Abstract
Purpose
The metabolic syndrome (MetS) is associated with a three-fold increase risk of cardiovascular (CV) morbidity and mortality, which is in part, due to a blunted CV reserve capacity, reflected by a reduced peak exercise left ventricular contractility and aerobic capacity, and a blunted peak arterial-ventricular coupling. To date, no study has examined whether aerobic exercise training in MetS can reverse the peak exercise CV dysfunction. Further, examining how exercise training alters CV function in a group of individuals with MetS prior to the development of diabetes and/or overt CVD, can provide insights into whether some of the pathophysiological changes to the CV can be delayed/reversed, lowering their CV risk. The objective of this study was to examine the effects of 8 weeks of aerobic exercise training in individuals with MetS on resting and peak exercise CV function.
Methods
Twenty MetS underwent either 8 weeks of aerobic exercise training (MetS-ExT; n=10) or remained sedentary (MetS-NonT; n=10) during this time period. Resting and peak exercise CV function was characterized using Doppler echocardiography and gas exchange.
Results
Exercise training did not alter resting left ventricular diastolic or systolic function and arterial-ventricular coupling in MetS. In contrast, at peak exercise an increase in LV contractility (40%, p<0.01), cardiac output (28%, p<0.05) and aerobic capacity (20%, p<0.01), while a reduction in vascular resistance (30%, p<0.05) and arterial-ventricular coupling (27%, p<0.01), were noted in the MetS-ExT but not the MetS-NonT group. Further, an improvement in Lifetime Risk Score was also noted in the MetS-ExT group.
Conclusions
These findings have clinical importance as they provide insight that some of the pathophysiological changes associated with MetS can be improved and lower the risk of CVD.
Keywords: cardiovascular disease, cardiac function, arterial function, obesity
Introduction
The metabolic syndrome (MetS) is a cluster of metabolic risk factors (abdominal obesity, elevated blood pressure and glucose, and dyslipidemia) that exerts a three-fold increased risk of cardiovascular disease (CVD) mortality compared to non-MetS individuals (26). Alarmingly, the prevalence of MetS in US adults is 34% and is on the rise due, in part, to rising rates of obesity (29). MetS has become a leading health concern because of its strong association with future myocardial/cerebral vascular events and CVD mortality (26).
The MetS-associated pathophysiological changes to the CV system contribute to the increased CVD risk. Such changes include an increase in arterial dysfunction (increased arterial stiffness, and endothelial dysfunction), left ventricular (LV) hypertrophy, and impaired CV reserve capacity (15, 33, 35). In particular, individuals with MetS demonstrate an impaired coupling between the heart and arterial system (15). This interaction, termed arterial-ventricular coupling (Ea/Ees), is an important determinant of net CV performance (9) and cardiac energetics (10). Ea/Ees is indexed by the ratio of effective arterial elastance (Ea; a measure of the net arterial load imposed on the left ventricle) to left ventricular end-systolic elastance (Ees; measure of LV chamber performance) (9). Impaired Ea/Ees is most readily evident when the CV system is under stress and/or with increasing age (31). For example, in MetS, the ability to reduce the Ea/Ees ratio is blunted during aerobic exercise (15). Further, this impaired coupling response coincides with a reduced peak aerobic capacity in MetS (15), which has been proposed to be an independent predictor of mortality relative to other risk factors (30).
In healthy older individuals, exercise training has been shown to increase aerobic capacity, improve peak LV performance (smaller end-systolic volume and increased stroke volume), and increase LV contractility (13, 37). In patients with coronary artery disease, Ea/Ees during handgrip isometric exercise was improved after exercise training. It is unknown whether exercise training in people with MetS (prior to the development of diabetes or overt CVD) can improve peak exercise LV-arterial coupling and CV function. An improvement in Ea/Ees and CV function in MetS would likely reflect a lower CVD risk. Thus, the objective of this study was to examine the effects of 8 weeks of exercise training on peak exercise arterial-ventricular coupling in clinically defined MetS patients without diabetes or clinical indications of CVD. We hypothesized that aerobic exercise training would improve peak Ea/Ees in MetS due to an improvement in peak exercise LV contractility.
Methods
Study Population
Twenty MetS subjects free from overt CVD and diabetes as determined by a detailed medical history, physical examination, and a normal resting and exercise electrocardiogram participated in this study. MetS was defined according to the updated National Cholesterol Education Program: Adult Treatment Panel III comprised of three out of the following five components: 1) obesity (waist men >102 cm, women >88 cm); 2) low HDL cholesterol (men<40 mg/dL; women<50 mg/dL); 3) hypertriglyceridemia (≥150 mg/dL); 4) elevated glucose (≥100 mg/dL & <126 mg/dL), and; 5) elevated BP (130/85 mmHg or use of hypertensive medications).
Exclusion criteria included diabetes mellitus (HbA1c ≥6.5 % or use of diabetic medications), pulmonary disease, angina, atrial fibrillation, aortic stenosis, anemia, myocardial infarction, stroke, or coronary revascularization as assessed by a detailed medical history physical examination and a resting and exercise electrocardiogram. Subjects who participated in regular exercise, defined as >30 minutes, 3 times/week were excluded. All subjects provided written informed consent to participate, that was approved by WVU Institutional Review Board.
Study Design
Physiological assessments were performed between 7:00–10:00 AM, in a quiet, temperature-controlled room, after a 12 hour fast and abstinence from alcohol, caffeine, and vitamins. CV medications were withheld 24 hours prior to assessments. After a minimum 15 minutes of quiet rest subjects underwent resting non-invasive assessments of arterial and cardiac structure and function.
Exercise Performance
Following assessment of resting CV measurement, subjects exercised to exhaustion on a modified cycle ergometer (Monarch 827, Sweden) equipped with a car seat to allow semi-recumbent exercise in seated upright position at approximately 130 degrees. This upright position is necessary and important to allow acquisition of optimal echocardiographic images during exercise. Throughout exercise the echo images were acquired approximately 60–90 seconds into each 3-minute exercise workload. If all images were not acquired within the time frame the duration of the exercise stage was extended to acquire those images. Pedal speed was maintained at 50 rpm, and workloads increased by 25 W every 3 minutes until exhaustion. Oxygen consumed (VO2), carbon dioxide produced (VCO2), respiratory exchange ratio (RER=VCO2/VO2), ventilation (Ve), ventilatory threshold (VT), and the ventilatory equivalent for CO2 (Ve/VCO2) were measured throughout exercise using a metabolic cart (TureOne 2400, ParvoMedics, Sandy, UT). Subjective symptoms of fatigue (BORG score 6 to 20), and blood pressure (sphygomomanometry) were recorded at the end of each workload.
Rest and Exercise Echocardiography
Echocardiograms were obtained using a GE Vivid i (GE Healthcare, Chalfont St. Giles, United Kingdom), portable ultrasound imaging system with a 5S-RS (2.0–5.0 MHz) Wideband Phased Array transducer. All echocardiograms were performed by experienced registered diagnostic cardiac sonographers. At rest, standard 2-dimensional images were obtained in the following acoustic views; parasternal long axis, and apical 4 chamber view. Pulsed wave Doppler tracings of the mitral valve inflow velocity (recorded at the leaflet’s tips) were recorded in the apical 4 chamber view. Continuous/pulse wave Doppler tracings of the LV outflow track velocity were obtained in the apical 5 chamber view positioned 5 mm proximal to the aortic valve. Spectral tissue Doppler imaging was performed in the apical 4 chamber view with the gate sample positioned in the lateral corner and septal side of the mitral annulus. During exercise, the sonographer quickly acquired a 2-dimensional image of the parasternal long axis view to obtain the size of the LV outflow tract diameter (base of the aortic leaflets). The sonographer then focused on capturing: 4-chamber views to obtain cardiac volumes and mitral flow velocities; and 5-chamber views to obtain pulse-and continuous-wave Doppler-flow from the LV outflow track.
Left Ventricular Geometry and Remodeling
In the supine position, LV dimensions, wall thickness, and chamber volumes were determined in triplicate from 2-dimensional, M-mode, and Doppler spectra echocardiography using standard methods (25). Sex-specific LV hypertrophy (LVH) and geometry patterns, based on LV mass index and relative wall thickness were defined as LV mass index >95 g/m2 for women or >115 g/m2 for men, and LV geometry was classified as normal, concentric remodeling, concentric LVH, or eccentric LVH (25).
Arterial-Ventricular Measurements
In the upright seated rest position and during exercise, LV end diastolic (EDV) and end-systolic (ESV) volumes, along with ejection fraction (EF) were determined from Simpson’s biplane method (25). Cardiac volumes were normalized to body surface area (BSA). Cardiac index (Ci) was determined from the product of heart rate (HR) and stroke volume index. Peak arteriovenous oxygen extraction was calculated from the Fick equation (VO2peak/cardiac output). Systemic vascular resistance index (SVRi) was calculated as mean arterial pressure (MAP) × 80/Ci. The indexes of arterial and ventricular elastance were calculated as; 1) arterial elastance (Ea) a measure of the net arterial load, was calculated as end-systolic pressure (ESP)/stroke volume (SV), where ESP is approximated as 0.9 × systolic blood pressure (SBP)(9). Of note, ESP calculated as 0.9 × SBP has previously been shown to closely approximate central ESP (21); 2) LV end-systolic elastance (Ees [calculated from BP, stroke volume, EF, and pre-ejection and systolic ejection time intervals from LV outflow Doppler]), was determined by the validated single-beat technique (12); and 3) Arterial ventricular coupling ratio was determined from Ea/Ees (9). Of note, Ea/Ees is mathematically related to EF via the formula Ea/Ees = (1/EF)−1. Reserve was defined as the difference in these variables between seated rest and peak exercise.
Diastolic Function
In the supine position, the medial mitral annular early diastolic velocity (e′) was determined by spectral tissue Doppler imaging (GE Vivid i) using standard methods. Early (E-wave) and late (A-wave) transmitral flow velocities, the isovolumetric relaxation time (IVRT), and the deceleration time of early filling velocity (Dec T) were measured by pulsed-wave Doppler (GE Vivid i). End-diastolic pressure was estimated as EDP = 11.96 + 0.596 × E/e′ (32). Because of the high incidence of fusion of the E- and A-wave during moderate/high intensity exercise, with the A-wave dominating, we are not able to measure Dec T- or E-wave. Therefore we were limited to measuring peak exercise IVRT and A-wave.
Body Anthropometry
Height, weight, and waist and hip circumference were measured using standard laboratory procedures. Fat distribution was assessed by measuring the waist circumference at the site of the smallest circumference between the rib cage and the iliac crest with subjects in a standing position. Hip circumference was measured at the site of the largest circumference between waist and thighs. Lean body mass and fat mass were measured using air displacement plethysmography (BodPod, COSMED Inc., USA). During assessment of body composition subjects wore close-fitting bathing suits and a swim cap. Body mass index (BMI) was calculated as weight (kg) divided by height (m) squared.
Blood Analyses
Venous blood sampling was obtained in the morning after a 12-hour overnight fast. Post training venous samples were collected at least 48 hours after the last exercise session. Plasma obtained from blood sampling was analyzed at West Virginia University Hospital’s central laboratory in Morgantown, West Virginia. Total cholesterol, high-density lipoprotein (HDL) cholesterol, triglycerides, and glucose were determined in plasma (lithium heparin, Becton Dickenson Plasma Separator Tubes) using Beckman-Coulter (CA, USA) DxC automated chemistry analyzers. Total cholesterol was measured using a cholesterol esterase/cholesterol oxidase/peroxidase-driven, timed-endpoint method (Coefficients of Variation <7%). HDL cholesterol was measured using a cholesterol esterase/cholesterol oxidase/peroxidase-driven, timed endpoint assay with automated initial homogeneous solubilization step (Coefficients of Variation <7%). Triglycerides were measured using a lipase/glycerol kinase/glycerophosphate oxidase/horseradish peroxidase-driven, timed-endpoint method (Coefficients of Variation 5–7%). Glucose was measured electrochemically using a glucose oxidase/catalase/molybdate-driven oxygen rate method (Coefficients of Variation <5%). Glycated hemoglobin (A1c fraction) was measured in whole blood (K2-EDTA, Becton Dickenson) using a BioRad (Hercules, CA, USA) Variant II Turbo High Performance Liquid Chromatography (HPLC) system (Coefficients of Variation <2%). Insulin was measured in serum (untreated/red-top tube, Becton Dickenson) on an Immulite 2000 immunochemistry system (Siemens, USA; Coefficients of Variation <10%). Homeostasis model assessment of insulin resistance (HOMA-IR) was estimated with the following formula: insulin resistance = fasting plasma insulin (in microunits per millileter, μU/mL) × fasting plasma glucose (FPG, in millimoles per liter, mmol/L) / 22.5.
Training Intervention
MetS were assigned into either an 8 week aerobic exercise intervention group (MetS-ExT) or an 8 week non-exercise control (MetS-NonT) group. Group assignments were made using a pseudo-random balance approach to ensure equal numbers enrolled in each group. The MetS-NonT exercise group (n=10; 50% female) were asked to maintain their normal sedentary lifestyle. The MetS-ExT group (n=10; 70% female) performed 8 weeks of supervised aerobic exercise in the Human Performance Lab at West Virginia University School of Medicine, 3 times per week for 60 minutes at a fixed exercise intensity. The intensity of prescribed exercise was based on individual results of maximal cardiopulmonary exercise tests. We used a ramp exercise protocol, whereby exercise training intensity started at 60% of heart rate reserve (heart rate range determined during exercise stress test) and increased every 2 weeks by 10%; from weeks 6–8 heart rate reserve was set at 85%. Adherence to the exercise prescription was documented through the use of wrist watch-style heart rate monitors (E600, Polar Electro OY, Oulu, Finland) and physical activity logs. Approved modalities included treadmills, elliptical machines, and cycle ergometers. All participants were instructed to maintain current eating behaviors for the duration of the 8-week intervention. All post-training measurements were performed 24–48 hours after the last exercise training session to avoid the immediate effects of a single bout of exercise. Measurements made before and after exercise training were obtained at the same time of day for each subject.
Lifetime Risk Score for CVD
The Lifetime Risk Score is a strong predictive capacity for future CV mortality and is based on an algorithm that incorporates sex (male/female), age (years), SBP (mmHg), diabetes mellitus (yes/no), total cholesterol (mg/dL), smoking (yes/no), BMI (kg/m2), and physical fitness (Metabolic equivalent; 1 MET = 3.5 ml/kg/min) (3, 4). Calculation of the score is available using a web-based interface (www.lifetimerisk.org).
Statistical analysis
Measurements of CV function were performed offline by a single investigator who was blinded to group allocation. The intra-class correlation coefficient (ICC) and coefficient of variations for all echocardiographic variables were derived in a subset of subjects (n=8). At rest, the ICC and the coefficient of variation for all variables, collected on two separate days, was >0.80 or between 7–12%, respectively. Similar results were obtained for echocardographic variables evaluated during peak exercise with all variables having an ICC>0.80 and coefficient of variation between 7–12% with the exception of the arterial-ventricular coupling ratio (ICC=0.63).
Normality was evaluated by the Kolmogorov-Smirnov test. Continuous variables were log transformed as necessary. To evaluate the effects of exercise training, paired t-tests and two-way repeated-measures ANOVA were used. We also ran a mixed effects models with a time-varying covariate to examine whether the change (pre vs post training intervention) in CV parameters where due to changes other CV parameters. All analyses were performed with the statistical package SPSS version 21 (SPSS, Chicago, IL). Values shown in the tables represent means ± SEM unless otherwise stated. A p ≤0.05 was defined as significant.
Results
Age, anthropometric, and metabolic characteristics of MetS individuals are shown in Table 1. The breakdown of metabolic components were: 100% had a waist circumference >102 cm (men) or >88 cm (women); 75% had elevated BP (130/85 mmHg or use of hypertensive medications); 70% had a low HDL cholesterol (men<40 mg/dL; women<50 mg/dL); 30% had hyper-triglyceridemia (≥150 mg/dL); and 50% had elevated glucose (≥100 mg/dL). In terms of LV remodeling, 15% had normal LV geometry and concentric remodeling, 50% had eccentric LV hypertrophy, and 20% had concentric LV hypertrophy.
Table 1.
Effects of exercise training on body composition and metabolic biomarkers in MetS
| MetS non-trained (n=10) | MetS trained (n=10) | |||
|---|---|---|---|---|
| Pre | Post | Pre | Post | |
| Age, years | 43 ± 3 | 48 ± 3 | ||
| Sex, female % | 60 | 70 | ||
| Height, cm | 171 ± 3 | 168 ± 3 | ||
| Weight, kg | 99 ± 7 | 99 ± 7 | 105 ± 6 | 105 ± 6 |
| Lean Mass, kg | 64 ± 4 | 64 ± 4 | 59 ± 5 | 59 ± 2 |
| Body fat, % | 35 ± 2 | 35 ± 3 | 43 ± 3† | 43 ± 2† |
| BSA, m2 | 2.10 ± 0.08 | 2.10 ± 0.09 | 2.13 ± 0.07 | 2.12 ± 0.07 |
| BMI, kg/m2 | 34 ± 2 | 34 ± 2 | 37 ± 2 | 37 ± 2 |
| Waist circumference, cm | 104 ± 3 | 103 ± 5 | 124 ± 10 | 122 ± 10 |
| Triglycerides, mg/dL | 149 ± 21 | 169 ± 20 | 116 ± 17 | 140 ± 24 |
| HDL, mg/dL | 41 ± 4 | 40 ± 2 | 47 ± 5 | 45 ± 4 |
| Glucose, mg/dL | 98 ± 2 | 97 ± 3 | 99 ± 3 | 97 ± 3 |
| HbA1c, % | 5.7 ± 0.1 | 5.5 ± 0.1 | 5.7 ± 0.1 | 5.6 ± 0.1 |
| Insulin, μIU/mL | 10.4 ± 2.4 | 10.7 ± 2.2 | 9.0 ± 1.5 | 9.0 ± 1.8 |
| HOMA-IR | 1.34 ± 0.30 | 1.27 ± 0.30 | 1.23 ± 0.19 | 1.45 ± 0.34 |
| Hypertensive (>140/90) % | 70 | 60 | ||
| Diabetes Mellitus % | 0 | 0 | ||
| Medications, % | ||||
| Hypertension | 20 | 30 | ||
| Cholesterol | 0 | 10 | ||
Values are mean ± sem; BSA: body surface area; BMI: body mass index; HDL: high density lipoprotein; HbA1c: hemoglobin A1c; HOMA-IR: homeostatic model assessment of insulin resistance.
p<0.05 vs, MetS non-trained group at specific visit (i.e., Pre or Post)
Of note no significant differences were found compare pre and post values within a group
Effects of exercise training on metabolic profile, body composition, and LV geometry
Eight weeks of exercise training did not alter basal fasting HbA1C, glucose, insulin, HDL, triglycerides, or HOMA-IR (Table 1). Further, exercise training was insufficient to significantly alter body composition (weight, lean mass or percent body fat), LV mass (in absolute terms and relative to BSA), the internal LV dimensions (internal diameter, septal and posterior wall thickness), and relative wall thickness in MetS (Table 2).
Table 2.
Effects of exercise training on supine left ventricular geometry and diastolic function in MetS
| MetS non-trained (n=10) | MetS trained (n=10) | |||
|---|---|---|---|---|
| Pre | Post | Pre | Post | |
| LV geometry | ||||
| Septal wall thickness, cm | 0.95 ± 0.05 | 0.96 ± 0.06 | 0.92 ± 0.06 | 0.92 ± 0.05 |
| Posterior wall thickness, cm | 0.87 ± 0.06 | 0.89 ± 0.06 | 0.86 ± 0.07 | 0.93 ± 0.06 |
| LV internal dimension, cm | 4.41 ± 0.13 | 4.38 ± 0.16 | 4.73 ± 0.16 | 4.67 ± 0.12 |
| LV Mass, g | 161 ± 8 | 164 ± 11 | 181 ± 21 | 187 ± 16 |
| LV Mass Index, g/m2 | 77 ± 3 | 78 ± 3 | 84 ± 9 | 88 ± 6 |
| Relative Wall Thickness | 0.40 ± 0.03 | 0.42 ± 0.04 | 0.36 ± 0.03 | 0.40 ± 0.02 |
| LV diastolic function | ||||
| E, m/s | 0.75 ± 0.03 | 0.75 ± 0.04 | 0.88 ± 0.05 | 0.92 ± 0.06 |
| A, m/s | 0.64 ± 0.03 | 0.60 ± 0.04 | 0.77 ± 0.07 | 0.74 ± 0.07 |
| E/A ratio | 1.21 ± 0.07 | 1.29 ± 0.07 | 1.24 ± 0.14 | 1.32 ± 0.13 |
| IVRT, m/s | 76 ± 5 | 71 ± 5 | 75 ± 8 | 61 ± 5 |
| Dec T, m/s | 203 ± 12 | 204 ± 14 | 193 ± 9 | 175 ± 8 |
| e′, m/s | 0.12 ± 0.01 | 0.13 ± 0.01 | 0.11 ± 0.01 | 0.11 ± 0.01 |
| E/e′ | 6.20 ± 0.26 | 6.21 ± 0.46 | 9.28 ± 1.05 | 9.13 ± 1.18 |
| LV EDP, mmHg | 16 ± 1 | 16 ± 1 | 18 ± 1 | 17 ± 1 |
Values are mean ± SEM; E: peak velocity of the early diastolic mitral flow; A: peak velocity of the late diastolic mitral flow; E/A: E divided by A; IVRT: isovolumetric relaxation time; Dec T: mitral flow deceleration time of early filling velocity; e′: mitral annular early diastolic velocity; E/e′: E divided by e′; LV EDP: left ventricular end-diastolic pressure. Of note no significant differences were found compare pre and post values within a group
Effects of exercise training on LV diastolic function
Resting LV diastolic function (i.e., E-wave, A-wave, E/A ratio, IVRT, Dec T, E/e′ and EDP) was not affected by 8 weeks of exercise training in MetS (Table 2). During exercise we were limited to examining changes in components of LV diastolic function namely IVRT, and A-wave. No significant differences in peak IVRT (MetS-ExT; 16±1 vs. 16±2; Non-T; 15±1 vs. 15±2), and A-wave (MetS-ExT; 1.38±0.06 vs. 1.28±0.11; Non-T; 1.48±0.08 vs. 1.53±0.06) were found in either MetS-ExT or Non-T pre and post intervention.
Effects of exercise training on arterial ventricular coupling
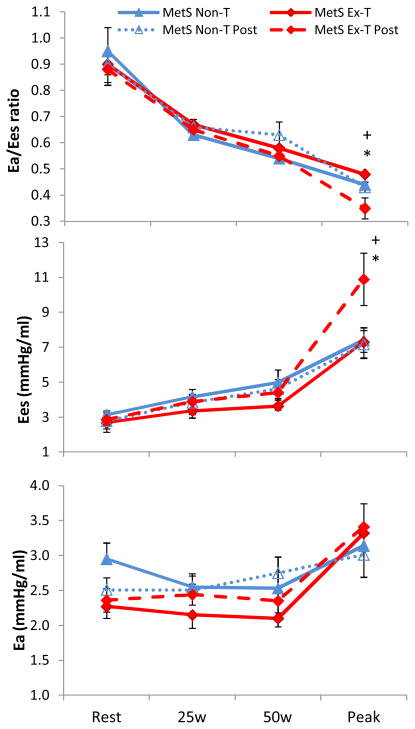
Exercise training in MetS did not alter resting Ea/Ees, Ea, or Ees (Figure 1). Further, with the exception of resting SVRi which was lower in the MetS-NonT, no differences in resting CV function were evident in either MetS group (Table 3). There were no significant time (pre and post) by group (MetS-ExT vs. NonT) interactions at rest for any CV parameter.
Figure 1.
Change in arterial-ventricular coupling (Ea/Ees), LV end-systolic elastance (Ees), and arterial elastance (Ea) from rest to peak exercise in Mets who underwent exercise training (MetS-ExT, diamond) and in MetS who remained inactive (MetS-NonT, triangles). Post intervention for both MetS group is depicted by a dashed line. Exercise training significantly reduced peak Ea/Ees, and increased peak Ees in MetS, and there was a significant time (pre and post intervention) by group (MetS-ExT vs. MetS-NonT) for Ea/Ees and Ees. *p<0.05 illustrates significant differences pre and post intervention in MetS Ex-T; +p<0.05 time by group interaction. Data presented as means ± SEM.
Table 3.
Effects of exercise training on cardiovascular function in MetS
| MetS non-trained (n=10) | MetS trained (n=10) | |||
|---|---|---|---|---|
| Pre | Post | Pre | Post | |
| EDVi, ml/m2 | ||||
| Seated | 47 ± 2 | 53 ± 3 | 47 ± 2 | 49 ± 2 |
| Peak | 49 ± 2 | 51 ± 2 | 46 ± 2 | 52 ± 3 |
| ESVi, ml/m2 | ||||
| Seated | 21 ± 1 | 23 ± 2 | 20 ± 1 | 20 ± 1 |
| Peak | 18 ± 1 | 17 ± 1 | 17 ± 2 | 14 ± 2* |
| SVi, ml/m2 | ||||
| Seated | 26 ± 2 | 30 ± 2 | 26 ± 2 | 29 ± 1 |
| Peak | 31 ± 2 | 35 ± 2 | 29 ± 2 | 38 ± 2* |
| HR, bpm | ||||
| Seated | 68 ± 4 | 67 ± 4 | 65 ± 3 | 64 ± 3 |
| Peak | 164 ± 4 | 157 ± 4* | 154 ± 6 | 151 ± 6 |
| CI, L·min/m2 | ||||
| Seated | 1.81 ± 0.16 | 2.05 ± 0.15 | 1.70 ± 0.11 | 1.85 ± 0.12 |
| Peak | 4.98 ± 0.27 | 5.39 ± 0.32 | 4.50 ± 0.42 | 5.78 ± 0.46* |
| EF. % | ||||
| Seated | 56 ± 2 | 58 ± 2 | 56 ± 2 | 60 ± 1 |
| Peak≠ | 62 ± 2 | 67 ± 3 | 63 ± 3 | 74 ± 2*† |
| SBP, mmHg | ||||
| Seated | 127 ± 4 | 122 ± 3 | 121 ± 4 | 122 ± 3 |
| Peak | 192 ± 6 | 190 ± 6 | 188 ± 5 | 185 ± 7 |
| DBP, mmHg | ||||
| Seated | 82 ± 3 | 80 ± 3 | 83 ± 2 | 80 ± 1 |
| Peak | 73 ± 8 | 76 ± 4 | 76 ± 5 | 64 ± 7 |
| ESP, mmHg | ||||
| Seated | 117 ± 4 | 110 ± 2 | 109 ± 4 | 110 ± 3 |
| Peak | 173 ± 5 | 171 ± 5 | 169 ± 4 | 167 ± 6 |
| SVRi, dyne·m2/s·cm−5 | ||||
| Seated | 4593 ± 381 | 3848 ± 256* | 4729 ± 360 | 4245 ± 301 |
| Peak≠ | 1877 ± 141 | 1755 ± 115 | 2243 ± 244 | 1565 ± 167* |
Values are mean ± SEM; EDVi: end-diastolic volume index; SVi: stroke volume index; ESVi: end-systolic volume index; Ci: cardiac output index; EF: ejection fraction; SBP: systolic blood pressure; DBP: diastolic blood pressure; ESP: end systolic blood pressure; SVRi: systemic vascular resistance index.
p≤0.05 compared to Pre-values within a group (MetS-NonT or MetS-ExT);
significant (p≤0.05) group (MetS-NonT vs. MetS-ExT) by time (pre to post) interaction.
p<0.05 vs, MetS non-trained group at specific visit (i.e., Pre or Post)
At peak exercise, aerobic training in MetS lowered peak Ea/Ees (−27%, p<0.01) by increasing peak Ees (+40%, p<0.01) and no change in peak Ea in MetS-ExT (Figure 1). In contrast, peak Ea/Ees, Ea, and Ees did not significantly differ between pre and post visits in MetS-NonT. Similarly, peak EF, and Ci were significantly (p<0.05) increased, and peak ESVi and SVRi were reduced (p<0.05) after exercise training in MetS-ExT, whereas no differences were found in MetS-NonT (Table 4). Furthermore, significant time (pre and post) by group (MetS-ExT vs. NonT) interactions for peak Ea/Ees, Ees, EF, and SVRi were evident.
Table 4.
Effects of exercise training on aerobic capacity in MetS
| MetS non-trained (n=10) | MetS trained (n=10) | |||
|---|---|---|---|---|
| Pre | Post | Pre | Post | |
| Ventilatory threshold, l/min | 1.13 ± 0.14 | 1.27 ± 0.12 | 1.20 ± 0.13 | 1.56 ± 0.21* |
| Ve/VCO2 slope | 34.8 ± 1.49 | 34.4 ± 1.27 | 34.4 ± 1.4 | 35.7 ± 1.2* |
| Respiratory exchange ratio | 1.13 ± 0.03 | 1.10 ± 0.01 | 1.10 ± 0.02 | 1.09 ± 0.02 |
| BORG scale | 19 ± 0.3 | 19 ± 0.2 | 19 ± 0.5 | 19 ± 0.4 |
| Peak VO2, (L/min) ≠ | 1.89 ± 0.20 | 1.84 ± 0.18 | 1.68 ± 0.16 | 2.00 ± 0.19* |
| Peak VO2, (ml/kg/min) LM ≠ | 29.4 ± 2.2 | 28.7 ± 1.5 | 29.4 ± 1.6 | 35.0 ± 1.7*† |
| Peak VO2, (ml/kg/min) BW ≠ | 19.1 ± 1.6 | 18.6 ± 1.2 | 16.2 ± 1.0 | 19.4 ± 1.0*† |
| Peak A-VO2 Diff, ml/100ml | 18.4 ± 2.0 | 16.4 ± 1.2 | 18.5 ± 1.5 | 16.2 ± 1.0 |
Values are mean ± SEM; A-VO2 Diff: arteriovenous oxygen difference.
p≤0.05 compared to Pre-values within a group (MetS-NonT or MetS-ExT);
significant (p≤0.05) group (MetS-NonT vs. MetS-ExT) by time (pre to post) interaction.
p<0.05 vs, MetS non-trained group at specific visit (i.e., Pre or Post)
Exercise capacity, in both absolute (L/min) and relative (ml/min/kg of body mass or lean mass) terms was improved in MetS after 8 weeks of exercise training as reflected by a 19–20% increase (p<0.01) in VO2peak and a 4% increase (p<0.05) in Ve/VCO2 (Table 4). Of note, a time (pre and post) by group (MetS-ExT vs. NonT) interaction for VO2peak was identified despite no differences in the respiratory exchange ratio, or perceived rate of exertion, in either MetS group. Further, no differences in peak arteriovenous oxygen difference were evident pre or post intervention in MetS (Table 4). Taking all individuals into consideration, an initial relationship between pre-intervention values for Ea/Ees delta (max-rest) and VO2peak (r=−0.58, p<0.01) was found. We then examined whether the difference in Ea/Ees delta from visit 1 and visit 2 was correlated with the change from visit 1 and 2 in VO2peak, however this relationship did not reach statistical significance (r=−0.40, p=0.09). In a stepwise mixed model approach with repeated measures and a time-varying covariate, we found that Ea/Ees delta contributed towards 16% of the variance on the effects of exercise training on peak VO2.
Lifetime Risk Score
In the MetS-ExT group, the Lifetime Risk Score was significantly reduced (20±7% to 16±6%, p=0.01) after training, whereas in the MetS-NonT group no difference was found (13±2% vs. 13±2%, p=0.8). Further, we identified a significant inverse correlation between the Lifetime Risk Score and peak VO2 (r=−0.53, p=0.02).
Discussion
This is the first study to examine the effects of aerobic exercise training on peak arterial-ventricular coupling in patients with the MetS. We report that following 8 weeks of aerobic exercise training, peak arterial-ventricular coupling, peak LV contractility and aerobic capacity were significantly improved and similar to levels noted in healthy untrained controls (15). Our unique patient population afforded the opportunity to assess whether exercise training can reverse impaired CV function reported in MetS patients prior to a diagnosis of CVD and/or diabetes. Since MetS is believed to be a harbinger in the pathogenesis of diabetes and CVD, these data provide evidence that exercise based interventions at this critical (preclinical disease) time point may help to reverse the pathophysiological progress of CVD, and/or delay the progression to overt CVD and/or diabetes.
The MetS is associated with pathophysiological changes to the CV system associated with an increased CVD mortality risk. Recently we reported that resting LV systolic function was well preserved in MetS patients who clearly manifested the symptoms of the metabolic disorder (including hypertension, insulin resistance, and hyperlipidemia) but who are free from overt CVD and/or diabetes (15). Conversely, in this same population we observed LV remodeling, LV hypertrophy and reduced LV diastolic function at rest (15). The progression from MetS to Type 2 Diabetes is characterized by additional LV enlargement and LV diastolic dysfunction, with evidence of LV systolic dysfunction and related sympathetic alterations (39). In our MetS population, exercise training was unable to reverse the LV structural changes or impairments in LV diastolic function. The lack of an observed effect of exercise training on LV mass is likely due to the duration of the exercise stimulus (8 weeks). Indeed, an intervention consisting of several months of aerobic training was found to be sufficient to exert physiological LV remodeling (16), reduce LV wall thickness, and reducd LV wall thickness-to-radius ratio (43). To what extent exercise training can improve LV diastolic function by improving the compliance of the heart remains controversial. Two recent studies examining a full year of exercise training, in MetS patients without Type 2 Diabetes or CVD (23) and healthy senior individuals (16), found no significant improvements in resting LV diastolic function. Advancing age and the development of CVD induce structural and functional alterations to the heart that is reflected by a reduction in the number of cardiomyocytes, an increase in connective tissue volume, and an increase in the formation of advanced glycation end products, which collectively result in impairment of LV diastolic function (22). Data would suggest that once these cross-linked collagen proteins are formed they are pathologically irreversible. Thus, any potential improvements in LV diastolic function to be gained from exercise training may be limited by cross-linked collagen (16).
The coupling between the heart and arterial system is an important and largely under appreciated determinant of cardiac performance and energetics (9). At rest, the coupling ratio is well maintained around 0.7–1.0 to optimize CV efficiency. This ratio is preserved with advancing age and in patients with heart failure (24, 31). Indeed, we have also recently shown that resting Ea/Ees is around 0.9 in MetS patients (15). In this study, exercise training in MetS did not alter resting Ea/Ees, or its components. While resting Ea/Ees is fairly well conserved, the ability of the CV system to respond to stress, in particular exercise, is blunted with age, and in heart failure patients (8, 31). We have previously shown that the Ea/Ees response to exercise is significantly impaired in MetS patients (15), which is, in part, due to impaired LV contractility and a blunted peripheral vasodilatory response to exercise (15). Importantly, this study indicates that 8 weeks of exercise training is sufficient to increase the peak LV contractile response and improve peak exercise Ea/Ees in MetS patients, as direct result of improved peak Ees.
Although we have shown that arterial-ventricular coupling is improved after exercise training in middle aged individuals with MetS, it is also important to identify whether older individuals with MetS would have the same beneficial effects of exercise training. In older (age >60 years) healthy individuals, peak exercise cardiac function is typically improved, at least up to the 7th decade, after exercise training depicted by increases in Ci, SV, EF, LV contractility, and VO2peak (2, 18, 34, 36). Future research should establish to what extent older individuals with MetS demonstrate improvements in arterial and cardiac function after exercise training. This is especially important given that the presence of MetS with older age is often accompanied by CVD (i.e., T2DM, coronary artery disease, etc.), and with increased formation of cross-linked advanced glycation end products in the LV and arterial walls, which may limit the physiological responses to exercise training (1).
A blunted Ea/Ees response during exercise has been shown to correspond with a decrease in VO2peak in MetS (15) and heart failure (8) patients. An inverse relationship has also been reported between VO2peak and Ea/Ees during exercise (14), which was confirmed in our study. In addition, acute infusion of verapamil improved arterial-ventricular coupling and resulted in a corresponding improvement in exercise capacity (11). These data suggest a direct link between Ea/Ees and aerobic capacity, in that an improvement in the coupling likely results in a more effective transfer of blood from the heart to periphery thereby increasing functional reserve capacity. Indeed, in the presence of a stiffer heart there is a greater change in BP for a given change in volume, which in a closed-system, can amplify the BP response and impair net cardiac ejection (11). In the current study, we found an increase in peak SV without a significant change in BP suggesting a more compliant cardiac response after exercise training. Further, the improvement in peak Ea/Ees was accompanied by a 20% increase in VO2peak, and that the change in Ea/Ees seemed to contribute (≈16%) towards the improved VO2peak. Given that aerobic capacity is determined by both central (HR and SV) and peripheral factors (skeletal muscle mass, calcium cycling, mitochondria capacity, capillary density, etc.), it is likely that the improved coupling, along with other physiological adaptations that were not examined in our study, contributed towards the improved VO2peak in MetS after exercise training. Indeed, Tjonna et al. (42) observed a 16% and 36% increase in VO2peak after 16 weeks of either continuous aerobic exercise or high intensity aerobic interval training in MetS, respectively. It was suggested that enhanced skeletal muscle capacity (improved calcium cycling and mitochondria capacity) contributed to the exercise-induced improvement in VO2peak (42). Although, we did not directly measure skeletal muscle oxidative capacity or oxygen extraction, these responses to exercise training have been reported elsewhere (42, 45). In contrast to these findings, we did not observe an improvement in peak arteriovenous oxygen difference (estimated from the Fick equation) after 8 weeks of exercise training in MetS, suggesting that muscle adaptation played a minor role in the reported improvements in aerobic exercise capacity in our patients. The effects of exercise training on improving peak arteriovenous oxygen difference in other populations are mixed with some reporting no changes (16, 34) and others showing an increase (27, 36). This lack of change may be related to the intensity of exercise training, whereby an increase arteriovenous oxygen difference was reported after high-intensity exercise training, but not after low-intensity training (36). An increase in exercise intensity and duration may be required to increase peripheral oxygen extraction in individuals with MetS. Further, research is needed to clarify the relationship between Ea/Ees, and VO2peak.
The improvement in LV contractility during exercise may have been due, in part, to an increased stroke volume, a decreased afterload, and improved arterial-ventricular coupling. Indeed, improvements in peak exercise SVi and ESVi were reported in MetS after exercise training. Similar improvements in cardiac volumes have been reported after exercise training in older sedentary individuals (16, 37). Improved peak LV performance post exercise training is unlikely to be due to enhanced myocardial β-adrenergic responses, as chronic exercise training has not been reported to alter β-adrenergic function (38). However, exercise training has been shown to improve calcium handling in experimental animal models, thereby improving cardiomyocyte function (46). Accordingly, improvements in calcium handling may have contributed to the improvement in LV contractility noted in MetS. The enhanced ability of the LV to empty as fitness increases may relate to a reduction in arterial stiffness/afterload in the conditioned state (44). However, following 8 weeks of exercise training, no improvements in Ea during exercise were reported in MetS. In contrast, a significant reduction in peak vascular resistance was established in the exercise trained MetS patients. Ea is an integrative index that incorporates the principal elements of arterial load, including systemic vascular resistance, total arterial compliance, characteristic impedance, and systolic and diastolic time intervals. Ea is therefore regarded a measure of the net arterial load that is imposed on the LV (9). Thus, the lack of change in Ea does not necessarily indicate that specific components of arterial load where not improved at peak exercise after training. This was clearly evident with the improvement in peak SVRi. Further, the improvement in SVRi or the lack of change in Ea, were not attributed to the slight changes in peak HR noted in the control group, as evident by similar findings after adjusting for HR as a time-vary covariate in a mixed effects model. Whether the improvement in SVRi at peak exercise is due to release of vasodilators causing vascular relaxation remains to be elucidated. Improvements have also been noted in resting endothelium-dependent vasodilatation in obese and MetS patients after exercise training (42). Accordingly, despite the lack of change in Ea, peak arterial function may have been improved in MetS, thus contributing to an improved aerobic capacity.
The beneficial CV effects at peak exercise attributed to exercise training were not a reflection of a change in body fat or lean mass. However, numerous studies have reported that regular moderate intensity exercise can result in reductions in weight and fat mass (20, 28). Thus, the lack of change in body composition in our study is likely due to the short duration of exercise training (8 weeks). Further, clinical metabolic biomarkers (HDL, triglycerides, glucose, etc.) remained unchanged. Although we did not find improvements in body composition or clinical blood biomarkers after training in MetS, we believe that improvements in peak CV function and aerobic capacity reflect a reduction in CV risk in MetS patients. A strong association exists between aerobic capacity and mortality with a positive correlation between improvements in aerobic capacity and an improved prognosis (7). A relationship that seems to be more robust than the relationship between weight loss and mortality (6). For example, for each MET (3.5 ml/kg/min) increase in exercise capacity confers a 12% improvement in survival (30). The average increase in peak VO2 (ml/kg/min) in the MetS-ExT group was 3.2 ml/kg/min, suggesting an improvement in survival. Further, the Lifetime Risk Score in MetS-ExT was significantly reduced by 4%, and the Lifetime Risk Score was inversely correlated with peak VO2. However, to fully prevent the progression to overt CVD and/or diabetes, and the pathophysiological changes to the CV system that accompanies this transition, persistent physical activity in combination with a nutritional dietary regimen (that includes optimal vitamin/mineral consumption) is required.
Study limitations
There are several limitations. First, the sample size for our training and non-training group is modest (n=10 in each). Although we find significant differences in peak Ea/Ees, this was due principally to a significant change in peak Ees (p<0.05 with statistical power >0.75), but we were underpowered in our statistical power for Ea (power=0.10). While it is evident that we have sufficient power in those CV variables where significance was observed, we cannot exclude the possibility of a type II statistical error (i.e. that we have falsely accepted the null hypothesis) in the CV variables that we not-significantly different where power was low. Therefore, these data should be regarded as preliminary, until we or others can obtain data on larger population sample. In the current study, we examined the effects of exercise training on Ea (net arterial load) as our arterial function parameter. Although it is important to study the interaction between the heart and arterial system in the same domain (i.e., elastance), measuring specific aspects of arterial function such as characteristic impedance, arterial stiffness (via pulse wave velocity), and intima medial thickness would provide additional insights into the beneficial effects of exercise training in MetS. Further, sex differences in the effects of exercise training on LV stiffness may have gone undetected given the small number of male vs. female subjects. Future research should examine whether there are sex-related differences in the coupling response, at rest and during exercise, after exercise training.
Second, pressure and flow were not directly measured, but rather estimated from non-invasive surrogates. However, the methods we used have been previously validated against invasive hemodynamic measurements (12). Our peak cardiac data may be underestimated due to a systematic underestimation of LV volumes from 2-D echocardiography (17, 41) and the challenge of acquiring echocardiograhic images during exercise. However, the technique we used has been successfully used by others (8, 40), and similar values have been observed suggesting fidelity in our data.
Third, we were limited in our ability to comprehensively characterize the extent of diastolic function during exercise because the focus of this study was to examine the impact of exercise training on peak exercise Ea/Ees and LV systolic function, and therefore echocardiographic views were optimized to examine systolic function. Further, the acquisition of LV diastolic parameters during exercise is challenging. Thus, we cannot rule out that exercise training in MetS also improved peak exercise LV diastolic function.
Fourth, peak arteriovenous oxygen difference was not measured but rather it was estimated using the Fick equation (VO2 divided by cardiac output). The Fick technique has been used to calculate arteriovenous oxygen difference in number of recent physiologic studies investigating mechanisms of exercise intolerance (5, 19). Our peak arteriovenous oxygen difference values were somewhat higher than reported by others (5), possibly due to underestimation of cardiac output. Most importantly, because key variables were measured at all testing times using identical methods in both groups, and because we assessed changes in reserve capacity (peak values – resting values) within individuals, comparisons of cardiac output and estimated arteriovenous oxygen difference between groups are valid.
Lastly, the short duration of exercise training (8 weeks) may have been insufficient to alter cardiac structure, body composition, and metabolic blood biomarkers. Thus, longer exercise training programs that incorporate different exercise modalities (interval, aerobic, and resistance training) are important to fully understand the role that exercise training has on improving CV structure/function in patients with MetS.
Conclusion
In conclusion, 8 weeks of aerobic exercise training of moderate-to-high intensity significantly improved peak exercise arterial-ventricular coupling, LV contractility, peripheral vascular resistance, and aerobic capacity in MetS individuals without overt CVD and Type 2 Diabetes. However, no improvements were evident in resting LV structure and diastolic function, metabolic profile or body composition after exercise training.
Acknowledgments
The authors thank Charles Murray and Diana Stofcheck for their help with the echocardiography.
Sources of Funding
This study was supported in part by the American Heart Association 11CRP7370056 (Dr Chantler), National Heart, Lung, Blood Institute T32- HL090610 (Sara Fournier), and the National Institute Of General Medical Sciences of the National Institutes of Health under Award Number U54GM104942.
Footnotes
Disclosures
All authors report that there are no conflicts of interest, financial or otherwise in connection with the submitted article to disclose. The results of the present study do not constitute endorsement by ACSM.
Disclosure of Funding
This study was supported in part by the American Heart Association 11CRP7370056 (Dr Chantler), National Heart, Lung, Blood Institute T32- HL090610 (Sara Fournier), and the National Institute Of General Medical Sciences of the National Institutes of Health under Award Number U54GM104942.
References
- 1.Aronson D. Cross-linking of glycated collagen in the pathogenesis of arterial and myocardial stiffening of aging and diabetes. J Hypertens. 2003;21(1):3–12. doi: 10.1097/00004872-200301000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Beere PA, Russell SD, Morey MC, Kitzman DW, Higginbotham MB. Aerobic Exercise Training Can Reverse Age-Related Peripheral Circulatory Changes in Healthy Older Men. Circulation. 1999;100(10):1085–94. doi: 10.1161/01.cir.100.10.1085. [DOI] [PubMed] [Google Scholar]
- 3.Berry JD, Dyer A, Cai X, et al. Lifetime Risks of Cardiovascular Disease. New England Journal of Medicine. 2012;366(4):321–9. doi: 10.1056/NEJMoa1012848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berry JD, Willis B, Gupta S, et al. Lifetime risks for cardiovascular disease mortality by cardiorespiratory fitness levels measured at ages 45, 55, and 65 years in men: The cooper center longitudinal study. Journal of the American College of Cardiology. 2011;57(15):1604–10. doi: 10.1016/j.jacc.2010.10.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhella PS, Prasad A, Heinicke K, et al. Abnormal haemodynamic response to exercise in heart failure with preserved ejection fraction. Eur J Heart Fail. 2011;13(12):1296–304. doi: 10.1093/eurjhf/hfr133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blair SN, Brodney S. Effects of physical inactivity and obesity on morbidity and mortality: current evidence and research issues. Med Sci Sports Exerc. 1999;31(11 Suppl):S646–62. doi: 10.1097/00005768-199911001-00025. [DOI] [PubMed] [Google Scholar]
- 7.Blair SN, Kohl HW, 3rd, Barlow CE, Paffenbarger RS, Jr, Gibbons LW, Macera CA. Changes in physical fitness and all-cause mortality. A prospective study of healthy and unhealthy men. JAMA. 1995;273(14):1093–8. [PubMed] [Google Scholar]
- 8.Borlaug BA, Olson TP, Lam CSP, et al. Global Cardiovascular Reserve Dysfunction in Heart Failure With Preserved Ejection Fraction. Journal of the American College of Cardiology. 2010;56(11):845–54. doi: 10.1016/j.jacc.2010.03.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chantler PD, Lakatta EG, Najjar SS. Arterial-ventricular coupling: mechanistic insights into cardiovascular performance at rest and during exercise. Journal of Applied Physiology. 2008;105(4):1342–51. doi: 10.1152/japplphysiol.90600.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chantler PD, Melenovsky V, Schulman SP, et al. The sex-specific impact of systolic hypertension and systolic blood pressure on arterial-ventricular coupling at rest and during exercise. American Journal of Physiology-Heart and Circulatory Physiology. 2008;295(1):H145–H53. doi: 10.1152/ajpheart.01179.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen C-H, Nakayama M, Talbot M, et al. Verapamil acutely reduces ventricular-vascular stiffening and improves aerobic exercise performance in elderly individuals. Journal of the American College of Cardiology. 1999;33(6):1602–9. doi: 10.1016/s0735-1097(99)00052-2. [DOI] [PubMed] [Google Scholar]
- 12.Chen CH, Fetics B, Nevo E, et al. Noninvasive single-beat determination of left ventricular end-systolic elastance in humans. J Am Coll Cardiol. 2001;38(7):2028–34. doi: 10.1016/s0735-1097(01)01651-5. [DOI] [PubMed] [Google Scholar]
- 13.Ehsani A, Ogawa T, Miller T, Spina R, Jilka S. Exercise training improves left ventricular systolic function in older men. Circulation. 1991;83(1):96–103. doi: 10.1161/01.cir.83.1.96. [DOI] [PubMed] [Google Scholar]
- 14.Fahs CA, Rossow LM, Yan H, et al. Resting and post exercise arterial-ventricular coupling in endurance-trained men and women. J Hum Hypertens. 2013;27(9):552–6. doi: 10.1038/jhh.2013.7. [DOI] [PubMed] [Google Scholar]
- 15.Fournier SB, Reger BL, Donley DA, et al. Exercise reveals impairments in left ventricular systolic function in patients with metabolic syndrome. Experimental Physiology. 2013;99(1):149–63. doi: 10.1113/expphysiol.2013.075796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fujimoto N, Prasad A, Hastings JL, et al. Cardiovascular Effects of 1 Year of Progressive and Vigorous Exercise Training in Previously Sedentary Individuals Older Than 65 Years of Age. Circulation. 2010;122(18):1797–805. doi: 10.1161/CIRCULATIONAHA.110.973784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gottdiener JS, Bednarz J, Devereux R, et al. American Society of Echocardiography recommendations for use of echocardiography in clinical trials. J Am Soc Echocardiogr. 2004;17(10):1086–119. doi: 10.1016/j.echo.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 18.Hartley LH, Grimby G, Kilbom Å, et al. Physical Training in Sedentary Middle-aged and Older Men III. Cardiac Output and Gas Exchange at Submaximal and Maximal Exercise. Scandinavian Journal of Clinical & Laboratory Investigation. 1969;24(4):335–44. doi: 10.3109/00365516909080170. [DOI] [PubMed] [Google Scholar]
- 19.Haykowsky MJ, Brubaker PH, John JM, Stewart KP, Morgan TM, Kitzman DW. Determinants of exercise intolerance in elderly heart failure patients with preserved ejection fraction. J Am Coll Cardiol. 2011;58(3):265–74. doi: 10.1016/j.jacc.2011.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Houmard JA, Tanner CJ, Slentz CA, Duscha BD, McCartney JS, Kraus WE. Effect of the volume and intensity of exercise training on insulin sensitivity. J Appl Physiol. 2004;96(1):101–6. doi: 10.1152/japplphysiol.00707.2003. [DOI] [PubMed] [Google Scholar]
- 21.Kelly RP, Ting CT, Yang TM, et al. Effective arterial elastance as index of arterial vascular load in humans. Circulation. 1992;86(2):513–21. doi: 10.1161/01.cir.86.2.513. [DOI] [PubMed] [Google Scholar]
- 22.Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part II: the aging heart in health: links to heart disease. Circulation. 2003;107(2):346–54. doi: 10.1161/01.cir.0000048893.62841.f7. [DOI] [PubMed] [Google Scholar]
- 23.Lalande S, Petrella RJ, Shoemaker JK. Effect of exercise training on diastolic function in metabolic syndrome. Applied Physiology, Nutrition, and Metabolism. 2012;38(5):545–50. doi: 10.1139/apnm-2012-0383. [DOI] [PubMed] [Google Scholar]
- 24.Lam CS, Roger VL, Rodeheffer RJ, et al. Cardiac structure and ventricular-vascular function in persons with heart failure and preserved ejection fraction from Olmsted County, Minnesota. Circulation. 2007;115(15):1982–90. doi: 10.1161/CIRCULATIONAHA.106.659763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification. Eur J Echocardiogr. 2006;7(2):79–108. doi: 10.1016/j.euje.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 26.Malik S, Wong ND, Franklin SS, et al. Impact of the Metabolic Syndrome on Mortality From Coronary Heart Disease, Cardiovascular Disease, and All Causes in United States Adults. Circulation. 2004;110(10):1245–50. doi: 10.1161/01.CIR.0000140677.20606.0E. [DOI] [PubMed] [Google Scholar]
- 27.McGuire DK, Levine BD, Williamson JW, et al. A 30-Year Follow-Up of the Dallas Bed Rest and Training Study. Circulation. 2001;104(12):1350–7. [PubMed] [Google Scholar]
- 28.McTiernan A, Sorensen B, Irwin ML, et al. Exercise effect on weight and body fat in men and women. Obesity (Silver Spring) 2007;15(6):1496–512. doi: 10.1038/oby.2007.178. [DOI] [PubMed] [Google Scholar]
- 29.Mozumdar A, Liguori G. Persistent Increase of Prevalence of Metabolic Syndrome Among U.S. Adults: NHANES III to NHANES 1999–2006. Diabetes Care. 2011;34(1):216–9. doi: 10.2337/dc10-0879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Myers J, Prakash M, Froelicher V, Do D, Partington S, Atwood JE. Exercise Capacity and Mortality among Men Referred for Exercise Testing. New England Journal of Medicine. 2002;346(11):793–801. doi: 10.1056/NEJMoa011858. [DOI] [PubMed] [Google Scholar]
- 31.Najjar SS, Schulman SP, Gerstenblith G, et al. Age and gender affect ventricular-vascular coupling during aerobic exercise. J Am Coll Cardiol. 2004;44(3):611–7. doi: 10.1016/j.jacc.2004.04.041. [DOI] [PubMed] [Google Scholar]
- 32.Ommen SR, Nishimura RA, Appleton CP, et al. Clinical utility of Doppler echocardiography and tissue Doppler imaging in the estimation of left ventricular filling pressures: A comparative simultaneous Doppler-catheterization study. Circulation. 2000;102(15):1788–94. doi: 10.1161/01.cir.102.15.1788. [DOI] [PubMed] [Google Scholar]
- 33.Pagé A, Dumesnil JG, Clavel M-A, et al. Metabolic Syndrome Is Associated With More Pronounced Impairment of Left Ventricle Geometry and Function in Patients With Calcific Aortic Stenosis: A Substudy of the ASTRONOMER (Aortic Stenosis Progression Observation Measuring Effects of Rosuvastatin) Journal of the American College of Cardiology. 2010;55(17):1867–74. doi: 10.1016/j.jacc.2009.11.083. [DOI] [PubMed] [Google Scholar]
- 34.Schulman SP, Fleg JL, Goldberg AP, et al. Continuum of Cardiovascular Performance Across a Broad Range of Fitness Levels in Healthy Older Men. Circulation. 1996;94(3):359–67. doi: 10.1161/01.cir.94.3.359. [DOI] [PubMed] [Google Scholar]
- 35.Scuteri A, Najjar SS, Muller DC, et al. Metabolic syndrome amplifies the age-associated increases in vascular thickness and stiffness. J Am Coll Cardiol. 2004;43(8):1388–95. doi: 10.1016/j.jacc.2003.10.061. [DOI] [PubMed] [Google Scholar]
- 36.Seals DR, Hagberg JM, Hurley BF, Ehsani AA, Holloszy JO. Endurance training in older men and women. I. Cardiovascular responses to exercise. Journal of Applied Physiology. 1984;57(4):1024–9. doi: 10.1152/jappl.1984.57.4.1024. [DOI] [PubMed] [Google Scholar]
- 37.Stratton J, Levy W, Cerqueira M, Schwartz R, Abrass I. Cardiovascular responses to exercise. Effects of aging and exercise training in healthy men. Circulation. 1994;89(4):1648–55. doi: 10.1161/01.cir.89.4.1648. [DOI] [PubMed] [Google Scholar]
- 38.Stratton JR, Cerqueira MD, Schwartz RS, et al. Differences in cardiovascular responses to isoproterenol in relation to age and exercise training in healthy men. Circulation. 1992;86(2):504–12. doi: 10.1161/01.cir.86.2.504. [DOI] [PubMed] [Google Scholar]
- 39.Straznicky NE, Grima MT, Sari CI, et al. The relation of glucose metabolism to left ventricular mass and function and sympathetic nervous system activity in obese subjects with metabolic syndrome. J Clin Endocrinol Metab. 2013;98(2):E227–37. doi: 10.1210/jc.2012-3277. [DOI] [PubMed] [Google Scholar]
- 40.Tartière-Kesri L, Tartière J-M, Logeart D, Beauvais F, Cohen Solal A. Increased Proximal Arterial Stiffness and Cardiac Response With Moderate Exercise in Patients With Heart Failure and Preserved Ejection Fraction. Journal of the American College of Cardiology. 2012;59(5):455–61. doi: 10.1016/j.jacc.2011.10.873. [DOI] [PubMed] [Google Scholar]
- 41.Tischler MD, Plehn JF. Applications of stress echocardiography: beyond coronary disease. J Am Soc Echocardiogr. 1995;8(2):185–97. doi: 10.1016/s0894-7317(05)80407-9. [DOI] [PubMed] [Google Scholar]
- 42.Tjonna AE, Lee SJ, Rognmo O, et al. Aerobic Interval Training Versus Continuous Moderate Exercise as a Treatment for the Metabolic Syndrome: A Pilot Study. Circulation. 2008;118(4):346–54. doi: 10.1161/CIRCULATIONAHA.108.772822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Turner MJ, Spina RJ, Kohrt WM, Ehsani AA. Effect of Endurance Exercise Training on Left Ventricular Size and Remodeling in Older Adults With Hypertension. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2000;55(4):M245–M51. doi: 10.1093/gerona/55.4.m245. [DOI] [PubMed] [Google Scholar]
- 44.Vaitkevicius PV, Fleg JL, Engel JH, et al. Effects of age and aerobic capacity on arterial stiffness in healthy adults. Circulation. 1993;88(4 Pt 1):1456–62. doi: 10.1161/01.cir.88.4.1456. [DOI] [PubMed] [Google Scholar]
- 45.van Tienen FHJ, Praet SFE, de Feyter HM, et al. Physical Activity Is the Key Determinant of Skeletal Muscle Mitochondrial Function in Type 2 Diabetes. Journal of Clinical Endocrinology & Metabolism. 2012;97(9):3261–9. doi: 10.1210/jc.2011-3454. [DOI] [PubMed] [Google Scholar]
- 46.Wisloff U, Loennechen JP, Falck G, et al. Increased contractility and calcium sensitivity in cardiac myocytes isolated from endurance trained rats. Cardiovasc Res. 2001;50(3):495–508. doi: 10.1016/s0008-6363(01)00210-3. [DOI] [PubMed] [Google Scholar]