Abstract
NK cells provide a vital surveillance against virally infected cells, tumor cells, and antibody-coated cells through the release of cytolytic mediators and gamma interferon (IFN-γ). Hexabromocyclododecane (HBCD) is a brominated flame retardant used primarily in expanded (EPS) and extruded (XPS) polystyrene foams for thermal insulation in the building and construction industry. Tetrabromobisphenol A (TBBPA) is used both as a reactive and an additive flame retardant in a variety of materials. HBCD and TBBPA contaminate the environment and are found in human blood samples. In previous studies, we have shown that other environmental contaminants, such as the dibutyltin (DBT) and tributyltin (TBT), decrease NK lytic function by activating mitogen-activated protein kinases (MAPKs) in the NK cells. HBCD and TBBPA also interfere with NK cell(s) lytic function. The current study evaluates whether HBCD and/or TBBPA have the capacity to activate MAPKs and MAPK kinases (MAP2Ks). The effects of concentrations of HBCD and TBBPA that inhibited lytic function on the phosphorylation state and total levels of the MAPKs (p44/42, p38, and JNK) and the phosphorylation and total levels of the MAP2Ks (MEK1/2 and MKK3/6) were examined. Results indicate that exposure of human NK cells to 10-0.5 µM HBCD or TBBPA activate MAPKs and MAP2Ks. This HBCD and TBBPA-induced activation of MAPKs may leave them unavailable for activation by virally infected or tumor target cells and thus contributes to the observed decreases in lytic function seen in NK cells exposed to HBCD and TBBPA.
Keywords: Hexabromocyclododecane, MAPK, MAP2K, MAP3K Tetrabromobisphenol A, NK cells
Introduction
Human NK cells are involved in the prevention of tumor development and viral infection due to their capacity to lyse mutated or infected cells and secrete cytokines (Lotzova, 1993). NK cells are a crucial immune defense against malignant neoplasms and viral infections (Lotzova, 1993; Vivier et al., 2004). Their role in protecting against cancers and viral infections is demonstrated by increased incidence of neoplasm and viral infection in individuals that lack NK cells (Ballas et al. 1990; Fleisher et al., 1982; Biron et al., 1989).
Mitogen activated protein kinases (MAPKs) and their upstream activators MAPK kinases (MAP2Ks) and MAP2K kinases (MAP3K) are part of the signaling pathways that regulate the function of human natural killer (NK) cells. The MAPK, p44/42 has been shown to be required for NK lysis of tumor target cells (Trotta et al., 1998; Vivier et al., 2004)), while both p44/42 and p38 activation appear to be needed for the secretion of cytokines (tumor necrosis factor alpha (TNFα) and interferon gamma (IFNγ) (Trotta et al., 1998; Trotta et al., 2000).
Hexabromocyclododecane (HBCD) and tetrabomobisphenol A (TBBPA) are widely used brominated flame retardants. TBBPA is used in plastics, paper, and textiles as a flame retardant (HSBD, 2001; IPCS/WHO, 1995). Human exposure occurs by inhalation, dermal contact, and ingestion (Birnbaum and Staskal, 2004; Peterman et al., 2000; HSBD, 2001; IPCS/WHO, 1995). TBBPA has additional uses as a plasticizer and as an intermediate in the production of other flame retardants (IPCS/WHO, 1995; HSBD, 2001, Gain, 1997). It has been found in commercial drinking water (Peterman et al., 2000) and in seafood (IPCS/WHO, 1995). TBBPA is found in human blood samples at levels of approximately 0.24 to 4.5 ng/g lipid which converts to about 0.002–0.034 nM, (Hagmar et al., 2000; Nagayama et al., 2001; Thomsen et al., 2002) indicating that a certain fraction of TBBPA accumulates in the body despite the fact that it appears to be eliminated to some extent from the human system (Schauer et al., 2006). HBCD is used as a flame retardant in polystyrene foams that are used as thermal insulation (Kajiwara et al. 2009). Upholstery materials such as those used in furniture and draperies are also treated with HBCD to reduce flammability (Kajiwara et al. 2009). It is not chemically bound to treated materials and thus can easily leach from their surface (de Wit, 2002). HBCD is found in dust particles in the air (Abdallah et al., 2008) and bioaccumulates in the food chain (Covaci et al., 2006). It has been detected in marine life (Peck et al., 2008) and bird eggs (Bustnes et al., 2007; Janak et al., 2008; Polder et al., 2008). Humans are exposed to HBCD through inhalation and diet (Abdallah et al., 2008; Knutsen et al., 2008; van Leeuwen et al., 2008). Due to these routes of exposure HBCD levels of 200 pg/g serum, which is approximately 0.3 nM, are found in human blood (Covaci et al., 2006; Knutsen et al., 2008). It is also found in human adipose tissue (Pulkrabova et al., 2009), and breast milk (Kakimoto et al., 2008; Thomsen et al., 2005; Thomsen et al., 2003).
There are limited studies on the toxicological effects of HBCD and TBBPA on humans (Hinkson and Whalen, 2009; Hinkson and Whalen, 2010; Kibakaya et al., 2009). However there have been studies on lab animals. van der Ven et al found when rats were exposed to HBCD, there was an increase in liver and pituitary weight, and also increased levels of cholesterol (van der Ven et al., 2006). HBCD has also been shown to have neurotoxic effects in mice (Eriksson et al., 2006) and to increase hepatic cytochrome P450 levels in rats (Germer et al., 2006). TBBPA was shown to increase the activity of glutathione reductase in rainbow trout (Ronisz et al., 2004). In mice it has been shown to cause decreases in serum proteins and red blood cells as well as increases in spleen weight (IPCS/WHO, 1995). Administration of TBBPA to newborn rats caused polycystic kidney lesions (Fukuda et al., 2004). Administration of TBBPA to Wistar rats showed effects on circulating thyroid hormone, T4, levels (Van der Ven et al., 2006). This same study showed that there were no effects of TBBPA exposure on the function of splenic natural killer (NK) cells in exposed rats or their offspring (Van der Ven et al., 2006). In vitro studies of TBBPA showed that it was able to compete with thyroid hormone, T4, for binding to human transthyretin (thyroid hormone transport protein) (Meerts et al., 2000).
Previous studies in our laboratory have shown that human NK cells exposed to HBCD or TPBPA exhibit significantly decreased lytic function and cell surface protein expression (Hinkson and Whalen, 2009; Hinkson and Whalen, 2010; Kibakaya et al., 2009; Hurd and Whalen, 2011). In the current study, we examine the activation states of the MAPK pathway in NK cells. If the functional status of this pathway were altered by either HBCD or TBBPA, then this could explain at least in part the loss of NK lytic function seen with exposure to these compounds.
Materials and Methods
Isolation of NK cells
Peripheral blood from healthy adult (male and female) donors was used for this study. Buffy coats (source leukocytes) obtained from Key Biologics, LLC (Memphis, TN) were used to prepare NK cells. Highly-purified NK cells were obtained using a rosetting procedure. Buffy coats were mixed with 0.8 ml of RosetteSep human NK cell enrichment antibody cocktail (StemCell Technologies, Vancouver, British Columbia, Canada) per 45 ml of buffy coat. The mixture was incubated for 20 min at room temperature (~25°C). Following the incubation, 7–8 ml of the mixture was layered onto 4 ml of Ficoll-Hypaque (1.077 g/ml; MP Biomedicals, Irvine, CA) and centrifuged at 1200 × g for 50 min. The cell layer was then collected and washed twice with phosphate-buffered saline (PBS; pH 7.2) and stored in complete media (RPMI-1640 supplemented with 10% heat-inactivated bovine calf serum [BCS], 2 mM l-glutamine, and 50 U penicillin G\50 µg streptomycin/ml) at 1 million cells/ml (Whalen et al., 2002).
Chemical preparation
TBBPA (purchased from Fisher Scientific, 97% pure) was dissolved in dimethyl sulfoxide (DMSO) (Sigma-Aldrich, St. Louis, MO) to yield a 100 mM stock solution. Desired concentrations of TBBPA were then prepared by dilution of the stock into complete media. The final concentration of DMSO in any of the TBBPA exposures did not exceed 0.01%.
Cell Treatments
NK cells (at a concentration of 3 million cells/ mL) were exposed in the following ways. 1. TBBPA or HBCD for 10 minutes: Cells were treated with the appropriate (DMSO) control or 10, 5, 2.5, 1, and 0.5 µM TBBPA or HBCD for 10 min at 37°C, 5%CO2. 2. TBBPA or HBCD for 1 hour: Cells were treated with the appropriate control or 10, 5, 2.5, 1, and 0.5 µM TBBPA or HBCD for 1 h at 37°C, 5%CO2. 3. TBBPA or HBCD for 6 hours: Cells were treated with the appropriate control or 10, 5, 2.5, 1, and 0.5 µM TBBPA or HBCD for 6 h at 37°C, 5%CO2. Following the above incubations the cells were washed twice and then lysed as described below.
Cell Viability
Cell viability was determined by trypan blue exclusion. Viability was determined at each concentration of TBBPA or HBCD. The viability of treated cells was then compared to that of control cells at each length of exposure. Viability of cells treated with the compounds was unchanged compared to controls. Additionally, activation of caspase-3 was monitored as an indicator of apoptosis.
Cell Lysates
Cell lysates were made using NK cells treated as described in the cell treatment section. Following the above treatments, the cells were centrifuged and the cell pellets were lysed using 500 µL of lysis buffer (Active motif, Carlsbad, CA) per 10 million cells. The cell lysates were stored frozen at −80°C up to the point when they were run on SDS-PAGE. All controls and TBBPA or HBCD-exposed cells for a given experimental set-up (described above) were from an individual donor. Each of the experimental set-ups was repeated a minimum of three times using cells from different donors.
Western Blot
Cell lysates were run on 10% sodium dodecylsulfate polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to polyvinylidene difluoride (PVDF) membrane. To analyze MAPKS, the PVDF was immunoblotted with antibodies to phospho-p38 (Thr180 and Tyr182), p38, phospho-p44/42 (Thr202/Tyr204), p44/42, phospho-JNK, JNK, and β-actin (Cell Signaling Technologies, Beverly, MA). For analysis of MAP2Ks, the membrane was blotted with antibodies to phospho-MEK, MEK, phosphor-MKK3/6, MKK3/6 and β-actin. Antibodies were visualized using ECL chemiluminescent detection system (Amersham, Piscataway, NJ) and Kodak Image Station (Kodak, Rochester, NY) or UVP imager (Upland, CA). To analyze for caspase 3 and cleaved caspase 3 levels, blots were probed with an antibody that recognizes both intact and cleaved caspase 3 (Cell Signaling Technologies). The density of each protein band was determined by densitometric analysis using the Kodak Image Station or UVP imager analysis software. The settings on the imagers were optimized to detect the largest possible signal range and to prevent saturation of the system. A given experimental set-up (as described in the cell treatment section) always had its own internal control. Thus, changes in protein expression were determined relative to the internal control. This determination provided relative quantitation by evaluating whether a given treatment changed expression of the specified protein relative to untreated cells. The density of each protein band was normalized to β-actin to correct for small differences in protein loading among the lanes.
Statistical analysis
Statistical analysis of the data for western blot analyses was carried out using ANOVA followed by pair-wise comparison using Student’s t test. A minimum of three separate determinations were carried out for each experimental set-up (n≥3) and statistical significance was noted at p<0.05.
RESULTS
Phospho-p44/42, p44/42, phospho-p38, p38, phospho-JNK, JNK in NK Cells Exposed to TBBPA for 10 minutes
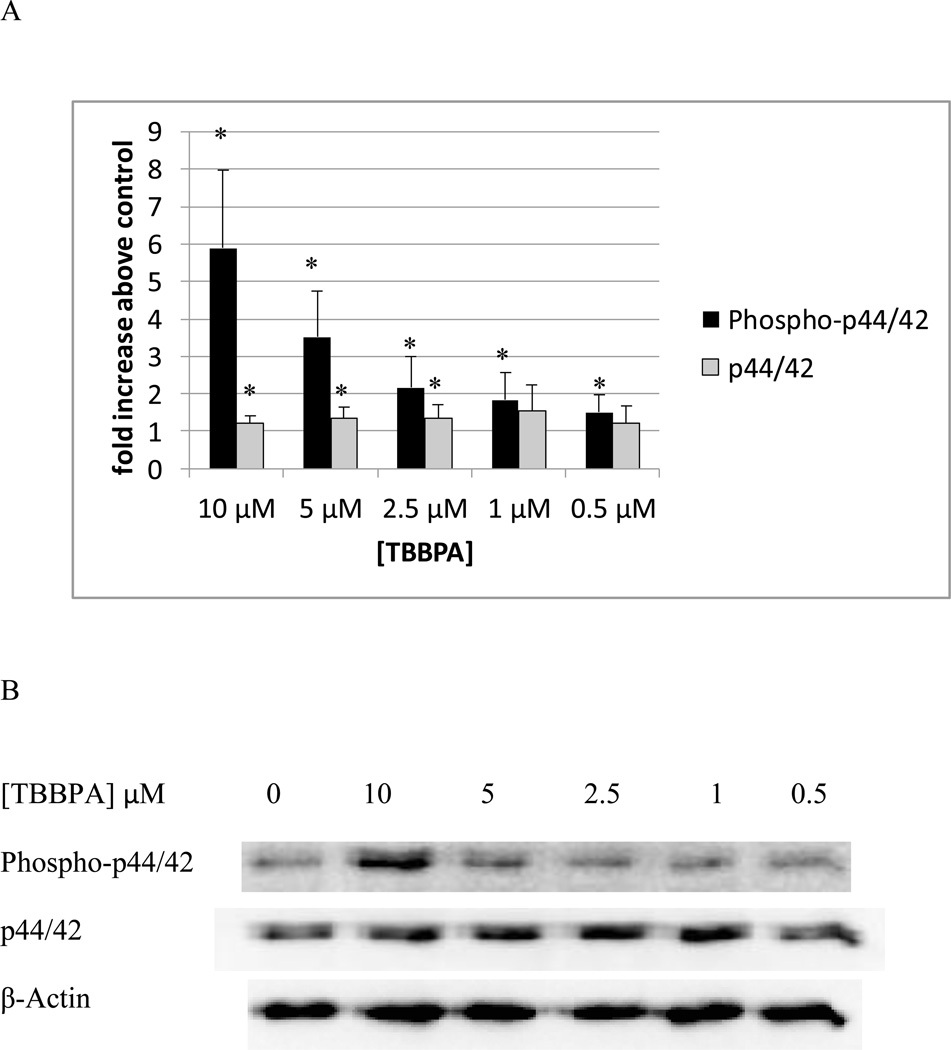
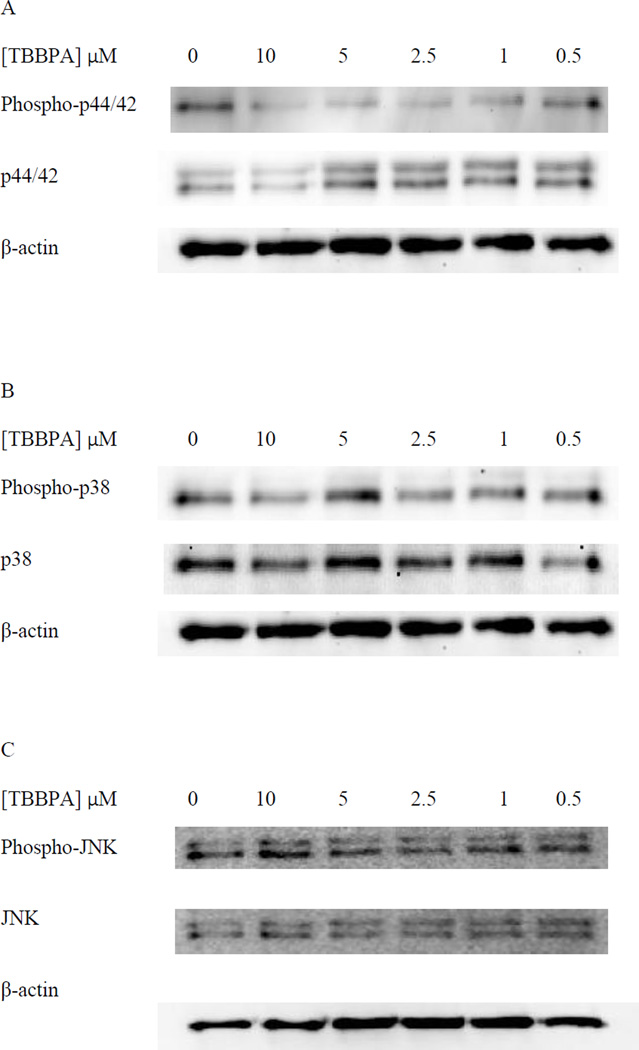
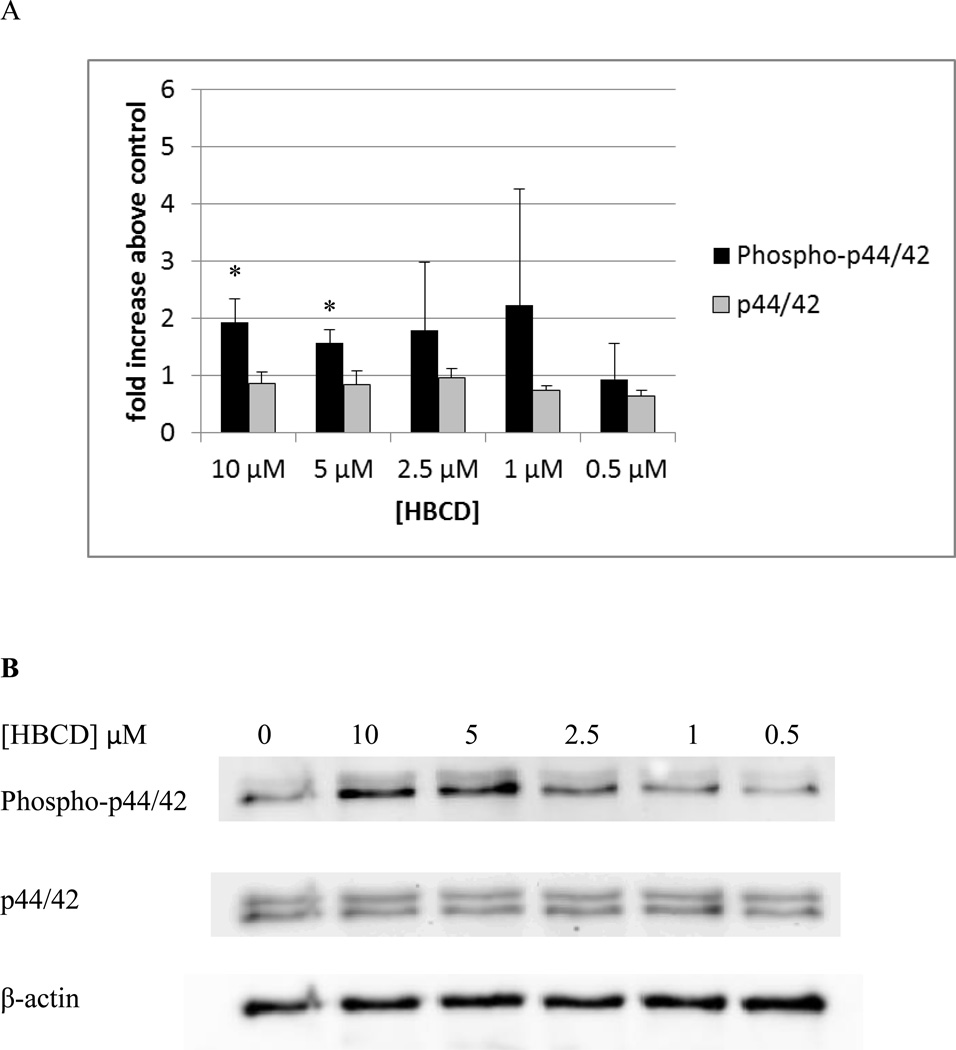
Figure 1A shows the levels of phospho-p44/42 and p44/42 in NK cells exposed to 10 µM – 0.5 µM TBBPA for 10 minutes relative to control levels. There was a statistically significant increase (p<0.05) in the phosphorylation (activation) of p44/42 at every concentration of TBBPA examined. The increase in phosphorylation was nearly 6 fold at the 10 µM concentration (p<0.001) at 5 µM the increase was 3.5 fold (p<0.001) while the increases at 2.5 and 1 µM were 2 fold (p<0.02). The increase seen at 0.05 µM TBBPA for 10 minutes was 1.5 fold (p<0.05). Small but significant increases in total p44 of about 1.2–1.3 fold were seen at 10 µM, 5 µM, and 2,5 µM (Figure 1A). Figure 1B shows data from a representative blot.
Figure 1. Effects of 10 minute exposures to 10- 0.5 µM TBBPA on phospho-p44/42 and total p44/42 in NK cells.
A) levels of phospho-p44/42 and total p44/42 normalized to control in pure NK. Values are mean ± S.D. from 8 separate experiments using cells from 8 different donors. * indicates significant difference compared to the control (p<0.05). The density of each protein band was normalized to β-actin to correct for small differences in protein loading among the lanes. B) Blot from a representative experiment.
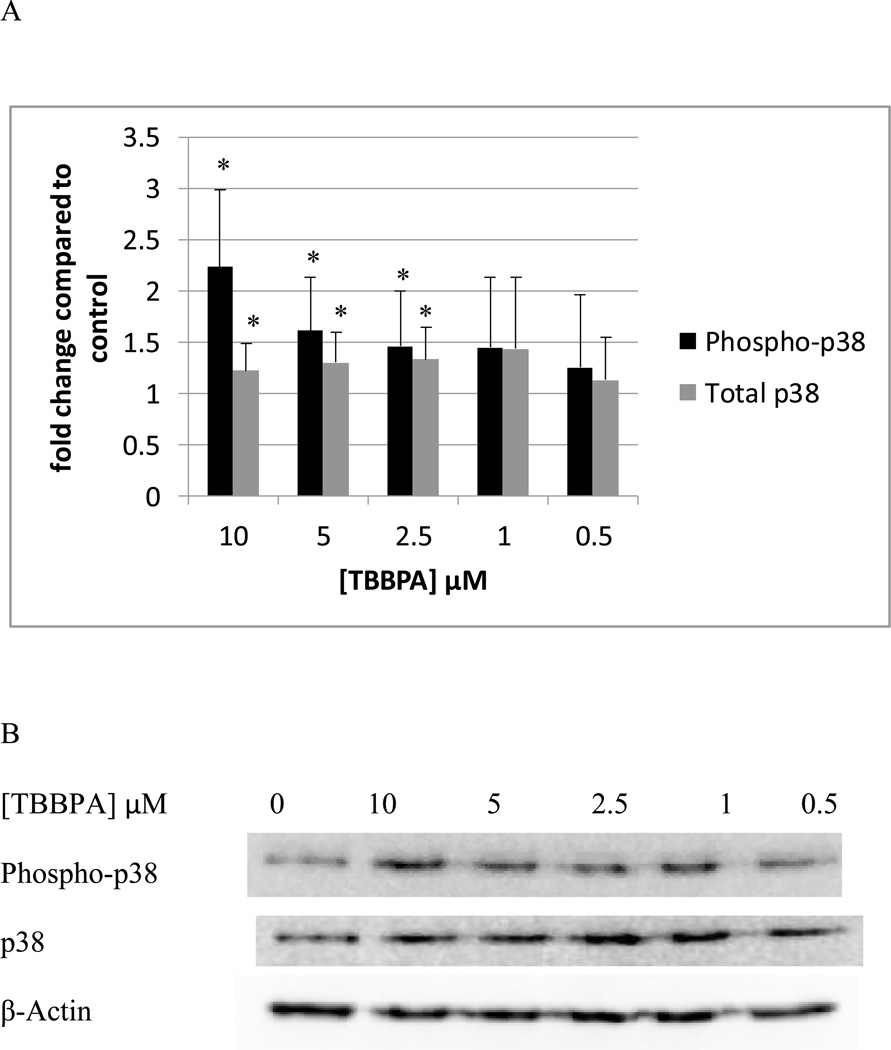
In NK cells exposed to TBBPA for 10 min, there were statistically significant increases in the phosphorylation of p38 at 10 µM, 5 µM, and 2.5 µM. 10 µM TBBPA caused an approximately 2 fold increase (p<0.01) in phosphorylation as compared to control cells, 5 and 2.5 µM TBBPA increased phosphorylation of p38 by about 1.5 fold (p<0.05) (Figure 2A). As was seen with p44/42, there were small but significant increases in total p38 of about 1.2– 1.3 fold at 10 µM, 5 µM, and 2,5 µM (p<0.05) (Figure 2A). Data from a representative blot is shown in Figure 2B.
Figure 2. Effects of 10 minute exposures to 10-0.5 µM TBBPA on phospho-p38 and total p38 in NK cells.
A) levels of phospho-p38 and total p38 normalized to control in pure NK. Values are mean ± S.D. from 8 separate experiments using cells from 8 different donors. * indicates significant difference compared to the control (p<0.05). Data was treated as described in Figure 1. B) Blot from a representative experiment.
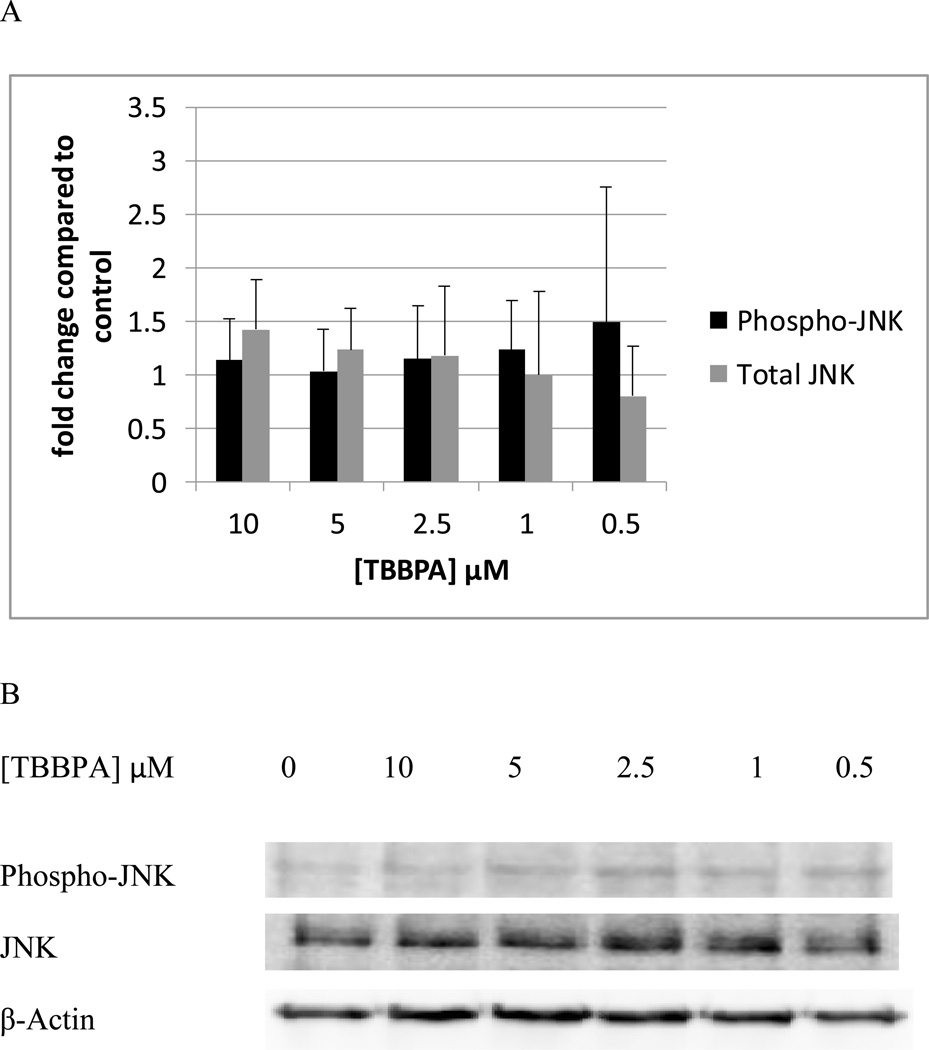
NK cells exposed to TBBPA for 10 min, showed no statistically significant changes in the phosphorylation of total JNK levels at any of the TBBPA concentrations that were tested (Figure 3A). Data from a representative blot is shown in Figure 3B.
Figure 3. Effects of 10 minute exposures to 10-0.5 µM TBBPA on phospho-JNK and total JNK in NK cells.
A) levels of phospho-JNK and total JNK normalized to control in pure NK. Values are mean ± S.D. from 8 separate experiments using cells from 8 different donors. * indicates significant difference compared to the control (p<0.05). Data was treated as described in Figure 1. B) Blot from a representative experiment.
Phospho-p44/42, p44/42, phospho-p38, p38, phospho-JNK, JNK in NK Cells Exposed to TBBPA for 1 hour
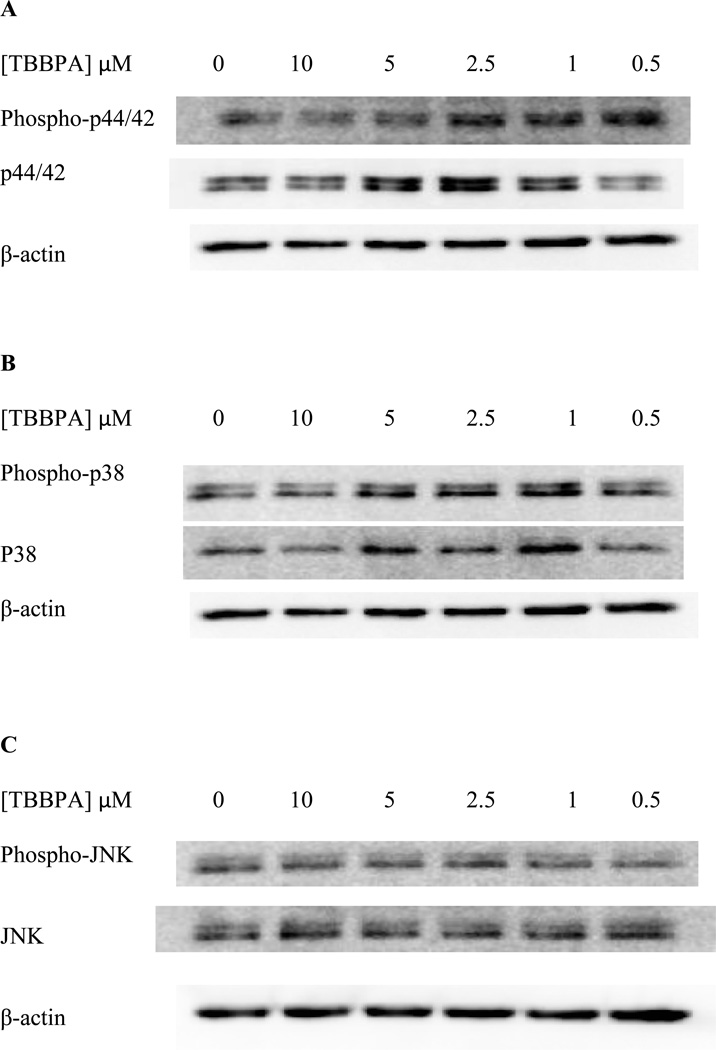
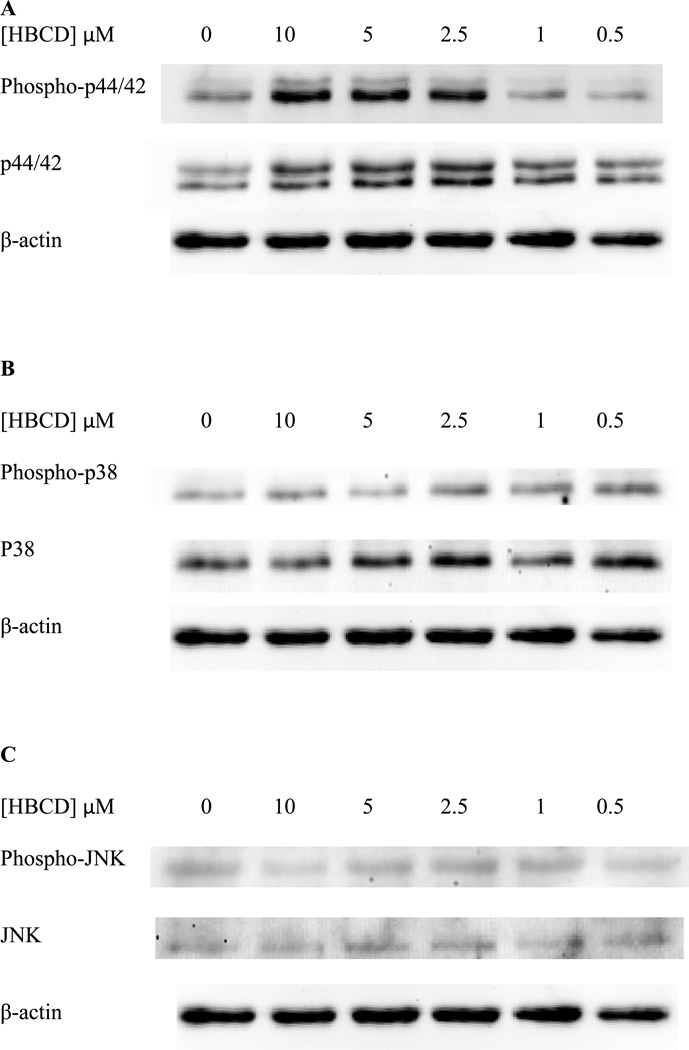
When NK cells were treated with TBBPA for 1 h, there were no statistically significant changes (p>0.05) in the phosphorylation (activation) of p44/42 at any concentration of TBBPA examined (Figure 4A). Cells from a total of 5 donors were examined in 5 separate experiments. The fold change was calculated compared to control (small differences in protein loading were corrected for by normalizing each band to β-actin). The 10 µM exposure showed fold changes of 0.8, 1.0, 0.9, 3.8, and 1.2 fold. As mentioned above, when statistical analysis was done there was no significant change compared to control (P>0.05). Exposure to 5 µM TBBPA for 1 h gave a range of fold changes of 0.7–1.4 fold, and again when the results from these 5 experiment using cells from 5 separate donors were combined there was no statistically significant change (p>0.05) in p44/42 phosphorylation compared to the control. All other concentrations of TBBPA also showed no statistically significant changes when the fold changes from 5 experiments were averaged. The same was true for p38 (Figure 4B). Phosphorylation of JNK was unaffected by TBBPA exposures except at the 2.5 µM concentration where there was a 1.8 fold over control cells (Figure 4C).
Figure 4. Effects of 1 hour exposures to 10-0.5 µM TBBPA on phospho-p44/42, p44/42, phospho-p38, p38, phospho-JNK, and JNK in NK cells.
A) representative blot of levels of phospho-p44/42 and total p44/42. B) representative blot of levels of phospho-p38 and total p38. C) representative blot of levels of phospho-JNK and total JNK. 5 separate experiments using cells from 5 different donors were carried out.
Phospho-p44/42, p44/42, phospho-p38, p38, phospho-JNK, JNK in NK Cells Exposed to TBBPA for 6 hours
Exposure of NK cells to 05–10 µM TBBPA for 6 hours caused no statistically significant changes in the phosphorylation state of any of the MAPKS. A total of 6 experiments using cells from 6 separate donors were carried out. The density of each protein band was normalized to β-actin to correct for small differences in protein loading among the lanes. As was described for the 1 hour data, there were a range of changes seen at each concentration that varied widely from one donor to the next (i.e 10 µM TBBPA caused fold changes ranging from 0.3–1.3 fold). Thus, when the data from all experiments was combined there were no statistically significant changes (p>0.05). Representative blots are shown in Figure 5.
Figure 5. Effects of 6 hour exposures to 10-0.5 µM TBBPA on phospho-p38, p38, phospho-JNK, and JNK in NK cells.
A) representative blot of levels of phospho-p44/42 and total p44/42. B) representative blot of levels of phospho-p38 and total p38. C) representative blot of levels of phospho-JNK and total JNK. 6 separate experiments using cells from 6 different donors were carried out.
Phospho-p44/42, p44/42, phospho-p38, p38, phospho-JNK, JNK in NK Cells Exposed to HBCD for 10 minutes
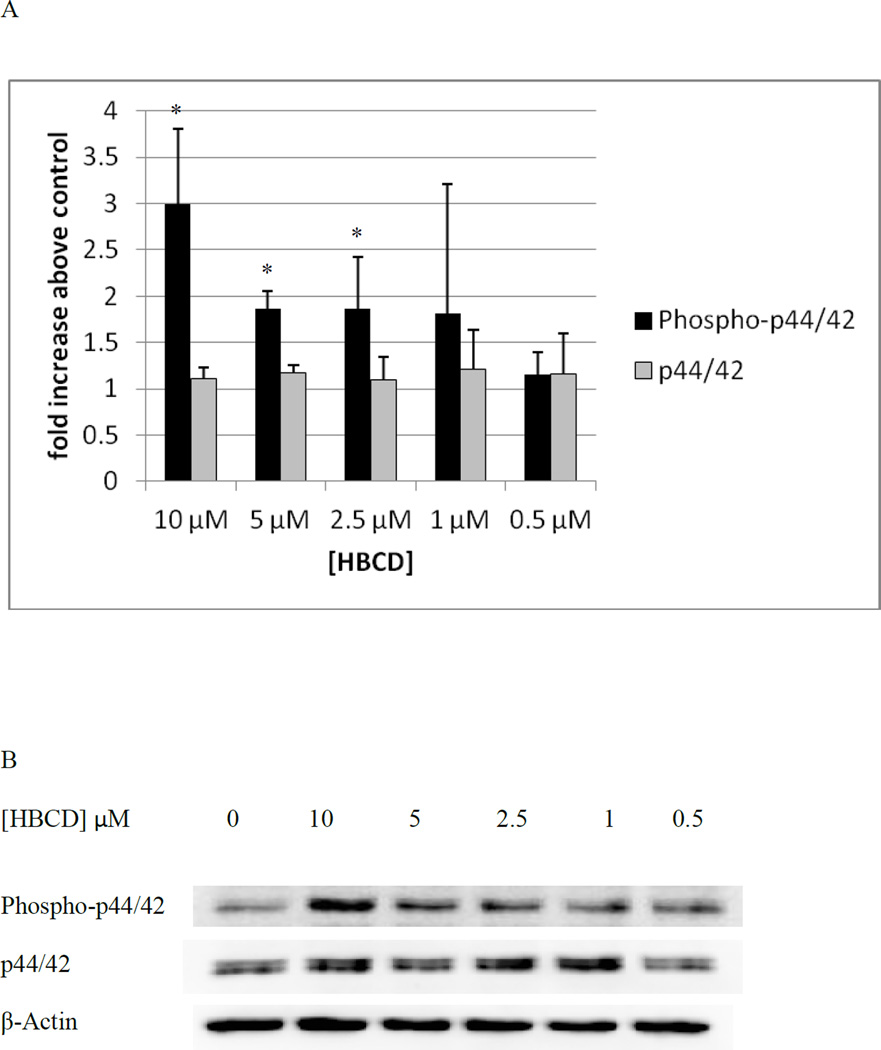
Figure 6A shows the levels of phospho-p44/42 and p44/42 in NK cells exposed to 10 µM – 0.5 µM HBCD for 10 minutes relative to control levels. There were statistically significant increases in the phosphorylation (activation) of p44/42 at 10, 5, and 2.5 µM. The increase in phosphorylation was 3 fold (p<0.01) at the 10 µM concentration, at 5 and 2.5 µM the increase was 1.9 fold (p<0.01 for 5 µM, p<0.03 for 2.5 µM). Figure 6B shows data from a representative blot.
Figure 6. Effects of 10 minute exposures to 10- 0.5 µM HBCD on phospho-p44/42 and total p44/42 in NK cells.
A) levels of phospho-p44/42 and total p44/42 normalized to control in pure NK. Values are mean ± S.D. from 5 separate experiments using cells from 5 different donors. * indicates significant difference compared to the control (p<0.05). Data was treated as described in Figure 1. B) Blot from a representative experiment.
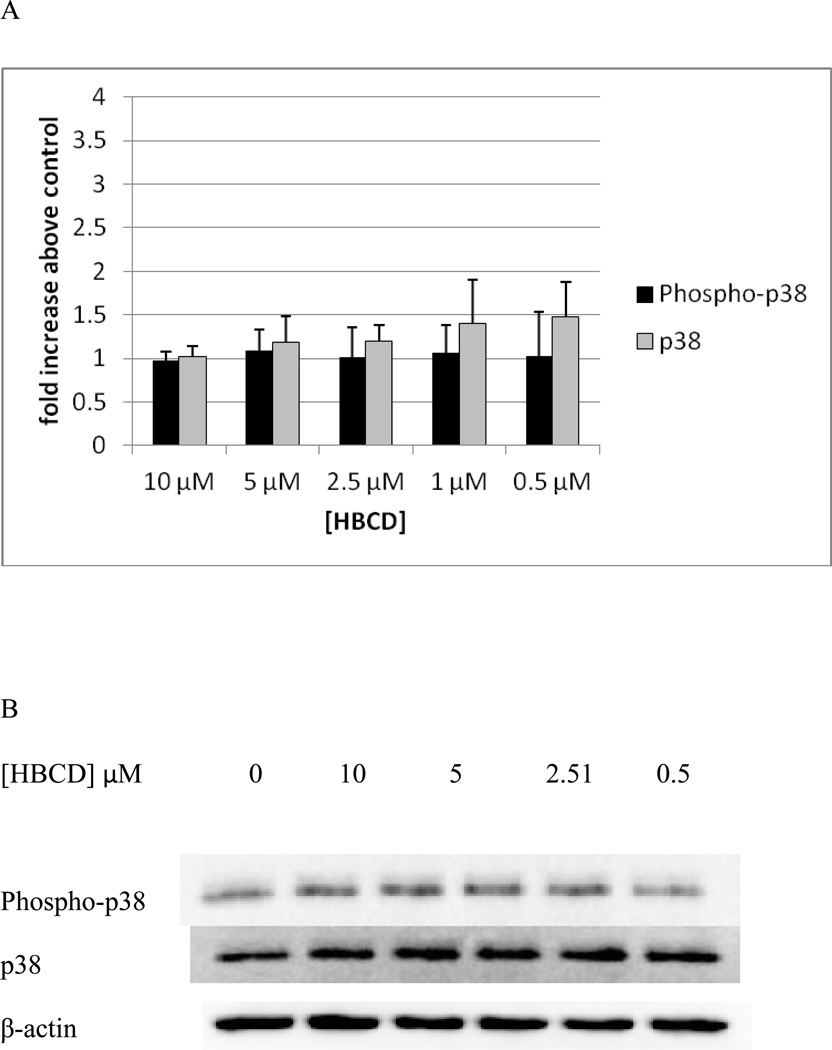
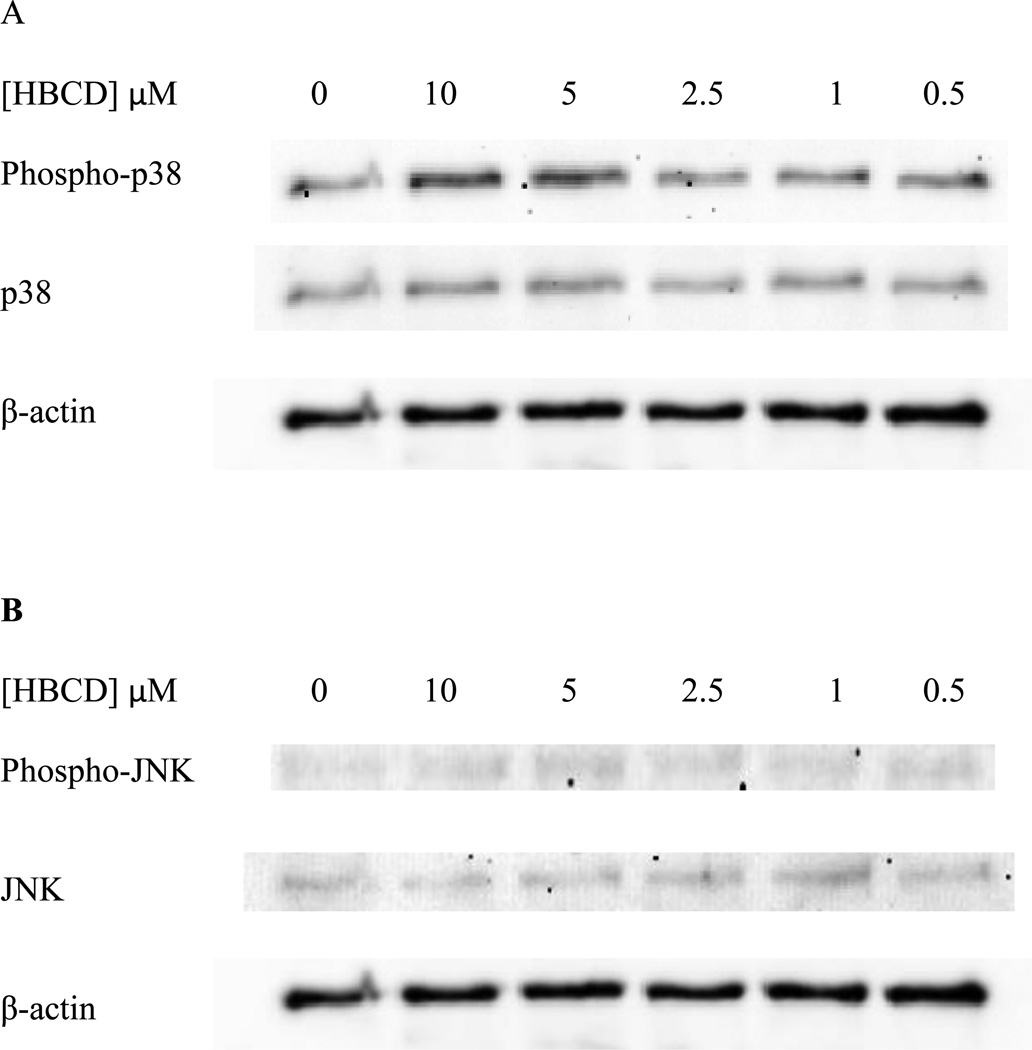
Exposure of NK cells HBCD for 10 min caused no significant increases in the phosphorylation of p38. (Figure 7A). Data from a representative blot is shown in Figure 7B.
Figure 7. Effects of 10 minute exposures to 10-0.5 µM HBCD on phospho-p38 and total p38 in NK cells.
A) levels of phospho-p38 and total p38 normalized to control in pure NK. Values are mean ± S.D. from 5 separate experiments using cells from 5 different donors. * indicates significant difference compared to the control (p<0.05). Data was treated as described in Figure 1. B) Blot from a representative experiment.
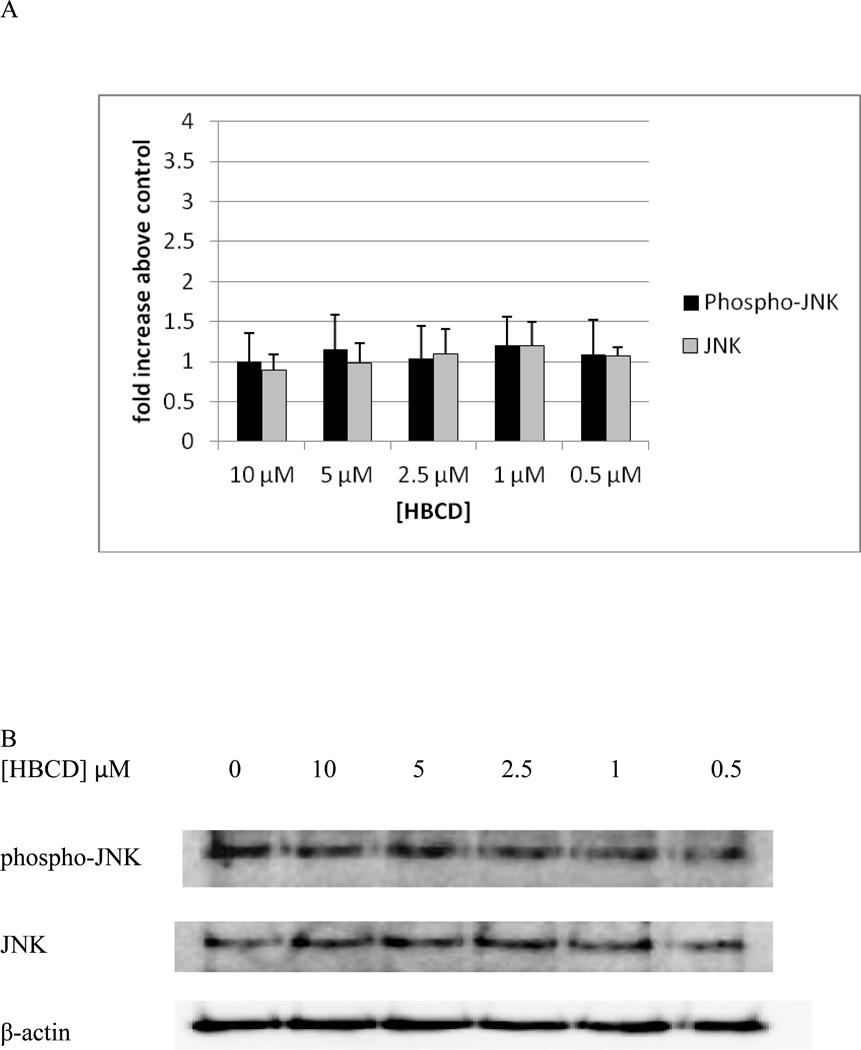
NK cells exposed to HBCD for 10 min, showed no statistically significant changes in the phosphorylation of total JNK levels at any of the HBCD concentrations that were tested (Figure 8A). Data from a representative blot is shown in Figure 8B.
Figure 8. Effects of 10 minute exposures to 10-0.5 µM HBCD on phospho-JNK and total JNK in NK cells.
A) levels of phospho-JNK and total JNK normalized to control in pure NK. Values are mean ± S.D. from 5 separate experiments using cells from 5 different donors. * indicates significant difference compared to the control (p<0.05). Data was treated as described in Figure 1. B) Blot from a representative experiment.
Phospho-p44/42, p44/42, phospho-p38, p38, phospho-JNK, JNK in NK Cells Exposed to HBCD for 1 hour
Exposure of NK cells to HBCD for 1 h caused statistically significant changes in the phosphorylation (activation) of p44/42 at the 10 and 5 µM exposures (p<0.03) Figure 9A. Figure 9 B shows data from a representative blot. p38 and JNK showed no significant changes in phosphorylation at any of the concentrations tested. As was described for the 1 hour and 6 h data for TBBPA, there were a range of changes seen in p38 phosphorylation at each HBCD concentration that varied widely from one donor (total of 4 donors tested) to the next (i.e 10 µM HBCD caused fold changes ranging from 0.7–1.4 fold and 5 µM HBCD caused fold changes ranging from 0.7–1.2 fold). Thus, when the data from all experiments was combined there were no statistically significant changes (p>0.05). Representative blots are shown in Figure 10.
Figure 9. Effects of 1 h exposures to 10- 0.5 µM HBCD on phospho-p44/42 and total p44/42 in NK cells.
A) levels of phospho-p44/42 and total p44/42 normalized to control in pure NK. Values are mean ± S.D. from 4 separate experiments using cells from 4 different donors. * indicates significant difference compared to the control (p<0.05). β-actin levels were determined for each condition to verify that equal amounts of protein were loaded. In addition, the density of each protein band was normalized to β-actin to correct for small differences in protein loading among the lanes. B) Blot from a representative experiment.
Figure 10. Effects of 1 hour exposures to 10-0.5 µM HBCD on phospho-p38, p38, phospho-JNK, and JNK in NK cells.
A) representative blot of levels of phospho-p38 and total p38. B) representative blot of levels of phospho-JNK and total JNK. 4 separate experiments using cells from 4 different donors were carried out.
Phospho-p44/42, p44/42, phospho-p38, p38, phospho-JNK, JNK in NK Cells Exposed to HBCD for 6 hours
As with TBBPA, NK cells exposed to 0.5–10 µM HBCD for 6 hours exhibited no significant changes in the phosphorylation state of any of the MAPKS. Representative blots are shown in Figure 11. However, as can be seen from the representative blot there were increases in phosphorylation of p44/42 at the 10 µM exposure. These increases varied widely from one donor to the next (10-1.4 fold) and were not statistically significant due to this. Only the 10 µM exposure showed increases in every experiment
Figure 11. Effects of 6 hour exposures to 10-0.5 µM HBCD on phospho-p44/42, p44/42, phospho-p38, p38, phospho-JNK, and JNK in NK cells.
A) representative blot of levels of phospho-p44/42 and total p44/42. B) representative blot of levels of phospho-p38 and total p38. C) representative blot of levels of phospho-JNK and total JNK. 4 separate experiments using cells from 4 different donors were carried out.
Effects of TBBPA and HBCD exposures on the activation states of MAP2Ks, MEK1/2 and MKK3/6 in NK cells
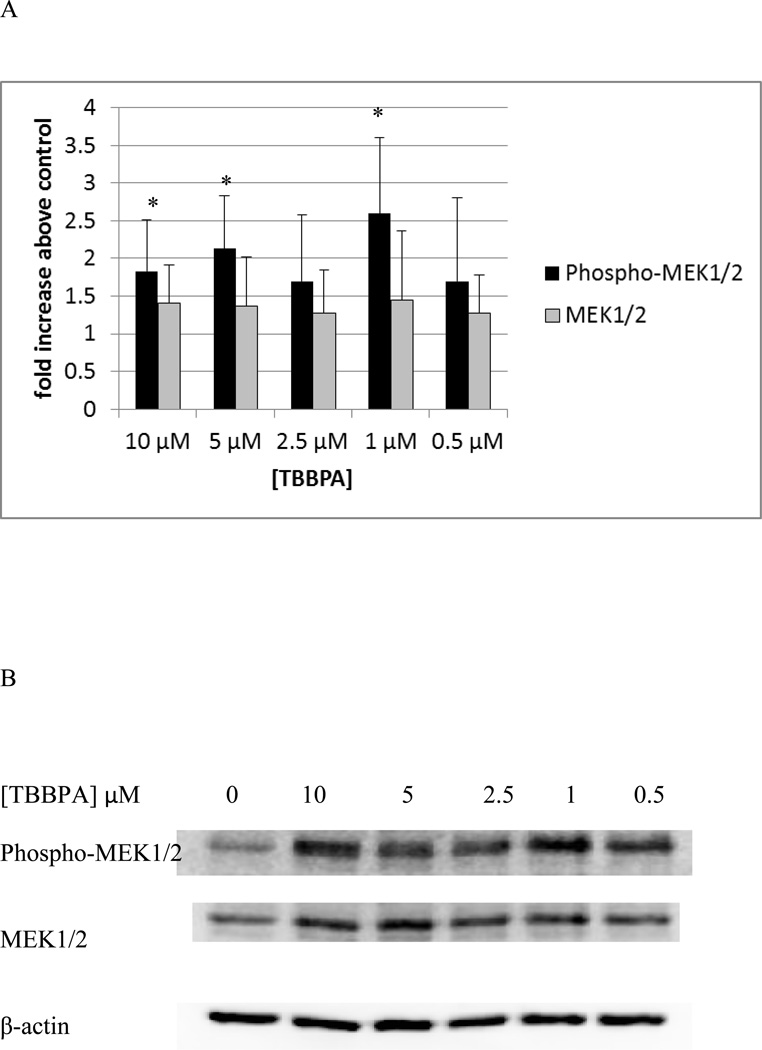
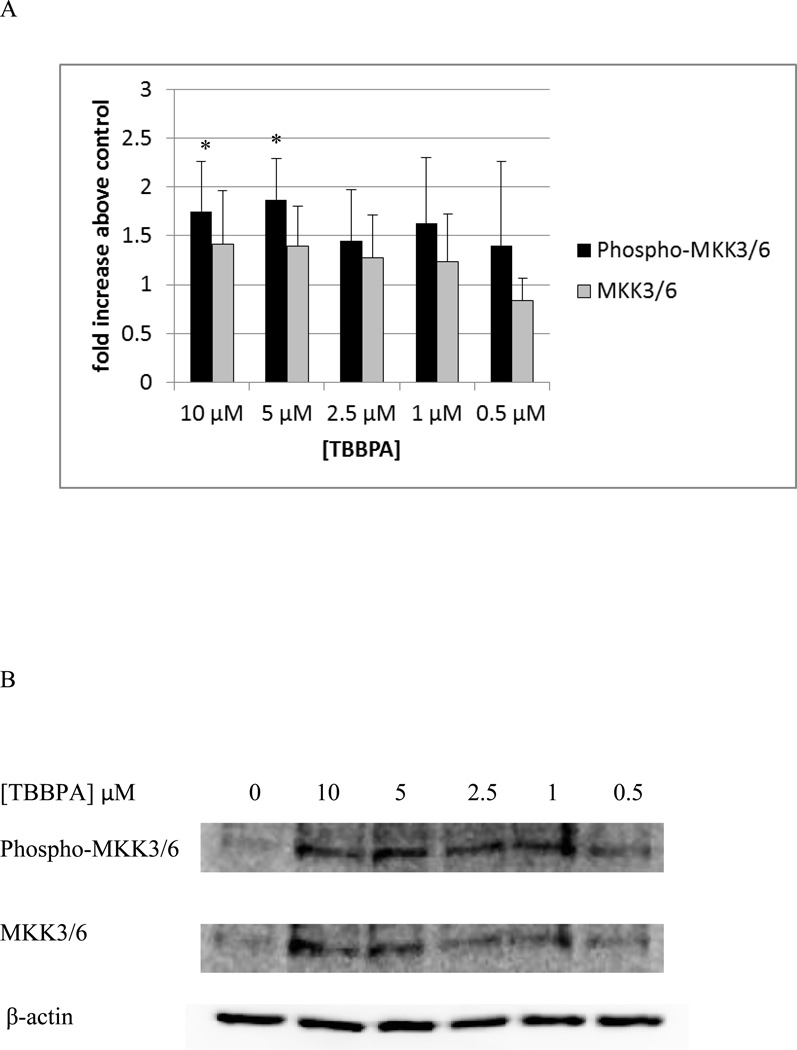
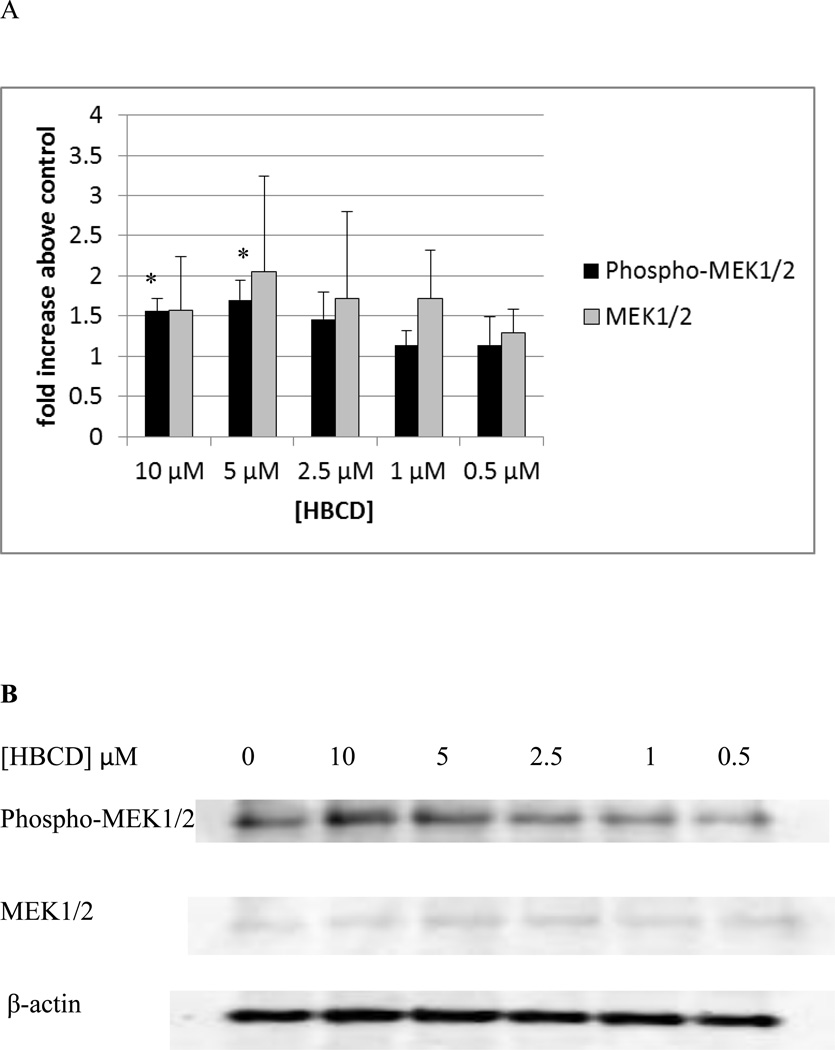
Ten minute exposures of NK cells to both TBBPA and HBCD caused substantial increases in the activation of p44/42. Thus, we examined if the immediate upstream activator of p44/42, MEK1/2, showed increased phosphorylation when exposed to these compounds. Figure 12 shows the effects of 10 minute exposures of NK cells to TBBPA on phosphorylation of MEK1/2. Phosphorylation was significantly increased about 1.8 and 2.1 fold (p<0.05) by exposures to 10 and 5 µM TBBPA. Exposure to 2.5 µM TBBPA also caused increased in phosphorylation of MEK in 6 out of 7 donors however the increases ranged rather broadly (1.1 to 3 fold) with an average increase of 1.7 fold. A ten minute exposure to 1 µM TBBPA also caused a statistically significant increase in phospho-MEK of 2.6 fold (p<0.05). TBBPA was also able to activate the immediate upstream activator of p38, MKK3/6. There was a 1.8 fold (p<0.05) increase in MKK3/6 phosphorylation when cells were exposed to 10 µM TBBPA for 10 min and a 1.9 fold (p<0.05) increase with a 5 µM exposure (Figure 13). The effects of 10 minute exposures to HBCD on the MEK1/2 are shown in Figure 14. Phosphorylation of MEK1/2 was significantly increased by 10 minute exposures to 10 and 5 µM HBCD, 1.6 and 1.7 fold respectively. We also examined the effects of TBBPA on the upstream activator of MEK, Raf-1, we found no significant activation of Raf-1 at any of the time points examined.
Figure 12. Effects of 10 minute exposures to 10- 0.5 µM TBBPA on phospho-MEK1/2 and total MEK1/2 in NK cells.
A) levels of phospho-MEK1/2 and total MEK normalized to control in pure NK. Values are mean ± S.D. from 7 separate experiments using cells from 7 different donors. * indicates significant difference compared to the control (p<0.05). Data was treated as described in figure 1. B) Blot from a representative experiment.
Figure 13. Effects of 10 minute exposures to 10- 0.5 µM TBBPA on phospho-MKK3/6 and total MKK3/6 in NK cells.
A) levels of phospho-MKK3/6 and total MKK3/6 normalized to control in pure NK. Values are mean ± S.D. from 5 separate experiments using cells from 5 different donors. * indicates significant difference compared to the control (p<0.05). Data was treated as described in figure 1. B) Blot from a representative experiment.
Figure 14. Effects of 10 minute exposures to 10- 0.5 µM HBCD on phospho-MEK1/2 and total MEK1/2 in NK cells.
A) levels of phospho-MEK1/2 and total MEK normalized to control in pure NK. Values are mean ± S.D. from 4 separate experiments using cells from 4 different donors. * indicates significant difference compared to the control (p<0.05). Data was treated as described in figure 1. B) Blot from a representative experiment.
Effects of TBBPA and HBCD exposures on Caspase-3
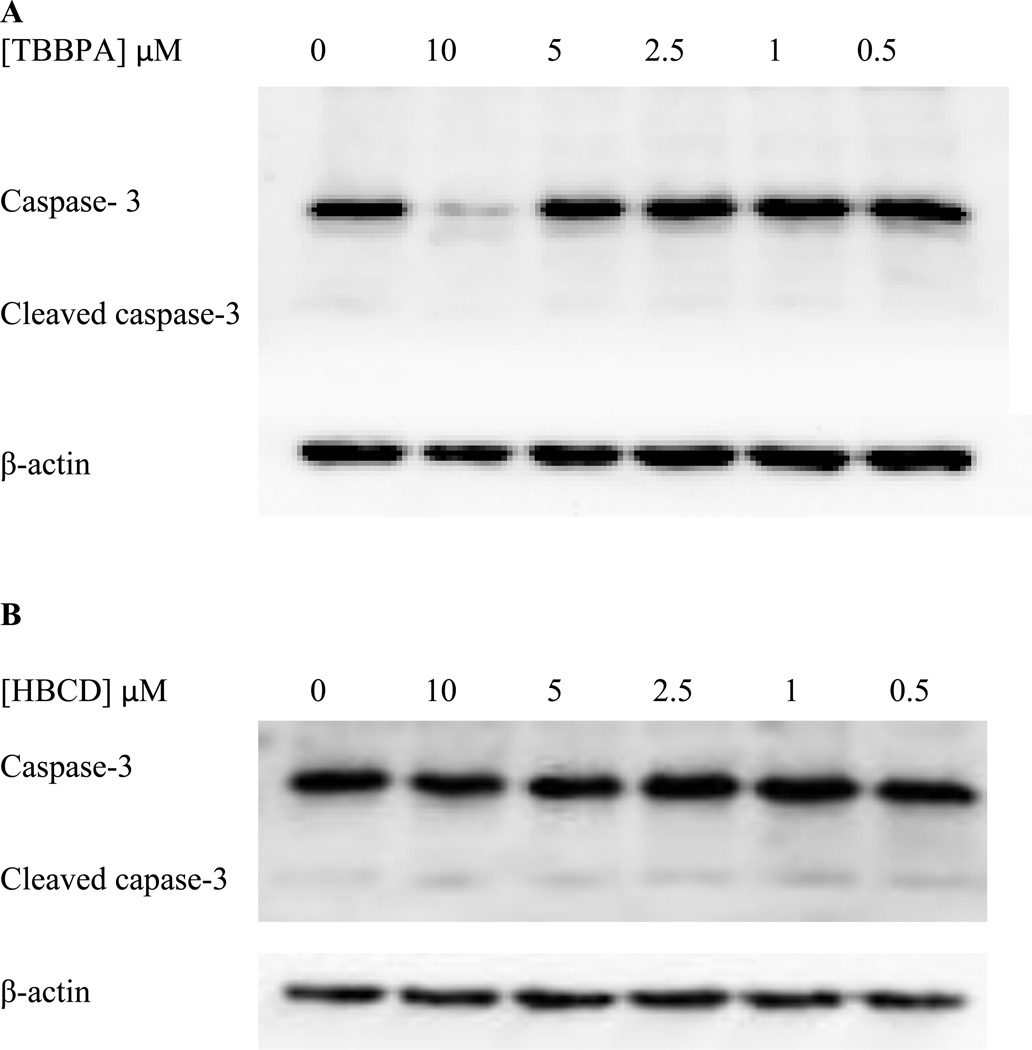
Lysates treated with TBBPA for 6 h were examined for the effects of exposures to TBBPA on caspase-3 levels. Caspase-3 cleavage to give cleaved caspase-3 is seen when apoptosis is activated. Thus, although TBBPA at the levels and length of exposure tested did not increase the necrosis of NK cells (measured by trypan blue exclusion), it was possible that TBBPA could be increasing apoptosis. Cells exposed to 0.5 – 5 µM TBBPA showed no statistically significant (p>0.05) changes in cleaved caspase-3 or intact caspase-3 levels as compared to control cells. Figure 15A shows a representative blot. However, exposure to 10 µM TBBPA greatly diminished the levels of intact caspase-3 (by 64%) compared to control cells (p<0.05). Interestingly, there was no increase in cleaved caspase-3 with this exposure. These data indicate that apoptosis is not being activated in NK cells by any level of TBBPA after 6 h however, 10 µM TBBPA is decreasing the levels of caspase 3 being expressed in the cell.
Figure 15. Effects of 6 hour exposures to 10-0.5 µM TBBPA and HBCD on Caspase-3 levels.
A) representative blot of levels of the effects of TBBPA exposures on Caspase-3 and cleaved Caspase-3. B) representative blot of levels of the effects of HBCD exposures on Caspase-3 and cleaved Caspase-3. 3 separate experiments using cells from 3 different donors were carried out.
Exposure of NK cells to 0.5–10 µM HBCD for 6 h caused no significant changes in the expression of either intact or cleaved caspase-3 as compared to the control cells. This indicated that HBCD was not activating apoptosis in NK cells over the time period that we studied. Figure 15B shows a representative blot.
Discussion
TBBPA and HBCD are two of the many chemicals known to contaminate the environment that are found in human tissues (Thornton et al. 2002; Sellstroem and Jansson, 1995) including human blood (Hagmar et al., 2000; Nagayama et al., 2001; Thomsen et al., 2002; Covaci et al., 2006; Knutsen et al., 2008; Pulkrabova et al., 2009; Kakimoto et al., 2008; Thomsen et al., 2005; Thomsen et al., 2003). NK cells are an essential component of the innate immune system providing protection from tumor and virally infected cells. When NK function is decreased in mice, it results in increased tumor formation (Wilson et al., 2001). Humans with defective NK cells have been shown to have an increased risk of developing certain tumors (Ortaldo et al., 1992) and individuals with absent or compromised NK lymphocytes experienced severe herpes virus and Epstein-Barr virus infections (Biron et al., 1989; Fleisher, et al., 1982). Compromise of NK cell function by chemical contaminant exposure could then lead to increased risk of both tumor development as well as viral infection as evidenced by decreased NK cell function resulting in increased tumor metastases in mice exposed to cigarette smoke (Lu et al., 2007). Additionally, exposure of mice to the chemical urethane causes tumors and has been shown to suppress NK function (Luebke et al., 1986). We have previously shown that exposures to TBBPA and HBCD inhibit the ability of human NK cells to bind and lyse tumor cells as well as decreasing expression of several cell surface proteins necessary for NK lytic function (Kibakaya et al., 2009; Hinkson and Whalen, 2009; Hinkson and Whalen, 2010; Hurd and Whalen, 2011). A brief (1 h) exposure to either TBBPA or HBCD (10-0.5 µM) followed by removal of the compound resulted in persistent loss of NK lytic function (Hinkson and Whalen, 2009; Kibakaya et al., 2009). Thus, a process was stimulated within 1 h of these exposures that caused sustained loss of NK lytic function. Based on these data it was hypothesized that a component of the lytic signaling pathway may be being activated by these compounds, which then resulted in persistent loss of NK lytic function. Activation of p44/42 is a necessary signal in the NK lytic pathway, whose activation occurs within a few minutes of exposure to targets (Trotta et al., 1998). Thus, we examined the effects of the compound at 10 minutes to see if such an early activation of p44/42 might be occurring. Longer incubations of 1 h and 6 h were also carried out to examine the length of any consistent activation of p44/42. The choice of exposure conditions was based on past studies including those where we examined the effect of other compounds known to inhibit NK lytic function on MAPK activation (Trotta et al., 1998; Aluoch and Whalen, 2005; Aluoch et al., 2006; Odman-Ghazi et al., 2010; Taylor and Whalen, 2011.) Those studies showed that the environmental contaminants tributyltin (TBT), dibutyltin (DBT), and ziram inhibit NK lytic function and cell surface-protein expression while altering the activation state of MAPK pathway components (Aluoch and Whalen, 2005; Aluoch et al., 2006; Odman-Ghazi et al., 2010; Taylor and Whalen, 2011).
Both TBBPA and HBCD caused activation of the MAPK p44/42 (ERK1/2). Activation of p44/42 was seen at concentrations of 2.5, 5, and 10 µM HBCD within 10 minutes. TBBPA caused a very robust activation of nearly 6 fold (above control) at 10 µM and induced smaller but still significant activations at all concentrations tested. Studies have shown that activation of p44/42 is necessary for NK-mediated destruction of tumor cells (Trotta et al., 1998; Vivier et al., 2004). Those studies showed that inhibition of the p44/42 pathway by the MEK inhibitor PD98059 blocked NK cell mediated destruction of tumor cells as well as target stimulation of interferon gamma (IFNγ) synthesis. Additionally, we have previously shown that activation of p44/42 in NK cells by phorbol 12 myristate 13 acetate (PMA) prior to their contact with target cells rendered the NK cell unable to lyse the target cells (Dudimah et al., 2010a). This loss of function was seen within an hour of p44/42 activation (Dudimah, et al., 2010a). Decreased expression of cell surface proteins involved in the cytotoxic process was also seen following activation of p44/42 by PMA (Dudimah et al., 2010b). Both TBBPA and HBCD were able to activate p44/42 and we have shown previously that activation of p44/42 decreases NK lytic function (Dudimah et al., 2010a). Thus, activation of this pathway appears to part of the mechanism by which these flame retardants diminish NK function. p44/42 activation is part of the lytic response signaling pathway (Vivier et al., 2004), thus if this signal is activated aberrantly by the flame retardant molecule then the cell will undergo changes that are consistent with having encountered and killed a tumor cell. It has been shown that once an NK cell has activated the lytic pathway it becomes inactivated to future encounters with tumor cells (Shenoy et al. 1993) Thus, the NK cell exposed to the flame retardants would be unresponsive to a subsequent encounter with an appropriate target cell, such as a tumor cell. These results are consistent with the fact that when NK cells exposed to either TBBPA or HBCD demonstrate marked inhibition of their ability to destroy tumor target cells (Kibakaya et al., 2009; Hinkson and Whalen, 2009).
The immediate upstream activator of p44/42, MEK, was also activated by TBBPA and HBCD. However no significant activation of Raf-1, the activator of MEK, was seen with these same exposures. A possible reason for this result was that our initial measurement was at 10 min and that activation of the MAP3K may no longer be detectable at that time point as turn-off processes may have already ensued.
TBBPA was also able to activate the p38 MAPK. Activation of p38 occurred after 10 minute exposures to 2.5, 5, and 10 µM TBBPA. HBCD did not activate p38. The MAP2K for p38 MKK3/6 was also activated by exposure to TBBPA.
The effects of TBBPA and HBCD on MAPK activation appear to occur rapidly. The effects of the compounds on MAPK phosphorylation were no longer consistently measurable after 1h. This too is consistent previous studies (Trotta et al., 1998; Aluoch and Whalen, 2005; Aluoch et al., 2006; Odman-Ghazi et al., 2010; Taylor and Whalen, 2011). Longer term consequences of this early activation would result in a number of cellular changes, which could include the types of effects on lytic function and protein expression that were described in previous studies (Hinkson and Whalen, 2009; Hinkson and Whalen, 2010; Kibakaya et al., 2009; Hurd and Whalen, 2011). Additionally, in the current study we saw a striking down regulation of caspase-3 protein levels in NK cells exposed to 10 µM TBBPA. This level of TBBPA also caused the greatest average activation (6 fold) of p44/42 after 10 min of exposure. Activation of p44/42 appears to be anti-apoptotic in many cells (Lu and Xu, 2006) and one study suggested that p44/42 activation may decrease caspase-3 levels (Xu et al., 2014). Further studies of effects of these compounds on caspase-3 expression are warranted.
In summary, the brominated flame retardants TBBPA and HBCD activated the MAPK p44/42 within 10 minutes. This activation appears to be an early part of the process by which these compounds diminish the lytic function of NK cells. Activation of p44/42 by both TBBPA and HBCD is at least in part due to the activation of the immediate upstream activator of p44/42. MEK1/2. TBBPA, and not HBCD, is able to activate the MAPK p38 within 10 minutes. This activation is also due to the activation of the immediate upstream activator of p38, MKK3/6. Alterations of these key signaling components in NK cells by exposures to TBBPA and HBCD may, at least in part, explain their negative effects on NK function.
Acknowledgements
This research was supported by Grant 2T34GM007663-33 and 5U54CA16066-03 from the National Institutes of Health.
Footnotes
Declaration of Interest
The Authors report no conflicts of interest. The Authors are alone responsible for the content and writing of the paper.
References
- Abdallah Mohamed AE, Harrad S, Ibarra C, Diamond M, Melymuk L, Robson M, Covaci A. Hexabromocyclododecanes in indoor dust from Canada, the United Kingdom, and the United States. Environmental Science & Technology. 2008;42:459–464. doi: 10.1021/es702378t. [DOI] [PubMed] [Google Scholar]
- Aluoch A, Whalen M. Tributyltin-induced effects on MAP kinases p38 and p44/42 in human natural killer cells. Toxicology. 2005;209:263–277. doi: 10.1016/j.tox.2004.12.034. [DOI] [PubMed] [Google Scholar]
- Aluoch A, Odman-Ghazi S, Whalen M. Alteration of an essential NK cell signaling pathway by low doses of tributyltin in human natural killer cells. Toxicology. 2006;224:229–237. doi: 10.1016/j.tox.2006.05.002. [DOI] [PubMed] [Google Scholar]
- Ballas ZK, Turner JM, Turner DA, Goetzman EA, Kemp JD. A patient with simultaneous absence of “classical”- natural killer cells (CD3−, CD16+, and NKH1+) and expansion of CD3+, CD4−, CD8−, NKH1+ subset. J. Allergy Clin. Immunol. 1990;85:453–459. doi: 10.1016/0091-6749(90)90155-w. [DOI] [PubMed] [Google Scholar]
- Birnbaum LS, Staskal DF. Brominated flame retardants: Cause for concern? Environ. Health Perspect. 2004;112:9–17. doi: 10.1289/ehp.6559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biron CA, Byron KS, Sullivan JL. Severe herpes virus in an adolescent without natural killer cells. New Engl. J. Med. 1989;320:1731–1735. doi: 10.1056/NEJM198906293202605. [DOI] [PubMed] [Google Scholar]
- Bustnes JO, Yoccoz NG, Bangjord G, Polder A, Skaare JU. Temporal trends (1986–2004) of organochlorines and brominated flame retardants in tawny owl eggs from northern Europe. Environmental Science & Technology. 2007;41:8491–8497. doi: 10.1021/es071581w. [DOI] [PubMed] [Google Scholar]
- Covaci A, Gerecke AC, Law RJ, Voorspoels S, Kohler M, Heeb NV, Leslie H, Allchin CR, De Boer J. Hexabromocyclododecanes (HBCDs) in the environment and humans: a review. Environmental Science & Technology. 2006;40:3679–3688. doi: 10.1021/es0602492. [DOI] [PubMed] [Google Scholar]
- de Wit CA. An overview of brominated flame retardants in the environment. Chemosphere. 2002;46:583–624. doi: 10.1016/s0045-6535(01)00225-9. [DOI] [PubMed] [Google Scholar]
- Dudimah FD, Griffey D, Wang X, Whalen MM. Activation of p44/42 MAK plays a role in TBT-induced loss of human natural killer (NK) cell function. Cell Biol. Toxicol. 2010a;26:435–444. doi: 10.1007/s10565-010-9154-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudimah FD, Abraha A, Wang X, Whalen MM. Activation of p44/42 MAPK in human natural killer cells decreases cell-surface protein expression: Relationship to tributyltin-induced alterations of protein expression. Toxicol. Mech. Meth. 2010a;20:544–555. doi: 10.3109/15376516.2010.518174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson P, Fischer C, Wallin M, Jakobsson E, Frederisson A. Impaired behaviour, Learning and memory, in adult mice neonatally exposed to hexabromocyclododecane (HBCDD) Environ. Toxicol. Pharmacol. 2006;21:317–322. doi: 10.1016/j.etap.2005.10.001. [DOI] [PubMed] [Google Scholar]
- Fleisher G, Koven N, Kamiya H, Henle W. A non-X-linked syndrome with susceptibility to severe Epstein-Bar virus infections. J. Pediatr. 1982;100:727–730. doi: 10.1016/s0022-3476(82)80572-6. [DOI] [PubMed] [Google Scholar]
- Fukuda N, Ito Y, Yamaguchi M, Mitumori K, Koizumi M, Hasegawa R, Kamata E, Ema M. Unexpected nephrotoxicity induced by tetrabromobisphenol A in newborn rats. Toxicol. Lett. 2004;150:145–155. doi: 10.1016/j.toxlet.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Gain B. Flame retardants’ Albemarle boosts capacity. Chem. Week. 1997 Jul 2;:88. 1997. Full text available from PROMT 97:369076. [Google Scholar]
- Germer S, Piersma AH, van der Ven L, Kamyschnikow A, Fery Y, Schmitz H-J, Schrenk D. Subacute effects of the brominated flame retardants hexabromocyclododecane and tetrabrombisphenol A on hepatic cytochrome P450 levels in rats. Toxicology. 2006;218:229–236. doi: 10.1016/j.tox.2005.10.019. [DOI] [PubMed] [Google Scholar]
- Hagmar L, Jakobsson K, Thuresson K, Rylander L, Sjodin A, Bergman A. Computer technicians are occupationally exposed to polybrominated diphenyl ethers and tetrabromobisphenol A. Organohalogen Comp. 2000;47:202–205. [Google Scholar]
- Hinkson NC, Whalen MM. Hexabromocyclododecane decreases the lytic function and ATP levels of human natural killer cells. J. Appl. Toxicol. 2009;29:656–661. doi: 10.1002/jat.1453. PMCID: PMC2788026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinkson NC, Whalen MM. Hexabromocyclododecane Decreases Tumor-cell-binding Capacity and Cell-Surface Protein Expression of Human Natural Killer Cells. J. Appl. Toxicol. 2010;30:302–309. doi: 10.1002/jat.1495. PMCID: PMC2876233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HSDB (Hazardous Substances Data Bank) 2,2´,6,6´-Tetrabromobisphenol A. Bethesda, MD: National Library of Medicine; 2001. http://www.toxnet.nlm.nih.gov/cgi-bin/sis/search. [Google Scholar]
- Hurd T, Whalen MM. Tetrabromobisphenol A decreases cell-surface proteins involved in human natural killer (NK) cell-dependent target cell lysis. Journal of Immunotoxicology. 2011;8:219–227. doi: 10.3109/1547691X.2011.580437. PMCID: PMC3145820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IPCS/WHO (International Program on Chemical Safety/World Health Organization) Environmental Health Criteria 172: Tetrabromobisphenol A and Derivatives. Geneva: World Health Organization; 1995. [Google Scholar]
- Janák K, Sellström U, Johansson AK, Becher G, de Wit CA, Lindberg P, Helander B. Enantiomer-specific accumulation of hexabromocyclododecanes in eggs of predatory birds. Chemosphere. 2008;73:S193–S200. doi: 10.1016/j.chemosphere.2007.03.077. [DOI] [PubMed] [Google Scholar]
- Kajiwara N, Sueoka M, Ohiwa T, Takigami H. Determination of flame-retardant hexabromocyclododecane diastereomers in textiles. Chemosphere. 2009;74:1485–1489. doi: 10.1016/j.chemosphere.2008.11.046. [DOI] [PubMed] [Google Scholar]
- Kakimoto K, Akutsu K, Konishi Y, Tanaka Y. Time trend of hexabromocyclododecane in the breast milk of Japanese women. Chemosphere. 2008;71:1110–1114. doi: 10.1016/j.chemosphere.2007.10.035. [DOI] [PubMed] [Google Scholar]
- Kibakaya EC, Stephen K, Whalen MM. Tetrabromobisphenol A has immunosuppressive effects on human natural killer cells. J. Immunotoxicology. 2009;6:285–292. doi: 10.3109/15476910903258260. PMCID: PMC2782892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutsen HK, Kvalem HE, Thomsen C, Froshaug M, Haugen M, Becher G, Alexander J, Meltzer HM. Dietary exposure to brominated flame retardants correlates with male blood levels in a selected group of Norwegians with a wide range of seafood consumption. Molecular Nutrition & Food Research. 2008;52:217–227. doi: 10.1002/mnfr.200700096. [DOI] [PubMed] [Google Scholar]
- Lotzova E. Definition and function of natural killer cells. Nat. Immunol. 1993;12:177–193. [PubMed] [Google Scholar]
- Lu LM, Zavitz CC, Chen B, Kianpour S, Wan Y, Stampfli MR. Cigarette smoke impairs NK cell-dependent tumor immune surveillance. J. Immunol. 2007;178:936–943. doi: 10.4049/jimmunol.178.2.936. [DOI] [PubMed] [Google Scholar]
- Lu Z, Xu S. ERK1/2 MAP kinases in cell survival and apoptosis. IUBMB Life. 2006;58:621–631. doi: 10.1080/15216540600957438. [DOI] [PubMed] [Google Scholar]
- Luebke RW, Riddle MM, Rogers RR, Rowe DG, Garner RJ, Smialowicz RJ. Immune function in adult C57BL/6J mice following exposure to urethane pre-or post natally. J. Immunopharmacol. 1986;8:243–257. [PubMed] [Google Scholar]
- Meerts IA, van Zanden JJ, Luijks EA, van Leeuwen-Bol I, Marsh G, Jakobsson E, Bergman A, Brouwer A. Potent competitive interactions of some brominated flame retardants and related compounds with human transthyretin in vitro. Toxicol. Sci. 2000;56:95–104. doi: 10.1093/toxsci/56.1.95. [DOI] [PubMed] [Google Scholar]
- Nagayama J, Takasuga T, Tsuji H, editors. Human Levels and Trends, Part 4. 2001. Contamination levels of brominated flame retardants, dioxins, and organochlorine compounds in the blood of Japanese adults; pp. 218–221. Located at http://www.kemi.se/aktuellt/BFR/bfr_del4.pdf. [Google Scholar]
- Odman-Ghazi S, Abraha A, Isom E, Whalen M. Dibutyltin activates MAP kinases in human natural killer cells, in vitro. Cell Biology and Toxicology. 2010;26:469–479. doi: 10.1007/s10565-010-9157-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortaldo JR, Glenn GM, Young HA, Frey JL. Natural killer (NK) cell lytic dysfunction and putative NK cell receptor expression abnormality in members of a family with chromosome 3p-linked von Hippel-Lindau disease. J. Natl. Cancer Inst. 1992;84:1897–1903. doi: 10.1093/jnci/84.24.1897. [DOI] [PubMed] [Google Scholar]
- Peck AM, Pugh RS, Moors A, Ellisor MB, Porter BJ, Becker PR, Kucklick JR. Hexabromocyclododecane in white-sided dolphins: temporal trend and stereoisomer distribution in tissues. Environmental Science & Technology. 2008;42:2650–2655. doi: 10.1021/es072052v. [DOI] [PubMed] [Google Scholar]
- Peterman PH, Orazio CE, Gale RW. Detection of tetrabromobisphenol A and formation of brominated [13C]-bisphenol A’s in commercial drinking water stored in reusable polycarbonate containers. ACS Div. Environ. Chem. Extended Abstr. 2000;40:431–433. [Google Scholar]
- Polder A, Venter B, Skaare JU, Bouwman H. Polybrominated diphenyl ethers and HBCD in bird eggs of South Africa. Chemosphere. 2008;73:148–154. doi: 10.1016/j.chemosphere.2008.03.021. [DOI] [PubMed] [Google Scholar]
- Pulkrabova J, Hradkova P, Hajslova J, Poustka J, Napravnikova M, Polacek V. Brominated flame retardants and other organochlorine pollutants in human adipose tissue samples from the Czech Republic. Environ. Intl. 2009;35:63–68. doi: 10.1016/j.envint.2008.08.001. [DOI] [PubMed] [Google Scholar]
- Ronisz D, Farmen Finne E, Karlsson H, Forlin L. Effects of the brominated flame retardants hexabromocyclododecane (HBCDD), and tetrabromobisphenol A (TBBPA), on hepatic enzymes and other biomarkers in juvenile rainbow trout and feral eelpout. Aquat. Toxicol. 2004;69:229–245. doi: 10.1016/j.aquatox.2004.05.007. [DOI] [PubMed] [Google Scholar]
- Schauer UM, Volkel W, Dekant W. Toxicokinetics of tetrabromobisphenol A in humans and rats after oral administration. Toxicol. Sci. 2006;91:49–58. doi: 10.1093/toxsci/kfj132. [DOI] [PubMed] [Google Scholar]
- Sellstroem U, Jansson B. Analysis of tetrabromobisphenol A in a product and environmental samples. Chemosphere. 1995;31:3085–3092. [Google Scholar]
- Shenoy AM, Sidner RA, Brahmi Z. Signal transduction in cytotoxic lymphocyte: decreased calcium influx in NK cell inactivated with sensitive target cells. Cell Immunol. 1993;147:294–301. doi: 10.1006/cimm.1993.1070. [DOI] [PubMed] [Google Scholar]
- Taylor TR, Whalen MM. Ziram Activates Mitogen-Activated Protein (MAP) Kinases and Decreases Cytolytic Protein Levels in Human Natural Killer Cells. Toxicology Mechanisms and Methods. 2011;21:577–584. doi: 10.3109/15376516.2011.578170. PMCID: PMC3183386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen C, Lundanes E, Becher G. Brominated flame retardants in archived serum samples from Norway: A study on temporal trends and the role of age. Environ. Sci. Technol. 2002;36:1414–1418. doi: 10.1021/es0102282. [DOI] [PubMed] [Google Scholar]
- Thomsen C, Froshaug M, Leknes H, Becher G. Brominated flame retardants in breast milk from Norway. Organohalogen Compd. 2003;64:33–36. [Google Scholar]
- Thomsen C, Froshaug M, Broadwell SI, Becher G, Eggesbo M. Levels of brominated flame retardants in milk from the Norwegian human milk study: HUMIS. Organohalogen Compd. 2005;67:509–512. [Google Scholar]
- Thornton JW, McCally M, Houlihan J. Biomonitoring of industrial pollutants: Health and policy implications of the chemical body burden. Public Health Rep. 2002;117:315–323. doi: 10.1016/S0033-3549(04)50167-X. www.ewg.org/reports/bodyburden/findings.php. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trotta R, Puorro KA, Paroli M, Azzoni L, Abebe B, Eisenlohr LC, Perussia B. Dependence of both spontaneous and antibody-dependent, granule exocytosis-mediated NK cell cytotoxicity on extracellular signal-regulated kinases. J Immunol. 1998;161:6648–6656. [PubMed] [Google Scholar]
- Trotta R, Fettuciari K, Azzoni L, Abebe B, Puorro KA, Eisenlohr LC, Perussia B. Differential role of p38 and c-Jun N-terminal kinase 1 mitogen-activated protein kinases in NK cell cytotoxicity. J Immunol. 2000;165:1782–1789. doi: 10.4049/jimmunol.165.4.1782. [DOI] [PubMed] [Google Scholar]
- van der Ven LT, Verhoef A, van de Kuil T, Slob W, Leonards PE, Visser TJ, Hamers T, Herlin M, Håkansson H, Olausson H, Piersma AH, Vos JG. A 28-day oral dose toxicity study enhanced to detect endocrine effects of hexabromocyclododecane in Wistar rats. Toxicol Sci. 2006;94:281–292. doi: 10.1093/toxsci/kfl113. [DOI] [PubMed] [Google Scholar]
- van Leeuwen SP, de Boer J. Brominated flame retardants in fish and shellfish - levels and contribution of fish consumption to dietary exposure of Dutch citizens to HBCD. Mol. Nutr. & Food Res. 2008;52:194–203. doi: 10.1002/mnfr.200700207. [DOI] [PubMed] [Google Scholar]
- Vivier E, Nunes JA, Vely F. Natural killer cell signaling pathways. Science. 2004;306:1517–1519. doi: 10.1126/science.1103478. [DOI] [PubMed] [Google Scholar]
- Whalen MM, Green SA, Loganathan BG. Brief butyltin exposure induces irreversible inhibition of the cytotoxic function on human natural killer cells in vitro. Environ. Res. 2002;88:19–29. doi: 10.1006/enrs.2001.4318. [DOI] [PubMed] [Google Scholar]
- Wilson SD, McCay JA, Butterworth LF, Munson AE, White KL., Jr Correlation of suppressed natural killer cell activity with altered host resistance models in B6C3F1 mice. Toxicol. Appl. Pharmacol. 2001;177:208–218. doi: 10.1006/taap.2001.9298. [DOI] [PubMed] [Google Scholar]
- Xu T, Wu X, Chen Q, Zhu S, Liu Y, Pan D, Chen X, Li D. The anti-apoptotic effects of salvianolic acid A on rat cardiomyocytes following ischemia/reperfusion by DUSP-mediated regulation of ERK1/2/JNK pathway. Plos One. 2014;9:1–14. doi: 10.1371/journal.pone.0102292. [DOI] [PMC free article] [PubMed] [Google Scholar]