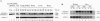Abstract
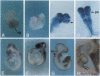
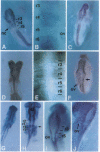
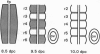
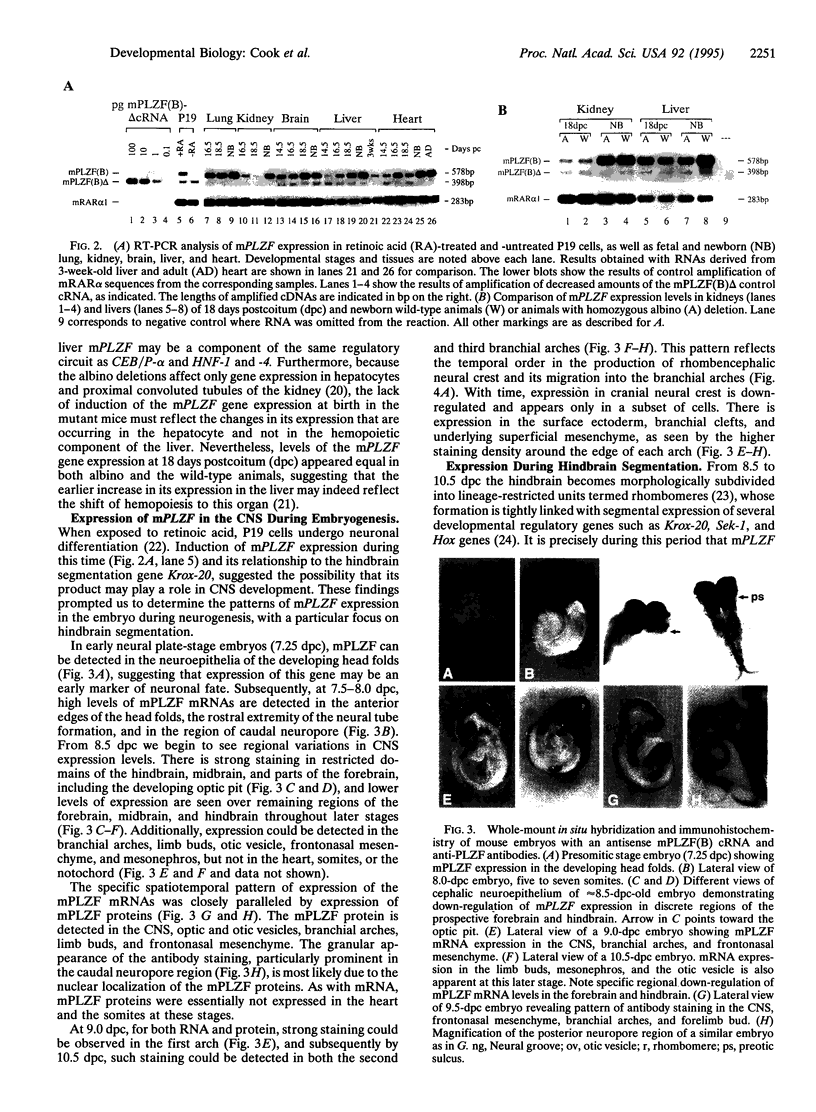
To investigate the potential biological role(s) of the PLZF gene, discovered as a fusion with the RARA locus in a patient with acute promyelocytic leukemia harboring a t(11;17) chromosomal translocation, we have isolated its murine homologue (mPLZF) and studied its patterns of developmental expression. The levels of mPLZF mRNAs increased perinatally in the liver, heart, and kidney, but with the exception of the heart, they were either absent or very low in the adult tissues. In situ analysis of mPLZF expression in mouse embryos between 7.0 and 10.5 days of development revealed that mPLZF mRNAs and proteins were coexpressed in spatially restricted and temporally dynamic patterns in the central nervous system. In the hindbrain region, a segmental pattern of expression correlated with the development of the rhombomeres. From 9.0 days of development, starting first in rhombomeres 3 and 5, there was an ordered down-regulation of expression in the center of each rhombomere, so that 1 day later elevated levels of mPLZF mRNAs and proteins were restricted to cells surrounding the rhombomeric boundaries. The chicken homologue of the PLZF gene, which we have also cloned, demonstrated a similar segmental pattern of expression in the hindbrain. To date, PLZF represents the only example of a transcription factor with elevated expression at rhombomeric boundaries. The high degree of evolutionary conservation between the patterns of PLZF expression during mammalian and avian central nervous system development suggests that it has an important functional role in the regionalization of the vertebrate hindbrain, potentially regulating boundary cell interactions.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bardwell V. J., Treisman R. The POZ domain: a conserved protein-protein interaction motif. Genes Dev. 1994 Jul 15;8(14):1664–1677. doi: 10.1101/gad.8.14.1664. [DOI] [PubMed] [Google Scholar]
- Benedyk M. J., Mullen J. R., DiNardo S. odd-paired: a zinc finger pair-rule protein required for the timely activation of engrailed and wingless in Drosophila embryos. Genes Dev. 1994 Jan;8(1):105–117. doi: 10.1101/gad.8.1.105. [DOI] [PubMed] [Google Scholar]
- Chen Z., Brand N. J., Chen A., Chen S. J., Tong J. H., Wang Z. Y., Waxman S., Zelent A. Fusion between a novel Krüppel-like zinc finger gene and the retinoic acid receptor-alpha locus due to a variant t(11;17) translocation associated with acute promyelocytic leukaemia. EMBO J. 1993 Mar;12(3):1161–1167. doi: 10.1002/j.1460-2075.1993.tb05757.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figdor M. C., Stern C. D. Segmental organization of embryonic diencephalon. Nature. 1993 Jun 17;363(6430):630–634. doi: 10.1038/363630a0. [DOI] [PubMed] [Google Scholar]
- Fraser S., Keynes R., Lumsden A. Segmentation in the chick embryo hindbrain is defined by cell lineage restrictions. Nature. 1990 Mar 29;344(6265):431–435. doi: 10.1038/344431a0. [DOI] [PubMed] [Google Scholar]
- Gluecksohn-Waelsch S., DeFranco D. Lethal chromosomal deletions in the mouse, a model system for the study of development and regulation of postnatal gene expression. Bioessays. 1991 Nov;13(11):557–561. doi: 10.1002/bies.950131102. [DOI] [PubMed] [Google Scholar]
- Jones-Villeneuve E. M., McBurney M. W., Rogers K. A., Kalnins V. I. Retinoic acid induces embryonal carcinoma cells to differentiate into neurons and glial cells. J Cell Biol. 1982 Aug;94(2):253–262. doi: 10.1083/jcb.94.2.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelsey G., Ruppert S., Beermann F., Grund C., Tanguay R. M., Schütz G. Rescue of mice homozygous for lethal albino deletions: implications for an animal model for the human liver disease tyrosinemia type 1. Genes Dev. 1993 Dec;7(12A):2285–2297. doi: 10.1101/gad.7.12a.2285. [DOI] [PubMed] [Google Scholar]
- Kozak M. Point mutations define a sequence flanking the AUG initiator codon that modulates translation by eukaryotic ribosomes. Cell. 1986 Jan 31;44(2):283–292. doi: 10.1016/0092-8674(86)90762-2. [DOI] [PubMed] [Google Scholar]
- Krumlauf R. Hox genes in vertebrate development. Cell. 1994 Jul 29;78(2):191–201. doi: 10.1016/0092-8674(94)90290-9. [DOI] [PubMed] [Google Scholar]
- Lumsden A., Keynes R. Segmental patterns of neuronal development in the chick hindbrain. Nature. 1989 Feb 2;337(6206):424–428. doi: 10.1038/337424a0. [DOI] [PubMed] [Google Scholar]
- Lumsden A. The cellular basis of segmentation in the developing hindbrain. Trends Neurosci. 1990 Aug;13(8):329–335. doi: 10.1016/0166-2236(90)90144-y. [DOI] [PubMed] [Google Scholar]
- Marshall H., Nonchev S., Sham M. H., Muchamore I., Lumsden A., Krumlauf R. Retinoic acid alters hindbrain Hox code and induces transformation of rhombomeres 2/3 into a 4/5 identity. Nature. 1992 Dec 24;360(6406):737–741. doi: 10.1038/360737a0. [DOI] [PubMed] [Google Scholar]
- Puelles L., Rubenstein J. L. Expression patterns of homeobox and other putative regulatory genes in the embryonic mouse forebrain suggest a neuromeric organization. Trends Neurosci. 1993 Nov;16(11):472–479. doi: 10.1016/0166-2236(93)90080-6. [DOI] [PubMed] [Google Scholar]
- Ruppert S., Kelsey G., Schedl A., Schmid E., Thies E., Schütz G. Deficiency of an enzyme of tyrosine metabolism underlies altered gene expression in newborn liver of lethal albino mice. Genes Dev. 1992 Aug;6(8):1430–1443. doi: 10.1101/gad.6.8.1430. [DOI] [PubMed] [Google Scholar]
- Schneider-Maunoury S., Topilko P., Seitandou T., Levi G., Cohen-Tannoudji M., Pournin S., Babinet C., Charnay P. Disruption of Krox-20 results in alteration of rhombomeres 3 and 5 in the developing hindbrain. Cell. 1993 Dec 17;75(6):1199–1214. doi: 10.1016/0092-8674(93)90329-o. [DOI] [PubMed] [Google Scholar]
- Sham M. H., Vesque C., Nonchev S., Marshall H., Frain M., Gupta R. D., Whiting J., Wilkinson D., Charnay P., Krumlauf R. The zinc finger gene Krox20 regulates HoxB2 (Hox2.8) during hindbrain segmentation. Cell. 1993 Jan 29;72(2):183–196. doi: 10.1016/0092-8674(93)90659-e. [DOI] [PubMed] [Google Scholar]
- Sham M. H., Vesque C., Nonchev S., Marshall H., Frain M., Gupta R. D., Whiting J., Wilkinson D., Charnay P., Krumlauf R. The zinc finger gene Krox20 regulates HoxB2 (Hox2.8) during hindbrain segmentation. Cell. 1993 Jan 29;72(2):183–196. doi: 10.1016/0092-8674(93)90659-e. [DOI] [PubMed] [Google Scholar]
- Sham M. H., Vesque C., Nonchev S., Marshall H., Frain M., Gupta R. D., Whiting J., Wilkinson D., Charnay P., Krumlauf R. The zinc finger gene Krox20 regulates HoxB2 (Hox2.8) during hindbrain segmentation. Cell. 1993 Jan 29;72(2):183–196. doi: 10.1016/0092-8674(93)90659-e. [DOI] [PubMed] [Google Scholar]
- Swiatek P. J., Gridley T. Perinatal lethality and defects in hindbrain development in mice homozygous for a targeted mutation of the zinc finger gene Krox20. Genes Dev. 1993 Nov;7(11):2071–2084. doi: 10.1101/gad.7.11.2071. [DOI] [PubMed] [Google Scholar]
- Topilko P., Schneider-Maunoury S., Levi G., Baron-Van Evercooren A., Chennoufi A. B., Seitanidou T., Babinet C., Charnay P. Krox-20 controls myelination in the peripheral nervous system. Nature. 1994 Oct 27;371(6500):796–799. doi: 10.1038/371796a0. [DOI] [PubMed] [Google Scholar]
- Topilko P., Schneider-Maunoury S., Levi G., Baron-Van Evercooren A., Chennoufi A. B., Seitanidou T., Babinet C., Charnay P. Krox-20 controls myelination in the peripheral nervous system. Nature. 1994 Oct 27;371(6500):796–799. doi: 10.1038/371796a0. [DOI] [PubMed] [Google Scholar]
- Topilko P., Schneider-Maunoury S., Levi G., Baron-Van Evercooren A., Chennoufi A. B., Seitanidou T., Babinet C., Charnay P. Krox-20 controls myelination in the peripheral nervous system. Nature. 1994 Oct 27;371(6500):796–799. doi: 10.1038/371796a0. [DOI] [PubMed] [Google Scholar]
- Tönjes R. R., Xanthopoulos K. G., Darnell J. E., Jr, Paul D. Transcriptional control in hepatocytes of normal and c14CoS albino deletion mice. EMBO J. 1992 Jan;11(1):127–133. doi: 10.1002/j.1460-2075.1992.tb05035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warrell R. P., Jr, de Thé H., Wang Z. Y., Degos L. Acute promyelocytic leukemia. N Engl J Med. 1993 Jul 15;329(3):177–189. doi: 10.1056/NEJM199307153290307. [DOI] [PubMed] [Google Scholar]
- Wilkinson D. G. Molecular mechanisms of segmental patterning in the vertebrate hindbrain and neural crest. Bioessays. 1993 Aug;15(8):499–505. doi: 10.1002/bies.950150802. [DOI] [PubMed] [Google Scholar]
- Wilkinson J. A. Etiologic factors in congenital displacement of the hip and myelodysplasia. Clin Orthop Relat Res. 1992 Aug;(281):75–83. [PubMed] [Google Scholar]
- Zelent A., Krust A., Petkovich M., Kastner P., Chambon P. Cloning of murine alpha and beta retinoic acid receptors and a novel receptor gamma predominantly expressed in skin. Nature. 1989 Jun 29;339(6227):714–717. doi: 10.1038/339714a0. [DOI] [PubMed] [Google Scholar]
- Zelent A. Translocation of the RAR alpha locus to the PML or PLZF gene in acute promyelocytic leukaemia. Br J Haematol. 1994 Mar;86(3):451–460. doi: 10.1111/j.1365-2141.1994.tb04773.x. [DOI] [PubMed] [Google Scholar]
- el-Baradi T., Pieler T. Zinc finger proteins: what we know and what we would like to know. Mech Dev. 1991 Nov;35(3):155–169. doi: 10.1016/0925-4773(91)90015-x. [DOI] [PubMed] [Google Scholar]