Summary
Simultaneous mutation of two peroxisomal thiolase enzymes shows that fatty acid β-oxidation is required for the normal development of inflorescences in Arabidopsis and for successful fertilization to produce seed.
Key words: 3-Ketoacyl-CoA thiolase, Arabidopsis thaliana, β-oxidation, flowering, germination, peroxisome, seed development.
Abstract
A specific function for peroxisomal β-oxidation in inflorescence development in Arabidopsis thaliana is suggested by the mutation of the ABNORMAL INFLORESCENCE MERISTEM 1 gene, which encodes one of two peroxisomal multifunctional proteins. Therefore, it should be possible to identify other β-oxidation mutants that recapitulate the aim1 phenotype. Three genes encode peroxisomal 3-ketoacyl-CoA thiolase (KAT) in Arabidopsis. KAT2 and KAT5 are present throughout angiosperms whereas KAT1 is a Brassicaceae-specific duplication of KAT2 expressed at low levels in Arabidopsis. KAT2 plays a dominant role in all known aspects of peroxisomal β-oxidation, including that of fatty acids, pro-auxins, jasmonate precursor oxophytodienoic acid, and trans-cinnamic acid. The functions of KAT1 and KAT5 are unknown. Since KAT5 is conserved throughout vascular plants and expressed strongly in flowers, kat2 kat5 double mutants were generated. These were slow growing, had abnormally branched inflorescences, and ectopic organ growth. They made viable pollen, but produced no seed indicating that infertility was due to defective gynaecium function. These phenotypes are strikingly similar to those of aim1. KAT5 in the Brassicaceae encodes both cytosolic and peroxisomal proteins and kat2 kat5 defects could be complemented by the re-introduction of peroxisomal (but not cytosolic) KAT5. It is concluded that peroxisomal KAT2 and KAT5 have partially redundant functions and operate downstream of AIM1 to provide β-oxidation functions essential for inflorescence development and fertility.
Introduction
Peroxisomes of higher plants display plasticity of function depending on plant growth stage and tissue localization. They play a major role in photorespiration in photosynthetic tissues while, in germinating seeds, these organelles mediate the catabolism of fatty acids from storage lipid to fuel post-germinative growth (Hu et al., 2012; Bussell et al., 2013), This catabolism is achieved by the β-oxidation pathway, which oxidizes activated fatty acids (acyl-CoA) to acetyl-CoA that may, in turn, enter the TCA cycle, the glyoxylate cycle, and gluconeogenesis (Graham, 2008). Arabidopsis mutants of many core β-oxidation proteins are unable to metabolize seed storage lipids and require an exogenous carbon source for seedling establishment. In addition, the plant hormones jasmonic acid (JA) and indole-acetic acid (IAA) are synthesized or matured by β-oxidation. An endogenous precursor to IAA, indole-butyric acid (IBA) and the synthetic auxin 2,4-dichlorobutyric acid (2,4-DB) undergo one cycle of β-oxidation to produce the biologically active auxins IAA and 2,4-dichlorophenoxy-acetic acid (2,4-D) (Baker et al., 2006). Genetic screens that have revealed β-oxidation mutants (Hayashi et al., 1998; Zolman et al., 2000; Eastmond, 2006) have used the sucrose dependence of seedling establishment or the resistance of seedlings to auxin precursors, the latter as assessed by root elongation of mutants on media containing the pro-auxins. Thus, the most readily observable phenotypes of plant β-oxidation mutants are obtained at the seedling stage of the life cycle. By contrast, despite significant impediments to seedling establishment, such β-oxidation mutants usually appear quite normal as mature plants.
In plants, import of all known β-oxidation substrates requires the ABC transporter COMATOSE (CTS), also known as PXA1, PED3, ACN2, and AtABCD1 (reviewed by Verrier et al., 2008). CoA-activated fatty acids are imported by CTS concomitant with CoA cleavage (De Marcos Lousa et al., 2013). Although it remains unknown whether this cleavage occurs on the matrix- or cytosolic side of peroxisomes, fatty acids must be re-activated by Long Chain Acyl-CoA Synthetase (LACS) proteins in peroxisomes in order to enter the β-oxidation cycle (Fulda et al., 2004). β-oxidation then proceeds via four different enzyme activities: (i) acyl-CoA oxidase (ACX), which oxidizes acyl-CoA to 2E-enoyl-CoA; (ii) an enoyl-CoA hydratase which oxidizes the 2E-enoyl-CoA to 3-hydroxyacyl-CoA, (iii) a 3-hydroxyacyl-CoA dehydrogenase producing 3-oxoacyl-CoA; (iv) 3-ketoacyl-CoA thiolase (KAT) which cleaves an acetyl-CoA molecule from 3-oxoacyl-CoA, leaving the acyl-CoA chain two carbons shorter (Graham, 2008).
Enzymes encoded by small multigene families catalyse each of these steps. In Arabidopsis thaliana the ACX family consists of six members that vary in substrate specificity according to acyl-CoA chain-length (Adham et al., 2005). In plants, activities (ii) and (iii) are typically carried out by a single-polypeptide multifunctional enzyme, of which there are two in A. thaliana named abnormal inflorescence meristem 1 (AIM1) and multifunctional protein 2 (MFP2) (Richmond and Bleecker, 1999; Rylott et al., 2006). In addition, there are a number of single function hydratases and dehydrogenases that may act on specific substrates, such as auxin precursors (Zolman et al., 2007, 2008; Wiszniewski et al., 2009; Strader et al., 2011). Finally, peroxisomal KAT is encoded by three genes in A. thaliana that are known as KAT1 (At1g04710), KAT2 (At2g33150), and KAT5 (At5g48880) (Germain et al., 2001).
Arabidopsis KAT2 has well-characterized roles throughout plant development. Mutant kat2 seedlings (also known as ped1) do not establish without an exogenous source of sugar and they are resistant to both IBA and 2,4-DB (Hayashi et al., 1998; Germain et al., 2001; Wiszniewski et al., 2009). Three cycles of β-oxidation are required for production of JA from 12-oxo-phytodienoic acid (OPDA), and KAT2 has been shown to contribute to JA production induced by wounding (Cruz Castillo et al., 2004; Afitlhile et al., 2005) and natural senescence (Castillo and Leon, 2008). KAT2 has recently been shown also to contribute significantly to peroxisomal benzoic acid (BA) synthesis (Bussell et al., 2014). By contrast, little is known about the functions of KAT1 and KAT5, although KAT5 does influence the composition of benzoylated metabolites in seeds (Bussell et al., 2014). Phylogenetic analysis of KAT proteins obtained from sequenced plant genomes showed that KAT2-like and KAT5-like proteins have been conserved in essentially all seed plant lineages, while the KAT1 gene is a duplication of KAT2 that is present only in the Brassicaceae family and expressed very weakly in Arabidopsis (Wiszniewski et al., 2012). The KAT5 gene produces two distinct transcripts that encode cytosolic and peroxisomal proteins (Carrie et al., 2007), but this trait also appears to be specific to species in the Brassicaceae family (Wiszniewski et al., 2012).
Amongst Arabidopsis β-oxidation mutants aim1 knockouts are unusual in expressing a strong phenotype in mature plants. Thus, as well as producing seedling roots resistant to pro-auxin, aim1 mutants have strongly altered leaf and inflorescence development resulting in highly reduced fecundity (Richmond and Bleecker, 1999). CTS and KAT2 have also been shown to be required for full fertility (Footitt et al., 2007a, b), but the mature plants lacking these genes do not exhibit any obvious morphological defects. KAT2 and KAT5 genes are expressed strongly in flowers and siliques indicating that thiolases may be functionally important in reproductive tissues (Kamada et al., 2003; Wiszniewski et al., 2012). KAT2 is co-expressed with genes of β-oxidation, but KAT5 unexpectedly is co-expressed (Carrie et al., 2007) and co-regulated (Stracke et al., 2007, 2010) with genes of flavonoid biosynthesis.
The dual targeting of KAT5 to peroxisomes and cytosol, the co-expression (and co-regulation) of the gene with those of flavonoid biosynthesis, and the apparent importance of β-oxidation in reproductive tissue suggest undiscovered functions for β-oxidation. The aim of the present work was to investigate KAT5 function, particularly in relation to reproduction and seed germination and to reveal that its function is partially redundant with that of KAT2 in inflorescence development and fertility.
Materials and methods
Growth conditions
Surface-sterilized seeds were scattered on 0.5× MS growth media supplemented with 1% (w/v) sucrose, hormones or selective herbicide as required. Seeds were imbibed and stratified for 48h at 4 °C, and grown under continuous light (~100 µE m–2 s–1) at 20 °C. Seedlings grown for approximately 10–15 d on 0.5× MS growth media were transferred to soil in pots. The soil mix was 2:1 v/v shamrock peat:vermiculite. Rooms were maintained at 22 °C and 60% relative humidity.
KAT mutants
The mutants used in this study are detailed in Table 1. These were obtained from FLAG (Samson et al., 2004) and CSHL (Sundaresan et al., 1995) T-DNA collections. kat2-1 (Ws-4) was first described in Germain et al. (2001), and kat1 (Ws-4) and kat5-1 (Ler) in Wiszniewski et al. (2009). kat2-5 (Ws-4, FLAG_307C02) and kat5-2 (Ws-4, FLAG_065D06) are new alleles described here. The T-DNA Express primer design server (http://signal.salk.edu/tdnaprimers.2.html) was used for primer design for the newly described lines. RT-PCR primers were designed to flank the insertion sites and used to screen each line for the absence of a full-length transcript as an indicator that they were transcript knockouts. Primer sequences are given in Supplementary Table S1 at JXB online. kat2 and kat5 mutants were crossed and the F2 generation segregated to make double mutants. As kat2 kat5 double mutants were infertile, they were maintained as sesquimutants (homozygous for one mutation and heterozygous for the other). kat2-1/kat2-1 kat5-2/KAT5 or kat2-1/KAT2 kat5-2/kat5-2 were analysed for segregation of kat2-1 and kat5-2 alleles. A Chi square test was used to test deviation from the expected segregation ratio (3:1) at each heterozygous locus.
Table 1.
Thiolase mutant lines used in this study
| Gene | Line | Allele | Ecotype | Reference |
|---|---|---|---|---|
| KAT1 (At1g04710) | FLAG_589G05 | kat1-1 | Ws-4 | Wiszniewski et al. (2009) |
| KAT2 (At2g33150) | T-DNA | kat2-1 | Ws-4 | Germain et al. (2001) |
| FLAG_307C02 | kat2-5 | Ws-4 | This study | |
| KAT5 (At5g48880) | CSHL_ET5406 | kat5-1 | Ler | Wiszniewski et al. (2009) |
| FLAG_065D06 | kat5-2 | Ws-4 | This study |
Seed and seedling phenotypic characterization
Hypocotyl elongation and germination were assayed to test for sucrose-dependence, and response to 2,4-DB was determined as described in Wiszniewski et al. (2009). Germination frequency was assayed using seed that had been after-ripened for at least 8 weeks. 250–300 seeds were scattered on water-agar (0.8% w/v) media and immediately placed under illumination with no stratification. Seeds were scored for germination every 24h and the experiment was replicated four times.
Fatty acid analysis
Seed weight and fatty acid composition were determined using seed harvested from soil-grown plants under long-day conditions. 500–600 seeds from individual plants were counted and weighed. For analysis of fatty acid contents of dry seeds, counted and weighed pools of seed (approximately 10mg) were extracted and measured by GC-MS as described in Wiszniewski et al. (2009). A similar protocol was used for analysis of fatty acids in germinating kat5-2 seedlings except that 50 seedlings were used for analysis.
Pollen viability
Pollen viability was assessed using the method of Alexander (1969). Pollen was spread on microscope slides and immersed in a drop of stain solution [9.5% (v/v) ethanol, 25% (v/v) glycerol, 2% (v/v) glacial acetic acid, 5% (w/v) phenol, 0.05% (w/v) malachite green, 0.05% (w/v) fuchsin acid, 0.005% (w/v) orange G]. Slides were briefly flamed (without boiling) prior to microscopy.
Flavonoid staining
Seeds were imbibed for 24h in water, then stained with 0.25% (w/v) diphenylboric acid 2-aminoethyl ester (DPBA) for 15min. The seed coat was removed, and embryos were viewed using an Olympus BX61 epifluorescence microscope with a FITC filter. tt4-1 seeds (Shirley et al., 1995) were used as a flavonoid-deficient control.
Plasmid construction and mutant complementation
KAT5.1 and KAT5.2 cDNAs, corresponding respectively to cytosolic (KAT5cyt) and peroxisomal (KAT5px) isoforms of the encoded protein, were isolated by RT-PCR (Biorad iScript select) from mature rosette leaf RNA, and ligated into the pCR2.1-TOPO vector (Invitrogen). Cloning primer sequences are given in Supplementary Table S1 at JXB online. Following sequencing to confirm error-free DNA replication, KAT5.1 and KAT5.2 cDNAs were cloned into pGREEN0180A, a vector based on pGREEN0179 (Hellens et al., 2000), but which had been modified for 35S over-expression and Gateway cloning (Wiszniewski et al., 2009). Agrobacterium tumefaciens strain GV3130 was used for A. thaliana floral dip (Clough and Bent, 1998) to introduce the constructs into kat sesquimutants. Primary transformants were selected using hygromycin resistance of the transgene, and PCR genotyping for the segregating thiolase locus.
Western blots
Western blotting was done essentially according to Germain et al. (2001). Twenty micrograms of soluble protein extracted from 7-d-old seedlings was separated on 12% pre-cast acrylamide gels (Bio-Rad Miniprotean TGX Cat #456–1043) and transferred to Hybond ECL membrane using a Bio-Rad mini-transfer cell. Blots were probed with 1/1000 dilution of KAT2 primary antibody (Germain et al., 2001). The 2° antibody was HRP-conjugated goat anti-rabbit (1/1000; Life Technologies, G21234), which was detected using Bio-Rad Clarity Western ECL substrate (Cat #170–5060). For a loading control, blots were re-probed with a 1/2000 dilution of α-tubulin (Sigma T-5168), for which the 2° antibody was a 1/5000 dilution of alkaline phosphatase goat anti-mouse (Sigma A-2179) and detection used Immune-Star AP (Bio-Rad #170–5018). Chemiluminescence was visualized using an ImageQuant RT ECL Imager.
Results
kat1 and kat5 mutants grow and develop normally
Knockout mutants in the Ws-4 background were available for each of the KAT genes, so the available kat mutants in this ecotype (Table 1) were obtained including new mutant alleles of KAT2 and KAT5 (kat2-5 and kat5-2, respectively), which were determined to be knockouts by the absence of transcripts (see Supplementary Fig. S1 at JXB online). Since kat5-2 is the only mutant in the Ws-4 background, for some experiments where it was desirable to include a second allele of kat5, the previously described kat5-1 (Ler) was used.
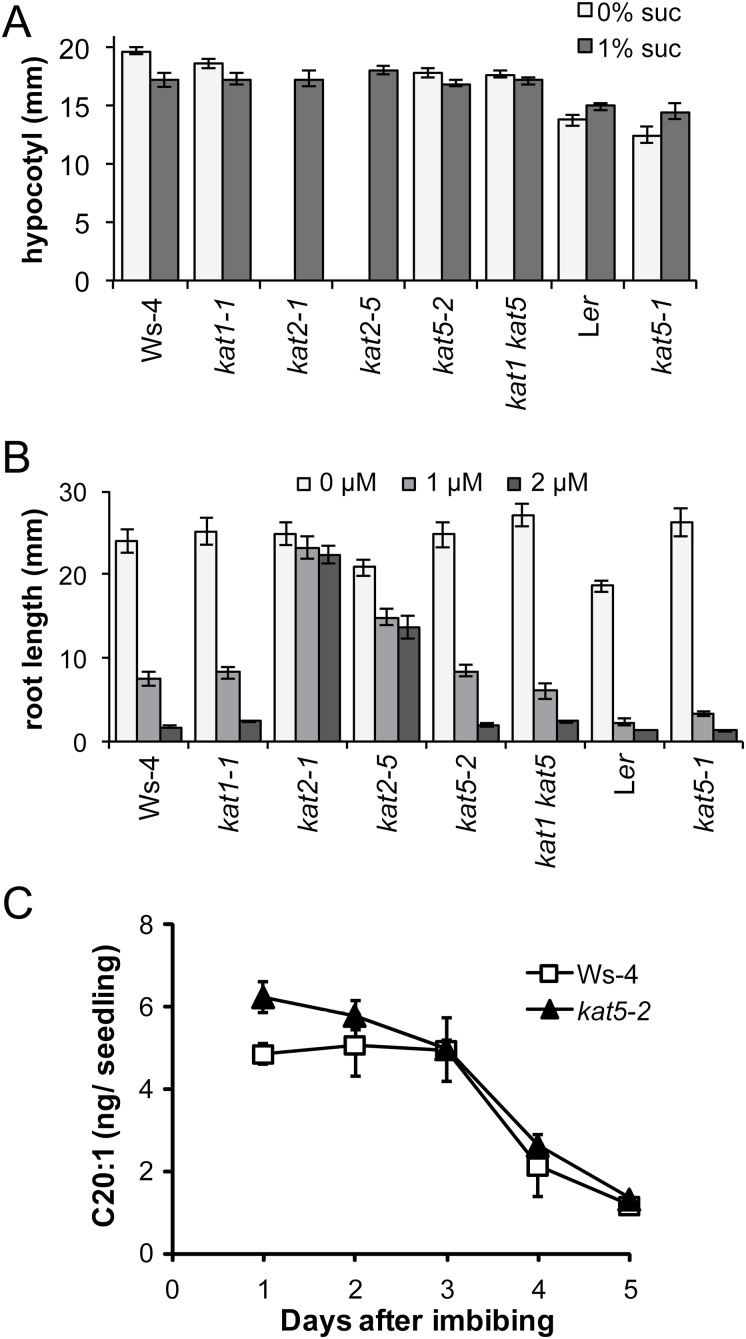
Like other kat2 mutants (Hayashi et al., 1998; Germain et al., 2001; Wiszniewski et al., 2009), kat2-5 was dependent on exogenous sugar supply for seedling establishment, was resistant to 2,4-DB (Fig. 1A, B), and exhibited reduced germination capacity (Fig. 2). For these experiments seeds had been after-ripened for 8 weeks and stored at –20 °C to maintain high viability, which overcomes the need to nick the seed coat as reported in some studies (Pinfield-Wells et al., 2005). kat1-1, kat5-2, and the double mutant kat1-1 kat5-2 seedlings were indistinguishable from the wild type (Fig. 1A, B). Fatty acid catabolism during the early growth of kat2-1 seedlings is retarded (Germain et al., 2001; Wiszniewski et al., 2009), but appears to proceed normally in kat5-2 (Fig. 1C). Thus, as expected, given the severity of kat2 phenotypes, KAT1 and KAT5 do not apparently play a significant role in oil catabolism or the processing of pro-auxins during seedling establishment.
Fig. 1.
β-oxidation phenotypes of kat mutants. (A) Length of etiolated hypocotyls. Plants were grown in the dark for 4 d on 0.5× MS media supplemented with or without 1% (w/v) sucrose (n ≥14). (B) Inhibition of root growth in response to varying concentrations of 2,4-DB of thiolase mutants after 8 d growth (n ≥13). (C) Degradation of the TAG fatty acid marker C20:1 in Ws-4 and kat5-2 from 2–5 d post-stratification. Seeds were germinated on plates and 50 seedlings were collected at 24h intervals. In each panel, values represent mean ±SE (n=4).
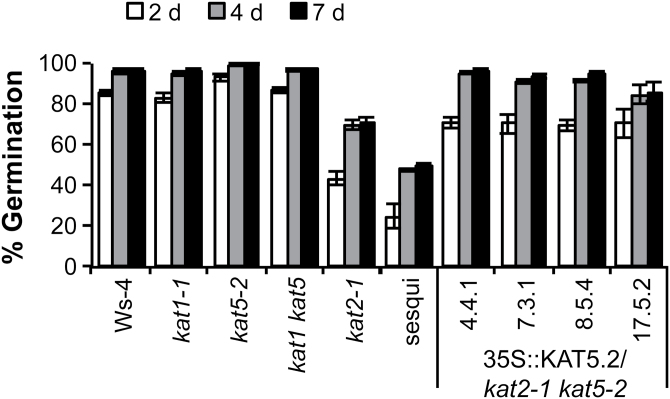
Fig. 2.
Analysis of germination of kat mutants. Germination frequency of seeds of kat mutants, a kat2 kat5 sesquimutant (kat2-1/kat2-1 kat5-2/KAT5), and a kat2-1 kat5-2 double homozygous mutant complemented with 35S::KAT5px (peroxisome-targeted KAT5). Approximately 200 after-ripened, age-matched seeds were scored daily for cumulative % germination on water-agar media. Values are mean ±SE (n=4). The kat2 kat5 double mutant can only be propagated with at least one wild-type KAT allele, and can only be complemented by peroxisome-targeted KAT (see text).
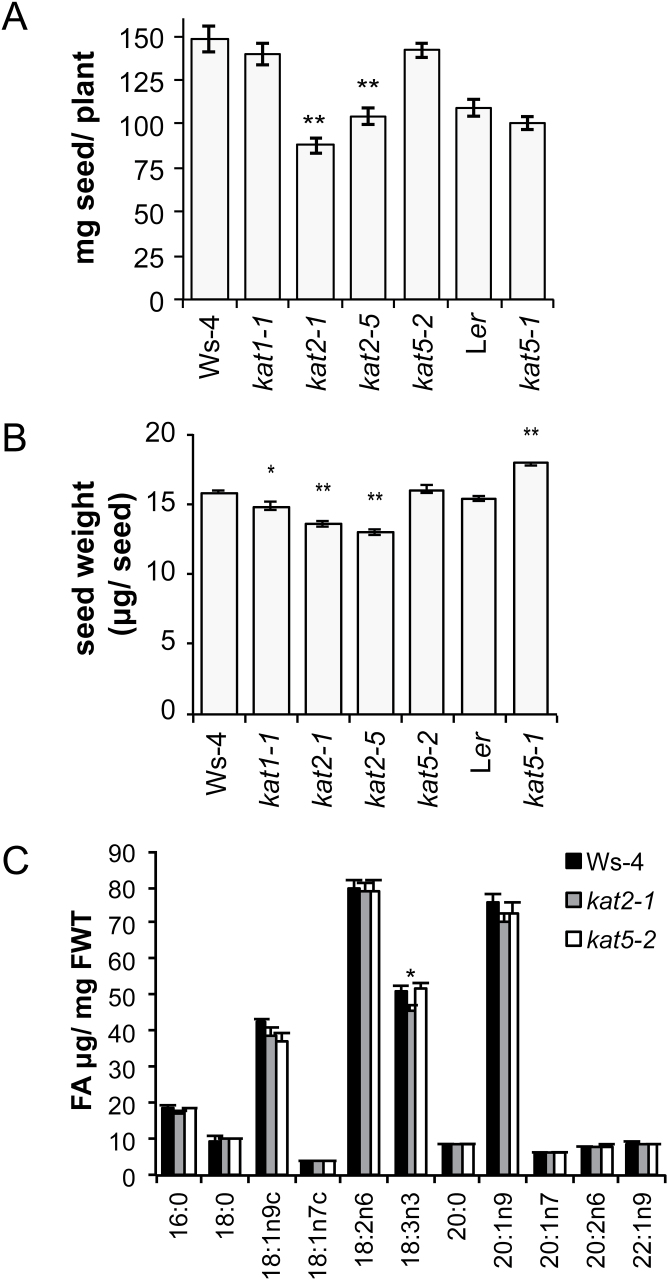
After seedling establishment, single mutants kat1-1, kat2-1, kat2-5, kat5-1, and kat5-2 appeared quite similar to wild-type plants (see Supplementary Fig. S2 at JXB online). Since KAT2 and KAT5 are highly expressed in developing seeds (Wiszniewski et al., 2012) and KAT2 is required for full fertility in A. thaliana (Footitt et al., 2007a) the effect of kat mutations on seed production was investigated. As reported previously (Footitt et al., 2007a), kat2 mutants produced lower seed yield per plant and the seeds were individually of lower weight than wild type (Fig. 3). For kat1-1, the total seed yield per plant was unaltered but individual seeds weighed slightly less than those of the wild type (Fig. 3A, B). Neither of the kat5 knockout mutants was altered in total seed mass produced per plant compared with their respective wild types, but the mass of individual kat5-1 seeds was greater than for Ler wild-type seeds (Fig. 3A, B). The seed oil content of kat2-1 and kat5-2 was quite similar (on a fresh-weight basis) to the wild type for a range of fatty-acid species, the main exception being C18:3n3 (linolenic acid) which was significantly lower in kat2-1 relative to the wild-type (P <0.05; Fig. 3C).
Fig. 3.
Seed mass and yield-per-plant of kat mutants. (A) Seed yield of kat mutant plants (n ≥7). (B) Mean seed weight of pools of 500–600 kat mutant seeds (n ≥7). (C) kat2-1 and kat5-2 seed oil content of individual fatty acid species normalized for seed weight (n=4). (Values are mean ±SE; *P <0.05, **P <0.01, ANOVA).
kat5 mutants exhibit normal flavonoid-related phenotypes
A role for KAT5 in flavonoid biosynthesis has previously been hypothesized based on co-expression and co-regulation of KAT5 with genes of that pathway (Carrie et al., 2007; Stracke et al., 2007, 2010) (see also Supplementary Fig. S3A, B at JXB online). To investigate a possible functional relationship between KAT5 and enzymes of flavonoid biosynthesis such as chalcone synthase (encoded by the TRANSPARENT TESTA 4 gene, TT4), the seed coat colour of kat mutants was compared with that of Ws-4, tt4, and Ler (the background of the tt4 mutant). All kat mutants and the wild type had pigmented seed coats while those of tt4 were transparent (see Supplementary Fig. S3C at JXB online). Seeds were imbibed for 24h, seed coats removed, and seedlings stained with diphenylboric acid 2-aminoethyl ester (DPBA), which fluoresces specifically in the presence of flavonoids (Peer et al., 2001). Fluorescence was clearly observed in the wild type and in kat2 and kat5 mutants, but was absent in tt4 (see Supplementary Fig. S3D at JXB online), indicating that the gross alteration in flavonoid content seen in flavonoid mutants was not apparent in kat5.
Analysis of kat double mutants
Three double mutant combinations were examined: kat1 kat2, kat1 kat5, and kat2 kat5. The kat1-1 kat5-2 double mutant was fully fertile and indistinguishable from the wild type in seed germination (Fig. 2) and seedling growth (Fig. 1) and so was not studied further. By contrast, many hundreds of F2 and F3 plants, generated by crossing kat1-1 with either kat2-1 or kat2-5, were screened but we were unable to obtain either sesquimutant (plants homozygous at one locus but heterozygous for the other) or double homozygote plants. It is concluded from this that KAT1, although barely expressed (Wiszniewski et al., 2012), may be partially redundant with KAT2 for some essential function and that the loss of both genes is lethal. Alternatively, the kat1-1 mutant carries a closely linked secondary mutation that is incompatible with kat2. However, alternative alleles of kat1 were not available to explore this further.
Crosses to generate the kat2 kat5 double mutant readily yielded fertile sesquimutant plants. Progeny from kat2-1 kat5-2 sesquimutant parents that were segregating at either the KAT2 or the KAT5 locus were analysed by PCR analysis of extracted DNA to determine the genotype at the heterozygous locus (Table 2). Seed for this experiment were cold-stratified and grown on MS medium containing sucrose to maximize germination and growth. The progeny of individual kat2-1/kat2-1 kat5-2/KAT5 plants did not differ significantly from the expected ratio of 1:2:1 for segregation at a single recessive locus (i.e. for KAT5/KAT5:kat5-2/KAT5:kat5-2/kat5-2). However, segregation analysis of kat2-1/KAT2-1 kat5-2/kat5-2 plants yielded a ratio at the KAT2 locus of about 4:4:1, significantly different from 1:2:1 (P <0.01) and under-represented in both homozygotes (kat2-1/kat2-1) and heterozygotes (kat2-1/KAT2-1).
Table 2.
Segregation analysis of kat2-1 kat5-2 mutants homozygous at one locus but segregating at the otherSegregating mutant seedlings were genotyped by PCR to determine their segregation ratio, and this was compared with the expected 1:2:1 ratio for a single recessive mutation by χ2 analysis. The frequency (%) of each genotype is given in brackets.
| Media | Background mutation | Segregating mutation | Total | χ2 (1:2:1 hypothesis) | ||
|---|---|---|---|---|---|---|
| Wild-type | Heterozygous | Homozygous | ||||
| 0.5× MS+1% suc | kat5-2 | KAT2/KAT2 | kat2-1/KAT2 | kat2-1/kat2-1 | ||
| 42 (46%) | 40 (44%) | 9 (9.9%) | 91 | 3.3×10–6 | ||
| 0.5× MS+1% suc | kat2-1 | KAT5/KAT5 | kat5-2/KAT5 | kat5-2/kat5-2 | ||
| 28 (30%) | 46 (49%) | 19 (20%) | 93 | 0.42 | ||
| Water–agar | kat2-1 | KAT5/KAT5 | kat5-2/KAT5 | kat5-2/kat5-2 | ||
| 50 (28%) | 105 (58%) | 26 (14%) | 181 | 0.0041 | ||
The data presented in Table 2 may be regarded as arising from maximum germination potential, due to the stratification of seeds and their incubation on medium containing both and sucrose, known germination promoters. To assess further the effect of kat2-1 and kat5-2 single and sesquimutants on germination potential, seed batches from plants that had been grown at the same time and under the same conditions were sown on water-agar and placed directly in the light without stratification. By 4 d, Ws-4 and kat5-2 had germination frequencies greater than 95% (Fig. 2). By contrast, germination at 7 d was 70% for kat2-1 (homozygous seed), and only 50% for kat2-1/kat2-1 kat5-2/KAT5 (note that single parent sesquimutant seed pools showed approximately 1:2:1 segregation ratio at the KAT5 locus, Table 2). It is inferred that the reduction in germination efficiency in the sesquimutant seed pools (Fig. 2) was due to double homozygotes among the progeny. Indeed, genotyping of seeds that had germinated after 7 d on water-agar indicated a deficiency of kat5-2 homozygotes such that the ratio of KAT5/KAT5:KAT5/kat5-2:kat5-2/kat5-2 was approximately 2:4:1, significantly different (P <0.01) from the expected 1:2:1 (Table 2). This implies that kat2-1 kat5-2 double homozygote seeds are less likely to germinate on water-agar than wild-type or sesquimutant seeds.
Double mutants of kat2 and kat5 recapitulate the aim1 phenotype
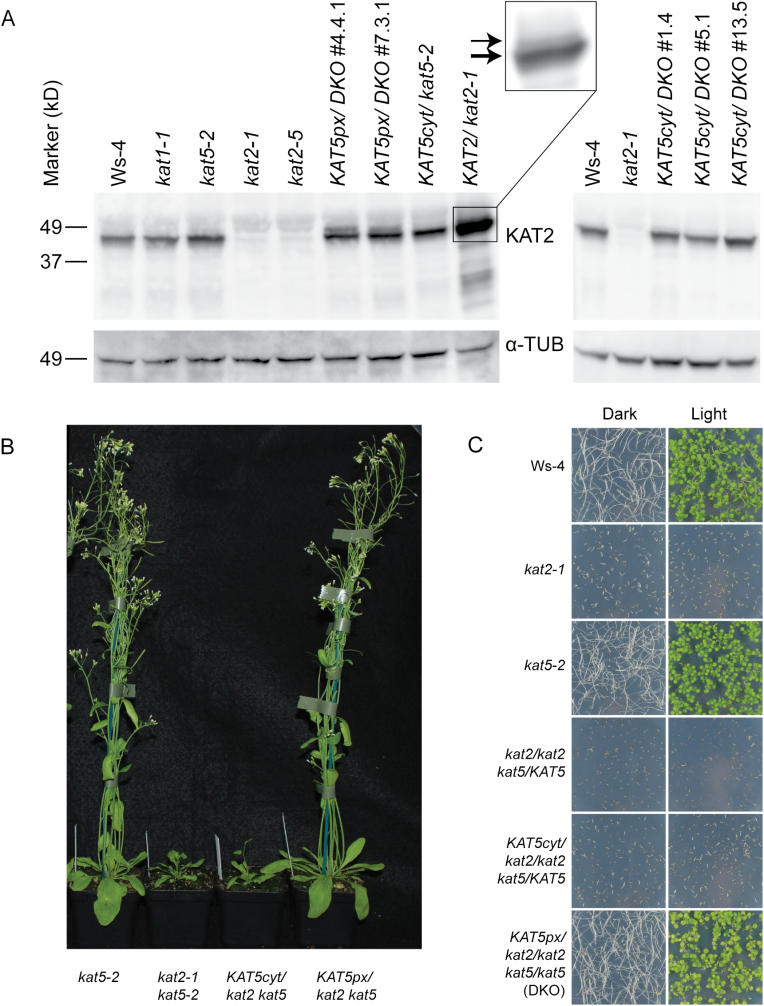
Homozygous kat2 kat5 (Ws-4) double knockout seedlings that had been identified by PCR genotyping were grown in soil under long-day conditions for further analysis. They exhibited severe growth defects (Fig. 4). These double mutant seedlings were paler green than the wild type (Fig. 4A). Throughout development, they were slower growing than the wild type or kat single mutants, displaying reduced rosette size and delayed flowering (Fig. 4B, C). Mature kat2 kat5 double knockout plants had reduced apical dominance and shorter inflorescences with atypical internode spacing and altered anatomy. These phenotypes are strongly reminiscent of the aim1-1 mutant (Richmond and Bleecker, 1999) (Fig. 4B, C). The kat2 kat5 double mutant was replicated using alternative alleles for each locus (ie. kat2-1 kat5-2, kat2-5 kat5-2, and kat2-1 kat5-1) and obtained similar phenotypes in each case (Figs 4, 5). As there was only one allele for kat5 in the Ws-4 background, the kat5 allele in the kat2-1 kat5-1 double mutant combination was in the Ler ecotype (Table 1), but this gave results consistent with crosses between Ws-4 mutants. Although kat2 kat5 mutants were slower growing than aim1-1 (also in the Ws-4 background), their flowers were similarly malformed, often exhibiting ectopic organ development (Fig. 4D).
Fig. 4.
kat2 kat5 double mutant phenotypes at various stages of plant development. (A) Ws-4 wild type (left) and kat2-1 kat5-2 double mutant (right) grown under continuous light for 14 d on 0.5× MS supplemented with 1% sucrose (scale bar, 10mm). (B) Phenotype of such plants grown for a further 22 d after transfer to soil and long days (16/8h light/dark; scale bar, 15mm). (C) Phenotype of 40-d-old Ws-4, 40-d-old aim1, and 55-d-old kat2-5 kat5-2 (scale bar, 30mm). Soil-grown plants were grown initially for 7 d on media containing sucrose to enable the establishment of double knockouts which were confirmed by PCR genotyping. (D) Close-up images of comparable lengths of typical inflorescences of the plants depicted in (C) which highlights the malformation and ectopic positioning of flowers, siliques, and cauline leaves in inflorescences of aim1 and kat2-5 kat5-2 mutants (scale bar, 30mm).
Fig. 5.
Peroxisomal KAT5 complements kat2 kat5 double knockouts. (A) Western blot of kat mutants. A KAT2 antibody (Germain et al., 2001) was used to probe protein extracted from 7-d-old whole seedlings that had been germinated on 0.5× MS media supplemented with 1% sucrose (left panel) or 2,4-DB (right panel, to select for double knockouts). α-TUB=α-tubulin loading control. The inset depicts resolution of a second, larger band originating in the KAT2 over-expressing line (see text). (B) Restoration of fertility in kat2 kat5 double knockouts by peroxisomal KAT5 (KAT5px). kat2 kat5 phenotypes were rescued by constitutive expression (by CAMV 35S promoter) of KAT5px, but not by a variant of the protein targeted to the cytosol (KAT5cyt). Plants were grown in long-day conditions for 35 d. (C) Complementation of the sucrose-dependent phenotype of kat2 kat5 double knockout. Seedlings were grown on 0.5× MS without sucrose supplement in the dark or in the light for 8 d. kat2-1/kat2-1 kat5-2/KAT5 sesquimutants were unable to establish on media lacking sucrose. The introduction of constitutively expressed KAT5px, but not KAT5cyt, allowed both the recovery of kat2 kat5 double knockouts and their establishment in the absence of sucrose. Scale bar, 20mm. For all panels, the kat2 kat5 mutant (DKO) was kat2-1 kat5-2.
At later stages of the life cycle, kat2 kat5 flowers developed more normally, appearing almost like the wild type. These late developing flowers produced a small amount of pollen, but the siliques of double mutant plants were always empty, even after manual self-pollination. Reciprocal crosses of kat2-1 kat5-2 and wild-type plants were conducted and wild-type pollen was unable to fertilize kat2-1 kat5-2 plants. However, Alexander staining showed that pollen from double mutants was viable (see Supplementary Fig. S4 at JXB online), and it was able to fertilize wild-type ovules, with such crosses producing heterozygous seed. Exogenous supply of JA has previously been show to restore fertility in the acx1 acx5 double mutant (Schilmiller et al., 2007), but treatment (as described in that work) with JA was unable to restore fertility to kat2-1 kat5-2 flowers despite several attempts. Thus there appears to be a defect in gynaecium function in kat2 kat5 mutants.
Peroxisomal rather than cytosolic KAT5 is essential
To determine if cytosolic or peroxisomal KAT5 activity could rescue these phenotypes, two transgenes were created, 35S::KAT5.1 and 35S::KAT5.2. These respectively encoded cytosolic (KAT5cyt) and peroxisomal (KAT5px) variants of KAT5 and were introduced into kat2-1 kat5-2 sesquimutant parent plants. PCR-genotyping of KAT2 and KAT5 loci in plants harbouring these transgenes revealed that KAT5px complemented the kat2 kat5 double mutants while KAT5cyt did not (Fig. 5). Expression of 35S::KAT5.2 in kat2-1 kat5-2 was detected by a KAT2 antibody that is reported to require a 5-fold greater amount of KAT5 than KAT2 protein to produce an equivalent signals (Germain et al., 2001) (Fig. 5A). Thus, although the band intensity is comparable to that seen for KAT2 in the wild type (or in the kat5-2 single mutant), it is likely that the 35S::KAT5.2 drives significant over-expression of KAT5. The mature KAT2 protein (after cleavage of the PTS2 targeting sequence) is expected to be 44.8kDa. A second band of approximately 49kDa was resolved in the 35S::KAT5.2 lines and this corresponds to the size predicted for the unprocessed peptide. The 35S::KAT2/ kat2-1 line described in Germain et al. (2001) over-expresses KAT2 and also had a heavier band that might correspond to excess unprocessed protein (Fig. 5A, inset). A protein corresponding to the expected size of the KAT5cyt protein is 43.2kDa can be seen in Western blots of confirmed kat2-1 kat5-2 double knockout plants transformed with 35S::KAT5.1 (Fig. 5A).
Wild-type-like plant habit and fertility was restored only in kat2-1 kat5-2 plants transformed with 35S::KAT5.2 (Fig. 5B). These results confirm that the infertility phenotype was due to the disruption of peroxisomal thiolase function. The 35S::KAT5.2 transgene also rescued seedling establishment in the absence of exogenous sucrose under both dark and light conditions, while the 35S::KAT5.1 transgene introduced in the kat2-1/kat2-1 kat5-2/KAT5 sesquimutant background did not (Fig. 5C). To determine if reduced germinability in sesquimutants was due to the absence of KAT5px (rather than of KAT5cyt), the germination frequency of kat2-1 kat5-2 double mutants complemented with 35S::KAT5.2 was assayed. Although germination was slightly delayed, expression of KAT5px in multiple independent lines restored germination frequency to almost 100% for double mutants (Fig. 2). Collectively, these data suggest that, while KAT2 is the major thiolase during germination, peroxisomal KAT5 also contributes to germination potential, development of reproductive tissue, and full fertility in A. thaliana.
Discussion
Functional analysis of KAT genes during development
Mutants have been studied to probe the functions of KAT genes during plant growth and development. Knockouts of KAT1 and KAT5 (and a kat1 kat5 double knockout) showed no obvious abnormal phenotypes during the life cycle. No evidence was found for a requirement for KAT5 in flavonoid synthesis in seeds, despite co-expression and co-regulation of KAT5 with genes of that pathway (Carrie et al., 2007; Stracke et al., 2007, 2010). KAT2 has previously been well characterized and is the primary isoform acting in β-oxidation of fatty acids during seed germination, and also in the processing of hormone precursors including IBA, 2,4-DB, and JA. Accordingly, kat2 seedlings require exogenous sucrose for establishment, are impaired in the breakdown of TAG and fatty acids, and are resistant to pro-auxins (Hayashi et al., 1998; Germain et al., 2001; Wiszniewski et al., 2009). It was also confirmed that the kat2 mutant produced fewer and smaller seeds than the wild type (or kat1 and kat5 plants). It was not possible to obtain kat1 kat2 mutants, and kat2 kat5 double knockout plants were infertile with gross morphological defects to vegetative and reproductive organs.
A role for β-oxidation in seed germination independent of oil breakdown has previously been described for other β-oxidation mutants (Russell et al., 2000; Pracharoenwattana et al., 2005; Pinfield-Wells et al., 2005). For example, it was recently shown that OPDA inhibits germination by acting synergistically with ABA to increase the levels of ABI5 protein (Dave et al., 2011). In β-oxidation mutants, including cts and kat2, the accumulation of OPDA (that is not converted to JA during seed development) consequently results in reduced germination frequency. kat2 seed is thus less likely to germinate than the wild type and our new results show that if kat2 homozygous seed also lacks at least one wild-type KAT5 allele, fewer seeds germinate and germination is slower.
β-oxidation enzymes are present in the endosperm and embryo during seed development (e.g. see http://bar.utoronto.ca/) and it has been observed that β-oxidation is active in the turnover of lipids in developing embryos (Arai et al., 2002; Chia et al., 2005). Indeed, gluconeogenesis appears to be disrupted in kat2-1 ovules and they accordingly exhibited reduced respiration rates that may impact embryo development (Footitt et al., 2007a). Alternatively, the accumulation of free-fatty acids may damage embryo cell membranes similar to the observations of damage in leaves of dark-treated kat2 and cts plants (Kunz et al., 2009). It remains unknown whether seed development in these mutants requires β-oxidation in the embryo, embryo sac, endosperm, ovule, ovary or a combination of these tissues. Indeed, seeds of kat2 are smaller than those of the wild type, kat1, or kat5 (Fig. 3; Footitt et al., 2007a) supporting the notion that β-oxidation plays a role in seed filling or maturation. Reduced individual seed mass has previously been reported in acx1-1, acx1-1 acx2-1, and cts-2 mutants (Pinfield-Wells et al., 2005) and in pex5 mutants (Khan and Zolman, 2010). The kat2 mutant also produced fewer seeds per plant than the wild type, probably as a result of the abortion of some seeds in each silique (Footitt et al., 2007a). Extremely compromised fertility is seen in some other β-oxidation mutants, including embryo lethality of aim1 mfp2 and acx3 acx4 double mutants (Rylott et al., 2003, 2006). It is interesting to note, however, that these reports are for mutants in the Ws-4 background and that a Col-0 acx3 acx4 double knockout mutant is fertile, pointing to ecotype-specific effects of altered β-oxidation capacity in Arabidopsis (Khan et al., 2012). While the present study has investigated the aim1 and kat2 kat5 double mutant phenotypes in the Ws-4 ecotype, equivalent studies of these genes in other Arabidopsis ecotypes would be valuable but have not yet been reported.
The kat2 kat5 double mutant is infertile, producing no seeds, even when treated with wild-type pollen. The KAT1 gene alone is thus insufficient for, or incapable of, conferring fertility. As kat2 kat5 can produce viable pollen, and fertility is not restored by the application of JA, its infertility may be due to compromised megagametophyte development or to an inability of the mutant style to support or direct fertilization. The latter has been reported for the abstinence by mutual consent (amc) mutant, an allele of pex13. However, amc can only exist as a heterozygote: homozygotes cannot be generated at all from heterozygous parent plants (Boisson-Dernier et al., 2008). Homozygous kat2 kat5 embryos are capable of developing into viable seeds if the parent plant contains one wild-type allele of either KAT2 or KAT5. Moreover, fertility (and germinability) was restored to kat2 kat5 double mutants by constitutive expression of peroxisome-targeted KAT5. This indicates that KAT2 and KAT5 genes are at least partially redundant in determining fertility and that the inability of native KAT5 to compensate for the loss of KAT2 in kat2 mutants may be due to a sub-threshold amount of KAT activity or to inappropriate timing and spatial expression patterns of KAT5. Peroxisomal KAT may supply essential metabolites to, or remove inhibitory metabolites from, the ovule sac or embryo. Alternatively, KAT activity may be required for proper gynaecium and ovule development, rather than play a direct role in the processes of fertilization, embryogenesis, and seed development.
β-oxidation is essential for normal inflorescence development and plant fertility
kat2 kat5 double mutants had slow growth, but exhibited proliferation of abnormal inflorescences including ectopic positioning of reproductive organs. The growth and development of kat2 kat5 plants was similar to that of the aim1 mutant. The possibility was considered that this may be due to impaired IAA synthesis via β-oxidation of IBA, since disruption to auxin metabolism can affect shoot branching (Bennett et al., 2006). However, this is not supported by an ech2 ibr1 ibr3 ibr10 quadruple mutant, which has severe defects in the conversion of IBA to IAA in peroxisomes. The quadruple mutant exhibits smaller cotyledons, slower leaf development, and delayed flowering, but it has no gross morphological defects at maturity and its inflorescences are fertile and appear similar to those of wild-type plants (Strader et al., 2011). The pale green leaves of kat2 kat5 plants were reminiscent of the reduced chlorophyll observed in the pex5-10 mutant that lacks a full-length PEX5 protein (Khan and Zolman, 2010). pex5-10 grew relatively normally after seedling establishment and was more similar to kat2 single mutants in that it exhibited poor germination and reduced weight of individual seeds, but the phenotype of mature plants did not resemble aim1.
By contrast, the ped1 ped3 double mutant combination (which is in the Ler background and is allelic to kat2 cts) had wavy irregular leaves and dwarfed, abnormal inflorescences (Hayashi et al., 2002) and appears to be very similar to aim1. An extreme case of impaired shoot growth of a β-oxidation mutant is the citrate synthase double mutant csy2 csy3 in which the accumulation of peroxisomal acetyl-CoA (rather than he absence of a particular product of β-oxidation) may explain the phenotype (Pracharoenwattana et al., 2005). Similarly, the shoot developmental abnormalities of kat2 kat5 (and/or aim1) could be due to the accumulation of a β-oxidation precursor or intermediate rather than due to the absence of a specific product such as IAA or JA. Given that, after seedling establishment, cts mutants exhibit normal shoot and inflorescence development, it seems unlikely that the build-up of extra-peroxisomal precursors is responsible for the altered inflorescence development. Alternatively, CTS-independent routes for the import of substrates such as the JA-precursor OPDA have been proposed (Theodoulou et al., 2005) and, if this is possible for other substrates, loss of CTS alone may be insufficient to produce severe phenotypes.
To test the possibility that intra-peroxisomal accumulation of a β-oxidation intermediate might explain these phenotypes, an attempt was made to make a cts aim1 double mutant. AIM1 and CTS genes are both located on chromosome 4 (AGIs At4g29010 and At4g39850, respectively). Despite readily deriving a crossover between these loci, and then screening in excess of 150 F3 plants, we were unable to generate double mutants, suggesting that this mutant combination is lethal. It is proposed that aim1-like phenotypes result from a blockage to β-oxidation caused by severe reduction of an essential metabolic capacity (e.g. aim1, or kat2 kat5) or that reduced metabolic capacity in combination with a loss of the primary peroxisome substrate import capability in ped1 ped3 (kat2 cts) or cts aim1, precludes the production of an essential metabolite or results in the accumulation of an intermediate that is toxic to plant development. The future identification of such intermediates may reveal a new metabolic function for peroxisomes.
Supplementary data
Supplementary data can be found at JXB online.
Supplementary Fig. S1. Identification of new KAT mutants.
Supplementary Fig. S2. Appearance of mature wild type and kat single mutants.
Supplementary Fig. S3. KAT5 is not required for flavonoid biosynthesis.
Supplementary Fig. S4. Viability of kat2 kat5 double mutant pollen
Supplementary Table S1. Oligonucleotide sequences.
Acknowledgements
We thank Matt Timmins and Ricarda Fenske of Metabolomics Australia (UWA, Perth) for help with fatty acid analysis. This work was supported by the Australian Research Council (Grant numbers FF0457721 and CE0561495) and an Australian Postgraduate Award to AAGW.
References
- Adham AR, Zolman BK, Millius A, Bartel B. 2005. Mutations in Arabidopsis acyl-CoA oxidase genes reveal distinct and overlapping roles in beta-oxidation. The Plant Journal 41, 859–874. [DOI] [PubMed] [Google Scholar]
- Afitlhile MM, Fukushige H, Nishimura M, Hildebrand DF. 2005. A defect in glyoxysomal fatty acid beta-oxidation reduces jasmonic acid accumulation in Arabidopsis. Plant Physiology and Biochemistry 43, 603–609. [DOI] [PubMed] [Google Scholar]
- Alexander MP. 1969. Differential staining of aborted and nonaborted pollen. Stain Technology 44, 117–122. [DOI] [PubMed] [Google Scholar]
- Arai Y, Nakashita H, Suzuki Y, Kobayashi Y, Shimizu T, Yasuda M, Doi Y, Yamaguchi I. 2002. Synthesis of a novel class of polyhydroxyalkanoates in Arabidopsis peroxisomes, and their use in monitoring short-chain-length intermediates of β-oxidation. Plant and Cell Physiology 43, 555–562. [DOI] [PubMed] [Google Scholar]
- Baker A, Graham IA, Holdsworth M, Smith SM, Theodoulou FL. 2006. Chewing the fat: beta-oxidation in signalling and development. Trends in Plant Science 11, 124–132. [DOI] [PubMed] [Google Scholar]
- Bennett T, Sieberer T, Willett B, Booker J, Luschnig C, Leyser O. 2006. The Arabidopsis MAX pathway controls shoot branching by regulating auxin transport. Current Biology 16, 553–563. [DOI] [PubMed] [Google Scholar]
- Boisson-Dernier A, Frietsch S, Kim TH, Dizon MB, Schroeder JI. 2008. The peroxin loss-of-function mutation abstinence by mutual consent disrupts male–female gametophyte recognition. Current Biology 18, 63–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussell JD, Behrens C, Ecke W, Eubel H. 2013. Arabidopsis peroxisome proteomics. Frontiers in Plant Science 4, 101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussell JD, Reichelt M, Wiszniewski AA, Gershenzon J, Smith SM. 2014. Peroxisomal ATP-binding cassette transporter COMATOSE and the multifunctional protein abnormal INFLORESCENCE MERISTEM are required for the production of benzoylated metabolites in arabidopsis seeds. Plant Physiology 164, 48–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrie C, Murcha MW, Millar AH, Smith SM, Whelan J. 2007. Nine 3-ketoacyl-CoA thiolases (KATs) and acetoacetyl-CoA thiolases (ACATs) encoded by five genes in Arabidopsis thaliana are targeted either to peroxisomes or cytosol but not to mitochondria. Plant Molecular Biology 63, 97–108. [DOI] [PubMed] [Google Scholar]
- Castillo MC, Leon J. 2008. Expression of the beta-oxidation gene 3-ketoacyl-CoA thiolase 2 (KAT2) is required for the timely onset of natural and dark-induced leaf senescence in Arabidopsis . Journal of Experimental Botany 59, 2171–2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chia TY, Pike MJ, Rawsthorne S. 2005. Storage oil breakdown during embryo development of Brassica napus (L.). Journal of Experimental Botany 56, 1285–1296. [DOI] [PubMed] [Google Scholar]
- Clough SJ, Bent AF. 1998. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana . The Plant Journal 16, 735–743. [DOI] [PubMed] [Google Scholar]
- Cruz Castillo M, Martínez C, Buchala A, Métraux JP, León J. 2004. Gene-specific involvement of beta-oxidation in wound-activated responses in Arabidopsis. Plant Physiology 135, 85–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dave A, Hernández ML, He Z, Andriotis VME, Vaistij FE, Larson TR, Graham IA. 2011. 12-Oxo-phytodienoic acid accumulation during seed development represses seed germination in Arabidopsis. The Plant Cell 23, 583–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Marcos Lousa C, van Roermund CW, Postis VL, Dietrich D, Kerr ID, Wanders RJ, Baldwin SA, Baker A, Theodoulou FL. 2013. Intrinsic acyl-CoA thioesterase activity of a peroxisomal ATP binding cassette transporter is required for transport and metabolism of fatty acids. Proceedings of the National Academy of Sciences, USA 110, 1279–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastmond PJ. 2006. SUGAR-DEPENDENT1 encodes a patatin domain triacylglycerol lipase that initiates storage oil breakdown in germinating Arabidopsis seeds. The Plant Cell 18, 665–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Footitt S, Cornah JE, Pracharoenwattana I, Bryce JH, Smith SM. 2007a. The Arabidopsis 3-ketoacyl-CoA thiolase-2 (kat2-1) mutant exhibits increased flowering but reduced reproductive success. Journal of Experimental Botany 58, 2959–2968. [DOI] [PubMed] [Google Scholar]
- Footitt S, Dietrich D, Fait A, Fernie AR, Holdsworth MJ, Baker A, Theodoulou FL. 2007b. The COMATOSE ATP-binding cassette transporter is required for full fertility in Arabidopsis. Plant Physiology 144, 1467–1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulda M, Schnurr J, Abbadi A, Heinz E, Browse J. 2004. Peroxisomal acyl-CoA synthetase activity is essential for seedling development in Arabidopsis thaliana . The Plant Cell 16, 394–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germain V, Rylott EL, Larson TR, Sherson SM, Bechtold N, Carde JP, Bryce JH, Graham IA, Smith SM. 2001. Requirement for 3-ketoacyl-CoA thiolase-2 in peroxisome development, fatty acid beta-oxidation and breakdown of triacylglycerol in lipid bodies of Arabidopsis seedlings. The Plant Journal 28, 1–12. [DOI] [PubMed] [Google Scholar]
- Graham IA. 2008. Seed storage oil mobilization. Annual Review of Plant Biology 59, 115–142. [DOI] [PubMed] [Google Scholar]
- Hayashi M, Nito K, Takei-Hoshi R, Yagi M, Kondo M, Suenaga A, Yamaya T, Nishimura M. 2002. Ped3p is a peroxisomal ATP-binding cassette transporter that might supply substrates for fatty acid beta-oxidation. Plant and Cell Physiology 43, 1–11. [DOI] [PubMed] [Google Scholar]
- Hayashi M, Toriyama K, Kondo M, Nishimura M. 1998. 2,4-Dichlorophenoxybutyric acid-resistant mutants of Arabidopsis have defects in glyoxysomal fatty acid beta-oxidation. The Plant Cell 10, 183–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellens RP, Edwards EA, Leyland NR, Bean S, Mullineaux PM. 2000. pGreen: a versatile and flexible binary Ti vector for Agrobacterium-mediated plant transformation. Plant Molecular Biology 42, 819–832. [DOI] [PubMed] [Google Scholar]
- Hu J, Baker A, Bartel B, Linka N, Mullen RT, Reumann S, Zolman BK. 2012. Plant peroxisomes: biogenesis and function. The Plant Cell 24, 2279–2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamada T, Nito K, Hayashi H, Mano S, Hayashi M, Nishimura M. 2003. Functional differentiation of peroxisomes revealed by expression profiles of peroxisomal genes in Arabidopsis thaliana . Plant and Cell Physiology 44, 1275–1289. [DOI] [PubMed] [Google Scholar]
- Khan BR, Adham AR, Zolman BK. 2012. Peroxisomal acyl-CoA oxidase 4 activity differs between Arabidopsis accessions. Plant Molecular Biology 78, 45–58. [DOI] [PubMed] [Google Scholar]
- Khan BR, Zolman BK. 2010. pex5 Mutants that differentially disrupt PTS1 and PTS2 peroxisomal matrix protein import in Arabidopsis. Plant Physiology 154, 1602–1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunz HH, Scharnewski M, Feussner K, Feussner I, Flugge UI, Fulda M, Gierth M. 2009. The ABC transporter PXA1 and peroxisomal beta-oxidation are vital for metabolism in mature leaves of Arabidopsis during extended darkness. The Plant Cell 21, 2733–2749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peer WA, Brown DE, Tague BW, Muday GK, Taiz L, Murphy AS. 2001. Flavonoid accumulation patterns of transparent testa mutants of arabidopsis. Plant Physiology 126, 536–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinfield-Wells H, Rylott EL, Gilday AD, Graham S, Job K, Larson TR, Graham IA. 2005. Sucrose rescues seedling establishment but not germination of Arabidopsis mutants disrupted in peroxisomal fatty acid catabolism. The Plant Journal 43, 861–872. [DOI] [PubMed] [Google Scholar]
- Pracharoenwattana I, Cornah JE, Smith SM. 2005. Arabidopsis peroxisomal citrate synthase is required for fatty acid respiration and seed germination. The Plant Cell 17, 2037–2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond TA, Bleecker AB. 1999. A defect in beta-oxidation causes abnormal inflorescence development in Arabidopsis. The Plant Cell 11, 1911–1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell L, Larner V, Kurup S, Bougourd S, Holdsworth M. 2000. The Arabidopsis COMATOSE locus regulates germination potential. Development 127, 3759–3767. [DOI] [PubMed] [Google Scholar]
- Rylott EL, Eastmond PJ, Gilday AD, Slocombe SP, Larson TR, Baker A, Graham IA. 2006. The Arabidopsis thaliana multifunctional protein gene (MFP2) of peroxisomal beta-oxidation is essential for seedling establishment. The Plant Journal 45, 930–941. [DOI] [PubMed] [Google Scholar]
- Rylott EL, Rogers CA, Gilday AD, Edgell T, Larson TR, Graham IA. 2003. Arabidopsis mutants in short- and medium-chain acyl-CoA oxidase activities accumulate acyl-CoAs and reveal that fatty acid beta-oxidation is essential for embryo development. Journal of Biological Chemistry 278, 21370–21377. [DOI] [PubMed] [Google Scholar]
- Samson F, Brunaud V, Duchene S, De Oliveira Y, Caboche M, Lecharny A, Aubourg S. 2004. FLAGdb++: a database for the functional analysis of the Arabidopsis genome. Nucleic Acids Research , 32 Database issue, D347–D350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilmiller AL, Koo AJ, Howe GA. 2007. Functional diversification of acyl-coenzyme a oxidases in jasmonic acid biosynthesis and action. Plant Physiology 143, 812–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirley BW, Kubasek WL, Storz G, Bruggemann E, Koornneef M, Ausubel FM, Goodman HM. 1995. Analysis of Arabidopsis mutants deficient in flavonoid biosynthesis. The Plant Journal 8, 659–671. [DOI] [PubMed] [Google Scholar]
- Stracke R, Favory JJ, Gruber H, Bartelniewoehner L, Bartels S, Binkert M, Funk M, Weisshaar B, Ulm R. 2010. The Arabidopsis bZIP transcription factor HY5 regulates expression of the PFG1/MYB12 gene in response to light and ultraviolet-B radiation. Plant Cell and Environment 33, 88–103. [DOI] [PubMed] [Google Scholar]
- Stracke R, Ishihara H, Huep G, Barsch A, Mehrtens F, Niehaus K, Weisshaar B. 2007. Differential regulation of closely related R2R3-MYB transcription factors controls flavonol accumulation in different parts of the Arabidopsis thaliana seedling. The Plant Journal 50, 660–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strader LC, Wheeler DL, Christensen SE, Berens JC, Cohen JD, Rampey RA, Bartel B. 2011. Multiple facets of Arabidopsis seedling development require indole-3-butyric acid-derived auxin. The Plant Cell 23, 984–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundaresan V, Springer P, Volpe T, Haward S, Jones JD, Dean C, Ma H, Martienssen R. 1995. Patterns of gene action in plant development revealed by enhancer trap and gene trap transposable elements. Genesand Development 9, 1797–1810. [DOI] [PubMed] [Google Scholar]
- Theodoulou FL, Job K, Slocombe SP, Footitt S, Holdsworth M, Baker A, Larson TR, Graham IA. 2005. Jasmonic acid levels are reduced in COMATOSE ATP-binding cassette transporter mutants. Implications for transport of jasmonate precursors into peroxisomes. Plant Physiology 137, 835–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verrier PJ, Bird D, Burla B, et al. 2008. Plant ABC proteins: a unified nomenclature and updated inventory. Trends in Plant Science 13, 151–159. [DOI] [PubMed] [Google Scholar]
- Wiszniewski AAG, Smith SM, Bussell JD. 2012. Conservation of two lineages of peroxisomal (Type I) 3-ketoacyl-CoA thiolases in land plants, specialization of the genes in Brassicaceae, and characterization of their expression in Arabidopsis thaliana . Journal of Experimental Botany 63, 6093–6103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiszniewski AAG, Zhou W, Smith SM, Bussell JD. 2009. Identification of two Arabidopsis genes encoding a peroxisomal oxidoreductase-like protein and an acyl-CoA synthetase-like protein that are required for responses to pro-auxins. Plant Molecular Biology 69, 503–515. [DOI] [PubMed] [Google Scholar]
- Zolman BK, Martinez N, Millius A, Adham AR, Bartel B. 2008. Identification and characterization of Arabidopsis indole-3-butyric acid response mutants defective in novel peroxisomal enzymes. Genetics 180, 237–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zolman BK, Nyberg M, Bartel B. 2007. IBR3, a novel peroxisomal acyl-CoA dehydrogenase-like protein required for indole-3-butyric acid response. Plant Molecular Biology 64, 59–72. [DOI] [PubMed] [Google Scholar]
- Zolman BK, Yoder A, Bartel B. 2000. Genetic analysis of indole-3-butyric acid responses in Arabidopsis thaliana reveals four mutant classes. Genetics 156, 1323–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.