Abstract
Strigolactones (SLs) or their derivatives have recently been defined as novel phytohormones that regulate root development. However, it remains unclear whether SLs mediate root growth in response to phosphorus (P) and nitrogen (N) deficiency. In this study, the responses of root development in rice (Oryza sativa L.) to different levels of phosphate and nitrate supply were investigated using wild type (WT) and mutants defective in SL synthesis (d10 and d27) or insensitive to SL (d3). Reduced concentration of either phosphate or nitrate led to increased seminal root length and decreased lateral root density in WT. Limitation of either P or N stimulated SL production and enhanced expression of D10, D17, and D27 and suppressed expression of D3 and D14 in WT roots. Mutation of D10, D27, or D3 caused loss of sensitivity of root response to P and N deficiency. Application of the SL analogue GR24 restored seminal root length and lateral root density in WT and d10 and d27 mutants but not in the d3 mutant, suggesting that SLs were induced by nutrient-limiting conditions and led to changes in rice root growth via D3. Moreover, P or N deficiency or GR24 application reduced the transport of radiolabelled indole-3-acetic acid and the activity of DR5::GUS auxin reporter in WT and d10 and d27 mutants. These findings highlight the role of SLs in regulating rice root development under phosphate and nitrate limitation. The mechanisms underlying this regulatory role involve D3 and modulation of auxin transport from shoots to roots.
Key words: Auxin, nitrate, phosphate, rice, root, strigolactone.
Introduction
The ability of plants to sense the availability of soil nutrients and to respond accordingly is of fundamental importance for their adaptation to the environment. The plasticity of root development in response to nitrogen (N) or phosphorus (P) deficiency is vital, as N and P are two major nutrients required for plant growth and development (Forde and Lorenzo, 2001; López-Bucio et al., 2003). Increased root-to-shoot ratio and root surface area induced by deficiency of N and P has been reported for several plant species (López-Bucio et al., 2003; Chun et al., 2005; Gruber et al., 2013). Changes in root morphology under conditions of nutrient deficiency are complex and vary according to experimental conditions and plant species. Various plant growth studies have concentrated on the responses of root systems of different species under conditions of phosphate deficiency. A significant phosphate deficiency-induced response in Arabidopsis thaliana involves reduction of primary root growth and enhancement of lateral root (LR) density (Williamson et al., 2001; Linkohr et al., 2002; Chevalier et al., 2003; López-Bucio et al., 2003; Pérez-Torres et al., 2008). In contrast to Arabidopsis, elongation of primary roots is a typical response to phosphate deprivation in other plant species (e.g. rice, Oryza sativa L.) (Yi et al., 2005; Niu et al., 2012). Little attention has been paid to root growth under conditions of N stress, possibly because of the inconsistent response of primary root length to nitrate deprivation depending on plant age and N concentration supplied in Arabidopsis (Zhang and Forde, 1998; Linkohr et al., 2002; Gruber et al., 2013). However, primary roots of rice and maize (Zea mays) typically elongate in response to N deprivation (Chun et al., 2005; Tian et al., 2008; Zhang et al., 2012). Relative to phosphate deficiency, root morphology in rice under conditions of N limitation has not yet been characterized in detail.
Root formation is regulated by both environmental conditions and intrinsic factors (e.g. plant hormones). Auxins play a key role in establishing and developing patterns of root morphology and are regulated by varying N and P supply (Forde, 2002; Chiou and Lin, 2011; Niu et al., 2012). Auxin signalling is associated with changes in root system architecture caused by phosphate deprivation; phosphate-deprived plants were shown to undergo primary root growth arrest and show formation of LR in the presence of exogenous auxin, while phosphate-supplied plants did not (López-Bucio et al., 2003; Chiou and Lin, 2011; Shen et al., 2013). In a previous study, inhibition of root growth in maize by high nitrate concentration was closely related to a reduction of auxin level in roots, and exogenous 1-naphthaleneacetic acid (NAA) and indole-3-acetic acid (IAA) restored primary root growth in the presence of high concentrations of nitrate (Tian et al., 2008). Few studies have evaluated the role of auxin in regulating root growth under low-N conditions.
In addition to auxin, strigolactones or their derivatives (SLs) play a pivotal role in modulation of root development (Kapulnik et al., 2011a; Ruyter-Spira et al., 2011; Rasmussen et al., 2012). In tomato and Arabidopsis, LR densities are increased in SL-deficient and SL-insensitive mutants, suggesting that SLs affect LR formation (Koltai et al., 2010; Kapulnik et al., 2011b; Ruyter-Spira et al., 2011). Application of the synthetic SL analogue GR24 resulted in decreased LR density via the signalling gene MAX2, which suppressed LR outgrowth. Ruyter-Spira et al. (2011) found that the net effect of SL on LRs is dependent on the auxin status of the plant. In contrast to findings in Arabidopsis, numbers of LRs on the seminal root did not differ among 14-d-old wild type (WT) and d10 and d14 rice mutants (Arite et al., 2012). The detailed biological mechanisms by which SLs function as plant hormones to regulate root growth remain unclear.
Strigolactones are closely regulated by phosphate availability in the soil, as demonstrated in both legumes and nonlegumes (Yoneyama et al., 2007, 2012). Strigolactones are thought to be involved in an adaptive response to phosphate deficiency because elevated SL levels in roots and root exudates under low-phosphate conditions may contribute to increased mycorrhizal colonization and nodulation, which enable plants to collect more nutrients (Yoneyama et al., 2007; Xie et al., 2010). Arite et al. (2012) reported that application of GR24 induced root elongation in rice, suggesting that positive regulation of rice root elongation by SLs might be another strategy by which rice plants adapt to phosphate deficiency. Further studies are needed to understand the mechanisms responsible for the effects of SLs on growth responses of rice roots under conditions of low phosphate availability. Similarly, the production of SLs by plants (primarily nonlegumes) is regulated by N availability regardless of different N forms (Yoneyama et al., 2007). Given elevated SL levels in rice and greater root elongation under conditions of N deficiency (Jamil et al., 2011a; Zhang et al., 2012), it is of interest to explore whether SLs contribute to the architecture of the rice root system under conditions of N starvation.
In this study, the role of SLs in regulating rice root growth in response to P- and N-limiting conditions was examined in SL-deficient and SL-insensitive mutants. Rice is an ideal model for SLs study because it has a range of well-characterized SL-synthesis mutants (d10, d17, and d27) and SL-response mutants (d3, d14, and d53) (Supplementary Fig. S1 available at JXB online; Xie et al., 2010; Waters et al., 2012; Jiang et al., 2013; Zhou et al., 2013). The current study found that root growth was related to elevated SL levels in roots and that exogenous GR24 restored seminal root length and reduced LR density under conditions of P and N deficiency in WT plants and SL-synthesis mutants, but not in the SL-signalling mutant d3. In addition, SL-deficient rice mutants carrying the auxin reporter construct DR5::GUS, and polar transport of radiolabelled [3H] IAA, revealed that SLs affect root growth in rice by decreasing auxin transport from shoots to roots.
Materials and methods
Plant growth
Wild-type rice (O. sativa L.) used in this study was the Shiokari ecotype. The SL-deficient mutants (d10 and d27, respectively) and the SL-signalling mutant (d3) were kindly provided by Shinjiro Yamaguchi of RIKEN Plant Science Center. Plants were grown in a greenhouse under natural light at day/night temperatures of 30/18°C. Seven-d-old seedlings of uniform size and vigour were transplanted into holes in a lid placed over the top of pots (four holes per lid and three seedlings per hole). Nutrient solutions varying from one-quarter to half strength were applied for 1 week and then full-strength nutrient solution was applied for an additional 2 weeks. Pots receiving phosphate treatments were filled with solutions lacking P or containing varying phosphate concentrations (1, 2, 10, 100, and 300 μM). Pots receiving nitrate treatments were filled with solutions lacking N or containing 0.01, 0.02, 0.1, 1, or 2.5mM nitrate. The full chemical composition of the International Rice Research Institute (IRRI) nutrient solution was 1.25mM NH4NO3, 0.3mM KH2PO4, 0.35mM K2SO4, 1.0mM CaCl2, 1.0mM MgSO4.7H2O, 0.5mM Na2SiO3, 20.0 μM Fe-EDTA, 9.0 μM MnCl2, 0.39 μM (NH4)6Mo7O24, 20.0 μM H3BO3, 0.77 μM ZnSO4, and 0.32 μM CuSO4 (pH 5.5). Each treatment consisted of four replicates arranged in a completely randomized design to avoid edge effects. The nutrient solution was replaced with fresh solution daily.
Phosphate was supplied in the nutrient medium as KH2PO4. To exclude potential effects of potassium (K+) on the treatments, the low-phosphate treatment solutions were supplemented with K+ to the same levels as those under sufficient phosphate conditions (300 μM) using K2SO4. Nitrate was supplied in the nutrient medium as Ca(NO3)2. Similarly, concentrations of Ca2+ in the low-nitrate treatment solutions were supplemented to the same levels as those under sufficient nitrate conditions (2.5mM) using CaCl2. In the experiments, the N forms, NH4 +/NO3 – in phosphate treatments and NO3 – in NO3 – treatments, were different from each other. As shown in Supplementary Fig. S2 available at JXB online, under two N concentrations (0.02 and 2.5mM), no difference was recorded in seminal root length and LR density between NH4 +/NO3 – and NO3 – treatments.
Hormonal treatments, including GR24 (dissolved in acetone) and NAA (dissolved in 1M NaOH), were applied to the plant-growth medium since P and N treatments starting. Seven-d-old rice seedlings were grown in solutions containing varying levels of phosphate and nitrate with 2.5 μM GR24 or 10nM NAA. GR24 treatments were applied in the same IRRI nutrient solution in 0.4% Phytogel medium. Rice seeds were germinated in trays for 3 d and then transferred into plant culture tubes and grown for 2 weeks. The control treatment for GR24 consisted of addition of 0.1% acetone to the medium.
All plant tissues were sampled 4h after the start of the light period to assay root morphology, N, P and auxin concentrations. For determination of IAA, roots samples were weighed, snap-frozen in liquid N2, and stored in a –80°C freezer.
Measurement of root system architecture
Seminal roots were significantly longer than adventitious roots under our experimental conditions. Our preliminary experiment showed that the responses of seminal roots to different treatments was similar to those of adventitious roots, and the number of adventitious root did not change significantly during experimental period. Therefore, seminal roots and LRs of seminal roots were chosen to study the effects of N and P on the rice root system. The length of seminal roots was measured with a ruler. Length and density of LRs were measured using the WinRhizo scanner-based image analysis system (Regent Instruments, Montreal, QC, Canada). The LR density was calculated as LR number divided by the length of the seminal roots.
Measurement of strigolactones
After rice plants were grown in the treatments for 2 weeks, root exudates (approximately 500ml) were collected at 24-h intervals as described previously (Yoneyama et al., 2012; Xie et al., 2013). Root exudates adsorbed on charcoal were eluted with acetone. After evaporation of the acetone in vacuo, the residue was dissolved in 50ml water and extracted three times with 50ml ethyl acetate. The ethyl acetate extracts were combined, washed with 0.2M K2HPO4 (pH 8.3), dried over anhydrous MgSO4, and concentrated in vacuo. These crude extracts were stored in sealed glass vials at 4 °C until use.
The concentrations of SLs in the root exudation were determined by LC-MS/MS as described previously (Xie et al., 2013). Data acquisition and analysis were performed with MassLynx version 4.1 (Waters, Milford, MA, USA). All experiments were repeated three times and the data are presented as mean±SE.
qRT-PCR analysis
Total RNA was isolated from the roots of rice seedlings. RNA extraction, reverse transcription, and quantitative reverse-transcription PCR (qRT-PCR) were performed as described previously (Chen et al., 2012). Primer sets for D and PIN are listed in Supplementary Tables S1 and S2 available at JXB online.
Determination of IAA
The concentrations of IAA in the first leaf, junction, and roots were determined as described previously (Song et al., 2013). Fresh weight of samples was determined, followed immediately by freezing in liquid N2. Sample measurement of free IAA by HPLC were carried out according to Song et al. (2013). A standard IAA sample was obtained from Sigma-Aldrich (St Louis, MO, USA).
To detect patterns of IAA distribution in rice plants, the pDR5::GUS construct was transformed into WT and d10 and d27 mutants using Agrobacterium tumefaciens EHA105; this construct was kindly provided by Professor Ping Wu’s group at Zhejiang University, Hangzhou, China. The samples used for IAA analysis were also used for histochemical GUS staining. Tissues were treated with ethanol prior to observation to remove chlorophyll pigmentation. The stained tissues were photographed using an Olympus SZX2-ILLK stereomicroscope with a colour CCD camera (Olympus).
[3H]IAA-transport assay
Shoot-to-root auxin transport was assayed according to Song et al. (2013). [3H]IAA solution was applied to the cut surface after rice shoots were removed 2cm above the root–shoot junction. The four root segments – i.e. root tip (RT, 0.5cm), the zone where LR initiation and emergence occurred (LI, 0.5–4.0cm), the LR elongation zone in which LRs were visible but their length was not as long as that of mature roots (LE, 4.0–8.0cm), and the mature LR zone (ML, 8.0–12.0cm), were incubated in scintillation solution for 18h in the dark and then sampled and weighed. [3H]IAA radioactivity was detected using a multipurpose scintillation counter (LS6500, Beckman-Coulter, Fullerton, CA, USA).
Data analysis
Experimental data were pooled for calculation of means and standard errors (SE) and analysed by one-way analysis of variance (ANOVA) followed by least significant difference (LSD) to determine the significance of differences between individual treatments. All statistical procedures were conducted with SPSS version 11.0 (SPSS, Chicago, IL, USA). In all analyses, P<0.05 was taken to indicate statistical significance.
Results
Low phosphate and nitrate concentrations enhanced seminal and lateral root elongation and inhibited lateral root density
The length of seminal roots increased significantly in WT plants with decreasing phosphate concentration, from 300 to 1 μM (Fig. 1A). Seminal root length was 26% greater with 10 μM and approximately 55% greater in 1 and 2 μM phosphate compared to that under sufficient phosphate conditions (SP, 300 μM) (Fig. 1C–E). The average length of LRs in WT plants increased by approximately 64% with 1 or 2 μM phosphate, while LR density in WT decreased by 40% with low-P treatment relative to SP treatment.
Fig. 1.
Effects of phosphate and nitrate availability on root morphology in wild-type rice seedlings. (A, B) Seedlings grown for 2 weeks in hydroponic media containing varying concentrations of phosphate (A) and nitrate (B). (C–E) Length of seminal root (C), lateral root (LR) length (D), and LR density (E) with containing varying concentrations of phosphate and nitrate. Red arrows indicate the four treatments for further study. Data are mean±SE. Different letters indicate significant differences (P<0.05, ANOVA) (this figure is available in colour at JXB online).
The changes in WT root architecture with decreasing nitrate concentration were similar to those observed for phosphate (Fig. 1B). For example, seminal root length was 22% larger with 0.1mM nitrate and about 33% greater with 0.01 and 0.02mM nitrate compared to that under sufficient nitrate conditions (SN, 2.5mM; Fig. 1C–E). A 22% increase in LR length and 20% decrease in LR density were observed in WT plants grown with 0.01 or 0.02mM nitrate compared with SN treatment. Based on the simplicity and precision of its measurement, LR density was chosen as an indicator of LR growth for the subsequent experiments. Compared with 2.5mM nitrate (Supplementary Fig. S2 available at JXB online), N deficiency increased seminal root length and decreased LR density regardless of N forms.
Nitrogen and phosphorus concentrations were examined in WT plants grown in the presence of varying concentrations of these nutrients (Supplementary Fig. S3 available at JXB online). A significant decrease in P concentration was observed at phosphate concentrations <10 μM and a significant decrease in N concentration was observed at nitrate concentrations <0.1mM (Supplementary Fig. S3A available at JXB online). Compared to SP and SN, low phosphate (LP, 2 μM) decreased P concentration in rice plants to the same extent as the decrease in N concentration by low nitrate (LN, 0.02mM) (Supplementary Fig. S3B available at JXB online).
Strigolactone evolution was enhanced in low-phosphate and low-nitrate media
To elucidate the role of SLs in the changes in root systems with low-phosphate and low-nitrate concentrations, SL exudates from WT roots were examined in WT plants. Compared with SP, the levels of 2′-epi-5-deoxystrigol, orobanchol, and orobanchyl acetate were increased by 63-, 33-, and 18-fold under LP conditions (Fig. 2A). Similarly, LN was associated with significantly enhanced levels of 2′-epi-5-deoxystrigol and orobanchyl acetate compared to SN. However, there was no difference in orobanchyl acetate content between LN and SN.
Fig. 2.
Levels of 2′-epi-5-deoxystrigol, orobanchol, and orobanchyl acetate exuded by wild-type rice plants (A) and qRT-PCR analysis of strigolactone-synthesis and -signalling genes (B) in wild-type rice plants. Seedlings were grown for 2 weeks in hydroponic media containing phosphate (LP, 2 μM; SP, 300 μM) and nitrate (LN, 0.02mM; SN, 2.5mM). Relative mRNA levels in B are normalized for individual gene relative to OsACT. Data are mean±SE. Different letters indicate significant differences (P<0.05, ANOVA).
The expression patterns of SL-synthesis and SL-signalling genes were analysed in WT plants. The relative levels of D10 and D17 expression were significantly enhanced by LP and LN compared with SP and SN, while the relative expression of D27 was enhanced only by LP (Fig. 2B). Interestingly, the expression levels of D14 were lower under LP and LN than under SP and SN, respectively. It was only with LP compared with SP that D3 expression was significantly lower. This effect may have been due to the higher SL levels in rice plants under LP and LN that downregulated the following signal gene as feedback, which is consistent with the previous report by Umehara et al. (2010).
The d10, d27, and d3 mutants were less sensitive than WT plants to low-phosphate and low-nitrate concentrations
In contrast to WT plants, the root architectures of d3, d10, and d27 were less responsive to LP and LN (Figs 3 and 4). For example, seminal root length was increased by 50% in WT plants and by approximately 18% in the three mutants when grown under LP (Fig. 3E). Seminal root length of WT plants grown in LN solution increased by 29%, while that of the mutants increased by approximately 14%. Similarly, LP and LN corresponded to reductions in LR density of 41 and 23%, respectively, in the WT (Fig. 4E). However, no differences in LR density were recorded in the three mutants under different nutrient conditions.
Fig. 3.
Effect of synthetic strigolactone analogue GR24 on seminal root length in wild type (WT, A), strigolactone-synthesis mutants d10 (B) and d27 (C), and the signalling mutant d3 (D). Rice seedlings were grown for 2 weeks in agar media containing varying concentrations of phosphate (LP, 2 μM; SP, 300 μM) and nitrate (LN, 0.02mM; SN, 2.5mM) with or without 2.5 μM GR24. (E) Seminal root length. Data are mean±SE. Different letters indicate significant differences (P<0.05, ANOVA) (this figure is available in colour at JXB online).
Fig. 4.
Effect of the-synthesis strigolactone analogue GR24 on lateral root (LR) density in wild type (WT, A), strigolactone-synthesis mutants d10 (B) and d27 (C), and the signalling mutant d3 (D). Rice seedlings were grown for 2 weeks in the agar media under phosphate (LP, 2 μM; SP, 300 μM) and nitrate (LN, 0.02mM; SN, 2.5mM) concentrations with or without 2.5 μM GR24. Bars, 4mm. (E) LR density. Data are mean±SE. Different letters in the same gene indicate significant differences (P<0.05, ANOVA).
Exogenous application of GR24 compensates for reduced response to low-phosphate and low-nitrate concentrations in mutants d10 and d27 but not in mutant d3
The effects of GR24 application to grown medium on root morphology were examined to assess whether the effects of LP and LN on rice root morphology were mediated by SLs. Rice plants grown under SP and SN in the presence of GR24 showed increased seminal root length and decreased LR density, starting from 1.25 μM GR24 (Supplementary Fig. S4 available at JXB online). Application of 2.5 μM GR24 had no effect on root morphology in WT plants under LP or LN conditions (Figs 3 and 4). However, application of GR24 in combination with SP and SN enhanced seminal root length and reduced LR density to the same extent as LP and LN. In d10 and d27 mutants, application of GR24 resulted in similar responses in seminal root length and LR density to those observed in WT plants under nutrient-deficient conditions. However, root morphology in the d3 mutant did not respond to GR24 application, indicating the involvement of the D3 gene family in the LP- and LN-regulating pathway.
Auxin reduces changes in root systems induced by low-phosphate and low-nitrate concentrations
A 66% increase in IAA concentration was observed in the first leaf of WT seedlings under LP compared to SP; however, IAA concentrations in the junction and root were 43 and 58% lower in the LP treatment group, respectively (Fig. 5A). Similar trends in IAA concentration in WT seedlings were observed for the LN and SN treatments (Fig. 5B). These results indicated that LP and LN downregulated polar auxin transport from shoots to roots. In contrast, similar IAA concentrations were found in the tissues of d10 and d27 mutants under low and sufficient N and P, and higher IAA concentrations were found in mutant roots under LP and LN relative to the WT, which probably led to the shorter seminal roots observed in mutants.
Fig. 5.
Auxin concentration in first leaf, junction, and root of wild type (WT) and strigolactone-synthesis mutants d10 and d27. Rice seedlings were grown for 2 weeks in hydroponic media containing phosphate (A; LP, 2 μM; SP, 300 μM) and nitrate (B; LN, 0.02mM; SN, 2.5mM) concentrations. Data are mean±SE. Different letters indicate significant differences (P<0.05, ANOVA).
Wild-type plants grown under LN in the presence of 1–1000nM NAA showed a decrease in seminal root length with increasing NAA except at 1nM, at which a slight increase in seminal root length was observed (Supplementary Fig. S5 available at JXB online). Application of 10nM NAA counteracted the effects of phosphate and nitrate deficiency on root morphology in WT rice seedlings (Fig. 6). However, root morphology of d10 and d27 mutants grown under LP and LN was less sensitive to application of NAA (Supplementary Fig. S5 available at JXB online), probably due to higher endogenous IAA concentrations in the roots.
Fig. 6.
Effect of exogenous NAA application on seminal root length and lateral root (LR) density in wild type (WT) and strigolactone-synthesis mutants d10 and d27. Seedlings were grown in hydroponic media containing phosphate (A: LP, 2 μM; SP, 300 μM) and nitrate (B: LN, 0.02mM; SN, 2.5mM) with or without 10nM NAA for 2 weeks. Data are mean±SE. Different letters indicate significant differences (P<0.05, ANOVA).
Application of exogenous GR24 significantly reduces auxin transport under sufficient phosphate and nitrate concentrations
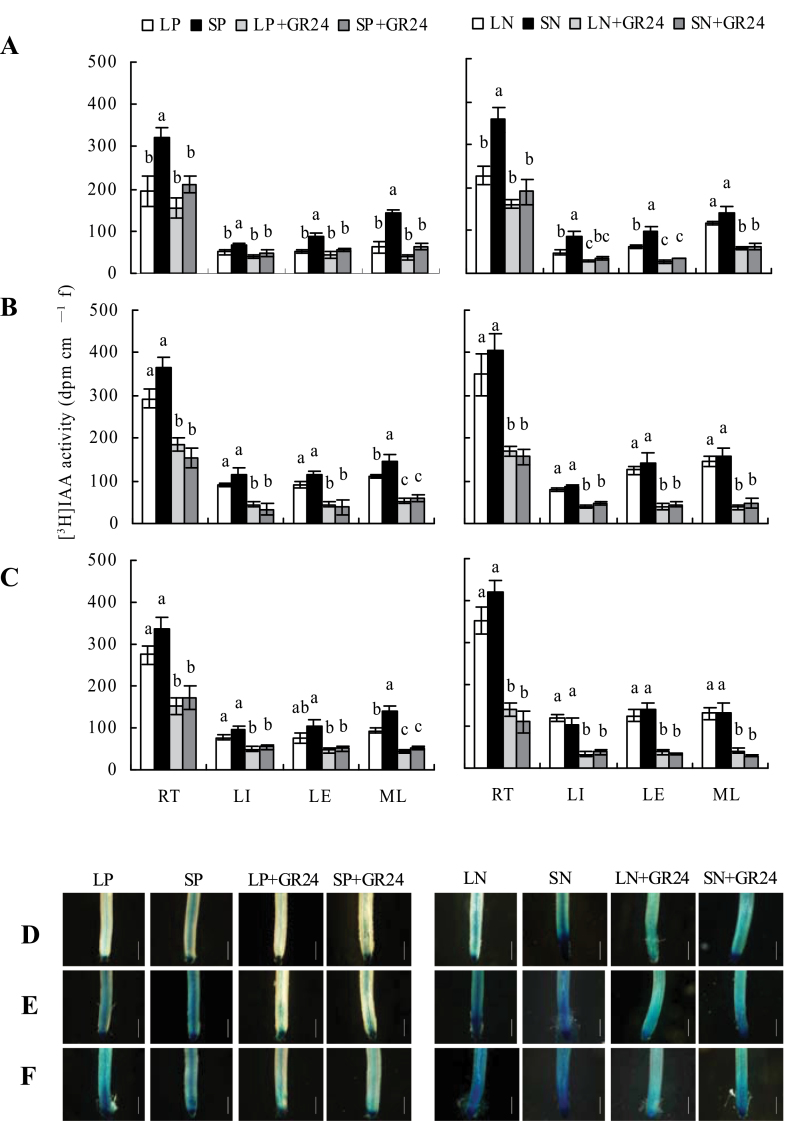
This study examined the effects of GR24 application on [3H]IAA transport (Fig. 7A–C). [3H]IAA transport from shoots to roots was significantly reduced under LP and LN in WT seedlings, which led to lower [3H]IAA activity in the four root zones. Application of GR24 to SP and SN treatments markedly reduced [3H]IAA transport in WT seedlings and in d10 and d27 mutants to the same extent as in WT seedlings under LP and LN.
Fig. 7.
[3H]IAA transport (A–C) and histochemical localization of DR5::GUS activity (2h at 37 °C) in root tips (D–F). Seedlings were grown for 2 weeks in hydroponic media containing phosphate (LP, 2 μM; SP, 300 μM) and nitrate (LN, 0.02mM; SN, 2.5mM) with or without 2.5 μM GR24. (A, D) Wild type; (B, E) strigolactone-synthesis mutant d10; (C, F) strigolactone-synthesis mutant d27. RT, root tip; LI, lateral root initiation and emergence zone; LE, lateral root elongation zone; ML, mature lateral root zone. Bars, 1mm. Data are mean±SE. Different letters in the same root zone indicate significant differences (P<0.05, ANOVA) (this figure is available in colour at JXB online).
The effects of GR24 on auxin status in rice were further investigated using transgenic plants expressing the DR5::GUS construct. In WT plants, DR5::GUS intensity was less widely distributed in root tips (Fig. 7D), 4 and 8cm from the root tip, junction, and stem (Supplementary Fig. S6 available at JXB online) under LP and LN conditions compared with SP and SN. No differences were recorded in mutants according to nutrient concentrations, and mutants showed higher DR5::GUS intensity than WT plants (Fig. 7D–F and Supplementary Fig. S6 available at JXB online), which was consistent with the results shown in Fig. 5. Application of GR24 under SP and SN significantly reduced IAA distribution in both WT and d10 and d27 mutants, and similar DR5::GUS intensity regulated by GR24 application was recorded in both WT and d10 and d27 mutants under respective nutrient-sufficient and -deficient conditions. These results further confirm that SLs induced by LP and LN downregulated polar auxin transport and consequently regulated root growth in rice.
qRT-PCR analysis showed that the expression levels of PIN1a-b, PIN5a, and PIN8–10a were significantly reduced under LP and LN compared to the respective nutrient-sufficient conditions (Fig. 8). Compared with respective nutrient-sufficient conditions, the levels of PIN1c and PIN10b expression were significantly reduced only in LP treatments, and PIN2 and PIN5b expression were significantly reduced only under LN. However, expression of PIN1d and PIN5c increased significantly under LP and expression of PIN1c-d increased significantly under LN. Application of GR24 under SP and SN markedly downregulated the levels of most PIN family genes to levels similar to those under LP and LN, with the exception of PIN5c and PIN8 under LP and PIN1c and PIN10b under LN. The expression of PIN1a-b, PIN5a, PIN9, and PIN10a was downregulated both by P and N deficiency and GR24 application.
Fig. 8.
qRT-PCR analysis of PIN family genes in wild-type rice seedlings. Rice seedlings were grown for 2 weeks in hydroponic media containing phosphate (A: LP, 2 μM; SP, 300 μM) and nitrate (B: LN, 0.02mM; SN, 2.5mM). Relative mRNA levels were normalized for individual genes relative to OsACT. Data are mean±SE. Different letters in the same gene indicate significant differences (P<0.05, ANOVA).
Discussion
Regulation of root growth in response to N and P deficiencies is essential for plants to optimize growth and productivity. Strigolactones have recently been defined as novel phytohormones that regulate root development (Kapulnik et al., 2011a; Ruyter-Spira et al., 2011; Mayzlish-Gati et al., 2012; Rasmussen et al., 2012). The present study provided evidence that the regulatory role played by SLs in rice root development under N- and P-limited conditions occurs via the D3 gene component of SL signalling and by modulation of auxin transport from shoots to roots. These findings suggest that regulation of root growth by SLs may be a strategy for adapting to conditions of P and N deficiency in rice plants.
Strigolactone exudation is enhanced by low-phosphate and low-nitrate concentrations
Unlike the universal promotion of SL production under conditions of P deficiency, increases in SLs under conditions of N deficiency rather than different N forms mostly occur in nonleguminous plants (Yoneyama et al., 2007, 2012). In this study, the levels of the three main SL fractions in rice were markedly higher under LP than under SP. LN conditions corresponded to significant increases in the levels of 2′-epi-5-deoxystrigol and orobanchyl acetate and the expression levels of D10 and D17 compared to SN conditions. These findings are consistent with the exudation of SLs enhanced by N deficiency in a susceptible rice cultivar reported by Jamil et al. (2011a). Jamil et al. (2011a, b) found that SL production in response to nutrient deficiency differed markedly among rice cultivars.
Exudation of SL in relation to availability of N appears to be part of the plant nutrient acquisition strategy. Yoneyama et al. (2012) reported N deficiency decreased shoot P concentration in four upland plant species, which resulted in increased SL exudation. Interestingly, in this study, LN corresponded to decreased N concentrations in WT plants, whereas LP corresponded to decreases in both N and P concentrations; the decrease in N concentration was similar in plants grown under LP and LN (Supplementary Fig. S3B available at JXB online), consistent with the results of Cai et al. (2012). The decrease in N concentration under LP probably resulted from the approximately 70% lower P concentration in LP treatment, which may have affected ATP synthesis and the electrochemical potential of plant cell membranes, thus decreasing N uptake by rice plants.
Rice root development is regulated by SLs via the D3 component of SL signalling
Several lines of evidence suggest that the SL pathway is involved in root growth under conditions of low-phosphate and low-nitrate concentrations. First, under conditions of phosphate and nitrate deficiency, elevated SL exudation paralleled the changes in root architecture in WT rice plants. For example, higher levels of SL exudation in WT seedlings grown under LP than under LN conditions corresponded to longer seminal roots and lower LR density under LP (Figs 1 and 2).
Second, in tomato and Arabidopsis, LR density was increased in SL mutants, suggesting that SLs affect LR formation (Koltai et al., 2010; Kapulnik et al., 2011b; Ruyter-Spira et al., 2011). In Arabidopsis, application of GR24 under SP conditions reduced LR formation by suppressing outgrowth of LRs, whereas application of GR24 under phosphate-limiting conditions induced LR formation (Kapulnik et al., 2011b; Ruyter-Spira et al., 2011). The density of LRs was affected by GR24 in WT seedlings and SL-synthesis mutants but not in the SL-signalling mutant, suggesting that the effect of SLs on LR density is mediated via the MAX2 F-box (Kapulnik et al., 2011b; Koltai, 2011; Ruyter-Spira et al., 2011). In contrast to findings in upland plants, LR density in rice did not differ among WT plants and d mutants in the present study (Fig. 4), consistent with the results of Arite et al. (2012). GR24 application under nutrient-sufficient conditions corresponded to reduced LR density, but the extent of the decrease did not change with increasing GR24 concentration (Supplementary Fig. S4 available at JXB online). Correspondingly, under nutrient-limiting conditions, application of GR24 did not lead to a further reduction in LR density, possibly because elevated SL levels under LN and LP may have been sufficient to affect LR initiation (Supplementary Fig. S4 available at JXB online). Moreover, application of GR24 caused recovery of the LR density phenotype induced by nutrient-limiting conditions in WT plants and d10 and d27 mutants but not in the d3 mutant, further suggesting that elevated SL levels under low-nutrient conditions may lead to a D3-dependent reduction in LR density.
Third, in Arabidopsis, the effects of GR24 application on primary root length were dependent on growth conditions, such as sugar availability. (Koltai, 2011). Ruyter-Spira et al. (2011) GR24 application led to a MAX2-dependent increase in primary root length only when sucrose was omitted from the medium. However, in Arabidopsis, the involvement of SLs in this primary root response may not be associated with P status (Koltai, 2011). Interestingly, in the present study, application of GR24 led to increased seminal root length. Moreover, application of GR24 to WT plants and d10 and d27 mutants, but not the d3 mutants under nutrient-sufficient conditions, led to complete recovery of the seminal root-length phenotype to that of WT plants under nutrient-limiting conditions, further confirming that elevation of SL levels under nutrient-limiting conditions can lead to a D3-dependent reduction in seminal root length.
Development of rice roots is regulated by SLs via reduction of auxin transport from shoots to roots
There is accumulating evidence that auxins act as signalling intermediates in the responses of root architecture to low phosphate supply (Niu et al., 2012). Polar auxin transport is essential for LR formation, but an auxin-transport-independent pathway is involved in changes in primary roots induced by phosphate stress in Arabidopsis (Williamson et al., 2001; Linkohr et al., 2002; López-Bucio et al., 2003; Ticconi et al., 2004; Jain et al., 2009). Studies of Arabidopsis have provided insight into the crosstalk between auxin and the root growth response to P deficiency (Pérez-Torres et al., 2008; Chiou and Lin, 2011). However, the mechanisms linking crosstalk between auxin and the response to N deficiency are poorly understood. In this study, IAA concentration was reduced under nutrient-limiting conditions in the junction and roots of WT plants, indicating that nutrient starvation prevented auxin transport from shoots to roots. In addition, application of NAA to WT plants under low-P and low-N conditions resulted in a phenotype that mimicked the root system architecture of WT plants under nutrient-sufficient conditions, indicating that auxin participated in regulation of root growth under nutrient-deficient conditions.
Strigolactones have been suggested to act as either modulators of auxin transport or as second messengers of auxin and to regulate shoot growth (Brewer et al., 2009; Crawford et al., 2010). In Arabidopsis, Ruyter-Spira et al. (2011) suggested that strigolactones are able to modulate local auxin levels and that the net result of strigolactone action is dependent on the auxin status of the plant. In present study, experiments with [3H]IAA transport and DR5::GUS activity further confirmed that application of GR24 markedly reduced auxin transport to levels equivalent to those under N- and P-deficient conditions, which in turn reduced the densities of LR and increased seminal root length.
Polar auxin transport is mediated primarily by PIN genes. Application of GR24 decreased the PIN1, PIN3, and PIN7-GFP gene expression levels in the primary root tip of Arabidopsis; however, PIN levels were unaffected when similar levels of GR24 were applied in the presence of exogenous auxin (Ruyter-Spira et al., 2011). In the current study, which examined expression of 12 PIN family genes in rice roots, the expression of PIN1a-b, PIN5a, PIN9, and PIN10a was downregulated both by P- and N-deficiency and by GR24 application, indicating that they involved auxin transport from shoots to roots downregulated by SLs under nutrient deficiency.
In conclusion, elevated exudation of strigolactones was observed in WT plants under phosphate- and nitrate-limiting conditions relative to those under conditions of sufficient respective nutrient availability. Strigolactones regulated the development of rice roots via the D3 component of SL signalling and by modulating transport of auxin from shoots to roots.
Supplementary material
Supplementary data are available at JXB online.
Supplementary Table S1. Primers for qRT-PCR of D genes.
Supplementary Table S2. Primers for qRT-PCR of PIN genes.
Supplementary Fig S1. Summary of SL biosynthetic and signalling pathways.
Supplementary Fig S2. Root morphology in response to different N forms.
Supplementary Fig S3. Effect of phosphate and nitrate availability on phosphorus and nitrogen concentrations in wild-type plants.
Supplementary Fig S4. Root architecture in response to GR24 application.
Supplementary Fig S5. Root architecture in response to differing NAA supplies.
Supplementary Fig S6. DR5::GUS activity in stem, junction, and root.
Acknowledgements
The SL mutants were kindly provided by Shinjiro Yamaguchi of RIKEN Plant Science Center. This work was funded by the Ministry of Science and Technology of China (2011CB100302), the National Nature Science Foundation of China (31071846 and 31172022), the State Key Laboratory of Soil and Sustainable Agriculture, Institute of Soil Science (Y052010013), Innovative Plan of JiangSu Province of China (CXLX13_280), the UU-COE Research Project from Utsunomiya University, the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD), the Innovative Research Team Development Plan of the Ministry of Education of China (IRT1256), and the China Scholarship Council.
References
- Arite T, Kameoka H, Kyozuka J. 2012. Strigolactone positively controls crown root elongation in rice. Journal of Plant Growth Regulation 31, 165–172. [Google Scholar]
- Brewer PB, Dun EA, Ferguson BJ, Rameau C, Beveridge CA. 2009. Strigolactone acts downstream of auxin to regulate bud outgrowth in pea and Arabidopsis . Plant Physiology 150, 482–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai J, Chen L, Qu H, Lian J, Liu W, Hu Y, Xu G. 2012. Alteration of nutrient allocation and transporter genes expression in rice under N, P, K, and Mg deficiencies. Acta Physiologiae Plantarum 34, 939–946. [Google Scholar]
- Chen Y, Fan X, Song W, Zhang Y, Xu G. 2012. Over-expression of OsPIN2 leads to increased tiller numbers, angle and shorter plant height through suppression of OsLAZY1 . Plant Biotechnology Journal 10, 139–149. [DOI] [PubMed] [Google Scholar]
- Chevalier F, Pata M, Nacry P, Doumas P, Rossignol M. 2003. Effects of phosphate availability on the root system architecture: large-scale analysis of the natural variation between Arabidopsis accessions. Plant, Cell and Environment 26, 1839–1850. [Google Scholar]
- Chiou TJ, Lin SI. 2011. Signaling network in sensing phosphate availability in plants. Annual Review of Plant Biology 62, 185–206. [DOI] [PubMed] [Google Scholar]
- Chun L, Mi G, Li J, Chen F, Zhang F. 2005. Genetic analysis of maize root characteristics in response to low nitrogen stress. Plant and Soil 276, 369–82. [Google Scholar]
- Crawford S, Shinohara N, Sieberer T, Williamson L, George G, Hepworth J, Müller D, Domagalska MA, Leyse O. 2010. Strigolactones enhance competition between shoot branches by dampening auxin transport. Development 137, 2905–2913. [DOI] [PubMed] [Google Scholar]
- Forde BG. 2002. The role of long-distance signalling in plant responses to nitrate and other nutrients. Journal of Experimental Botany 53, 39–43. [PubMed] [Google Scholar]
- Forde B, Lorenzo H. 2001. The nutritional control of root development. Plant and Soil 232, 51–68. [Google Scholar]
- Gruber BD, Giehl RFH, Friedel S, Wirén N. 2013. Plasticity of the Arabidopsis root system under nutrient deficiencies. Plant Physiology 163, 161–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain A, Poling MD, Smith AP, Nagarajan VK, Lahner B, Meagher RB, Raghothama KG. 2009. Variations in the composition of gelling agents affect morphophysiological and molecular responses to deficiencies of phosphate and other nutrients. Plant Physiology 150, 1033–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamil M, Charnikhova T, Cardoso C, Jamil T, Ueno K, Verstappen F, Asami T, Bouwmeester HJ. 2011a. Quantification of the relationship between strigolactones and Striga hermonthica infection in rice under varying levels of nitrogen and phosphorus. Weed Research 51, 373–385. [Google Scholar]
- Jamil M, Rodenburg J, Charnikhova T, Bouwmeester HJ. 2011b. Pre-attachment Striga hermonthica resistance of New Rice for Africa (NERICA) cultivars based on low strigolactone production. New Phytologist 192, 964–975. [DOI] [PubMed] [Google Scholar]
- Jiang L, Liu X, Xiong G, et al. 2013. DWARF 53 acts as a repressor of strigolactone signalling in rice. Nature 504, 401–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapulnik Y, Delaux PM, Resnick N, et al. 2011b. Strigolactones affect lateral root formation and root-hair elongation in Arabidopsis . Planta 233, 209–216. [DOI] [PubMed] [Google Scholar]
- Kapulnik Y, Resnick N, Mayzlish-Gati E, Kaplan Y, Wininger S, Hershenhorn J, Koltai H. 2011a. Strigolactones interact with ethylene and auxin in regulating root-hair elongation in Arabidopsis . Journal of Experimental Botany 62, 2915–2924. [DOI] [PubMed] [Google Scholar]
- Koltai H. 2011. Strigolactones are regulators of root development. New Phytologist 190, 545–549. [DOI] [PubMed] [Google Scholar]
- Koltai H, Dor E, Hershenhorn J, et al. 2010. Strigolactones’ effect on root growth and root-hair elongation may be mediated by auxin-efflux carriers. Journal of Plant Growth Regulation 29, 129–136. [Google Scholar]
- Linkohr BI, Williamson LC, Fitter AH, Leyser HMO. 2002. Nitrate and phosphate availability and distribution have different effects on root system architecture of Arabidopsis . The Plant Journal 29, 751–760. [DOI] [PubMed] [Google Scholar]
- López-Bucio J, Cruz-Ramírez A, Herrera-Estrella L. 2003. The role of nutrient availability in regulating root architecture. Current Opinion in Plant Biology 6, 280–287. [DOI] [PubMed] [Google Scholar]
- Mayzlish-Gati E, De-Cuyper C, Goormachtig S, et al. 2012. Strigolactones are involved in root response to low phosphate conditions in Arabidopsis . Plant Physiology 160, 1329–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu YF, Chai RS, Jin GL, Wang H, Tang CX, Zhang YS. 2012. Responses of root architecture development to low phosphorus availability: a review. Annals of Botany 112, 391–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Torres CA, López-Bucio J, Cruz-Ramírez A, Ibarra-Laclette E, Dharmasiri S, Estelle M, Herrera-Estrella L. 2008. Phosphate availability alters lateral root development in Arabidopsis by modulating auxin sensitivity via a mechanism involving the TIR1 auxin receptor. The Plant Cell 20, 3258–3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen A, Mason MG, De Cuyper C, et al. 2012. Strigolactones suppress adventitious rooting in Arabidopsis and pea. Plant Physiology 158, 1976–1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruyter-Spira C, Kohlen W, Charnikhova T, et al. 2011. Physiological effects of the synthetic strigolactone analog GR24 on root system architecture in Arabidopsis: another belowground role for strigolactones? Plant Physiology 155, 721–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen C, Wang S, Zhang S, Xu Y, Qian Q, Qi Y, Jiang DA. 2013. OsARF16, a transcription factor, is required for auxin and phosphate starvation response in rice (Oryza sativa L.). Plant, Cell and Environment 36, 607–620. [DOI] [PubMed] [Google Scholar]
- Song W, Sun H, Li J, Gong X, Huang S, Zhu X, Zhang Y, Xu G. 2013. Auxin distribution is differentially affected by nitrate in roots of two rice cultivars differing in responsiveness to nitrogen nutrients. Annals of Botany 112, 1383–1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian Q, Chen F, Liu J, Zhang F, Mi G. 2008. Inhibition of maize root growth by high nitrate supply is correlated to reduced IAA levels in roots. Journal of Plant Physiology 165, 942–951. [DOI] [PubMed] [Google Scholar]
- Ticconi CA, Delatorre CA, Lahner B, Salt DE, Abel S. 2004. Arabidopsis pdr2 reveals a phosphate-sensitive checkpoint in root development. The Plant Journal 37, 801–814. [DOI] [PubMed] [Google Scholar]
- Umehara M, Hanada A, Magome H, Takeda-Kamiya N, Yamaguchi S. 2010. Contribution of strigolactones to the inhibition of tiller bud outgrowth under phosphate deficiency in rice. Plant Cell Physiology 51, 1118–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters MT, Brewer PB, Bussell JD, Smith SM, Beveridge CA. 2012. The Arabidopsis ortholog of rice DWARF27 acts upstream of MAX1 in the control of plant development by strigolactones. Plant Physiology 159, 1073–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson LC, Ribrioux SPCP, Fitter AH, Leyser HMO. 2001. Phosphate availability regulates root system architecture in Arabidopsis . Plant Physiology 126, 875–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie X, Yoneyama K, Yoneyama K. 2010. The strigolactone story. Annual Review of Phytopathology 48, 93–117. [DOI] [PubMed] [Google Scholar]
- Xie X, Yoneyama K, Kisugia T, Uchidab K, Itoc S, Akiyamac K, Hayashic H, Yokotab T, Nomura T, Yoneyama K. 2013. Confirming stereochemical structures of strigolactones produced by rice and tobacco. Molecular Plant 6, 153–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi K, Wu Z, Zhou J, Du L, Guo L, Wu Y, Wu P. 2005. OsPTF1, a novel transcription factor involved in tolerance to phosphate starvation in rice. Plant Physiology 138, 2087–2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneyama K, Xie X, Kim H, Kisugi T, Nomura T, Sekimoto H, Yokota T, Yoneyama K. 2012. How do nitrogen and phosphorus deficiencies affect strigolactone production and exudation? Planta 235, 1197–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneyama K, Yoneyama K, Takeuchi Y, Sekimoto H. 2007. Phosphorus deficiency in red clover promotes exudation of orobanchol, the signal for mycorrhizal symbionts and germination stimulant for root parasites. Planta 225, 1031–1038. [DOI] [PubMed] [Google Scholar]
- Zhang J, Xu L, Wang F, Deng M, Yi K. 2012. Modulating the root elongation by phosphate/nitrogen starvation in an OsGLU3 dependant way in rice. Plant Signaling and Behavior 7, 1144–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Forde BG. 1998. An Arabidopsis MADS box gene that controls nutrient-induced changes in root architecture. Science 279, 407–409. [DOI] [PubMed] [Google Scholar]
- Zhou F, Lin Q, Zhu L, et al. 2013. D14–SCFD3-dependent degradation of D53 regulates strigolactone signaling. Nature 504, 406–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.