Background: The antigen translocation complex TAP plays a crucial role in adaptive immunity.
Results: TAP reconstituted in nanodiscs shows a tight coupling between peptide binding and ATP hydrolysis, which is blocked by herpesviral ICP47.
Conclusion: An annular lipid belt is essential for TAP function and high affinity inhibition by ICP47.
Significance: Nanodiscs are a promising approach to dissect the function, lipid interaction, and modulation of membrane proteins.
Keywords: ATP-binding Cassette Transporter, Antigen Processing, Herpesvirus, Lipid Bilayer, Membrane Protein, Membrane Transport, Membrane Transporter Reconstitution, Peptide Transport, Transporter
Abstract
The transporter associated with antigen processing (TAP) constitutes a focal element in the adaptive immune response against infected or malignantly transformed cells. TAP shuttles proteasomal degradation products into the lumen of the endoplasmic reticulum for loading of major histocompatibility complex (MHC) class I molecules. Here, the heterodimeric TAP complex was purified and reconstituted in nanodiscs in defined stoichiometry. We demonstrate that a single heterodimeric core-TAP complex is active in peptide binding, which is tightly coupled to ATP hydrolysis. Notably, with increasing peptide length, the ATP turnover was gradually decreased, revealing that ATP hydrolysis is coupled to the movement of peptide through the ATP-binding cassette transporter. In addition, all-atom molecular dynamics simulations show that the observed 22 lipids are sufficient to form an annular belt surrounding the TAP complex. This lipid belt is essential for high affinity inhibition by the herpesvirus immune evasin ICP47. In conclusion, nanodiscs are a powerful approach to study the important role of lipids as well as the function, interaction, and modulation of the antigen translocation machinery.
Introduction
The adaptive immune system eliminates virally or malignantly transformed cells when cytotoxic T-lymphocytes recognize antigenic peptides presented by MHC class I molecules (MHC I) on the cell surface. The transporter associated with antigen processing (TAP)2 is one of the key players in this process. Degradation products of the ubiquitin-proteasome pathway are recognized by TAP and further shuttled into the lumen of the endoplasmic reticulum. TAP is the central junction of a multisubunit machinery, which orchestrates the efficient peptide editing and loading of MHC I molecules. Peptide-MHC I complexes traffic to the cell surface and present their antigenic cargo to CD8+ cytotoxic T-lymphocytes (1–3).
TAP belongs to the family of ATP-binding cassette proteins, which transport a wide range of solutes across cell membranes driven by ATP binding and hydrolysis (4). The heterodimeric complex consists of TAP1 (ABCB2) and TAP2 (ABCB3). Each half-transporter contains a transmembrane domain of six transmembrane helices (TMs), which are involved in peptide binding and translocation (5, 6), followed by a C-terminal nucleotide-binding domain, which coordinates ATP binding and hydrolysis (4). This core-TAP complex is essential and sufficient for peptide binding and translocation (7). In addition, an extra 4-TM bundle at each TAP subunit provides a membrane interaction platform for the MHC I-specific chaperone tapasin and assembly of the peptide-loading complex (7–10). In our working model, peptide and ATP binding occurs independently from each other but together induce an allosteric coupling between transmembrane domain and the nucleotide-binding domain, leading to a dimerization of the two nucleotide-binding domains and a switch of the transmembrane domains from the inward- to the outward-facing conformation, thus moving peptides in the lumen of the endoplasmic reticulum. Finally, ATP hydrolysis resets the transport complex back to the inward-facing, pre-translocation state (11, 12).
TAP translocates most efficiently peptides with 8–12 amino acids in length and displays a specificity by recognizing the three N- and C-terminal residues in the binding pocket of TAP (13–17). Notably, even peptides of 40 amino acids in length and peptides with bulky side chains, such as fluorophores, chemical proteases, or polylysine chains, can be transported, however with much lower efficiency (6, 17–19). Various viral proteins, among others such as ICP47 from herpes simplex virus, inhibit TAP. ICP47, which blocks peptide binding to TAP, is the only soluble viral TAP inhibitor known so far (20, 21). Interestingly, the active domain ICP47(3–34), which constitutes two amphipathic α-helices linked by a flexible loop (22–25), inhibits TAP in microsomes with a 50–100-fold higher affinity than TAP solubilized in detergent (24).
A detailed mechanistic understanding of peptide transport and viral inhibition requires the investigation of the TAP complex in an isolated but membrane-embedded state because associated lipids have been found to be essential for TAP function (26). It is further worth mentioning that only a very limited number of detergents maintains the lipid environment crucial for TAP function (26, 27). Here, we report on the tandem affinity purification and first functional reconstitution of the heterodimeric TAP complex in nanodiscs (Nd). These disc-like membrane particles, ranging from 8 to 16 nm in diameter, consist of two membrane scaffold proteins (MSPs) enclosing a lipid bilayer and the membrane protein of interest (28, 29). The design of the MSPs is based on the amphipathic helically structured serum protein apolipoprotein A-1.
Nanodiscs provide several key advantages (29) as follows: (i) small size compared with liposomes (30, 31); (ii) stoichiometry and composition of membrane proteins and lipids can be controlled precisely (28); (iii) substrate, ligand, and protein interactions can be studied in a close-to-native lipid environment with access to both sides of the membrane protein complex (32–36). Here, we demonstrate that TAP reconstituted in nanodiscs displays an allosteric coupling between peptide binding and ATP hydrolysis. The lipid environment, in particular the annular lipid belt, is essential for the high affinity interaction and inhibition of TAP by the viral inhibitor ICP47.
EXPERIMENTAL PROCEDURES
Materials
Escherichia coli polar lipid was purchased from Avanti Polar Lipids. Digitonin was from Carl Roth or Calbiochem. Chemicals were ordered from Sigma and Carl Roth.
Protein Expression and Membrane Preparation
TAP was expressed in Pichia pastoris as described recently (26, 37). Briefly, P. pastoris strain SMD1163 was co-transformed with two pPICZC plasmids (Invitrogen) harboring either core-TAP1 (Q03518; residues 227–808) followed by a tobacco etch virus protease cleavage site, mVenus, and a His10 tag or core-TAP2 (Q59H06; residues 124–704) fused to a tobacco etch virus cleavage site and mCerulean followed by a StrepII tag. Yeast cells were resuspended in breaking buffer containing 50 mm sodium phosphate, pH 7.4, 1 mm EDTA, 100 mm aminohexanoic acid, 5% (v/v) glycerol, and protease inhibitors (protease inhibitor mix HP, Serva). The suspension was mixed 3:1 with ice-cold glass beads (0.5 mm diameter). Membranes were prepared by using a FastPrep-24 (MP Biomedicals) three times for 45 s and 6 m/s to break the cells, followed by centrifugation at 4000 × g for 15 min. The supernatant was further separated by ultracentrifugation (45 min, 100,000 × g). Membrane pellets were resuspended in 20 mm Hepes, pH 7.4, 200 mm NaCl, 50 mm KCl, 15% (v/v) glycerol.
Solubilization and Tandem Affinity Purification
TAP-containing membranes (10 mg/ml of total protein) were incubated in solubilization buffer (20 mm Hepes, pH 7.4, 200 mm NaCl, 50 mm KCl, 10 mm imidazole, 15% (v/v) glycerol, 2% (w/v) digitonin, and protease inhibitors (protease inhibitor mix HP)) for 90 min at 4 °C. Solubilized proteins were separated by ultracentrifugation at 100,000 × g for 45 min at 4 °C. TAP was first purified via immobilized metal affinity chromatography using the His10 tag at the C terminus of TAP1. Here, the solubilizate was mixed overnight with His-select nickel affinity matrix (Sigma). The beads were washed with 10 column volumes of 40 mm imidazole in purification buffer (20 mm Hepes, pH 7.4, 200 mm NaCl, 50 mm KCl, 15% (v/v) glycerol, 0.05% (w/v) digitonin) and eluted with 3 column volumes of 200 mm imidazole in purification buffer. Eluted proteins were loaded to high capacity Strep-Tactin-Sepharose (IBA), washed with 5 column volumes of the purification buffer, and eluted by 10 mm d-desthiobiotin (IBA) in purification buffer. All steps were performed at 4 °C. The concentration was determined by the absorbance (ϵ280 170,045 m−1 cm−1 and ϵ515 92,000 m−1 cm−1). Purified TAP complexes were analyzed by multicolor fluorescence size exclusion chromatography (MC-FSEC (37)), SDS-PAGE, and immunoblotting. In-gel fluorescence was detected with a Typhoon scanner (GE Healthcare). For detection of TAP1mVenus, the 488-nm laser and a band path 555-nm filter were used. TAP2mCerulean was monitored using the 457-nm laser and a short path 526-nm filter.
Multicolor Fluorescence Size Exclusion Chromatography
MC-FSEC analysis of the TAP complex in digitonin (TAP/Dig) or reconstituted in nanodiscs (TAP/Nd) was performed in SEC buffer (20 mm Hepes, pH 7.4, 200 mm NaCl, 50 mm KCl, 0.05% (w/v) digitonin or 10 mm Tris/HCl, pH 8.0, 150 mm NaCl, respectively) using a Shodex semi-micro KW404–4F (4.6 × 300 mm) column on an Agilent 1200 HPLC system (flow 0.3 ml/min) at 10 °C. TAP1 and TAP2 were detected via mVenus and mCerulean fluorescence, using two fluorescence detectors set to λex/em 515/535 and 435/470 nm, respectively (37). In addition, the absorbance was recorded at 280 nm.
Assembly of Nanodiscs
Membrane scaffold proteins MSP1 and MSP1E3D1 were expressed and purified as described (38). Both MSPs carry an N-terminal His6 tag and a subsequent tobacco etch virus protease cleavage site without a linker region (MGSSHHHHHHENLYFQG-MSP). Purified TAP complexes and MSPs were mixed with either sodium cholate- (100/25 mm lipid) or digitonin (4/25 mm lipid)-solubilized E. coli polar lipid at a TAP/MSP/lipid molar ratio of 1:10:300 for MSP1 and 1:10:850 for MSP1E3D1. Empty nanodiscs were formed by MSPs and E. coli polar lipid in a molar ratio of 1:35 (MSP1) or 1:90 (MSP1E3D1). If the final detergent concentration was less than 12 mm (cholate) or 0.5 mm (digitonin), the corresponding detergent was added. After incubation at 4 °C for 30 min, 100 mg of SMD-2 Biobeads (Bio-Rad) (dry weight) per mg of protein were added in four portions (1 h, overnight, two times for 2 h). After removal of the Biobeads, TAP complexes reconstituted in nanodiscs were concentrated to 2–5 μm at 1500 × g in Amicon Ultra 0.5-ml centrifugal filters with a 100-kDa cutoff (Millipore).
Electron Microscopy
Samples (3 μl) of SEC-purified empty nanodiscs and TAP/Nd were applied to a carbon-coated and glow-discharged EM grid for 1 min and subsequently stained with 3 μl of 1% uranyl acetate for 1 min. EM was performed with a CM120 (Philips) electron microscope equipped with a 2k x 2k CCD camera (Gatan).
Peptide and ICP47 Binding
TAP/Dig (4 μg, 310 nm) was incubated with 1 μm C4F peptide (RRYCFKSTEL, cysteine-labeled with fluorescein) in the presence and absence of the unlabeled competitor peptide RRYQKSTEL (R9LQK, 400 μm) in binding buffer (PBS, pH 7.4, 0.05% (w/v) digitonin). After incubation at 4 °C for 15 min, the mixture was transferred onto a filter plate (0.65 μm; Hydrophilic Low Protein Binding Durapore® membrane, Millipore) and washed three times with 250 μl of binding buffer at 4 °C. Bound peptides were solubilized on the filter with PBS, pH 7.4, 1% SDS. After 15 min of incubation and denaturation of the fluorescent fusion proteins at 95 °C for 5 min, the eluted peptides were quantified by fluorescence at λex/em 485/520 nm. Peptides were synthesized by Fmoc (N-(9-fluorenyl)methoxycarbonyl) solid-phase chemistry.
Alternatively, peptide and ICP47 binding to TAP solubilized in digitonin and TAP reconstituted in nanodiscs were analyzed by MC-FSEC. TAP/Dig or TAP/NdS were incubated with 1 μm of the fluorescent peptide RRYCAtto565KSTEL (C4Atto565) for 30 min on ice in either 20 mm Hepes, pH 7.4, 200 mm NaCl, 50 mm KCl, 15% (v/v) glycerol, 0.05% (w/v) digitonin (TAP/Dig), or 10 mm Tris/HCl, pH 8.0, 150 mm NaCl, 10% (v/v) glycerol (TAP/Nd). 1 or 12 μm of full-length ICP47H45C-Atto565 were incubated for 60 min with TAP/Nd or TAP/Dig, respectively. The samples were analyzed by MC-FSEC (Shodex KW404–4F column) in SEC buffer complemented with 10% glycerol at 0 °C to minimize ligand dissociation. Fluorescence detectors were set to λex/em 435/470 and 563/589 nm, respectively.
Microscale Thermophoresis
Microscale thermophoresis (MST) is based on the movement of fluorescent peptides along a temperature gradient induced by an infrared laser (39, 40). The change in fluorescence was monitored using a Monolith NT.115 (pico) MST with red LED Laser (λex/em 598–650/674–693 nm). The fluorescent peptide C4Atto655 (25 nm) was titrated with increasing concentrations of TAP/Dig or TAP/Nd at 22 °C and transferred into standard capillaries. The dilution buffer for TAP/Dig was 20 mm Hepes, pH 7.4, 200 mm NaCl, 50 mm KCl, 15% (v/v) glycerol, 0.05% (w/v) digitonin and for TAP/Nd was 10 mm Tris/HCl, pH 8.0, 150 mm NaCl. The change in fluorescence ΔF was derived from Fhot/Fcold, where Fhot and Fcold are the mean fluorescence at the end of the measurement and directly (0.6 s) after turning on the infrared laser, respectively. To determine the equilibrium dissociation constant (KD), the data were fitted to Equation 1, with [A0] and [T0] representing the known concentration of C4Atto655 and TAP, respectively.
 |
Hence, B represents the concentration of peptide C4Atto655-bound TAP complex. In addition, the IC50 value of TAP inhibition by ICP47 was determined. TAP/Dig or TAP/Nd (500 nm each) was incubated with different concentrations of ICP47(2–34) for 30 min. C4Atto655 was added (25 nm final) and incubated for another 30 min. Thermophoresis was conducted at 22 °C for 30 s. Background binding was determined with a 300-fold excess of unlabeled peptide R9LQK. Ki values were calculated with Equation 2, where B is the concentration of bound C4Atto655 (41).
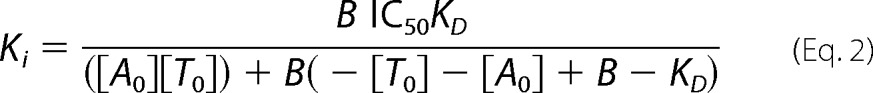 |
ATP Hydrolysis
The ATPase activity of TAP was determined by a colorimetric assay based on the complex formation of free inorganic phosphate and ammonium molybdate with malachite green (42). The assay was performed with purified TAP/Dig and TAP/Nd as described (27). TAP (500 nm) was incubated with 3 mm ATP and 5 mm MgCl2 as well as either the high affinity epitope R9LQK, the nonbinder peptide EPGYTNSTD (E9D, 1 μm each) or the 9-mer RRYCFKSTEL, 18-mer RRYQKSTELRRYCFKSTEL, and 27-mer RRYQKSTELRRYQKSTELRRYCFKSTEL peptides (10 μm each, cysteine labeled with fluorescein). TAP/Dig samples additionally contain 20 μm MSP1. The peptide-stimulated ATPase activity was inhibited by 10 μm ICP47(2–34). The assay was conducted in 25 μl for 30 min at 37 °C in corresponding binding buffer supplemented with a final concentration of 1 mm ouabain, 50 μm EGTA, and 5 mm NaN3. The reaction was stopped with 175 μl of 20 mm H2SO4. The release of inorganic phosphate was visualized by adding 50 μl of malachite green solution (3.5 mm malachite green, 0.2% (v/v) Tween 20, 0.8% (w/v) ammonium molybdate in 20% (v/v) H2SO4) followed by absorbance at 620 nm.
Peptide Transport
Peptide transport was analyzed in microsomes isolated from human B-cell lymphoma Raji cells. Rough microsomes were prepared as described (43). 10 μm RRYQNSTCFL (9-mer), RRYQKSTELRRYQNSTCFL (18-mer), or RRYQKSTELRRYQKSTELRRYQNSTCFL (27-mer; cysteine labeled with fluorescein; N-core glycosylation site is underlined) were incubated with 40 μg of microsomes and 5 mm MgCl2 in PBS, pH 7.0. Peptide transport was started by adding 3 mm ATP and was performed for 2 min at 37 °C. The transport reaction was stopped with 10 mm EDTA in PBS, pH 8.0, supplemented with 500 μm R9LQK to block any fluorescent peptide bound to TAP. Microsomes were pelleted (20,000 × g, 8 min) and washed with PBS, pH 8.0, 10 mm EDTA. Pellets were lysed in 50 mm Tris/HCl, pH 7.5, 150 mm NaCl, 5 mm KCl, 1 mm CaCl2, 1 mm MnCl2, 1% Nonidet P-40 for 15 min at room temperature. Debris was pelleted and the supernatant was bound to concanavalin A beads for 1 h at 4 °C. Bound peptides were eluted with lysis buffer, 200 mm methyl-α-d-mannopyranoside for 30 min at room temperature. Fluorescence was detected at λex/em 485/520 nm.
Phospholipid Quantification in Nanodiscs
SEC-purified nanodisc samples were heated above 200 °C in 450 μl of 8.9 n H2SO4 for 25 min. Brown color, resulting from proteins, was bleached by 150 μl of 30% H2O2 heated above 200 °C for 30 min. Total phosphorus was visualized by adding 500 μl of 2.5% (w/v) ammonium molybdate and 500 μl 10% (w/v) ascorbic acid in 3.9 ml of deionized water followed by absorbance at 820 nm.
Computational Techniques
Molecular dynamics (MD) simulations were run with GROMACS 4.6.5 (44), using the Amber99SB-ILDN protein (45, 46) and Stockholm lipid (47, 48) force fields, and TIP3P water (49). Periodic boundary conditions were used and long range Coulomb interactions were treated with a smooth particle mesh Ewald method (50). Temperature and pressure were kept constant at 300 K and 1 bar, respectively. To set up the simulation systems, coordinates of MSP1 and core-TAP were taken from a cryo-EM structure (51) and homology model (52), respectively. Starting from a 96-lipid POPE/POPG (3:1) NdS with embedded core-TAP, we removed 74 lipids in seven consecutive steps (8–12 lipids per step) to finally arrive at a 22-lipid TAP/NdS. Each of these systems was simulated for 50 ns prior to the next lipid removal step, for a total simulation time of 400 ns. Total system size was about 192,000 atoms. The chosen lipid mixture mimics the composition of the E. coli lipid used for TAP reconstitution. In addition, we repeated all simulations with a POPC/POPG (3:1) mixture. For reference, we also carried out a 50-ns MD simulation of core-TAP in a POPC/POPG (3:1) bilayer.
RESULTS
Tandem Affinity Purification of the TAP Complex
We first used an orthogonal purification procedure to prepare stoichiometrically well defined transport complexes. TAP was first purified by metal affinity chromatography via the His10 tag of TAP1 and subsequently via the StrepII tag of TAP2 using a Strep-Tactin matrix. TAP1mVenus and TAP2mCerulean were detected by MC-FSEC calibrated with equal concentrations of soluble mVenus and mCerulean (Fig. 1A). After immobilized metal affinity chromatography, 30% of the eluted proteins corresponded to TAP1 homodimers, which are inactive in peptide binding and transport, and were removed by the second purification step. Following the tandem affinity purification, the monodispersity and 1:1 stoichiometry of TAP1/2 complexes were shown by MC-FSEC (Fig. 1A) as well as SDS-PAGE in-gel fluorescence, Coomassie staining, and immunoblotting (Fig. 1B). Using a filter binding assay, we demonstrate stoichiometric peptide binding (87 ± 7%) to the heterodimeric TAP complexes (Fig. 1C).
FIGURE 1.
Tandem affinity purification of the TAP complex. A, 500 mg of crude membranes were solubilized at 10 mg/ml in 2% (w/v) digitonin. TAP was purified by immobilized metal affinity chromatography and subsequently via a Strep-Tactin resin. Monodispersity and stoichiometry of TAP1mVenus (yellow-orange) and TAP2mCerulean (blue) was analyzed by MC-FSEC (A280 black line). The fluorescence intensities of mVenus and mCerulean are calibrated to equal concentrations (37). The apparent molecular mass of TAP/Dig (red arrow) was estimated by the elution (black arrows) of dextran blue (2 MDa), bovine thyroglobulin (669 kDa), and β-amylase (200 kDa). B, purified TAP complexes were analyzed by SDS-PAGE (9%, Coomassie), in-gel fluorescence, and immunoblotting using TAP1- and TAP2-specific antibodies. C, activity of purified TAP was determined by a peptide binding filter assay. 4 μg of TAP (310 nm) was incubated with 1 μm C4F peptide. Binding was competed with a 400-fold molar excess of unlabeled peptide R9LQK. a.u., arbitrary unit; mAU, milli-absorption unit.
Reconstitution of the TAP Complex in Nanodiscs
Purified TAP complexes were reconstituted in small (TAP/NdS) and large nanodiscs (TAP/NdL) assembled by MSP1 and MSP1E3D1, respectively. Nanodiscs were formed by mixing TAP with cholate or digitonin-solubilized E. coli polar lipid and MSPs, followed by detergent removal with polystyrene beads, schematically illustrated in Fig. 2A. Based on systematic screening, a molar TAP/MSP/lipid ratio of 1:10:300 for TAP/NdS and 1:10:850 for TAP/NdL was found to be optimal for reconstitution. After self-assembly, the lipid particles were analyzed by MC-FSEC. TAP1 and TAP2 in nanodiscs were detected via mVenus and mCerulean, respectively (Fig. 2, B and C). The major elution peak corresponds to heterodimeric TAP1-TAP2 complexes in nanodiscs as confirmed by SDS-PAGE (Fig. 2D). As expected, the apparent molecular mass slightly shifts with the size of nanodiscs (320 and 340 kDa for TAP/NdS and TAP/NdL, respectively). Negative stain electron microscopy confirms the assembly of disc-like nanoparticles. EM images illustrate that SEC-purified TAP/NdL are reasonably homogeneous particles with the expected diameter of ∼12 nm (Fig. 2E). In contrast, TAP/NdS particles appeared in a larger disc size than expected (data not shown). Similar results were reported for ATP-binding cassette importers, including MalFGK2 (53) or OpuA (54) incorporated in nanodiscs.
FIGURE 2.
Reconstitution of the TAP complex in nanodiscs. A, scheme of the reconstitution of TAP into nanodiscs using a TAP model described previously (52) and MSP1 (Protein Data Bank code 1AV1). Purified TAP was mixed with MSPs and E. coli polar lipids solubilized in cholate. Nanodiscs are assembled by detergent removal using Biobeads. TAP/NdS formed by MSP1 or MSP1ED1 in a molar TAP/MSP/lipid ratio of 1:10:300 (B) or 1:10:850 (C) were analyzed by MC-FSEC. Arrows indicate the apparent molecular weight of TAP in NdS and NdL and the volume where empty NdS and NdL would typically elute. D, reconstitution and protein composition of the SEC-purified TAP-Nd complexes were analyzed by SDS-PAGE (12%, Coomassie). Coomassie staining of MSP1 is significantly lower than of MSP1E3D1 as seen previously (33). E, negative-stain electron microscopy of TAP/NdL complexes. The bars scale to 10 nm. a.u., arbitrary unit; mAU, milli-absorption unit.
TAP in Nanodiscs Is Active in Peptide Binding
After successful reconstitution, we analyzed the peptide binding activity of TAP/NdS by MC-FSEC (Fig. 3). Binding of the fluorescent high affinity peptide C4Atto565 was detected by the overlapping TAP signal detected via mCerulean, whereas free peptides elute at larger volumes (Fig. 3A). Under saturating conditions, peptide binding to TAP in nanodiscs was similar to TAP solubilized in digitonin (Fig. 3C). We next determined the peptide binding affinity to TAP reconstituted in nanodiscs. Because TAP/Nd could not be separated from free peptides by filter or centrifugation assays, we had to establish a new homogeneous interaction assay. MST reports on a direct ligand-protein interaction by minute changes in the diffusion of particles in a microscopic temperature gradient (39, 40). Accordingly, the change in fluorescence (ΔF) of C4Atto655 reflects the concentration of peptide-TAP complexes (decrease in fluorescence signal, Fig. 4A). The equilibrium dissociation constants KD determined by MST are in the same range, with 54 ± 12 nm for TAP/Dig, 91 ± 15 nm for TAP/NdS, and 120 ± 16 nm for TAP/NdL (Fig. 4, B–D). Competition with an excess of unlabeled peptides demonstrates that the binding is peptide-specific (see Fig. 6B).
FIGURE 3.
Peptide binding to TAP in nanodiscs analyzed by MC-FSEC. Nanodiscs were generated with lipids solubilized in digitonin. A, binding of the fluorescent peptide C4Atto565 to TAP/NdS. TAP/NdS was incubated with 1 μm C4Atto565 for 30 min and analyzed at 0 °C by MC-FSEC. The correlated fluorescence emission of the TAP complex detected via mCerulean (blue) and C4Atto565 (red) reflects the bound peptide C4Atto565. The dotted line represents the binding in the presence of 400 μm unlabeled peptide R9LQK. Peptide binding to TAP/Dig was performed the same way. B, C4Atto565 binding to empty nanodiscs was performed as described in A. C, peptide binding to TAP/Dig and TAP/NdS. Bound peptide C4Atto565 was calculated from peak areas (black); background is determined in excess of unlabeled peptide R9LQK (white) shown in A. Error bars are defined by standard deviation of the TAP signal. a.u., arbitrary unit.
FIGURE 4.
Binding affinities of TAP analyzed by MST. A, normalized MST traces of the fluorescent peptide C4Atto655 (25 nm) with increasing concentrations of TAP/Dig are shown as representative examples. Dotted lines indicate data points used for the calculation of the binding curves. Peptide binding to TAP/Dig (B), TAP/NdS (C), and TAP/NdL (D) is shown. KD values were calculated by Equation 1. Peptide binding was measured in triplicate. Error bars, S.D. a.u., arbitrary unit.
FIGURE 6.
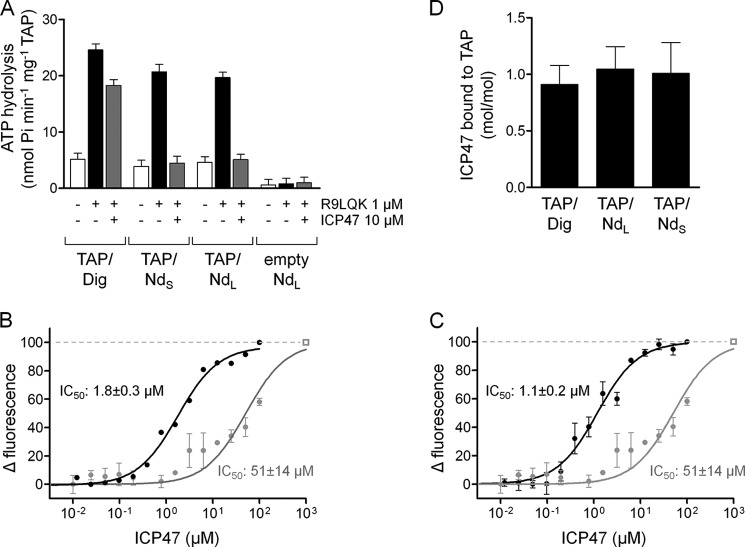
Nanodiscs mimic the lipid environment required for high affinity viral inhibition of TAP. A, inhibition of ATP hydrolysis by the viral TAP inhibitor ICP47. TAP/Dig, TAP/NdS, or TAP/NdL was incubated with 5 mm MgCl2, 3 mm ATP, and either 1 μm R9LQK, 20 μm ICP47(2–34), or both. ATPase activity was measured for 30 min at 37 °C. B and C, competitive binding of peptide C4Atto655 and ICP47 was measured by MST. TAP/NdS (B) or TAP/NdL (C) (500 nm each) was incubated with C4Atto655 (25 nm) and increasing concentrations of ICP47(2–34). The IC50 values were calculated by the change in fluorescence with 1.8 ± 0.3 μm and c.i. 1.4–2.3 μm (TAP/NdS) and 1.1 ± 0.2 μm and c.i. 0.9–1.6 μm (TAP/NdL). The competitive peptide binding of ICP47 was compared with TAP/Dig, leading to an IC50 value of 51 ± 14 μm and c.i. of 34–76 μm (TAP/Dig, gray curves, identical data in B and C). The rectangle indicates the competition with a 300-fold excess of unlabeled peptide R9LQK. D, stoichiometry of ICP47 binding to TAP/Dig and TAP/Nd was determined by MC-FSEC at 0 °C. 1 or 12 μm of ICP47Atto565 were incubated with 500 nm TAP/Nd or TAP/Dig, respectively.
Allosteric Coupling between Peptide Binding and ATP Hydrolysis
We investigated the ATPase activity of TAP reflecting peptide translocation because it is not possible to follow the transport process directly in nanodiscs. ATP hydrolysis by TAP was determined in the presence and absence of peptides. Notably, the high affinity peptide R9LQK stimulated ATP hydrolysis of TAP reconstituted in NdS and NdL (Fig. 5A). In the absence of peptides or in the presence of the nonbinder peptide E9D (16), TAP/Nd and TAP/Dig show only background levels of the ATPase activity. This behavior was similar to TAP solubilized in digitonin. The ATPase activity of TAP was not affected by the size of the nanodiscs, indicating that the amount of lipids does not strongly influence the peptide-stimulated ATPase activity.
FIGURE 5.
Allosteric coupling between peptide binding and ATP hydrolysis. A, TAP/Dig, TAP/NdS, TAP/NdL, or empty nanodiscs were incubated with 3 mm MgATP and 1 μm of the high affinity peptide R9LQK or nonbinding peptide E9D. Background ATPase activity is given in the absence of peptide. ATP hydrolysis was measured in triplicate. Error bars, S.D. B, TAP/NdL was incubated with 5 mm MgCl2, 3 mm ATP, as well as 10 μm 9-, 18-, and 27-mer, respectively. The peptide stimulated ATP hydrolysis was determined in triplicate. Error bars, S.D. C, peptide transport of the 9-, 18-, and 27-mer. TAP-containing microsomes were incubated with 10 μm of each peptide, 5 mm MgCl2, and 3 mm ATP. Peptide transport was followed for 2 min at 37 °C. Error bars, S.D. D, peptide binding (9-, 18-, and 27-mer) to TAP/NdL was measured by MST. Increasing concentrations of peptide were incubated with 200 nm TAP/NdL and 25 nm of C4Atto655. The IC50 values were calculated by the change in fluorescence. The IC50 values are 0.9 μm with a confidence interval (c.i.) of 0.8–1 μm for 9-mer, 1.6 μm with a c.i. of 1.3–2 μm for 18-mer, and 0.7 μm with a c.i. of 0.5–0.8 μm for 27-mer. All binding data were measured in triplicate. Error bars, S.D.
We next examined the ATP turnover of TAP/NdL stimulated by peptides of different lengths (9, 18, and 27 amino acids) (Fig. 5B). These peptides are concatemers of the peptide epitope R9LQK, preserving all high affinity binding motifs (16). By competition MST (increase in fluorescence signal), their binding affinity was determined to be similar (IC50 of 0.9 μm for 9-mer, 1.6 μm for 18-mer, and 0.7 μm for 27-mer; Fig. 5D). Peptide-stimulated ATP hydrolysis was measured at peptide concentrations of 10 μm, which is well above the KD values of all three peptides. The 9-mer peptide leads to an ATP turnover of 3.9 ± 0.2/min per TAP/NdL. Notably, the 18- and 27-mer peptides induce an ATP turnover of 1.1 ± 0.1/min and 0.5 ± 0.3/min, respectively (Fig. 5B). Following these results, we investigated peptide transport by TAP in microsomes at initial rate conditions. The transport of the 18- and 27-mer was only 61 and 23% of the 9-mer, respectively (Fig. 5C). Thus, the length of peptides strongly influenced the ATP turnover and transport rates. These findings show that the ATPase activity reflects peptide translocation by TAP, which is slowed down with increasing peptide length.
A Lipid Interface Is Essential for High Affinity TAP Inhibition
We also examined the effect of the viral TAP inhibitor ICP47 (active domain, amino acids 3–34) (22) on the allosteric coupling between peptide binding and ATP hydrolysis. ICP47 encoded by HSV-1 blocks the peptide-stimulated ATP hydrolysis by competing with R9LQK for the binding pocket (Fig. 6A). ICP47 alone does not induce ATP hydrolysis of either TAP/Dig or TAP/Nd. Upon addition of ICP47 (10 μm), the peptide-induced ATP hydrolysis of TAP/NdS and TAP/NdL was blocked to background levels, although TAP/Dig was only partially (33%) inhibited. These results point to the important role of the lipid interface, without an influence of the membrane scaffold protein itself, in the mechanism of how ICP47 blocks peptide binding to TAP. For this reason, the inhibition of peptide binding was analyzed by competition microscale thermophoresis (increase in fluorescence signal) with rising concentrations of ICP47 (Fig. 6, B and C). 100% competition was recorded at a 300-fold excess of unlabeled peptide (Fig. 6, B and C, dashed line, open rectangle). Remarkably, ICP47 has a drastically higher potency to inhibit TAP in nanodiscs (IC50 1.8 ± 0.3 μm for TAP/NdS and 1.1 ± 0.2 μm for TAP/NdL; Fig. 6, C and D) as compared with detergent-solubilized TAP (IC50 51 ± 14 μm for TAP/Dig). These results show that the lipids surrounding the TAP complex are essential for high affinity ICP47-TAP interaction. We further wondered whether the different IC50 values are explained by an accumulation at the membrane or a correct folding of ICP47 triggered by the lipid environment in nanodiscs. Therefore, we determined the stoichiometry of ICP47Atto565 binding to TAP/Dig and TAP/Nd by MC-FSEC (37) under saturating conditions (10-fold above the KD value). As shown in Fig. 6D, ICP47 binds to TAP solubilized in digitonin or TAP reconstituted in nanodiscs in a 1:1 stoichiometry. These findings lead us to the assumption that the membrane environment in nanodiscs facilitates correct folding of ICP47 and high affinity interaction with TAP.
An Annular Lipid Belt Remains Around TAP in Small Nanodiscs
With respect to the remarkable difference in the inhibition pattern of ICP47 to TAP, the amount of phospholipids per nanodisc after reconstitution of TAP was determined. We defined that 22 ± 2 and 113 ± 3 lipids remain associated with TAP/NdS and TAP/NdL, respectively. In empty MSP1 nanodiscs, depending on the lipid species, ∼120 (POPC) to 160 (DPPC) phospholipids have been found (28). Based on the cross-sectional area of the transmembrane domain of the homology model (52), core-TAP can replace ∼60 phospholipids. Although the E. coli polar lipids used in this study clearly represent a more complex lipid mixture as compared with the pure phospholipid nanodiscs, the observation that the transporter replaces more than 100 lipids in NdS thus cannot be understood solely by simple geometric considerations. Instead, other mechanisms are at play, such as possible changes in lipid affinity due to the presence of TAP. In conclusion, 22 lipids in TAP/NdS seem to be sufficient for high affinity TAP inhibition by ICP47.
The surprisingly small number of lipids found in the NdS complex (22 ± 2) raises questions about its structure at the atomic level and, in particular, whether as little as 22 lipids are sufficient to keep up a lipid belt around TAP. To address these questions, we used all-atom MD simulations in explicit solvent. The final structure of TAP/NdS obtained from our simulations is shown in Fig. 7A. The POPE and POPG lipids indeed form a belt that entirely encircles the TM region of TAP1/2, isolating the transporter from MSP1. Only a small number of residues on the TAP complex and MSP1 are in direct contact (Fig. 7B). The scaffold proteins accommodate the small nanodisc size by partial unfolding, yet retain a largely helical conformation (average helicity is 50%, compared with 72% in the starting cryo-EM structure). In our reference simulation of TAP in a conventional lamellar bilayer, 50 lipids are in direct contact with TAP. The striking difference with the 22 lipids strongly suggests that structural changes of the lipids in TAP/NdS are required to maintain a complete belt around TAP. We observed tilting of the lipids with respect to the TM helices and displacement of some phosphate headgroups toward the center of the membrane (Fig. 7C), allowing the lipids to cover more surface of TAP in the plane of the Nd. The classical bilayer structure is lost (for Nd < 50–60 lipids), and the resulting lipid belt is substantially thinner. Our simulations of the POPC/POPG NdS yielded similar results (Fig. 7C), including the formation of a lipid belt around TAP.
FIGURE 7.
Molecular dynamics simulations of the annular lipid belt around the TAP complex in small nanodiscs. A, structure of TAP/NdS after 400 ns of MD simulation. TAP is colored green and MSP1 salmon. The 22-lipid belt contains 17 POPE (blue) and 5 POPG (dark yellow) molecules; phosphate headgroup atoms are shown as spheres. B, time-averaged minimal TAP-MSP1 distances in the 22-lipid nanodisc. C, phosphate density along the normal to the nanodisc plane, for a TAP-containing lamellar POPE/POPG bilayer (black line) and the 22-lipid TAP/NdS systems with POPE/POPG (dashed blue line) and POPC/POPG (dash-dotted red line) membranes.
DISCUSSION
Functional and structural analyses of membrane proteins are often difficult, especially in the case of multisubunit complexes, where the correct assembly, stoichiometry, and stability must be carefully controlled. In addition, the local lipid environment is emerging as one of the most important of the many factors that govern their stability and function. As TAP1 and TAP2 can form inactive homodimers, we used a tandem affinity purification strategy via a His10 tag at TAP1 and a StrepII-tag at TAP2. C-terminal tagging with mVenus (TAP1) and mCerulean (TAP2) facilitated sensitive quantification of TAP1/2 complexes. Based on the calibrated fluorescence, a monodisperse heterodimeric TAP complex was isolated. TAP function was preserved, resulting in stoichiometric peptide binding. The purified TAP complex was reconstituted in small (9.8 nm) and large nanodiscs (12 nm) formed by MSP1 and MSP1E3D1, respectively. Typically, membrane proteins are reconstituted in liposomes to study their function in a physiologically relevant membrane environment. Proteoliposomes are at least 10-fold larger than nanodiscs and display a mixed protein orientation (26). In addition, in nanodiscs the number of lipids and protein subunits is well defined. The limited number of lipids in nanodiscs mimics molecular crowding as recently observed by solid-state NMR, which shows a reduced dynamic of proteorhodopsin in nanodiscs compared with lamellar preparations (31). Therefore, nanodiscs display a more native-like environment compared with liposomes. We demonstrated by MC-FSEC that the apparent molecular mass of 320–340 kDa corresponds to one heterodimeric TAP complex per nanodisc (TAP 200 kDa, MSPs 50–60 kDa and lipids 25–100 kDa). TAP in nanodiscs is active in peptide binding and allosteric coupling between substrate binding and ATP hydrolysis.
In this study, we have established several approaches to investigate peptide-TAP, protein-TAP, and lipid-TAP interaction using nanodiscs, microscale thermophoresis, MC-FSEC, and MD simulations. The affinity and maximal binding of antigenic peptides to TAP/Nd is comparable with TAP in digitonin. Hence, the reconstitution procedure maintains the function of peptide binding. Furthermore, the high affinity peptide R9LQK stimulated ATP hydrolysis of TAP in nanodiscs, whereas the nonbinder peptide E9D (16) did not induce ATP hydrolysis above background. These data demonstrate the allosteric coupling between peptide binding and ATP hydrolysis of TAP reconstituted in nanodiscs.
It is known that TAP can translocate peptides of different length, however, with much lower efficiency (15, 19). Here, we show that a 9-, 18-, and 27-mer peptide, bound to the TAP complex with similar affinity, displayed a gradually decreased transport and ATPase activity. At saturating concentrations (5–10 times above KD values), the 9-mer peptide led to a turnover of 4 ATP/min, whereas the 18- and 27-mer peptides reduced the turnover to 1 ATP/min and 0.5 ATP/min, respectively. These results were confirmed by a reduced peptide transport (61 and 23%) of the 18-mer and 27-mer, respectively, in comparison with the 9-mer peptide. Assuming that the 9- to 27-mer peptides have similar association rates based on the measured coincident KD values, these results show that the movement of longer peptides through the TAP complex becomes rate-limiting.
In addition, we examined whether lipids in nanodiscs are sufficient to promote high affinity protein interaction with the viral TAP inhibitor ICP47. Based on previous findings that ICP47 inhibits TAP in microsomes with a 50–100-fold higher affinity than TAP solubilized in detergent, we speculate that the lipid interface promotes ICP47 binding to TAP via a correct folding of ICP47 by interacting with the lipid-TAP interface (24). Here, we observed a full inhibition of peptide-induced ATP hydrolysis for TAP in nanodiscs, although the peptide-stimulated ATPase of TAP/Dig was only partially blocked by ICP47 (10 μm). These observations were confirmed by competition binding assays, revealing inhibition constants Ki of 0.28 and 0.21 μm of TAP/NdS and TAP/NdL, respectively, which are significantly higher than for TAP in digitonin (5.0 μm). ICP47 binds in a 1:1 stoichiometry to TAP reconstituted in nanodiscs. Thus, the lipid interface in nanodiscs does not lead to an accumulation of the viral factor but may promote the correct folding of ICP47 for the high affinity inhibition of TAP. We therefore determined the number of phospholipids in small and large nanodiscs containing reconstituted TAP. Our MD simulations show that, by tilting and displacement of their phosphate headgroups toward the center of the membrane, the 22 lipids in TAP/NdS still allow for the formation of an annular lipid belt around the core-TAP complex. This finding is consistent with our experimental observation that the ICP47-TAP interaction is not significantly affected by higher amounts of lipids in TAP/NdL. Thus, the annular lipid belt surrounding the TAP complex in small nanodiscs is essential and sufficient to promote correct folding of ICP47 for high affinity TAP inhibition.
The allosteric coupling between peptide binding/translocation and ATP hydrolysis is not significantly altered between TAP reconstituted in nanodiscs or TAP in digitonin. We used digitonin for solubilization, which is known to optimally preserve TAP activity (26, 27), forming large lipid-detergent micelles of 75–100 kDa (55, 56). Although these associated lipids are sufficient to preserve TAP function, a lipid interface of 22 annular lipids is essential for high affinity ICP47-TAP interaction.
In conclusion, one stoichiometrically defined heterodimeric TAP complex was reconstituted in nanodiscs. TAP/Nd is active in high-affinity binding of peptides. Furthermore, a peptide-stimulated ATP hydrolysis was observed, reflecting the allosteric coupling between peptide binding and ATP hydrolysis. Steric constraints, such as longer peptides and bulky side chains, which hinder peptide translocation but not binding (18), decelerated the translocation and ATP hydrolysis cycle of TAP. Thus, the peptide-stimulated ATPase activity indirectly reports on the translocation process of TAP in nanodiscs. The peptide-stimulated ATP hydrolysis was blocked by the herpesvirus immune evasin ICP47 by a 1:1 interaction. This allosteric inhibition is significantly decreased in detergent micelles. Thus, nanodiscs provide this essential lipid interface for high affinity ICP47-TAP interaction. The small size, the lipid environment, and the accessibility to both sides of the membrane protein complex make nanodiscs a powerful approach to study the dynamics and interaction mechanism of TAP by biophysical techniques, including single molecule studies.
Acknowledgments
We thank Katrin Rohde and Dr. Christine Ziegler (Max Planck Institute of Biophysics, Frankfurt/M.) for their support in electron microscopy. We are grateful to Elisa Lehnert for peptide synthesis of ICP47(2–34). We also thank Christine Le Gal, Drs. Rupert Abele, Inga Hänelt, Peter Mayerhofer, and Fabian Seyffer for helpful comments on the manuscript.
This work was supported by German Research Foundation Grant SFB 807 (Transport and Communication across Biological Membranes (to R. T. and L. V. S.), Emmy Noether grant (to L. V. S.), Cluster of Excellence RESOLV EXC 1069 (to L. V. S.), and the European Drug Initiative on Channels and Transporters EDICT (to R. T.) funded by the European Commission Seventh Framework Program.
- TAP
- transporter associated with antigen processing
- MD
- molecular dynamics
- TM
- transmembrane
- Dig
- digitonin
- MSP
- membrane scaffold protein
- c.i.
- confidence interval
- POPC
- 1-palmitoyl-2-oleoyl-l-phosphatidylcholine
- POPG
- 1-palmitoyl-2-oleoyl-l-phosphatidylglycerol
- POPE
- 1-palmitoyl-2-oleoyl-l-phosphatidylethanolamine
- MST
- microscale thermophoresis
- MC-FSEC
- multicolor fluorescence-size exclusion chromatography
- SEC
- size exclusion chromatography
- Nd
- nanodisc
- MSP
- membrane scaffold protein.
REFERENCES
- 1. Hulpke S., Tampé R. (2013) The MHC I loading complex: a multitasking machinery in adaptive immunity. Trends Biochem. Sci. 38, 412–420 [DOI] [PubMed] [Google Scholar]
- 2. Procko E., O'Mara M. L., Bennett W. F., Tieleman D. P., Gaudet R. (2009) The mechanism of ABC transporters: general lessons from structural and functional studies of an antigenic peptide transporter. FASEB J. 23, 1287–1302 [DOI] [PubMed] [Google Scholar]
- 3. Parcej D., Tampé R. (2010) ABC proteins in antigen translocation and viral inhibition. Nat. Chem. Biol. 6, 572–580 [DOI] [PubMed] [Google Scholar]
- 4. Rees D. C., Johnson E., Lewinson O. (2009) ABC transporters: the power to change. Nat. Rev. Mol. Cell Biol. 10, 218–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nijenhuis M., Hämmerling G. J. (1996) Multiple regions of the transporter associated with antigen processing (TAP) contribute to its peptide binding site. J. Immunol. 157, 5467–5477 [PubMed] [Google Scholar]
- 6. Herget M., Oancea G., Schrodt S., Karas M., Tampé R., Abele R. (2007) Mechanism of substrate sensing and signal transmission within an ABC transporter: use of a Trojan horse strategy. J. Biol. Chem. 282, 3871–3880 [DOI] [PubMed] [Google Scholar]
- 7. Koch J., Guntrum R., Heintke S., Kyritsis C., Tampé R. (2004) Functional dissection of the transmembrane domains of the transporter associated with antigen processing (TAP). J. Biol. Chem. 279, 10142–10147 [DOI] [PubMed] [Google Scholar]
- 8. Procko E., Raghuraman G., Wiley D. C., Raghavan M., Gaudet R. (2005) Identification of domain boundaries within the N termini of TAP1 and TAP2 and their importance in tapasin binding and tapasin-mediated increase in peptide loading of MHC class I. Immunol. Cell Biol. 83, 475–482 [DOI] [PubMed] [Google Scholar]
- 9. Koch J., Guntrum R., Tampé R. (2006) The first N-terminal transmembrane helix of each subunit of the antigenic peptide transporter TAP is essential for independent tapasin binding. FEBS Lett. 580, 4091–4096 [DOI] [PubMed] [Google Scholar]
- 10. Hulpke S., Tomioka M., Kremmer E., Ueda K., Abele R., Tampé R. (2012) Direct evidence that the N-terminal extensions of the TAP complex act as autonomous interaction scaffolds for the assembly of the MHC I peptide-loading complex. Cell. Mol. Life Sci. 69, 3317–3327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Abele R., Tampé R. (2004) The ABCs of immunology: structure and function of TAP, the transporter associated with antigen processing. Physiology 19, 216–224 [DOI] [PubMed] [Google Scholar]
- 12. Seyffer F., Tampé R. (2014) ABC transporters in adaptive immunity. Biochim. Biophys. Acta 10.1016/j.bbagen.2014.05.022 [DOI] [PubMed] [Google Scholar]
- 13. Neefjes J. J., Momburg F., Hämmerling G. J. (1993) Selective and ATP-dependent translocation of peptides by the MHC-encoded transporter. Science 261, 769–771 [DOI] [PubMed] [Google Scholar]
- 14. van Endert P. M., Tampé R., Meyer T. H., Tisch R., Bach J. F., McDevitt H. O. (1994) A sequential model for peptide binding and transport by the transporters associated with antigen processing. Immunity 1, 491–500 [DOI] [PubMed] [Google Scholar]
- 15. Androlewicz M. J., Cresswell P. (1994) Human transporters associated with antigen processing possess a promiscuous peptide-binding site. Immunity 1, 7–14 [DOI] [PubMed] [Google Scholar]
- 16. Uebel S., Kraas W., Kienle S., Wiesmüller K. H., Jung G., Tampé R. (1997) Recognition principle of the TAP transporter disclosed by combinatorial peptide libraries. Proc. Natl. Acad. Sci. U.S.A. 94, 8976–8981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Herget M., Baldauf C., Schölz C., Parcej D., Wiesmüller K. H., Tampé R., Abele R., Bordignon E. (2011) Conformation of peptides bound to the transporter associated with antigen processing (TAP). Proc. Natl. Acad. Sci. U.S.A. 108, 1349–1354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gorbulev S., Abele R., Tampé R. (2001) Allosteric crosstalk between peptide-binding, transport, and ATP hydrolysis of the ABC transporter TAP. Proc. Natl. Acad. Sci. U.S.A. 98, 3732–3737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Koopmann J. O., Post M., Neefjes J. J., Hämmerling G. J., Momburg F. (1996) Translocation of long peptides by transporters associated with antigen processing (TAP). Eur. J. Immunol. 26, 1720–1728 [DOI] [PubMed] [Google Scholar]
- 20. Ahn K., Meyer T. H., Uebel S., Sempé P., Djaballah H., Yang Y., Peterson P. A., Früh K., Tampé R. (1996) Molecular mechanism and species specificity of TAP inhibition by herpes simplex virus ICP47. EMBO J. 15, 3247–3255 [PMC free article] [PubMed] [Google Scholar]
- 21. Tomazin R., Hill A. B., Jugovic P., York I., van Endert P., Ploegh H. L., Andrews D. W., Johnson D. C. (1996) Stable binding of the herpes simplex virus ICP47 protein to the peptide binding site of TAP. EMBO J. 15, 3256–3266 [PMC free article] [PubMed] [Google Scholar]
- 22. Neumann L., Kraas W., Uebel S., Jung G., Tampé R. (1997) The active domain of the herpes simplex virus protein ICP47: a potent inhibitor of the transporter associated with antigen processing. J. Mol. Biol. 272, 484–492 [DOI] [PubMed] [Google Scholar]
- 23. Beinert D., Neumann L., Uebel S., Tampé R. (1997) Structure of the viral TAP-inhibitor ICP47 induced by membrane association. Biochemistry 36, 4694–4700 [DOI] [PubMed] [Google Scholar]
- 24. Aisenbrey C., Sizun C., Koch J., Herget M., Abele R., Bechinger B., Tampé R. (2006) Structure and dynamics of membrane-associated ICP47, a viral inhibitor of the MHC I antigen-processing machinery. J. Biol. Chem. 281, 30365–30372 [DOI] [PubMed] [Google Scholar]
- 25. Pfänder R., Neumann L., Zweckstetter M., Seger C., Holak T. A., Tampé R. (1999) Structure of the active domain of the herpes simplex virus protein ICP47 in water/sodium dodecyl sulfate solution determined by nuclear magnetic resonance spectroscopy. Biochemistry 38, 13692–13698 [DOI] [PubMed] [Google Scholar]
- 26. Schölz C., Parcej D., Ejsing C. S., Robenek H., Urbatsch I. L., Tampé R. (2011) Specific lipids modulate the transporter associated with antigen processing (TAP). J. Biol. Chem. 286, 13346–13356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Herget M., Kreissig N., Kolbe C., Schölz C., Tampé R., Abele R. (2009) Purification and reconstitution of the antigen transport complex TAP: a prerequisite for determination of peptide stoichiometry and ATP hydrolysis. J. Biol. Chem. 284, 33740–33749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Denisov I. G., Grinkova Y. V., Lazarides A. A., Sligar S. G. (2004) Directed self-assembly of monodisperse phospholipid bilayer nanodiscs with controlled size. J. Am. Chem. Soc. 126, 3477–3487 [DOI] [PubMed] [Google Scholar]
- 29. Bayburt T. H., Sligar S. G. (2010) Membrane protein assembly into nanodiscs. FEBS Lett. 584, 1721–1727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hagn F., Etzkorn M., Raschle T., Wagner G. (2013) Optimized phospholipid bilayer nanodiscs facilitate high resolution structure determination of membrane proteins. J. Am. Chem. Soc. 135, 1919–1925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mörs K., Roos C., Scholz F., Wachtveitl J., Dötsch V., Bernhard F., Glaubitz C. (2013) Modified lipid and protein dynamics in nanodiscs. Biochim. Biophys. Acta 1828, 1222–1229 [DOI] [PubMed] [Google Scholar]
- 32. Ritchie T. K., Kwon H., Atkins W. M. (2011) Conformational analysis of human ATP-binding cassette transporter ABCB1 in lipid nanodiscs and inhibition by the antibodies MRK16 and UIC2. J. Biol. Chem. 286, 39489–39496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kawai T., Caaveiro J. M., Abe R., Katagiri T., Tsumoto K. (2011) Catalytic activity of MsbA reconstituted in nanodisc particles is modulated by remote interactions with the bilayer. FEBS Lett. 585, 3533–3537 [DOI] [PubMed] [Google Scholar]
- 34. Mishra S., Verhalen B., Stein R. A., Wen P. C., Tajkhorshid E., Mchaourab H. S. (2014) Conformational dynamics of the nucleotide binding domains and the power stroke of a heterodimeric ABC transporter. Elife 3, e02740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nasr M. L., Singh S. K. (2014) Radioligand binding to nanodisc-reconstituted membrane transporters assessed by the scintillation proximity assay. Biochemistry 53, 4–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bao H., Duong F. (2014) Nucleotide-free MalK drives the transition of the maltose transporter to the inward-facing conformation. J. Biol. Chem. 289, 9844–9851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Parcej D., Guntrum R., Schmidt S., Hinz A., Tampé R. (2013) Multicolour fluorescence-detection size-exclusion chromatography for structural genomics of membrane multiprotein complexes. PLoS One 8, e67112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Roos C., Zocher M., Müller D., Münch D., Schneider T., Sahl H. G., Scholz F., Wachtveitl J., Ma Y., Proverbio D., Henrich E., Dötsch V., Bernhard F. (2012) Characterization of co-translationally formed nanodisc complexes with small multidrug transporters, proteorhodopsin and with the E. coli MraY translocase. Biochim. Biophys. Acta 1818, 3098–3106 [DOI] [PubMed] [Google Scholar]
- 39. Wienken C. J., Baaske P., Rothbauer U., Braun D., Duhr S. (2010) Protein-binding assays in biological liquids using microscale thermophoresis. Nat. Commun. 1, 100. [DOI] [PubMed] [Google Scholar]
- 40. Jerabek-Willemsen M., Wienken C. J., Braun D., Baaske P., Duhr S. (2011) Molecular interaction studies using microscale thermophoresis. Assay Drug Dev. Technol. 9, 342–353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Swillens S. (1995) Interpretation of binding curves obtained with high receptor concentrations: practical aid for computer analysis. Mol. Pharmacol. 47, 1197–1203 [PubMed] [Google Scholar]
- 42. Henkel R. D., VandeBerg J. L., Walsh R. A. (1988) A microassay for ATPase. Anal. Biochem. 169, 312–318 [DOI] [PubMed] [Google Scholar]
- 43. Walter P., Blobel G. (1983) Preparation of microsomal membranes for cotranslational protein translocation. Methods Enzymol. 96, 84–93 [DOI] [PubMed] [Google Scholar]
- 44. Pronk S., Páll S., Schulz R., Larsson P., Bjelkmar P., Apostolov R., Shirts M. R., Smith J. C., Kasson P. M., van der Spoel D., Hess B., Lindahl E. (2013) GROMACS 4.5: a high throughput and highly parallel open source molecular simulation toolkit. Bioinformatics 29, 845–854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hornak V., Abel R., Okur A., Strockbine B., Roitberg A., Simmerling C. (2006) Comparison of multiple Amber force fields and development of improved protein backbone parameters. Proteins 65, 712–725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lindorff-Larsen K., Piana S., Palmo K., Maragakis P., Klepeis J. L., Dror R. O., Shaw D. E. (2010) Improved side-chain torsion potentials for the Amber ff99SB protein force field. Proteins 78, 1950–1958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Jämbeck J. P., Lyubartsev A. P. (2012) Derivation and systematic validation of a refined all-atom force field for phosphatidylcholine lipids. J. Phys. Chem. B 116, 3164–3179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Jämbeck J. P., Lyubartsev A. P. (2012) An extension and further validation of an all-atomistic force field for biological membranes. J. Chem. Theory Comput. 8, 2938–2948 [DOI] [PubMed] [Google Scholar]
- 49. Jorgensen W. L., Chandrasekhar J., Madura J. D., Impey R. W., Klein M. L. (1983) Comparison of simple potential functions for simulating liquid water. J. Chem. Phys. 79, 926–935 [Google Scholar]
- 50. Essmann U., Perera L., Berkowitz M. L., Darden T., Lee H., Pedersen L. G. (1995) A smooth particle mesh Ewald method. J. Chem. Phys. 103, 8577–8593 [Google Scholar]
- 51. Frauenfeld J., Gumbart J., Sluis E. O., Funes S., Gartmann M., Beatrix B., Mielke T., Berninghausen O., Becker T., Schulten K., Beckmann R. (2011) Cryo-EM structure of the ribosome-SecYE complex in the membrane environment. Nat. Struct. Mol. Biol. 18, 614–621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Oancea G., O'Mara M. L., Bennett W. F., Tieleman D. P., Abele R., Tampé R. (2009) Structural arrangement of the transmission interface in the antigen ABC transport complex TAP. Proc. Natl. Acad. Sci. U.S.A. 106, 5551–5556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Bao H., Duong F. (2012) Discovery of an auto-regulation mechanism for the maltose ABC transporter MalFGK2. PLoS One 7, e34836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Karasawa A., Swier L. J., Stuart M. C., Brouwers J., Helms B., Poolman B. (2013) Physicochemical factors controlling the activity and energy coupling of an ionic strength-gated ATP-binding cassette (ABC) transporter. J. Biol. Chem. 288, 29862–29871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Smith E. L., Pickels E. G. (1940) Micelle formation in aqueous solutions of digitonin. Proc. Natl. Acad. Sci. U.S.A. 26, 272–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kelleher D. J., Gilmore R. (1994) The Saccharomyces cerevisiae oligosaccharyltransferase is a protein complex composed of Wbp1p, Swp1p, and four additional polypeptides. J. Biol. Chem. 269, 12908–12917 [PubMed] [Google Scholar]