Abstract
A tumor-selective non-lytic retroviral replicating vector (RRV), Toca 511, and an extended-release formulation of 5-fluorocytosine (5-FC), Toca FC, are currently being evaluated in clinical trials in patients with recurrent high-grade glioma (NCT01156584, NCT01470794, NCT01985256). Tumor-selective propagation of this RRV enables highly efficient transduction of glioma cells with cytosine deaminase (CD), which serves as a prodrug activator for conversion of the anti-fungal prodrug 5-FC to the anti-cancer drug 5-fluorouracil (5-FU) directly within the infected cells. We investigated whether, in addition to its direct cytotoxic effects, 5-FU generated intracellularly by RRV-mediated CD/5-FC prodrug activator gene therapy could also act as a radiosensitizing agent. Efficient transduction by RRV and expression of CD was confirmed in the highly aggressive, radioresistant human glioblastoma cell line U87-EGFRvIII and its parental cell line U87MG (U87). RRV-transduced cells showed significant radiosensitization even after transient exposure to 5-FC. This was confirmed both in vitro by a clonogenic colony survival assay and in vivo by bioluminescence imaging analysis. These results provide a convincing rationale for development of tumor-targeted radiosensitization strategies utilizing the tumor-selective replicative capability of RRV, and incorporation of radiation therapy into future clinical trials evaluating Toca 511 and Toca FC in brain tumor patients.
INTRODUCTION
Conventional replication-defective vectors, which are incapable of further propagation beyond initial infection, do not diffuse far from the injection site and therefore are inadequate for gene delivery into large solid tumors 1. Thus, the major obstacle has been the low efficiency of delivering prodrug activator genes to sufficient numbers of cancer cells in vivo using non-replicating vectors. To address such issues, we have developed retroviral replicating vectors (RRV), which we and others have shown in a variety of preclinical models to be capable of highly efficient gene delivery throughout solid tumor masses concomitant with viral replication 2-10. RRV-mediated gene delivery is tumor-selective in vivo because of the inability of RRV to infect non-dividing cells, and because anti-viral defense mechanisms active in normal cells are frequently mutated in cancer cells. We and others have confirmed that RRV are highly effective vectors for prodrug activator therapy in experimental models of glioma 7-13, and unlike oncolytic replicating viruses currently being tested in the clinic, RRV replication is intrinsically non-lytic and so the immunosuppressive tumor environment continues to shield the virus until extensive spread is attained, after which infected cells can be killed selectively and synchronously upon prodrug administration. Moreover, as the retrovirus integrates its transgene permanently into the cancer cell genome, residual infected glioma cells can become a reservoir for re-infection of malignant cells upon recurrence, enabling repeated cycles of prodrug administration to achieve further therapeutic benefit even after only a single injection of RRV 8, 12.
An improved version of one such RRV, Toca 511 (vocimagene amiretrorepvec), is currently being evaluated in multi-center Phase I dose-escalation trials for patients with recurrent high-grade glioma (www.clinicaltrials.gov, NCT01156584, NCT01470794, and NCT01985256). This vector delivers a human codon-optimized and thermo-stabilized version of the yeast cytosine deaminase (CD) prodrug activator gene 14. Its corresponding prodrug, 5-fluorocytosine (5-FC), is an orally-bioavailable FDA-approved anti-fungal agent that efficiently crosses the blood-brain barrier. The CD enzyme then converts 5-FC into 5-fluorouracil (5-FU), which has a very short half-life and is therefore localized primarily within the RRV-infected tumor. Thus, the adverse effects of systemic 5-FU chemotherapy can be minimized, while still achieving very high local concentrations of 5-FU generated directly within the tumor. 5-FU, one of the most commonly used chemotherapeutic drugs, has been widely combined with radiation to obtain radiosensitization and synergistic killing in several human cancers 15.
Radiation is an important component of conventional first-line treatment of glioma and is used clinically for small recurrent gliomas, but to date, no studies have been reported evaluating RRV-mediated CD/5-FC prodrug activator gene therapy in combination with radiotherapy. Accordingly, here we investigated whether the high levels of intracellular 5-FU generated by the clinical vector Toca 511 followed by a short pulse of 5-FC might exert radiosensitizing effects on the human glioma cell line U87MG and its radio-resistant variant U87-EGFRvIII, both in cell culture as well as in a unique tumor establishment challenge model scored by bioluminescent imaging. Our results indicate that combining RRV-mediated CD/5-FC prodrug activator gene therapy with radiation therapy can achieve even greater efficacy than either modality alone.
MATERIALS AND METHODS
Vectors
Plasmids encoding the RRV constructs pAC3-emd and pAC3-yCD2 (Toca 511) have been described previously 14. Both vectors are based on Moloney murine leukemia virus with an amphotropic envelope (4070A), and an internal ribosome entry site (IRES) linked to green fluorescent protein (emd) or an optimized yeast cytosine deaminase (yCD2), respectively, has been inserted into 3’-untranslated region of the viral genome (Fig. 1A, B). To produce AC3-emd virus, 293T cells were transiently transfected with pAC3-emd as previously described 3, 16. Supernatants were harvested and filtered 48 hours later, and stored at −80 °C. Preparations of AC3-yCD2 virus manufactured with the same process used for clinical vector production were provided by Tocagen Inc. Also, a self-inactivating (SIN) lentiviral vector expressing firefly luciferase (pRRL-sin-cPPT-hCMV-Fluc2) was provided from the UCLA Vector Core & Shared Resource Facility, and used in generating the luciferase-marked glioblastoma cell lines used in this study. Firefly luciferase cDNA is inserted into the multiple cloning site of the vector. Lentiviral vector titers were determined by measuring viral p24 antigen concentration by ELISA as described previously 17.
Figure 1.
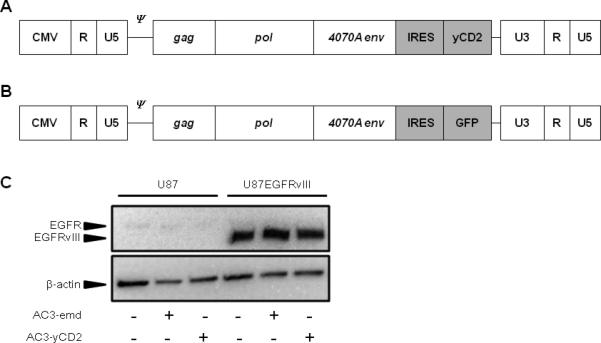
A, B: Design of retroviral replicating vectors AC3-yCD2 (Toca 511) (A) and AC3-emd (B). Both vectors are derived from amphotropic (4070A) MLV, in which an IRES-yCD2 or IRES-GFP cassette has been inserted downstream of the envelope sequence. CMV, cytomegalovirus promoter; U3/R/U5, domains of viral long terminal repeat; ψ, packaging signal; gag/pol/4070A env, viral coding sequences. C, EGFR protein expression in U87 and U87EGFRvIII cells, with and without AC3-emd or AC3-yCD2 transduction. Upper panel: Total protein extracts from cells stably transduced with either RRV and from uninfected control cells were analyzed by Western blot with anti-EGFR antibody (Millipore, Billerica, MA, USA). Lower panel: β-actin antibody (Abcam, Cambridge, MA, USA) was used as an internal loading control.
Cell lines
The human glioma cell line U87EGFRvIII (18; generously provided by P. Mischel, UCSD) and parental cell line U87MG (U87; American Type Culture Collection (ATCC)# HTB-14) were maintained in exponential growth as monolayer cultures by serial passage at 37 °C / 5% CO2, in Dulbecco'smodified Eagle'smedium (DMEM) with 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin. To establish RRV-producing cell lines, U87EGFRvIII or U87 cells were infected with AC3-yCD2 or AC3-emd at a multiplicity of infection (MOI) of 0.1 and cultured for >30 days. To confirm presence of yeast CD gene in these stably transduced cell lines (U87-AC3-yCD2 and U87EGFRvIII-AC3-yCD2), quantitative real-time PCR analysis was performed as described previously 2, 19. For confirmation of GFP expression in U87-AC3-emd and U87EGFRvIII-AC3-emd, the GFP positive cell fraction was monitored at serial time points by flow cytometry (Beckman Coulter, CA, USA) 16. For in vivo bioluminescence imaging studies, U87EGFRvIII and U87EGFRvIII-AC3-yCD2 cells were transduced at a multiplicity of infection (MOI) of 2 using lentiviral vector RRL-sin-cPPT-hCMV-Fluc2, and luciferase expression was confirmed by optical imaging (Xenogen IVIS, Alameda, CA, USA; Suppl. Fig. 1).
Western blot analysis
Total protein extracts were prepared from U87 and U87EGFRvIII cells infected with RRV(AC3-emd or AC3-yCD2) or uninfected, in RIPA buffer containing protease inhibitor (Sigma-Aldrich, St. Louis, MO, USA). Proteins contents were quantified using the Bradford assay. Proteins (20 μg) were resolved in Laemmli buffer by SDS-PAGE and transferred to an Immobilon-P Transfer Membrane (Millipore, Billerica, MA, USA). Western blotting was performed using rabbit polyclonal anti-EGFR antibody (Millipore, Billerica, MA, USA) and rabbit polyclonal anti-beta actin antibody (Abcam, Cambridge, MA, USA). Membranes were treated with peroxidase-conjugated secondary antibodies (Bio-Rad Laboratories, Hercules, CA, USA) and analyzed with SuperSignal West Femto Chemiluminescent Substrate (Thermo Scientific, Fremont, CA, USA).
5-FC treatment
Stocks of 5-FC (Sigma-Aldrich, St. Louis, MO, USA) were stored at −70 °C. At the time of exposure, the cells were grown in T-75 flasks and harvested by removal of the growth medium and addition of 0.25 % trypsin. Cells were transiently exposed to 5-FC for 1 hour, based on their reported human circulating half-life values 20, 21, in 1.5 mL microfuge tubes at 37 °C, then suspended in fresh medium without 5-FC.
In vitro clonogenic assays
For drug dose-response experiments with or without irradiation, U87 cells received a transient pulse of drug treatment with 5-FC at different concentrations (0, 0.1, or 1 mM) for one hour. For irradiated groups, immediately after one-hour exposure, the cells were irradiated at the dose of 2 Gy or 4 Gy and re-plated at different dilutions in DMEM with 10% FBS. The cells were incubated for 14 days at 37 °C, then fixed and stained. Only colonies with >50 cells were counted, and the clonogenic survival fraction was calculated by normalizing against the unirradiated control. A RS320 Irradiation System (Gulmay Medical, Bethel, CT, USA) was used with the following parameters: 300 kVp, HVL 3 mmCu, 10 mA, 1.743 Gy / min at 34.7 cm FSD.
In vivo tumor establishment assay
Female Foxn1 nu/nu athymic mice (6~8 weeks old, Harlan Laboratories Inc.) were housed under specific pathogen-free conditions, and all animal studies were conducted under protocols approved by the UCLA Animal Research Committee. U87EGFRvIII-Fluc2 cells and U87EGFRvIII-AC3-yCD2-Fluc2 cells in culture were exposed to DMEM / 10% FBS with 0.1 mM 5-FC for two hours, and then replaced with fresh complete medium without 5-FC. An aliquot of each cell line was irradiated with 2 Gy or 4 Gy, and remainder was unirradiated. Cell viability was then quantitated for each treatment group with trypan blue, and an equal number of viable cells was inoculated into the right cerebral hemisphere of athymic nude mice with a Hamilton syringe and stereotactic micro-injector (Narishige, Tokyo, Japan) (1 × 105 viable cells / 2 μl PBS). Injection site coordinates were 1 mm anterior and 1.5 mm lateral to the bregma and 3 mm deep from the dura mater. Luciferase signals from brain tumors were examined by optical in vivo imaging every 3 or 4 days until one mouse in each group died due to tumor progression. Bioluminescence data was analyzed by Living Image™ Software (Xenogen IVIS, Alameda, CA, USA).
Statistical analysis
For in vitro survival data, Two-Way ANOVA was performed for statistical comparison of the radiosensitivity between groups, and p values < 0.05 were considered significant. For in vivo data, ANOVA was performed for statistical comparison of bioluminescence signals between groups, and p values < 0.05 were considered significant. All analysis were performed using GraphPad Prism 5 software (GraphPad Software, La Jolla, CA, USA).
RESULTS
RRV transduction does not affect the EGFR expression status of U87EGFRvIII cells
The parental U87 glioblastoma cell line expresses low levels of wild-type epidermal growth factor receptor (EGFR), and does not express the EGFR variant vIII (EGFRvIII). Overexpression of EGFRvIII in this cell line results in a highly aggressive and radioresistant phenotype 18, 22, 23 . Therefore, we first sought to determine whether the radiation sensitivity of these glioma cells might be affected by RRV transduction per se. To this end, we examined the expression levels of EGFRvIII protein before and after transduction with RRV in both parental U87 cells and U87EGFRvIII cells. Both cell lines were inoculated with AC3-yCD2 or AC3-emd (Fig. 1A & 1B, respectively) at an MOI of 0.1 and cultured for 30 days to allow adequate time for viral spread throughout the culture, confirming stable transduction by flow cytometry for AC3-emd or quantitative real-time PCR for AC3-yCD2 (data not shown). Western blot analysis of total protein extracts from both cell lines, with or without RRV transduction, confirmed that EGFR protein was detected in U87EGFRvIII cells but not in parental U87 cells, and transduction with AC3-emd or AC3-yCD2 did not detectably alter EFRvIII expression status in either cell line (Fig. 1C).
Highly effective radiosensitization of AC3-yCD2 (Toca 511) transduced cells with a single short exposure to 5-FC prodrug
We evaluated whether RRV-mediated transduction of the CD gene would change the radiosensitivity of U87 cells after exposure to a short pulse of high-concentration 5-FC in vitro. Clonogenic survival curves were generated of U87 and U87-AC3-yCD2 cells, without or with exposure to 5-FC (0.1 or 1 mM) for 1 hour, immediately prior to irradiation at 2 Gy or 4 Gy, and normalized against their respective controls (unirradiated group) (Fig. 2). As expected, the survival curves for transduced or untransduced U87 cells without 5-FC exposure were not significantly different (p=0.1226). However, cells that have been transduced with RRV expressing the CD gene showed reduced clonogenic survival after the transient exposure to 5-FC at 0.1 mM or 1 mM concentration compared to the control group at any radiation dose tested. Two-way ANOVA revealed that the effect of 5-FC concentration and radiation dose both affected the results significantly (p value = 0.0121 and < 0.0001, respectively).
Figure 2.
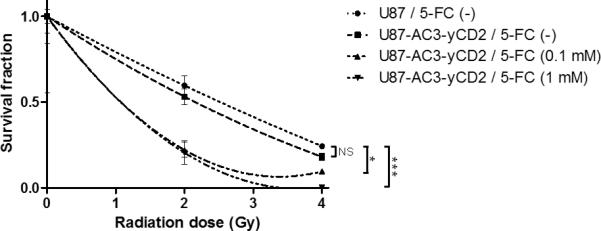
In vitro clonogenic survival fractions of U87 and U87-AC3-yCD2 cells with or without short exposure to 5-FC at different concentrations. In order to investigate the radiosensitization activity of 5-FU, which was intracellulary converted from 5-FC, the cells were pulsed with 5-FC (0.1 mM or 1 mM) for 1 hour, then followed by 2 Gy or 4 Gy irradiation. Control cells were not irradiated in each group. After 14 days, colonies were fixed, stained and counted. Each point represents the average of three replicates, bars indicate SD, NS: not significant, *: p < 0.05, ***: p < 0.001.
Radiosensitization effect of CD prodrug activator gene transduction with 5-FC pulse confirmed in vivo by intracranial tumor establishment assay
To next evaluate whether combining CD/5-FC prodrug activator gene therapy with irradiation would exert an enhanced cytotoxic effect in vivo, we used a radioresistant U87 subline, U87EGFRvIII, and devised an intracranial tumor establishment assay that employs glioma cell lines expressing the firefly luciferase reporter gene, which provides a quantitative bioluminescence readout that can be monitored in real-time by non-invasive optical imaging. Transduction with a conventional replication-defective lentiviral vector expressing luciferase resulted in a comparable level of bioluminescence from both U87EGFRvIII-FLuc2 and U87EGFRvIII-AC3-yCD2-FLuc2 cells upon exposure to luciferin, and a highly robust quantitative correlation between cell number and bioluminescent signal intensity was confirmed for both cell lines by optical imaging in vitro (Supplementary Fig. 1).
Prior to tumor establishment and optical imaging analysis in vivo, radiosensitization procedures were performed ex vivo, enabling precise control over prodrug concentration, exposure time, and radiation dose. Accordingly, U87EGFRvIII-Fluc2 or U87EGFRvIII-AC3-yCD2-Fluc2 cells in culture were first exposed for 2 hours to 0.1 mM 5-FC, which approximates the lowest 5-FC concentration measured in cerebrospinal fluid from patients undergoing treatment for CNS fungal infection 24. After this short pulse of 5-FC followed by washout, aliquots from each cell culture were given either 2 Gy or 4 Gy irradiation, and the remaining cells served as unirradiated controls. After stereotactic implantation into athymic mouse brain (1 × 105 cells / mouse), bioluminescence signals during tumor establishment and progression were monitored by in vivo optical imaging at serial time points (Fig. 3A and 3B).
Figure 3.
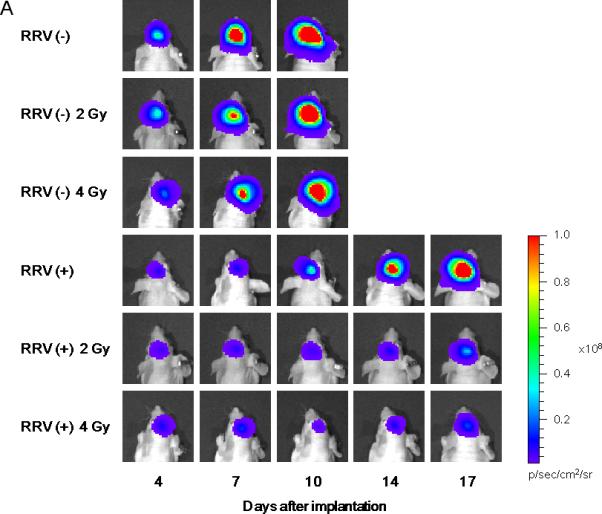
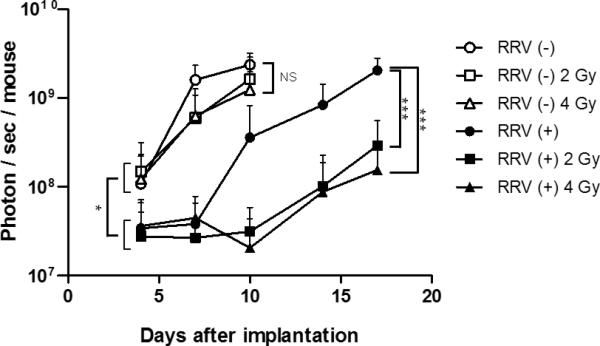
Brain tumor establishment and growth in vivo monitored by bioluminescence optical imaging after intracerebral implantation of glioma cells exposed to prodrug pulse and irradiation ex vivo. Uninfected and AC3-yCD2-infected U87EGFRvIII human glioma cells were pulsed with 0.1 mM 5-FC for 2 hours ex vivo, then unirradiated or irradiated with 2 Gy or 4 Gy, prior to stereotactic intracerebral implantation in athymic nude mice on Day 0 (1 × 105 cells / mouse). Bioluminescence signals from brain tumors were monitored every 3 or 4 days by in vivo optical imaging until one mouse in each group died due to tumor progression. A: Representative bioluminescence images of established brain tumors for each group on days 4, 7, 10, 14 and 17 are paneled. Mice implanted with the cells infected with RRV and irradiated at 2 Gy or 4 Gy showed marked tumor growth inhibition compared to other groups. B: Average signal intensities in each group were plotted; bars indicate SD. NS: not significant, *: p < 0.05, ***: p < 0.001.
RRV(−): U87EGFRvIII-Fluc2 cells without irradiation, represented as white circles (○)
RRV(+): U87EGFRvIII-AC3-yCD2-Fluc2 cells without irradiation, black circles (●)
RRV(−) 2Gy: U87EGFRvIII-Fluc2 cells irradiated with 2 Gy, white rectangles (□)
RRV(+) 2Gy: U87EGFRvIII-AC3-yCD2-Fluc2 cells irradiated with 2 Gy, black rectangles (■);
RRV(−) 4Gy: U87EGFRvIII-Fluc2 cells irradiated with 4 Gy, white triangles (Δ)
RRV(+) 4Gy: U87EGFRvIII-AC3-yCD2-Fluc2 cells irradiated with 4 Gy, black triangles (▲).
Initial bioluminescence signal intensities of uninfected U87EGFRvIII-FLuc2 cells without irradiation, or with either 2 Gy or 4 Gy irradiation, were all closely clustered together at the first time point (Day 4), indicating that there were no significant disparities in the implanted cell dose among these groups. Among these groups, there was some reduction in bioluminescence signal intensity from tumors established with irradiated uninfected cells as compared to unirradiated control tumors at the next time point (Day 7), however, bioluminescence signals in all of these groups continued to increase thereafter, indicating progressive tumor growth. By the third time point (Day 10), all of the uninfected U87EGFRvIIIFluc2 tumor groups showed high signal intensities, and there were no significant differences between them. This is consistent with previous reports characterizing U87EGFRvIII cells which have been reported to form radioresistant and aggressive tumors, and indeed, imaging could not be performed beyond Day 10 due to symptoms of tumor progression necessitating animal termination, as expected from the shorter survival time of controls implanted with intracerebral U87EGFRvIII tumors as compared to parental U87 tumors (Supplementary Fig. 2).
Similarly, initial bioluminescence signal intensities of infected U87EGFRvIII-AC3-yCD2-Fluc2 cells, either without irradiation or with 2 Gy or 4 Gy irradiation, were all closely clustered together at the first time point (Day 4), again indicating that there were no significant disparities in the implanted cell dose among these groups. However, all three of these infected U87EGFRvIII-AC3-yCD2-Fluc2 tumor groups, whether unirradiated or irradiated, showed significantly reduced signal intensities compared to the uninfected U87EGFRvIII-FLuc2 tumor groups (Fig. 3B, p < 0.05), even though the same number of viable cells had been inoculated intracerebrally on Day 0 for all groups, suggesting that even a short pulse of 5-FC prodrug had significantly impacted the post-implantation viability or growth rate of infected glioma cells expressing yCD2, regardless of irradiation. Subsequently, bioluminescence from animals implanted with infected U87EGFRvIII-AC3-yCD2-Fluc2 cells exposed to 5-FC but without irradiation showed increasing signal intensities after Day 7, indicating tumor progression. In contrast, bioluminescence after Day 7 was consistently lower in infected U87EGFRvIII-AC3-yCD2-Fluc2 groups with 5-FC and either 2 Gy or 4 Gy irradiation as compared to unirradiated infected controls, and these statistically significant reductions in signal intensities of the irradiated groups continued through Day 17 (Fig. 3B, p < 0.001), when the unirradiated infected control animals had to be terminated. Taken together with the relative lack of tumor growth inhibition in uninfected irradiated groups, this indicates a potent radiosensitization effect of yCD2 / 5-FC prodrug activator gene therapy, even when pre-implantation exposure was limited to a lower concentration of 5-FC for only 2 hours prior to irradiation.
DISCUSSION
AC3-yCD2 represents an improved RRV exhibiting enhanced genomic stability during serial replication, and with enhanced potency due to the use of yeast CD rather than bacterial CD25, and extensive sequence modifications for codon optimization and thermostabilization at mammalian body temperature 14. As noted, AC3-yCD2 (Toca 511) in combination with Toca FC is being evaluated in clinical trials in patients with recurrent high-grade glioma (www.clinicaltrials.gov: NCT01156584, NCT01470794, and NCT01985256). To investigate if concomitant radiation therapy would be compatible with this approach and enhance glioma cell killing, in the present study we evaluated whether 5-FC would radiosensitize glioma cells that are infected by AC3-yCD2, while sparing uninfected cells.
In clonogenic survival assays, we found that 1 hour of exposure to 5-FC resulted in considerably enhanced killing of U87 glioma cells infected with AC3-yCD2 after irradiation, and this effect was more apparent at the lower radiation dose. The data therefore suggest that rapid and efficient conversion of 5-FC to 5-FU intracellularly even after a relatively short pulse of 5-FC in an infected tumor would be sufficient to achieve a significant radiosensitization effect.
To evaluate the effects of prior ex vivo exposure to prodrug and/or radiation, we employed a novel bioluminescence optical imaging-based method for non-invasive in vivo monitoring. Performing these treatments ex vivo enabled precise control over dosage and timing of exposure, while bioluminescence imaging provided a quantitative, consistent, and reproducible readout in vivo. Using this assay, AC3-yCD2 & 5-FC-mediated radiosensitization was confirmed to significantly impact subsequent establishment of radioresistant U87EGFRvIII tumors in vivo, even with transient pulse of 5-FC.
After a single pulse of 5-FC prodrug for 2 hours, RRV-transduced U87EGFRvIII-AC3-yCD2-Fluc2 cells showed significantly reduced bioluminescent tumor signals as compared to untransduced U87EGFRvIII-Fluc2 cells even at the first imaging time point (Day 4), even though the number of injected viable cells on Day 0 had been equal (1 × 105 cells). This indicates that, even within 2 hours, the cytotoxic effects of prodrug uptake and conversion in the infected cells was sufficient to cause eventual lethality in a majority of these cells (~90% based on bioluminescence signal quantitation, see Fig. 3B), when the cells attempted to divide after implantation in vivo.
Furthermore, while bioluminescence imaging showed comparable signal intensity from unirradiated and irradiated groups at the first imaging time point, indicating that there was no significant difference in initial tumor establishment among these groups, subsequent tumor growth was significantly delayed in the RRV-transduced groups that were also irradiated following the short pulse of 5-FC prodrug. This suggests that even short-term exposure of glioma cells expressing yCD2 to 5-FC prodrug may have contributed to blocking these cells from radiation-induced Potentially Lethal Damage Repair, thereby significantly impacting their post-implantation survival or proliferation rate.
It should also be noted that only a single dose of prodrug was used in the present study, and while this was found to be sufficient to mediate both transient tumor growth inhibition and radiosensitization effects, longer-term survival has been achieved by repeated prodrug administration in intracranial glioma models. Accordingly, multiple cycles of 5-FC prodrug administration are employed in current clinical protocols, and this may provide on-going sensitization effects for multiple fractionated doses of radiation as well. These encouraging results therefore support the evaluation of a combined treatment regimen incorporating radiation therapy into future clinical trials employing AC3-yCD2 (Toca 511) and Toca FC in patients with gliomas. Unlike radiosensitizing agents which are systemically active, the local production and action of 5-FU by RRV-mediated CD / 5-FC prodrug activator therapy should allow for radiation synergy selectively in the tumor, while sparing nearby healthy tissues.
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. Christopher R. Logg for advice and suggestions, and the UCLA Vector Core & Shared Resource Facility (supported by P30 DK041301 and P30 CA016042) for assistance with lentiviral vector production, and the UCLA Virology Core for assistance with p24 ELISA. This work was supported in part by NIH grant # U01 NS059821 and Tocagen Inc. The work was also enabled by support to Tocagen from Accelerate Brain Cancer Cure, National Brain Tumor Society, American Brain Tumor Association, Musella Foundation, and Voices Against Brain Cancer.
Funding:
This work was supported in part by NIH grant # U01 NS059821 and a research award from Tocagen Inc.
Footnotes
Current address:
University of Miami Miller School of Medicine 1550 NW 10th Ave, Papanicolaou Bldg., Room 410 (M-877) Miami, FL, 33136 Tel: 305-243-3243 FAX: 305-243-5885 nkasahara@med.miami.edu
CONFLICT OF INTEREST
HG, JR and DJ are employees and/or shareholders of Tocagen, Inc. NK is a consultant, has ownership interest, and is the recipient of a research grant from Tocagen, Inc. The research conducted under this grant award has been reviewed and approved by the UCLA Conflict of Interest Review Committee.
References
- 1.Rainov NG, Ren H. Clinical trials with retrovirus mediated gene therapy--what have we learned? J Neurooncol. 2003;65(3):227–36. doi: 10.1023/b:neon.0000003652.71665.f2. [DOI] [PubMed] [Google Scholar]
- 2.Hiraoka K, Kimura T, Logg CR, Kasahara N. Tumor-selective gene expression in a hepatic metastasis model after locoregional delivery of a replication-competent retrovirus vector. Clin Cancer Res. 2006;12(23):7108–16. doi: 10.1158/1078-0432.CCR-06-1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hiraoka K, Kimura T, Logg CR, Tai CK, Haga K, Lawson GW, et al. Therapeutic efficacy of replication-competent retrovirus vector-mediated suicide gene therapy in a multifocal colorectal cancer metastasis model. Cancer Res. 2007;67(11):5345–53. doi: 10.1158/0008-5472.CAN-06-4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kikuchi E, Menendez S, Ozu C, Ohori M, Cordon-Cardo C, Logg CR, et al. Highly efficient gene delivery for bladder cancers by intravesically administered replication-competent retroviral vectors. Clin Cancer Res. 2007;13(15 Pt 1):4511–8. doi: 10.1158/1078-0432.CCR-07-0151. [DOI] [PubMed] [Google Scholar]
- 5.Kikuchi E, Menendez S, Ozu C, Ohori M, Cordon-Cardo C, Logg CR, et al. Delivery of replication-competent retrovirus expressing Escherichia coli purine nucleoside phosphorylase increases the metabolism of the prodrug, fludarabine phosphate and suppresses the growth of bladder tumor xenografts. Cancer Gene Ther. 2007;14(3):279–86. doi: 10.1038/sj.cgt.7701013. [DOI] [PubMed] [Google Scholar]
- 6.Logg CR, Tai CK, Logg A, Anderson WF, Kasahara N. A uniquely stable replication-competent retrovirus vector achieves efficient gene delivery in vitro and in solid tumors. Hum Gene Ther. 2001;12(8):921–32. doi: 10.1089/104303401750195881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tai CK, Wang W, Lai YH, Logg CR, Parker WB, Li YF, et al. Enhanced efficiency of prodrug activation therapy by tumor-selective replicating retrovirus vectors armed with the Escherichia coli purine nucleoside phosphorylase gene. Cancer Gene Ther. 2010;17(9):614–23. doi: 10.1038/cgt.2010.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tai CK, Wang WJ, Chen TC, Kasahara N. Single-shot, multicycle suicide gene therapy by replication-competent retrovirus vectors achieves long-term survival benefit in experimental glioma. Mol Ther. 2005;12(5):842–51. doi: 10.1016/j.ymthe.2005.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang W, Tai CK, Kershaw AD, Solly SK, Klatzmann D, Kasahara N, et al. Use of replication-competent retroviral vectors in an immunocompetent intracranial glioma model. Neurosurg Focus. 2006;20(4):E25. doi: 10.3171/foc.2006.20.4.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang WJ, Tai CK, Kasahara N, Chen TC. Highly efficient and tumor-restricted gene transfer to malignant gliomas by replication-competent retroviral vectors. Hum Gene Ther. 2003;14(2):117–27. doi: 10.1089/104303403321070810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hlavaty J, Jandl G, Liszt M, Petznek H, Konig-Schuster M, Sedlak J, et al. Comparative evaluation of preclinical in vivo models for the assessment of replicating retroviral vectors for the treatment of glioblastoma. J Neurooncol. 2011;102(1):59–69. doi: 10.1007/s11060-010-0295-5. [DOI] [PubMed] [Google Scholar]
- 12.Ostertag D, Amundson KK, Lopez Espinoza F, Martin B, Buckley T, Galvao da Silva AP, et al. Brain tumor eradication and prolonged survival from intratumoral conversion of 5-fluorocytosine to 5-fluorouracil using a nonlytic retroviral replicating vector. Neuro Oncol. 2012;14(2):145–59. doi: 10.1093/neuonc/nor199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Solly SK, Trajcevski S, Frisen C, Holzer GW, Nelson E, Clerc B, et al. Replicative retroviral vectors for cancer gene therapy. Cancer Gene Ther. 2003;10(1):30–9. doi: 10.1038/sj.cgt.7700521. [DOI] [PubMed] [Google Scholar]
- 14.Perez OD, Logg CR, Hiraoka K, Diago O, Burnett R, Inagaki A, et al. Design and selection of toca 511 for clinical use: modified retroviral replicating vector with improved stability and gene expression. Mol Ther. 2012;20(9):1689–98. doi: 10.1038/mt.2012.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McGinn CJ, Lawrence TS. Recent advances in the use of radiosensitizing nucleosides. Semin Radiat Oncol. 2001;11(4):270–80. doi: 10.1053/srao.2001.26002. [DOI] [PubMed] [Google Scholar]
- 16.Logg CR, Kasahara N. Retrovirus-mediated gene transfer to tumors: utilizing the replicative power of viruses to achieve highly efficient tumor transduction in vivo. Methods Mol Biol. 2004;246:499–525. doi: 10.1385/1-59259-650-9:499. [DOI] [PubMed] [Google Scholar]
- 17.Stripecke R, Koya RC, Ta HQ, Kasahara N, Levine AM. The use of lentiviral vectors in gene therapy of leukemia: combinatorial gene delivery of immunomodulators into leukemia cells by state-of-the-art vectors. Blood Cells Mol Dis. 2003;31(1):28–37. doi: 10.1016/s1079-9796(03)00062-7. [DOI] [PubMed] [Google Scholar]
- 18.Wang MY, Lu KV, Zhu S, Dia EQ, Vivanco I, Shackleford GM, et al. Mammalian target of rapamycin inhibition promotes response to epidermal growth factor receptor kinase inhibitors in PTEN-deficient and PTEN-intact glioblastoma cells. Cancer research. 2006;66(16):7864–9. doi: 10.1158/0008-5472.CAN-04-4392. [DOI] [PubMed] [Google Scholar]
- 19.Logg CR, Robbins JM, Jolly DJ, Gruber HE, Kasahara N. Retroviral replicating vectors in cancer. Methods Enzymol. 2012;507:199–228. doi: 10.1016/B978-0-12-386509-0.00011-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Finch RE, Bending MR, Lant AF. Plasma levels of 5-fluorouracil after oral and intravenous administration in cancer patients. Br J Clin Pharmacol. 1979;7(6):613–7. doi: 10.1111/j.1365-2125.1979.tb04651.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vermes A, Guchelaar HJ, Dankert J. Flucytosine: a review of its pharmacology, clinical indications, pharmacokinetics, toxicity and drug interactions. J Antimicrob Chemother. 2000;46(2):171–9. doi: 10.1093/jac/46.2.171. [DOI] [PubMed] [Google Scholar]
- 22.Kim K, Brush JM, Watson PA, Cacalano NA, Iwamoto KS, McBride WH. Epidermal growth factor receptor vIII expression in U87 glioblastoma cells alters their proteasome composition, function, and response to irradiation. Mol Cancer Res. 2008;6(3):426–34. doi: 10.1158/1541-7786.MCR-07-0313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Valdes G, Iwamoto KS. Re-evaluation of cellular radiosensitization by 5-fluorouracil: High-dose, pulsed administration is effective and preferable to conventional low-dose, chronic administration. Int J Radiat Biol. 2013;89(10):851–62. doi: 10.3109/09553002.2013.797620. [DOI] [PubMed] [Google Scholar]
- 24.Block ER, Bennett JE. Pharmacological studies with 5-fluorocytosine. Antimicrob Agents Chemother. 1972;1(6):476–82. doi: 10.1128/aac.1.6.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kievit E, Bershad E, Ng E, Sethna P, Dev I, Lawrence TS, et al. Superiority of yeast over bacterial cytosine deaminase for enzyme/prodrug gene therapy in colon cancer xenografts. Cancer Res. 1999;59(7):1417–21. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


