Abstract
BACKGROUND
The “anticonvulsant face”, comprised of a short nose, low nasal bridge, epicanthal folds, and wide mouth, was suggested in the 1970s to indicate teratogenesis caused by the anticonvulsant drugs phenytoin and phenobarbital. However, these were based on subjective clinical observations. In the present study we have applied objective and reliable quantitative measures to the operational definitions of craniofacial features in anticonvulant-exposed cases. We have adopted anthropometric analysis based on image analysis of laser light scans. Using morphometric methods, we established the positions of physical features and objectively determined the changes in the size and shape of affected soft tissues of the faces of children exposed to those anticonvulsant drugs during pregnancy.
METHODS
Thirteen individuals, exposed throughout pregnancy to phenytoin as either monotherapy or polytherapy, were identified in a previous analysis as having significant changes in their craniofacial features based on measurements of cephalometric radiographs, changes associated with “the anticonvulsant face”.. The soft tissues of their faces were imaged by 3D laser (structured light) scanning.
RESULTS
The notable changes in soft tissues identified by laser light scans were a wide philtrum (between the left and right cristae philtri), narrow mouth (between the left and right cheilions), short nasal bridge (between nasale and pronasale), short nose height (between the nasale and subnasale), and flat orbits (based on the orbital protrusion index).
CONCLUSIONS
This analysis of phenytoin-exposed individuals is the first anthropometric analysis of the craniofacial surface, designed to render the identification of abnormal features both objective and realiable. These analyses demonstrated that there were several significant changes in the soft tissue of the face, corroborating earlier studies of alterations of the craniofacial skeleton in the anticonvulsant face. Two of the features identified in the current study as aberrant in the exposed individuals -- widening of the philtrum and narrowing of the mouth -- have not been described previously as part of this phenotype.
Keywords: laser light scanner, anthropometry, 3D imaging, anticonvulsants, the “anticonvulsant face”
INTRODUCTION
Facial features, including those of hypertelorism, broad bridge of nose, short nose, and wide mouth, were described as characteristics of the fetal hydantoin syndrome in the clinical reports in the 1970s (Hill R et al, 1974; Hanson and Smith, 1975; Hanson JW et al., 1976). This phenotype was referred to as “the fetal hydantoin syndrome” (Hanson and Smith, 1975). The children described in these reports had been exposed during pregnancy primarily to anticonvulsants, primarily phenytoin (brand name Dilantin) as monotherapy or to polytherapy with phenytoin and barbiturates, either phenobarbital or primidone.
The initial descriptions (Hanson JW et al., 1976; Jones KL et al., 1989; Ardinger H, 1988) of the changes in the facial features of children exposed prenatally to several different anticonvulsant drugs, including phenytoin, carbamazepine and valproate, were based on clinical impressions. More objective, reliable methods for describing craniofacial features have been developed, and these include measurements from photographs (called photogrammetry), direct anthropometry during examination, laser (structured light) scanning, and three-dimensional (3D) stereophotogrammetric morphology (Allanson, 1997; Altobelli, 1994; Astley and Clarren, 1996; Bookstein, 1991; Deutsch et al, 2013; Hammond P et al, 2005; Wong et al, 2008).
The development of laser scanning made it possible to create a reliable spatial coordinate system of the surface of the face with minimal distortion (Altobelli, 1994; Baca et al, 1994). This provides a more objective method for determining the changes in facial features to be looked for by clinicians. We present here the findings in the soft tissue landmarks of anticonvulsant-exposed children and adults with “the anticonvulsant face”, using a laser light surface scanner to establish three-dimensional positions and to determine changes in size and shape.
METHODS
Subjects
The anticonvulsant-exposed children and teenagers, between ages 5.6 and 17.6 years, were recruited from three sources:
a cohort identified at birth in a study of consecutive newborn infants (Leppig et al, 1987);
children identified from a review of the medical records of women with epilepsy at a large health maintenance organization; and
children whose mothers were referred by their neurologists who had monitored their treatment with anticonvulsants to prevent seizures during pregnancy.
The criteria for inclusion were exposure to either phenytoin monotherapy or polytherapy throughout pregnancy. The exclusion criteria were: exposure to another teratogen, such as maternal diabetes mellitus, during pregnancy; a multiple gestation birth; individuals less than 5.5 years of age; individuals not considered to have European, Caucasian ancestry.
Twenty-eight individuals, between 5.6 and 17.6 years of age, had had previous evaluations by physical examinations, skull cephalometric, hand and wrist radiographs, photographs and dental molds. Several findings in these and additional anticonvulsant-exposed individuals have been published: a description of the changes identified in the cephalometric radiographs (VanLang et al, 1984; Orup Jr HI et al, 2003); mesiodistal tooth crown dimensions (Orup Jr HI et al, 1998); changes in fingers (Lu et al, 2000), toes (Bokhari et al, 1999) and dermal ridge patterns (Bokhari et al, 1999); and the correlation of midface and digit hypoplasia with deficits in cognitive function (Holmes et al, 2005).
We present here the findings by laser scan analysis in a subset of 13 of these 28 individuals. This subset was identified from a review of the findings in previous analyses of the changes in craniofacial dimensions in frontal and lateral cephalometric radiographs (Orup Jr HI et al, 2003). These 13 individuals had shown on their previous radiographs shortening of at least one of these dimensions, N – Ans, S – N, S –Ba or BIOD by at least one standard deviation. These are measurements, respectively, of the height of the maxilla (N-Ans), length of anterior cranial base (S-N), the length from sella turcica to basion (S-Ba), that is the posterior cranial base and the width of the bony interorbital distance (BIOD).
Each individual was re-contacted and asked to return for a physical examination, photographs, radiographs and to have laser light scanning of their facial features. Each was asked to review and sign an informed consent document.
Imaging and morphometry
Prior to scanning, soft tissue landmarks were marked with a small dot using a cosmetic marking pencil, such as crista philtri, nasion, orbitale, pronasale and subnasale (Figures 1 and 2; Table 1). Imaging was performed by a Cyberware ECHO 3030 surface scanner (Cyberware, Monterey, CA) with a Silicon Graphics Iris Indigo XS24 computer. Nineteen landmarks, six of which are bilateral, were selected for this analysis (Table 1). The subjects were scanned with their eyes closed, their teeth in centric occlusion and their lips relaxed. Each was asked to remain still during the entire scan, which lasted approximately 17 seconds. The soft tissue landmarks were digitized directly on the computer monitor and three-dimensional Cartesian coordinates were obtained from these discrete points. These three-dimensional coordinates (x, y, z) values were stored in a file and exported into Microsoft Excel spreadsheets for calculations, analysis, and comparison to normal values. Each individual scan was digitized three times, so each three-dimensional x, y, z coordinate represented an average across three digitized coordinates from the same scan. Conventional scalar measurements were calculated and used in the reported dimensions and proportional indices. Twelve dimensions and twelve proportional indices were calculated (Table 2).
Figure 1.
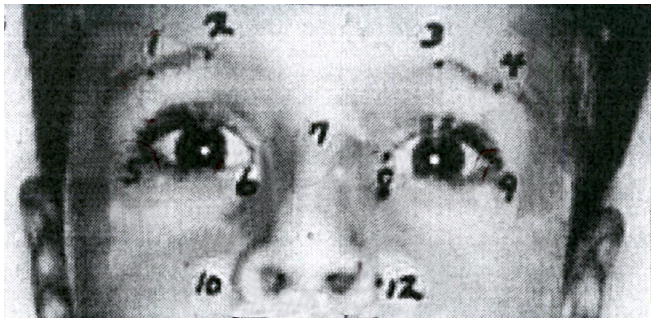
A view of part of the face of a participant, showing landmark placement with numbered dots. The numbers refer to landmarks listed in Table 1.This child has the anteverted nostrils, short nose and long upper lip of the “anticonvulsant face”.
Figure 2.
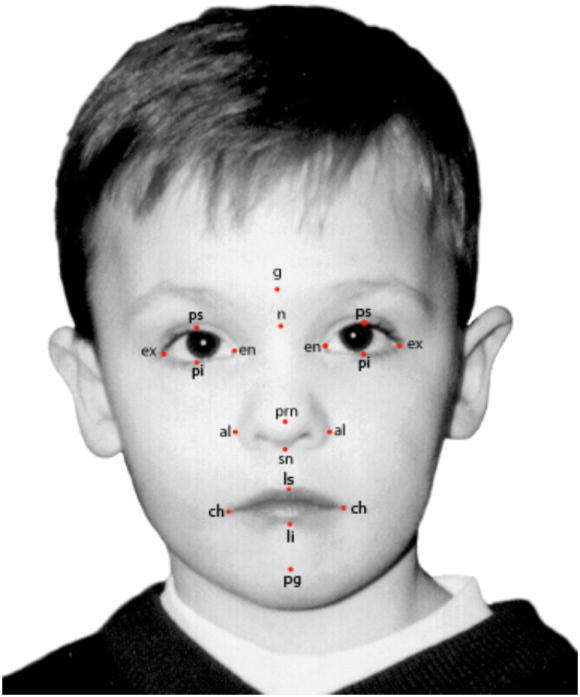
Shows the abbreviations of the soft tissue landmarks, listed in Table 1, in a frontal photograph. This child had not been exposed during pregnancy to an anticonvulsant drug.
Table 1. Anthropometric Landmarks Utilized in Analysis(revised 24 June 2013).
| ID | Landmark | Definition |
|---|---|---|
|
| ||
| al | Alare | The most lateral point on each alar contour (R, L)* (10,12)** |
| ch | Cheilion | The point located at each labial commissure (R, L)* |
| cph | Crista Philtri | The point on the elevated philtral margin above the vermilion (R, L)* |
| en | Endocanthion | The point at the inner commissure of the eye fissure (R, L)* (6,8)** |
| ex | Exocanthion | The point at the outer commissure of the eye fissure (R, L)* (5,9)** |
| Is | LabialeSuperius | The midpoint of the upper vermilion line* |
| n | Nasion | Point at the midline of both the nasal root and the nasofrontal suture* (7)** |
| or | Orbitale | Lowest point on the lower margin of each orbit* |
| prn | Pronasale | The most anterior point (tip) of the apex nasi* |
| sci | Superciliare | The highest point on eyebrow midportion upper borderline (R, L)* (1,4)** |
| mci | Medial Ciliare | The medial point on the eyebrow upper borderline (R, L) (2,3)** |
| sn | Subnasale | The midpoint at the columella base at which the lower border of the nasal columella and the surface of the cutaneous upper lip meet* (11)** |
Table 2. Laser Scan Analysis of Soft Tissue Dimensions and Proportions.
| Facial Dimensions Obtained front Laser Scan | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Average Values | Standard Score (Z-score) | t-Test** | ||||||||
| Facial Dimensions | Control | Subjects | N | Mean | S. D. | S.E. | Max. | Min. | Median | Significance |
| Length of Eye Fissure R (ex-en) | 30.41 | 29.49 | 13 | -0.69 | 1.40 | 0.39 | 2.63 | -2.99 | -0.82 | NS |
| Length of Eye Fissure L (ex-en) | 30.40 | 29.13 | 13 | -1.13 | 1.32 | 0.37 | 1.08 | -3.30 | -1.07 | p<.01 |
| Intercanthal Width (en-en) | 32.56 | 32.52 | 13 | 0.07 | 1.27 | 0.35 | 3.05 | -1.30 | -0.30 | NS |
| Biocular width (ex-ex) | 87.66 | 89.36 | 13 | 0.52 | 1.41 | 0.39 | 3.51 | -1.61 | 0.24 | NS |
| Height of Orbit & Eyebrow R (or-sci) | 38.22 | 35.38 | 13 | -0.83 | 0.57 | 0.16 | 0.12 | -1.87 | -0.81 | p<.001*** |
| Height of Orbit & Eyebrow L (or-sci) | 38.16 | 36.19 | 13 | -0.60 | 0.88 | 0.24 | 1.59 | -1.68 | -0.57 | p<.05 |
| Length of Nasal Bridge (n-prn) | 45.57 | 37.27 | 13 | -2.42 | 1.12 | 0.31 | -0.35 | -4.99 | -2.32 | p<.00001*** |
| Philtrum width (cph-cph) | 9.85 | 13.26 | 13 | 2.74 | 1.79 | 0.50 | 6.15 | 0.51 | 3.09 | p<.001** |
| Mouth Width (ch-ch)* | 50.32 | 45.41 | 13 | -1.58 | 1.98 | 0.55 | 0.77 | -7.55 | -1.38 | P<.05 |
| Upper Lip Length (sn-ls) | 14.15 | 13.24 | 13 | -0.41 | 1.34 | 0.37 | 1.23 | -2.81 | -0.39 | NS |
| Nose width (al-al)* | 32.06 | 32.57 | 8 | 0.33 | 1.45 | 0.51 | 2.29 | -2.29 | 0.52 | NS |
| Nose Height (n-sn) | 50.59 | 45.01 | 13 | -1.77 | 2.13 | 0.59 | 1.16 | -7.37 | -1.31 | p<.05 |
| Proportional Indices for facial dimensions Obtained from Laser Scan (Z-score) | ||||||||||
| Proportional Index X 100 | ||||||||||
| Intercanthal Width/Biocular Width (en-en/ex-ex) | 37.17 | 36.44 | 13 | -0.33 | 1.35 | 0.37 | 3.45 | -1.68 | -0.65 | NS |
| Orbital Protrusion Index (ex-ex/[ex-en R & L + en-en] | 93.46 | 98.01 | 13 | 2.34 | 1.13 | 0.31 | 5.58 | 0.91 | 2.04 | p<.00001*** |
| Orbital Width Index (ex-en/en-en) L | 94.38 | 90.02 | 13 | -0.53 | 1.05 | 0.29 | 0.37 | -3.29 | -0.22 | NS |
| Orbital Width Index (ex-en/en-en) R | 94.38 | 91.06 | 13 | -0.43 | 1.06 | 0.29 | 1.03 | -2.77 | -0.11 | NS |
| Intercanthal Width/Mouth Width (en-en/ch-ch) | 65.10 | 72.49 | 13 | 1.26 | 2.06 | 0.57 | 6.60 | -0.86 | 0.82 | p<.05 |
| Nose Width/Mouth Width (al-al/ch-ch) | 65.39 | 70.05 | 8 | 0.95 | 1.32 | 0.47 | 2.69 | -0.75 | 0.98 | NS |
| Philtrum Width/Mouth Width (cph-cph/ch-ch) | 20.06 | 29.66 | 13 | 3.63 | 2.28 | 0.63 | 8.87 | 0.84 | 3.63 | p<.0001*** |
| Nasal Index (al-al/n-sn) | 65.24 | 69.11 | 8 | 0.69 | 1.05 | 0.37 | 2.27 | -1.10 | 0.86 | NS |
| Nasal Tip Protrusion/Nose width (sn-prn/al-al) | 58.51 | 54.35 | 8 | -0.86 | 1.34 | 0.47 | 0.78 | -2.69 | -0.72 | NS |
| Nasal Tip Protrusion/Nose Height (sn-prn/n-sn) | 38.39 | 40.63 | 13 | 0.51 | 1.92 | 0.53 | 6.16 | -2.14 | 0.32 | NS |
| Nasal Bridge Length/Nose Height (n-prn /n-sn) | 89.98 | 83.37 | 13 | -1.81 | 1.68 | 0.47 | 2.98 | -3.69 | -2.19 | p<.01 |
| Intercanthal Width/Nose Width (en-en/al-al) | 101.83 | 99.60 | 8 | -0.22 | 1.00 | 0.35 | 1.60 | -1.10 | -0.54 | NS |
Legends:
Obtained from direct measure
One sample two-tailed t-test of standard scores
Statistically significant after Bonferroni correction for multiple comparisons
NS – Not Significant
Also, frontal and lateral photographs were obtained a Minolta Maxxum 7000 camera with a 100 mm lens mounted on a tripod. The camera was positioned at eye-level. Each subject was asked to have centric occlusion of teeth, lips relaxed and head in a natural position (Moorees, 1985). The lens was set at a magnification scale of 1/10, and the frontal plane of the face was parallel to the camera lens. Black and white prints (5 × 7) were made from film (100 Ektachrome) used for the photographs. The direct measurement of the intercanthal width with a sliding digital caliper (CEN-TECH, Accuracy: 0.03 mm, Resolution: 0.01 mm) was used to calculate the specific photographic enlargement to be utilized in all relevant calculations for each subject’s prints. The average of two direct measures was used in these calculations.
Thirteen frontal and thirteen lateral (left) prints were digitized directly utilizing the sixteen specific landmarks. These prints were digitized using an Accugrid Translucent (Numonics Corporation, Montgomeryville, PA; Resolution: 0.0127 mm, Accuracy: 0.127 mm) connected to a Toshiba T2105 486DX2 personal computer. A computer program was developed to establish a Cartesian coordinate system for each print, yielding two-dimensional spatial coordinates (x, y) for each digitized landmark. Each print was digitized three times by the first author (H.I.O.). These coordinates were then entered into Microsoft Excel spreadsheets where averages for each specific landmark coordinate were calculated. Specific scalar (linear, angular, proportional) measures were calculated using geometric and trigonometric methods. In addition, proportional indices, standard (z) score calculations, and statistical analyses were performed. The dimensions in Table 2 were selected for analysis, based on the findings by Farkas (1994) that they are reliable and accurate. Dimensions involving the anatomic landmarks in the medial end of the eyebrow (points 2 and 3 in Figure 1) were not used. Farkas’ normative anthropometric measures and proportional indices served as the control population for the soft tissue analysis in this study (Farkas, 1994). The ethnic background of the subjects in this study was similar to that of Farkas’ North American Caucasian sample of individuals in the general population.
Metric properties
Since live subjects were scanned, a slight tremor or sway could have been introduced during the scan. Landmark identification (marking with cosmetic marker) [Figure 1] prior to scanning aided in the location of facial landmarks on the computer screen. In a separate validation study to assess the reliability of the Cyberware ECHO 3030 surface scanner, Baca et al (1994) compared direct anthropometric measurements on moulages to those obtained digitally with the surface scanner. Baca and his colleagues found excellent agreement between the two methods, with the mean differences for these measures ranging from 1.9 millimeters (for facial height) to 0.0 millimeters (for cutaneous upper lip height). Baca et al found approximately 83% of the surface scan measurements made on the moulages are within 1.0 millimeter of the direct caliper measurements and 98% are within 2.0 millimeters. In this study, the average of the mean difference scores approximated zero (0.03 mm).
The reliability of the Farkas measurements of the type used here, obtained either through digital or direct anthropometry, has also been established (e.g., Heike et al, 2010; Lauer et al, 2001; Weinberg et al, 2006; Wong et al, 2008).
Difficulty in scanning or locating certain landmarks due to areas of intense curvature or limited monitor resolution warranted some additional direct measures as well as the utilization of photographs as supplements to the laser scan where appropriate. In addition to citing the reliability of the laser surface scan, as found by Baca et al, Dahlberg’s error equation was used to determine the root mean square error for ten randomly selected scans. Two digitizations of each of the ten scans were used to calculate dimensions (listed in Table 2) for comparison in the error calculation. For these laser scan dimensions, the error values ranged from .20 (en-en) to .85 (or-sci) millimeters. Interobserver variability was eliminated by having a single individual (H.I.O.) digitize all scans.
The error of the method for the photogrammetry aspect of the soft tissue analysis was similar to that employed for those previously mentioned. The landmarks were marked on the face with a cosmetic marker where appropriate; only readily identifiable landmarks were utilized. In addition, the photographic method was standardized and the magnification factor was further individualized for increased accuracy. The error of method was calculated using the root mean square equation of Dahlberg. Ten randomly selected frontal and lateral photographs were utilized for this determination. The landmarks (listed in Table 1) were digitized on two separate occasions and the corresponding Cartesian coordinates were utilized to calculate the dimensions (outlined in Table 2) for comparisons. For both the frontal and the lateral photographic measures outlined in Table 2, the error values ranged from .22 (en – en) to 1.29 (nasolabial angle) millimeters or degrees.
Two dimensions (Nose width: Alare [al]-Alare and Mouth Width: Cheilion-[ch] Cheilion) [Figure 1] were measured directly with a sliding digital caliper (CEN-TECH, Accuracy: 0.003 mm, Resolution: 0.01 mm). These areas were not always readily discernible on the laser scan and were not obtained reliably from photographs. An average of two separate measurements was used in any calculations.
Statistical Analysis
Standardized (Z-) scores were calculated for the measures and proportional indices of the laser scan and photographs conditioning on age, gender, and and ethnicity based on Farkas’ population values (Farkas, 1994; see Deutsch et al, 2012). For each measurement the following data was derived (Table 2):
Exposed population’s average value
Control population’s average value (age, sex, and race specific)
Exposed population’s average standard (z) score
Exposed population’s standard deviation for the mean standard (z) score
Exposed population’s standard error for the mean standard (z) score
Exposed population’s maximum, minimum, and median standard (z) score
For each dimension, one sample two-tailed t-tests at the 5 percent confidence level were performed, using the standard (z) scores. The patients studied were separated into monotherapy and polytherapy-exposed groups for further analysis. One sample two-tailed t-tests at the 5 % confidence level were performed using the standard (z) scores for each exposed group separately. In addition, the standard (z) scores of the monotherapy and polytherapy-exposed subjects were compared to each other utilizing an unpaired, two-tailed t-test at the 5% confidence level.
To correct for alpha-inflation due to multiple comparisons, the Bonferroni correction was conducted (Shaffer, 1995). The results that were significant statistically after the Bonferroni correction are noted in Table 2.
RESULTS
Several notable soft tissue changes were identified. The philtrum width (cph – cph) was increased dramatically, in contrast to a decreased mouth width (ch – ch) (Table 2). Additionally, the nasal bridge (n – prn) was short, as was the nose height (n – sn); the nasal bridge was short for the given nose height (nasal bridge height/nose height index) , suggesting a shorter upturned nose. The orbits were found to be flattened significantly (as defined by the orbital protrusion index). The intercanthal distance (en-en) was not increased, indicating no evidence of either telecanthus or hypertelorism.
DISCUSSION
This analysis, employing laser light scans with objective measurement, identified two features of the anticonvulsant face that had not been appreciated previously: a wide philtrum and the narrow/small mouth. It also confirmed features that had been reported earlier: shortening of the nose, in particular the short nasal bridge and nose height, which had been determined in the previous analysis of the changes in radiographs of these children (Orup et al, 2003).
One limitation of prior clinical descriptions is their subjective nature. Because respected clinicians, such as David Smith and James Hanson (Hanson et al, 1975), had reported in their initial seminal descriptions the distinctive findings of a wide mouth and hypertelorism, it was likely that subsequent observers would “see” the same findings. This “observer bias” causes the examiners to see what he/she expects to see (Harvey et al, 2003). Subsequent objective measurements in cephalometric radiographs (Orup et al, 2003) showed that hypertelorism was not a feature of the anticonvulsant embryopathy. However, it is also possible that a single anticonvulsant-exposed individual will have hypertelorism, while most of the exposed group will not. Likewise, measurements from laser light scans in this study showed that the wide mouth, described by Hanson et al (1975), was not a feature of the “anticonvulsant face,” but rather the opposite: a narrow or small mouth. These novel insights underscore the value of using objective measurements, by whatever method, to characterize a phenotype.
We have tested these findings in the laser light scan analysis in two studies of infants and children exposed during pregnancy to the anticonvulsant drugs phenytoin and phenobarbital. Eighty-seven newborn infants exposed during pregnancy to phenytoin and 64 exposed to Phenobarbital were examined for three of these facial features: short nose, long upper lip (or long philtrum), and an increased intercanthal distance (Holmes et al., 2001). Subjective impressions were recorded (e.g., nose short and long upper lip) and measurements were made by two study physicians who did not know the exposure status of each infant. A total of 13.2% of the phenytoin-exposed and 15.2% of the phenobarbital-exposed infants had “midface hypoplasia,” defined as having two of the three measurements more than one standard deviation from the mean for length of nose, height (or length) of upper lip and inner-canthal distance. The width of philtrum and mouth were not examined in these newborn infants.
In a second follow-up study of children between 6.5 and 16 years of age who had been exposed to either phenytoin or phenobarbital, the facial features identified in this laser light analysis were measured (Nasri et al., in press). In addition, the masked examiner assessed whether or not the child had the “anticonvulsant face.” The analysis of the findings showed that the length and width of the philtrum, length of nose, inter-innercanthal distance, and width of the mouth were significantly more common in the children considered by the masked examiner (L.B.H.) to have “the anticonvulsant face”.
Does an accurate identification of “the anticonvulsant face” matter? It does matter, if the child with these features has an increased risk for medical complications, such as cognitive dysfunction. This correlation has been suggested in the evaluation of children who had been exposed during pregnancy to carbamazepine (Ornoy and Cohen, 1996), valproate (Mawer et al., 2002) and phenytoin and phenobarbital (Holmes et al., 2005).
The reported correlation between the presence of subtle changes in facial features with cognitive dysfunction makes it important for the examining pediatrician to ask the new mother about exposure to anticonvulsant drugs in pregnancy. In a review of the newborn examination findings in a consecutive series of 207 anticonvulsant-exposed infants, 74% of the pediatricians noted that they knew the infant had been exposed to one of these drugs (Peller et al., 2006). However, those pediatricians rarely noted that they had examined the infant for the physical features of anticonvulsant embryopathy. This underscores the need to make pediatricians aware of the potential significance of these findings in counseling the parents of anticonvulsant drug-exposed newborn infants.
This report concerns individuals exposed during pregnancy to the anticonvulsant drug phenytoin alone or in polytherapy with phenobarbital. These drugs are used much less often, now, by pregnant women with epilepsy.Similar studies are needed in individuals exposed during pregnancy to the “newer” anticonvulsant drugs, such as lamotrigine, levetiracetam, and topiramate, as these are more common current exposures (Hernandez-Diaz et al., 2012). In one early report (Holmes et al., 2008), it was suggested that lamotrigine-exposed infants have had an increased frequency of cleft lip and palate and cleft palate alone (Holmes and Hernandez-Diaz, 2012). Other studies have not shown an increase in oral clefts, a difference that will require a much larger number of lamotrigine-exposed infants to resolve. Topiramate-exposed infants have been found to have a significant higher frequency of malformations, in general, and oral clefts, in particular, with and without other anomalies (Holmes and Hernandez-Diaz, 2012). Do these lamotrigine and topiramate-exposed children with craniofacial abnormalities have changes in craniofacial structures, as were observed in the phenytoinexposed children described in this report? If they have such changes, are they similar or different? Do these lamotrigine-exposed and topiramate-exposed infants with craniofacial abnormalities have a higher frequency of deficits in cognitive function in comparison to the children exposed to the same drugs who do not have craniofacial abnormalities? Systematic evaluations are needed to answer these important questions.
References
- Allanson JE. Objective techniques for craniofacial assessment: what are the choices? Amer J Med Gen. 1997;70:1–5. doi: 10.1002/(sici)1096-8628(19970502)70:1<1::aid-ajmg1>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Altobelli DE. Computer-assisted acquisition of facial surface topography. In: Farkas LG, editor. Anthropometry of head and face. Second edition. Raven Press; New York, NY: 1994. pp. 225–226. [Google Scholar]
- Ardinger HH, Atkin JF, Blackston RD, et al. Verification of the fetal valproate syndrome phenotype. Am J Med Genet. 1988;29:171–185. doi: 10.1002/ajmg.1320290123. [DOI] [PubMed] [Google Scholar]
- Astley SJ, Clarren SK. A case definition and photographic screening tool for the facial phenotype of fetal alcohol syndrome. J Pediatr. 1996;129:33–41. doi: 10.1016/s0022-3476(96)70187-7. [DOI] [PubMed] [Google Scholar]
- Baca DB, Deutsch CK, D’Agostino RB., Jr . Correspondence between direct anthropometry and structured light digit measurement. In: Farkas LG, editor. Anthropometry of the Head and Face. Second edition. Raven Press; New York: 1994. pp. 235–237. [Google Scholar]
- Bokhari A, Coull B, Holmes LB. Effect of prenatal exposure to anticonvulsant drugs on dermal ridge patterns of fingers. Teratology. 2006;66:19–23. doi: 10.1002/tera.10044. [DOI] [PubMed] [Google Scholar]
- Bokhari A, Connolly S, Harvey EA, Holmes LB. Effects on toes from prenatal exposure to anticonvulsants. Teratology. 1998;66:122–126. doi: 10.1002/tera.10085. [DOI] [PubMed] [Google Scholar]
- Bookstein FL. Morphometric tools for landmark data: geometry and biology. Cambridge University Press; Cambridge: 1991. [Google Scholar]
- Deutsch CK, Shell AR, Bird BD. The Farkas system of craniofacial anthropometry: Methodology and normative databases. In: Preedy VR, editor. Handbook of Anthropometry: Physical Measures of Human Form in Health and Disease. Springer-Verlag; London: 2012. [Google Scholar]
- Farkas LG. Anthropometry of the Head and Face. Second Edition. Raven Press; New York: 1994. [Google Scholar]
- Hammond P, Hutton TJ, Allanson JE, et al. Discriminating power of localized three-dimensional facial morphology. Am J Hum Genet. 2005;77:999–1010. doi: 10.1086/498396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson JW, Smith DW. The fetal hydantoin syndrome. J Pediatr. 1975;87:285–290. doi: 10.1016/s0022-3476(75)80604-4. [DOI] [PubMed] [Google Scholar]
- Hanson JW, Myrianthopoulos NC, Sedgwick Harvey MA, Smith DW. Risks of the offspring of women treated with hydantoin anticonvulsants, with emphasis on the fetal hydantoin syndrome. J Pediatr. 1976;89:662–668. doi: 10.1016/s0022-3476(76)80414-3. [DOI] [PubMed] [Google Scholar]
- Harvey EA, Coull BA, Holmes LB. Anticonvulsant teratogenesis 5: Observer bias in a cohort study. Birth Defects Research (Part A): Clin Mol Teratology. 2003;67:452–456. doi: 10.1002/bdra.10055. [DOI] [PubMed] [Google Scholar]
- Heike CL, Upson K, Stuhaug E, Weinberg SM. 3D digital stereophotogrammetry: a practical guide to facial image acquisition. Head Face Med. 2010;28:6–18. doi: 10.1186/1746-160X-6-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Diaz S, Smith CR, Shen A, et al. Comparative safety of antiepileptic drugs during pregnancy. Neurology. 2012;78:1692–1699. doi: 10.1212/WNL.0b013e3182574f39. [DOI] [PubMed] [Google Scholar]
- Hill RM, Verniaud WN, Horning MG, McCulley LB, Morgan NF. Infants exposed in utero to antiepileptic drugs. N Engl J Med. 2001;344:1132–1138. doi: 10.1001/archpedi.1974.02110240031002. [DOI] [PubMed] [Google Scholar]
- Holmes LB, Coull BA, Dorfman J, Rosenberger PB. The correlation of deficits in IQ with midface and digit hypoplasia in children exposed in utero to anticonvulsant drugs. J Pediatr. 2005;146:118–122. doi: 10.1016/j.jpeds.2004.08.048. [DOI] [PubMed] [Google Scholar]
- Holmes LB, Hernandez-Diaz S. Newer anticonvulsants: lamotrigine, topiramate and gabapentin. Birth Defects Research (Part A): Clin Mol Teratology. 2012;94:599–606. doi: 10.1002/bdra.23028. [DOI] [PubMed] [Google Scholar]
- Jones KL, Lacro RV, Johnson KA, Adams J. Pattern of malformations in the children of women treated with carbamazepine during pregnancy. N Engl J Med. 1989;320:1661–1666. doi: 10.1056/NEJM198906223202505. [DOI] [PubMed] [Google Scholar]
- Lauer EA, Corner BD, Lim P, Beecher RM, Deutsch CK. Repeated-measure validation of craniofacial metrics from 3D surface scans: application to medical genetics. J Internat Soc Optical Engineering. 2002;4661:177–181. [Google Scholar]
- Leppig KA, Werler MM, Cann CI, Cook CA, Holmes LB. Predictive value of minor anomalies in association with major malformations. J Pediatr. 1987;110:531–537. doi: 10.1016/s0022-3476(87)80543-7. [DOI] [PubMed] [Google Scholar]
- Lu MCK, Sammel MD, Cleveland RH, Ryan LM, Holmes LB. Digit effects produced by prenatal exposure to antiepileptic drugs. Teratology. 2000;61:277–283. doi: 10.1002/(SICI)1096-9926(200004)61:4<277::AID-TERA6>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Mawer G, Clayton-Smith J, Coyle H, Kini U. Outcome of pregnancy in women attending an out-patient epilepsy clinic: adverse features associated with higher doses of sodium valproate. Seizure. 2002;11:512–518. doi: 10.1016/s1059-1311(02)00135-8. [DOI] [PubMed] [Google Scholar]
- Moorrees CFA. Natural head position. In: Jacobson A, Caulfield PW, editors. Introduction of Radiographic Cephalometry. Lea and Febiger; 1985. [Google Scholar]
- Nasri H, Deutsch CK, Holmes LB. Analyzing “The anticonvulsant face”. Birth Defects Res A Clin Mol Teratol. 2014 doi: 10.1002/bdra.23250. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ornoy A, Cohen E. Outcome of children born to epileptic mothers treated with carbamazepine during pregnancy. Arch Dis Child. 1996;75:517–520. doi: 10.1136/adc.75.6.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orup HI, Jr, Keith DA, Holmes LB. Prenatal anticonvulsant drug exposure: teratogenic effect on the dentition. J Craniofacial Genetics Devel Biol. 1998;18:129–137. [PubMed] [Google Scholar]
- Orup HI, Jr, Holmes LB, Keith DA, Coull BA. Craniofacial skeletal deviations following in utero exposure to the anticonvulsant phenytoin: monotherapy and polytherapy. Orthod Craniofacial Res. 2003;6:2–19. doi: 10.1046/j.1439-0280.2003.2o212.x. [DOI] [PubMed] [Google Scholar]
- Peller AJ, Holmes LB. Study exam vs. medical record review for fetal effects of anticonvulsant drugs: interrater disagreement. Birth Defects Research (Part A): Clinical and Molecular Teratology. 2006;76:373. [Google Scholar]
- Shaffer JP. Multiple Hypothesis Testing. Ann Rev Psych. 1995;46:561–584. [Google Scholar]
- VanLang QN, Tassinari MS, Keith DA, Holmes LB. Effect of in utero exposure to anticonvulsants on craniofacial development and growth. J Craniofacial Genet Dev Biol. 1984;4:115–133. [PubMed] [Google Scholar]
- Weinberg SM, Naidoo S, Govier DP, Martin RA, Kane AA, Marazita ML. Anthropometric precision and accuracy of digital three-dimensional photogrammetry: Comparing the Genex and 3dMD imaging systems with one another and with direct anthropometry. J Craniofac Surg. 2006;17:477–483. doi: 10.1097/00001665-200605000-00015. [DOI] [PubMed] [Google Scholar]
- Wong JY, Oh AK, Ohta E, Hunt AT, Rogers GF, Mulliken JB, Deutsch CK. Validity and Reliability of Craniofacial Anthropometric Measurements of 3D Digital Photogrammetric Images. Cleft-Lip Craniofacial Journal. 2008;45:232–239. doi: 10.1597/06-175. [DOI] [PubMed] [Google Scholar]


