shown decrease apoptotic cell death by promoting the expression of bcl-2 (Paterniti et al., 2010; Yin et al., 2010) and attenuating caspase-3 activity both in vitro (Iwashita et al., 2007; Yin et al., 2010) and in vivo in ischemic conditions (Yin et al., 2010) as well as in spinal cord injury (Paterniti et al., 2010). Interestingly, PPARδ upregulation in SRC-3 deficient mice has been shown to promote alternative activation of microglia in a mouse model of EAE, indicating another mechanism by which it modulates microglial activity (Xiao et al., 2010).
So far only one study has assessed the possible protection of PPARδ agonists in a transgenic mouse model of Alzheimer’s disease. In a paper by Kalinin et al. (Kalinin et al., 2009) long term PPARδ agonist treatment reduced the subicular Aβ load and reduced astrocytic activation in 5xFAD mice. The reduction in the levels of Aβ was associated with increased expression of Aβ degrading enzymes neprilysin and insulin degrading enzyme, however, the role of microgliosis in this context was not analyzed. An additional in vitro study indicates that PPARδ agonists also protect primary neurons from Aβ induced cell death (Madrigal et al., 2007).
The majority of studies have attributed the neuroprotective properties of PPARδ to its role in ameliorating inflammatory reactions. Indeed, the most well defined effect of PPARδ is its ability to suppress inflammation in macrophages. The evidence for a direct neuroprotective role of PPARδ in vitro (Smith et al., 2004) is somewhat controversial. In one study PPARδ ligand GW0742 alone was not able to rescue SH-SY5Y cells from MPP+ induced cell death as measured by LDH release, although in vivo administration attenuated MPTP induced neurotoxicity (Martin et al., 2013). In contrast, other PPARδ activators L-165041 and GW501516 were shown to be directly neuroprotective in vitro at very high concentrations (Iwashita et al., 2007). Treatment with GW0742 protected cerebellar granule neurons from low-KCl induced toxicity only during a 12-hour exposure period in high concentrations (Smith et al., 2004) and also protected a mouse hippocampal cell line against glutamate toxicity during the same 12 hour period of exposure (Jin et al., 2012). The treatment during a longer exposure period was no longer protective and longer exposure times (48 hours) to GW0742 actually induced cell death (Smith et al., 2004).
Overall, accumulating data supports the role for PPARδ in controlling inflammatory reactions. Whether PPARδ activation is directly neuroprotective or if its neuroprotective effects are mediated through suppression of inflammation needs clarification.
RXR
The actions of RXR agonists are diverse, owing to the ability of RXR to form permissive heterodimers with other type II nuclear receptors, and their complexity is poorly understood. In addition to acting as heterodimerization partners for type II nuclear receptors, RXRs can act as homotetramers to modulate DNA 3D architecture or as homodimers that associate with their target genes in the presence or absence of ligand (Dawson and Xia, 2012).
Only a few genes have been shown to be regulated by the RXR homodimer complex (IJpenberg et al., 2004), most prominently a subset of chemokines (Nunez et al., 2010). RXR heterodimers are characterized as ‘permissive’ or ‘non-permissive’ based on whether ligation of either member of the heterodimer can elicit transcription. In the brain, permissive receptors include the PPARs, LXRs and the NR4A receptors (Rőszer et al., 2013) while non-permissive complexes are formed with RARs, thyroid and vitamin D receptors. Thus, RXR agonists can elicit pleiotropic actions through their actions on RXR homodimers as well as heterodimers containing permissive receptors. Recent work has shown that RXR agonists only regulate a subset of genes controlled by permissive receptors and that this subset is cell type-specific (Szeles et al., 2010). The basis for this restriction is not understood but explains why a broader range of effects of RXR agonists in the brain is not observed.
Alzheimer’s disease
There are a limited number of studies investigating RXR actions in neurodegenerative disease, although these receptors have well established roles during development. The literature on RXR activation is confused by a number of studies which investigate the actions of docasohexanoic acid (DHA), an omega 3 –polyunsaturated fatty acid, and attribute its actions to its binding to RXRs (de Urquiza et al., 2000). However, DHA also binds to PPARs (Kliewer et al., 1997) and two GPCRs (Im, 2012), thus it is not possible to conclude that these are RXR-specific actions. RXRα levels have been found to be elevated in dementia in a manner correlated with cognitive impairment (Akram et al., 2010). Cramer et al. (Cramer et al., 2012) reported that the RXR agonist bexarotene resulted in the rapid reduction in soluble forms of Aβ in the brains of mouse models of AD, owing to the induction of the LXR target genes apoE and Abca1 and elevation of brain high density lipoprotein levels (Ulrich et al., 2013). The reduction in soluble Aβ species was associated with improved neural network function and reversal of behavioral deficits. This effect on soluble Aβ levels was also reported by Fitz (Fitz et al., 2013a), Veeraraghavalu (Veeraraghavalu et al., 2013) and Ulrich (Ulrich et al., 2013), but not others (Table I; see below). Importantly, bexarotene-mediated behavioral improvement was observed by Fitz (Fitz et al., 2013b), Boehm-Cagan (Boehm-Cagan and Michaelson, 2014) and Tesseur (Tesseur et al., 2013). Cramer et al. reported that bexarotene treatment also resulted in the rapid reduction in amyloid plaque burden (Cramer et al., 2012) and this finding has been controversial (Landreth et al., 2013) (see below).
Parkinson’s disease
In a remarkable paper, McFarland reported that in two rodent models of Parkinson’s bexarotene acted to prevent neuronal loss and prevented functional impairment (McFarland et al., 2013). These effects were achieved at very low drug levels. Bexarotene was postulated to act through the ability of RXRs to heterodimerize with Nurr1 and drive its transcriptional activities.
Nurr1
The NR4A receptors were first identified as immediate-early genes induced in the nervous system in response to a wide variety of extracellular stimuli such as seizures (de Ortiz and Jamieson, 1996), stress (Garcia-Yague et al., 2013) and neurotransmitters (Barneda-Zahonero et al., 2012; Debernard et al., 2012). The NR4A family, including Nur77 (NR4A1; NGF-IA), Nurr1 (NR4A2; NGF-IB) and Nor-1 (NR4A3), are unique among type II nuclear receptors because the steric hindrance in their nominal ligand binding domains prevents them from accepting ligands. They were long thought to be constitutively active, but it has been recently shown that they can also play a role in transcriptional repression in a context dependent manner. NR4A transcriptional activity depends mainly on gene expression, miRNA targeting, alternative splicing, posttranslational modification, subcellular localization, and interactions with other nuclear receptors (Michelhaugh et al., 2005; Maxwell and Muscat, 2006; Sacchetti et al., 2006; Mohan et al., 2012; Yang et al., 2012).
All three family members can signal at NBREs (or NurREs) as monomers or homo- or heterodimers with other NR4As. Importantly, Nurr1 and Nur77 can also signal in complex with RXRs at DR5 repeats, and NR4A/RXR heterodimers can be activated by RXR ligands. Synthetic ligands that bind directly to NR4A:RXR heterodimers and drive transcriptional activity have also been described (Morita et al., 2005; Ishizawa et al., 2012). Development of specific ligands for NR4As could be therapeutically relevant for a variety of diseases. Aside from their critical role in nervous system function, NR4As are implicated as regulators of glucose homeostasis (Close et al., 2013), fatty acid metabolism (Volakakis et al., 2009; Holla et al., 2011), cellular proliferation (Sirin et al., 2010), cancer (Mohan et al., 2012), and immune regulation both in the periphery and in the brain.
Parkinson’s disease
Nurr1 mutations are associated with rare genetic forms of PD, but are not a major genetic risk factor for PD (see Decressac 2013 for an excellent review of Nurr1 in PD) (Decressac et al., 2013). A substantial body of evidence indicates that Nurr1 is downregulated in sporadic PD patients. This downregulation is selective for neurons with α-synuclein inclusions, and decreased Nurr1 correlates with decreased dopaminergic signaling markers in these cells. Rodents highly express Nurr1 in the ventral tegmental area (VTA) and substantia nigra pars compacta (SNpc) throughout development and into adulthood (Saucedo-Cardenas and Conneely, 1996; Zetterstrom et al., 1996). Nurr1 overexpression directs the differentiation of mesodiencephalic dopaminergic neurons (mdDAs) in vitro by controlling transcription of the dopaminergic genes tyrosine hydroxylase (TH), dopamine transporter (DAT), vesicular monoamine transporter 2 (VMAT2), and RET receptor tyrosine kinase (cRET) (Decressac et al., 2013). In vivo, Nurr1 knockouts develop mdDAs, but these neurons exhibit dysfunctional neurotransmission during embryogenesis and are not maintained in newborn animals. Interestingly, mdDAs in Nurr1 heterozygous mice have an increased vulnerability to stressors including MPTP and also exhibit an age-dependent dysfunction that correlates with decreasing motor abilities (Jiang et al., 2005). Studies utilizing conditional knockouts of Nurr1 in mature dopaminergic neurons indicate that these effects cannot entirely be attributed to developmental Nurr1, but that Nurr1 plays an important ongoing role in the maintenance of mdDAs.
The role of Nurr1 in mdDA neuronal survival is probably due to a combination of factors, including maintenance of dopaminergic neurotransmission machinery expression and facilitating the response of mdDAs to GDNF (Decressac et al., 2012). Nurr1 has also been shown to mediate cAMP-response element binding protein (CREB)-induced neuroprotection in response to stress, by upregulating an anti-apoptotic gene program in hippocampal neurons (Volakakis et al., 2010) and increasing BDNF expression in cerebellar granule cells (CGCs) (Barneda-Zahonero et al., 2012). In fact, Nurr1 is transcriptionally regulated in a CREB-dependent manner (Altarejos and Montminy, 2011). Additionally, Nurr1 plays a role in DNA repair of double strand breaks in neurons (Malewicz et al., 2011) and nucleotide excision repair in melanoma cells (Jagirdar et al., 2013). In both cell types Nurr1 is recruited to nuclear foci containing DNA repair proteins via a mechanism involving poly(ADP-ribose) polymerase-1 (PARP-1) and has a non-transcriptional critical role in DNA repair. Nurr1 is also induced by inflammatory stimuli in microglia and astrocytes and downregulates inflammatory gene transcription by CoREST-dependent transrepression at NFκB target gene promoters (Saijo et al., 2009). Knockdown of Nurr1 in the SN resulted in an enhanced glial inflammatory response to LPS or α-synuclein and increased death of dopaminergic neurons, suggesting that anti-inflammatory activities of Nurr1 may also be important for PD pathogenesis.
McFarland et al. reported that bexarotene, acting though its ability to stimulate Nurr1:RXR heterodimers, prevented dopaminergic cell loss, and impairment of both motor and cognitive function in two different rodent models of PD (McFarland et al., 2013).
Alzheimer’s disease
Recent evidence from the Saura group indicates that Nurr1 levels are decreased in an AD mouse model as well as in late-stage AD patients (España et al., 2010; Parra-Damas et al., 2014), and it has been reported that Nur77 levels decrease with age in an APP/PS1 mouse model of AD (Dickey et al., 2003). However, a direct role of Nurr1 in AD pathogenesis has yet to be studied. The involvement of Nurr1 in regulating neuronal survival, neuroinflammation, and hippocampal function and plasticity (Volakakis et al., 2010; Hawk et al., 2012; Bridi and Abel, 2013), however, makes it an attractive target for further study in AD and other neurodegenerative diseases.
Reproducibility of nuclear receptor effects in mouse models of neurodegenerative disease
The first study of the effects of nuclear receptor agonists in AD models was published in 2003 (Yan et al., 2003). Subsequently, 39 additional studies have been reported in 14 genetic animal models using agonists to PPARγ, LXRs and RXRs. From this body of work it is quite clear that, in the context of AD models, nuclear receptor agonists have their most consistent and robust effects on cognition and learning. Of the studies that have evaluated behavioral endpoints, there are 31 reports of behavioral improvements, one report where drug toxicity precluded interpretation of behavior (Tesseur et al., 2013) and 5 reports of failure to observe behavioral improvements. Similarly, these agents reproducibly reduced inflammation and the number and activation status of microglia, and in some cases, astrocytes. In the 17 studies which examined this endpoint, only two did not observe these effects.
Other endpoints were less consistent and have generated substantial controversy. Aβ reduction following nuclear receptor agonist treatment was observed in 61% of the 36 studies that measured deposited amyloid burden in 12 animal models of AD and 72% of 33 studies found reductions in soluble Aβ species. A clear example of the variability of plaque reduction is provided by the 12 studies examining the effect of LXR agonists. Plaque loss varied from 0–65% in these studies. These studies differed in the animal models used, the LXR agonist, and the formulation used to treat the mice, as well as the length of the treatment period, but in all but one study behavioral improvement was observed. Recently, the ability of the RXR agonist bexarotene to reduce plaque burden has been called into question. Cramer et al. reported that brief treatments with bexarotene reduced plaque burden, however, chronic drug treatment was not associated with plaque loss (Cramer et al., 2012). Five reports have challenged the former finding in 3 different mouse AD models (LaClair et al., 2013; Price et al., 2013; Tesseur et al., 2013; Veeraraghavalu et al., 2013; Fitz et al., 2013a). These studies used a very different drug formulation in their treatments, in which bexarotene was administered solubilized in DMSO (or in a cyclodextrin vehicle) rather than as the clinical formulation (TargretinTM) as micronized crystals used by Cramer et al. (Cramer et al., 2012). New work has compared these preparations and shown that they yield very different pharmacodynamics and overall drug exposure (Chen et al., 2013).
Amyloid plaques are removed from the brain principally by microglial-mediated phagocytosis and there is now good evidence that nuclear receptor agonists promote phagocytosis. However, we currently have no reliable markers for drug action within these cells in the brain. Phagocytosis of fAβ is stimulated by bexarotene and this effect is reliant upon RXRα (Yamanaka et al., 2012). Clearly, sustained activation of RXR fails to maintain a phagocytically active population of microglia, as plaque loads rebound to normal levels after several months of drug treatment and the basis of this effect is unknown. Importantly, plaque burden is unrelated to cognitive improvement in both mice and men.
It should be noted that LaClair et al. reported that bexarotene failed to improve behavior in APP/PS1 mice, but the conclusions of this study are invalidated by the absence of any behavioral deficits in their untreated AD transgenic model (LaClair et al., 2013). Indeed, cognition and learning are the most critical measure of efficacy for AD-directed therapies and the highly reproducible effects of nuclear receptor agonists on behavior support the translation of these studies into clinical trials in AD.
The outcomes of nuclear receptor treatment in rodent models of other neurodegenerative diseases have been less variable. For instance, of 27 studies using agonists for PPARγ, PPARα, PPARδ, LXR, or Nurr1 in the treatment of Parkinson’s mouse or rat models, 23 reported a positive outcome. One additional study in MPTP-treated monkeys (Swanson et al., 2011) reported no effects at 2.5mg/kg/day Pioglitazone, but a positive outcome was seen when that dose was doubled. The small number of studies performed in ALS and Huntington’s mouse models using nuclear receptor agonists also show promise, with all three studies in ALS models and 2 of 3 studies in Huntington’s models reporting positive outcomes.
While some outcome variability exists in nuclear receptor agonist studies, a strong persistence of positive outcomes in animal studies indicates that nuclear receptor agonists may be of therapeutic benefit in several neurodegenerative diseases. Many questions about the role of nuclear receptors in neurodegenerative disease remain unanswered, and further studies are required to elucidate the complex mechanisms behind the salutary actions of nuclear receptor agonists.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- A-Gonzalez N, et al. Apoptotic Cells Promote Their Own Clearance and Immune Tolerance through Activation of the Nuclear Receptor LXR. Immunity. 2009;31:245–258. doi: 10.1016/j.immuni.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akram A, Schmeidler J, Katsel P, Hof PR, Haroutunian V. Increased expression of RXRα in dementia: an early harbinger for the cholesterol dyshomeostasis? Molecular Neurodegeneration. 2010;5:36. doi: 10.1186/1750-1326-5-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alaynick WA. Nuclear receptors, mitochondria and lipid metabolism. Mitochondrion. 2008;8:329–337. doi: 10.1016/j.mito.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altarejos JY, Montminy M. CREB and the CRTC co-activators: sensors for hormonal and metabolic signals. Nature Reviews Molecular Cell Biology. 2011;12:141–151. doi: 10.1038/nrm3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson S, Andersson R, Gustafsson N, Warner M, Gustafsson J. Inactivation of liver X receptor beta leads to adult-onset motor neuron degeneration in male mice. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:3857–3862. doi: 10.1073/pnas.0500634102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arsenijevic D, de Bilbao F, Plamondon J, Paradis E, Vallet P, Richard D, Langhans W, Giannakopoulos P. Increased infarct size and lack of hyperphagic response after focal cerebral ischemia in peroxisome proliferator-activated receptor beta-deficient mice. Journal of cerebral blood flow and metabolism. 2006;26:433–445. doi: 10.1038/sj.jcbfm.9600200. [DOI] [PubMed] [Google Scholar]
- Barbiero JK, Santiago R, Tonin FS, Boschen S, Da Silva LM, de Werner MFP, Da Cunha C, Lima MMS, Vital MABF. PPAR-α agonist fenofibrate protects against the damaging effects of MPTP in a rat model of Parkinson’s disease. Progress in neuro-psychopharmacology & biological psychiatry. 2014;53C:35–44. doi: 10.1016/j.pnpbp.2014.02.009. [DOI] [PubMed] [Google Scholar]
- Barneda-Zahonero B, Servitja J, Badiola N, Miñano-Molina AJ, Fadó R, Saura CA, Rodríguez-Alvarez J. Nurr1 protein is required for N-methyl-D-aspartic acid (NMDA) receptor-mediated neuronal survival. The Journal of biological chemistry. 2012;287:11351–11362. doi: 10.1074/jbc.M111.272427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barroso E, del Valle J, Porquet D, Santos AMV, Salvadó L, Rodríguez-Rodríguez R, Gutiérrez P, Anglada-Huguet M, Alberch J, Camins A, Palomer X, Pallàs M, Michalik L, Wahli W, Vázquez-Carrera M. Tau hyperphosphorylation and increased BACE1 and RAGE levels in the cortex of PPARβ/δ-null mice. Biochimica et biophysica acta. 2013;1832:1241–1248. doi: 10.1016/j.bbadis.2013.03.006. [DOI] [PubMed] [Google Scholar]
- Beaven SW, Tontonoz P. Nuclear receptors in lipid metabolism: targeting the heart of dyslipidemia. Annual review of medicine. 2006;57:313–329. doi: 10.1146/annurev.med.57.121304.131428. [DOI] [PubMed] [Google Scholar]
- Boehm-Cagan A, Michaelson DM. Reversal of apoE4-driven brain pathology and behavioral deficits by Bexarotene. The Journal of Neuroscience. 2014 doi: 10.1523/JNEUROSCI.5198-13.2014. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braissant O, Foufelle F, Scotto C, Dauça M, Wahli W. Differential expression of peroxisome proliferator-activated receptors (PPARs): tissue distribution of PPAR-alpha, -beta, and -gamma in the adult rat. Endocrinology. 1996;137:354–366. doi: 10.1210/endo.137.1.8536636. [DOI] [PubMed] [Google Scholar]
- Breidert T, Callebert J, Heneka MT, Landreth G, Launay JM, Hirsch EC. Protective action of the peroxisome proliferator-activated receptor-gamma agonist pioglitazone in a mouse model of Parkinson’s disease. Journal of neurochemistry. 2002;82:615–624. doi: 10.1046/j.1471-4159.2002.00990.x. [DOI] [PubMed] [Google Scholar]
- Bridi MS, Abel T. The NR4A orphan nuclear receptors mediate transcription-dependent hippocampal synaptic plasticity. Neurobiology of learning and memory. 2013;105:151–158. doi: 10.1016/j.nlm.2013.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J, 3rd, Theisler C, Silberman S, Magnuson D, Gottardi-Littell N, Lee JM, Yager D, Crowley J, Sambamurti K, Rahman MM, Reiss AB, Eckman CB, Wolozin B. Differential Expression of Cholesterol Hydroxylases in Alzheimer’s Disease. Journal of Biological Chemistry. 2004;279:34674–34681. doi: 10.1074/jbc.M402324200. [DOI] [PubMed] [Google Scholar]
- Carta AR, Frau L, Pisanu A, Wardas J, Spiga S, Carboni E. Rosiglitazone decreases peroxisome proliferator receptor-γ levels in microglia and inhibits TNF-α production: new evidences on neuroprotection in a progressive Parkinson’s disease model. Neuroscience. 2011;194:250–261. doi: 10.1016/j.neuroscience.2011.07.046. [DOI] [PubMed] [Google Scholar]
- Carta AR, Pisanu A. Modulating microglia activity with PPAR-γ agonists: a promising therapy for Parkinson’s disease? Neurotoxicity Research. 2013;23:112–123. doi: 10.1007/s12640-012-9342-7. [DOI] [PubMed] [Google Scholar]
- Castrillo A, Tontonoz P. Nuclear receptors in macrophage biology: at the crossroads of lipid metabolism and inflammation. Annual review of cell and developmental biology. 2004;20:455–480. doi: 10.1146/annurev.cellbio.20.012103.134432. [DOI] [PubMed] [Google Scholar]
- Chawla A, Repa JJ, Evans RM, Mangelsdorf DJ. Nuclear receptors and lipid physiology: opening the X-files. Science (New York, N.Y.) 2001;294:1866–1870. doi: 10.1126/science.294.5548.1866. [DOI] [PubMed] [Google Scholar]
- Chen L, Wang Y, Zhang J, Hao L, Guo H, Lou H, Zhang D. Bexarotene nanocrystal-Oral and parenteral formulation development, characterization and pharmacokinetic evaluation. European journal of pharmaceutics and biopharmaceutics e.V. 2013 doi: 10.1016/j.ejpb.2013.12.005. [DOI] [PubMed] [Google Scholar]
- Chiang M, Chen C, Lee M, Chen H, Chen H, Wu Y, Hung C, Kang J, Chang C, Chang C, Wu Y, Tsai Y, Chern Y. Modulation of energy deficiency in Huntington’s disease via activation of the peroxisome proliferator-activated receptor gamma. Human molecular genetics. 2010;19:4043–4058. doi: 10.1093/hmg/ddq322. [DOI] [PubMed] [Google Scholar]
- Close AF, Rouillard C, Buteau J. NR4A orphan nuclear receptors in glucose homeostasis: a minireview. Diabetes & Metabolism. 2013;39:478–484. doi: 10.1016/j.diabet.2013.07.005. [DOI] [PubMed] [Google Scholar]
- Colton CA. Heterogeneity of microglial activation in the innate immune response in the brain. Journal of neuroimmune pharmacology: the official journal of the Society on NeuroImmune Pharmacology. 2009;4:399–418. doi: 10.1007/s11481-009-9164-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combs CK, Johnson DE, Karlo JC, Cannady SB, Landreth GE. Inflammatory mechanisms in Alzheimer’s disease: inhibition of beta-amyloid-stimulated proinflammatory responses and neurotoxicity by PPARgamma agonists. The Journal of neuroscience. 2000;20:558–567. doi: 10.1523/JNEUROSCI.20-02-00558.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craft S, Cholerton B, Baker LD. Insulin and Alzheimer’s disease: untangling the web. J. Alzheimers Dis. 2013;1(33 Suppl):S263–S275. doi: 10.3233/JAD-2012-129042. [DOI] [PubMed] [Google Scholar]
- Cramer PE, Cirrito JR, Wesson DW, Lee CY, Karlo JC, Zinn AE, Casali BT, Restivo JL, Goebel WD, James MJ, Brunden KR, Wilson DA, Landreth GE. ApoE-directed therapeutics rapidly clear β-amyloid and reverse deficits in AD mouse models. Science (New York, N.Y.) 2012;335:1503–1506. doi: 10.1126/science.1217697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui W, Sun Y, Wang Z, Xu C, Peng Y, Li R. Liver X receptor activation attenuates inflammatory response and protects cholinergic neurons in APP/PS1 transgenic mice. Neuroscience. 2012;210:200–210. doi: 10.1016/j.neuroscience.2012.02.047. [DOI] [PubMed] [Google Scholar]
- Czirr E, Wyss-Coray T. The immunology of neurodegeneration. The Journal of clinical investigation. 2012;122:1156–1163. doi: 10.1172/JCI58656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai Y, Tan X, Wu W, Warner M, Gustafsson J. Liver X receptor β protects dopaminergic neurons in a mouse model of Parkinson disease. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:13112–13117. doi: 10.1073/pnas.1210833109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson MI, Xia Z. The retinoid X receptors and their ligands. Biochim Biophys Acta. 2012;1821:21–56. doi: 10.1016/j.bbalip.2011.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Ortiz SP, Jamieson GA., Jr HZF-3, an immediate-early orphan receptor homologous to NURR1/NOT: induction upon membrane depolarization and seizures. Molecular brain research. 1996;38:1–13. doi: 10.1016/0169-328x(95)00263-r. [DOI] [PubMed] [Google Scholar]
- de Urquiza AM, Liu S, Sjöberg M, Zetterström RH, Griffiths W, Sjövall J, Perlmann T. Docosahexaenoic acid, a ligand for the retinoid X receptor in mouse brain. Science (New York, N.Y.) 2000;290:2140–2144. doi: 10.1126/science.290.5499.2140. [DOI] [PubMed] [Google Scholar]
- Debernard KAB, Mathisen GH, Paulsen RE. Differences in NGFI-B, Nurr1, and NOR-1 expression and nucleocytoplasmic translocation in glutamate-treated neurons. Neurochem. Int. 2012;61:79–88. doi: 10.1016/j.neuint.2012.04.002. [DOI] [PubMed] [Google Scholar]
- Decressac M, Kadkhodaei B, Mattsson B, Laguna A, Perlmann T, Björklund A. α-Synuclein-induced down-regulation of Nurr1 disrupts GDNF signaling in nigral dopamine neurons. Science Translational Medicine. 2012;4:163ra156. doi: 10.1126/scitranslmed.3004676. [DOI] [PubMed] [Google Scholar]
- Decressac M, Volakakis N, Björklund A, Perlmann T. NURR1 in Parkinson disease—from pathogenesis to therapeutic potential. Nature Reviews Neurology. 2013;9:629–636. doi: 10.1038/nrneurol.2013.209. [DOI] [PubMed] [Google Scholar]
- Dehmer T, Heneka MT, Sastre M, Dichgans J, Schulz JB. Protection by pioglitazone in the MPTP model of Parkinson’s disease correlates with I kappa B alpha induction and block of NF kappa B and iNOS activation. Journal of neurochemistry. 2004;88:494–501. doi: 10.1046/j.1471-4159.2003.02210.x. [DOI] [PubMed] [Google Scholar]
- Denner LA, Rodriguez-Rivera J, Haidacher SJ, Jahrling JB, Carmical JR, Hernandez CM, Zhao Y, Sadygov RG, Starkey JM, Spratt H, Luxon BA, Wood TG, Dineley KT. Cognitive enhancement with rosiglitazone links the hippocampal PPARγ and ERK MAPK signaling pathways. The Journal of neuroscience. 2012;32:16725a–16735a. doi: 10.1523/JNEUROSCI.2153-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickey CA, Loring JF, Montgomery J, Gordon MN, Eastman PS, Morgan D. Selectively reduced expression of synaptic plasticity-related genes in amyloid precursor protein + presenilin-1 transgenic mice. The Journal of neuroscience. 2003;23:5219–5226. doi: 10.1523/JNEUROSCI.23-12-05219.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donkin JJ, Stukas S, Hirsch-Reinshagen V, Namjoshi D, Wilkinson A, May S, Chan J, Fan J, Collins J, Wellington CL. ATP-binding cassette transporter A1 mediates the beneficial effects of the liver X receptor agonist GW3965 on object recognition memory and amyloid burden in amyloid precursor protein/presenilin 1 mice. The Journal of biological chemistry. 2010;285:34144–34154. doi: 10.1074/jbc.M110.108100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont M, Stack C, Elipenahli C, Jainuddin S, Gerges M, Starkova N, Calingasan NY, Yang L, Tampellini D, Starkov AA, Chan RB, Di Paolo G, Pujol A, Beal MF. Bezafibrate administration improves behavioral deficits and tau pathology in P301S mice. Human molecular genetics. 2012;21:5091–5105. doi: 10.1093/hmg/dds355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupuis L, et al. A randomized, double blind, placebo-controlled trial of pioglitazone in combination with riluzole in amyotrophic lateral sclerosis. PLoS ONE. 2012;7:e37885. doi: 10.1371/journal.pone.0037885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escribano L, et al. Rosiglitazone Rescues Memory Impairment in Alzheimer’s Transgenic Mice: Mechanisms Involving a Reduced Amyloid and Tau Pathology. Neuropsychopharmacology. 2010;35:1593–1604. doi: 10.1038/npp.2010.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escribano L, Simón A, Pérez-Mediavilla A, Salazar-Colocho P, del Río J, Frechilla D. Rosiglitazone reverses memory decline and hippocampal glucocorticoid receptor down-regulation in an Alzheimer’s disease mouse model. Biochemical and Biophysical Research Communications. 2009;379:406–410. doi: 10.1016/j.bbrc.2008.12.071. [DOI] [PubMed] [Google Scholar]
- España J, Valero J, Miñano-Molina AJ, Masgrau R, Martín E, Guardia-Laguarta C, Lleó A, Giménez-Llort L, Rodríguez-Alvarez J, Saura CA. beta-Amyloid disrupts activity-dependent gene transcription required for memory through the CREB coactivator CRTC1. The Journal of neuroscience. 2010;30:9402–9410. doi: 10.1523/JNEUROSCI.2154-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira ST, Clarke JR, Bomfim TR, de Felice FG. Inflammation, defective insulin signaling, and neuronal dysfunction in Alzheimer’s disease. Alzheimer’s & dementia : the journal of the Alzheimer’s Association. 2014;10:S76–S83. doi: 10.1016/j.jalz.2013.12.010. [DOI] [PubMed] [Google Scholar]
- Fitz NF, Castranio EL, Carter AY, Kodali R, Lefterov I, Koldamova R. Improvement of Memory Deficits and Amyloid-β Clearance in Aged APP23 Mice Treated with a Combination of Anti-Amyloid-β Antibody and LXR Agonist. J. Alzheimers Dis. 2014 doi: 10.3233/JAD-132789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitz NF, Cronican A, Pham T, Fogg A, Fauq AH, Chapman R, Lefterov I, Koldamova R. Liver X receptor agonist treatment ameliorates amyloid pathology and memory deficits caused by high-fat diet in APP23 mice. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30:6862–6872. doi: 10.1523/JNEUROSCI.1051-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitz NF, Cronican AA, Lefterov I, Koldamova R. Comment on “ApoE-directed therapeutics rapidly clear β-amyloid and reverse deficits in AD mouse models”. Science (New York, N.Y.) 2013;340:924. doi: 10.1126/science.1235809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Yagüe ÁJ, Rada P, Rojo AI, Lastres-Becker I, Cuadrado A. Nuclear import and export signals control the subcellular localization of Nurr1 protein in response to oxidative stress. The Journal of biological chemistry. 2013;288:5506–5517. doi: 10.1074/jbc.M112.439190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghisletti S, Huang W, Jepsen K, Benner C, Hardiman G, Rosenfeld MG, Glass CK. Cooperative NCoR/SMRT interactions establish a corepressor-based strategy for integration of inflammatory and anti-inflammatory signaling pathways. Genes & Development. 2009;23:681–693. doi: 10.1101/gad.1773109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass CK, Saijo K. Nuclear receptor transrepression pathways that regulate inflammation in macrophages and T cells. Nature reviews. Immunology. 2010;10:365–376. doi: 10.1038/nri2748. [DOI] [PubMed] [Google Scholar]
- Gold M, Alderton C, Zvartau-Hind M, Egginton S, Saunders AM, Irizarry M, Craft S, Landreth G, Linnamägi U, Sawchak S. Rosiglitazone monotherapy in mild-to-moderate Alzheimer’s disease: results from a randomized, double-blind, placebo-controlled phase III study. Dementia and geriatric cognitive disorders. 2010;30:131–146. doi: 10.1159/000318845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon S, Martinez FO. Alternative activation of macrophages: mechanism and functions. Immunity. 2010;32:593–604. doi: 10.1016/j.immuni.2010.05.007. [DOI] [PubMed] [Google Scholar]
- Hawk JD, Bookout AL, Poplawski SG, Bridi M, Rao AJ, Sulewski ME, Kroener BT, Manglesdorf DJ, Abel T. NR4A nuclear receptors support memory enhancement by histone deacetylase inhibitors. The Journal of clinical investigation. 2012;122:3593–3602. doi: 10.1172/JCI64145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heneka MT, Landreth GE, Hüll M. Drug insight: effects mediated by peroxisome proliferator-activated receptor-gamma in CNS disorders. Nature clinical practice. Neurology. 2007;3:496–504. doi: 10.1038/ncpneuro0586. [DOI] [PubMed] [Google Scholar]
- Heneka MT, Sastre M, Dumitrescu-Ozimek L, Hanke A, Dewachter I, Kuiperi C, O’Banion K, Klockgether T, van Leuven F, Landreth GE. Acute treatment with the PPARgamma agonist pioglitazone and ibuprofen reduces glial inflammation and Abeta1-42 levels in APPV717I transgenic mice. Brain : a journal of neurology. 2005;128:1442–1453. doi: 10.1093/brain/awh452. [DOI] [PubMed] [Google Scholar]
- Holla VR, Wu H, Shi Q, Menter DG, DuBois RN. Nuclear orphan receptor NR4A2 modulates fatty acid oxidation pathways in colorectal cancer. The Journal of biological chemistry. 2011;286:30003–30009. doi: 10.1074/jbc.M110.184697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hondares E, Mora O, Yubero P, La Concepción de MR, Iglesias R, Giralt M, Villarroya F. Thiazolidinediones and rexinoids induce peroxisome proliferator-activated receptor-coactivator (PGC)-1alpha gene transcription: an autoregulatory loop controls PGC-1alpha expression in adipocytes via peroxisome proliferator-activated receptor-gamma coactivation. Endocrinology. 2006;147:2829–2838. doi: 10.1210/en.2006-0070. [DOI] [PubMed] [Google Scholar]
- Hong C, Tontonoz P. Coordination of inflammation and metabolism by PPAR and LXR nuclear receptors. Current Opinion in Genetics & Development. 2008;18:461–467. doi: 10.1016/j.gde.2008.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, Yang Y, Yu Y, Wen G, Shang N, Zhuang W, Lu D, Zhou B, Liang B, Yue X, Li F, Jun du, Bu X. Synthesis and Identification of New Flavonoids Targeting Liver X Receptor β Involved Pathway as Potential Facilitators of Aβ Clearance with Reduced Lipid Accumulation. Journal of medicinal chemistry. 2013;56:6033–6053. doi: 10.1021/jm301913k. [DOI] [PubMed] [Google Scholar]
- IJpenberg A, Tan NS, Gelman LS, Kersten S, Kersten E, Seydoux J, Xu J, Metzger D, Canaple L, Chambon P, Wahli W, Desvergne B. In vivo activation of PPAR target genes by RXR homodimers. The EMBO journal. 2004;23:2083–2091. doi: 10.1038/sj.emboj.7600209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Im D. Omega-3 fatty acids in anti-inflammation (pro-resolution) and GPCRs. Progress in lipid research. 2012;51:232–237. doi: 10.1016/j.plipres.2012.02.003. [DOI] [PubMed] [Google Scholar]
- Inestrosa NC, Carvajal FJ, Zolezzi JM, Tapia-Rojas C, Serrano F, Karmelic D, Toledo EM, Toro A, Toro J, Santos MJ. Peroxisome proliferators reduce spatial memory impairment, synaptic failure, and neurodegeneration in brains of a double transgenic mice model of Alzheimer’s disease. J. Alzheimers Dis. 2013;33:941–959. doi: 10.3233/JAD-2012-120397. [DOI] [PubMed] [Google Scholar]
- Ishizawa M, Kagechika H, Makishima M. NR4A nuclear receptors mediate carnitine palmitoyltransferase 1α gene expression by the rexinoid HX600. Biochemical and Biophysical Research Communications. 2012;418:780–785. doi: 10.1016/j.bbrc.2012.01.102. [DOI] [PubMed] [Google Scholar]
- Iwashita A, Muramatsu Y, Yamazaki T, Muramoto M, Kita Y, Yamazaki S, Mihara K, Moriguchi A, Matsuoka N. Neuroprotective efficacy of the peroxisome proliferator-activated receptor delta-selective agonists in vitro and in vivo. The Journal of pharmacology and experimental therapeutics. 2007;320:1087–1096. doi: 10.1124/jpet.106.115758. [DOI] [PubMed] [Google Scholar]
- Jagirdar K, Yin K, Harrison M, Lim W, Muscat GEO, Sturm RA, Smith AG. The NR4A2 nuclear receptor is recruited to novel nuclear foci in response to UV irradiation and participates in nucleotide excision repair. PLoS ONE. 2013;8:e78075. doi: 10.1371/journal.pone.0078075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang C, Wan X, He Y, Pan T, Jankovic J, Le Weidong Age-dependent dopaminergic dysfunction in Nurr1 knockout mice. Experimental Neurology. 2005;191:154–162. doi: 10.1016/j.expneurol.2004.08.035. [DOI] [PubMed] [Google Scholar]
- Jiang Q, Heneka M, Landreth GE. The role of peroxisome proliferator-activated receptor-gamma (PPARgamma) in Alzheimer’s disease: therapeutic implications. CNS drugs. 2008;22:1–14. doi: 10.2165/00023210-200822010-00001. [DOI] [PubMed] [Google Scholar]
- Jiang Q, Lee CYD, Mandrekar S, Wilkinson B, Cramer P, Zelcer N, Mann K, Lamb B, Willson TM, Collins JL. ApoE Promotes the Proteolytic Degradation of Aβ. Neuron. 2008;58:681–693. doi: 10.1016/j.neuron.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin H, Ham SA, Kim MY, Woo IS, Kang ES, Hwang JS, Lee K, Kim HJ, Roh GS, Lim D, Kang D, Seo HG. Activation of peroxisome proliferator-activated receptor-δ attenuates glutamate-induced neurotoxicity in HT22 mouse hippocampal cells. Journal of Neuroscience Research. 2012;90:1646–1653. doi: 10.1002/jnr.23053. [DOI] [PubMed] [Google Scholar]
- Jin J, Albertz J, Guo Z, Peng Q, Rudow G, Troncoso JC, Ross CA, Duan W. Neuroprotective effects of PPAR-γ agonist rosiglitazone in N171-82Q mouse model of Huntington’s disease. Journal of neurochemistry. 2013;125:410–419. doi: 10.1111/jnc.12190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johri A, Calingasan NY, Hennessey TM, Sharma A, Yang L, Wille E, Chandra A, Beal MF. Pharmacologic activation of mitochondrial biogenesis exerts widespread beneficial effects in a transgenic mouse model of Huntington’s disease. Human molecular genetics. 2012;21:1124–1137. doi: 10.1093/hmg/ddr541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johri A, Chandra A, Beal MF. PGC-1α, mitochondrial dysfunction, and Huntington’s disease. Free radical biology & medicine. 2013;62:37–46. doi: 10.1016/j.freeradbiomed.2013.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalinin S, Richardson JC, Feinstein DL. A PPARdelta agonist reduces amyloid burden and brain inflammation in a transgenic mouse model of Alzheimer’s disease. Current Alzheimer research. 2009;6:431–437. doi: 10.2174/156720509789207949. [DOI] [PubMed] [Google Scholar]
- Katsouri L, Blondrath K, Sastre M. Peroxisome proliferator-activated receptor-γ cofactors in neurodegeneration. IUBMB life. 2012;64:958–964. doi: 10.1002/iub.1097. [DOI] [PubMed] [Google Scholar]
- Kiaei M. Peroxisome Proliferator-Activated Receptor-gamma in Amyotrophic Lateral Sclerosis and Huntington’s Disease. PPAR research. 2008;2008:418765. doi: 10.1155/2008/418765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiaei M, Kipiani K, Chen J, Calingasan NY, Beal MF. Peroxisome proliferator-activated receptor-gamma agonist extends survival in transgenic mouse model of amyotrophic lateral sclerosis. Experimental Neurology. 2005;191:331–336. doi: 10.1016/j.expneurol.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Kim H, Fan X, Gabbi C, Yakimchuk K, Parini P, Warner M, Gustafsson J. Liver X receptor beta (LXRbeta): a link between beta-sitosterol and amyotrophic lateral sclerosis-Parkinson’s dementia. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:2094–2099. doi: 10.1073/pnas.0711599105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Basak JM, Holtzman DM. The role of apolipoprotein E in Alzheimer’s disease. Neuron. 2009;63:278–303. doi: 10.1016/j.neuron.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliewer SA, Sundseth SS, Jones SA, Brown PJ, Wisely GB, Koble CS, Devchand P, Wahli W, Willson TM, Lenhard JM, Lehmann JM. Fatty acids and eicosanoids regulate gene expression through direct interactions with peroxisome proliferator-activated receptors alpha and gamma. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:4318–4323. doi: 10.1073/pnas.94.9.4318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koldamova RP, Lefterov IM, Staufenbiel M, Wolfe D, Huang S, Glorioso JC, Walter M, Roth MG, Lazo JS. The liver X receptor ligand T0901317 decreases amyloid beta production in vitro and in a mouse model of Alzheimer’s disease. The Journal of biological chemistry. 2005;280:4079–4088. doi: 10.1074/jbc.M411420200. [DOI] [PubMed] [Google Scholar]
- Kreisler A, Gelé P, Wiart J, Lhermitte M, Destée A, Bordet R. Lipid-lowering drugs in the MPTP mouse model of Parkinson’s disease: fenofibrate has a neuroprotective effect, whereas bezafibrate and HMG-CoA reductase inhibitors do not. Brain research. 2007;1135:77–84. doi: 10.1016/j.brainres.2006.12.011. [DOI] [PubMed] [Google Scholar]
- Kumar P, Kaundal RK, More S, Sharma SS. Beneficial effects of pioglitazone on cognitive impairment in MPTP model of Parkinson’s disease. Behavioural brain research. 2009;197:398–403. doi: 10.1016/j.bbr.2008.10.010. [DOI] [PubMed] [Google Scholar]
- La Monte de SM, Tong M, Lester-Coll N, Plater M, Jr, Wands JR. Therapeutic rescue of neurodegeneration in experimental type 3 diabetes: relevance to Alzheimer’s disease. J. Alzheimers Dis. 2006;10:89–109. doi: 10.3233/jad-2006-10113. [DOI] [PubMed] [Google Scholar]
- LaClair KD, Manaye KF, Lee DL, Allard JS, Savonenko AV, Troncoso JC, Wong PC. Treatment with bexarotene, a compound that increases apolipoprotein-E, provides no cognitive benefit in mutant APP/PS1 mice. Molecular Neurodegeneration. 2013;8:18. doi: 10.1186/1750-1326-8-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacombe P, Mathews PM, Schmidt SD, Breidert T, Heneka MT, Landreth GE, Feinstein DL, Galea E. Effect of anti-inflammatory agents on transforming growth factor beta over-expressing mouse brains: a model revised. J Neuroinflammation. 2004;1:11. doi: 10.1186/1742-2094-1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laloux C, Petrault M, Lecointe C, Devos D, Bordet R. Differential susceptibility to the PPAR-γ agonist pioglitazone in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine and 6-hydroxydopamine rodent models of Parkinson’s disease. Pharmacological research: the official journal of the Italian Pharmacological Society. 2012;65:514–522. doi: 10.1016/j.phrs.2012.02.008. [DOI] [PubMed] [Google Scholar]
- Landreth G, Jiang Q, Mandrekar S, Heneka M. PPARgamma agonists as therapeutics for the treatment of Alzheimer’s disease. Neurotherapeutics : the journal of the American Society for Experimental NeuroTherapeutics. 2008;5:481–489. doi: 10.1016/j.nurt.2008.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landreth GE, Cramer PE, Lakner MM, Cirrito JR, Wesson DW, Brunden KR, Wilson DA. Response to comments on “ApoE-directed therapeutics rapidly clear β-amyloid and reverse deficits in AD mouse models”. Science (New York, N.Y.) 2013;340:924. doi: 10.1126/science.1234114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee EY, Lee JE, Park JH, Shin IC, Koh HC. Rosiglitazone, a PPAR-γ agonist, protects against striatal dopaminergic neurodegeneration induced by 6-OHDA lesions in the substantia nigra of rats. Toxicology letters. 2012;213:332–344. doi: 10.1016/j.toxlet.2012.07.016. [DOI] [PubMed] [Google Scholar]
- Lee JH, Park SM, Kim OS, Lee CS, Woo JH, Park SJ, Joe E, Jou I. Differential SUMOylation of LXRalpha and LXRbeta mediates transrepression of STAT1 inflammatory signaling in IFN-gamma-stimulated brain astrocytes. Molecular Cell. 2009;35:806–817. doi: 10.1016/j.molcel.2009.07.021. [DOI] [PubMed] [Google Scholar]
- Lefterov I, Bookout A, Wang Z, Staufenbiel M, Mangelsdorf D, Koldamova R. Expression profiling in APP23 mouse brain: inhibition of Abeta amyloidosis and inflammation in response to LXR agonist treatment. Molecular Neurodegeneration. 2007;2:20. doi: 10.1186/1750-1326-2-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madrigal JLM, Kalinin S, Richardson JC, Feinstein DL. Neuroprotective actions of noradrenaline: effects on glutathione synthesis and activation of peroxisome proliferator activated receptor delta. Journal of neurochemistry. 2007;103:2092–2101. doi: 10.1111/j.1471-4159.2007.04888.x. [DOI] [PubMed] [Google Scholar]
- Malewicz M, Kadkhodaei B, Kee N, Volakakis N, Hellman U, Viktorsson K, Leung CY, Chen B, Lewensohn R, van Gent DC, Chen DJ, Perlmann T. Essential role for DNA-PK-mediated phosphorylation of NR4A nuclear orphan receptors in DNA double-strand break repair. Genes & Development. 2011;25:2031–2040. doi: 10.1101/gad.16872411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandrekar-Colucci S, Karlo JC, Landreth GE. Mechanisms underlying the rapid peroxisome proliferator-activated receptor-γ-mediated amyloid clearance and reversal of cognitive deficits in a murine model of Alzheimer’s disease. The Journal of neuroscience. 2012;32:10117–10128. doi: 10.1523/JNEUROSCI.5268-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandrekar-Colucci S, Landreth GE. Nuclear receptors as therapeutic targets for Alzheimer’s disease. Expert Opinion on Therapeutic Targets. 2011;15:1085–1097. doi: 10.1517/14728222.2011.594043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin HL, Mounsey RB, Mustafa S, Sathe K, Teismann P. Pharmacological manipulation of peroxisome proliferator-activated receptor γ (PPARγ) reveals a role for anti-oxidant protection in a model of Parkinson’s disease. Experimental Neurology. 2012;235:528–538. doi: 10.1016/j.expneurol.2012.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin HL, Mounsey RB, Sathe K, Mustafa S, Nelson MC, Evans RM, Teismann P. A peroxisome proliferator-activated receptor-δ agonist provides neuroprotection in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine model of Parkinson’s disease. Neuroscience. 2013;240:191–203. doi: 10.1016/j.neuroscience.2013.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masciopinto F, Di Pietro ND, Corona C, Bomba M, Pipino C, Curcio M, Di Castelnuovo AD, Ciavardelli D, Silvestri E, Canzoniero LM, Sekler I, P A, olfi, Sensi SL, Pandolfi A. Effects of long-term treatment with pioglitazone on cognition and glucose metabolism of PS1-KI, 3xTg-AD, and wild-type mice. Cell Death and Disease. 2012;3:e448. doi: 10.1038/cddis.2012.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell MA, Muscat GEO. The NR4A subgroup: immediate early response genes with pleiotropic physiological roles. Nuclear Receptor Signaling. 2006;4:e002. doi: 10.1621/nrs.04002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland K, Spalding TA, Hubbard D, Ma J, Olsson R, Burstein ES. Low Dose Bexarotene Treatment Rescues Dopamine Neurons and Restores Behavioral Function in Models of Parkinson’s Disease. ACS Chemical Neuroscience. 2013;4:1430–1438. doi: 10.1021/cn400100f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelhaugh SK, Vaitkevicius H, Wang J, Bouhamdan M, Krieg AR, Walker JL, Mendiratta V, Bannon MJ. Dopamine neurons express multiple isoforms of the nuclear receptor nurr1 with diminished transcriptional activity. Journal of neurochemistry. 2005;95:1342–1350. doi: 10.1111/j.1471-4159.2005.03458.x. [DOI] [PubMed] [Google Scholar]
- Mohan HM, Aherne CM, Rogers AC, Baird AW, Winter des C, Murphy EP. Molecular pathways: the role of NR4A orphan nuclear receptors in cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2012;18:3223–3228. doi: 10.1158/1078-0432.CCR-11-2953. [DOI] [PubMed] [Google Scholar]
- Moreno S, Farioli-Vecchioli S, Cerù MP. Immunolocalization of peroxisome proliferator-activated receptors and retinoid X receptors in the adult rat CNS. Neuroscience. 2004;123:131–145. doi: 10.1016/j.neuroscience.2003.08.064. [DOI] [PubMed] [Google Scholar]
- Morita K, Kawana K, Sodeyama M, Shimomura I, Kagechika H, Makishima M. Selective allosteric ligand activation of the retinoid X receptor heterodimers of NGFI-B and Nurr1. Biochemical Pharmacology. 2005;71:98–107. doi: 10.1016/j.bcp.2005.10.017. [DOI] [PubMed] [Google Scholar]
- Morris GP, Clark IA, Zinn R, Vissel B. Microglia: a new frontier for synaptic plasticity, learning and memory, and neurodegenerative disease research. Neurobiology of learning and memory. 2013;105:40–53. doi: 10.1016/j.nlm.2013.07.002. [DOI] [PubMed] [Google Scholar]
- Mosher KI, Wyss-Coray T. Microglial dysfunction in brain aging and Alzheimer’s disease. Biochemical Pharmacology. 2014;88:594–604. doi: 10.1016/j.bcp.2014.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudò G, Mäkelä J, Di Liberto V, Tselykh TV, Olivieri M, Piepponen P, Eriksson O, Mälkiä A, Bonomo A, Kairisalo M, Aguirre JA, Korhonen L, Belluardo N, Lindholm D. Transgenic expression and activation of PGC-1α protect dopaminergic neurons in the MPTP mouse model of Parkinson’s disease. Cellular and molecular life sciences. 2012;69:1153–1165. doi: 10.1007/s00018-011-0850-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukundan L, Odegaard JI, Morel CR, Heredia JE, Mwangi JW, Ricardo-Gonzalez RR, Goh YPS, Eagle AR, Dunn SE, Awakuni JUH, Nguyen KD, Steinman L, Michie SA, Chawla A. PPAR-delta senses and orchestrates clearance of apoptotic cells to promote tolerance. Nature Medicine. 2009;15:1266–1272. doi: 10.1038/nm.2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayak D, Roth TL, McGavern DB. Microglia development and function. Annual Review of Immunology. 2014;32:367–402. doi: 10.1146/annurev-immunol-032713-120240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nenov MN, Laezza F, Haidacher SJ, Zhao Y, Sadygov RG, Starkey JM, Spratt H, Luxon BA, Dineley KT, Denner L. Cognitive enhancing treatment with a PPARγ agonist normalizes dentate granule cell presynaptic function in Tg2576 APP mice. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2014;34:1028–1036. doi: 10.1523/JNEUROSCI.3413-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolakakis N, Aboulkassim T, Ongali B, Lecrux C, Fernandes P, Rosa-Neto P, Tong X, Hamel E. Complete rescue of cerebrovascular function in aged Alzheimer’s disease transgenic mice by antioxidants and pioglitazone, a peroxisome proliferator-activated receptor gamma agonist. The Journal of neuroscience. 2008;28:9287–9296. doi: 10.1523/JNEUROSCI.3348-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolakakis N, Hamel E. The Nuclear Receptor PPARgamma as a Therapeutic Target for Cerebrovascular and Brain Dysfunction in Alzheimer’s Disease. Frontiers in Aging Neuroscience. 2010;2 doi: 10.3389/fnagi.2010.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Núñez V, Alameda D, Rico D, Mota R, Gonzalo P, Cedenilla M, Fischer T, Boscá L, Glass CK, Arroyo AG, Ricote M. Retinoid X receptor alpha controls innate inflammatory responses through the up-regulation of chemokine expression. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:10626–10631. doi: 10.1073/pnas.0913545107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odegaard JI, Chawla A. Alternative macrophage activation and metabolism. Annual review of pathology. 2011;6:275–297. doi: 10.1146/annurev-pathol-011110-130138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odegaard JI, Chawla A. The immune system as a sensor of the metabolic state. Immunity. 2013;38:644–654. doi: 10.1016/j.immuni.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odegaard JI, Ricardo-Gonzalez RR, Goforth MH, Morel CR, Subramanian V, Mukundan L, Eagle AR, Vats D, Brombacher F, Ferrante AW, Chawla A. Macrophage-specific PPARgamma controls alternative activation and improves insulin resistance. Nature. 2007;447:1116–1120. doi: 10.1038/nature05894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Reilly J, Lynch M. Rosiglitazone improves spatial memory and decreases insoluble Aβ(1–42) in APP/PS1 mice. Journal of neuroimmune pharmacology : the official journal of the Society on NeuroImmune Pharmacology. 2012;7:140–144. doi: 10.1007/s11481-011-9282-7. [DOI] [PubMed] [Google Scholar]
- Ouk T, Potey C, Gautier S, Bastide M, Deplanque D, Staels B, Duriez P, Leys D, Bordet R. PPARs: a potential target for a disease-modifying strategy in stroke. Current drug targets. 2013;14:752–767. doi: 10.2174/1389450111314070005. [DOI] [PubMed] [Google Scholar]
- Papadopoulos P, Rosa-Neto P, Rochford J, Hamel E. Pioglitazone improves reversal learning and exerts mixed cerebrovascular effects in a mouse model of Alzheimer’s disease with combined amyloid-β and cerebrovascular pathology. PLoS ONE. 2013;8:e68612. doi: 10.1371/journal.pone.0068612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park L, Wang G, Zhou P, Zhou J, Pitstick R, Previti ML, Younkin L, Younkin SG, van Nostrand WE, Cho S, Anrather J, Carlson GA, Iadecola C. Scavenger receptor CD36 is essential for the cerebrovascular oxidative stress and neurovascular dysfunction induced by amyloid-beta. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:5063–5068. doi: 10.1073/pnas.1015413108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parra-Damas A, Valero J, Chen M, España J, Martín E, Ferrer I, Rodríguez-Alvarez J, Saura CA. Crtc1 activates a transcriptional program deregulated at early Alzheimer’s disease-related stages. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2014;34:5776–5787. doi: 10.1523/JNEUROSCI.5288-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterniti I, Esposito E, Mazzon E, Galuppo M, Di Paola R, Bramanti P, Kapoor A, Thiemermann C, Cuzzocrea S. Evidence for the role of peroxisome proliferator-activated receptor-beta/delta in the development of spinal cord injury. The Journal of pharmacology and experimental therapeutics. 2010;333:465–477. doi: 10.1124/jpet.110.165605. [DOI] [PubMed] [Google Scholar]
- Pedersen WA, McMillan PJ, Kulstad JJ, Leverenz JB, Craft S, Haynatzki GR. Rosiglitazone attenuates learning and memory deficits in Tg2576 Alzheimer mice. Experimental Neurology. 2006;199:265–273. doi: 10.1016/j.expneurol.2006.01.018. [DOI] [PubMed] [Google Scholar]
- Peters JM, Lee SS, Li W, Ward JM, Gavrilova O, Everett C, Reitman ML, Hudson LD, Gonzalez FJ. Growth, adipose, brain, and skin alterations resulting from targeted disruption of the mouse peroxisome proliferator-activated receptor beta(delta) Molecular and Cellular Biology. 2000;20:5119–5128. doi: 10.1128/mcb.20.14.5119-5128.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pialat J, Cho T, Beuf O, Joye E, Moucharrafie S, Moucharaffie S, Langlois J, Nemoz C, Janier M, Berthezene Y, Nighoghossian N, Desvergne B, Wiart M. MRI monitoring of focal cerebral ischemia in peroxisome proliferator-activated receptor (PPAR)-deficient mice. NMR in biomedicine. 2007;20:335–342. doi: 10.1002/nbm.1157. [DOI] [PubMed] [Google Scholar]
- Polak PE, Kalinin S, Russo CD, Gavrilyuk V, Sharp A, Peters JM, Richardson J, Willson TM, Weinberg G, Feinstein DL. Protective effects of a peroxisome proliferator-activated receptor-beta/delta agonist in experimental autoimmune encephalomyelitis. Journal of neuroimmunology. 2005;168:65–75. doi: 10.1016/j.jneuroim.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Price AR, Xu G, Siemienski ZB, Smithson LA, Borchelt DR, Golde TE, Felsenstein KM. Comment on “ApoE-directed therapeutics rapidly clear β-amyloid and reverse deficits in AD mouse models”. Science (New York, N.Y.) 2013;340:924. doi: 10.1126/science.1234089. [DOI] [PubMed] [Google Scholar]
- Quinn LP, Crook B, Hows ME, Vidgeon-Hart M, Chapman H, Upton N, Medhurst AD, Virley DJ. The PPARgamma agonist pioglitazone is effective in the MPTP mouse model of Parkinson’s disease through inhibition of monoamine oxidase B. British Journal of Pharmacology. 2008;154:226–233. doi: 10.1038/bjp.2008.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddell DR, et al. The LXR agonist TO901317 selectively lowers hippocampal Abeta42 and improves memory in the Tg2576 mouse model of Alzheimer’s disease. Molecular and cellular neurosciences. 2007;34:621–628. doi: 10.1016/j.mcn.2007.01.011. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Rivera J, Denner L, Dineley KT. Rosiglitazone reversal of Tg2576 cognitive deficits is independent of peripheral gluco-regulatory status. Behavioural brain research. 2011;216:255–261. doi: 10.1016/j.bbr.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rőszer T, Menéndez-Gutiérrez MP, Cedenilla M, Ricote M. Retinoid X receptors in macrophage biology. Trends in Endocrinology & Metabolism. 2013;24:460–468. doi: 10.1016/j.tem.2013.04.004. [DOI] [PubMed] [Google Scholar]
- Sacchetti P, Carpentier R, Ségard P, Olivé-Cren C, Lefebvre P. Multiple signaling pathways regulate the transcriptional activity of the orphan nuclear receptor NURR1. Nucleic acids research. 2006;34:5515–5527. doi: 10.1093/nar/gkl712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacchetti P, Sousa KM, Hall AC, Liste I, Steffensen KR, Theofilopoulos S, Parish CL, Hazenberg C, Richter LA, Hovatta O, Gustafsson J, Arenas E, Richter LÄ, Gustafsson J. Liver X Receptors and Oxysterols Promote Ventral Midbrain Neurogenesis In Vivo and in Human Embryonic Stem Cells. Cell stem cell. 2009;5:409–419. doi: 10.1016/j.stem.2009.08.019. [DOI] [PubMed] [Google Scholar]
- Sadeghian M, Marinova-Mutafchieva L, Broom L, Davis JB, Virley D, Medhurst AD, Dexter DT. Full and partial peroxisome proliferation-activated receptor-γ agonists, but not δ agonist, rescue of dopaminergic neurons in the 6-OHDA parkinsonian model is associated with inhibition of microglial activation and MMP expression. Journal of neuroimmunology. 2012;246:69–77. doi: 10.1016/j.jneuroim.2012.03.010. [DOI] [PubMed] [Google Scholar]
- Saijo K, Crotti A, Glass CK. Regulation of microglia activation and deactivation by nuclear receptors. Glia. 2013;61:104–111. doi: 10.1002/glia.22423. [DOI] [PubMed] [Google Scholar]
- Saijo K, Winner B, Carson CT, Collier JG, Boyer L, Rosenfeld MG, Gage FH, Glass CK. A Nurr1/CoREST pathway in microglia and astrocytes protects dopaminergic neurons from inflammation-induced death. Cell. 2009;137:47–59. doi: 10.1016/j.cell.2009.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sastre M, Dewachter I, Rossner S, Bogdanovic N, Rosen E, Borghgraef P, Evert BO, Dumitrescu-Ozimek L, Thal DR, Landreth G, Walter J, Klockgether T, van Leuven F, Heneka MT. Nonsteroidal anti-inflammatory drugs repress beta-secretase gene promoter activity by the activation of PPARgamma. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:443–448. doi: 10.1073/pnas.0503839103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saucedo-Cardenas O, Conneely OM. Comparative distribution of NURR1 and NUR77 nuclear receptors in the mouse central nervous system. Journal of molecular neuroscience : MN. 1996;7:51–63. doi: 10.1007/BF02736848. [DOI] [PubMed] [Google Scholar]
- Schintu N, Frau L, Ibba M, Caboni P, Garau A, Carboni E, Carta AR. PPAR-gamma-mediated neuroprotection in a chronic mouse model of Parkinson’s disease. The European journal of neuroscience. 2009;29:954–963. doi: 10.1111/j.1460-9568.2009.06657.x. [DOI] [PubMed] [Google Scholar]
- Schütz B, Reimann J, Dumitrescu-Ozimek L, Kappes-Horn K, Landreth GE, Schürmann B, Zimmer A, Heneka MT. The oral antidiabetic pioglitazone protects from neurodegeneration and amyotrophic lateral sclerosis-like symptoms in superoxide dismutase-G93A transgenic mice. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2005;25:7805–7812. doi: 10.1523/JNEUROSCI.2038-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Searcy JL, Phelps JT, Pancani T, Kadish I, Popovic J, Anderson KL, Beckett TL, Murphy MP, Chen K, Blalock EM, L PW, Landfield PW, field Porter NM, Thibault O. Long-term pioglitazone treatment improves learning and attenuates pathological markers in a mouse model of Alzheimer’s disease. J. Alzheimers Dis. 2012;30:943–961. doi: 10.3233/JAD-2012-111661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semple BD, Noble-Haeusslein LJ. Broad-spectrum neuroprotection against traumatic brain injury by agonism of peroxisome proliferator-activated receptors. Experimental Neurology. 2011;229:195–197. doi: 10.1016/j.expneurol.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata N, Kawaguchi-Niida M, Yamamoto T, Toi S, Hirano A, Kobayashi M. Effects of the PPARgamma activator pioglitazone on p38 MAP kinase and IkappaBalpha in the spinal cord of a transgenic mouse model of amyotrophic lateral sclerosis. Neuropathology : official journal of the Japanese Society of Neuropathology. 2008;28:387–398. doi: 10.1111/j.1440-1789.2008.00890.x. [DOI] [PubMed] [Google Scholar]
- Shin J, Ko HS, Kang H, Lee Y, Lee Y, Pletinkova O, Troconso JC, Dawson VL, Dawson TM. PARIS (ZNF746) repression of PGC-1α contributes to neurodegeneration in Parkinson’s disease. Cell. 2011;144:689–702. doi: 10.1016/j.cell.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirin O, Lukov GL, Mao R, Conneely OM, Goodell MA. The orphan nuclear receptor Nurr1 restricts the proliferation of haematopoietic stem cells. Nature cell biology. 2010;12:1213–1219. doi: 10.1038/ncb2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SA, Monteith GR, Robinson JA, Venkata NG, May FJ, Roberts-Thomson SJ. Effect of the peroxisome proliferator-activated receptor beta activator GW0742 in rat cultured cerebellar granule neurons. Journal of Neuroscience Research. 2004;77:240–249. doi: 10.1002/jnr.20153. [DOI] [PubMed] [Google Scholar]
- Sodhi RK, Singh N, Jaggi AS. Neuroprotective mechanisms of peroxisome proliferator-activated receptor agonists in Alzheimer’s disease. Naunyn-Schmiedeberg’s archives of pharmacology. 2011;384:115–124. doi: 10.1007/s00210-011-0654-6. [DOI] [PubMed] [Google Scholar]
- Swanson CR, Eric du, Johnson DA, Johnson JA, Emborg ME. Neuroprotective Properties of a Novel Non-Thiazoledinedione Partial PPAR-γ Agonist against MPTP. PPAR research. 2013;2013:582809. doi: 10.1155/2013/582809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson CR, Joers V, Bondarenko V, Brunner K, Simmons HA, Ziegler TE, Kemnitz JW, Johnson JA, Emborg ME. The PPAR-γ agonist pioglitazone modulates inflammation and induces neuroprotection in parkinsonian monkeys. J Neuroinflammation. 2011;8:91. doi: 10.1186/1742-2094-8-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Széles L, Póliska S, Nagy G, Szatmari I, Szanto A, Pap A, Lindstedt M, Santegoets SJAM, Rühl R, Dezsö B, Nagy L. Research resource: transcriptome profiling of genes regulated by RXR and its permissive and nonpermissive partners in differentiating monocyte-derived dendritic cells. Molecular endocrinology (Baltimore, Md) 2010;24:2218–2231. doi: 10.1210/me.2010-0215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terwel D, Steffensen KR, Verghese PB, Kummer MP, Gustafsson J, Holtzman DM, Heneka MT. Critical role of astroglial apolipoprotein E and liver X receptor-α expression for microglial Aβ phagocytosis. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:7049–7059. doi: 10.1523/JNEUROSCI.6546-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesseur I, Lo AC, Roberfroid A, Dietvorst S, van Broeck B, Borgers M, Gijsen H, Moechars D, Mercken M, Kemp J, D’Hooge R, de Strooper B. Comment on “ApoE-directed therapeutics rapidly clear β-amyloid and reverse deficits in AD mouse models”. Science (New York, N.Y.) 2013;340:924. doi: 10.1126/science.1233937. [DOI] [PubMed] [Google Scholar]
- Theofilopoulos S, et al. Brain endogenous liver X receptor ligands selectively promote midbrain neurogenesis. Nature Chemical Biology. 2012;9:126–133. doi: 10.1038/nchembio.1156. [DOI] [PubMed] [Google Scholar]
- Toledo EM, Inestrosa NC. Activation of Wnt signaling by lithium and rosiglitazone reduced spatial memory impairment and neurodegeneration in brains of an APPswe/PSEN1ΔE9 mouse model of Alzheimer’s disease. Molecular Psychiatry. 2009;15:272–285. doi: 10.1038/mp.2009.72. [DOI] [PubMed] [Google Scholar]
- Ulrich JD, Burchett JM, Restivo JL, Schuler DR, Verghese PB, Mahan TE, Landreth GE, Castellano JM, Jiang H, Cirrito JR, Holtzman DM. In vivo measurement of apolipoprotein E from the brain interstitial fluid using microdialysis. Molecular Neurodegeneration. 2013;8:13. doi: 10.1186/1750-1326-8-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulusoy GK, Celik T, Kayir H, Gürsoy M, Isik AT, Uzbay TI. Effects of pioglitazone and retinoic acid in a rotenone model of Parkinson’s disease. Brain research bulletin. 2011;85:380–384. doi: 10.1016/j.brainresbull.2011.05.001. [DOI] [PubMed] [Google Scholar]
- Uppalapati D, R das N, Gangwal RP, Damre MV, Sangamwar AT, Sharma SS. Neuroprotective Potential of Peroxisome Proliferator Activated Receptor-α Agonist in Cognitive Impairment in Parkinson’s Disease: Behavioral, Biochemical, and PBPK Profile. PPAR research. 2014;2014:753587. doi: 10.1155/2014/753587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanmierlo T, Rutten K, Dederen J, Bloks VW, van der Zee LCV, Kuipers F, Kiliaan A, Blokland A, Sijbrands EJG, Steinbusch H, Prickaerts J, Lütjohann D, Mulder M. Liver X receptor activation restores memory in aged AD mice without reducing amyloid. Neurobiol. Aging. 2011;32:1262–1272. doi: 10.1016/j.neurobiolaging.2009.07.005. [DOI] [PubMed] [Google Scholar]
- Veeraraghavalu K, Zhang C, Miller S, Hefendehl JK, Rajapaksha TW, Ulrich J, Jucker M, Holtzman DM, Tanzi RE, Vassar R, Sisodia SS. Comment on “ApoE-directed therapeutics rapidly clear β-amyloid and reverse deficits in AD mouse models”. Science (New York, N.Y.) 2013;340:924. doi: 10.1126/science.1235505. [DOI] [PubMed] [Google Scholar]
- Volakakis N, Joodmardi E, Perlmann T. NR4A orphan nuclear receptors influence retinoic acid and docosahexaenoic acid signaling via up-regulation of fatty acid binding protein 5. Biochemical and Biophysical Research Communications. 2009;390:1186–1191. doi: 10.1016/j.bbrc.2009.10.116. [DOI] [PubMed] [Google Scholar]
- Volakakis N, Kadkhodaei B, Joodmardi E, Wallis K, Panman L, Silvaggi J, Spiegelman BM, Perlmann T. NR4A orphan nuclear receptors as mediators of CREB-dependent neuroprotection. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:12317–12322. doi: 10.1073/pnas.1007088107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Schuster GU, Hultenby K, Zhang QS, Andersson S, Andersson R, Gustafsson J. Liver X receptors in the central nervous system: from lipid homeostasis to neuronal degeneration. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:13878–13883. doi: 10.1073/pnas.172510899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesson DW, Borkowski AH, Landreth GE, Nixon RA, Levy E, Wilson DA. Sensory network dysfunction, behavioral impairments, and their reversibility in an Alzheimer’s β-amyloidosis mouse model. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2011;31:15962–15971. doi: 10.1523/JNEUROSCI.2085-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitney KD, Watson MA, Collins JL, Benson WG, Stone TM, Numerick MJ, Tippin TK, Wilson JG, Winegar DA, Kliewer SA. Regulation of cholesterol homeostasis by the liver X receptors in the central nervous system. Molecular endocrinology (Baltimore, Md) 2002;16:1378–1385. doi: 10.1210/mend.16.6.0835. [DOI] [PubMed] [Google Scholar]
- Xiao Y, Xu J, Wang S, Mao C, Jin M, Ning G, Xu J, Zhang Y. Genetic ablation of steroid receptor coactivator-3 promotes PPAR-beta-mediated alternative activation of microglia in experimental autoimmune encephalomyelitis. Glia. 2010;58:932–942. doi: 10.1002/glia.20975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka M, Ishikawa T, Griep A, Axt D, Kummer MP, Heneka MT. PPARγ/RXRα-induced and CD36-mediated microglial amyloid-β phagocytosis results in cognitive improvement in amyloid precursor protein/presenilin 1 mice. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32:17321–17331. doi: 10.1523/JNEUROSCI.1569-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Q, Zhang J, Liu H, Babu-Khan S, Vassar R, Biere AL, Citron M, Landreth G. Anti-inflammatory drug therapy alters beta-amyloid processing and deposition in an animal model of Alzheimer’s disease. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2003;23:7504–7509. doi: 10.1523/JNEUROSCI.23-20-07504.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D, Li T, Wang Y, Tang Y, Cui H, Tang Y, Zhang X, Chen D, Shen N, Le Weidong miR-132 regulates the differentiation of dopamine neurons by directly targeting Nurr1 expression. Journal of cell science. 2012;125:1673–1682. doi: 10.1242/jcs.086421. [DOI] [PubMed] [Google Scholar]
- Yin K, Deng Z, Hamblin M, Xiang Y, Huang H, Zhang J, Jiang X, Wang Y, Chen YE. Peroxisome proliferator-activated receptor delta regulation of miR-15a in ischemia-induced cerebral vascular endothelial injury. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30:6398–6408. doi: 10.1523/JNEUROSCI.0780-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin K, Deng Z, Hamblin M, Zhang J, Chen YE. Vascular PPARδ protects against stroke-induced brain injury. Arteriosclerosis, Thrombosis, and Vascular Biology. 2011;31:574–581. doi: 10.1161/ATVBAHA.110.221267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelcer N, Khanlou N, Clare R, Jiang Q, Reed-Geaghan EG, Landreth GE, Vinters HV, Tontonoz P. Attenuation of neuroinflammation and Alzheimer’s disease pathology by liver x receptors. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:10601–10606. doi: 10.1073/pnas.0701096104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelcer N, Tontonoz P. Liver X receptors as integrators of metabolic and inflammatory signaling. The Journal of clinical investigation. 2006;116:607–614. doi: 10.1172/JCI27883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zetterström RH, Williams R, Perlmann T, Olson L. Cellular expression of the immediate early transcription factors Nurr1 and NGFI-B suggests a gene regulatory role in several brain regions including the nigrostriatal dopamine system. Brain research. Molecular brain research. 1996;41:111–120. doi: 10.1016/0169-328x(96)00074-5. [DOI] [PubMed] [Google Scholar]
- Zhang-Gandhi CX, hi, Drew PD. Liver X receptor and retinoid X receptor agonists inhibit inflammatory responses of microglia and astrocytes. Journal of neuroimmunology. 2007;183:50–59. doi: 10.1016/j.jneuroim.2006.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlokovic BV. Cerebrovascular effects of apolipoprotein E: implications for Alzheimer disease. JAMA Neurology. 2013;70:440–444. doi: 10.1001/jamaneurol.2013.2152. [DOI] [PMC free article] [PubMed] [Google Scholar]