Background: Receptor endocytosis mediating LDL uptake needs ARH or Dab2, and NPC1L1 endocytosis for accomplishing intestinal cholesterol absorption involves Numb.
Results: Numb cannot mediate LDLR endocytosis. ARH and Dab2 cannot promote NPC1L1 internalization.
Conclusion: ARH, Dab2, and Numb play distinct roles in LDL and free cholesterol uptakes.
Significance: Defining roles of the adaptors further elucidates regulation of cholesterol metabolism.
Keywords: Cholesterol, Endocytosis, Lipid Absorption, Lipoprotein Receptor, Low-density Lipoprotein (LDL), ARH, Dab2, NPC1L1, Numb
Abstract
The uptake of circulating low density lipoproteins (LDL) is mediated by LDL receptor (LDLR) through clathrin-dependent endocytosis. At the early stage of this process, adaptor proteins ARH and Dab2 specifically bind the endocytic signal motif in LDLR and recruit clathrin/AP2 to initiate internalization. On the other hand, intestinal cholesterol is absorbed by Niemann-Pick C1-Like 1 (NPC1L1) through clathrin-dependent endocytosis. Another adaptor protein, Numb recognizes the endocytic motif in NPC1L1 C terminus and couples NPC1L1 to endocytic machinery. The ARH, Dab2, and Numb proteins contain a homogeneous phosphotyrosine binding (PTB) domain that directly binds endocytic motifs. Because ARH, Dab2, and Numb are all PTB domain family members, the emerging mystery is whether these adaptors act complementally in LDLR and NPC1L1 endocytosis. Here, we found that ARH and Dab2 did not bind NPC1L1 and were not required for NPC1L1 internalization. Similarly, Numb lacked the ability to interact with the LDLR C terminus and was dispensable for LDL uptake. Only the Numb isoforms with shorter PTB domain could facilitate NPC1L1 endocytosis. Besides the reported function in intestinal cholesterol absorption, Numb also mediated cholesterol reabsorption from bile in liver. We further identified a Numb variant with G595D substitution in humans of low blood LDL-cholesterol. The G595D substitution impaired NPC1L1 internalization and cholesterol reabsorption, due to attenuating affinity of Numb to clathrin/AP2. These results demonstrate that Numb specifically regulates NPC1L1-mediated cholesterol absorption both in human intestine and liver, distinct from ARH and Dab2, which selectively participate in LDLR-mediated LDL uptake.
Introduction
Cholesterol is a critical component of most biological membranes in higher eukaryotes and serves as a precursor for synthesis of bile acids and steroid hormones. Besides de novo biosynthesis, mammalian cells also acquire cholesterol from two sources, lipoprotein particles in circulation and free cholesterol in diet and bile. Both the lipoprotein and free cholesterol uptakes are modulated in accord with cellular requirement for cholesterol to maintain homeostasis (1–3).
As the major cholesterol transport vehicles in plasma, low density lipoprotein (LDL) particles carry cholesterol through the body for use by various cells. LDL receptor (LDLR), a single transmembrane glycoprotein ubiquitously expressed on the cell surface, mediates LDL entry into cells via clathrin-dependent vesicular endocytosis (4, 5). The cytoplasmic tail of LDLR contains a single NPX(Y/F) (where χ stands for any amino acid) motif (6, 7) that is required for endocytosis of the receptor. Following the attachment of LDL to the extracellular domain of LDLR, the adaptor proteins autosomal recessive hypercholesterolemia (ARH) and Disabled homolog 2 (Dab2) directly bind the NPX(Y/F) motif and further recruit clathrin and AP2 to initiate the endocytosis (8–10). Involvement of ARH and Dab2 in LDLR endocytosis exhibits in a tissue-specific manner. ARH is required for LDLR function in liver, and its mutations markedly reduce hepatic uptake of LDL, resulting in elevated circulating LDL-cholesterol levels (8, 11). However, deficiency of ARH in fibroblasts has little or no effect on LDLR function (12). Instead, Dab2 was identified to play a major role in LDLR internalization in fibroblasts independently of ARH (10).
In human intestine and liver, Niemann-Pick C1-Like 1 (NPC1L1),4 a multitransmembrane protein, facilitates dietary and biliary cholesterol absorption (13). Previous studies have shown that, similar to LDLR, NPC1L1 mediates cholesterol uptake through clathrin-dependent endocytosis (3, 14). Recently, we identified an endocytic signal sequence, YVNXXF (1306–1311 aa), in the cytoplasmic C-terminal part of NPC1L1, which is critical for NPC1L1 internalization (15). Deletion or mutations of this motif markedly compromise NPC1L1 endocytosis. Remarkably, another adaptor protein, Numb, was identified to function in NPC1L1 endocytosis. Numb directly binds the signal (YVNXXF) motif and recruits clathrin/AP2 to drive the internalization of NPC1L1 with cholesterol. Intestine-specific Numb knock-out mice exhibit significant lower cholesterol absorption than wild-type.
ARH, Dab2, and Numb, performing important functions in cholesterol uptake, are members of a class of clathrin-associated sorting proteins (CLASPs). Interestingly, the three CLASPs possess similar architecture featuring an N-terminal phosphotyrosine-binding (PTB) domain and a C-terminal clathrin-interaction segment (16). It has been known that the PTB domain usually binds to substrates that do not have a phosphorylated tyrosine, allowing the CLASPs to recognize proteins containing endocytic signals (17). Furthermore, the YVNXXF signal motif of NPC1L1 resembles the (F/Y)XNPX(F/Y) signal sequence in LDLR (6). Notably, previous studies have shown that different adaptors may be complementary in the endocytosis of some proteins, such as ARH and Dab2 in LDLR endocytosis (9, 10) and Dab2 and Numb in integrin endocytosis (18, 19). Given the described comparability of ARH, Dab2, and Numb, the emerging questions are whether ARH and Dab2 can bind NPC1L1 to modulate cholesterol absorption, and whether Numb can be used to promote LDLR internalization. In addition, there are four Numb isoforms in mammals due to alternative splicing (20). Although isoforms of Numb are functional in NPC1L1, endocytosis is still unknown. Despite requirement of Numb for intestinal cholesterol uptake (15), it remains to be determined whether Numb acts similarly in biliary cholesterol reabsorption accomplished by NPC1L1 in human liver.
In the current study, we investigated the functions of ARH, Dab2, and Numb in receptor-mediated LDL uptake versus NPC1L1-dependent cholesterol absorption, and found that these adaptors play different roles. Our results demonstrate that Numb regulates cholesterol absorption in an isoform-specific manner and also extend Numb function to human liver. Establishment of the association between a Numb polymorphism impairing NPC1L1 internalization and human low LDL-cholesterol validates the role of Numb in NPC1L1-mediated cholesterol absorption.
EXPERIMENTAL PROCEDURES
Materials
We obtained filipin and lovastatin from Sigma; methyl-β-cyclodextrin (MCD) from Cyclodextrin Technologies Development Inc., cholesterol from Steraloids Inc., and Dil stain was from Invitrogen. Lipoprotein-deficient serum (d > 1.215 g/ml) was prepared from newborn calf serum by ultracentrifugation as reported previously (21). Primary antibodies used for immunoblots were as follows: mouse monoclonal anti-actin (Sigma), rabbit monoclonal anti-Numb (Cell Signaling), rabbit polyclonal to ARH (Abcam), rabbit polyclonal anti-Dab2 (Santa Cruz), mouse monoclonal anti-His (Santa Cruz), and mouse monoclonal anti-adaptin (BD Transduction Laboratories). Mouse monoclonal anti-Myc IgG (clone 9E10) was prepared from hybridomas (ATCC); polyclonal antibodies against GST, EGFP, and mouse NPC1L1 were produced by immunizing rabbits in our laboratory. Alexa Fluor 488 donkey anti-rabbit IgG and Alexa Fluor 555 donkey anti-mouse IgG were purchased from Invitrogen; horseradish peroxidase-conjugated donkey anti-mouse and anti-rabbit IgG were from Jackson ImmunoResearch Laboratories.
Construction of Plasmids
Coding regions of human Numb and Dab2 were PCR amplified from Huh7 cells cDNA and cloned into pcDNA3.0 vector containing a Myc epitope tag. DNA fragment encoding full-length LDLR protein was amplified from mouse liver cDNA and cloned into plasmid pEGFP-N1 with an EGFP at C terminus. The plasmid of ARH was a gift from Paul A. Welling and was also subcloned into pcDNA3.0 vector containing a Myc epitope tag (22). Fragments encoding ARH, Numb, Dab2-PTB (1–220 aa), and Numb-PTB (1–176 aa) were subcloned in-frame to a His6 tag in the pRSET vector. NPC1L1-C (1266–1332 aa), LRP-C (4445–4544 aa), and LDLR-C (813–862 aa) were cloned into pGEX-4T-3 vector.
Cell Culture and Transfection
CRL1601 (a McArdle RH7777 rat hepatoma cell line) cells were grown in monolayer at 37 °C in 5% CO2. The cells were maintained in medium A (Dulbecco's modified Eagle's medium containing 100 units/ml of penicillin and 100 mg/ml of streptomycin sulfate) supplemented with 10% FBS. Low cholesterol medium was medium A supplemented with 5% lipoprotein-deficient serum, 1 μm lovastatin, 50 μm mevalonate, and 1.5% MCD. Cholesterol-replenishing medium contained medium A supplemented with 5% lipoprotein-deficient serum, 1 μm lovastatin, 50 μm mevalonate, and 15 μg/ml of cholesterol-MCD. Cholesterol-MCD inclusion complexes were prepared as described previously (23). CRL1601 cells stably expressing NPC1L1-EGFP were generated as previous description (3).
In Vitro Pull-down Assay
In vitro pull-down was performed as previously described with minor modifications. GST C-terminal recombinant proteins and His-PTB adaptor proteins were expressed in Escherichia coli (BL21; Invitrogen) and purified under nondenaturing conditions, using glutathione-SepharoseTM 4 Fast Flow (GE Healthcare) or Ni-NTA-agarose (Qiagen), as recommended by the manufacturer. Aliquots of Ni-NTA beads containing 50 μm of the recombinant His-tagged adaptor proteins or PTB domains were mixed with 20 μm of each GST fusion protein in 300 μl of binding buffer (20 mm HEPES, 20 mm imidazole, 120 mm potassium acetate, 10% glycerol, and 0.1% Triton X-100, pH 7.5) and incubated for 60 min on a rotating platform at 4 °C. Then the beads were collected by centrifugation (2,000 × g for 5 min). Supernatants were removed by aspiration, and beads were washed 5 times with binding buffer. The proteins bound to beads were eluted in loading buffer, resolved by SDS-PAGE, and then immunoblot analysis with anti-His and anti-GST antibodies.
siRNA Knockdown
Duplexes of siRNA were synthesized by Genepharma (Shanghai, China). The sequence of siRNA targeting rat CHC was 5′-CAUCUCACUUGCUCAACGU-3′, ARH was 5′-CCUGGUUUAUGCUCGGAAA-3′, Dab2 was 5′-CCUGUUGUCUUCACUCCUU-3′, and Numb was 5′-GCUGUCCCUACGCAUCAAU-3′. Control siRNA was 5′-UUCUCCGAACGUGUCACGUUU-3′. CRL1601 cells were plated in 12-well plates (day 0). 24 h later, the cells were separately transfected with siRNA oligonucleotides (50 nmol) using Oligofectamine (Invitrogen). Transfection was performed twice on days 1 and 3. On day 4, the cells were used for assays.
Immunostaining and Fluorescence Quantification
To visualize intracellular-localized protein, cells on glass coverslips were fixed by 4% paraformaldehyde/PBS for 20 min at room temperature and washed with PBS. Next, the cells were permeabilized in PBS containing 0.1% Triton X-100 for 5 min and blocked with 1% BSA for 1 h at room temperature. The cells were then incubated in blocking buffer containing primary antibodies for 1 h, followed by three times washing with PBS and additional incubation with fluorescent secondary antibodies for 1 h at room temperature. For quantification of intracellular NPC1L1 abundance in NPC1L1-EGFP stable cell line, the method was applied as previously described (3).
Adenovirus-mediated NPC1L1 Overexpression in Mice Livers
Human NPC1L1 cDNA (or Numb cDNA) with CMV promoter was subcloned to pShuttle vector followed by recombination with pAdEasy vector. After pAdEasy plasmid was transfected into HEK293 cells, adenoviruses were packaged and amplified and then purified with CsCl ultracentrifugation (24). The viruses were titered and administrated via caudal vein injection (2 × 108 pfu viruses per mouse). 4 days after injection, mice were sacrificed and the bile, liver, and plasma were collected for analyses.
Blood and Liver Chemistry
Serum total cholesterol, biliary phospholipid, and biliary total cholesterol levels were determined according to the manufacturer's instructions (Wako, Japan).
Immunoblot Analysis
For whole cell lysate, the treated cells were harvested and suspended with 120 μl of RIPA buffer supplemented with protease inhibitors and then passed 10 times through a number 7 needle. Protein concentration of the extracts was determined according to the Lowry method (Bio-Rad), and then the extracts were mixed with SDS loading buffer. After boiling for 10 min, the extracts were subjected to SDS-PAGE, transferred to nitrocellulose filters (Whatman), and subjected to immunoblot analysis.
RNA Extraction and Semi-quantitative RT-PCR
Total RNAs were isolated using TRIzol reagent (Sigma) and then reverse transcribed with Moloney murine leukemia virus reverse transcriptase (Promega). The cDNAs were amplified by semi-quantitative RT-PCR. The primers used were as follows: mouse forward, 5′-GAGTCAAGAGGAATGCACATC-3′; mouse reverse, 5′-CACAACTCTGAGCCCATCC-3′; human forward, 5′-GATGAATCAAGAGGAATGCAC-3′; and human reverse, 5′-CACAACTCTGAGTCCATCT-3′.
LDL Internalization Assay
Human LDL was isolated from serum by ultracentrifugation according to the method as described (25). Fluorescent 1,1′-dioactadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (Invitrogen)-labeled LDL (Dil-LDL) was prepared as described by Pitas et al. (26). The siRNA-treated cells, grown on glass coverslips, were placed at 4 °C for 30 min and then incubated in cold medium (DMEM, 5% lipoprotein-deficient serum) supplemented with 10 μg/ml of Dil-LDL at 4 °C for 1 h. Next, the cells were rinsed twice with cold PBS prior to transferring cells to 37 °C medium without Dil-LDL to allow internalization for either 0 or 1 h. The cells were then fixed in 4% paraformaldehyde/PBS for 25 min.
Immunoprecipitation
After treatment, cells were harvested and suspended with 500 μl of 0.5% Nonidet P-40 (5 mm EDTA, 5 mm EGTA, 0.5% Nonidet P-40 in PBS buffer) containing protease inhibitors and then passed 10 times through a number 7 needle. Solubilized cell lysates were incubated with beads conjugated with Myc antibody at 4 °C for 2 h. Then the beads were washed 5 times with buffer. The proteins bound to beads were eluted in loading buffer at 95 °C for 10 min and then analyzed by immunoblot.
Statistical Analyses
The data are presented as mean ± S.D.; analyses were performed with unpaired two-tailed Student's t test or one-way analysis of variance as described.
RESULTS
NPC1L1 Selectively Interacts with Numb but Not ARH or Dab2
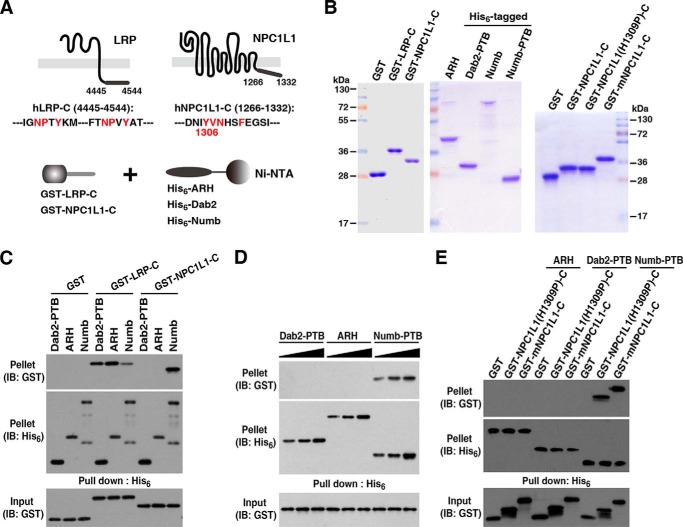
To investigate whether PTB-domain containing adaptors ARH and Dab2 are involved in NPC1L1-mediated cholesterol absorption, we first tested their interactions with the NPC1L1 C terminus (NPC1L1-C, 1266–1332 aa) carrying the endocytic signal motif YVNXXF by employing an in vitro pulldown assay (Fig. 1A). Glutathione S-transferase (GST)-fused NPC1L1-C and His6-tagged CLASPs including ARH, Dab2, and Numb were prepared from E. coli (Fig. 1B). The C-terminal tail of low density lipoprotein receptor-related protein (LRP) (GST-LRP-C, 4445–4544 aa) was used as a positive control because it contains two functional NPXY motifs and interacts with all these CLASPs (27). After incubation with each of the His6-tagged CLASPs previously immobilized on Ni-NTA-agarose beads and followed washing, specifically bound NPC1L1-C were eluted and analyzed by Western blotting. Compared with LRP-C, NPC1L1-C preferentially binds to Numb, not ARH or Dab2-PTB (Fig. 1C). Even with higher concentrations, ARH or Dab2 still could not interact with NPC1L1-C (Fig. 1D). These results demonstrated that NPC1L1-C selectively binds Numb in vitro.
FIGURE 1.
NPC1L1 C terminus binds Numb, but not ARH or Dab2. A, schematic illustration of the in vitro pulldown assay. Recombinant His6-tagged CLASPs (ARH, Dab2, and Numb) or/and their PTBs were bound to Ni-NTA beads and tested for interaction with GST fusion proteins of either the NPC1L1 or LRP C terminus (positive control). B, purified recombinant proteins from E. coli for the binding assay shown by SDS-PAGE and Coomassie Brilliant Blue staining. C, interactions between His6-tagged CLASPs and GST-fused NPC1L1-C or LRP-C analyzed by in vitro pulldown. The specifically bound proteins (pellet) were assessed by Western blots with anti-GST antibody compared with input proteins (Input). D, increasing concentrations (50, 100, and 150 μm) of CLASPs were used in a pulldown assay. E, binding of Numb to GST-fused NPC1L1(H1309P)-C and mNPC1L1-C. IB, immunoblot.
The YVNXXF endocytic motif identified in NPC1L1 is similar to the (F/Y)XNPX(F/Y) motif that was found in LDLR (6) and other membrane proteins including LRP, APP, Notch/Sanpodo, and Integrin. As previous reports showed that the core NPX(F/Y) motif of LDLR could be recognized by ARH and Dab2, we changed the YVNHSF sequence in NPC1L1 to YVNPSF according with the (F/Y)XNPX(F/Y) motif by replacement of His-1309 with proline (H1306P) and examined their interactions with ARH, Dab2, and Numb. Numb bound to both forms of NPC1L1-C, but this alteration did not enable ARH or Dab2 interaction (Fig. 1E). In addition, mouse NPC1L1 (mNPC1L1), containing the corresponding sequence YVNYGF, also bound Numb but not ARH or Dab2 (Fig. 1E). These results suggest that the YVNXXF motif in NPC1L1 is distinct from the classical (F/Y)XNPX(F/Y) sequon.
ARH and Dab2 Cannot Mediate NPC1L1 Internalization
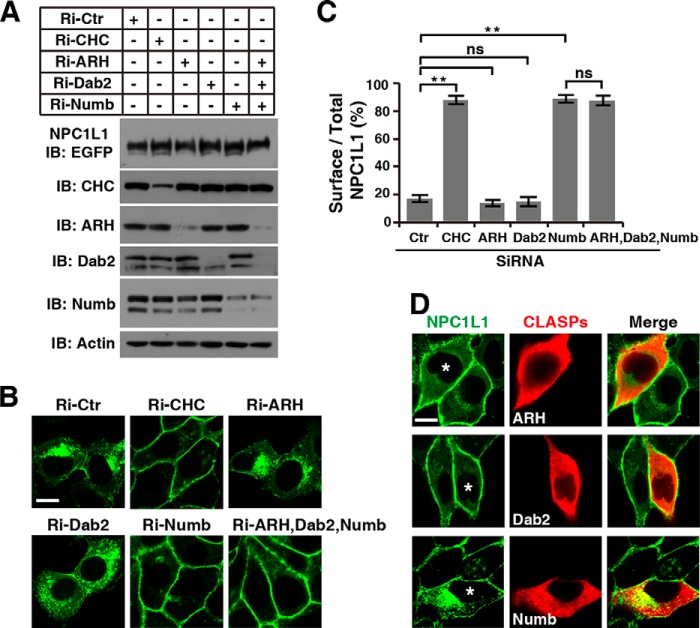
To directly determine the requirement of these CLASPs for cholesterol-induced NPC1L1 endocytosis, subcellular localization of NPC1L1 was analyzed in cell knockdown of individual ARH, Dab2, or Numb. CRL1601 cells stably expressing the NPC1L1-EGFP fusion protein (CRL1601/NPC1L1-EGFP) were transfected with various short interfering RNA (siRNA) targeting ARH, Dab2, or Numb. Clathrin heavy chain (CHC) has been shown to be required for NPC1L1 endocytosis (3) and its siRNA was used as a positive control here. The CHC, ARH, Dab2, and Numb proteins were substantially reduced by the corresponding siRNAs (Fig. 2A). As shown in Fig. 2B and quantified in C, NPC1L1 was completely internalized to the perinuclear compartment in cells transfected with non-relevant control siRNA. Similar to previously reported data (3, 15), knockdown of Numb or CHC led to predominant cell surface localization of NPC1L1 due to attenuated internalization. By contrast, the majority of NPC1L1 was internalized into the perinuclear region in ARH or Dab2 RNAi cells. To exclude the complementary effect or the effect of the low expression level of certain CLASPs in cells, we performed RNAi-rescue experiments. Plasmids encoding ARH, Dab2, or siRNA-resistant Numb were transfected into Numb knockdown cells (CRL1601 cells transfected with siRNA targeting Numb) separately (Fig. 2D). Exogenous Numb completely rescued the endocytosis of NPC1L1 (bottom panel). However, ARH and Dab2 failed to restore NPC1L1 internalization harmed by defect of Numb (top and middle panels). Together with the fact that ARH and Dab2 cannot bind NPC1L1-C, these data demonstrate that ARH and Dab2 are not required for NPC1L1 endocytosis.
FIGURE 2.
Numb, but not ARH or Dab2, is required for the NPC1L1 endocytosis. A, Western blotting to measure the RNAi efficiency in CRL1601/NPC1L1-EGFP cells. Ctr, control. B, the cellular localization of NPC1L1-EGFP. The cells receiving siRNA were incubated in low cholesterol medium to reduce cellular cholesterol for 60 min, and then refed with cholesterol-replenishing medium to deliver cholesterol for 60 min. Then the cells were fixed and examined by confocal microscopy. C, quantification of the plasma membrane-localized NPC1L1-EGFP shown in B. Data are shown as mean ± S.D., n = 20. ns, not significant; **, p < 0.01. D, internalization of NPC1L1 in Numb RNAi cells transfected with plasmids encoding Myc-tagged ARH, Dab2, or Numb (marked by asterisk). The sequence of Numb in plasmid was siRNA-resistant. The cells were treated as B and immunofluorescent staining was performed to label ARH, Dab2, or Numb. Scale bars, 10 μm. IB, immunoblot.
Numb Facilitates NPC1L1 Endocytosis in an Isoform-specific Manner
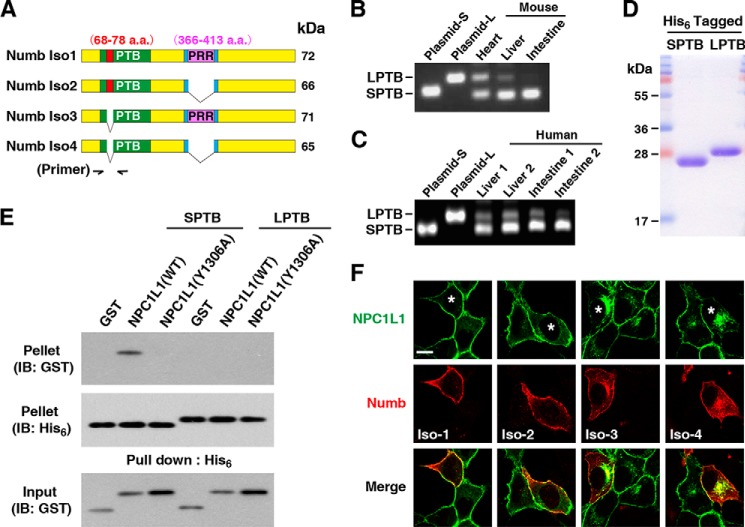
Mammalian Numb gene gives rise to four alternatively spliced transcripts (28) that produce four protein isoforms, ranging from 65 to 72 kDa. They are different in the length of their PTB (lacking or containing an 11-amino acid insert, 68–78 aa) and PRR (proline-rich region) (lacking or containing a 48-amino acid insert, 366–413 aa) domains (Fig. 3A). The expression pattern and functions of various isoforms are distinct (20). Which isoforms are responsible for cholesterol absorption is still unknown. Because the PTB domain that recognizes the YVNXXF endocytic motif of NPC1L1 determines binding specificity, we focused on distinguishing the functions of Numb isoforms containing different PTB domains.
FIGURE 3.
Numb isoforms with shorter PTB facilitate NPC1L1 internalization in mammals. A, schematic of Numb isoforms in mammals. Lengths of their PTB and PRR domains are various. B, the mRNA expressions of Numb isoforms with different PTB domains analyzed by semi-quantitative RT-PCR in mouse tissues. Primers used were indicated in A. SPTB, shorter PTB; LPTB, longer PTB. C, the mRNA expressions of Numb isoforms with different PTB domains in human liver and intestine (two samples of each tissue). D, purified His6-tagged shorter and longer Numb-PTB proteins from E. coli. E, in vitro pulldown assay of Numb-PTB with GST-NPC1L1-C (WT or Y1306A). After incubation of GST fusion proteins with Numb-PTBs, the pellets and inputs were assessed by Western blots with the indicated antibodies. F, internalization of NPC1L1 in Numb RNAi cells transfected with plasmids encoding Myc-tagged Numb isoforms (marked by asterisk). The sequences of Numb isoforms in plasmids were siRNA-resistant. The cells were treated as described in the legend Fig. 2B and immunofluorescent staining was performed to label exogenous Numb. Scale bars, 10 μm. IB, immunoblot.
The mRNA expressing patterns of Numb with different PTB domains in mouse (Fig. 3B) and human (Fig. 3C) tissues were analyzed by semi-quantitative RT-PCR using a pair of oligonucleotide primers that match flanking sequences of the 11-amino acid insertion in the PTB domain (Fig. 3A). The expected sizes of amplified products from Numb isoforms lacking the insertion in the PTB (SPTB) and isoforms with the insertion (LPTB) transcripts were 114 and 147 base pairs (bp), respectively. Intensity of the 114-bp band amplified from mouse heart mRNA is almost the same as that of the 147-bp band, revealing that Numb with LPTB and SPTB exist almost equally in mouse heart (Fig. 3B). Interestingly, Numb with shorter PTB was predominantly expressed in liver and intestine of both mouse and human (Fig. 3, B and C), the main organs for cholesterol absorption.
Next we used in vitro pulldown assay to test the NPC1L1-binding capacities of LPTB and SPTB. His6-tagged Numb-LPTB and Numb-SPTB were purified from E. coli (Fig. 3D). WT NPC1L1-C bound Numb-SPTB, whereas the Y1306A mutation completely ablated this association. In contrast, there was no interaction between NPC1L1-C and Numb-LPTB (Fig. 3E). To distinguish the functions of various Numb isoforms in NPC1L1 endocytosis, plasmids encoding four Numb isoforms were transfected into Numb knockdown cells, respectively. Numb with SPTB (isoforms 3 and 4) completely rescued NPC1L1 endocytosis (Fig. 3F). However, Numb with LPTB (isoforms 1 and 2) had no effect on NPC1L1 endocytosis, consistent with the above result that Numb-LPTB cannot bind to NPC1L1. Taken together, Numb regulates NPC1L1 endocytosis in an isoform-specific manner in which Numb isoforms 3 and 4 with shorter PTB domains, the dominant forms in liver and intestine, are involved in this process.
Numb Is Required for Cholesterol Reabsorption from Bile in Liver
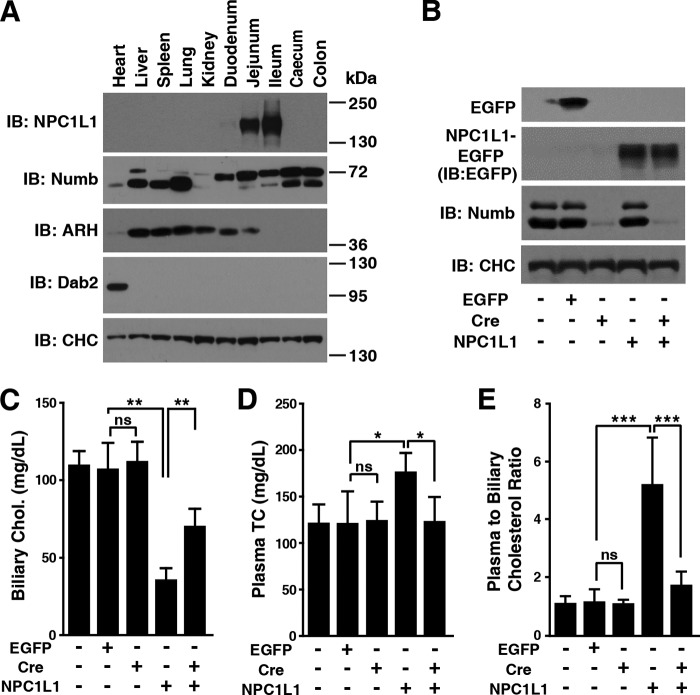
We have shown that Numb is required for intestinal cholesterol absorption (15). Does Numb also regulate cholesterol absorption from bile in liver? Notably, the expression of NPC1L1 in liver is species-specific. Unlike in humans that express a high level of NPC1L1 both in the intestine and liver, mouse only expresses NPC1L1 in intestine (29). Studies with transgenic mice have shown that hepatic NPC1L1 promotes cholesterol reabsorption from bile (30, 31).
As Numb is expressed in many tissues including liver (Fig. 4A), to explore the role of Numb in cholesterol reabsorption from bile in liver, we generated liver-specific Numb-deficient (L-Numb−/−) mice by adenovirus expressing Cre recombinase (Ad-Cre) injection to Numbflox/flox mice and used Ad-NPC1L1 expression in mouse liver to mimic human hepatic NPC1L1 (30, 31) (Fig. 4B). The biliary cholesterol (Fig. 4C) and plasma total cholesterol (Fig. 4D) were measured, and the plasma to biliary cholesterol ratio (Fig. 4E) was taken as an indicator of cholesterol reabsorption. Before expressing NPC1L1 in mouse liver, there was no significant difference of biliary cholesterol, plasma cholesterol, and the plasma to biliary cholesterol ratio between WT and L-Numb−/− mice (Fig. 4, C–E, compare second and third columns), indicating that Numb had no effect on cholesterol absorption without NPC1L1. Exogenous NPC1L1 expression in WT mice led to lower biliary, higher plasma cholesterol concentration and a dramatically elevated plasma to biliary cholesterol ratio due to the cholesterol reabsorption from bile to blood (Fig. 4, C–E, compare second and fourth columns). However, the reabsorption was dramatically impaired when liver Numb was depleted (Fig. 4, C–E, compare fourth and fifth columns). As a control, there was no significant difference in biliary phospholipid among these mice (data not shown). These data suggest that Numb also regulates NPC1L1-mediated biliary cholesterol reabsorption in human liver.
FIGURE 4.
Numb is required for NPC1L1-mediated cholesterol reabsorption from bile in mouse liver. A, tissue expression pattern of different CLASPs in mouse. The lysate of various tissue samples from C57/B6 mouse were subjected to SDS-PAGE and then immunoblot with indicated antibodies. B–E, 8-week-old Numbflox/flox mice fed on chow diet were injected with adenovirus expressing Cre recombinase (2 × 109 pfu/mouse) or PBS (−). After 2 days, the mice were administrated with adenovirus expressing NPC1L1-EGFP or EGFP (2 × 108 pfu/mouse). After an additional 4 days, the mice were fasted overnight, sacrificed, and subjected to various analyses. Livers were collected and subjected to immunoblot analysis (B). Biliary cholesterol (C), plasma total cholesterol (D), and plasma to biliary cholesterol ratio (E) were determined. Values are reported as mean ± S.D., n = 5. ns, not significant; *, p < 0.05; **, p < 0.01; ***, p < 0.001. IB, immunoblot.
Numb Does Not Participate in LDL Uptake
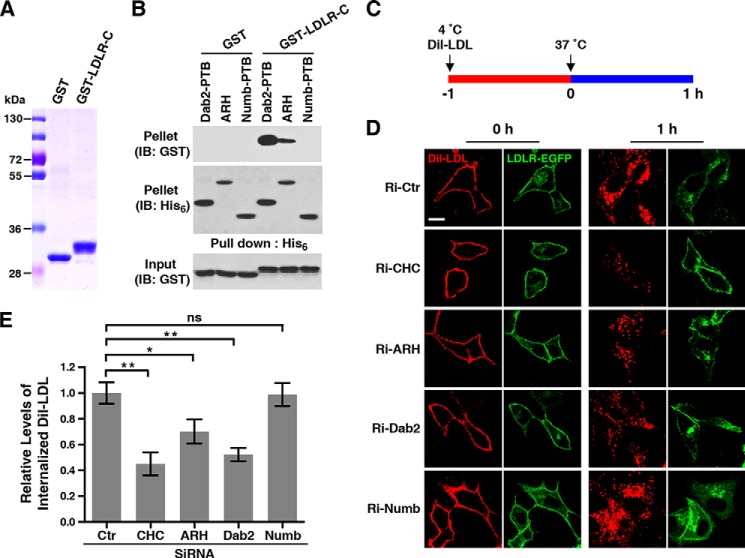
To investigate whether Numb is involved in LDLR-mediated LDL uptake, an in vitro pulldown assay was first used to examine the binding of Numb-PTB to LDLR-C (Fig. 5, A and B). Consistent with previous reports (8, 10), both ARH and Dab2-PTB bound to GST-LDLR-C. By contrast, no detectable interaction between Numb-PTB and GST-LDLR-C was found (Fig. 5B), indicating that LDLR-C preferentially binds ARH and Dab2, not Numb.
FIGURE 5.
Numb does not bind LDLR-C and is dispensable for LDL uptake. A, purified GST and GST-tagged LDLR C terminus (GST-LDLR-C) from E. coli. B, interaction of the LDLR C terminus with His6-tagged Dab2-PTB, ARH, or Numb-PTB analyzed by in vitro binding. C, diagram showing the procedure of the LDL internalization assay. Cells were incubated with Dil-LDL (10 μg/ml) for 1 h (time point 0 h) at 4 °C, allowing binding of Dil-LDL to cell surface LDLR, followed by internalization for another 1 h (time point 1 h) at 37 °C in Dil-LDL-free medium. At the indicated time points, cells were fixed and examined by confocal microscopy. D, LDL internalization in CRL1601/LDLR-EGFP cells transfected with the indicated siRNA. E, densitometric quantification of the internalized Dil-LDL at the 1-h time point. Results were normalized to the mean of control (Ctr) and shown as mean ± S.D., n = 10. ns, not significant; *, p < 0.05; **, p < 0.01. Scale bars, 10 μm. IB, immunoblot.
Next, we performed a LDL internalization assay using fluorescent Dil-labeled LDL (Dil-LDL). CRL1601 cells expressing LDLR-EGFP were transfected with siRNA targeting CHC, ARH, Dab2, or Numb separately. As illustrated in Fig. 5C, the transfected cells were incubated with Dil-LDL for 1 h at 4 °C, permitting Dil-LDL bound to LDL receptor and synchronizing the internalization, and then shifted to 37 °C in Dil-LDL-free medium to initiate endocytosis. After 1 h, Dil-LDL·LDLR complexes were internalized and the cells were fixed for observation. As shown in a representative experiment (Fig. 5D), at the 0 h time point, Dil-LDL was restrictively bound to the cell surface without internalization in all cells. After chasing for 1 h at 37 °C, internalized Dil-LDL was observed and quantified. In CHC, ARH, and Dab2 knockdown cells, the internalized Dil-LDL was significantly less than control cells. But in Numb knockdown cells, the uptake of LDL was unaffected (Fig. 5, D and E). Collectively, these data demonstrate that Numb does not participate in LDLR-mediated LDL uptake.
G595D Numb Polymorphism Is Associated with Low LDL-Cholesterol in Humans
In the light of the fact that Numb functions in cholesterol absorption, we attempted to study the influence of Numb polymorphism on blood cholesterol in human populations. As patterns of linkage disequilibrium vary among populations with distinct demographic histories, studies in different populations can assist in mapping the actual susceptibility variants. The samples in our study were selected from three ethnic groups, including 1928 Kazakh men, 2043 Kazakh women, 1941 Uygur men, 2596 Uygur women, 2649 Han men, and 2811 Han women in Xinjiang, China, whose genetic backgrounds and the risks of cardiovascular disease are distinct (32, 33). The samples included 466 subjects with low LDL-cholesterol (LDL-C) (<1.87 mmol/liter) and 494 subjects with high LDL-C (>3.11 mmol/liter) (data not shown).
We sequenced all exons and exon-intron junctions of the Numb gene in 960 total samples and uncovered one nonsynonymous variant (rs17781919, 1784G>A), encoding a G595D amino acid substitution. Five samples with low LDL-C in contrast to no sample with high LDL-C were identified carrying this SNP from Kazakh and Uygur (p = 0.0274, two-tailed Fisher's exact test) (Table 1). Table 2 detailing the clinical characteristics of individuals with the G595D variation shows that the LDL-C values of the carriers are far below the averages of corresponding ethnicity and gender (compare LDL-C and Cohort LDL-C) and are ranked in the lowest 20th percent (rank). These results indicate that the G595D Numb polymorphism is associated with low LDL-C in humans.
TABLE 1.
Distribution of the Numb(G595D) variant in three human populations
| Ethnicity | Nucleotide | Amino acid | na | Frequency |
|---|---|---|---|---|
| Low LDL-Cb (<1.87 mmol/liter) | ||||
| Kazakh (n = 168) | 1784G>A | G595D | 2 | 0.006 |
| Uygur (n = 152) | 1784G>A | G595D | 3 | 0.010 |
| Han (n = 146) | 0 | |||
| High LDL-C (>3.11 mmol/liter) | ||||
| Kazakh (n = 170) | 0 | |||
| Uygur (n = 184) | 0 | |||
| Han (n = 140) | 0 | |||
a n is the number of people carrying the Numb(G595D) variant with low or high LDL-C.
b LDL-C, low density lipoprotein cholesterol. Normal LDL-C range: 2.07–3.11 mmol/liter.
TABLE 2.
Characteristics of individuals with the Numb(G595D) variant
| Ethnicity | Gender | Variants | LDL-Ca | Cohort LDL-C | Rank | HDL-C | TC | TG | FBG | BMI | Age |
|---|---|---|---|---|---|---|---|---|---|---|---|
| mmol/liter | mmol/liter | mmol/liter | mmol/liter | mmol/liter | mmol/liter | kg/m2 | yr | ||||
| #1 Kazakh | Man | G595D | 1.38 | 2.89 ± 0.92 (n = 1928) | 1872 | 1.15 | 3.01 | 0.58 | 3.70 | 24.2 | 56 |
| #2 Kazakh | Woman | G595D | 1.05 | 2.92 ± 0.94 (n = 2043) | 2018 | 1.07 | 2.54 | 0.60 | 3.89 | 19.9 | 40 |
| #3 Uygur | Man | G595D | 1.23 | 2.87 ± 0.91 (n = 1941) | 1898 | 0.84 | 2.65 | 0.73 | 3.87 | 21.6 | 35 |
| #4 Uygur | Woman | G595D | 1.23 | 2.86 ± 0.93 (n = 2596) | 2535 | 0.85 | 2.30 | 0.70 | 4.10 | 25.0 | 30 |
| #5 Uygur | Man | G595D | 1.32 | 2.87 ± 0.91 (n = 1941) | 1898 | 0.83 | 2.50 | 0.82 | 4.70 | 20.3 | 42 |
a The following abbreviations are used: LDL-C, low density lipoprotein cholesterol; Cohort LDL-C, average value of LDL-C for the corresponding ethnicity and gender shown as mean ± S.D.; n is the sample size; Rank, position of the LDL-C value for individuals carrying the Numb(G595D) variation in the corresponding cohort that was arranged in descending order; HDL-C, high density lipoprotein cholesterol; TC, serum total cholesterol; TG, triglyceride; FBG, fasting blood glucose; and BMI, body mass index.
Numb G595D Variation Attenuates NPC1L1-mediated Cholesterol Absorption
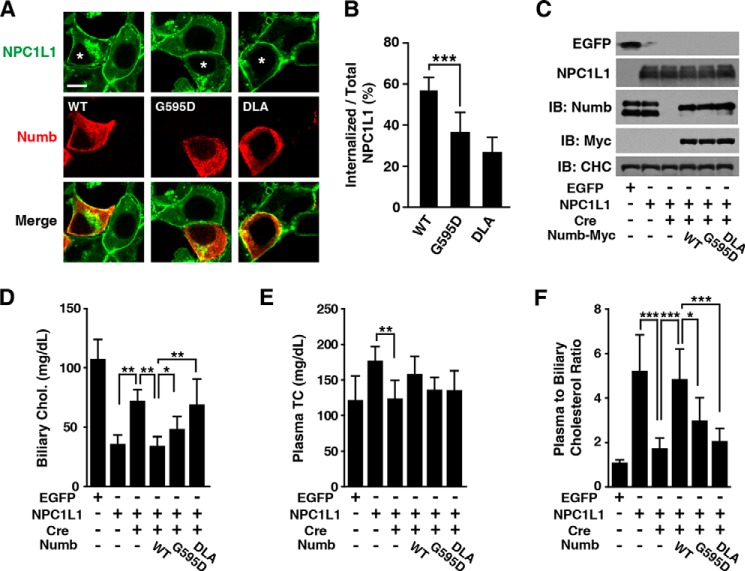
Because Numb is required for NPC1L1 endocytosis, it is possible that the low LDL-C levels in people carrying the Numb G595D substitution were caused by deficiency of NPC1L1-mediated cholesterol absorption. To validate this hypothesis, we first assessed the effect of the Numb(G595D) variant on NPC1L1 endocytosis by performing a rescue experiment. Numb-DLA, a mutation of the DPF motif (Asp-Pro-Phe, residue 555–557) that abolishes clathrin/AP2 binding, was used as a positive control. Compared with Numb(WT), Numb(G595D) drove significantly less internalization of NPC1L1 in Numb knockdown cells (Fig. 6, A and B). To test influence of the G595D variation on cholesterol absorption in vivo, adenoviruses expressing NPC1L1 and Numb variants (WT, G595D, and DLA) were applied to L-Numb−/− mice generated as described in Fig. 4 (see also Fig. 6C). In contrast to the control Ad-EGFP, Ad-NPC1L1-EGFP offered mice effective cholesterol reabsorption from bile (Fig. 6, D–F, compare first and second columns), and co-injection of Ad-Cre, which excised Numb in liver (L-Numb−/−) (Fig. 6C), compromised the cholesterol reabsorption (Fig. 6, D–F, compare second and third columns). Under such background, additional expression of Numb(WT) (Fig. 6, D–F, fourth column) almost restored the biliary cholesterol absorption to the same level as that in mice only receiving Ad-NPC1L1-EGFP (Fig. 6, D–F, second column). In contrast, the cholesterol reabsorption of mice receiving Numb(G595D) was significantly lower than that of mice with Numb(WT) (Fig. 6, D–F, compare fourth and fifth columns). As positive control, mice expressing Numb-DLA also displayed lower biliary cholesterol absorption (Fig. 6, D–F, compare fourth and sixth columns). Collectively, these results demonstrate that the Numb(G595D) variant is deficient in NPC1L1-mediated cholesterol absorption.
FIGURE 6.
The G595D substitution of Numb impairs NPC1L1-mediated cholesterol reabsorption from bile. A, internalization of NPC1L1 in Numb RNAi cells transfected with plasmids encoding Myc-tagged Numbs (WT, G595D, and DLA) (marked by asterisk). The cells were treated as Fig. 2B and immunofluorescent staining was performed to label exogenous Numb variants. Scale bars, 10 μm. B, quantification of the internalized NPC1L1-EGFP shown in A. Data are shown as mean ± S.D., n = 10. ***, p < 0.001. C–F, liver-specific Numb-deficient mice were generated by adeno-Cre (2 × 109 pfu/mouse) injection to 8-week-old male Numbflox/flox mice. After 2 days, the mice were administrated with adenovirus expressing NPC1L1-EGFP (2 × 108 pfu/mouse) and various Numb-Myc (WT, G595D, and DLA) (2 × 109 pfu/mouse). After an additional 4 days, the mice were fasted overnight before sacrificing. Livers were collected and subjected to immunoblot (C). Biliary cholesterol (D), plasma total cholesterol (E), and plasma to biliary cholesterol ratio (F) were measured. Data are mean ± S.D., n = 6. *, p < 0.05; **, p < 0.01; ***, p < 0.001. DLA, mutation of the Numb DPF motif (Asp-Pro-Phe). IB, immunoblot.
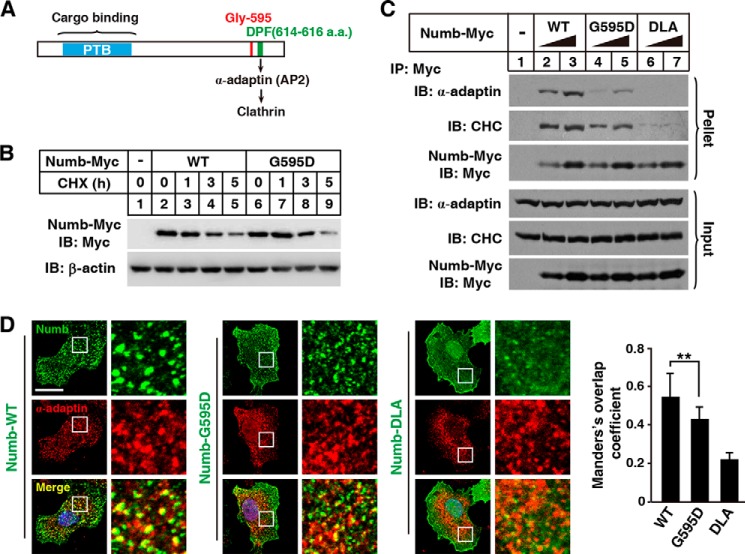
Next, we investigated the molecular mechanism responsible for impaired cholesterol absorption caused by the G595D substitution. We first measured protein stability and found that Numb(WT) and Numb(G595D) had similar half-lives (Fig. 7B). As the Gly-595 locates closely to the DPF motif that directly binds the AP2 subunit α-adaptin to bridge cargoes to endocytic machinery for internalization (Fig. 7A) (34), we then tested the possibility that the G595D substitution, like Numb-DLA, may alter the affinity of Numb to clathrin and AP2, impairing recruitment of NPC1L1 to clathrin-coated pits. The co-immunoprecipitation assay was employed to appraise the association of clathrin/AP2 with Numb variants including WT, G595D, and Numb-DLA. The results showed that Numb(G595D) co-precipitated with less α-adaptin and CHC than WT Numb. As control, Numb-DLA completely abolished the binding of clathrin/AP2 to Numb (Fig. 7C). Consistently, WT Numb preferentially localized to the AP2 positive clathrin-coated structures in cells, whereas G595D substitution dramatically decreased the localization of Numb to clathrin-coated structures (Fig. 7D). Collectively, these data demonstrate that the attenuated affinity of Numb(G595D) to clathrin and AP2 caused the defect of NPC1L1 internalization, leading to low cholesterol absorption.
FIGURE 7.
The G595D substitution attenuates the affinity of Numb to clathrin/AP2. A, domain structure of Numb. B, the protein levels of Numb (WT or G595D) expressed in CRL1601 cells after cycloheximide (CHX) treatment for the indicated times. C, the interaction of Numb variants (WT, G595D, and DLA) with α-adaptin and CHC analyzed by co-immunoprecipitation. D, immunofluorescent staining of Numbs (α-Myc) and clathrin-coated structures (α-adaptin) in CRL1601 cells. The Numb colocalized with α-adaptin was measured by ImageJ (Manders's overlap coefficient). Values are mean ± S.D., n = 20. **, p < 0.01. Scale bars, 10 μm. IP, immunoprecipitation; IB, immunoblot.
DISCUSSION
Both the LDLR-mediated LDL-cholesterol uptake and the NPC1L1-mediated free cholesterol absorption are controlled by clathrin-dependent endocytosis. Here we investigated the roles of adaptor proteins ARH, Dab2, and Numb in these two processes. We found that Numb, rather than ARH and Dab2, bind NPC1L1 to facilitate its endocytosis. Conversely, ARH and Dab2, not Numb, are required for receptor-mediated LDL uptake. Numb stimulates NPC1L1 internalization in an isoform-specific manner, in which only the isoforms with short PTB domains are functional. Besides mediating intestinal cholesterol absorption, Numb also participates in biliary cholesterol reabsorption in human liver. Furthermore, the Numb G595D variation, which impairs NPC1L1 internalization, is associated with human low blood LDL-cholesterol. These findings demonstrate that ARH, Dab2, and Numb modulate cholesterol homeostasis through performing distinct roles in LDLR- and NPC1L1-mediated cholesterol uptake processes.
The specific employment of adaptor proteins by LDLR and NPC1L1 is strictly determined by recognition of endocytic signals. Recently, the Numb-dependent endocytic signal sequence (YVNXXF) in the C-terminal portion of NPC1L1 has been defined (15). Although this sequence is similar to the classical endocytic sequon (F/Y)XNPX(F/Y) of LDLR, we found that the NPC1L1 C terminus cannot bind ARH or Dab2. This observation suggests that the YVNXXF motif in NPC1L1 represents a novel class of endocytic sequon, distinguished from the classical one in LDLR and preferentially recognized by Numb. Prior to definition of this novel binding motif, Numb had been reported to interact with several NPXY motif-containing proteins, such as Sanpodo (YTNPAF) (34) and integrin-β (FKNPNY) (18). Interestingly, we observed that the LDLR cytoplasmic tail almost cannot bind Numb. Similarly, substitution of the fourth histidine residue in the NPC1L1 endocytic signal with proline mimicking the NPXY motif of LDLR still does not enable the interaction with ARH and Dab2. It is likely that the endocytic signal motif of either NPC1L1 or LDLR is not a unique recognition site for binding to adaptor proteins. Additional residues neighboring the motif may also contribute to the specificity of binding. In addition, we predict that other YVNXXF-bearing proteins may also function via engagement of Numb. Further studies discovering novel Numb-bound proteins will expand the physiological functions of Numb.
The presence of insert within PRR domains caused by alternative splicing makes Numb exhibit distinct functions in some biological processes. For example, in the neuronal lineage, human Numb isoforms with a short PRR promote differentiation; conversely, isoforms containing a long PRR direct proliferation (28). A neuron-specific RNA-binding protein, Rbfox3, has been identified to promote neuronal differentiation during development by modulating alternative splicing of Numb (35). Here, our observations reveal that Numb stimulates NPC1L1 endocytosis in a PTB domain-specific manner with only isoforms 3 and 4 possessing shorter PTB domains that can bind NPC1L1 and are functional in the internalization, independent on the length of PRR domain. An existing enigma is why only the shorter PTB domain can bind the NPC1L1 C terminus and insertion of the 11-amino acid insertion (ERKFFKGFFGK) abrogates this binding. Previous structural analyses of Drosophila Numb have revealed that the Numb PTB domain recognizes distinct target peptides using a common hydrophobic groove and diverse interactions originate from a flexible usage of this groove (36, 37). Sequence alignments of the mammalian Numb PTB domains indicated the insert is not included in the sites for direct interaction with multiple targets (36, 37). The 11-amino acid insertion might alter conformation of the PTB domain, especially the hydrophobic groove, and consequently change its binding specificity. Significantly, Numb with shorter PTB is predominantly expressed in human liver and intestine, which is consistent with the requirement of active Numb for NPC1L1-mediated cholesterol absorption in these organs. It is possible that cholesterol absorption might be regulated at the pre-mRNA level by modulating alternative splicing of Numb. Regrettably, there is a lack of available information about the PTB isoforms switch, because all identified regulators of Numb alternative splicing, including Rbfox3 (35), QKI-5 (38), RBM5, RBM6, and RBM10 (39), are concentrated in switching PRR isoforms and exert no effect on the splicing pattern of PTB.
Although the specific function of Numb in NPC1L1-dependent cholesterol absorption of mouse has been solidly attested by studies using the mouse model, in consideration of the disparity among species, the role of Numb in human cholesterol absorption is not definite. Establishment of a correlation between gene variants and clinical traits will provide a critical insight into the function of genes in humans. Based on this strategy, we sequenced the coding regions of the Numb gene from 960 samples with aberrant LDL-cholesterol levels and identified a variation (1784G>A, G595D) that specifically appears in humans with low LDL-cholesterol. An analogous strategy has been utilized by Cohen et al. (40, 41) identifying many nonsense or missense variants in the coding region of PCSK9, an enzyme that degrades LDLR, in samples with the lowest and highest plasma levels of LDL-cholesterol. Variants that contribute to the trait will be enriched in frequency in such a population with the trait, so the extreme population distribution of Numb(G595D) raises the possibility that this variation is directly responsible for the decreased LDL-cholesterol. A reasonable explanation is that the variation influences Numb activity of driving NPC1L1 internalization and decreases cholesterol absorption. Indeed, we demonstrated that Numb (G595D) impaired cholesterol absorption, further strengthening the association between Numb and the LDL-cholesterol level. Collectively, these findings offer strong evidence that Numb is also essential for maintaining cholesterol homeostasis in humans via regulating cholesterol absorption.
It is well known that liver plays a pivotal role in maintaining whole body cholesterol homeostasis. In addition to being the primary organ for cholesterol de novo synthesis, production, and uptake of lipoprotein cholesterol, liver consumes cholesterol by excreting unesterified cholesterol and cholesterol-derived bile acids into bile. In human liver, NPC1L1 residing in bile canalicular membrane transports biliary cholesterol back to hepatocytes, preventing excessive loss of cholesterol (14, 30). Given the specific binding of NPC1L1 to the shorter PTB domain, predominate expression of Numb with shorter PTB in liver implies a potential function in NPC1L1-dependent biliary cholesterol reabsorption. By manipulating the Numb gene in mouse liver expressing exogenous NPC1L1, we verified the supposition above. Combined with the identified role of intestinal Numb, we conclude that Numb is required for NPC1L1-mediated cholesterol absorption both in human intestine and liver. It seems that intestinal and hepatic Numb cooperatively regulate the cholesterol level of the body through modulating dietary cholesterol absorption and biliary cholesterol excretion. These data indicate disruption of the Numb-NPC1L1 interaction, which will effectively reduce dietary cholesterol absorption and elevate biliary cholesterol excretion, may offer a potential therapy for hypercholesterolemia.
Acknowledgments
We are grateful to Yu-Xiu Qu, Jie Xu, and Jie Qin for technical assistance, Paul A. Welling for the gift of ARH-plasmid.
This work was supported by Ministry of Science and Technology of China Grants 2011CB910900 and 2012CB524900, National Natural Science Foundation of China Grants 30925012, 31230020, 81260041, 81270155, and 91213306, and Xinjiang Science and Technology Department Grant 2013911111.
- NPC1L1
- Niemann-Pick C1-Like 1
- LDLR
- LDL receptor
- ARH
- autosomal recessive hypercholesterolemia
- Dab2
- Disabled homolog 2
- CLASP
- class of clathrin-associated sorting protein
- PTB
- phosphotyrosine-binding domain
- MCD
- methyl-β-cyclodextrin
- LRP
- low density lipoprotein receptor-related protein
- CHC
- clathrin heavy chain
- LDL-C
- LDL-cholesterol
- PRR
- proline-rich region
- SPTB
- shorter PTB
- LPTB
- longer PTB
- aa
- amino acid
- EGFP
- enhanced green fluorescent protein
- Ni-NTA
- nickel-nitrilotriacetic acid
- Dil-LDL
- 1,1′-dioactadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate-labeled LDL.
REFERENCES
- 1. Abifadel M., Varret M., Rabès J. P., Allard D., Ouguerram K., Devillers M., Cruaud C., Benjannet S., Wickham L., Erlich D., Derré A., Villéger L., Farnier M., Beucler I., Bruckert E., Chambaz J., Chanu B., Lecerf J. M., Luc G., Moulin P., Weissenbach J., Prat A., Krempf M., Junien C., Seidah N. G., Boileau C. (2003) Mutations in PCSK9 cause autosomal dominant hypercholesterolemia. Nat. Genet. 34, 154–156 [DOI] [PubMed] [Google Scholar]
- 2. Brown M. S., Goldstein J. L. (1999) A proteolytic pathway that controls the cholesterol content of membranes, cells, and blood. Proc. Natl. Acad. Sci. U.S.A. 96, 11041–11048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ge L., Wang J., Qi W., Miao H. H., Cao J., Qu Y. X., Li B. L., Song B. L. (2008) The cholesterol absorption inhibitor ezetimibe acts by blocking the sterol-induced internalization of NPC1L1. Cell Metab. 7, 508–519 [DOI] [PubMed] [Google Scholar]
- 4. Goldstein J. L., Brown M. S. (2009) The LDL receptor. Arterioscler. Thromb. Vasc. Biol. 29, 431–438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brown M. S., Goldstein J. L. (1986) A receptor-mediated pathway for cholesterol homeostasis. Science 232, 34–47 [DOI] [PubMed] [Google Scholar]
- 6. Chen W. J., Goldstein J. L., Brown M. S. (1990) NPXY, a sequence often found in cytoplasmic tails, is required for coated pit-mediated internalization of the low density lipoprotein receptor. J. Biol. Chem. 265, 3116–3123 [PubMed] [Google Scholar]
- 7. Davis C. G., Lehrman M. A., Russell D. W., Anderson R. G., Brown M. S., Goldstein J. L. (1986) The J.D. mutation in familial hypercholesterolemia: amino acid substitution in cytoplasmic domain impedes internalization of LDL receptors. Cell 45, 15–24 [DOI] [PubMed] [Google Scholar]
- 8. Garcia C. K., Wilund K., Arca M., Zuliani G., Fellin R., Maioli M., Calandra S., Bertolini S., Cossu F., Grishin N., Barnes R., Cohen J. C., Hobbs H. H. (2001) Autosomal recessive hypercholesterolemia caused by mutations in a putative LDL receptor adaptor protein. Science 292, 1394–1398 [DOI] [PubMed] [Google Scholar]
- 9. He G., Gupta S., Yi M., Michaely P., Hobbs H. H., Cohen J. C. (2002) ARH is a modular adaptor protein that interacts with the LDL receptor, clathrin, and AP-2. J. Biol. Chem. 277, 44044–44049 [DOI] [PubMed] [Google Scholar]
- 10. Maurer M. E., Cooper J. A. (2006) The adaptor protein Dab2 sorts LDL receptors into coated pits independently of AP-2 and ARH. J. Cell Sci. 119, 4235–4246 [DOI] [PubMed] [Google Scholar]
- 11. Jones C., Hammer R. E., Li W. P., Cohen J. C., Hobbs H. H., Herz J. (2003) Normal sorting but defective endocytosis of the low density lipoprotein receptor in mice with autosomal recessive hypercholesterolemia. J. Biol. Chem. 278, 29024–29030 [DOI] [PubMed] [Google Scholar]
- 12. Zuliani G., Arca M., Signore A., Bader G., Fazio S., Chianelli M., Bellosta S., Campagna F., Montali A., Maioli M., Pacifico A., Ricci G., Fellin R. (1999) Characterization of a new form of inherited hypercholesterolemia: familial recessive hypercholesterolemia. Arterioscler. Thromb. Vasc. Biol. 19, 802–809 [DOI] [PubMed] [Google Scholar]
- 13. Altmann S. W., Davis H. R., Jr., Zhu L. J., Yao X., Hoos L. M., Tetzloff G., Iyer S. P., Maguire M., Golovko A., Zeng M., Wang L., Murgolo N., Graziano M. P. (2004) Niemann-Pick C1 Like 1 protein is critical for intestinal cholesterol absorption. Science 303, 1201–1204 [DOI] [PubMed] [Google Scholar]
- 14. Wang L. J., Song B. L. (2012) Niemann-Pick C1-Like 1 and cholesterol uptake. Biochim. Biophys. Acta 1821, 964–972 [DOI] [PubMed] [Google Scholar]
- 15. Li P. S., Fu Z. Y., Zhang Y. Y., Zhang J. H., Xu C. Q., Ma Y. T., Li B. L., Song B. L. (2014) The clathrin adaptor Numb regulates intestinal cholesterol absorption through dynamic interaction with NPC1L1. Nat. Med. 20, 80–86 [DOI] [PubMed] [Google Scholar]
- 16. Traub L. M. (2009) Tickets to ride: selecting cargo for clathrin-regulated internalization. Nat. Rev. Mol. Cell Biol. 10, 583–596 [DOI] [PubMed] [Google Scholar]
- 17. Uhlik M. T., Temple B., Bencharit S., Kimple A. J., Siderovski D. P., Johnson G. L. (2005) Structural and evolutionary division of phosphotyrosine binding (PTB) domains. J. Mol. Biol. 345, 1–20 [DOI] [PubMed] [Google Scholar]
- 18. Nishimura T., Kaibuchi K. (2007) Numb controls integrin endocytosis for directional cell migration with aPKC and PAR-3. Dev. Cell 13, 15–28 [DOI] [PubMed] [Google Scholar]
- 19. Teckchandani A., Toida N., Goodchild J., Henderson C., Watts J., Wollscheid B., Cooper J. A. (2009) Quantitative proteomics identifies a Dab2/integrin module regulating cell migration. J. Cell Biol. 186, 99–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gulino A., Di Marcotullio L., Screpanti I. (2010) The multiple functions of Numb. Exp. Cell Res. 316, 900–906 [DOI] [PubMed] [Google Scholar]
- 21. Goldstein J. L., Basu S. K., Brown M. S. (1983) Receptor-mediated endocytosis of low-density lipoprotein in cultured cells. Methods Enzymol. 98, 241–260 [DOI] [PubMed] [Google Scholar]
- 22. Fang L., Garuti R., Kim B. Y., Wade J. B., Welling P. A. (2009) The ARH adaptor protein regulates endocytosis of the ROMK potassium secretory channel in mouse kidney. J. Clin. Invest. 119, 3278–3289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Brown A. J., Sun L., Feramisco J. D., Brown M. S., Goldstein J. L. (2002) Cholesterol addition to ER membranes alters conformation of SCAP, the SREBP escort protein that regulates cholesterol metabolism. Mol. Cell 10, 237–245 [DOI] [PubMed] [Google Scholar]
- 24. Luo J., Deng Z. L., Luo X., Tang N., Song W. X., Chen J., Sharff K. A., Luu H. H., Haydon R. C., Kinzler K. W., Vogelstein B., He T. C. (2007) A protocol for rapid generation of recombinant adenoviruses using the AdEasy system. Nat. Protoc. 2, 1236–1247 [DOI] [PubMed] [Google Scholar]
- 25. Redgrave T. G., Roberts D. C., West C. E. (1975) Separation of plasma lipoproteins by density-gradient ultracentrifugation. Anal. Biochem. 65, 42–49 [DOI] [PubMed] [Google Scholar]
- 26. Pitas R. E., Innerarity T. L., Weinstein J. N., Mahley R. W. (1981) Acetoacetylated lipoproteins used to distinguish fibroblasts from macrophages in vitro by fluorescence microscopy. Arteriosclerosis 1, 177–185 [DOI] [PubMed] [Google Scholar]
- 27. Ranganathan S., Liu C. X., Migliorini M. M., Von Arnim C. A., Peltan I. D., Mikhailenko I., Hyman B. T., Strickland D. K. (2004) Serine and threonine phosphorylation of the low density lipoprotein receptor-related protein by protein kinase Cα regulates endocytosis and association with adaptor molecules. J. Biol. Chem. 279, 40536–40544 [DOI] [PubMed] [Google Scholar]
- 28. Verdi J. M., Bashirullah A., Goldhawk D. E., Kubu C. J., Jamali M., Meakin S. O., Lipshitz H. D. (1999) Distinct human NUMB isoforms regulate differentiation versus proliferation in the neuronal lineage. Proc. Natl. Acad. Sci. U.S.A. 96, 10472–10476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Davies J. P., Scott C., Oishi K., Liapis A., Ioannou Y. A. (2005) Inactivation of NPC1L1 causes multiple lipid transport defects and protects against diet-induced hypercholesterolemia. J. Biol. Chem. 280, 12710–12720 [DOI] [PubMed] [Google Scholar]
- 30. Temel R. E., Tang W., Ma Y., Rudel L. L., Willingham M. C., Ioannou Y. A., Davies J. P., Nilsson L. M., Yu L. (2007) Hepatic Niemann-Pick C1-like 1 regulates biliary cholesterol concentration and is a target of ezetimibe. J. Clin. Invest. 117, 1968–1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wang L. J., Wang J., Li N., Ge L., Li B. L., Song B. L. (2011) Molecular characterization of the NPC1L1 variants identified from cholesterol low absorbers. J. Biol. Chem. 286, 7397–7408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Li N., Wang H., Yan Z., Yao X., Hong J., Zhou L. (2012) Ethnic disparities in the clustering of risk factors for cardiovascular disease among the Kazakh, Uygur, Mongolian and Han populations of Xinjiang: a cross-sectional study. BMC Public Health 12, 499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tao J., Ma Y. T., Xiang Y., Xie X., Yang Y. N., Li X. M., Fu Z. Y., Ma X., Liu F., Chen B. D., Yu Z. X., Chen Y. (2013) Prevalence of major cardiovascular risk factors and adverse risk profiles among three ethnic groups in the Xinjiang Uygur Autonomous Region, China. Lipids Health Dis. 12, 185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Santolini E., Puri C., Salcini A. E., Gagliani M. C., Pelicci P. G., Tacchetti C., Di Fiore P. P. (2000) Numb is an endocytic protein. J. Cell Biol. 151, 1345–1352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kim K. K., Nam J., Mukouyama Y. S., Kawamoto S. (2013) Rbfox3-regulated alternative splicing of Numb promotes neuronal differentiation during development. J. Cell Biol. 200, 443–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Li S. C., Zwahlen C., Vincent S. J., McGlade C. J., Kay L. E., Pawson T., Forman-Kay J. D. (1998) Structure of a Numb PTB domain-peptide complex suggests a basis for diverse binding specificity. Nat. Struct. Biol. 5, 1075–1083 [DOI] [PubMed] [Google Scholar]
- 37. Zwahlen C., Li S. C., Kay L. E., Pawson T., Forman-Kay J. D. (2000) Multiple modes of peptide recognition by the PTB domain of the cell fate determinant Numb. EMBO J. 19, 1505–1515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zong F. Y., Fu X., Wei W. J., Luo Y. G., Heiner M., Cao L. J., Fang Z., Fang R., Lu D., Ji H., Hui J. (2014) The RNA-binding protein QKI suppresses cancer-associated aberrant splicing. PLoS Genet. 10, e1004289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bechara E. G., Sebestyén E., Bernardis I., Eyras E., Valcárcel J. (2013) RBM5, 6, and 10 differentially regulate NUMB alternative splicing to control cancer cell proliferation. Mol. Cell 52, 720–733 [DOI] [PubMed] [Google Scholar]
- 40. Cohen J., Pertsemlidis A., Kotowski I. K., Graham R., Garcia C. K., Hobbs H. H. (2005) Low LDL cholesterol in individuals of African descent resulting from frequent nonsense mutations in PCSK9. Nat. Genet. 37, 161–165 [DOI] [PubMed] [Google Scholar]
- 41. Kotowski I. K., Pertsemlidis A., Luke A., Cooper R. S., Vega G. L., Cohen J. C., Hobbs H. H. (2006) A spectrum of PCSK9 alleles contributes to plasma levels of low-density lipoprotein cholesterol. Am. J. Hum. Genet. 78, 410–422 [DOI] [PMC free article] [PubMed] [Google Scholar]