Significance
Intracellular accumulation of the abnormally modified tau is hallmark pathology of AD, but the mechanism leading to tau aggregation is not fully characterized. In the present study, we studied the effects of tau SUMOylation on its phosphorylation, ubiquitination, degradation, and aggregation. We discovered that sumoylation competes with ubiquitination in modifying tau, correlating with tau hyperphosphorylation. Identification of the posttranslational modification on tau provides the new insight into the molecular mechanism in tau aggregation.
Keywords: SUMOylation, tau, phosphorylation, ubiquitination, degradation
Abstract
Intracellular accumulation of the abnormally modified tau is hallmark pathology of Alzheimer’s disease (AD), but the mechanism leading to tau aggregation is not fully characterized. Here, we studied the effects of tau SUMOylation on its phosphorylation, ubiquitination, and degradation. We show that tau SUMOylation induces tau hyperphosphorylation at multiple AD-associated sites, whereas site-specific mutagenesis of tau at K340R (the SUMOylation site) or simultaneous inhibition of tau SUMOylation by ginkgolic acid abolishes the effect of small ubiquitin-like modifier protein 1 (SUMO-1). Conversely, tau hyperphosphorylation promotes its SUMOylation; the latter in turn inhibits tau degradation with reduction of solubility and ubiquitination of tau proteins. Furthermore, the enhanced SUMO-immunoreactivity, costained with the hyperphosphorylated tau, is detected in cerebral cortex of the AD brains, and β-amyloid exposure of rat primary hippocampal neurons induces a dose-dependent SUMOylation of the hyperphosphorylated tau. Our findings suggest that tau SUMOylation reciprocally stimulates its phosphorylation and inhibits the ubiquitination-mediated tau degradation, which provides a new insight into the AD-like tau accumulation.
Alzheimer’s disease (AD) is the most common neurodegenerative disorder in the elderly. Intracellular accumulation of neurofibrillary tangles (NFTs) and extracellular precipitation of senile plaques are the most prominent pathological hallmarks of AD (1–3). The clinical-to-pathological correlation studies have demonstrated that the number of NFTs consisting of hyperphosphorylated tau correlates with the degree of dementia in AD (4–6). Tau is the major microtubule-associated protein that normally contains 2–3 mol of phosphate per mole of tau protein. In AD brains, tau is abnormally hyperphosphorylated (namely AD-P-tau) and the phosphate level increases to 5–9 mol phosphate per mole tau (4). AD-P-tau does not bind to tubulin and become incompetent in promoting microtubule assembly and maintaining the stability of the microtubules. The AD-P-tau also sequesters normal tau from microtubules (7), and serves as a template for the conversion of normal tau into misfolded protein in a prion-like manner (8). In addition to hyperphosphorylation, tau is also contains other posttranslational modifications, such as ubiquitination and SUMOylation (5, 9–11). The abnormal modification of tau also decreases its solubility, and ∼40% of the hyperphosphorylated tau in AD brains has been isolated as sedimentable nonfibril cytosolic protein (1, 12). Although the mechanisms underlying the formation of the NFTs remain unclear, the altered tau modifications and impaired degradation are believed to play a role. Therefore, clarifying the mechanism that may cause tau accumulation is of great significance for understanding the pathogenesis of AD and for developing new therapeutics.
Like other proteins, tau can be degraded by autophagy-lysosomal and ubiqutin-proteasomal systems under physiological conditions. In mouse cortical neurons, a C-terminal–truncated form of tau that mimics tau cleaved at Asp421 (tauΔC) is removed by macroautophagic and lysosomal mechanisms (13). Lysosomal perturbation inhibits the clearance of tau with accumulation and aggregation of tau in M1C cells (14). Cathepsin D released from lysosome can degrade tau in cultured hippocampal slices (15). Inhibition of the autophagic vacuole formation leads to a noticeable accumulation of tau (14). Studies also suggest that tau protein is degraded in an ubiquitin-, ATP-, and 26S proteasome-, but not a 20S proteasome-dependent manner under normal conditions (16). When the cells are exposed to the stresses, CHIP, a ubiquitin ligase that interacts directly with Hsp70/90, can induce tau ubiquitination and thus selectively reduce the level of detergent insoluble tau (17). The compensatory activation of autophagy-lysosomal or ubiqutin-proteasomal system can antagonize tau aggregation; therefore, tau accumulation does not show in the early stage of AD. During the evolution of AD, a gradual impairment of autophagy-lysosomal system and ubiqutin-proteasomal system has been detected at later stage of the disease (18–20). Studies suggest that the ubiquitin-mediated degradation pathway seems ineffective in removing the tau-positive fibrillar structures in the AD brains (21–23); however, the mechanisms underlying the impairment of the ubiqutin-proteasomal system are elusive.
Ubiquitin is an important component of the cellular defense system that tags abnormal proteins for their degradation by ATP-dependent nonlysosomal proteases (24). Monoclonal antibodies 3-39 and 5-25 raised against paired helical filaments of NFTs have been shown to recognize ubiquitin (25). Meanwhile, tau can be sumoylated at K340 in vitro by SUMO-1 (small ubiquitin-like modifier protein-1) and to a lesser extent by SUMO2 and SUMO3 (9–11). Moreover, SUMO-1 immunoreactivity was colocalized with tau aggregates in neuritic plaques of APP transgenic mice (11). It is well known that SUMO share similarities with ubiquitin in both the structure and the biochemistry of their conjugation (26). Therefore, tau SUMOylation may compete against its ubiquitination and thus suppress tau degradation. In the present study, we found that tau SUMOylation reciprocally stimulates its phosphorylation and thus inhibits the ubiquitination and degradation of tau proteins.
Results
Tau SUMOylation Promotes Tau Phosphorylation.
To explore the effect of tau SUMOylation on its phosphorylation, we first overexpressed eGFP-labeled SUMO-1 or the vector in HEK293/tau cells that stably express the longest isoform of human tau for 24 h and analyzed the phosphorylation level of tau by Western blotting. Compared with the controls, expression of SUMO-1 significantly increased tau phosphorylation at Thr-205, Ser-214, Thr-231, Ser-262, Ser-396, and Ser-404 sites, and a 95-kDa band was found (Fig. 1 A and B).
Fig. 1.
Tau SUMOylation at K340 promotes tau phosphorylation at multiple AD-associated sites. (A and B) HEK293 cells with stable expression of human tau441 (HEK293/tau) were transfected with eGFP–SUMO-1 or the vector for 24 h, or the wild-type HEK293 cells were transiently cotransfected with tau K340R and eGFP-–SUMO-1. The expression of eGFP–SUMO-1 (∼39 kDa) and the phosphorylation level of tau at multiple AD-associated sites as labeled were measured by Western blotting and quantitative analyses. (C) Further justification of tau SUMOylation by staining tau and SUMO-1 in the same blot. The cell extracts were isolated on the same SDS/PAGE and transferred with the same nitrocellulose membrane, the membrane was then cut into two parts and blotted with total tau antibody (Tau5) and SUMO-1 antibody, respectively. The SUMOylation of tau was justified by the appearance of 95-kDa tau bands with both antibodies. (D and E) HEK293/tau cells were transfected with eGFP–SUMO-1 (green) for 24 h and the phosphorylation level of tau at Ser396 (red) were compared in the cells with and without expression of SUMO-1 by immunofluorescence staining. *P < 0.05, **P < 0.01 vs. vector in B; #P < 0.05, ##P < 0.01 vs. SUMO-1 in B. (Scale bar in D, 20 μm.)
To verify the effect of tau SUMOylation on its phosphorylation, we mutated tau lysine 340 to arginine (K340R), the reported SUMOylation site of tau (9). We found that expression of K340R tau abolished its hyperphosphorylation (Fig. 1 A and B). We also treated the cells with different concentrations of ginkgolic acid (GA), an inhibitor of protein SUMOylation (27), after transfection of SUMO-1 into HEK293/tau cells. Phosphorylation of tau at Thr205, Ser262, and Ser396 was gradually reduced with a decreasing SUMO-1 staining after GA treatment (Fig. S1). Interestingly, the ∼95-kDa (55 kDa tau + 40 kDa GFP-SUMO) band was only seen in the cells expressing wild-type tau but not those expressing K340R tau (Fig. 1A), indicating that the ∼95-kDa band may represent the SUMOylated tau. Both antibodies against total tau antibody (Tau5) and SUMO-1 antibody reacted with the 95-kDa band, confirming that the 95-kDa band represented the SUMOylated tau (Fig. 1C). Immunofluorescence staining demonstrated that the cells transfected with SUMO-1 (green) showed higher level of tau phosphorylation compared with untransfected cells (Fig. 1 D and E). These data together strongly suggest that SUMOylation promotes tau phosphorylation.
Tau Phosphorylation Stimulates Tau SUMOylation.
To explore whether tau phosphorylation reciprocally affects its SUMOylation, we induced tau hyperphosphorylation by treated the cells with okadaic acid (OA; 20 nM) to inhibit PP2A (28) [the crucial phosphatase involved in tau hyperphosphorylation (29)]. We found that inhibition of PP2A significantly increased the immunoreactivity of SUMO-1 that costained with the phosphorylated tau at ∼95-kDa bands highlighted with red frames (Fig. 2 A–C). Coimmunoprecipitation (co-IP) revealed a correlative tau hyperphosphorylation with an enhanced tau SUMOylation after OA treatment (Fig. 2D, bottom panel, third lane). Remarkably, mutation of tau at K340R abolished both hyperphosphorylation and SUMOylation of tau (Fig. 2 D and E). Thus, these data suggest that tau hyperphosphorylation stimulates its SUMOylation at K340.
Fig. 2.
Tau phosphorylation stimulates tau SUMOylation. (A–C) HEK293/tau cells were transfected with SUMO-1 for 24 h and then treated with 20 nM OA (inhibitor of PP2A) for 16 h before harvested for measurement of SUMOylation and phosphorylation of tau by Western blotting. For quantitative analyses, the bands around molecular mass of 55 kDa for tau protein and 95 kDa for both SUMO-1 and tau proteins were included. (D and E) HEK293/wild-type cells were transfected with wild-type tau (tau) or tau K340R plus eGFP–SUMO-1 or the vector for 24 h, and then the cells were treated with 20 nM OA for 16 h. The cell extracts were immunoprecipitated and analyzed by Western blotting using AT8 (to p-tau) and anti–SUMO-1 antibodies, reciprocally. *P < 0.05, **P < 0.01 vs. SUMO-1 in B and C, vs. tau in E; ##P < 0.01 vs. tau plus SUMO-1 in E.
Tau SUMOylation Decreases Solubility and the Degradation of Tau Proteins.
SUMOylation can stabilize the protein and thus promote aggregation (30–32). To assess the effect of SUMOylation on the solubility of tau, we transiently cotransfected the cells with SUMO-1 and tau or tau K340R, and analyzed the level of tau in sarkosyl-soluble and the insoluble fractions. Overexpression of SUMO-1 induced a remarkable shift of total tau and the phosphorylated tau at pThr231, pSer396, and pSer404 from sarkosyl-soluble to the insoluble fraction in the cells expressing wild-type tau, whereas coexpression of SUMO-1 did not increase the insoluble level of the K340R tau (Fig. 3 A and B). To better preserve the 95-kDa bands, which were vanished in sarkosyl-treated samples, we used a mild RIPA buffer to prepare the soluble and insoluble fractions (Materials and Methods). The results showed that expression of SUMO-1 significantly increased the levels of high molecular weight total tau and the phosphorylated tau (95-kDa) at Ser396 and Ser404 with much stronger signals in the insoluble fractions than the soluble ones (Fig. 3 C and D). These data suggest that SUMOylation decreases the solubility of tau.
Fig. 3.
Overexpression of SUMO-1 decreases solubility of tau and inhibits tau degradation. (A and B) HEK293 cells were transiently cotransfected with SUMO-1 and human tau or tau K340R for 48 h. After lysed with sarkosyl buffer, the sarkosyl-soluble and insoluble fractions were obtained by centrifugation as described in Materials and Methods, and then the levels of total tau (Tau5) and the phosphorylated tau at Thr231, Ser396, and Ser404 were detected by Western blotting and quantitative analysis. (C and D) HEK293 cells were transiently cotransfected with SUMO-1 or the vector and human tau for 48 h, and then the RIPA soluble and insoluble tau proteins isolated from the same SDS-PAGE gel were analyzed. (E and F) HEK293 cells were transiently cotransfected with SUMO-1 or the vector and wild-type human tau or tau K340R for 24 h, and then the cells were treated with 100 μg/mL CHX for another 0, 1, 3, 7, 12, and 24 h, respectively. The degradation of tau was detected by Western blotting and quantitative analysis. Tau5 reacts with total tau. The data were expressed as mean ± SD of three independent experiments. *P < 0.05, **P < 0.01 vs. vector plus tau, ##P < 0.01 vs. SUMO-1 plus tau.
To further verify the effect of tau SUMOylation on its degradation, we transiently transfected the cells with wild-type tau alone or cotransfected the wild-type tau with SUMO-1 for 24 h, and then treated the cells with cycloheximide (CHX), an inhibitor of protein biosynthesis, for different time points. We found that level of tau at 55 kDa was significantly decreased at 12 h and it was completely disappeared at 24 h in the cells expressing tau alone, suggesting a time-dependent degradation of tau proteins (Fig. 3 E and F). Cotransfection of SUMO-1 blocked tau degradation with a significant accumulation of the SUMOylated tau at 95 kDa, and the degradation of tau proteins was observed again when SUMO-1 was coexpressed with the tau K340R mutant (Fig. 3 E and F). These data strongly suggest that tau SUMOylation at K340 inhibits tau degradation.
SUMOylation Inhibits the Ubiquitination of Tau.
SUMO shares similarities with ubiquitin in both structure and the biochemistry for the substrates (26, 33). To verify the effect of SUMOylation on the ubiquitination of tau, we transfected HEK293/tau cells with SUMO-1 or the vector for 24 h, and then treated the cells with 100 μg/mL CHX for another 1 h. We found that level of total ubiquitinated proteins was significantly decreased in the cells expressing SUMO-1 (Fig. 4 A and B). By immunoprecipitation, we further confirmed that SUMOylation decreased ubiquitination of tau (Fig. 4 C and D). These data suggest that SUMOylation inhibits ubiquitination of the phosphorylated tau, which may cause tau aggregation.
Fig. 4.
Overexpression of SUMO-1 inhibits ubiquitination of the phosphorylated tau. HEK293/tau cells were transfected with SUMO-1 or the vector for 24 h and then treated with 100 μg/mL CHX for 1 h. The ubiquitination of total protein was analyzed by Western blotting (A and B), and ubiquitination of tau proteins was measured by immunoprecipitation using anti-tau antibody (Tau 5) and antiubiquitin antibody (C and D). **P < 0.01, vs. CHX in B, vs. vector in D.
SUMO-1 Immunoreactivity Is Colocalized with the Phosphorylated Tau in AD Brain.
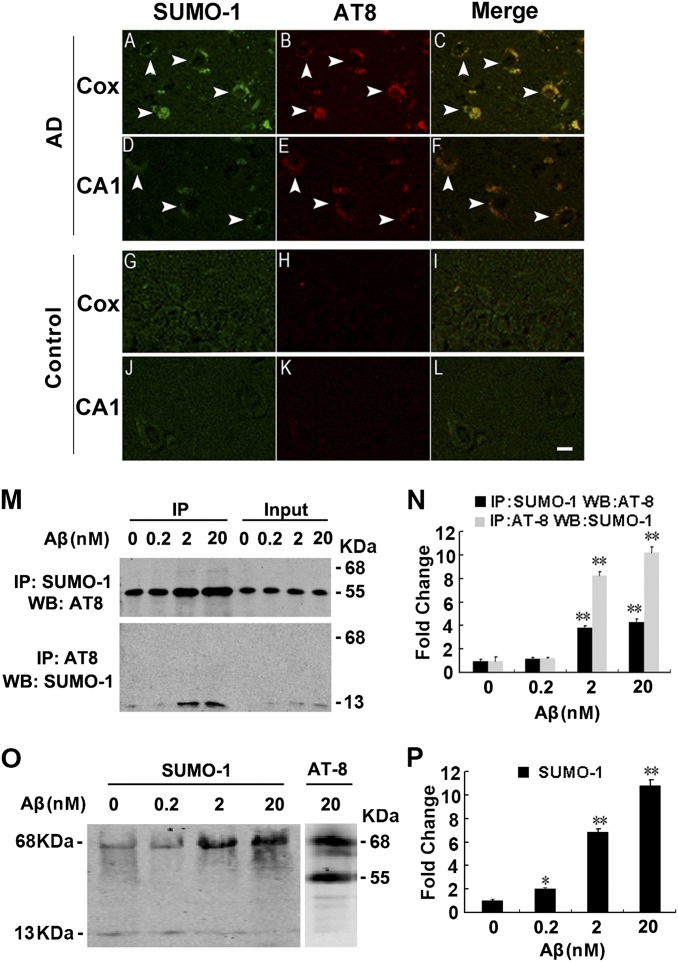
To verify the SUMOylation and its relationship with the phosphorylated tau in the AD brains, we performed a costaining using anti–SUMO-1 and AT8 antibody that reacts with the phosphorylated tau at Ser198/Ser199/Ser202/Thr205. Compared with the age-matched controls (Fig. 5 G–L), the significantly enhanced immunoreactivity of SUMO-1 (Fig. 5 A and D, arrowheads) and the phosphorylated tau (Fig. 5 B and E, arrowheads) were detected in the cortex and the hippocampal CA1 region of the AD brain, and the SUMO-1 immunoreactivity was colocalized with the phosphorylated tau (Fig. 5 C and F, arrowheads), suggesting that the hyperphosphorylated tau is SUMOylated in the AD brains.
Fig. 5.
Colocalization of SUMO-1 with the phosphorylated tau in the AD brains. (A–L) Double-staining of SUMO-1 and the phosphorylated tau (AT8) in the cerebral cortex (Cox) and hippocampal CA1 of the AD brains (A–F) and the age-matched controls (G–L). (Scale bar, 20 μm.) (M and N) Rat primary hippocampal neurons (14 DIV) were treated with 0, 0.2, 2, 20 nM of Aβ1–40 for 24 h. Co-IP)/Western blotting (WB) (M) and quantitative analyses (N) were performed by using AT8 and SUMO-1 antibodies (note that sample boiling required in the co-IP procedure causes dropout SUMO, therefore, only ∼13-kDa band of endogenous SUMO-1 was observed in M). (O and P) Rat primary hippocampal neurons (14 DIV) were treated as described above, then the samples were extracted with RIPA buffer without boiling for Western blotting. After separated by SDS/PAGE on the same gel, the membrane was cut into two and immunostained respectively with SUMO-1 and AT8 (note that the 68-kDa band stained by both SOMU-1 and AT8 represents the endogenously SUMOylated tau induced by Aβ exposure). *P < 0.05, **P < 0.01 vs. DMSO (0 nM Aβ).
β-Amyloid Exposure Increased SUMOylation of the Hyperphosphorylated Tau.
To explore the upstream factors that may cause tau SUMOylation, we treated the primary cultured rat hippocampal neurons (14 d in vitro, DIV) with different concentrations of β-amyloid (Aβ40) for 24 h. Coimmunoprecipitation assay showed that Aβ exposure induced a dose-dependent increase of tau phosphorylation with a correlated elevation of SUMOylation of endogenous tau (55 kDa tau + 13 kDa SUMO) (Fig. 5 M and N). Because SUMO seemed easily disconnected from endogenous tau proteins when the samples were boiled for dissociation of complex proteins from the coimmunoprecipitated beads, we could only detect the ∼13-kDa SUMO-1 band but not SUMO-conjugated tau, when AT8 antibody was used for immunoprecipitation (Fig. 5M, Lower). To further confirm tau SUMOylation, we did comparative Western blotting again with mild RIPA buffer without boiling of the samples. The results showed that Aβ exposure induced a dose-dependent elevation of SUMO-1 immunoreactive band at 68 kDa that was also stained with AT8 (Fig. 5 O and P). These data strongly suggest that Aβ may be upstream of the SUMOylation of the hyperphosphorylated tau, as seen in the neurons of the AD brain.
Discussion
Increasing studies show that a gradual impairment of ubiqutin-proteasomal system at later stages of AD is at least partially responsible for the reduced degradation of tau proteins (15–17). In the present study, we demonstrate: (i) that SUMOylation of tau increases its phosphorylation; on the other hand, tau phosphorylation promotes its SUMOylation; (ii) that SUMOylation competes against ubiquitination that may result in the reduced tau degradation; (iii) that SUMOylation enhances tau aggregation, all of which can induce the formation of neurofibrillary tangles as seen in the AD brains; (iv) that the SUMOylated tau is increased and colocalized in the AD brains, and Aβ treatment causes a dose-dependent SUMOylation of the abnormally hyperphosphorylated tau (Fig. S2). To our knowledge, this is the first report showing a reciprocal stimulation of tau SUMOylation and phosphorylation, and the effects of tau SUMOylation on its degradation/aggregation. Several types of posttranslational modifications of tau, including phosphorylation, glycosylation, glycation, prolyl-isomerization, truncation, nitration, polyamination, ubiquitination, SUMOylation, oxidation, and aggregation, with a particular interest toward their relevance in AD, have been reviewed (34), but the relationship between these posttranslational modifications has not been characterized. In the present study, we demonstrate that tau SUMOylation promotes its phosphorylation.
Currently, it is not fully understood how SUMOylation may affect phosphorylation of tau. We speculate that SUMOylation may alter the conformation of tau and thus render tau a better substrate of phosphorylation by some protein kinases. A previous study showed that SUMOylation of GSK-3β could induce phosphorylation of the kinase at Tyr216 (35), which activates the kinase, suggesting that SUMOylation of protein kinases or phosphatases may also contribute to the increased tau phosphorylation. To clarify this theory, we measured the activity of GSK-3β and PP2A, the most implicated protein kinase and protein phosphatase in tau phosphorylation (36–40). We found that overexpression of SUMO-1 could activate GSK-3β with no effect on PP2A activity, and activation of GSK-3β via using the mixture of wortmannin and GF-109203X (GFX) (41, 42) significantly enhanced immunoreactivity of SUMO-1 (Fig. S3), suggesting that GSK-3β activation may also contribute to the SUMO-1–induced tau hyperphosphorylation. We also found that tau hyperphosphorylation promoted tau SUMOylation in HEK293 cells with stable or transient expression of wild-type human tau40. A previous study also demonstrated that treatment of the cells with protein phosphatase inhibitor (OA) could stimulate tau SUMOylation (9). Together, these data suggest that tau SUMOylation reciprocally stimulates tau phosphorylation.
Given that tau is hyperphosphorylated in AD patients (43) and hyperphosphorylation of tau promotes its SUMOylation as observed in the current study, we wonder that the hyperphosphorylated tau might be SUMOylated in the AD brains. Conflict results were observed regarding SUMOylation of the phosphorylated tau/NFTs: Pountney et al. reported that NFTs were not observed by SUMO-1 immunostaining (44), whereas Dorval’s and Takahashi’s studies showed that SUMO-1 immunoreactivity was colocalized with the phosphorylated tau in APP transgenic mice (9, 11). By using an optimized immunohistochemical condition (Materials and Methods), we observed in the present study that the enhanced SUMO-1 immunoreactivity was colocalized with the hyperphosphorylated tau in the AD brains (Fig. 5), which supports SUMOylation of the hyperphosphorylated tau.
Aberrant accumulation of the hyperphosphorylated tau as neurofibrillary tangles in the AD brains is implicated in the pathogenesis of the disease (45, 46). The mechanism underlying the shift of soluble tau to insoluble tau is still not clarified. It is well known that tau hyperphosphorylation promotes tau aggregation. Other posttranslational modifications could also contribute to aggregation; for example, tau acetylation has been reported to be associated with insoluble, thioflavin-positive neurofibrillary tangles in tau transgenic mouse models (47). We demonstrate here that tau SUMOylation reduced the solubility of both total and the phosphorylated tau proteins. With β-sheet structure, soluble tau tends to form tau aggregates that are insoluble in sarkosyl solution (48, 49). However, a SUMO-binding motif forms an extended structure that binds between the α-helix and β-strand of SUMO-1 (50), implying that the alteration of tau conformation by SUMOylation may not explain the increased insolubility of tau here. A recent study suggests that monomeric- and poly-SUMOylated proteins represent different solubility on poly-SUMOylation chains that are assembled with alternative linkages (51). Dorval et al. reported that colchicine-induced microtubule depolymerization could markedly increase tau SUMOylation, which suggests that once released from the microtubules, the soluble tau is free to interact with SUMO enzymes and undergo covalent modifications (9). Because tau SUMOylation reciprocally stimulates tau phosphorylation, and it is well known that hyperphosphorylation decreases the solubility of tau; therefore, the poly-SUMOylation at least contributes to the reduced solubility of tau.
SUMO displays similarities to ubiquitin in both structure and the biochemistry of the conjugation with their substrate proteins (26, 30). Lysine residues are common targets for ubiquitination and SUMOylation. Previous studies show that SUMO-1 modification of Mdm2 and IκBα prevents their self-ubiquitination (52) and are resistant to proteasome-mediated degradation (53). Therefore, SUMOylation may counteract protein ubiquitination. In the current study we provide direct evidence demonstrating that tau SUMOylation obstructs its ubiquitination that can results in decreased tau degradation and an increased tau aggregation, as observed in the AD brains. Because tau phosphorylation promotes conversely its SUMOylation and the latter competes for ubiquitination, therefore, the inhibited ubiquitination of tau may be dependent on its phosphorylation. A previous study shows that ubiquitination of IkBa is dependent on its prior phosphorylation (54), which supports our speculation.
Taking these data together, we have found in the present study that tau SUMOylation promotes reciprocally its hyperphosphorylation, which results in reduced tau ubiquitination and degradation with an increased tau aggregation.
Materials and Methods
Plasmids, Chemicals, and Antibodies.
The plasmids encoding eGFP-tagged full-length SUMO-1 were kindly provided by Guanghui Wang (Soochow University, Suzhou, China). Mutation of tau lysine 340 into arginine (K340R) was carried out using the QuikChange site-directed mutagenesis kit by following the manufacturer’s instructions (GeneChem). The mutant was generated by PCR and cloned in a His-tagged pcDNA3.1 (-) vector in XhoI and KpnI restriction sites.
CHX and OA were purchased from Merck Chemical. Bicinchoninic acid protein detection kit was from Pierce. Reagents for cell culture were from Gibco. Antibodies used in this study are listed in Table S1.
Cell Culture, Transfection, and Drug Treatment.
The HEK293 cells were cultured in DMEM supplemented with 10% (vol/vol) FBS (Gibco) and grown at 37 °C in a humid atmosphere containing 5% (vol/vol) CO2. Transfection of the human tau441 cDNA in HEK293 cells was carried out with Lipofectamine 2000 transfection kit according to the manufacturer’s instructions, and the cells with stable expression of tau (HEK293/tau) cells were selected, cloned by dilution, and maintained in the presence of G418 (250 μg/mL). The expression of tau was identified by Western blot.
For the transfection of pEGFP-C2–SUMO-1 or its empty vector pEGFP-C2 in HEK293/tau or wild-type HEK293 cells, lipofectamine 2000 kit (Invitrogen; 10 μL) and SUMO-1 DNA plasmid or its vector pEGFP-C2 (2.5 μg) were mixed in 200 μL of OPTI-MEM followed by equilibration at 15–25 °C for 10 min, and the lipofectamine-DNA complex was added to the cells at 37 °C for further treatment. For double-transfection and the protein degradation assay, the SUMO-1 and tau or tau K340R plasmids were mixed with the ratio of 1:1. The medium was replaced 6 h after the transfection and the cells were incubated for an additional 18 h. Then the cells were treated with 100 μg/mL CHX for different times and the degradation of tau was identified by Western blotting.
To explore the effect of tau phosphorylation on its SUMOylation, the HEK293 cells with expression of tau or K340R tau were treated with 20 nM OA to inhibit PP2A for 16 h. To inhibit SUMOylation of tau, the HEK293/tau cells transfected with SUMO-1 plasmids for 48 h were incubated with ginkgolic acid for 2 h.
Primary Cultures of Hippocampal Neurons and Aβ Treatment.
Primary cultures of rat hippocampal neurons were prepared from E18 Sprague–Dawley rat embryos. Briefly, hippocampi were dissected in D-Hanks supplemented with glucose (18 mM). Then mechanically dissociated in minimum essential medium (MEM) and seeded onto poly-l-lysine (25 μg/mL) coated six-well plates at a density of 8 × 104 cells per well in neurobasal medium containing 2% (vol/vol) B-27, glutamax (2 mM), penicillin (50 U/mL), and streptomycin (50 μg/mL) (Gibco). Hippocampal neurons were cultured for 14 d at 37 °C in a humidified 5% (vol/vol) CO2 incubator before treatment.
Aβ1–40 peptide (64130-05, AnaSpec) was resuspended with anhydrous DMSO (Sigma) at 40 μM as final concentration. Aliquot Aβ peptide was stored at −80 °C in a deep freezer before use. To investigate the neurotoxicity of Aβ on primary cultures of hippocampal neurons, Aβ was added to the cell culture at 14 DIV for 24 h. Then the samples were subjected to immunoprecipitation and Western blotting.
Other Methods.
See SI Materials and Methods for animal housing and hippocampal injection, sample preparation and Western blotting, immunoprecipitation, immunohistochemistry, and confocal microscopy.
Statistical Analysis.
Data were analyzed using SPSS 18.0 statistical software. The one-way ANOVA procedure followed by least-square difference’s post hoc tests was used to determine the statistical significance of differences of the means. For a single comparison, the significance of differences between means was determined by the t test. P < 0.05 was accepted as statistically significant.
Supplementary Material
Acknowledgments
We thank Dr. Inge Grundke-Iqbal and Khalid Iqbal (New York State Institute for Basic Research) for R134d antibody and the Alzheimer’s disease brain sections, and Dr. Guanghui Wang (Soochow University) for the small ubiquitin-like modifier protein 1 plasmid. This work was supported in part by Grants 81271405 and 91132305 from the National Natural Science Foundation of China, and Grant 2013DFG32670 from Ministry of Science and Technology of the People's Republic of China.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1417548111/-/DCSupplemental.
References
- 1.Iqbal K, et al. Defective brain microtubule assembly in Alzheimer’s disease. Lancet. 1986;2(8504):421–426. doi: 10.1016/s0140-6736(86)92134-3. [DOI] [PubMed] [Google Scholar]
- 2.Koh JY, Yang LL, Cotman CW. Beta-amyloid protein increases the vulnerability of cultured cortical neurons to excitotoxic damage. Brain Res. 1990;533(2):315–320. doi: 10.1016/0006-8993(90)91355-k. [DOI] [PubMed] [Google Scholar]
- 3.Selkoe DJ. Alzheimer’s disease: A central role for amyloid. J Neuropathol Exp Neurol. 1994;53(5):438–447. doi: 10.1097/00005072-199409000-00003. [DOI] [PubMed] [Google Scholar]
- 4.Iqbal K, Alonso AdelC, Grundke-Iqbal I. Cytosolic abnormally hyperphosphorylated tau but not paired helical filaments sequester normal MAPs and inhibit microtubule assembly. J Alzheimers Dis. 2008;14(4):365–370. doi: 10.3233/jad-2008-14402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iqbal K, Grundke-Iqbal I. Ubiquitination and abnormal phosphorylation of paired helical filaments in Alzheimer’s disease. Mol Neurobiol. 1991;5(2-4):399–410. doi: 10.1007/BF02935561. [DOI] [PubMed] [Google Scholar]
- 6.Iqbal K, Grundke-Iqbal I. Alzheimer neurofibrillary degeneration: Significance, etiopathogenesis, therapeutics and prevention. J Cell Mol Med. 2008;12(1):38–55. doi: 10.1111/j.1582-4934.2008.00225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alonso AC, Zaidi T, Grundke-Iqbal I, Iqbal K. Role of abnormally phosphorylated tau in the breakdown of microtubules in Alzheimer disease. Proc Natl Acad Sci USA. 1994;91(12):5562–5566. doi: 10.1073/pnas.91.12.5562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alonso AC, Grundke-Iqbal I, Iqbal K. Alzheimer’s disease hyperphosphorylated tau sequesters normal tau into tangles of filaments and disassembles microtubules. Nat Med. 1996;2(7):783–787. doi: 10.1038/nm0796-783. [DOI] [PubMed] [Google Scholar]
- 9.Dorval V, Fraser PE. Small ubiquitin-like modifier (SUMO) modification of natively unfolded proteins tau and alpha-synuclein. J Biol Chem. 2006;281(15):9919–9924. doi: 10.1074/jbc.M510127200. [DOI] [PubMed] [Google Scholar]
- 10.Dorval V, Fraser PE. SUMO on the road to neurodegeneration. Biochim Biophys Acta. 2007;1773(6):694–706. doi: 10.1016/j.bbamcr.2007.03.017. [DOI] [PubMed] [Google Scholar]
- 11.Takahashi K, Ishida M, Komano H, Takahashi H. SUMO-1 immunoreactivity co-localizes with phospho-Tau in APP transgenic mice but not in mutant Tau transgenic mice. Neurosci Lett. 2008;441(1):90–93. doi: 10.1016/j.neulet.2008.06.012. [DOI] [PubMed] [Google Scholar]
- 12.Köpke E, et al. Microtubule-associated protein tau. Abnormal phosphorylation of a non-paired helical filament pool in Alzheimer disease. J Biol Chem. 1993;268(32):24374–24384. [PubMed] [Google Scholar]
- 13.Dolan PJ, Johnson GV. A caspase cleaved form of tau is preferentially degraded through the autophagy pathway. J Biol Chem. 2010;285(29):21978–21987. doi: 10.1074/jbc.M110.110940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hamano T, et al. Autophagic-lysosomal perturbation enhances tau aggregation in transfectants with induced wild-type tau expression. Eur J Neurosci. 2008;27(5):1119–1130. doi: 10.1111/j.1460-9568.2008.06084.x. [DOI] [PubMed] [Google Scholar]
- 15.Bednarski E, Lynch G. Cytosolic proteolysis of tau by cathepsin D in hippocampus following suppression of cathepsins B and L. J Neurochem. 1996;67(5):1846–1855. doi: 10.1046/j.1471-4159.1996.67051846.x. [DOI] [PubMed] [Google Scholar]
- 16.Grune T, et al. Tau protein degradation is catalyzed by the ATP/ubiquitin-independent 20S proteasome under normal cell conditions. Arch Biochem Biophys. 2010;500(2):181–188. doi: 10.1016/j.abb.2010.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Petrucelli L, et al. CHIP and Hsp70 regulate tau ubiquitination, degradation and aggregation. Hum Mol Genet. 2004;13(7):703–714. doi: 10.1093/hmg/ddh083. [DOI] [PubMed] [Google Scholar]
- 18.Butler D, Nixon RA, Bahr BA. Potential compensatory responses through autophagic/lysosomal pathways in neurodegenerative diseases. Autophagy. 2006;2(3):234–237. doi: 10.4161/auto.2729. [DOI] [PubMed] [Google Scholar]
- 19.Nixon RA, Cataldo AM, Mathews PM. The endosomal-lysosomal system of neurons in Alzheimer’s disease pathogenesis: A review. Neurochem Res. 2000;25(9-10):1161–1172. doi: 10.1023/a:1007675508413. [DOI] [PubMed] [Google Scholar]
- 20.Nixon RA, et al. Extensive involvement of autophagy in Alzheimer disease: An immuno-electron microscopy study. J Neuropathol Exp Neurol. 2005;64(2):113–122. doi: 10.1093/jnen/64.2.113. [DOI] [PubMed] [Google Scholar]
- 21.Grundke-Iqbal I, et al. Microtubule-associated polypeptides tau are altered in Alzheimer paired helical filaments. Brain Res. 1988;464(1):43–52. doi: 10.1016/0169-328x(88)90017-4. [DOI] [PubMed] [Google Scholar]
- 22.Mori H, Kondo J, Ihara Y. Ubiquitin is a component of paired helical filaments in Alzheimer’s disease. Science. 1987;235(4796):1641–1644. doi: 10.1126/science.3029875. [DOI] [PubMed] [Google Scholar]
- 23.Perry G, Friedman R, Shaw G, Chau V. Ubiquitin is detected in neurofibrillary tangles and senile plaque neurites of Alzheimer disease brains. Proc Natl Acad Sci USA. 1987;84(9):3033–3036. doi: 10.1073/pnas.84.9.3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hershko A, Eytan E, Ciechanover A, Haas AL. Immunochemical analysis of the turnover of ubiquitin-protein conjugates in intact cells. Relationship to the breakdown of abnormal proteins. J Biol Chem. 1982;257(23):13964–13970. [PubMed] [Google Scholar]
- 25.Wang GP, Grundke-Iqbal I, Kascsak RJ, Iqbal K, Wisniewski HM. Alzheimer neurofibrillary tangles: monoclonal antibodies to inherent antigen(s) Acta Neuropathol. 1984;62(4):268–275. doi: 10.1007/BF00687608. [DOI] [PubMed] [Google Scholar]
- 26.Hay RT. SUMO: A history of modification. Mol Cell. 2005;18(1):1–12. doi: 10.1016/j.molcel.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 27.Fukuda I, et al. Ginkgolic acid inhibits protein SUMOylation by blocking formation of the E1-SUMO intermediate. Chem Biol. 2009;16(2):133–140. doi: 10.1016/j.chembiol.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 28.Gong CX, et al. Phosphorylation of microtubule-associated protein tau is regulated by protein phosphatase 2A in mammalian brain. Implications for neurofibrillary degeneration in Alzheimer’s disease. J Biol Chem. 2000;275(8):5535–5544. doi: 10.1074/jbc.275.8.5535. [DOI] [PubMed] [Google Scholar]
- 29.Wang JZ, Liu F. Microtubule-associated protein tau in development, degeneration and protection of neurons. Prog Neurobiol. 2008;85(2):148–175. doi: 10.1016/j.pneurobio.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 30.Glickman MH, Ciechanover A. The ubiquitin-proteasome proteolytic pathway: Destruction for the sake of construction. Physiol Rev. 2002;82(2):373–428. doi: 10.1152/physrev.00027.2001. [DOI] [PubMed] [Google Scholar]
- 31.Jentsch S, Pyrowolakis G. Ubiquitin and its kin: How close are the family ties? Trends Cell Biol. 2000;10(8):335–342. doi: 10.1016/s0962-8924(00)01785-2. [DOI] [PubMed] [Google Scholar]
- 32.Kimura T, et al. Aggregation of detergent-insoluble tau is involved in neuronal loss but not in synaptic loss. J Biol Chem. 2010;285(49):38692–38699. doi: 10.1074/jbc.M110.136630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dohmen RJ. SUMO protein modification. Biochim Biophys Acta. 2004;1695(1-3):113–131. doi: 10.1016/j.bbamcr.2004.09.021. [DOI] [PubMed] [Google Scholar]
- 34.Martin L, Latypova X, Terro F. Post-translational modifications of tau protein: Implications for Alzheimer’s disease. Neurochem Int. 2011;58(4):458–471. doi: 10.1016/j.neuint.2010.12.023. [DOI] [PubMed] [Google Scholar]
- 35.Eun Jeoung L, et al. Regulation of glycogen synthase kinase 3beta functions by modification of the small ubiquitin-like modifier. Open Biochem J. 2008;2:67–76. doi: 10.2174/1874091X00802010067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gong CX, Iqbal K. Hyperphosphorylation of microtubule-associated protein tau: A promising therapeutic target for Alzheimer disease. Curr Med Chem. 2008;15(23):2321–2328. doi: 10.2174/092986708785909111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mattson MP. Neuronal death and GSK-3beta: A tau fetish? Trends Neurosci. 2001;24(5):255–256. doi: 10.1016/s0166-2236(00)01838-5. [DOI] [PubMed] [Google Scholar]
- 38.Takashima A. GSK-3 is essential in the pathogenesis of Alzheimer’s disease. J Alzheimers Dis. 2006;9(3, suppl):309–317. doi: 10.3233/jad-2006-9s335. [DOI] [PubMed] [Google Scholar]
- 39.Voronkov M, Braithwaite SP, Stock JB. Phosphoprotein phosphatase 2A: A novel druggable target for Alzheimer’s disease. Future Med Chem. 2011;3(7):821–833. doi: 10.4155/fmc.11.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang JZ, Grundke-Iqbal I, Iqbal K. Kinases and phosphatases and tau sites involved in Alzheimer neurofibrillary degeneration. Eur J Neurosci. 2007;25(1):59–68. doi: 10.1111/j.1460-9568.2006.05226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peng JH, et al. Dehydroevodiamine attenuates tau hyperphosphorylation and spatial memory deficit induced by activation of glycogen synthase kinase-3 in rats. Neuropharmacology. 2007;52(7):1521–1527. doi: 10.1016/j.neuropharm.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 42.Zhu LQ, et al. Activation of glycogen synthase kinase-3 inhibits long-term potentiation with synapse-associated impairments. J Neurosci. 2007;27(45):12211–12220. doi: 10.1523/JNEUROSCI.3321-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Geschwind DH. Tau phosphorylation, tangles, and neurodegeneration: The chicken or the egg? Neuron. 2003;40(3):457–460. doi: 10.1016/s0896-6273(03)00681-0. [DOI] [PubMed] [Google Scholar]
- 44.Pountney DL, et al. SUMO-1 marks the nuclear inclusions in familial neuronal intranuclear inclusion disease. Exp Neurol. 2003;184(1):436–446. doi: 10.1016/j.expneurol.2003.07.004. [DOI] [PubMed] [Google Scholar]
- 45.Grundke-Iqbal I, et al. Abnormal phosphorylation of the microtubule-associated protein tau (tau) in Alzheimer cytoskeletal pathology. Proc Natl Acad Sci USA. 1986;83(13):4913–4917. doi: 10.1073/pnas.83.13.4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee VM, Goedert M, Trojanowski JQ. Neurodegenerative tauopathies. Annu Rev Neurosci. 2001;24:1121–1159. doi: 10.1146/annurev.neuro.24.1.1121. [DOI] [PubMed] [Google Scholar]
- 47.Cohen TJ, et al. The acetylation of tau inhibits its function and promotes pathological tau aggregation. Nat Commun. 2011;2:252. doi: 10.1038/ncomms1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kimura T, et al. GSK-3beta is required for memory reconsolidation in adult brain. PLoS ONE. 2008;3(10):e3540. doi: 10.1371/journal.pone.0003540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Maeda S, et al. Granular tau oligomers as intermediates of tau filaments. Biochemistry. 2007;46(12):3856–3861. doi: 10.1021/bi061359o. [DOI] [PubMed] [Google Scholar]
- 50.Song J, Zhang Z, Hu W, Chen Y. Small ubiquitin-like modifier (SUMO) recognition of a SUMO binding motif: A reversal of the bound orientation. J Biol Chem. 2005;280(48):40122–40129. doi: 10.1074/jbc.M507059200. [DOI] [PubMed] [Google Scholar]
- 51.Mullen JR, Das M, Brill SJ. Genetic evidence that polysumoylation bypasses the need for a SUMO-targeted Ub ligase. Genetics. 2011;187(1):73–87. doi: 10.1534/genetics.110.124347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Buschmann T, Fuchs SY, Lee CG, Pan ZQ, Ronai Z. 2000. SUMO-1 modification of Mdm2 prevents its self-ubiquitination and increases Mdm2 ability to ubiquitinate p53. Cell 101(7):753–762. Retraction in Fuchs SY, Lee CG, Pan ZQ, Ronai Z (2002) Cell 110(4):531. [DOI] [PubMed]
- 53.Desterro JM, Rodriguez MS, Hay RT. SUMO-1 modification of IkappaBalpha inhibits NF-kappaB activation. Mol Cell. 1998;2(2):233–239. doi: 10.1016/s1097-2765(00)80133-1. [DOI] [PubMed] [Google Scholar]
- 54.Ulrich HD. Mutual interactions between the SUMO and ubiquitin systems: A plea of no contest. Trends Cell Biol. 2005;15(10):525–532. doi: 10.1016/j.tcb.2005.08.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.