Background: mRNA transcription and decay are coordinated processes.
Results: The Rpb4/7 module of RNA polymerase II is required for the transcription and mRNA decay factor Ccr4-Not to associate with elongation complexes.
Conclusion: Association between these two entities is required for Ccr4-Not to promote transcription elongation.
Significance: Our work provides molecular insights into how transcription and mRNA decay are linked.
Keywords: mRNA Decay, RNA Polymerase II, RNA Synthesis, Transcription Elongation Factor, Transcription Regulation, Ccr4-Not
Abstract
Gene expression relies on the balance between mRNA synthesis in the nucleus and decay in the cytoplasm, processes once thought to be separate. We now know that transcription and decay rates are coordinated, but the factors or molecular mechanisms are unclear. The Ccr4-Not complex regulates multiple stages of gene expression, from mRNA synthesis to protein destruction. One of its functions is to promote RNA polymerase II elongation by reactivating arrested elongation complexes. Here we explored the features of polymerase required for Ccr4-Not to promote elongation and found that the Rpb4/7 module is important for Ccr4-Not to associate with elongation complexes and stimulate elongation. Rpb4/7 has also been implicated in coordinating mRNA synthesis and decay, but its role in this process is controversial. The interplay between Ccr4-Not and Rpb4/7 described here suggests a mechanism for how the cell coordinates mRNA synthesis and decay.
Introduction
Achieving proper gene expression is a complex process involving the control of a gene product from mRNA synthesis to protein destruction. Although the pathways used to regulate the expression of a gene are believed to be highly coordinated, some steps are separated by the formidable barrier of the nuclear envelope (e.g. transcription and translation). Furthermore, some pathways once thought to be antagonistic and distinct from each other, such as transcription and cytoplasmic mRNA decay, are now known to undergo crosstalk (1, 2). However, the mechanism of how this occurs is unknown and is under intense investigation.
Recent advances in genomic analysis has led to the concept of gene expression “buffering,” or the idea that mRNA synthesis and destruction rates are coordinated (3, 4). Counterintuitively, an increase in synthesis rate leads to a corresponding increase in decay rates during stress or when transcription or decay is perturbed genetically. This buffering response may fine-tune gene expression and prepare the cell to adapt to changing environments. Buffering occurs during stress responses as well as during the course of yeast species evolution (2, 5). The molecular players in gene buffering are largely unknown, but several studies have implicated candidates involved in transcription and decay (4, 6–8).
mRNA decay is initiated by the removal of the poly(A) tail by cytoplasmic deadenylases. This process is carried out by Pan2/3 and Ccr4 in yeast, with Ccr4 appearing to play the major role (9–11). Ccr4 resides in a multisubunit complex called Ccr4-Not, which was first described as a transcriptional activator/repressor (9, 12). In addition to regulating the TATA-binding protein at the initiation stage, it directly associates with elongating RNA polymerase II (RNAPII)2 and can reactivate arrested polymerase in vitro (13, 14). Ccr4-Not has pivotal roles in both mRNA production and decay. Another factor participating in transcription that has been implicated in decay is the Rpb4/7 module of RNAPII. Deleting Rpb4 or using mutants in the core RNA polymerase that weaken the interaction with Rpb4/7 caused reduced decay of mRNAs. Interestingly, deleting RPB4 or mutating RPB7 affected the deadenylation step in decay (6, 15). However, a recent study suggested that Rpb4 primarily promotes transcription in the nucleus (16); thus, it is controversial whether Rbp4 plays a direct role in decay. Curiously, Ccr4-Not mutants display many phenotypes observed in the RPB4-deleted strain including elongation defects, stress sensitivity, impaired transcription-coupled repair, and reduced turnover of mRNAs (9, 17). Although significant strides have been made in understanding how Ccr4-Not and Rpb4/7 individually contribute to regulating gene expression, a functional connection between Ccr4-Not and the Rpb4/7 remained elusive.
Here, we used a highly purified in vitro reconstitution system to study the functional and physical interaction between Ccr4-Not and the Rpb4/7 module of RNA polymerase II. We demonstrate that Rpb4/7 is required for Ccr4-Not to bind elongation complexes and rescue arrested RNAPII. These results provide, for the first time, a molecular basis for the relationship between these entities and suggest a possible mechanism for how transcription and mRNA decay are coordinated.
EXPERIMENTAL PROCEDURES
Strains and Protein Purification
Yeast strains are listed in Table 1. Ccr4-Not complex was purified from yeast from a Caf40- or Not4-TAP strain containing a deletion of DST1 by tandem affinity purification (TAP) as described in a previous publication (13). RNAPII and its mutant derivatives were purified through Rpb4-TAP or Rpb8-TAP (for 4/7Δ RNAPII) (18). The amount of RNAPII was estimated by Bradford assay using bovine serum albumin as a standard. To produce recombinant Rpb4/Rpb7, RPB4 and RPB7 were cloned into and co-expressed from a polycistronic vector (19), producing N-terminal hexahistidine-tagged RPB4 and C-terminal Strep-tag II-tagged RPB7. Details are available upon request. The dimer was purified by sequential affinity chromatography on Ni-NTA- and Strep-Tactin-agarose.
TABLE 1.
Strains used in this study
| Strain | Genotype | Source or Reference |
|---|---|---|
| CB010ΔRPB4 | MATa RPB9::TAP::TRP1, pep4::HIS3prb1::LEU2, prc1::HIS3, rpb4::URA3, ade2-11, his3-11,15, leu2-3,112, ura3-1, trp1-1, can1-100 | Ref. 26 |
| JR1414 | MATa RPB4::TAP::TRP1, rpb9Δ::KanMx, pep4::HIS3prb1::LEU2, prc1::HIS3, ade2-11, his3-11,15, leu2-3,112, ura3-1, trp1-1, can1-100 | This study |
| JR1408 | MATa, NOT4-TAP::HIS3, dst1Δ::URA3, his3Δ1, leu2Δ0, met15Δ0, ura3Δ0 | Ref. 13 |
| JR1519 | MATa, NOT4-TAP::HIS3, dst1Δ::URA3, CAF1-13MYC::KanMx, his3Δ1, leu2Δ0, met15Δ0, ura3Δ0 | This study |
| JR1523 | MATa; CAF40-TAP::HIS3, dst1Δ::URA3, his3Δ1, leu2Δ0, met15Δ0, ura3Δ0 | This study |
Elongation Complex Formation and Run-off Assays
Radiolabeled elongation complexes were formed on 100 ng of 3′-tailed templates by incubating ∼0.250 pmol of RNAPII (estimated by protein content), [32P]UTP, ATP, CTP, and UpG dinucleotide as described in our previous study (13). Ccr4-Not (amounts indicated in the figure legends) was added for 5 min. To measure the binding of Ccr4-Not to elongation complexes (ECs), the mixture was separated on native gels. For run-off assays, after incubation with Ccr4-Not, GTP and cold UTP were added, and aliquots were taken at the time points described in the figure legends. RNA products were analyzed on urea-PAGE gels. The gels were dried, exposed to a PhosphorImager screen for 12–16 h, and scanned using the Typhoon system (Molecular Dynamics). The data are presented as the percentage of run-off (run-off product divided by the sum of the run-off and remaining EC).
Rpb4/7 Binding Assays
Approximately 5 μg of Rpb4/7 dimer immobilized on Strep-Tactin beads (IBA Life Sciences) or Ni-NTA-agarose, depending on the experiment, was incubated with 1 μg of Ccr4-Not complex for 1 h at 4C in binding buffer (20 mm Tris-Cl, pH 7.5, 100 mm NaCl, 10% glycerol, 0.01% Nonidet P-40). The unbound fraction was saved, the beads were washed with binding buffer, and bound proteins were eluted in SDS-PAGE loading buffer. Ccr4-Not binding was detected by Western blotting through an epitope tag incorporated into the C terminus of Caf1. A Cy-5 labeled secondary antibody (GE Healthcare Life Sciences) was used, and the images were recorded on a Typhoon system and quantified using ImageJ software. Binding was quantified relative to a defined amount of input.
RESULTS
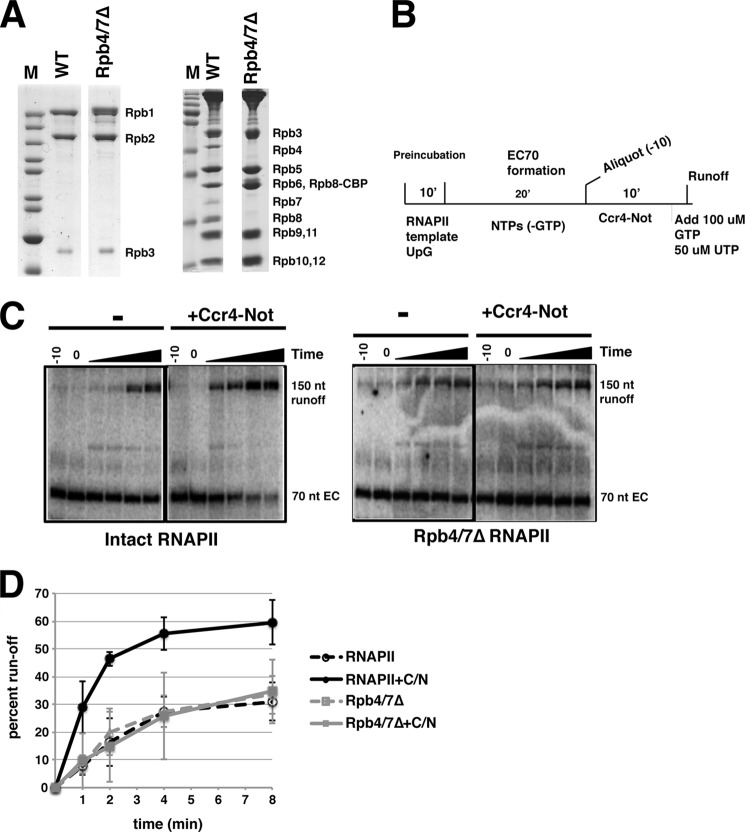
The Ccr4-Not complex binds to RNAPII elongation complexes in vitro, and its association with elongating RNAPII is stabilized by the nascent transcript (13), but the structural features of RNAPII required for this interaction are not known. The Rpb4/7 module and Rpb9 are not essential for viability and have been implicated in elongation (20); therefore, they are excellent candidates for playing a role in the interaction between RNAPII and Ccr4-Not. Although Ccr4-Not immunoprecipitated with RNAPII in crude extracts from an rpb4Δ mutant, the interaction with other elongation factors in the extract could have stabilized an otherwise weakened interaction between the complex and the mutant RNAPII (13). To definitively address the role of the Rpb4/7 module in mediating the interaction between Ccr4-Not and ECs, we purified RNAPII lacking the Rpb4/7 module (4/7Δ RNAPII) (Fig. 1A). In vitro transcription reactions were initiated on a previously characterized 3′-tailed template using UpG dinucleotide (13, 21). This template produces transcripts resistant to RNase H digestion, suggesting that this in vitro system is not prone to the artifact of R-loop formation (21). In addition, transcripts in the ECs are sensitive to RNase, and permanganate footprinting experiments indicate that the transcription bubble is closing behind RNAPII.3 Intact RNAPII and the Rpb4/7Δ form of RNAPII were used to transcribe across a G-less cassette, which arrested at four G bases forming ECs with a 70-nucleotide transcript. Ccr4-Not was incubated with the EC, and then GTP was added to initiate run-off transcription (Fig. 1B). The percentage of run-off transcription was plotted versus time. Consistent with our previous results (13), adding Ccr4-Not stimulated the production of run-off transcripts ∼2–3-fold (Fig. 1, C and D). Next, we examined the activity of the 4/7Δ RNAPII. The mutant RNAPII displayed a similar level of transcription run-off as the wild type polymerase in the absence of Ccr4-Not, which is consistent with previous studies suggesting that the 4/7 module is not required for elongation in vitro (22). Strikingly, the rescue of arrested Rpb4/7Δ RNAPII by Ccr4-Not was greatly reduced when compared with that observed for intact RNAPII (Fig. 1, C and D). As a control, we confirmed that the arrested 4/7-less polymerase could be stimulated by transcription elongation factor IIS (TFIIS), suggesting that the mutant polymerase responds to elongation factors that work through a different mechanism.4
FIGURE 1.
Rpb4/7 is required for Ccr4-Not to rescue arrested RNAPII. A, purification of intact (WT) and Rpb4/7-less RNA polymerase (Rpb4/7Δ). Gels were stained with Coomassie Blue. Rpb8 migrates differently in the last lane because of the calmodulin binding peptide (CBP) tag. M, molecular size markers. B, schematic diagram of elongation complex formation and transcription run-off assays. C, representative transcription run-off gels using intact (12-subunit) and Rpb4/7Δ RNAPII. Reactions were stopped at 1, 2, 4, and 8 min after the addition of nucleotides. Where indicated, 1.5 μg of Ccr4-Not was added to the reactions. D, averages of the quantification of three separate experiments. Error bars represent S.D. Intact RNAPII (black lines) or Rpb4/7Δ RNAPII (gray lines) reactions were incubated with Ccr4-Not (C/N), (solid lines), or an equivalent amount of bovine serum albumin carrier protein (dashed lines). The percentage of run off of ECs was plotted over time.
Rpb9 is required for proper elongation by RNAPII (20), and as such, it could be important for Ccr4-Not to stimulate elongation. RNAPII was purified from a strain containing a complete deletion of the RPB9 gene (Fig. 2A). Rpb9-less RNAPII (Rpb9Δ RNAPII) was used in transcription run-on assays, and the mutant polymerase produced run-off transcripts to the same extent as wild type polymerase (compare Fig. 1D with Fig. 2C). Importantly, Ccr4-Not was able to stimulate elongation from Rpb9Δ RNAPII similar to intact RNAPII (Fig. 2C). Thus, Rpb9 is not required for Ccr4-Not to stimulate elongation. These results suggest that the Rpb4/7 module is particularly important for Ccr4-Not to stimulate transcription.
FIGURE 2.
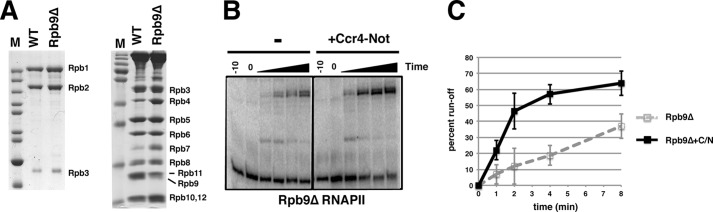
Rpb9 is not required for Ccr4-Not to stimulate elongation. A, RNAPII was isolated from wild type cells (WT) and from an rpb9Δ mutant (Rpb9Δ). Gels were stained by Coomassie Blue. Rpb9 and Rpb11 could not be resolved in the gels and co-migrate as a doublet. A reduction in the lower band is observed in preparations of Rpb9Δ polymerase, indicating an absence of Rpb9. M, molecular size markers. B, a representative run-off gel using Rpb9-less polymerase. Assays were performed as described for Fig. 1C. C, as described for Fig. 1D, except that Rpb9Δ polymerase was used. Transcription reactions with carrier protein (gray dashed lines) or Ccr4-Not (C/N), (black solid lines) is shown. Error bars represent S.D.
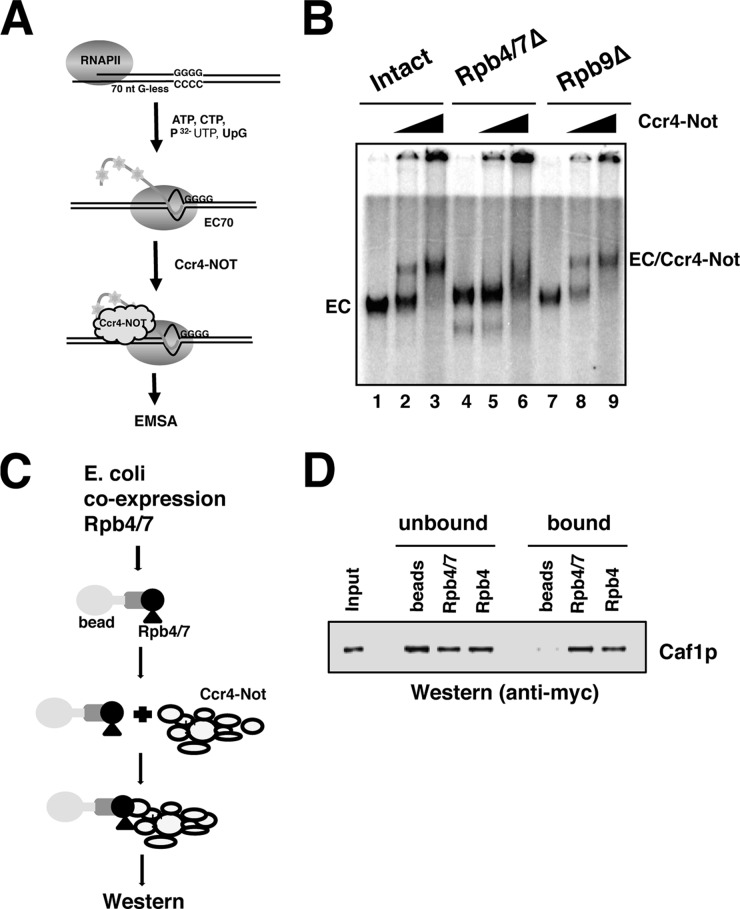
We examined why the loss of Rpb4/7 from RNAPII reduced the ability of Ccr4-Not to rescue arrested ECs. One explanation is that a physical interaction between the Rpb4/7 module and Ccr4-Not stabilizes the association of Ccr4-Not with the EC. To test this, we employed an EMSA to analyze the binding of Ccr4-Not to the mutant ECs. Both intact and mutant RNAPII were used to reconstitute ECs with radiolabeled UTP incorporated into the transcript, and Ccr4-Not was titrated into the binding assay (Fig. 3A). Titrating in Ccr4-Not into ECs formed with intact RNAPII produced a supershift, and essentially all of the EC was bound by Ccr4-Not to an RNAPII ratio of 3:1 (Fig. 3B, lane 3). We have previously shown that the binding of Ccr4-Not to ECs is not mediated exclusively by nucleic acid interactions, suggesting direct contact between RNAPII and Ccr4-Not (13). However, titrating the same amount of Ccr4-Not failed to produce a significant discreetly shifted species of EC formed with Rpb4/7Δ RNAPII. A smear was detected only at the highest concentration of Ccr4-Not (Fig. 3B, lane 6) that migrated in a position not correlating to an EC-Ccr4-Not complex. This result was observed in multiple gels using different preparations of Ccr4-Not. We speculate that a weak interaction between Ccr4-Not and the mutant RNAPII, which cannot withstand the gel conditions, delayed the entry of the EC into the gel. In contrast, Ccr4-Not was capable of supershifting ECs formed with Rpb9Δ RNAPII (Fig. 3B, lanes 8 and 9). Thus, the Rpb4/7 module is required for Ccr4-Not to associate stably with elongating RNAPII.
FIGURE 3.
Rpb4/7 is required for the stable association of Ccr4-Not with RNAPII elongation complexes. A, schematic of EC formation and binding assay. Approximately 250 fmol of intact or mutant RNAPII (Rpb4/7Δ and Rpb9Δ) was used to form ECs on 100 ng of template (EC70). ECs were incubated for 10 min with 0.5 and 1.5 μg of Ccr4-Not complex or carrier protein (RNAPII-only lanes). nt, nucleotides. B, products were separated on native polyacrylamide gels and visualized by the incorporation of radiolabeled UTP into the nascent transcript (see “Experimental Procedures”). C, schematic of the pulldown assay to measure the binding between recombinant Rpb4/7 and Ccr4-Not. Rpb4/7 were co-expressed using a bicistronic expression system, and Rpb4 was purified separately. Proteins (∼5 μg) were immobilized on Ni-NTA-agarose by a His6 tag incorporated into the Rpb4 subunit. Beads were incubated for 1 h with TAP-purified Ccr4-Not containing Myc-tagged Caf1. D, the fractions were analyzed by Western blotting using the 9E10 antibody. Naked beads were used as a control. 200 ng of purified Ccr4-Not is shown in the input lane. Bands were detected using a Cy-5-conjugated secondary antibody. Half of the total of each fraction was loaded onto the gel.
The most straightforward explanation for why Rpb4/7 is required for Ccr4-Not binding is that the two interact directly. Alternatively, a conformational change in the core of RNAPII caused by the loss of Rpb4/7 could weaken Ccr4-Not binding. Comparison of the 10-subunit crystal structure of core RNAPII and the complete 12-subunit version indicates that Rpb4/7 moves the clamp domain of RNAPII into a closed position similar to what is observed for the elongation complex (20). Demonstrating the latter mechanism would require a co-crystal structure of Ccr4-Not in an RNAPII EC, which has not been attainable through crystallography. For testing the binding of Ccr4-Not to Rpb4/7 at a biochemical level, Rpb4/7 was co-expressed in E. coli and purified by two-step affinity purification. Each subunit had a unique affinity tag; thus, the dimer was purified away from excess individual subunits (Fig. 3C and see “Experimental Procedures”). The recombinant dimer was used in pulldown assays with purified Ccr4-Not containing a Myc tag on the Caf1 subunit of the complex. The results indicate that Ccr4-Not directly binds to the Rpb4/7 dimer (Fig. 3D). We attempted to identify which subunit(s) of the 4/7 module mediates this interaction. Rpb7 could not be expressed in a soluble form alone (not shown); however, a hexahistidine-tagged version of Rpb4 could be purified, and this was used in the pulldown assay. Ccr4-Not bound to Rpb4, but not as well as it did to the 4/7 dimer (Fig. 3D). In this experiment, Rpb4/7 bound ∼41% of the input complex, whereas Rpb4 bound ∼16%. The binding of Ccr4-Not to Rpb4/7 was also observed when the dimer was immobilized through a Strep-tag in the Rpb7 subunit.4 Collectively, the data suggest that the Rpb4/7 module of RNAPII is important for Ccr4-Not to associate with ECs.
DISCUSSION
The coupling of the rates of mRNA transcription and decay is a newly identified response to changes in cell physiology. For example, as the transcription of a gene increases, so does its decay rate in a compensatory response called buffering (3, 10). The factors and pathways involved in this process are starting to be identified, but the mechanism of this phenomenon is not known. One of the first factors suggested to play a role in the coupling of transcription and decay was the Rpb4/7 module of RNA polymerase (7). Deleting RPB4 or mutating RPB7 reduces mRNA decay, including the deadenylation step in decay (1, 6). Ccr4 is the major mRNA deadenylase, and it was a candidate to be involved in this process. Furthermore, a comparative study of the synthesis and decay rates of mRNAs between two related yeast species, Saccharomyces cerevisiae and Saccharomyces paradoxus, identified Rpb4 and Ccr4-Not as candidates in the adaptation process (5). The relationship between Ccr4-Not and Rpb4/7 was not known. Here, we provide a molecular explanation for the role of Rpb4/7 in mRNA decay and potentially an important piece of the puzzle in defining how transcription and decay are coordinated to balance the level of gene expression.
A prevailing model to explain the coordination between transcription and decay invokes the “imprinting” of RNAs with factors involved in transcription in the nucleus, which can then impart the regulation of decay in the cytoplasm (1, 2). In particular, the imprinting of mRNAs with Rpb4/7 during the process of transcription was proposed (1). This model logically involves the shuttling of transcription and decay factors in and out of the nucleus. There is an opposing view questioning whether or not mRNAs are imprinted with Rpb4/7 and whether the shuttling of Rpb4/7 out of the nucleus is required for its control over decay. In this latest study, it was determined that covalently tethering Rpb4 to the core of RNAPII mostly rescued the synthesis and decay defects in the rpb4Δ mutant (16). The authors of that study argue that the disassociation of Rpb4/7 from RNAPII, and thus, nuclear cytoplasmic shuttling and imprinting, are not required for Rpb4 to function in decay. Our results here suggest an alternative mechanism that unifies both points of view and explains the relationship between Rpb4/7 and Ccr4-Not in transcription and decay. Rpb4/7 may serve as a scaffold to recruit mRNA transport and decay factors, such as Ccr4-Not, co-transcriptionally. An attractive model based on the results described here is that Rpb4/7 recruits Ccr4-Not to elongating RNA polymerase and the nascent transcript (this work and Ref. 13). Ccr4-Not travels with RNAPII during elongation by contacting 4/7 and the transcript, and could remain with mRNAs after termination, where it can impart regulation on those mRNAs in the cytoplasm. If such a model is correct, this could explain why Rpb4 is required for mRNA decay (6), but such regulation does not necessitate the disassociation of Rpb4/7 from RNAPII (16). It is also possible that Ccr4-Not and Rpb4/7 form a complex on mRNAs in the cytoplasm.
Although our data suggest that Rpb4/7 is an important point of contact between RNAPII and Ccr4-Not, clearly surfaces on the 10-subunit core of polymerase also participate in the interaction. Ccr4-Not still binds weakly to 4/7-less RNAPII, as evidenced by the delayed migration of RNAPIIΔ4/7 ECs into native gels (Fig. 3), and weak stimulation of elongation by Ccr4-Not (Fig. 1). The possibility that Ccr4-Not makes contacts with more than Rpb4/7 in ECs is not surprising considering the size of the complex, 1 MDa.
The association of Ccr4-Not with Rpb4/7 may be important for other transcription-associated functions. As mentioned above, Rpb4 plays multiple roles in the transcription process, including initiation, elongation, and 3′ end processing (9, 14, 17, 23). It is possible that Rpb4/7 recruits Ccr4-Not and other elongation factors to promote transcription. Likewise, both Ccr4-Not and Rpb4 have been implicated in transcription-coupled DNA repair (24, 25), and this physical interaction may play an important role here, too. These possibilities remain to be tested. One could think of Rpb4/7 as a second platform on RNA polymerase, the first being the well characterized C-terminal domain of Rpb1, for the assembly of regulatory factors onto mRNAs co-transcriptionally. Unfortunately, attempts to analyze the requirement for Rpb4/7 in the recruitment of Ccr4-Not to genes were not successful because deleting RPB4 reduced the expression of, and RNAPII loading at, genes known to be dependent on Ccr4-Not; thus, it was not possible to attribute reduced Ccr4-Not recruitment to the loss of Rpb4 specifically versus a total loss of RNAPII (not shown and Ref. 13). Nonetheless, our work described here provides molecular insights into why Ccr4-Not and Rpb4/7 are involved in many of the same processes and may suggest a mechanism for how these two factors regulate the coordination between transcription and decay.
Acknowledgments
We thank J. Brooks Crickard and Jason Miller for comments on this manuscript. Song Tan is acknowledged for providing the polycistronic expression vectors. The members of the Center for Eukaryotic Gene Regulation are recognized for their comments and feedback during the completion of this work.
This work was supported, in whole or in part, by National Institutes of Health Grants GM58672 (to J. C. R.) and GM100997 (to J. F.).
J. B. Crickard and J. C. Reese, unpublished data.
V. Babbarwal and J. C. Reese, unpublished data.
- RNAPII
- RNA polymerase II
- Ccr4-Not
- carbon catabolite repressor protein 4-negative on TATA complex
- EC
- elongation complexes
- TAP
- tandem affinity purification
- Ni-NTA
- nickel-nitrilotriacetic acid.
REFERENCES
- 1. Haimovich G., Choder M., Singer R. H., Trcek T. (2013) The fate of the messenger is pre-determined: a new model for regulation of gene expression. Biochim. Biophys. Acta 1829, 643–653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pérez-Ortín J. E., de Miguel-Jiménez L., Chávez S. (2012) Genome-wide studies of mRNA synthesis and degradation in eukaryotes. Biochim. Biophys. Acta 1819, 604–615 [DOI] [PubMed] [Google Scholar]
- 3. Shalem O., Dahan O., Levo M., Martinez M. R., Furman I., Segal E., Pilpel Y. (2008) Transient transcriptional responses to stress are generated by opposing effects of mRNA production and degradation. Mol. Syst. Biol. 4, 223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sun M., Schwalb B., Pirkl N., Maier K. C., Schenk A., Failmezger H., Tresch A., Cramer P. (2013) Global analysis of eukaryotic mRNA degradation reveals Xrn1-dependent buffering of transcript levels. Mol. Cell 52, 52–62 [DOI] [PubMed] [Google Scholar]
- 5. Dori-Bachash M., Shema E., Tirosh I. (2011) Coupled evolution of transcription and mRNA degradation. PLoS Biol. 9, e1001106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lotan R., Bar-On V. G., Harel-Sharvit L., Duek L., Melamed D., Choder M. (2005) The RNA polymerase II subunit Rpb4p mediates decay of a specific class of mRNAs. Genes Dev. 19, 3004–3016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shalem O., Groisman B., Choder M., Dahan O., Pilpel Y. (2011) Transcriptome kinetics is governed by a genome-wide coupling of mRNA production and degradation: a role for RNA Pol II. PLoS Genet. 7, e1002273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Haimovich G., Medina D. A., Causse S. Z., Garber M., Millán-Zambrano G., Barkai O., Chávez S., Pérez-Ortín J. E., Darzacq X., Choder M. (2013) Gene expression is circular: factors for mRNA degradation also foster mRNA synthesis. Cell 153, 1000–1011 [DOI] [PubMed] [Google Scholar]
- 9. Miller J. E., Reese J. C. (2012) Ccr4-Not complex: the control freak of eukaryotic cells. Crit. Rev. Biochem. Mol. Biol. 47, 315–333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Schwalb B., Schulz D., Sun M., Zacher B., Dümcke S., Martin D. E., Cramer P., Tresch A. (2012) Measurement of genome-wide RNA synthesis and decay rates with Dynamic Transcriptome Analysis (DTA). Bioinformatics 28, 884–885 [DOI] [PubMed] [Google Scholar]
- 11. Tucker M., Valencia-Sanchez M. A., Staples R. R., Chen J., Denis C. L., Parker R. (2001) The transcription factor associated Ccr4 and Caf1 proteins are components of the major cytoplasmic mRNA deadenylase in Saccharomyces cerevisiae. Cell 104, 377–386 [DOI] [PubMed] [Google Scholar]
- 12. Collart M. A., Panasenko O. O. (2012) The Ccr4-Not complex. Gene 492, 42–53 [DOI] [PubMed] [Google Scholar]
- 13. Kruk J. A., Dutta A., Fu J., Gilmour D. S., Reese J. C. (2011) The multifunctional Ccr4-Not complex directly promotes transcription elongation. Genes Dev. 25, 581–593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Reese J. C. (2013) The control of elongation by the yeast Ccr4-Not complex. Biochim. Biophys. Acta 1829, 127–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lotan R., Goler-Baron V., Duek L., Haimovich G., Choder M. (2007) The Rpb7p subunit of yeast RNA polymerase II plays roles in the two major cytoplasmic mRNA decay mechanisms. J. Cell Biol. 178, 1133–1143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Schulz D., Pirkl N., Lehmann E., Cramer P. (2014) Rpb4 subunit functions mainly in mRNA synthesis by RNA polymerase II. J. Biol. Chem. 289, 17446–17452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Choder M. (2004) Rpb4 and Rpb7: subunits of RNA polymerase II and beyond. Trends Biochem. Sci 29, 674–681 [DOI] [PubMed] [Google Scholar]
- 18. Suh M. H., Ye P., Zhang M., Hausmann S., Shuman S., Gnatt A. L., Fu J. (2005) Fcp1 directly recognizes the C-terminal domain (CTD) and interacts with a site on RNA polymerase II distinct from the CTD. Proc. Natl. Acad. Sci. U.S.A. 102, 17314–17319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Selleck W., Tan S. (2008) Recombinant protein complex expression in E. coli. Curr. Protoc. Protein Sci., Chapter 5, Unit 5.21, 10.1002/0471140864.ps0521s52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cramer P., Armache K. J., Baumli S., Benkert S., Brueckner F., Buchen C., Damsma G. E., Dengl S., Geiger S. R., Jasiak A. J., Jawhari A., Jennebach S., Kamenski T., Kettenberger H., Kuhn C. D., Lehmann E., Leike K., Sydow J. F., Vannini A. (2008) Structure of eukaryotic RNA polymerases. Annu. Rev. Biophys. 37, 337–352 [DOI] [PubMed] [Google Scholar]
- 21. Zhang Z., Wu C. H., Gilmour D. S. (2004) Analysis of polymerase II elongation complexes by native gel electrophoresis: evidence for a novel carboxyl-terminal domain-mediated termination mechanism. J. Biol. Chem. 279, 23223–23228 [DOI] [PubMed] [Google Scholar]
- 22. Edwards A. M., Kane C. M., Young R. A., Kornberg R. D. (1991) Two dissociable subunits of yeast RNA polymerase II stimulate the initiation of transcription at a promoter in vitro. J. Biol. Chem. 266, 71–75 [PubMed] [Google Scholar]
- 23. Runner V. M., Podolny V., Buratowski S. (2008) The Rpb4 subunit of RNA polymerase II contributes to cotranscriptional recruitment of 3′ processing factors. Mol. Cell. Biol. 28, 1883–1891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gaillard H., Tous C., Botet J., González-Aguilera C., Quintero M. J., Viladevall L., García-Rubio M. L., Rodríguez-Gil A., Marín A., Ariño J., Revuelta J. L., Chávez S., Aguilera A. (2009) Genome-wide analysis of factors affecting transcription elongation and DNA repair: a new role for PAF and Ccr4-Not in transcription-coupled repair. PLoS Genet. 5, e1000364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Li S., Smerdon M. J. (2002) Rpb4 and Rpb9 mediate subpathways of transcription-coupled DNA repair in Saccharomyces cerevisiae. EMBO J. 21, 5921–5929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jensen G. J., Meredith G., Bushnell D. A., Kornberg R. D. (1998) Structure of wild-type yeast RNA polymerase II and location of Rpb4 and Rpb7. EMBO J. 17, 2353–2358 [DOI] [PMC free article] [PubMed] [Google Scholar]